Abstract
Rapidly characterizing the three-dimensional structures of proteins and the multimeric machines they form remains one of the great challenges facing modern biological and medical sciences. Ion mobility-mass spectrometry based techniques are playing an expanding role in characterizing these functional complexes, especially in drug discovery and development workflows. Despite this expansion, ion mobility-mass spectrometry faces many challenges, especially in the context of detecting small differences in protein tertiary structure that bear functional consequences. Collision induced unfolding is an ion mobility-mass spectrometry method that enables the rapid differentiation of subtly-different protein isoforms based on their unfolding patterns and stabilities. In this review, we summarize the modern implementation of such gas-phase unfolding experiments and provide an overview of recent developments in both methods and applications.
Introduction
The fundamental relationship between protein structure and function makes their study critical in ongoing efforts to understand both fundamental elements of biochemistry and human disease [1]. In order to understand protein structure, its role in defining function, and any changes that may occur in disease states, it is essential to explore the connective biophysical parameters that link such elements of biophysics together [2]. One such element is protein stability, often reported as a free energy of protein unfolding and represents one of the most widely utilized descriptors of protein structure [3]. Given the significance of protein stability in the framework of understanding protein structure and function, new experimental techniques that can extract such values with improved figures of merit are clearly needed.
Mass spectrometry (MS) has recently experienced a proliferation of structural biology related research, focusing primarily on heterogeneous proteins, protein complexes, and protein-ligand complexes due to its ability to access such mixtures with sensitivity, speed, and low limits of detection [4]. Ion mobility (IM), which separates ions based on their size to charge ratio and reports ion size in terms of an orientationally-averaged collision cross section (CCS), has also been widely deployed in combination with MS as a platform for structural biology [5]. Prior to this recent period of expansion, IM-MS was used to primarily assign the conformations of peptides [6] and small proteins [7] in the gas phase. However, as the size and complexity of biomolecules increases, IM-derived CCS values alone often yield insufficient information to define the structures of proteins in detail [7].
Collisional activation has long been used to probe the structure and stability of protein ions in the gas phase [8,9]. Collision induced unfolding (CIU) represents an extension of this earlier work, and is best viewed as a gas-phase analog of differential scanning calorimetry experiments often carried out in solution. In a typical CIU experiment, isolated biomolecular ions are activated through energetic collisions with a background gas (e.g. Argon) in order to increase their internal energy and cause them to change conformation (unfold) in the gas-phase, without providing sufficient energy to cause the significant dissociation of covalent bonds [10]. The progress of this CIU process is followed through IM-MS, with the former stage providing direct measurement of protein unfolding through changes in ion CCS and the latter analyzing the composition of the isolated biomolecules and enabling any collision induced dissociation (CID) products to be excluded from the analysis. Early examples of CIU include the observation of cytochrome c [7] and apomyoglobin unfolding in the gas phase [11]. Modern implementations of the technology have been extended well beyond these examples, to include detailed analyses of the CIU mechanism and applications to a range of therapeutically-relevant targets.
The potential of CIU as an analytical fingerprinting technique to study the structures and stabilities of proteins, protein complexes, and protein-ligand complexes is now emerging. The collisional activation of protein assemblies often yields a multitude of partially folded intermediates stable on the millisecond time scale that can provide a range of diagnostic information related to the structures of the isolated protein complexes [12,13]. In addition, CIU has been used to assay the stabilities of proteins and protein-ligand complexes in the gas phase. Although the stability measurements offered by CIU data for biomolecular ions are relative, and allow for comparisons of protein states rather than determination of absolute thermodynamic properties, they also provide valuable insight into the structure and native binding interactions of proteins and their complexes [9,14–17]. In this review, we recount past examples of CIU as a means of illuminating both current and future applications of the technology.
