Abstract
Background
Mast cells serve an important sentinel function at mucosal barriers and have been implicated as key early inducers of Type 2 immune responses in food allergy. The generation of Th2 and IgE following food allergen ingestion is inhibited in the absence of mast cells. Group 2 innate lymphoid cells are also thought to play an important early role in nascent allergic responses.
Objective
To test whether IgE-mediated mast cell activation promotes intestinal ILC2 responses following ingestion of food allergens and whether ILC2 amplify food allergy.
Methods
Two different mouse models of food allergy, one using intraperitoneally ovalbumin (OVA) primed BALB/c animals and the other using enterally peanut-sensitized inherently atopic IL4raF709 mice, were applied to test the contributions of IgE antibodies and mast cells to ILC2 responses. The effect of ILC2 on mast cell activation and on anaphylaxis was tested.
Results
ILC2 responses were significantly impaired in both models of food allergy in Igh7−/− mice harboring a targeted deletion of the gene encoding IgE. A similar reduction in food allergen-induced ILC2 was observed in mast cell deficient Il4raF709 KitW-sh mice and this was partially corrected by reconstituting these animals using cultured bone marrow mast cells. Mast cells activated ILC2 for IL-13 production in an IL-4Rα-dependent manner. Activated ILC2 amplified systemic anaphylaxis by increasing target tissue sensitivity to mast cell mediators.
Conclusions & clinical relevance
These findings support an important role for IgE-activated mast cells in driving intestinal ILC2 expansion in food allergy and reveal that ILC2, in turn, can enhance responsiveness to the mediators of anaphylaxis produced by mast cells. Strategies designed to inhibit IgE signaling or mast cell activation are likely to inhibit both Type 2 immunity and immediate hypersensitivity in food allergy.
Keywords: mast cell, IgE, ILC2, food allergy
Introduction
Food allergy is a currently intractable disease in which affected individuals exhibit dysregulated immunological responses to normally innocuous ingested protein allergens, resulting in sensitization with the production of allergen-specific IgE antibodies. In its most severe form, the resultant IgE-mediated hypersensitivity activated upon subsequent food ingestion can result in life-threatening anaphylactic shock, and food allergy is thus responsible for a substantial fraction of emergency hospital visits [1–4]. Incidence of the disease has risen dramatically in the last couple of decades, and current estimates suggest that around 6–8% of children are sensitized to one or more foods in westernized nations [5]. A recent study in JAMA Pediatrics calculated the economic burden of food allergy at around $25 billion a year, most of which is due to indirect costs and changes in lifestyle rather than direct medical care [6]. The need for constant vigilance against allergen exposure in the course of everyday life along with the ever present fear of reaction are sources of significant anxiety [7].
The factors predisposing some individuals to the development of anaphylactic sensitivity to food allergens have not been fully elucidated. It is known that mast cells and basophils promote the induction of pro-allergic adaptive immune responses by providing cytokines, including IL-4 and IL-9, that drive Th2 expansion and inhibit the generation of regulatory T (Treg) cells in the intestinal mucosa [8–11]. This immunological environment is conducive to the production of food-specific IgE antibodies that then bind to tissue mast cells via the high-affinity IgE receptor, FcεRI, and lead to activation following re-exposure to allergens. Activated mast cells release preformed and newly synthesized vasoactive amines and lipid mediators that act on vascular endothelium and a number of other target tissues to cause anaphylaxis [12]. Although the presence of food-specific IgE antibodies is required to trigger this reaction, there is a poor correlation between IgE levels and severity of anaphylaxis. For instance, some individuals testing positive for IgE will pass oral food challenges while others with similar IgE levels will develop severe reactions [5]. A number of other factors affecting mast cell homeostasis and triggering threshold or the sensitivity of target tissues to the mediators of anaphylaxis are likely to regulate the severity of reactions.
The contributions of other intestinal innate immune cells to allergic reactions to foods have not been fully explored. The presence of type 2 innate lymphoid cells (ILC2) at intestinal mucosal surfaces as well as their capacity to produce significant amounts of IL-4 and IL-13 implicates them as potential collaborators of mast cells in the sensitization and effector phases of allergic responses. ILC2 are rare lymphocytes that develop from common lymphoid progenitors in an Id2 and Rora-dependent manner [13–16]. They are identified by the absence of markers that define other leukocyte lineages combined with expression of the transcription factor GATA-3 or surface markers such as Thy1 (CD90), the IL-2 receptor CD25, the IL-7 receptor CD127, the IL-33 receptor ST2, the IL-25 receptor IL-17RB, ICOS and Sca-1 [15]. Since being described in 2010, ILC2 have been implicated in the pathology of allergic diseases as well as productive immunity against helminth parasites [17–25]. The exceptionally high level of IL-13 secreted by ILC2 leads to subepithelial collagen deposition and airway hyperreactivity in murine models of asthma, and to worm expulsion through goblet cell hyperplasia and smooth muscle contraction in parasite-infested mice [26]. ILC2 and mast cells exhibit coordinate increases during allergic responses (e.g., in the asthmatic lung and in the skin in atopic dermatitis) and both are found basally at relatively high frequencies in the lamina propria of the small intestine [18, 20, 27]. Recent studies have demonstrated that ILC2 and ILC2-associated cytokines favor allergic sensitization to foods [24, 25, 28–30]. As mast cells serve as innate mucosal sentinels for allergens and danger signals and have been shown to exhibit marked IgE-dependent upregulation of ILC2-inducing cytokines, we hypothesized that IgE and mast cells might directly promote ILC2 accumulation.
In this report, using two models of food allergy, we found that intestinal ILC2 increased in response to sensitization in an IgE-dependent manner. In inherently atopic IL4raF709 mice, peanut ingestion resulted in over-representation of ILC2, which was reduced in mast cell-deficient mice. In a cell culture system, IgE-activated mast cells induced the secretion of IL-13 by ILC2. Adoptive transfer experiments demonstrated that ILC2-derived IL-13 enhanced sensitivity to mast cell mediators, thereby complementing the effects of activated mast cells in IgE-mediated anaphylactic shock.
Results
ILC2 exacerbate allergic sensitization to foods in murine models, but the mechanisms driving their expansion remain unclear. Recent reports have demonstrated the importance of IL-25 and IL-33 [24, 29, 31, 32]. While these are both generally considered to be epithelial-derived cytokines [30], several groups have shown that they can be produced at high levels by hematopoietic cells, including mast cells [33, 34]. Furthermore, additional cytokines including IL-2, IL-4 and IL-7 stimulate ILC2 growth and cytokine production, raising the possibility that food allergen-driven ILC2 activation may not be exclusively a response to damage of the epithelial barrier. We have demonstrated that IgE, mast cells and ILC2 are all important for achieving robust Th2 responses and IgE production in response to ingested food allergen [8, 9, 24]. We therefore hypothesized that IgE-mediated mast cell activation, might drive expansion of or activate cytokine production by intestinal ILC2 in murine models of food allergy.
