Abstract
Individuals on probation and parole are disproportionately at high risk for HIV infection and experience significant barriers to accessing health care. This study was a two-group randomized controlled trial conducted at a community corrections office and was designed to link HIV positive probationers/parolees to HIV treatment in the community. HIV positive participants were assigned to one of the two treatment conditions: 1) Project Bridge (PB), an intensive case-management intervention; or 2) treatment as usual (TAU), involving standard referral to treatment. We hypothesized that PB would be more effective than TAU in terms of initiating individuals in community HIV treatment. We found no difference in rates of, or time to, treatment initiation when comparing PB to TAU (all ns>0.05). Additionally, there was no statistically significant difference between HIV medication regiment initiation by treatment arm (P>0.05). Despite limitations, we found that probationers and parolees were willing to be screened and linked to treatment.
Keywords: Community HIV treatment, probation, parole, case management
INTRODUCTION
Nearly 4.7 million adults were under criminal justice supervision in the community in 2014, of which 82 percent were on probation [1]. Although the overall rate of individuals under correctional supervision has declined since 2007, probationers and parolees still represent the largest group within the total correctional population, and the number of parolees has actually grown slightly since 2007 [1]. There is wealth of evidence that criminal justice populations in the community are at disproportionately high risk for HIV infection [2, 3] and experience substantial barriers to accessing health care [4, 5].
The time immediately following incarceration, when individuals are likely to be engaged in community supervision, represents a high risk period. Probationers and parolees report engaging in risky sexual behaviors during this time, including unprotected sex, transactional sex, and sex with multiple partners [4, 2, 3, 6, 7]. In a study examining dissolution of primary intimate relationships during incarceration, Khan et al. [8] found that ending a primary relationship while incarcerated was associated with almost three times the prevalence of having two or more new partners within four weeks of release. Intravenous drug use is also a prevalent HIV risk factor amongst the community corrections population [4, 2, 3, 9].
Following release, probationers and parolees are tasked with immediately establishing basic necessities such as housing, employment, social supports, insurance, medical care as well as compliance with any community corrections requirements. In a qualitative study assessing former inmate post-release behaviors, participants acknowledged the challenge of community reentry and reported engaging in drug use and sex for drugs, money, or transportation as a means of coping [10].
Community reentry challenges also pose a health risk for HIV positive probationers and parolees who fail to engage in HIV treatment following release. Studies examining health outcomes for inmates receiving HIV treatment suggest the ability to achieve high rates of viral suppression during incarceration, but those health gains are not sustained post-release [11–14].
Failure to enroll with an HIV clinic within 30 days of release [15] and to fill antiretroviral therapy (ART) prescriptions before medication interruption occurs [11, 12] are major treatment retention challenges, and are linked to poor clinical outcomes. Individuals with intermittent access to ART experience similar health outcomes as those without access to the medication and HIV positive individuals who never initiate ART therapy, including diminished CD4 cell counts [16]. Recent research demonstrates that engagement in pre-release activities including discharge planning [17, 15, 18], disease management and HIV education, as well as completing a needs assessment, are associated with retention in HIV treatment following release [17]. However, access to such services pre-release is not guaranteed and additional community-based supports are needed to link the HIV-positive probation population, who do not experience a long-term jail or prison incarceration, with treatment.
Studies involving case management with HIV populations to date have shown mixed results. Previous studies examining successful facilitators to community HIV treatment for criminal justice populations have emphasized the use of enhanced case management [19, 20] and patient navigation services [21]. However, these studies have focused on providing linkage to care for individuals making an immediate transition from incarceration to the community. Within the general population, research demonstrates a higher efficacy for case management in linking individuals to HIV treatment versus passive referral [22–24]. Furthermore, these studies are largely focused on individuals who were recently diagnosed as being HIV-positive.
Thus, the current study sought to identify an effective linkage to care intervention that would facilitate the initiation of HIV treatment services and ART medication for HIV-positive probationers and parolees already in the community. Using a randomized controlled trial design, we examined the feasibility of linking individuals who identified as HIV-positive at community corrections, either by testing positive for HIV or by self-reporting their positive status, to community-based medical services (see Gordon et al., 2013 for further study details). The experimental intervention utilized the Project Bridge (PB) team-based model, which was originally designed to assist HIV-positive prisoners transitioning back into the community by providing intensive case management, medical, and social support to HIV infected individuals after their release from prison (see below for further description on PB). This model was applied to the community corrections population for the current study. The larger goals of this study were: (1) to determine if PB can be effectively utilized by individuals with HIV who are recruited through community corrections; and (2) to determine the impact of PB versus treatment as usual (TAU) on initiation of HIV treatment.