Generation and Analysis of CIU data
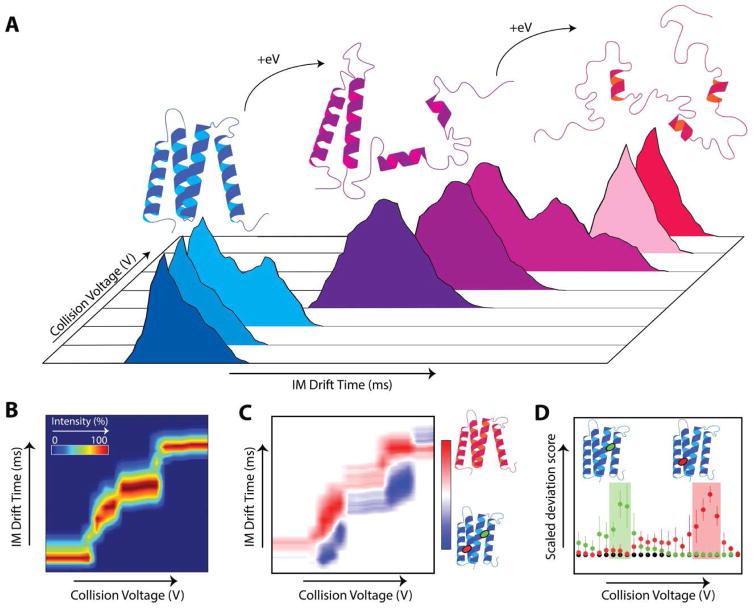
Typical CIU experiments are performed by sequentially increasing an accelerating potential difference that serves to activate ions prior to ion mobility separation. As such, IM arrival time distributions (ATDs) are acquired at each stepped potential (Fig. 1A), creating a large multidimensional dataset. The changes in measured ATD correspond to structural transitions of the protein ion in the gas phase which, while not directly assessing solution phase structures, can be used to generate unique fingerprints (Fig. 1B) that can reflect such native state structure information. Several methods to generate these fingerprints have been described [15]*[18]**[19,20], offering quantitative metrics for rapidly distinguishing subtle structural changes in proteins and protein complexes.
Figure 1.
A: Diagrams and cartoons depicting the CIU of proteins and common methods of analysis. As collision energy (eV) is increased, an isolated protein ion unfolds in the gas phase. B: CIU fingerprint with collision voltage on the x-axis, arrival time on y-axis, and intensity shown using a color scale. C: CIU comparison plot analysis depicting an apo and a doubly bound protein-ligand complex (red and green oval) with collision voltage on x-axis, arrival time on y-axis, and color scheme representing the differential intensities of the apo (red) and ligand bound (blue) states. D: A scaled deviation score analysis depicting a comparison of two different ligand bound states with CIU data acquired for the apo protein A score is computed that statistically assess fingerprint similarity at each voltage, enabling a narrow the window of collision voltage to be defined that maximizes dissimilarity between analytes, as shown by green shaded area for
 ligand and red shaded area for
ligand and red shaded area for
 ligand.
ligand.
To generate a CIU fingerprint, the arrival time distribution of the m/z corresponding to the analyte ion must be extracted from the raw data at each collision voltage applied to create a matrix for analysis. Manual generation of this matrix can be time-consuming, and recent CIU experiments have relied more heavily upon automated extraction tools capable of creating such file structures rapidly [15]*[20]. Once generated, replicates can be used to assign statistical confidence to observed deviations between fingerprints representing different protein forms, e.g. between ligand-bound and unbound states. These quantitative comparisons can be leveraged to classify binding events and structural changes into biologically relevant categories, such as differentiating functional from nonspecific lipids bound to membrane proteins [15]*, or determining the binding site of a ligand in systems with multiple known binding pockets [17]. As these workflows become routine and advance towards automated and high-throughput analyses, continued development of automated extraction and processing tools will be essential to realizing the full potential of CIU experiments.
Probing Protein Structure and Stability using CIU
Collisional activation followed by IM-MS has been used to probe the conformations of proteins in the gas phase for nearly two decades [10,11]. For example, early CIU experiments probed the activation energy barriers associated the gas-phase folding and unfolding of apomyoglobin following charge manipulation, revealing clear evidence of both Coulombic and structural components for the barriers detected between the gas-phase conformers [11]. Tandem IM technology [21,22] combined with collisional activation has been used to examine similar activation energy barriers in greater detail, revealing connectivity maps between the multitude of intermediate states populated during the CIU of small proteins [23]. Overall, these early CIU experiments were aimed primarily at uncovering the biophysical rules governing gas-phase protein ions, and succeeded in significantly advancing our understanding of protein stability and structure in a solvent-free environment.
Following on from this earlier work, CIU has been implemented to study the structure and dissociation behavior of protein complexes [24]. For example, early work [25] proposed an unfolding-based mechanism for protein complex CID, in which a single subunit unfolds and is ejected bearing a large portion of the total charge of the assembly, largely through collecting indirect evidence of protein CIU. The introduction of CIU enabled the direct observation of collisionally-activated protein assemblies, confirming that they populate partially folded intermediates that are stable on the millisecond timescale [8,12,26]. Other structural rearrangements of protein complexes have been shown in the gas phase via collisional activation and IM-MS. For instance, many reports have shown evidence of compaction upon the activation of ring-like protein complexes that contain significant internal cavities [26,27]. Moreover, computational chemistry has been used to probe protein complex CIU, reproducing many of the general features of experimental data [28–30]. Recent computational approaches in this area incorporate charge hopping within coarse-grained models and mobile protons within all-atom MD simulations [31]*. Despite these recent advances, however, a complete model capable of predicting the unfolding transitions of heated protein complexes in CIU remains elusive.