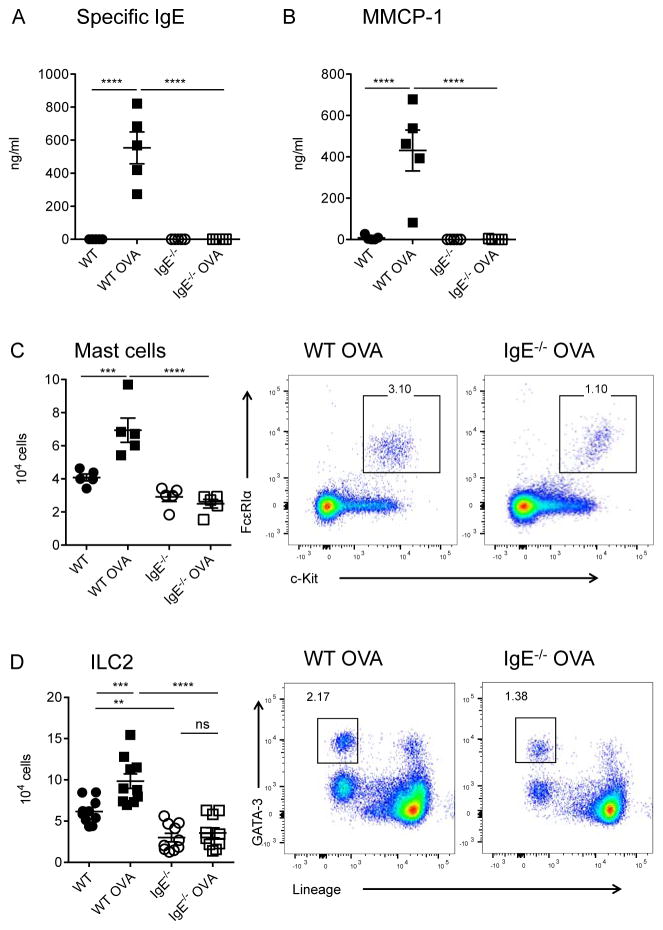
We first interrogated the impact of allergen sensitization on ILC2 in the “intestinal anaphylaxis” model developed by Rothenberg and colleagues, which is both IgE and mast cell-dependent [35]. After intraperitoneal immunization with ovalbumin (OVA) adsorbed to alum and repeated intragastric OVA feedings, WT but not IgE-deficient mice developed robust serum levels of OVA-specific IgE (Figure 1A). Enteral challenge with OVA provoked dramatic IgE-mediated increases in the mast cell granule protease, mouse mast cell protease-1 (MMCP-1) in the serum of sensitized mice (Figure 1B). Flow cytometric analysis of jejunal lamina propria leukocytes revealed a significant expansion of c-Kit+FcεRIα+ mast cells in WT but not IgE−/− mice (Figure 1C). We are aware that this finding contrasts with previous reports using the same model with FcεRIα−/− mice, a difference that may be related to the technique used to measure mast cells. We used flow cytometry rather than histology to quantify intestinal mast cell because flow cytometry is an inherently quantitative technique capable of assessing the entire tissue and does not fail to detect mast cells following IgE-dependent loss of granule contents, which can artificially reduce apparent mast cell levels observed by histology following anaphylaxis [9, 35–37]. It is also possible that alterations in microbial colonization or immunological differences between FcεRIα−/− and IgE−/− mice, including CD23 feedback, could indirectly influence mechanisms of allergen-induced mast cell expansion, giving rise to disparate results in the different experimental systems. In some settings, the primary allergenic stimulus, in the context of a particular microbial environment might be so strong that it overwhelms the amplifying effects of IgE. Our results show that, under certain defined conditions, IgE can play an important role in amplifying mast cell expansion.
Figure 1.
Requirement for IgE in expansion of intestinal mast cells and ILC2 in OVA-immunized mice on the BALB/c background. A) Serum OVA-specific IgE levels. B) Serum MMCP-1 post-challenge. C) Jejunal mast cells: summary plot and representative flow diagrams. D) Jejunal ILC2: summary plot and representative flow diagrams. Statistical analysis by two-way ANOVA with Sidak’s post-test. Summary data are from one of two independent experiments (A–C) or pooled from both experiments (D).
We identified ILC2 on the basis of the absence of lineage (Lin)− markers combined with the presence of intracellular GATA-3+ (Supplementary Figure 1). ILC2 also displayed a largely IgE-dependent expansion in food allergic mice (Figure 1D). We also consistently observed non-significant trends for ILC2 expansion in food allergic IgE−/− mice, suggesting additional mechanisms, such as a contribution from epithelial or tuft cells. Interestingly, intestinal ILC2 numbers were reduced at baseline in IgE−/− mice relative to WT controls (Figure 1D). Since ILC2 can promote mast cell growth and activation, lowered steady state ILC2 numbers in IgE−/− mice may contribute to the lack of mast cell expansion in these mice.
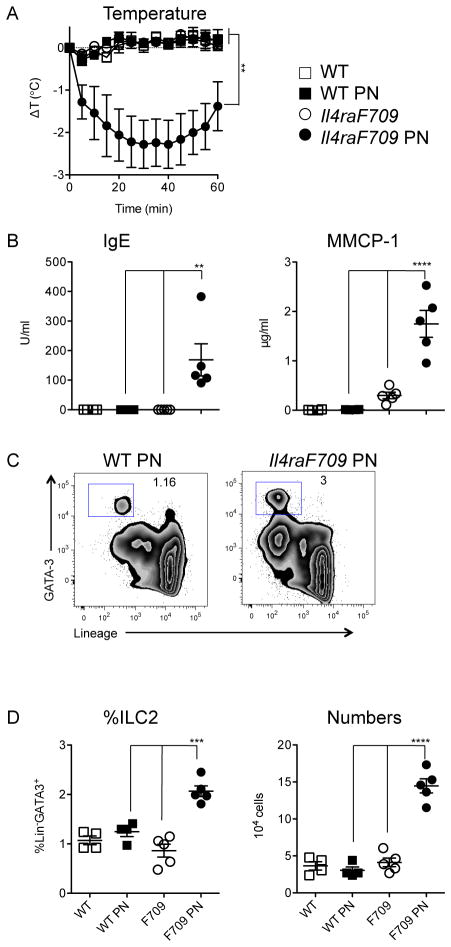
While the intestinal anaphylaxis model provided evidence that IgE was required for ILC2 expansion in food allergy, we reasoned that it would be important to corroborate the result using an approach in which sensitization could be achieved by the more physiologic route of ingestion. We therefore investigated the impact of peanut gavage on ILC2 homeostasis in Il4raF709 mice, rendered inherently atopic by knock-in of an unconstrained form (lacking the ITIM motif) of the IL-4Rα chain. We previously reported that dramatic intestinal mast cell expansion and anaphylactic sensitivity to foods can be induced by repeated enteral exposure to allergen in the absence of adjuvant [36]. Following peanut feeding, allergy-prone Il4raF709 mice, but not tolerant WT controls, developed hypersensitivity to peanut with increased levels of peanut-specific IgE along with challenge-induced hypothermia, a sensitive indicator of anaphylaxis in mice (Figure 2A, B). Elevated serum levels of MMCP-1 were evident in reacting mice consistent with mast cell degranulation (Figure 2B). Peanut-sensitized Il4raF709 mice exhibited increased frequencies (roughly double) and total numbers (approximately three-fold) of ILC2 in the small intestine (Figure 2C, D). Compared to IgE-sufficient animals, IgE-deficient Il4raF709 mice exhibited reduced frequencies and numbers of ILC2 following peanut feeding (Figure 3).
Figure 2.