METHODS
Participants
Participants were recruited between April 2011 and May 2015 from the Central Intake Unit of the Baltimore, Maryland Probation/Parole Offices. To be eligible for entry into the intervention study, participants had to be a male or female adult on probation or parole in Baltimore City, with plans to reside in Baltimore City for the duration of the study, and be HIV positive. Furthermore, these individuals needed to be out of care, or disengaging with current treatment due to low or no satisfaction with their current provider, as demonstrated by not attending treatment within the past 30 days. Individuals who did not meet these criteria or were unable to provide informed consent were excluded.
HIV positive individuals were identified either via self-report, with a confirmatory rapid oral swab HIV test, or via participation in a randomized HIV testing study assessing the willingness of probationers and parolees to be tested for HIV in community corrections or a local clinic. Individuals participating in the randomized testing study agreed to be tested for HIV using a rapid oral swab test and were randomly assigned to receive the HIV test immediately on-site in a private office within the community corrections unit or at a Baltimore City walk-in clinic (see Gordon et al., 2013 & Gordon et al. 2016 for further study description)[25, 26]. Participants tested on-site at community corrections with reactive test results, who were not previously aware of his/her HIV status were immediately referred to a community health center in Baltimore City for confirmatory testing. Additionally, participants randomized for primary testing at the local clinic with reactive test results were immediately administered a confirmatory test. All newly diagnosed HIV positive participants were subsequently contacted regarding their willingness to engage in the PB model. Participants with reactive test results who admitted to being aware of his/her status only after completing primary testing were also offered enrollment in the intervention study.
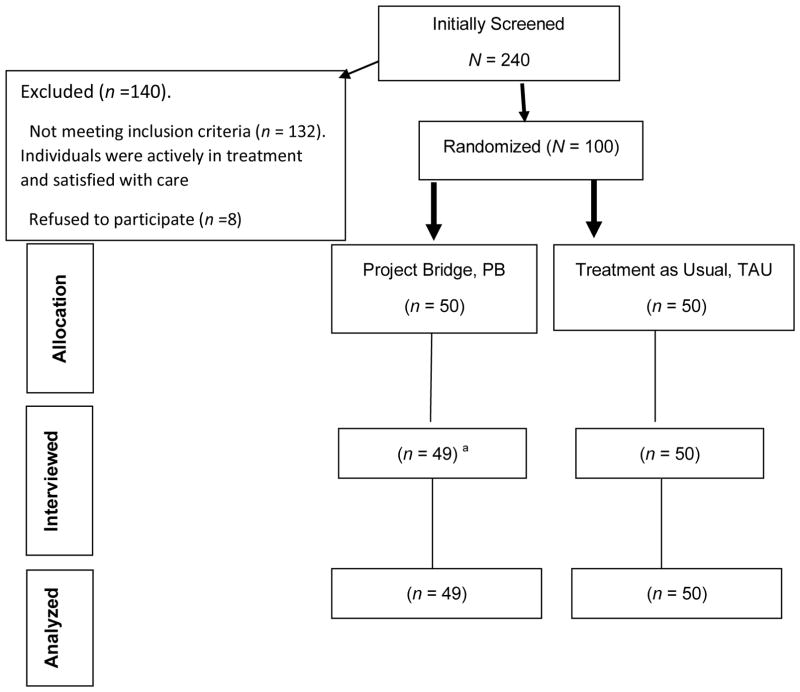
As shown in the consort diagram (see Figure I), of the 240 probationers and parolees who self-reported being HIV positive and were initially screened, 140 (58%) were excluded after screening. Of those 140, 132 did not meet inclusion criteria as they were actively in HIV treatment and satisfied with their current provider and 8 refused to provide consent to participate. The remaining 100 individuals were consented and randomized.
Figure 1.
Consort Diagram
Explanatory Footnote
aone participant was not located for their 3-month follow-up assessment.
Project Bridge (PB): Intensive case management
Treatment as Usual (TAU): Passive referral to treatment
Study design
This study was a two-group randomized controlled trial. Participants were assigned, within gender, to one of the two treatment conditions. A random permutation procedure was used to ensure that participants had an equal chance of being assigned to either PB or TAU. For each block of four participants, two were assigned at random to the condition receiving PB, and two to the condition receiving TAU. Blocks of four participants rather than blocks of two participants were used in order to thwart any attempt by an interested observer, such as a clinic staff member, to deduce the random assignment procedure. Immediately following random assignment, the research assistant reiterated the treatment condition requirements, which were initially described in the informed consent process. Assessments were conducted at baseline and three months post-randomization (only 3-month outcomes are reported).