Extending from these mechanistically-framed studies, CIU has been used to quantify shifts in protein complex stability upon binding large populations of both anions and cations. Early CIU work in this area indicated that buffer components of low volatility bound to intact protein complexes can act to stabilize protein complex in the gas phase [32]. These initial results were expanded upon by screening a wider range of solution additives for their ability to stabilize gas-phase protein complexes [12,33–35]. For example, CIU and CID studies incorporating a broad range anions and cations bound to significant number of protein complexes revealed that stabilizing anions act primarily through evaporative cooling, whereas stabilizing cations act to bind tightly to protein complexes and limit charge mobility [34,35]. More direct efforts to stabilize protein complexes have been prosecuted through chemical cross-linking, where the CIU of intact protein complexes modified with charge-bearing chemical agents revealed significant increases in gas-phase stabilities [36]. Overall, this work demonstrates the clear utility of CIU data in building next-generation IM-MS technologies aimed at measuring labile protein complexes and structures.
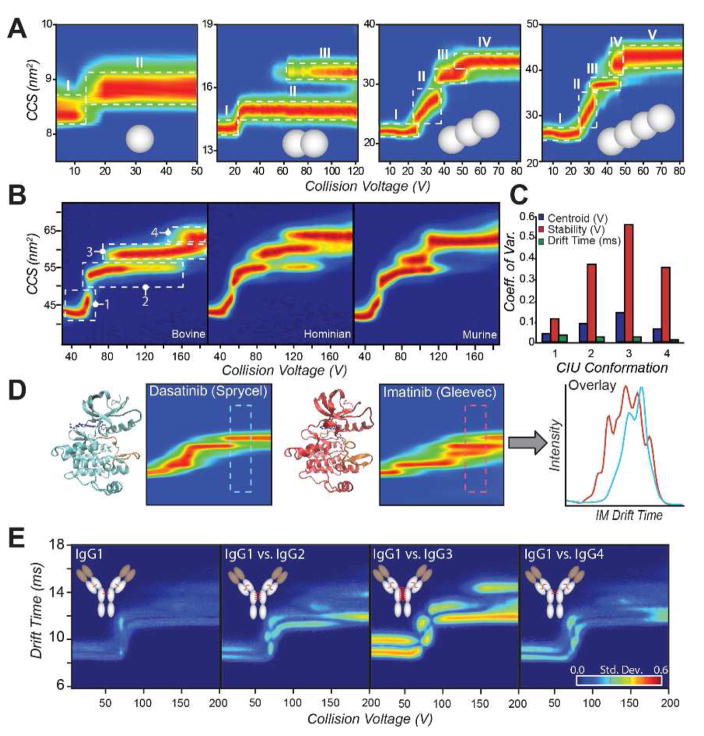
More recent experiments have aimed provide a detailed mechanism for CIU in the context of monomeric proteins. For instance, a survey of proteins ranging from 8 to 78 kDa, and containing between one and four domains, produced evidence of a strong correlation between native domain structure and the number of CIU transitions observed for low charge state protein ions (Fig. 2A) [37]**. A follow-on study in this area used both domain-specific ligand binding and noncovalent constructs to build the first detailed CIU mechanism for a multi-domain protein [13]**. This same report highlighted both the similarities and differences in the CIU of iso-CCS homologous protein variants, demonstrating both a strong correlation between quantified CIU similarity and sequence identity as well as identifying the stability of CIU features as the main element of variation in unfolding data acquired across sequence variants (Fig. 2B). Similarly, the structural differences of ubiquitin ions having similar ground state CCS values produced from cation-to-anion proton transfer reaction (CAPTR) experiments targeting a broad range of precursor ion charge states were detected by CIU [38]. These studies, taken together, begin to paint a detailed picture of the CIU mechanism as well as point toward future applications in protein engineering, where the stability of individual protein domains within larger constructs can be measured without need of labelling or surface attachment.
Figure 2.
A: A series of covalently linked poly-ubiquitin proteins (1–4 ubiquitins, gray spheres) is probed by CIU [37]**. Single domain ubiquitin results in a single CIU transition, from an initial native-like state (I) to a more extended state (II) upon collisional activation. Each additional domain added results in an additional CIU transition, indicating that the transitions are representative of the domain structure of the protein in solution. B: Bovine, human, and murine serum albumin proteins CIU fingerprints are compared. Despite high sequence homology and globally similar three-dimensional structures, CIU readily distinguishes each variant, demonstrating sensitivity towards subtle alterations in protein isoforms [13]**. C: Coefficient of variation (CV) across the bovine, human, and murine albumins represented in (B) for centroid voltage (blue), stability or horizontal length (red), and center drift time (green) for each feature. High CVs indicate significant differences between fingerprints. D: Comparison of type I (Dasantinib, left) and type II (Imatinib, right) inhibitors bound to Abl kinase. CIU distinguishes the binding location of inhibitors to the kinase, enabling a screening assay based on the region of maximal difference in the CIU fingerprint (far right) [17]. E: IgG subtypes 1–4 (left to right) are quantitatively distinguished by CIU [45]**. Each subtype exhibits different patterns of disulfide bonding in a broadly conserved overall structure, resulting in different CIU fingerprints.