Peanut ingestion and its effects on ILC2 in food allergy-prone Il4raF709 mice on the BALB/c background. A) Anaphylaxis following enteral PN sensitization and challenge. B) Serum PN-specific IgE and MMCP-1. C) Representative flow plots showing jejunal ILC2 (Lin−GATA-3+) frequencies. D) Summary plots showing ILC2 frequency and numbers. Data are from one of three independent experiments. Statistical analysis by repeated measures two-way ANOVA (A) and two-way ANOVA with Sidak’s post-tests (B, D).
Figure 3.
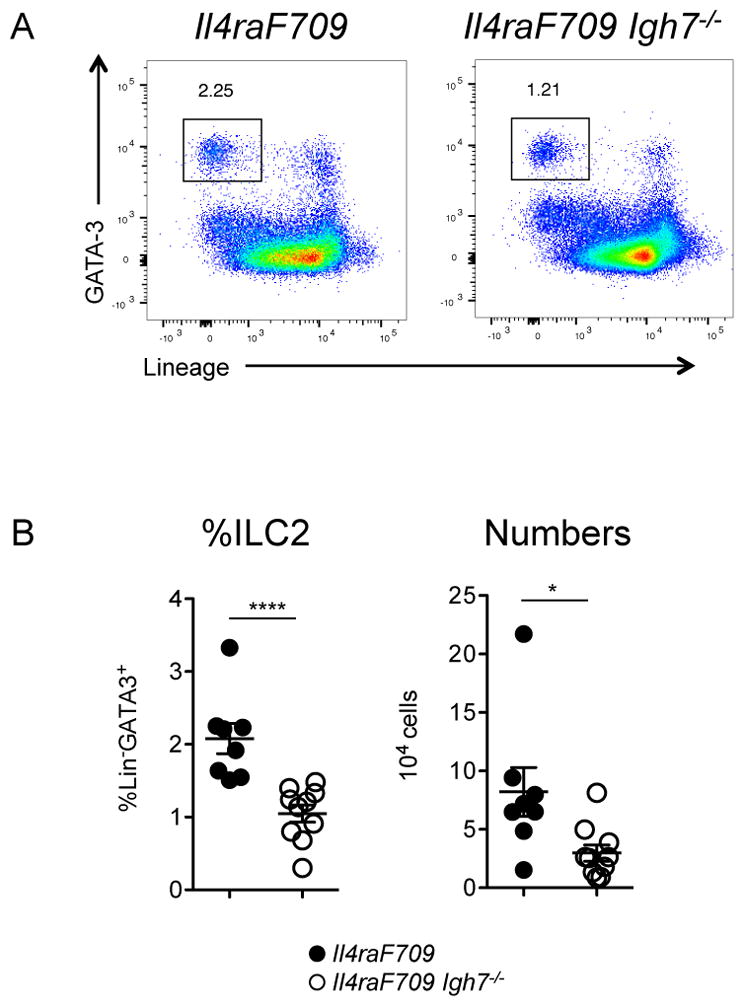
Requirement for IgE in intestinal ILC2 expansion. A) Representative flow cytometry plots depicting ILC2 frequencies in the small intestine of peanut-fed mice on the BALB/c background. B) Summary plots showing ILC2 frequencies and numbers in the jejunum of peanut-sensitized, IgE-sufficient or IgE-deficient Il4raF709 mice. Statistical analysis by Mann Whitney unpaired t test. Data shown depict one of three experiments, n=8–10 per group.
We have previously employed a number of strategies to establish that the presence of functional mast cells is required both for allergic sensitization (Th2 and IgE responses) and for anaphylactic reactions in peanut-fed IL4raF709 mice [8]. In order to test for a contribution of mast cells to ILC2 expansion and allergic sensitization, we used KitW-sh mice in which an inversion upstream of the c-Kit promoter region disrupts mast cell development. As expected, mast cell-deficient IL4raF709 KitW-sh mice exhibited impaired anaphylactic reactions accompanied by reduced IgE production compared with mast cell-sufficient IL4raF709 controls (Figure 4A, B). Reconstitution of the mast cell compartment in KitW-sh mice by i.p. injection of BMMC from WT C57BL/6 donors led to a restoration of IgE production and anaphylactic sensitivity. Compared with normal IL4raF709 controls, mast cell-deficient IL4raF709 KitW-sh mice had reduced frequencies of ILC2 following sensitization, which were partially restored by reconstitution with mast cells (Figure 4C).
Figure 4.
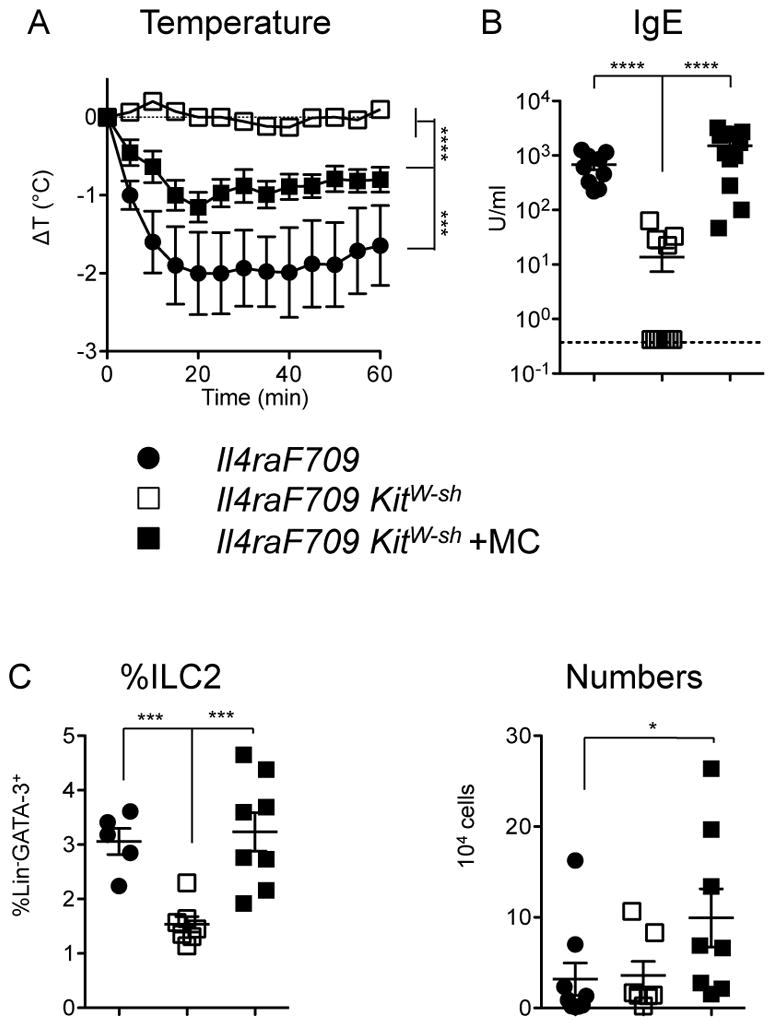
Contributions of mast cells to ILC2 expansion and allergic sensitization on the C57BL/6 background. A) Anaphylaxis after gavage challenge with PN in sensitized mice. Statistical analysis by repeated measures two-way ANOVA. B) Serum levels of PN-specific IgE. C) Frequencies and absolute numbers of ILC2 in the jejunum as assessed by flow cytometry. Data are combined from three independent experiments assessing mast cell reconstitution, n=5–14 per group. Statistical analyses in B–C performed using ANOVA with Bonferroni post-test on log-transformed values.