Human subjects
The study was approved by the Institute’s Institutional Review Board (IRB) and the state correctional research committee. The study investigators obtained a Federal Certificate of Confidentiality in order to protect participants from being subpoenaed for the purposes of releasing sensitive information. The study was prospectively registered at ClinicalTrials.gov.
Intervention
Participants were randomly assigned, within gender, to receive either the PB or the TAU model of care for twelve months. The TAU model represented the standard referral to treatment, providing participants with a list of HIV treatment providers in Baltimore City where they would receive the standard level of care (i.e., not a PB intervention model). Participants were informed that they would be contacted for follow-up visits at three, six, and twelve months following randomization. Any participants randomized to TAU who did not initiate and/or engage in treatment by the six-month follow-up were offered a “rescue” opportunity to cross-over to the PB intervention group. Participants were deemed to have failed to initiate in treatment if by month six they had not scheduled or attended a medical or counseling appointment with a provider or at a clinic that included having their viral load measured at least once, and did not plan to visit a provider or clinic. Participants crossing over to the PB model of care were eligible to receive intervention services for twelve months.
Project Bridge
PB is a team-based model of care providing intensive case management for individuals with HIV as they transition back into the community from incarceration. The primary goal of PB is to increase continuity of medical care through social stabilization. The PB intervention initiated as a Ryan White Comprehensive AIDS Resources Emergency Act-funded Special Project of National Significance (SPNS) research and demonstration program to provide 12-months of intensive case management, medical, and social support to HIV infected individuals after their release from prison or jail in Rhode Island [20, 27]. Despite completion of SPNS funding in 2002, the Rhode Island Department of Health and Ryan White Part B funds have continued to support the PB model with HIV positive prisoners transitioning back into the community. The current study aimed to apply this model to the HIV positive community corrections population in Baltimore, Maryland.
Study participants receiving the PB model in the experimental arm received twelve months of services coordinated by case management teams comprised of a Master’s level social worker serving as the case manager, as well as a social work assistant acting as an outreach worker. PB case management teams assisted participants with many of their social and practical needs, with an emphasis on facilitating participation in HIV medical care. Participants also received support to engage in addiction and mental health treatment, other support services, and entitlement. Case managers conducted an initial psycho-social assessment with each participant to better understand the participant’s unique history and needs, which was then used to develop an individualized service plan. The service plan initially addressed immediate concerns including medications, medical coverage, shelter/housing, medical care, substance use treatment, mental health treatment, income and social benefits, and legal obligations.
Throughout the intervention, case managers were responsible for overall case planning, and conducting case reviews and facilitating case conferences for all decisions requiring clinical judgement. Case managers were also available to attend all medical exams, as requested by the participant, to confer with the doctor, assist in communication, and addressing adherence concerns. Participants were requested to attend weekly sessions with the case manager for the first three months of the intervention. Participants could also attend additional meetings with the outreach worker. This care management team member was responsible for assisting participants with appointment registration, negotiating aspects of the healthcare visit, accompanying participants to appointments as requested, supporting attendance and participation in 12-Step Programs, seeking out drug-free social and recreational opportunities, and teaching participants how to utilize social service resources. Additionally, all participants receiving the PB intervention were eligible to receive bus tokens to facilitate access to their health care and support services appointments. Participants randomized to TAU were free to enter any community health clinic.
Assessments
All participants received the same assessments at baseline covering core domains across Seek, Test, Treat, and Retain Criminal Justice grants that focused on the following (see Chandler et al., 2015 for further description of the measures; and Chandler et al. 2017. Three-month follow-up interviews assessed self-reported recent substance use, substance use treatment adherence, crime and legal activities, sexual risk behavioral and injection drug use, access to medical care, general health and depression risk, HAART medication adherence, and service utilization adherence.
Outcome Measures
The primary short-term outcome measure examined during the three-month post randomization period was participant initiation in community HIV treatment (yes vs. no). A secondary outcome measure of initiation in an HIV medication regiment (yes vs. no) was assessed for participants who were prescribed HIV medication in their community HIV treatment setting.
Hypotheses
We hypothesized that PB would be more effective than TAU in terms of initiating HIV positive probationers and parolees into community-based HIV treatment. A second hypothesis suggested that participants randomized to PB would be more likely to initiate an HIV medication regiment than those in TAU, if prescribed medications in that treatment condition.
Statistical Analysis
The 100 participants (PB, n = 50, TAU, n= 50) were compared by treatment condition with regard to each outcome variable listed above using logistic regression analysis for the binary outcome measures [28]. The explanatory variable in the model was treatment condition (PB vs. TAU), and gender was included as a predictor variable in view of the need to examine differences in responsiveness to treatment by men and women.