Developing CIU for the High Throughput Assessment of Protein-Ligand Interactions
Characterizing the binding of ligands to proteins and protein complexes is a rapidly growing application area for CIU measurements, as the information content of such experiments can be used to rapidly provide binding affinities, inform on the nature of ligand attachment, and elucidate the location of binding. Binding locations can be differentiated by CIU, as the binding of a ligand to different sites in a protein results in differential alterations to its unfolding pathway. By comparing against CIU fingerprints acquired for ligands with known binding sites, specific binding locations for uncharacterized ligands can be determined. Building on early work in this area [9], a number of reports now utilize CIU to probe allosteric and conformationally-selective binding modes in the context of both inhibitor screening and analysis as well as probing membrane protein-lipid interactions [16,39]. Because discrete ligand bound states can be resolved and analyzed separately in CIU, stability shifts detected and compared between binding states can be used as evidence to support a cooperative stabilization mechanism. For example, such a mechanism was detected in the Concanavalin A tetramer upon polysaccharide binding [16]*. In another report, CIU indicated that a compact conformation of a ligand-bound protein was highly stabilized, suggesting a possible allosteric binding mode in the context of the protein system in question, which was confirmed by hydrogen-deuterium exchange [39].
CIU has been widely utilized to assess binding of inhibitors and drugs to enzymes [17,40,41]. For example, the kinase domain of BCR-Abl, a target implicated in chronic myeloid leukemia, was screened against a small library of kinase inhibitors using CIU [17]. Inhibitors having known selectivities for the active or inactive states of the kinase produced significantly different CIU fingerprints (Fig. 2D), enabling the development of a classification system based on narrow regions of the acquired fingerprints where the two classes produced maximally different unfolding patterns. Another kinase, protein kinase A (PKA), was probed by CIU [40], similarly revealing significant differences in gas-phase unfolding upon binding different kinase inhibitor classes. CIU was also used to probe binding of HIV drugs to the membrane protein ZMPSTE24 [41], demonstrating that shifts in gas-phase protein stability can be directly correlated to solution phase Kd values.
CIU measurements are uniquely suited to analyze the role of lipids and other stabilizing molecules bound to heterogeneous membrane proteins. An early example of such work utilized CIU to classify a range of lipids interacting with membrane protein channels, where gas-phase unfolding data provided predictive information allowing the authors to identify functional lipids that bore structural and functional consequences when attached to the proteins studied [42]**. This type of CIU assessment is now part of an automated workflow [15]*, enabling the rapid quantitative analysis of membrane protein stabilization through lipid and ligand binding. Most CIU studies of membrane protein lipid binding are carried out over the entire ensemble of binding stoichiometries detected, leading to uncertainty surrounding the role of individual lipid bound states in contributing to overall protein stability. However, recent work utilizing a heated electrospray ion source coupled to IM-MS demonstrates that most lipids dissociate from model membrane proteins in CIU experiments as neutral species, confirming the validity of such ensemble analysis and pointing the way toward improved IM-MS instrumentation tailored for the CIU analysis of membrane protein ligand binding [43].
CIU for the Rapid Characterization of Biotherapeutics
As biotherapeutics have emerged as a multibillion dollar industry, their analytical characterization has received proportional interest. Characterization of monoclonal antibodies is highly challenging given their size and the dynamic nature of their post-translational modifications, the state of which directly influences their function and efficacy. Given their critical importance and complex nature, as well as the need for high-throughput analysis and quality control metrics, CIU is ideally poised to be a part of future biotherapeutic analysis workflows. Recently, CIU methods have been applied to the characterization of the NIST monoclonal antibody standard [44], comparative analyses of immunoglobulins [45], active innovator and biosimilar therapeutics [46,47], and antibody-drug conjugates (ADCs) [48], indicating the rapid expansion of CIU applications in this area.