Since our findings from two independent models of food allergy implicated mast cells as drivers of intestinal ILC2 accumulation, we hypothesized that IgE-mediated activation of mast cells elicited production of cytokine(s) favoring the activation and expansion of ILC2. When ILC2 were cultured in the presence of mast cells undergoing IgE-mediated activation, IL-13 secretion increased approximately 5-fold (Figure 5A). Control experiments using IL-13-deficient ILC2 cultured with WT mast cells validated that the observed IL-13 was almost entirely ILC2-derived (Figure 5A). Eosinophil-derived IL-4 has been shown to stimulate proliferation and cytokine secretion by ILC2 [38], and since mast cells can also produce IL-4 [39, 40], we tested whether IL-4 was required for mast cell-mediated enhancement of ILC2 IL-13 secretion. When co-cultured with purified ILC2, IgE-activated IL-4-deficient mast cells displayed a reduced ability to stimulate IL-13 production by ILC2. Similarly, IL-4Rα-deficient ILC2 exhibited minimal responses to mast cell activation (Figure 5A). On its own, recombinant IL-4 was sufficient to induce IL-13 secretion by ILC2 (Figure 5A). Taken together, our in vivo and tissue culture data suggest that mast cells sustain and activate ILC2, likely via actions of IL-4.
Figure 5.
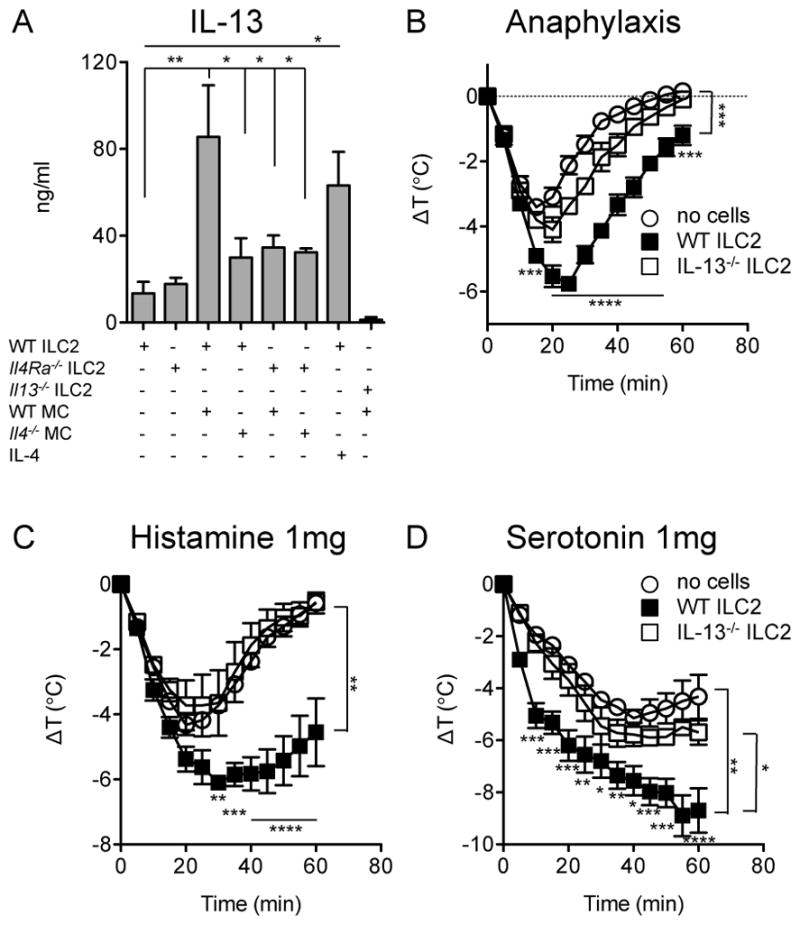
Induction of ILC2-derived IL-13 and its impact on anaphylaxis. A) IL-13 secretion by ILC2 stimulated by IgE+Ag-activated BMMC. Role of IL-4 in MC-stimulated ILC2 IL-13 secretion. Statistical analysis by one-way ANOVA with Bonferroni post-tests. B) Anaphylaxis in mice passively sensitized with IgE anti-TNP (2μg) at −16h and challenged with TNP-OVA (20μg) by i.p. injection (n=3–4). Activated, flow-sorted ILC2 (2×105) were transferred via i.v. injection at −16h. C) Core body temperature loss after administration of 1mg histamine (n=4–5). D) Core body temperature loss after administration of 1mg serotonin (n=4–5). Statistical analysis by repeated measures 2-way ANOVA with Bonferroni post-tests at individual time points. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Data are representative of two independent experiments each.
As IL-13 has been shown by Strait and colleagues to potently enhance the effects of mast cell mediators on vascular tissues, thus increasing sensitivity for anaphylactic shock [41], we examined whether IL-13 released by activated ILC2 could modulate the severity of anaphylaxis. ILC2 were enriched from intestinal leukocytes through cytokine-driven expansion, and then flow sorted to high purity. Adoptive transfer of purified, in vitro activated ILC2 into mice that were passively sensitized with IgE resulted in enhanced loss of core body temperature during anaphylaxis as well as delayed recovery from hypothermia (Figure 5B). In order to test the hypothesis that the exacerbated hypothermia was due to secretion of IL-13 by ILC2, we investigated whether ILC2 from IL-13-deficient mice would retain the ability shown by WT cells. Under the conditions tested, IL-13−/− ILC2 were unable to exacerbate IgE-mediated anaphylaxis to a statistically significant degree (Figure 5B). IL-13 has been shown to increase the response of vascular tissue to the mast cell mediators that cause anaphylaxis [41]. We investigated whether IL-13 from ILC2 would also exacerbate the shock resulting from administration of histamine or serotonin. Injection of activated WT ILC2 greatly magnified the loss of core body temperature in response to histamine (average Δ9C vs. 4.5C) (Figure 5C). A similar effect was observed in response to serotonin (Figure 5D). These exacerbations were largely due to IL-13 produced by ILC2. Taken together, these observations demonstrate that ILC2 can amplify the intensity of IgE-mediated anaphylaxis by IL-13 induced increases in the sensitivity of target tissues to the mediators of hypersensitivity.
Discussion
The lack of consistent correlation between food-specific IgE levels in allergic patients and the severity of hypersensitivity reactions they exhibit when challenged strongly implicates the presence of additional mechanisms that contribute to the severity of anaphylaxis. Here we considered that mast cells, which are the source of the vasoactive mediators of anaphylaxis, arise in the same mucosal sites where ILC2 are induced following food allergen exposure and that they favor both the expansion and activation of ILC2. This led us to hypothesize that there might be a close relationship between these two innate immune cell types in which mast cells might promote ILC2 activation and ILC2, which are strong IL-13 producers, could in turn act on target tissues to regulate physiologic responses to mast cell mediators. Our findings support such a model and these observations represent an important advance in our understanding of the interactions and complementary functions of these two enigmatic cell types.