Cox regression survival curves were used to assess whether the time until first medical care visit, representing initiation in treatment, differed between the two study conditions. The Cox regression for survival analysis method was employed based on the assumption of independence in the time to treatment initiation between participants, and a multiplicative relationship between predictor variables and treatment initiation. The researchers also assumed a constant hazard ratio over the three-month period, suggesting that the effect of the treatment condition was constant during the follow-up time.
RESULTS
Participant characteristics
Baseline demographic information is presented in Table I. In general, participants in both the control and intervention group had similar demographic characteristics. The average age of participants in the total sample was 46 years (SD=7.6), 78% self-identified as male and 93% identified as Black or African American, with only 3% identifying as Hispanic or Latino. More than half of all study participants were high school graduates or were general educational development equivalent (64%), while one-third (34%) indicated having not completed high school. Additionally, 94% of the study sample was unemployed. Thirty-four percent reported having no health insurance and 39% considered themselves homeless. The majority of the community corrections population sampled (70%) were on probation, 25% were on parole and 5% reported supervision from both probation and parole.
Table 1.
Participant Baseline Characteristics by Treatment Condition (N=100)
| Project Bridge (PB) (n=50) | Treatment as Usual (TAU) (n =50) | Total Sample (N=100) | |
|---|---|---|---|
| Age, m (sd) | 46.8 (6.5) | 45.1 (8.6) | 45.9 (7.6) |
| Gender, male, n (%) | 39 (78) | 39 (78) | 78 (78) |
| Race, black, n (%) | 47 (94) | 46 (92) | 93 (93) |
| Hispanic/Latino, n (%) | 1 (2) | 2 (4) | 3 (3) |
| Education Level, n (%)c | |||
| less than high school | 18 (36.7) | 16 (32) | 34 (34.3) |
| high school graduate/GED | 30 (61.2) | 33 (66) | 63 (63.6) |
| college or higher | 1 (2.1) | 1 (2) | 2 (2.1) |
| Unemployed, n (%) | 49 (98) | 45 (90) | 94 (94) |
| Income, m (sd) | |||
| Health Insurance, n (%) | 32 (64) | 34 (68) | 66 (66) |
| Homeless, n (%) | 16 (32) | 23 (46) | 39 (39) |
| Any Drug Use, m (sd)a | 17.8 (30.3) | 20.2 (32.8) | 19.0 (31.4) |
| Heroin Use, m (sd)a | 5.7 (18.5) | 6.0 (21.6) | 5.8 (20.0) |
| Heroin Use, n (%)2 | 35 (70) | 40 (80) | 75 (75) |
| Cocaine Use, n (%)b | 27 (54) | 27 (54) | 54 (54) |
| IV Drug use, n (%)b, | 23 (46) | 28 (56) | 51 (51) |
| CJ Status, n (%) | |||
| Probation | 34 (68) | 36 (72) | 70 (70) |
| Parole | 13 (26) | 12 (24) | 25 (25) |
| Both | 3 (6) | 2 (4) | 5 (5) |
| Crime days, m (sd)a | 6.3 (20.1) | 2.1 (12.8) | 4.2 (16.9) |
| Onset criminal activity, m (sd) | 15.1 (6.1) | 14.0 (4.6) | 14.6 (5.4) |
| Crime severity, m (sd)d | 5.6 (.6) | 5.6 (.7) | 5.6 (.7) |
| Prison/Jail days, m (sd)a | 15.0 (29.1) | 21.2 (32.2) | 18.1 (30.7) |
| Age first arrested, m (sd) | 18.2 (6.3) | 18.3 (5.1) | 18.3 (5.7) |
| Lifetime incarcerations, m(sd)b | 11.4 (10.5) | 10.7 (8.8) | 11.0 (9.6) |
| Year with HIV, m (sd)f | 14.3 (7.4) | 13.5 (8.3) | 13.9 (7.8) |
| HCV+, n (%)g | 24 (48) | 28 (56) | 52 (52) |
| HBV+, n (%)g | 2 (4) | 4 (8) | 6 (6) |
| Drug Treatment, m (sd)b | 2.9 (2.7) | 2.9 (3.0) | 2.9 (2.8) |
| methadone (ever), n (%) | 12 (24) | 16 (32) | 29 (29) |
| buprenorphine (ever), n (%) | 17 (34) | 13 (26) | 30 (30) |
| vaginal sex w/out condom, m (sd)e | 6.7 (21.5) | 2.9 (11.6) | 4.8 (17.3) |
| anal sex w/out a condom, m (sd),5 | 1.8 (7.7) | .6 (3.5) | 1.2 (5.9) |
Explantory footnote
past 90 days
Lifetime
1 person did not report education level
crime severity scored 1 (low) to 7 (high)
number of times during the past 90 days
year of diagnosis was only reported (no month/day)
Self-report positive
On average, participants reported living with HIV for approximately 14 years when they entered the study, and 52% reported having Hepatitis C. History of substance use was prevalent with 75% of the sample reporting some lifetime heroin use, and nearly half (54%) reporting lifetime cocaine use and injection drug use (51%). On average, participants had engaged in three drug treatment programs in their lifetime, and equal percentages of the sample had reported receiving methadone (30%) and buprenorphine (30%) medication addiction treatment in their lifetime. In the 90 days preceding randomization, the total sample had a mean of 19 days (SD=31.4) of self-reported drug use, during which time nearly 6 days included heroin use. The sample mean also averaged 4.8 (17.3) heterosexual vaginal sex encounters and 1.2 (5.9) anal sex encounters without a condom during this time period. The mean age for onset of criminal activity in the study sample was approximately 15 years (5.4). Additionally, the study sample reported an mean average of 4.2 days (16.9) of crime and 18.1 incarceration days (30.7) during the 90 days prior to randomization.