CIU has been used to rapidly distinguish subtle differences in large antibodies, such as between IgG subclasses with different disulfide bonding patterns [45]**. The differences in disulfide bridging, despite identical sequences and other post-translational modifications, resulted in nearly identical mass and arrival time information, but could be quantitatively differentiated using CIU (Fig. 2E). Glycosylated and deglycosylated IgGs were also distinguished by CIU, indicating that CIU has broad applicability to rapidly distinguish subtle changes to large proteins that are otherwise highly challenging to characterize. More recent work compares an innovator biotherapeutic, Remicade, with Remsima, the first FDA-approved antibody-based biosimilar [47]. CIU was used as part of a multi-attribute monitoring (MAM) workflow, and provided a rapid assessment of therapeutic similarity, with the differences detected amongst biotherapeutic lots of Remsima linked to variations in antibody glycoforms using bottom-up proteomics. Other recent studies have extended CIU into the analysis of antibody-drug conjugates (ADCs), an area of intense pharmaceutical interest. Recent work has demonstrated drug conjugation serves to stabilize monoclonal antibodies in a manner readily detectable by CIU [48]. As such, the rapid analysis of biotherapeutics is clearly a growth area for CIU methods, where the technique promises to provide key solutions to the growing challenges surrounding the quality control and similarity assessment of intact protein therapeutics.
The Future of CIU
Future advances in CIU look to generate more accurate and specific measurements of proteins and complexes as improvements to instrumentation and analysis methods continue. As these measurements improve, CIU will provide greater detail on the structures of gas phase biomolecules and thus further improve our understanding of gas-phase protein biophysics. CIU fingerprinting has already shown great promise for screening protein-ligand interactions, as well as for protein structural analysis and biotherapeutic characterization. Development of CIU into a true high-throughput screening technology, using optimized assays of only a few activation energies alongside high-throughput sample introduction tools and automated data processing, holds great potential for many applications. The high information content of CIU experiments, compared to many common screening techniques, has the potential to allow for more targeted drug screening and improved protein engineering methodologies.
In the longer term, it is possible to envision CIU being used in multi-stage analyses of intact protein complexes, comprising unfolding, complex dissociation, and top-down down sequencing into a single workflow aimed at comprehensive protein complex discovery and identification. CIU transitions could also be developed into sensitive detection assays, in which specific unfolding transitions could be uniquely associated with a protein isoform and monitored to determine the presence of this conformational state within a complex mixture. In general, CIU methods represent a promising approach in our ever expanding needs surrounding the structural characterization of proteins, protein complexes and protein therapeutics.
Highlights.
Collision induced unfolding (CIU) refers primarily to use of biomolecular unfolding in the gas phase to detect subtle changes in protein structure, stability or composition.
CIU can probe a wide range of biomolecules, from multiprotein complexes to biotherapeutics.
CIU has great potential as a high throughput technique, capable of detecting changes in protein structure, or in protein-ligand binding mode, in seconds.
Acknowledgments
Ruotolo lab research efforts aimed at understanding the fundamental principles of CIU are supported by the National Science Foundation (CAREER 1253384), while efforts aimed at developing CIU methods applied directly to protein topology mapping is supported by the National Institutes of Health (NIGMS, R01 GM095832). CIU of Biotherapeutics in the Ruotolo lab is supported, in part, by both Eli Lilly and Company and Agilent Technologies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Osadchy M, Kolodny R. Maps of protein structure space reveal a fundamental relationship between protein structure and function. Proceedings of the National Academy of Sciences. 2011;108:12301–12306. doi: 10.1073/pnas.1102727108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redfern OC, Dessailly B, Orengo CA. Exploring the structure and function paradigm. Current Opinion in Structural Biology. 2008;18:394–402. doi: 10.1016/j.sbi.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nick Pace C, Scholtz JM, Grimsley GR. Forces stabilizing proteins. FEBS Lett. 2014;588:2177–2184. doi: 10.1016/j.febslet.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konermann L, Vahidi S, Sowole MA. Mass Spectrometry Methods for Studying Structure and Dynamics of Biological Macromolecules. Analytical Chemistry. 2014;86:213–232. doi: 10.1021/ac4039306. [DOI] [PubMed] [Google Scholar]
