Abstract
Tissues present complex biochemical and morphological composition associated with their various cell types and physiological functions. Mass spectrometry (MS) imaging technologies are powerful tools to investigate the molecular information from biological tissue samples and visualize their complex spatial distributions. Coupling of gas-phase ion mobility spectrometry (IMS) technologies to MS imaging has been increasingly explored to improve performance for biological tissue imaging. This approach allows improved detection of low abundance ions and separation of isobaric molecular species, thus resulting in more accurate determination of the spatial distribution of molecular ions. In this review, we highlight recent advances in the field focusing on promising applications of these technologies for metabolite, lipid and protein tissue imaging.
Introduction
Imaging technologies provide transformative capabilities to investigate and visualize the complexity and cellular heterogeneity of biological systems [1]. Spatial and molecular complexity of biological systems are intrinsically associated to many physiological processes related to normal organ development as well as aberrant disease progression [2–4]. Cancer initiation and promotion, for example, involve multistep cellular and genetic changes that result in complex tumorigenic state and tissue features [5]. Molecular imaging technologies including MS imaging are key approaches for investigating complex biological tissues, allowing spatial characterization of molecular components associated to heterotypic interactions exhibited by different cell types and their diverse microenvironment [6–8].
MS imaging allows untargeted analysis of hundreds of molecular species directly from a tissue sample, providing direct spatial correlation between their abundances and histological features. Molecular species are chemically identified based on high mass accuracy measurements of their mass-to-charge ratios (m/z), isotopic distributions, and tandem MS fragmentation patterns [9,10]. The speed, sensitivity, and specificity of MS imaging techniques have been widely explored for in depth investigation of the cellular environment of healthy and diseased thin tissue sections [11–13]. Despite its powerful analytical capabilities, direct tissue analysis by MS imaging presents challenges including matrix interferences and chemical noise, which can hinder detection and identification of less abundant molecular species. Moreover, the inability of MS imaging techniques to separate isomeric molecular ions prior to mass analysis can lead to inaccurate assessment of their spatial distribution within the tissue samples. In an attempt to address these challenges, gas-phase ion mobility spectrometry (IMS) technologies have been integrated into MS imaging workflows, allowing rapid separation of molecular ions post ionization and prior to mass analysis. In this review, we provide a brief overview of MS imaging and IMS techniques, focusing on recent advances coupling these approaches for enhanced imaging of metabolites, lipids and proteins (Figure 1). Promising applications to tissue imaging are highlighted to demonstrate the value of these methods in biomedical research.
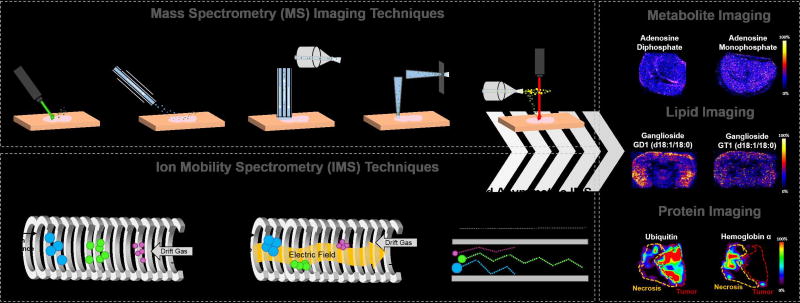
Figure 1.
Schematic representation of the MS imaging and IMS techniques that have been integrated for enhanced imaging of metabolites, lipids and proteins from biological tissues. MS imaging techniques highlighted include MALDI, DESI, LMJ-SSP, LESA and LAESI. IMS techniques include DTIMS, TWIMS and FAIMS. 2D ion images of metabolites (adenosine diphosphate and adenosine monophosphate), lipids (Gangliosides GD1 (d18:1/18:0) and GT1 (d18:1/18:0)), and proteins (ubiquitin and hemoglobin α) obtained using MS imaging and IMS are shown [26,28,56].
Mass Spectrometry Imaging Techniques
MS imaging techniques provide a range of analytical and imaging capabilities for biological tissue imaging. Secondary ion MS (SIMS) was the first ionization technique introduced for surface imaging [14]. However, the molecular fragmentation processes inherent to SIMS analysis at that time hindered biomolecule analysis for tissue imaging [15,16]. Following SIMS, matrix assisted laser desorption/ionization (MALDI) was developed for surface analysis, enabling softer ionization of molecular ions and imaging of intact biomolecules with molecular weight up to thousands of Daltons. MALDI-MS typically utilizes a UV laser beam to transfer energy to a matrix-embedded analyte surface, desorbing molecules into the gas phase and promoting molecular ionization [17]. MALDI has been extensively used for biological tissue imaging of metabolites, lipids and proteins with spatial resolution ranging from ~5–200 µm [18–24], and has been integrated with IMS for biological applications [25–36]. The development of cluster SIMS allowed softer ionization of molecular ions and thus its application for imaging of intact metabolites and lipids from biological samples [16,37,38], with sub-micrometer spatial resolution [39]. Nevertheless, no approaches to couple SIMS imaging with IMS have been reported.