Experiments using adoptive transfer of highly purified activated ILC2 revealed that ILC2 given before antigen challenge in passively sensitized mice directly enhanced the severity of anaphylaxis, and further that IL-13 secreted by ILC2 increased the sensitivity of vascular target tissue to the effects of the vasoactive amines, histamine and serotonin. Our finding that IL-13 secreted by ILC2 can regulate the severity of anaphylactic shock is consistent both with the work of Strait et al. demonstrating the potent augmenting effect of IL-13 on anaphylaxis, as well as with studies that have shown ILC2 to be a major source of IL-13 in vivo [20, 31, 41]. In mice, IL-13 lacks the abilities of IL-4 to stimulate mast cell growth or B cell switching to IgE, but does have strong indirect effects that amplify Th2 responses. Conflicting data have been presented on the role of IL-13 in the immune sensitization phase versus effector phase (degranulation, anaphylaxis) of food allergy, in part due to the difficulty in disentangling the two phases and in selectively manipulating IL-13 independently from IL-4 [25, 42, 43]. Brandt et al. reported that IL-13Rα1−/− mice developed minimal allergic reactions despite showing similar levels of allergen-specific IgE, and Lee et al. found that ILC2 enhanced allergic symptoms in an IL-13-dependent manner. As our studies do not include experiments using mice with a lineage-specific disruption of IL-13 production in ILC2 we cannot conclude that ILC2-derived IL-13 is essential for the elicitation of anaphylaxis. However our data support the conclusion that ILC2-derived IL-13 can enhance anaphylaxis driven by mast cells. The target of ILC2-derived IL-13 appears to be the vascular tissue, which expresses receptors for IL-13 [44]. The literature on allergic asthma and atopic dermatitis also supports a predominantly tissue-focused impact of IL-13 from ILC2 on allergic pathology, and suggests that additional IL-13 effects may be exerted on tissue remodeling and on intestinal permeability to macromolecules [18, 45, 46].
In this study we examined one aspect of the interaction between mast cells and ILC2, focusing on the ability of mast cells to stimulate ILC2 expansion and activation. We unexpectedly found that the absence of IgE led to a reduction in intestinal ILC2 numbers, which is consistent with a role for IgE, and perhaps mast cells, in ILC2 homeostasis. It remains to be seen whether FcεRIα−/− mice also exhibit reduced ILC2 numbers. We have previously reported that the mast cell-ILC2 interaction operates in the opposite direction as well, and that ILC2 can promote mast cell growth and degranulation [24]. Recent work in disparate areas of immunology has highlighted the two-way nature of the mast cell-ILC2 interactions [25, 27, 47], with an essential role for mast cell activation in driving a host-protective ILC2 response in expulsion of gastrointestinal helminths [48]. Modest increases in intestinal ILC2 numbers have been reported previously in some models of food allergy, but frequently ILC2 secretion of IL-13 increases to a greater extent than total cell numbers [24, 25, 43, 48, 49]. At present, we suspect that inconsistent findings regarding ILC2 expansion likely relate to the lack of a standard approach for identifying these cells, but may also be due differences in the sites (lymph nodes or intestine) and timing (pre- or post-challenge) of analysis within the various food allergy models. It is plausible that ILC2, as innate cells, may reach peak frequency during the early stages of sensitization, before standard experimental endpoints. The fact that we observed IgE-related differences in our model suggest that under certain conditions of allergen exposure, timing and analysis, there is an effect on the ILC2 response. Experimental animal studies have also illustrated roles for basophils in the pathology of food allergy [11, 50, 51] and although interactions between basophils and ILC2 have not been addressed in these studies, basophil-derived IL-4 could theoretically also support ILC2 growth and activation.
This report defines a key link between innate cell types and the IgE-mediated adaptive response that drives anaphylaxis, and describes a central mechanism that may alter the balance between non-reactive sensitization and lethal anaphylaxis. We suggest that reducing ILC2 activity, perhaps via IgE blockade, may be an effective means of limiting severe food reactions.
Methods
Mice
Wild-type BALB/c mice were bred at Boston Children’s Hospital. Inherently atopic Il4raF709 mice on BALB/c and C57BL/6 backgrounds as well as Il4raF709 KitW-sh mice (B6 background) have been previously described [8, 36, 52]. IgE-deficient Igh7−/− mice were previously described [53]. IL-13-deficient mice have been described [54, 55]. IL-4−/− and IL-4Rα−/− mice were purchased from the Jackson Laboratory. Mice were housed in isolator cages under specific pathogen-free conditions. All experiments were conducted under procedures approved by the Institutional Animal Care and Use Committees of Boston Children’s Hospital and Boston University Medical Center, protocol number 14-09-2780R.
Cell culture
Cell culture was performed using RPMI-1640 medium supplemented with penicillin, streptomycin, gentamicin, 2-mercaptoethanol, 2mM sodium pyruvate, glutamine, 10mM HEPES (all Life Technologies) and 10% fetal calf serum (Atlanta Biologicals). Cells were incubated at 37°C humidified atmosphere with 5% CO2. Mast cells (BMMC) were cultured from bone marrow precursors in the presence of IL-3 and SCF (both 20ng/ml, Shenandoah Biotechnology) as previously described [56]. ILC2 were expanded from lamina propria leukocytes with 20ng/ml IL-33 (Biolegend), 25ng/ml IL-25 (Biolegend), 10ng/ml IL-4 (Shenandoah Biotechnology) and 5ng/ml IL-2 (as IL-2-transfected X63 supernatant) combined with 5μg/ml αIFNγ (XMG1.2, Biolegend) for 3–7 days prior to cell sorting, and were subsequently maintained in IL-2-supplemented medium.
Co-culture of ILC2 and mast cells
BMMC sensitized for 24h with 1μg/ml IgE anti-DNP (clone SPE-7, Sigma-Aldrich) in the presence of IL-3 and SCF. BMMC were then washed to remove unbound IgE, and cultured with ILC2 at a 1:1 ratio (2×104 cells in 200μl) for 48h in round-bottom 96-well plates. DNP-albumin (100ng/ml, Sigma-Aldrich), IL-2 (2ng/ml, Shenandoah Biotechnology), IL-3 (10ng/ml, Shenandoah Biotechnology), and SCF (10ng/ml, Shenandoah Biotechnology) were included in all conditions. Where indicated, recombinant IL-4 (Shenandoah Biotechnology) was included at 20ng/ml.
Isolation of intestinal leukocytes
Leukocytes were isolated from the jejunal lamina propria for analysis by flow cytometry and cell culture according to previously described procedures [56, 57]. The intestinal epithelium was removed from 1cm intestinal segments with a 30min incubation at 37°C in a rotary incubator in PBS containing 2% FCS, 10mM EDTA, and 1.5mM dithioerythritol. Remaining tissue was chopped finely prior to digestion for 30–45min with 1.6mg/ml collagenase IV (Worthington Biochemicals), 0.3mg/ml hyaluronidase (Worthington Biochemicals) and 100μg/ml DNAse I (Applichem) in complete RPMI supplemented with 20% FCS, 2mM CaCl2 and 2mM MgCl2 (Sigma Aldrich). After passage through a 70μm cell strainer (BD Pharmingen), cell suspensions were separated using 40% Percoll underlain with 70% Percoll (GE Healthcare). Leukocytes were collected from the interface.
Food allergen sensitization and anaphylaxis
OVA sensitization
Mice were immunized i.p. with 100μg OVA adsorbed to 1mg alum hydroxide gel (Sigma Aldrich), and again 10 days later with 10μg OVA adsorbed to 1mg alum. On days 20, 22, 24, 27, 29 and 31, mice were gavaged with 50mg OVA. On day 34, mice were challenged with 150mg OVA i.g.