- 5.Lanucara F, Holman SW, Gray CJ, Eyers CE. The power of ion mobility-mass spectrometry for structural characterization and the study of conformational dynamics. Nat Chem. 2014;6:281–294. doi: 10.1038/nchem.1889. [DOI] [PubMed] [Google Scholar]
- 6.Wyttenbach T, von Helden G, Bowers MT. Gas-Phase Conformation of Biological Molecules: Bradykinin. Journal of the American Chemical Society. 1996;118:8355–8364. [Google Scholar]
- 7.Clemmer DE, Hudgins RR, Jarrold MF. Naked Protein Conformations. Cytochrome c in the Gas Phase. Journal of the American Chemical Society. 1995;117:10141–10142. [Google Scholar]
- 8.Benesch JLP, Aquilina JA, Ruotolo BT, Sobott F, Robinson CV. Tandem Mass Spectrometry Reveals the Quaternary Organization of Macromolecular Assemblies. Chemistry & Biology. 2006;13:597–605. doi: 10.1016/j.chembiol.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Hyung SJ, Robinson CV, Ruotolo BT. Gas-phase unfolding and disassembly reveals stability differences in ligand-bound multiprotein complexes. Chemistry & biology. 2009 doi: 10.1016/j.chembiol.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Shelimov KB, Clemmer DE, Hudgins RR, Jarrold MF. Protein structure in Vacuo: Gas-phase conformations of BPTI and cytochrome c. Journal of the American Chemical Society. 1997;119:2240–2248. [Google Scholar]
- 11.Shelimov KB, Jarrold MF. Conformations, Unfolding, and Refolding of Apomyoglobin in Vacuum: An Activation Barrier for Gas-Phase Protein Folding. Journal of the American Chemical Society. 1997;119:2987–2994. [Google Scholar]
- 12.Ruotolo BT, Hyung S-J, Robinson PM, Giles K, Bateman RH, Robinson CV. Ion mobility-mass spectrometry reveals long-lived, unfolded intermediates in the dissociation of protein complexes. Angewandte Chemie (International ed in English) 2007;46:8001–8004. doi: 10.1002/anie.200702161. [DOI] [PubMed] [Google Scholar]
- 13**.Eschweiler JD, Martini RM, Ruotolo BT. Chemical Probes and Engineered Constructs Reveal a Detailed Unfolding Mechanism for a Solvent-Free Multidomain Protein. Journal of the American Chemical Society. 2016 doi: 10.1021/jacs.6b11678. jacs.6b11678-jacs.11676b11678. This study reports for the first time a detailed mechanism of multi domain protein, serum albumin, unfolding in the gas phase. Overlaying the dissociation curve of domain specific ligands to CIU fingerprint helped correlate domain unfolding to transitions observed in CIU. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopper JTS, Oldham NJ. Collision Induced Unfolding of Protein Ions in the Gas Phase Studied by Ion Mobility-Mass Spectrometry: The Effect of Ligand Binding on Conformational Stability. Journal of the American Society for Mass Spectrometry. 2009;20:1851–1858. doi: 10.1016/j.jasms.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 15*.Allison TM, Reading E, Liko I, Baldwin AJ, Laganowsky A, Robinson CV. Quantifying the stabilizing effects of protein-ligand interactions in the gas phase. Nature communications. 2015;6:8551–8551. doi: 10.1038/ncomms9551. The Pulsar software package provided in this report standardizes the analysis of membrane protein-ligand interactions, enabling quantitative comparisons to distinguish between functional and non-specific binding events observed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niu S, Ruotolo BT. Collisional unfolding of multiprotein complexes reveals cooperative stabilization upon ligand binding. Protein Science. 2015;24:1272–1281. doi: 10.1002/pro.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabuck JN, Hyung SJ, Ko KS, Fox CC, Soellner MB, Ruotolo BT. Activation state-selective kinase inhibitor assay based on ion mobility-mass spectrometry. Analytical Chemistry. 2013;85:6995–7002. doi: 10.1021/ac4012655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18**.Eschweiler JD, Rabuck-Gibbons JN, Tian Y, Ruotolo BT. CIUSuite: A Quantitative Analysis Package for Collision Induced Unfolding Measurements of Gas-Phase Protein Ions. Analytical Chemistry. 2015;87:11516–11522. doi: 10.1021/acs.analchem.5b03292. The CIUSuite software package reported here provides, in our view, the most versatile quantitative analysis package for CIU data currently available, particularly when coupled to automated data extraction [20]. The capability of CIU to highlight and quantify subtle differences in protein structure and modifications are explored, as well as the potential to build high-throughput screens using CIU as a classification technique. [DOI] [PubMed] [Google Scholar]