At the three-month follow-up visit, most participants had established insurance with 85% of the PB and 92% of the TAU group reporting current coverage (three-month data not shown). PB participants reported average of 3.0 (13.5) crime days, which was significantly less than the TAU group average of 7.2 days (21.5); p = 0.027. PB participants also reported significantly less drug use in the follow-up period with an average 8.9 days (21.5) of illicit substance use compared to the TAU groups average of 19.4 days (29.6); p = 0.002. However, both groups reported initiating in substance use treatment at equal rates (54%) and there was not a significant difference in uptake of medication-assisted treatment (MAT) between the groups (PB = 65%; TAU = 78%).
Outcomes
There was no statistically significant difference in participant initiation in community HIV treatment by treatment condition (χ2 (1) = .079, p = 0.778). Of the 99 participants who were interviewed at three month follow-up, 69% entered HIV treatment (PB, men=26/38, women=7/11 vs. TAU, men=28/39, women=7/11, X2 (1) = 0.333, p = 0.564). No statistically significant difference was identified between PB and TAU regarding the number of days from randomization to treatment initiation. The survival curve demonstrates that PB participants had an average of 27.2 days (31.4) between randomization and treatment initiation, compared to 26.1 days (30.6) for TAU participants (X2 (1) = .024; p=.877). The primary reasons for failure to enter HIV treatment were as follows: missed appointment (n=10), too many issues to deal with in their life (n=10), work conflict (n=3), no insurance (n=3), scared of taking medication (n=1), medication left over from prison (n=1), not accepting that they are HIV positive (n=1), and no reason (n=2).
There was no statistically significant difference in terms of those who were prescribed HIV medication and those who actually initiated an HIV medication regiment (X2 (1)=1.55, p=.166). Of the 68 participants who entered HIV treatment, 50 (74%) began HIV medication (PB=22/33; 67%) vs. TAU=28/35; 80%). Moreover, it should be noted that of the 100 randomized participants, three were newly identified with HIV, having tested positive during the initial rapid HIV testing process at the parole and probation setting (see Gordon et al. 2013). All three of the newly diagnosed participants were successfully linked to HIV treatment within the first three months (two received TAU and one received PB).
DISCUSSION
The majority of HIV positive parolees and probationers identified at a community supervision office in this randomized controlled trial were successfully linked to HIV treatment. We found no difference in rates of, or time to HIV treatment initiation when comparing the PB intensive case management strategy to the TAU passive referral. Additionally, there were no statistically significant differences between HIV medication regiment initiation by treatment condition. Thus the study results provide evidence that it is feasible to identify and link HIV-positive individuals in the community corrections population, who are not currently in treatment, to medical services in the community. The present study also demonstrates that a less intensive level of services may be sufficient to initiate treatment abstaining parolees and probationers who are aware of their HIV status into medical care.
Both study conditions demonstrated high rates of HIV treatment initiation, with 69% of the sample initiating treatment within three months of randomization. This was surprising given our hypothesis that the PB intervention would provide a more supportive and thus immediate linkage to HIV treatment compared to TAU. One possible explanation as to why there were no differences between the comparison groups is that most of the study participants had been living with HIV for a long time and case management services merely provided an opportunity to supplement what they already knew with regard to re-initiating care. Previous studies examining successful facilitators to community HIV treatment for criminal justice populations have emphasized the use of similar enhanced case management [19, 20] and patient navigation services [21]. However, these studies have focused on providing linkage to care for individuals making an immediate transition from incarceration to the community. Participants in the present study were already living in the community when PB services were offered, so the facilitators to their engagement may differ from individuals with pre-release status. Thus it is possible that the critical facilitating intervention to initiate treatment for HIV positive parolees and probationers in this study was not providing intensive case management, but simply identifying and referring them to community-based HIV treatment.