Peanut sensitization
Mice were intragastrically fed with 22.5mg peanut butter (containing 5mg protein) diluted in 250μl 0.2M sodium bicarbonate pH 8 weekly for four weeks. One week after the final sensitization, mice were challenged by gavage with 400mg peanut butter (Skippy, Hormel Foods).
Mast cell reconstitution
Mast cell-deficient Il4raF709 KitW-sh mice were injected i.p. with 5×106 BMMC prepared from WT C57BL/6 mice at 12-8 weeks prior to sensitization and again at 4 weeks prior to sensitization.
For passive anaphylaxis studies, mice were injected with 2μg IgE anti-TNP (38.2, BD Biosciences) and challenged 16hrs later with 20μg TNP-OVA (Biosearch Technologies) as previously described [8, 58]. Responsiveness to mast cell mediators was assessed by injection with 1mg of histamine or serotonin (both Sigma Aldrich) as previously described [41, 56]. Core body temperature was monitored using subcutaneously implanted temperature transponders, and data were acquired using a DAS-6001 console (Bio Medic Data Systems) as previously described [36].
Flow cytometry and cell sorting
Antibodies against the following molecules were used to identify ILC populations: CD45 (clone 30-F11, Biolegend), GATA-3 (TWAJ, eBioscience) and RORγt (B2D, eBioscience). Lineage marker-expressing cells were excluded using a cocktail of FITC-conjugated antibodies purchased from Biolegend against B220 (RA3-6B2), CD3ε (145-2C11), CD4 (RM4-5), CD8α (53-6.7), CD11b (M1/70), CD11c (N418), CD19 (6D5), CD49b (DX5), FcεRIα (MAR-1), Gr-1 (RB6-8C5), NKp46 (29A1.4), TCRβ (H57-597) and TCRγδ (UC7-13D5). Intracellular staining was performed using the Foxp3/Transcription Factor staining kit (eBioscience). Cultured ILC2 were surface stained with Thy1.2 (53-2.1, BD Biosciences), Sca-1 (D7, Biolegend), c-Kit (2B8, Biolegend) and lineage markers, then sorted on a BD FACSAria II on the basis of Lin− Thy1+ Sca-1+ and c-Kitlow/−. Dead cells were excluded from all experiments based on staining with eFluor 780 fixable viability dye (eBioscience), non-specific Fcγ receptor interactions were blocked with anti-CD16/32 (clone 93, Biolegend), and analyses were restricted to single cells by FSC-H and FSC-W signals. CountBright beads (ThermoFisher) were used to determine cellularity. Cells were analyzed on an LSRII Fortessa or a FACSCalibur cytometer (BD Biosciences) and data were processed using FlowJo version 10.0.8 (Tree Star Inc.).
ILC2 adoptive transfer
Leukocytes were isolated from the intestinal lamina propria, and cultured under ILC2-expanding conditions (IL-2, IL-4, IL-33, IL-25) for 5 days, as described above. ILC2 were then purified by flow sorting as described above, and expanded in culture for an additional 3–7 days. The cells were then washed, counted, and 2×105 were injected i.v. via the retro-orbital sinus of unmanipulated (unirradiated) WT BALB/c mice. Adoptive transfers were performed 16h prior to challenge.
ELISAs
Sandwich ELISAs were performed using a kit for MMCP-1 (eBioscience). IL-13 was assessed using matched antibody pairs: clones eBio13A and eBio1316H from eBioscience. OVA- and peanut-specific IgE were measured by capturing IgE with 3μg/ml goat anti-mouse IgE (Southern Biotech) followed by detection with 200ng/ml biotinylated OVA or peanut extract (as appropriate) as previously described [8, 36].
Statistical analysis
Data were plotted and analyzed using Prism GraphPad (version 5.0f). Temperature curves were analyzed using repeated measures two-way ANOVA to calculate P values between groups. All other data were analyzed using Mann Whitney unpaired t tests (for two groups), ANOVA with Bonferroni post-tests (for three or more groups) or 2-way ANOVA with Sidak’s multiple comparison testing (two variables). In cases where values were spread across multiple orders of magnitude, data were log-transformed prior to analysis with parametric tests. Two-tailed P values are summarized as follows: *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001.
Supplementary Material
Acknowledgments
We are grateful for funding received from the NIH, including grants 1R01AI119918-01 (HCO), 5T32AI007512-28 (OTB) and 1K01DK106303-01 (OTB). Additional generous support was provided by the Rao Chakravorti Family fund, the Christine Olsen and Robert Small Food Allergy Research Fund, the Bunning Family Foundation, and the Nanji Family Fund for Food Allergy Research.
Abbreviations
- ILC2
Type 2 innate lymphoid cell
- Th
T helper
- BMMC
bone marrow-derived mast cell
- Treg
regulatory T cell
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- MLN
mesenteric lymph nodes
- TNP
trinitrophenyl
- KO
knockout
- WT
wild-type
- OVA
ovalbumin
- PN
peanut
Footnotes
Disclosure
The authors declare that they have no conflicts of interest.
References
- 1.Mullins RJ, Dear KB, Tang ML. Time trends in Australian hospital anaphylaxis admissions in 1998–1999 to 2011–2012. The Journal of allergy and clinical immunology. 2015;136:367–75. doi: 10.1016/j.jaci.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Nocerino R, Leone L, Cosenza L, Berni Canani R. Increasing rate of hospitalizations for food-induced anaphylaxis in Italian children: An analysis of the Italian Ministry of Health database. The Journal of allergy and clinical immunology. 2015;135:833–5. e3. doi: 10.1016/j.jaci.2014.12.1912. [DOI] [PubMed] [Google Scholar]
- 3.Tejedor-Alonso MA, Moro-Moro M, Mosquera Gonzalez M, Rodriguez-Alvarez M, Perez Fernandez E, Latasa Zamalloa P, Farias Aquino E, Gil Prieto R, Gil de Miguel A. Increased incidence of admissions for anaphylaxis in Spain 1998–2011. Allergy. 2015;70:880–3. doi: 10.1111/all.12613. [DOI] [PubMed] [Google Scholar]
- 4.Turner PJ, Gowland MH, Sharma V, Ierodiakonou D, Harper N, Garcez T, Pumphrey R, Boyle RJ. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: an analysis of United Kingdom national anaphylaxis data, 1992–2012. The Journal of allergy and clinical immunology. 2015;135:956–63. e1. doi: 10.1016/j.jaci.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. The Journal of allergy and clinical immunology. 2014;133:291–307. doi: 10.1016/j.jaci.2013.11.020. quiz 08. [DOI] [PubMed] [Google Scholar]
- 6.Gupta R, Holdford D, Bilaver L, Dyer A, Holl JL, Meltzer D. The economic impact of childhood food allergy in the United States. JAMA pediatrics. 2013;167:1026–31. doi: 10.1001/jamapediatrics.2013.2376. [DOI] [PubMed] [Google Scholar]
- 7.DunnGalvin A, Dubois AE, Flokstra-de Blok BM, Hourihane JO. The effects of food allergy on quality of life. Chemical immunology and allergy. 2015;101:235–52. doi: 10.1159/000375106. [DOI] [PubMed] [Google Scholar]