- 19.Sivalingam GN, Yan J, Sahota H, Thalassinos K. Amphitrite: A program for processing travelling wave ion mobility mass spectrometry data. International Journal of Mass Spectrometry. 2013;345–347:54–62. doi: 10.1016/j.ijms.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haynes SE, Polasky DA, Dixit SM, Majmudar JD, Neeson K, Ruotolo BT, Martin BR. Variable-Velocity Traveling-Wave Ion Mobility Separation Enhancing Peak Capacity for Data-Independent Acquisition Proteomics. Analytical Chemistry. 2017 doi: 10.1021/acs.analchem.7b00112. acs.analchem.7b00112-acs.analchem.00117b00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koeniger SL, Merenbloom SI, Valentine SJ, Jarrold MF, Udseth HR, Smith RD, Clemmer DE. An IMS-IMS Analogue of MS-MS. Analytical Chemistry. 2006;78:4161–4174. doi: 10.1021/ac051060w. [DOI] [PubMed] [Google Scholar]
- 22.Merenbloom SI, Koeniger SL, Valentine SJ, Plasencia MD, Clemmer DE. IMS-IMS and IMS-IMS-IMS/MS for Separating Peptide and Protein Fragment Ions. Analytical Chemistry. 2006;78:2802–2809. doi: 10.1021/ac052208e. [DOI] [PubMed] [Google Scholar]
- 23.Pierson NA, Clemmer DE. An IMS–IMS threshold method for semi-quantitative determination of activation barriers: Interconversion of proline cis↔trans forms in triply protonated bradykinin. International Journal of Mass Spectrometry. 2015;377:646–654. doi: 10.1016/j.ijms.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehmood S, Allison TM, Robinson CV. Mass Spectrometry of Protein Complexes: From Origins to Applications. Annual Review of Physical Chemistry. 2015;66:453–474. doi: 10.1146/annurev-physchem-040214-121732. [DOI] [PubMed] [Google Scholar]
- 25.Felitsyn N, Kitova EN, Klassen JS. Thermal decomposition of a gaseous multiprotein complex studied by blackbody infrared radiative dissociation. Investigating the origin of the asymmetric dissociation behavior. Analytical Chemistry. 2001;73:4647–4661. doi: 10.1021/ac0103975. [DOI] [PubMed] [Google Scholar]
- 26.Ruotolo BT, Giles K, Campuzano I, Sandercock AM, Bateman RH, Robinson CV. Evidence for macromolecular protein rings in the absence of bulk water. Science (New York, NY) 2005;310:1658–1661. doi: 10.1126/science.1120177. [DOI] [PubMed] [Google Scholar]
- 27.Hall Z, Politis A, Bush MF, Smith LJ, Robinson CV. Charge-state dependent compaction and dissociation of protein complexes: Insights from ion mobility and molecular dynamics. Journal of the American Chemical Society. 2012;134:3429–3438. doi: 10.1021/ja2096859. [DOI] [PubMed] [Google Scholar]
- 28.Fegan SK, Thachuk M. A Charge Moving Algorithm for Molecular Dynamics Simulations of Gas-Phase Proteins. Journal of Chemical Theory and Computation. 2013;9:2531–2539. doi: 10.1021/ct300906a. [DOI] [PubMed] [Google Scholar]
- 29.Fegan SK, Thachuk M. Controlling Dissociation Channels of Gas-Phase Protein Complexes Using Charge Manipulation. Journal of The American Society for Mass Spectrometry. 2014;25:722–728. doi: 10.1007/s13361-014-0831-1. [DOI] [PubMed] [Google Scholar]
- 30.Thachuk M, Fegan SK, Raheem N. Description and control of dissociation channels in gas-phase protein complexes. The Journal of Chemical Physics. 2016;145:65101–65101. [Google Scholar]
- 31*.Popa V, Trecroce DA, McAllister RG, Konermann L. Collision-Induced Dissociation of Electrosprayed Protein Complexes: An All-Atom Molecular Dynamics Model with Mobile Protons. Journal of Physical Chemistry B. 2016;120:5114–5124. doi: 10.1021/acs.jpcb.6b03035. This study reports the first attempt at implementing mobile proton model to all-atom MD simulations for examining the CIU behavior of protein complexes in vacuo. The results of the study are consistent with the asymmetric charge partitioning caused by subunit unfolding and charge migration, which is in good agreement with experimental studies. [DOI] [PubMed] [Google Scholar]
- 32.Freeke J, Robinson CV, Ruotolo BT. Residual counter ions can stabilise a large protein complex in the gas phase. International Journal of Mass Spectrometry. 2010;298:91–98. [Google Scholar]
- 33.Wagner ND, Kim D, Russell DH. Increasing Ubiquitin Ion Resistance to Unfolding in the Gas Phase Using Chloride Adduction: Preserving More “Native-Like” Conformations Despite Collisional Activation. Analytical Chemistry. 2016;88:5934–5940. doi: 10.1021/acs.analchem.6b00871. [DOI] [PubMed] [Google Scholar]