The present study contributes to the literature that seeks to understand optimal strategies for engaging the community-based criminal justice population in HIV treatment. Our study revealed a community-dwelling population of individuals aware of their HIV positive status, but not currently seeking any medical treatment. Given the high and comparable rates of initiative in both treatment conditions, it is unclear which aspects of the PB intensive case management, rather than the program taken as a whole, may facilitate treatment and/or medication regiment initiation for this population. One common element between PB and TAU services reported by participants was the receipt of regular appointment reminders and outreach calls, which may be sufficient for individuals with longer-term awareness of their HIV status than individuals who are newly diagnosed.
Previous research evidencing the effectiveness of case management on HIV treatment initiation has largely focused on linking a newly diagnosed population to treatment [24–26]. The present study only included three participants with recent HIV diagnoses. On average, our study participants lived 13.9 years (PB: 14.3 years (7.8); TAU: 13.5 years (8.3)) with their diagnosis prior to study involvement. The current study’s participants were not selected based on their undergoing a significant life event such as new diagnosis or incarceration release. Thus, it may be that intensive case management services were not necessary to stimulate the same linkage to care as needed by populations in the midst of major change. The measured impact of PB case management services may change as future analyses examine the effect of such services on sustained engagement in care and medication adherence six and twelve months post initiation.
Previous research examining the impact of intensive post-release motivational case management services found their intervention to be no more effective than comprehensive pre-release discharge planning in achieving linkage to community HIV care services [18]. As Wohl et al. [18] speculated, it may be that once a basic threshold for service initiation is met, more resource intensive efforts add little to health outcomes. Establishing insurance coverage may be one component of that basic threshold. Despite low overall rates of insurance at baseline, nearly all the PB and TAU participants were insured by the three-month follow-up. Thus is may be that having administrative support from either PB or any TAU provider to aid in establishing this basic access requirement was sufficient to complete the qualifying viral load testing medical appointment and medication initiation. In a study of HIV-positive individuals in the criminal justice system, Chen et al. [29] found health insurance to be the most significant enabling factor associated with individuals engaging in HIV care.
Participants in this study also initiated ART medication regiments at a high rate across both treatment conditions. Of the 68 individuals who initiated treatment, 74% initiated an ART medication regiment. ART medication initiation and persistence within the community dwelling criminal justice involved population is generally low [11, 30, 31, 12]. Prior research examining uptake of ART demonstrate significant associations between alcohol and illicit substance use and decreased initiation and adherence to medication [32, 29, 33, 30, 34]. In a qualitative study of recently released prisoners’ HIV treatment adherence, Haley et al. [30] found that substance use relapse was a major indicator for treatment delay and prolonged periods of ART nonadherence.
In the present study, the PB group reported significantly fewer days of substance use than the comparison group. The PB intake assessment inquires about patient’s substance use behavior and its affect on other aspects of daily life, and with case management services may have influenced PB participants’ decision to reduce substance use. However, it is unknown what specific substance use reduction services were offered to those in TAU. Both treatment groups initiated drug treatment programs, including MAT services, at similar rates. It is possible that initiation in substance use treatment services, either through PB or TAU case management, was a significant enabling factor for study participants to both initiate HIV provider services and ART.
A cautious interpretation of results is recommended because of several limitations in the current study. First, the study only involved probationers and parolees from Baltimore, so the findings are not necessarily generalizable to other geographic locations and/or populations. Second, the sample size was smaller than anticipated because we had to discontinue recruitment at one of our sites. Third, we did not obtain baseline viral loads and had to rely on clinic records for follow-up viral loads, which were often inconsistent, and thus not included in the present analysis. Fourth, we had to rely on self-report for many of the Treatment as Usual participants because we were unable to obtain clinic records. Fifth, the baseline time period and follow-up time period covered a short window of 90 days. Sixth, we were unable to collect additional process data such as dose of care for those who initiated community HIV treatment.
In conclusion, we found that probationers and parolees were willing to be screened and linked to treatment. This is notable since these activities took place in an applied setting (probation and parole office in Baltimore City). While there were no significant differences between the two treatment conditions both had high rates of treatment initiation. Moreover, it should be noted that PB (intensive case management) may have other benefits that were not measured in this current study. The individual connection with a near peer or case manager is a critical component. This can lead to assistance with the follow up and improvement and health literacy. This translate well to the criminal justice setting as is done with the Transitions clinic approach http://transitionsclinic.org. However, one should be cautious about the impact of intensive case management services for the established community corrections population.
Acknowledgments
We would like to thank the Maryland Department of Public Safety and Correctional Services (DPSCS), Chase Brexton Health Services (CBHS) and all of the participants in the study.