- 8.Burton OT, Noval Rivas M, Zhou JS, Logsdon SL, Darling AR, Koleoglou KJ, Roers A, Houshyar H, Crackower MA, Chatila TA, Oettgen HC. Immunoglobulin E signal inhibition during allergen ingestion leads to reversal of established food allergy and induction of regulatory T cells. Immunity. 2014;41:141–51. doi: 10.1016/j.immuni.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noval Rivas M, Burton OT, Wise P, Charbonnier LM, Georgiev P, Oettgen HC, Rachid R, Chatila TA. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity. 2015;42:512–23. doi: 10.1016/j.immuni.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CY, Lee JB, Liu B, Ohta S, Wang PY, Kartashov AV, Mugge L, Abonia JP, Barski A, Izuhara K, Rothenberg ME, Finkelman FD, Hogan SP, Wang YH. Induction of Interleukin-9-Producing Mucosal Mast Cells Promotes Susceptibility to IgE-Mediated Experimental Food Allergy. Immunity. 2015;43:788–802. doi: 10.1016/j.immuni.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noti M, Kim BS, Siracusa MC, Rak GD, Kubo M, Moghaddam AE, Sattentau QA, Comeau MR, Spergel JM, Artis D. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin-basophil axis. The Journal of allergy and clinical immunology. 2014;133:1390–9. 99 e1–6. doi: 10.1016/j.jaci.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogan SP, Wang YH, Strait R, Finkelman FD. Food-induced anaphylaxis: mast cells as modulators of anaphylactic severity. Seminars in immunopathology. 2012;34:643–53. doi: 10.1007/s00281-012-0320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508:397–401. doi: 10.1038/nature13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klose CS, Flach M, Mohle L, Rogell L, Hoyler T, Ebert K, Fabiunke C, Pfeifer D, Sexl V, Fonseca-Pereira D, Domingues RG, Veiga-Fernandes H, Arnold SJ, Busslinger M, Dunay IR, Tanriver Y, Diefenbach A. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340–56. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 15.McKenzie AN, Spits H, Eberl G. Innate lymphoid cells in inflammation and immunity. Immunity. 2014;41:366–74. doi: 10.1016/j.immuni.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, Barlow JL, Neill DR, Panova V, Koch U, Radtke F, Hardman CS, Hwang YY, Fallon PG, McKenzie AN. Transcription factor RORalpha is critical for nuocyte development. Nature immunology. 2012;13:229–36. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scanlon ST, McKenzie AN. Type 2 innate lymphoid cells: new players in asthma and allergy. Current opinion in immunology. 2012;24:707–12. doi: 10.1016/j.coi.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Kim HY, Chang YJ, Subramanian S, Lee HH, Albacker LA, Matangkasombut P, Savage PB, McKenzie AN, Smith DE, Rottman JB, DeKruyff RH, Umetsu DT. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. The Journal of allergy and clinical immunology. 2012;129:216–27. e1–6. doi: 10.1016/j.jaci.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, Dekruyff RH, Umetsu DT. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nature immunology. 2011;12:631–8. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–70. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, Huang LC, Johnson D, Scanlon ST, McKenzie AN, Fallon PG, Ogg GS. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. The Journal of experimental medicine. 2013;210:2939–50. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–4. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 23.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, Bhandoola A, Artis D. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–6. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noval Rivas M, Burton OT, Oettgen HC, Chatila T. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. The Journal of allergy and clinical immunology. 2016;138:801–11. e9. doi: 10.1016/j.jaci.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JB, Chen CY, Liu B, Mugge L, Angkasekwinai P, Facchinetti V, Dong C, Liu YJ, Rothenberg ME, Hogan SP, Finkelman FD, Wang YH. IL-25 and CD4(+) TH2 cells enhance type 2 innate lymphoid cell-derived IL-13 production, which promotes IgE-mediated experimental food allergy. The Journal of allergy and clinical immunology. 2016;137:1216–25. e1–5. doi: 10.1016/j.jaci.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tait Wojno ED, Artis D. Innate lymphoid cells: balancing immunity, inflammation, and tissue repair in the intestine. Cell host & microbe. 2012;12:445–57. doi: 10.1016/j.chom.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, Mitchell AJ, Tay SS, Jain R, Forbes-Blom E, Chen X, Tong PL, Bolton HA, Artis D, Paul WE, Fazekas de St Groth B, Grimbaldeston MA, Le Gros G, Weninger W. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nature immunology. 2013;14:564–73. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Wang Y, Tang L, de Villiers WJ, Cohen D, Woodward J, Finkelman FD, Eckhardt ER. Dietary medium-chain triglycerides promote oral allergic sensitization and orally induced anaphylaxis to peanut protein in mice. The Journal of allergy and clinical immunology. 2013;131:442–50. doi: 10.1016/j.jaci.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galand C, Leyva-Castillo JM, Yoon J, Han A, Lee MS, McKenzie AN, Stassen M, Oyoshi MK, Finkelman FD, Geha RS. IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells. The Journal of allergy and clinical immunology. 2016;138:1356–66. doi: 10.1016/j.jaci.2016.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tordesillas L, Goswami R, Benede S, Grishina G, Dunkin D, Jarvinen KM, Maleki SJ, Sampson HA, Berin MC. Skin exposure promotes a Th2-dependent sensitization to peanut allergens. The Journal of clinical investigation. 2014;124:4965–75. doi: 10.1172/JCI75660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JB, Chen CY, Liu B, Mugge L, Angkasekwinai P, Facchinetti V, Dong C, Liu YJ, Rothenberg ME, Hogan SP, Finkelman FD, Wang YH. IL-25 and CD4 T2 cells enhance type 2 innate lymphoid cell-derived IL-13 production, which promotes IgE-mediated experimental food allergy. The Journal of allergy and clinical immunology. 2015 doi: 10.1016/j.jaci.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muto T, Fukuoka A, Kabashima K, Ziegler SF, Nakanishi K, Matsushita K, Yoshimoto T. The role of basophils and proallergic cytokines, TSLP and IL-33, in cutaneously sensitized food allergy. International immunology. 2014;26:539–49. doi: 10.1093/intimm/dxu058. [DOI] [PubMed] [Google Scholar]
- 33.Hsu CL, Neilsen CV, Bryce PJ. IL-33 is produced by mast cells and regulates IgE-dependent inflammation. PloS one. 2010;5:e11944. doi: 10.1371/journal.pone.0011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikeda K, Nakajima H, Suzuki K, Kagami S, Hirose K, Suto A, Saito Y, Iwamoto I. Mast cells produce interleukin-25 upon Fc epsilon RI-mediated activation. Blood. 2003;101:3594–6. doi: 10.1182/blood-2002-09-2817. [DOI] [PubMed] [Google Scholar]