- 34.Han L, Hyung SJ, Mayers JJS, Ruotolo BT. Bound anions differentially stabilize multiprotein complexes in the absence of bulk solvent. Journal of the American Chemical Society. 2011;133:11358–11367. doi: 10.1021/ja203527a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han L, Hyung SJ, Ruotolo BT. Bound cations significantly stabilize the structure of multiprotein complexes in the gas phase. Angewandte Chemie - International Edition. 2012;51:5692–5695. doi: 10.1002/anie.201109127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samulak BM, Niu S, Andrews PC, Ruotolo BT. Ion Mobility-Mass Spectrometry Analysis of Crosslinked Intact Multiprotein Complexes: Enhanced Gas-phase Stabilities and Altered Dissociation Pathways. Analytical Chemistry. 2016:5290–5298. doi: 10.1021/acs.analchem.6b00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Zhong Y, Han L, Ruotolo BT. Collisional and Coulombic unfolding of gas-phase proteins: high correlation to their domain structures in solution. Angewandte Chemie (International ed in English) 2014;53:9209–9212. doi: 10.1002/anie.201403784. This study demonstrates positive correlation between number of domains in a group of sixteen proteins with molecular weight ranging from 8–78 kDa and the number of unfolding transitions observed in the gas phase studied using CIU IM-MS. [DOI] [PubMed] [Google Scholar]
- 38.Laszlo KJ, Munger EB, Bush MF. Folding of Protein Ions in the Gas Phase after Cation-to-Anion Proton-Transfer Reactions. Journal of the American Chemical Society. 2016;138:9581–9588. doi: 10.1021/jacs.6b04282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beveridge R, Migas LG, Payne KAP, Scrutton NS, Leys D, Barran PE. Mass spectrometry locates local and allosteric conformational changes that occur on cofactor binding. Nature Communications. 2016;7:12163–12163. doi: 10.1038/ncomms12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Byrne DP, Vonderach M, Ferries S, Brownridge PJ, Eyers CE, Eyers PA. cAMP-dependent protein kinase (PKA) complexes probed by complementary differential scanning fluorimetry and ion mobility–mass spectrometry. Biochemical Journal. 2016;473:3159–3175. doi: 10.1042/BCJ20160648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehmood S, Marcoux J, Gault J, Quigley A, Michaelis S, Young SG, Carpenter EP, Robinson CV. Mass spectrometry captures off-target drug binding and provides mechanistic insights into the human metalloprotease ZMPSTE24. Nat Chem. 2016;8:1152–1158. doi: 10.1038/nchem.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42**.Laganowsky A, Reading E, Allison TM, Ulmschneider MB, Degiacomi MT, Baldwin AJ, Robinson CV. Membrane proteins bind lipids selectively to modulate their structure and function. Nature. 2014;510:172–175. doi: 10.1038/nature13419. This report lays the foundation for defining the selectively of lipid binding to membrane proteins using a CIU-based stability shift assay. Lipids that have functional relevance to membrane proteins were found to have enhanced CIU stability compared to lipids that were bound indiscriminantly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Cong X, Liu W, Laganowsky A. Characterization of Membrane Protein–Lipid Interactions by Mass Spectrometry Ion Mobility Mass Spectrometry. Journal of The American Society for Mass Spectrometry. 2016 doi: 10.1007/s13361-016-1555-1. [DOI] [PubMed] [Google Scholar]
- 44*.Campuzano IDG, Larriba C, Bagal D, Schnier PD. Ion Mobility and Mass Spectrometry Measurements of the Humanized IgGk NIST Monoclonal Antibody. ACS Symposium Series. 2015;1202:75–112. A complete overview of ion mobility and mass spectrometry based techniques for evaluation of antibodies, focused on characterization of the NIST monoclonal antibody standard. CIU curves for the NIST standard are combined with molecular dynamics simulations to provide characterization of structure and properties. [Google Scholar]
- 45**.Tian Y, Han L, Buckner AC, Ruotolo BT. Collision Induced Unfolding of Intact Antibodies: Rapid Characterization of Disulfide Bonding Patterns, Glycosylation, and Structures. Analytical Chemistry. 2015;87:11509–11515. doi: 10.1021/acs.analchem.5b03291. This report demonstrates the potential of CIU as a tool for rapid characterization of biotherapeutics. CIU is used to differentiate IgG subclasses with different disulfide bonding patterns, as well as differences in glycosylation state, all in rapid fashion on intact monoclonal antibodies. [DOI] [PubMed] [Google Scholar]
- 46.Ferguson CN, Gucinski-Ruth AC. Evaluation of Ion Mobility-Mass Spectrometry for Comparative Analysis of Monoclonal Antibodies. Journal of the American Society for Mass Spectrometry. 2016;27:822–833. doi: 10.1007/s13361-016-1369-1. [DOI] [PubMed] [Google Scholar]
- 47.Pisupati K, Tian Y, Okbazghi S, Benet A, Ackermann R, Ford M, Saveliev SV, Hosfield CM, Urh M, Carlson E, et al. A Multidimensional Analytical Comparison of Remicade and the Biosimilar Remsima. Analytical Chemistry. 2017 doi: 10.1021/acs.analchem.6b04436. acs.analchem.6b04436-acs.analchem.04436b04436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Botzanowski T, Erb S, Hernandez-Alba O, Ehkirch A, Colas O, Wagner-Rousset E, Rabuka D, Beck A, Drake PM, Cianferani S. Insights from native mass spectrometry approaches for top- and middle- level characterization of site-specific antibody-drug conjugates. mAbs. 2017:0–0. doi: 10.1080/19420862.2017.1316914. [DOI] [PMC free article] [PubMed] [Google Scholar]