Funding
This research was funded by the National Institute on Drug Abuse, Grant R01 DA 16237 (Principal Investigators: Michael S. Gordon, D.P.A. & Josiah D. Rich, M.D.). Dr. Rich’s involvement in this project was also supported by NIH grants K24 DAO22112 and P30-A1-42853.
Footnotes
Conflict of Interest
The authors report no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Kaeble D, Glaze L, Tsoutis A, Minton T. Correctional Populations in the United States, 2014. Bureau of Justice Statistics; 2016. Available at: http://www.bjs.gov/content/pub/pdf/cpus14.pdf. [Google Scholar]
- 2.Adams LM, Kendall S, Smith A, Quigley E, Stuewig JB, Tangney JP. HIV risk behaviors of male and female jail inmates prior to incarceration and one year post-release. AIDS Behav. 2013;17(8):2685–94. doi: 10.1007/s10461-011-9990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark CB, McCullumsmith CB, Waesche MC, Islam MA, Francis R, Cropsey KL. HIV-risk characteristics in community corrections. J Addict Med. 2013;7(1):45–51. doi: 10.1097/ADM.0b013e3182781806. [DOI] [PubMed] [Google Scholar]
- 4.Adams J, Nowels C, Corsi K, Long J, Steiner JF, Binswanger IA. HIV risk after release from prison: a qualitative study of former inmates. J Acquir Immune Defic Syndr. 2011;57(5):429–34. doi: 10.1097/QAI.0b013e31821e9f41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cropsey KL, Binswanger IA, Clark CB, Taxman FS. The unmet medical needs of correctional populations in the United States. J Natl Med Assoc. 2012;104(11–12):487–92. doi: 10.1016/s0027-9684(15)30214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green TC, Pouget ER, Harrington M, et al. Limiting options: sex ratios, incarceration rates, and sexual risk behavior among people on probation and parole. Sex Transm Dis. 2012;39(6):424–30. doi: 10.1097/OLQ.0b013e318254c81a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrow KM, Project SSG. HIV, STD, and hepatitis risk behaviors of young men before and after incarceration. AIDS Care. 2009;21(2):235–43. doi: 10.1080/09540120802017586. [DOI] [PubMed] [Google Scholar]
- 8.Khan MR, Behrend L, Adimora AA, Weir SS, White BL, Wohl DA. Dissolution of primary intimate relationships during incarceration and implications for post-release HIV transmission. J Urban Health. 2011;88(2):365–75. doi: 10.1007/s11524-010-9538-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Havens JR, Oser CB, Leukefeld CG. Injection risk behaviors among rural drug users: implications for HIV prevention. AIDS Care. 2011;23(5):638–45. doi: 10.1080/09540121.2010.516346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luther JB, Reichert ES, Holloway ED, Roth AM, Aalsma MC. An exploration of community reentry needs and services for prisoners: a focus on care to limit return to high-risk behavior. AIDS Patient Care STDS. 2011;25(8):475–81. doi: 10.1089/apc.2010.0372. [DOI] [PubMed] [Google Scholar]
- 11.Baillargeon J, Giordano TP, Rich JD, et al. Accessing antiretroviral therapy following release from prison. JAMA. 2009;301(8):848–57. doi: 10.1001/jama.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Springer SA, Pesanti E, Hodges J, Macura T, Doros G, Altice FL. Effectiveness of antiretroviral therapy among HIV-infected prisoners: reincarceration and the lack of sustained benefit after release to the community. Clin Infect Dis. 2004;38(12):1754–60. doi: 10.1086/421392. [DOI] [PubMed] [Google Scholar]
- 13.Springer SA, Friedland GH, Doros G, Pesanti E, Altice FL. Antiretroviral treatment regimen outcomes among HIV-infected prisoners. HIV Clin Trials. 2007;8(4):205–12. doi: 10.1310/hct0804-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephenson BL, Wohl DA, Golin CE, Tien HC, Stewart P, Kaplan AH. Effect of release from prison and re-incarceration on the viral loads of HIV-infected individuals. Public Health Rep. 2005;120(1):84–8. doi: 10.1177/003335490512000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baillargeon JG, Giordano TP, Harzke AJ, Baillargeon G, Rich JD, Paar DP. Enrollment in outpatient care among newly released prison inmates with HIV infection. Public Health Rep. 2010;125(Suppl 1):64–71. doi: 10.1177/00333549101250S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pai NP, Estes M, Moodie EE, Reingold AL, Tulsky JP. The impact of antiretroviral therapy in a cohort of HIV infected patients going in and out of the San Francisco county jail. PLoS One. 2009;4(9):e7115. doi: 10.1371/journal.pone.0007115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Althoff AL, Zelenev A, Meyer JP, et al. Correlates of retention in HIV care after release from jail: results from a multi-site study. AIDS Behav. 