- 35.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, Zimmermann N, Finkelman FD, Rothenberg ME. Mast cells are required for experimental oral allergen-induced diarrhea. The Journal of clinical investigation. 2003;112:1666–77. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathias CB, Hobson SA, Garcia-Lloret M, Lawson G, Poddighe D, Freyschmidt EJ, Xing W, Gurish MF, Chatila TA, Oettgen HC. IgE-mediated systemic anaphylaxis and impaired tolerance to food antigens in mice with enhanced IL-4 receptor signaling. The Journal of allergy and clinical immunology. 2011;127:795–805. e1–6. doi: 10.1016/j.jaci.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahrens R, Osterfeld H, Wu D, Chen CY, Arumugam M, Groschwitz K, Strait R, Wang YH, Finkelman FD, Hogan SP. Intestinal mast cell levels control severity of oral antigen-induced anaphylaxis in mice. Am J Pathol. 2012;180:1535–46. doi: 10.1016/j.ajpath.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bal SM, Bernink JH, Nagasawa M, Groot J, Shikhagaie MM, Golebski K, van Drunen CM, Lutter R, Jonkers RE, Hombrink P, Bruchard M, Villaudy J, Munneke JM, Fokkens W, Erjefalt JS, Spits H, Ros XR. IL-1beta, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nature immunology. 2016;17:636–45. doi: 10.1038/ni.3444. [DOI] [PubMed] [Google Scholar]
- 39.Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. Journal of immunology. 2005;174:1063–72. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- 40.Oettgen HC, Burton OT. IgE and Mast Cells: The Endogenous Adjuvant. Advances in immunology. 2015;127:203–56. doi: 10.1016/bs.ai.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Strait RT, Morris SC, Smiley K, Urban JF, Jr, Finkelman FD. IL-4 exacerbates anaphylaxis. Journal of immunology. 2003;170:3835–42. doi: 10.4049/jimmunol.170.7.3835. [DOI] [PubMed] [Google Scholar]
- 42.Brandt EB, Munitz A, Orekov T, Mingler MK, McBride M, Finkelman FD, Rothenberg ME. Targeting IL-4/IL-13 signaling to alleviate oral allergen-induced diarrhea. The Journal of allergy and clinical immunology. 2009;123:53–8. doi: 10.1016/j.jaci.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chu DK, Mohammed-Ali Z, Jimenez-Saiz R, Walker TD, Goncharova S, Llop-Guevara A, Kong J, Gordon ME, Barra NG, Gillgrass AE, Van Seggelen H, Khan WI, Ashkar AA, Bramson JL, Humbles AA, Kolbeck R, Waserman S, Jordana M. T helper cell IL-4 drives intestinal Th2 priming to oral peanut antigen, under the control of OX40L and independent of innate-like lymphocytes. Mucosal immunology. 2014;7:1395–404. doi: 10.1038/mi.2014.29. [DOI] [PubMed] [Google Scholar]
- 44.Schnyder B, Lugli SM, Schnyder-Candrian S, Eng VM, Moser R, Banchereau J, Ryffel B, Car BD. Biochemical and morphological characterization of vascular and lymphocytic interleukin-4 receptors. Am J Pathol. 1996;149:1369–79. [PMC free article] [PubMed] [Google Scholar]
- 45.Madden KB, Whitman L, Sullivan C, Gause WC, Urban JF, Jr, Katona IM, Finkelman FD, Shea-Donohue T. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. Journal of immunology. 2002;169:4417–22. doi: 10.4049/jimmunol.169.8.4417. [DOI] [PubMed] [Google Scholar]
- 46.Zheng T, Oh MH, Oh SY, Schroeder JT, Glick AB, Zhu Z. Transgenic expression of interleukin-13 in the skin induces a pruritic dermatitis and skin remodeling. The Journal of investigative dermatology. 2009;129:742–51. doi: 10.1038/jid.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moretti S, Renga G, Oikonomou V, Galosi C, Pariano M, Iannitti RG, Borghi M, Puccetti M, De Zuani M, Pucillo CE, Paolicelli G, Zelante T, Renauld JC, Bereshchenko O, Sportoletti P, Lucidi V, Russo MC, Colombo C, Fiscarelli E, Lass-Florl C, Majo F, Ricciotti G, Ellemunter H, Ratclif L, Talesa VN, Napolioni V, Romani L. A mast cell-ILC2-Th9 pathway promotes lung inflammation in cystic fibrosis. Nat Commun. 2017;8:14017. doi: 10.1038/ncomms14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimokawa C, Kanaya T, Hachisuka M, Ishiwata K, Hisaeda H, Kurashima Y, Kiyono H, Yoshimoto T, Kaisho T, Ohno H. Mast Cells Are Crucial for Induction of Group 2 Innate Lymphoid Cells and Clearance of Helminth Infections. Immunity. 2017;46:863–74. e4. doi: 10.1016/j.immuni.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 49.Khodoun MV, Tomar S, Tocker JE, Wang YH, Finkelman FD. Prevention of food allergy development and suppression of established food allergy by neutralization of thymic stromal lymphopoietin, IL-25, and IL-33. The Journal of allergy and clinical immunology. 2017 doi: 10.1016/j.jaci.2017.02.046. [DOI] [PubMed] [Google Scholar]
- 50.Hussain M, Borcard L, Walsh KP, Pena Rodriguez M, Mueller C, Kim BS, Kubo M, Artis D, Noti M. Basophil-derived IL-4 promotes epicutaneous antigen sensitization concomitant with the development of food allergy. The Journal of allergy and clinical immunology. 2017 doi: 10.1016/j.jaci.2017.02.035. [DOI] [PubMed] [Google Scholar]
- 51.Reber LL, Marichal T, Mukai K, Kita Y, Tokuoka SM, Roers A, Hartmann K, Karasuyama H, Nadeau KC, Tsai M, Galli SJ. Selective ablation of mast cells or basophils reduces peanut-induced anaphylaxis in mice. The Journal of allergy and clinical immunology. 2013;132:881–8. e1–11. doi: 10.1016/j.jaci.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tachdjian R, Al Khatib S, Schwinglshackl A, Kim HS, Chen A, Blasioli J, Mathias C, Kim HY, Umetsu DT, Oettgen HC, Chatila TA. In vivo regulation of the allergic response by the IL-4 receptor alpha chain immunoreceptor tyrosine-based inhibitory motif. The Journal of allergy and clinical immunology. 2010;125:1128–36. e8. doi: 10.1016/j.jaci.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oettgen HC, Martin TR, Wynshaw-Boris A, Deng C, Drazen JM, Leder P. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370:367–70. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 54.McKenzie GJ, Bancroft A, Grencis RK, McKenzie AN. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Current biology: CB. 1998;8:339–42. doi: 10.1016/s0960-9822(98)70134-4. [DOI] [PubMed] [Google Scholar]
- 55.McKenzie GJ, Emson CL, Bell SE, Anderson S, Fallon P, Zurawski G, Murray R, Grencis R, McKenzie AN. Impaired development of Th2 cells in IL-13-deficient mice. Immunity. 1998;9:423–32. doi: 10.1016/s1074-7613(00)80625-1. [DOI] [PubMed] [Google Scholar]
- 56.Burton OT, Darling AR, Zhou JS, Noval-Rivas M, Jones TG, Gurish MF, Chatila TA, Oettgen HC. Direct effects of IL-4 on mast cells drive their intestinal expansion and increase susceptibility to anaphylaxis in a murine model of food allergy. Mucosal immunology. 2013;6:740–50. doi: 10.1038/mi.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lefrancois L, Lycke N. Isolation of mouse small intestinal intraepithelial lymphocytes, Peyer’s patch, and lamina propria cells. Current protocols in immunology/edited by John E Coligan [et al] 2001;Chapter 3(Unit 3):19. doi: 10.1002/0471142735.im0319s17. [DOI] [PubMed] [Google Scholar]
- 58.Burton OT, Logsdon SL, Zhou JS, Medina-Tamayo J, Abdel-Gadir A, Noval Rivas M, Koleoglou KJ, Chatila TA, Schneider LC, Rachid R, Umetsu DT, Oettgen HC. Oral immunotherapy induces IgG antibodies that act through FcgammaRIIb to suppress IgE-mediated hypersensitivity. The Journal of allergy and clinical immunology. 2014;134:1310–17. e6. doi: 10.1016/j.jaci.2014.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.