2013;17(Suppl 2):S156–70. doi: 10.1007/s10461-012-0372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wohl DA, Scheyett A, Golin CE, et al. Intensive case management before and after prison release is no more effective than comprehensive pre-release discharge planning in linking HIV-infected prisoners to care: a randomized trial. AIDS Behav. 2011;15(2):356–64. doi: 10.1007/s10461-010-9843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordan AO, Cohen LR, Harriman G, Teixeira PA, Cruzado-Quinones J, Venters H. Transitional care coordination in New York City jails: facilitating linkages to care for people with HIV returning home from Rikers Island. AIDS Behav. 2013;17(Suppl 2):S212–9. doi: 10.1007/s10461-012-0352-5. [DOI] [PubMed] [Google Scholar]
- 20.Rich JD, Holmes L, Salas C, et al. Successful linkage of medical care and community services for HIV-positive offenders being released from prison. J Urban Health. 2001;78(2):279–89. doi: 10.1093/jurban/78.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koester KA, Morewitz M, Pearson C, et al. Patient navigation facilitates medical and social services engagement among HIV-infected individuals leaving jail and returning to the community. AIDS Patient Care STDS. 2014;28(2):82–90. doi: 10.1089/apc.2013.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craw JA, Gardner LI, Marks G, et al. Brief strengths-based case management promotes entry into HIV medical care: results of the antiretroviral treatment access study-II. J Acquir Immune Defic Syndr. 2008;47(5):597–606. doi: 10.1097/QAI.0b013e3181684c51. [DOI] [PubMed] [Google Scholar]
- 23.Gardner LI, Metsch LR, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;19(4):423–31. doi: 10.1097/01.aids.0000161772.51900.eb. [DOI] [PubMed] [Google Scholar]
- 24.Katz MH, Cunningham WE, Fleishman JA, et al. Effect of case management on unmet needs and utilization of medical care and medications among HIV-infected persons. Ann Intern Med. 2001;135(8 Pt 1):557–65. doi: 10.7326/0003-4819-135-8_part_1-200110160-00006. [DOI] [PubMed] [Google Scholar]
- 25.Gordon MS, Kinlock TW, McKenzie M, Wilson ME, Rich JD. Rapid HIV testing for individuals on probation/parole: outcomes of an intervention trial. AIDS Behav. 2013;17(6):2022–30. doi: 10.1007/s10461-013-0456-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon MS, Carswell SB, Wilson M, et al. Factors Associated With Receiving Rapid HIV Testing Among Individuals on Probation or Parole. J Correct Health Care. 2016;22(4):290–9. doi: 10.1177/1078345816669347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaller ND, Holmes L, Dyl AC, et al. Linkage to treatment and supportive services among HIV-positive ex-offenders in Project Bridge. J Health Care Poor Underserved. 2008;19(2):522–31. doi: 10.1353/hpu.0.0030. [DOI] [PubMed] [Google Scholar]
- 28.Hosmer DW, Lemenshow S. Applied logistic regression. New York: Wiley; 1989. [Google Scholar]
- 29.Chen NE, Meyer JP, Avery AK, et al. Adherence to HIV treatment and care among previously homeless jail detainees. AIDS Behav. 2013;17(8):2654–66. doi: 10.1007/s10461-011-0080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haley DF, Golin CE, Farel CE, et al. Multilevel challenges to engagement in HIV care after prison release: a theory-informed qualitative study comparing prisoners’ perspectives before and after community reentry. BMC Public Health. 2014;14:1253. doi: 10.1186/1471-2458-14-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer JP, Cepeda J, Springer SA, Wu J, Trestman RL, Altice FL. HIV in people reincarcerated in Connecticut prisons and jails: an observational cohort study. Lancet HIV. 2014;1(2):e77–e84. doi: 10.1016/S2352-3018(14)70022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azar MM, Springer SA, Meyer JP, Altice FL. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend. 2010;112(3):178–93. doi: 10.1016/j.drugalcdep.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman MS, Marshal MP, Stall R, et al. Associations between substance use, sexual risk taking and HIV treatment adherence among homeless people living with HIV. AIDS Care. 2009;21(6):692–700. doi: 10.1080/09540120802513709. [DOI] [PubMed] [Google Scholar]
- 34.Malta M, Strathdee SA, Magnanini MM, Bastos FI. Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: a systematic review. Addiction. 2008;103(8):1242–57. doi: 10.1111/j.1360-0443.2008.02269.x. [DOI] [PubMed] [Google Scholar]