Ambient ionization MS techniques were introduced in 2004 with the development of desorption electrospray ionization (DESI) [40]. Ambient ionization MS enables real-time and in-situ analysis of samples at atmospheric pressure conditions with minimal to no-sample preparation, and was thus rapidly adapted for imaging applications [41]. DESI utilizes a spray of charged solvent droplets to desorb and ionize analyte molecules from a sample surface, allowing efficient analysis of metabolites and lipids from biological samples [40]. The demonstration of DESI-MS for tissue imaging in 2006 drove its use in biomedical research [42], and it is now broadly used for tissue imaging with a typical spatial resolution of 150 µm [43–48]. The increased application of DESI led to the development of tens of other ambient ionization MS techniques, many of which have been successfully adapted for tissue imaging [49]. Among these, liquid extraction based techniques, such as liquid extraction surface analysis (LESA) [50], liquid microjunction surface sampling (LMJ-SSP) [51], and nanoDESI [52], employ a solvent ‘liquid microjunction’ that interacts with a sample surface to extract molecules in a pulsed or continuous approach [53]. Extracted molecules are re-aspirated, ionized and introduced into the mass spectrometer by electrospray ionization. LESA, LMJ-SSP and nanoDESI have been used for biological tissue imaging providing moderate to high spatial resolution (500–1000 µm, 630 µm and 15 µm, respectively)[54–56]. Other ambient ionization MS techniques have employed hybrid methods for desorption and ionization. Laser desorption electrospray ionization (LAESI), for example, combines a laser pulse to ablate material from a tissue surface and ESI to intercept the resulting plume for ionization [57].
Ion Mobility Spectrometry
IMS technologies are well suited for coupling with MS imaging techniques as they provide high-throughput separation capabilities (milliseconds), without significant increase in typical imaging analysis time (<1s/pixel), which is infeasible with other separation techniques such as liquid-chromatography (LC) approaches (minutes to hours). IMS are used for gas phase separation of molecular ions based on their size, shape, and charge within an electric field, also defined as their “mobility” (K) [58]. The most established form of IMS, named drift tube ion mobility spectrometry (DTIMS) employs a constant low electric field used to propel ions through a drift tube cell, while a high pressure buffer gas (typically helium or nitrogen) flows in the opposite direction of the ions, slowing their acceleration [59]. The number of collisions an ion experiences, dictated by its collisional cross section (CCS), influences its velocity through the drift cell, thus allowing separation from other ions. Besides DTIMS, traveling wave ion mobility spectrometry (TWIMS) is a widespread IMS technology that also employs an electric field to drive ions through a traveling wave ion guide. Contrary to DTIMS, a pulsed DC voltage is applied to each electrode in succession throughout the TWIMS device creating a travelling wave that the ions “surf” proportionally to K [59]. DTIMS and TWIMS have been widely integrated to LC-ESI-MS workflows to separate metabolites, lipids and protein molecular ions [60], with typical separation resolution of 60 and 40, respectively, and total separation time of less than 200 ms. DTIMS and TWIMS require complex hardware operated under low pressure conditions, and are commonly commercialized as part of mass spectrometer systems in tandem with time of flight (TOF) mass analyzers. On the other hand, differential mobility spectrometry (DMS), or field asymmetric ion mobility spectrometry (FAIMS), are smaller and simpler IMS devices that can be incorporated to the interface of various mass spectrometers [61]. In FAIMS, an alternating electric field is applied perpendicularly to the ion path such that the ions experience both high and low field environments. The mobility of ions in a high field (Kh) is different from their mobility in a low field (KL), thus causing ions to drift towards the walls of the ion mobility cell. This drift can be counteracted by a compensating voltage applied parallel to the alternating electric field, allowing for ions with a particular Kh/KL ratio to traverse through the device towards the mass analyzer. FAIMS has also been widely employed in LC-ESI-MS applications, providing typical separation resolution of 15 at no significant increase in analysis time when operated at fixed field values [62]. In the last 10 years, DTIMS, TWIMS, and FAIMS have been integrated to MS imaging techniques such as MALDI, LESA, DESI, LMJ-SS, and LAESI for applications in metabolites, lipids and proteins imaging, few of which we highlight next.
Imaging of Small metabolites and Synthetic Drugs
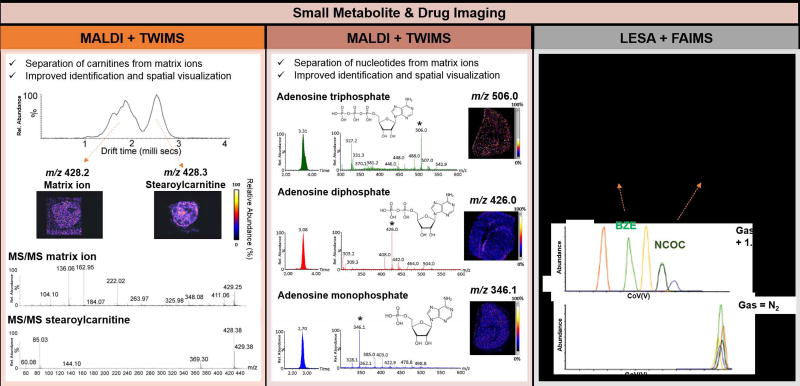
Small primary metabolites have important regulatory roles in many biochemical pathways including glycolysis, glutaminolysis, and the tricarboxylic acid cycle, and are highly representative of cellular state. Dysregulated expression and distribution of metabolites have been explored by MS imaging to identify potential disease markers in biological tissue samples [63]. However, mass spectral interferences, which are prominent in MALDI experiments that present matrix ions at the same m/z range, as well as the inability to separate structural isomers have hindered broader use of MS imaging for metabolite imaging. Integration with IMS has thus been explored to provide improved chemical specificity and imaging accuracy for metabolite imaging. For example, MALDI and TWIMS have been used to image carnitines and acylcarnitines, essential compounds involved in cellular metabolism, in hypoxic tumor tissue regions of a breast cancer xenograft model. Separation of the stearoylcarnitine ion [M+H]+ at m/z 428.3 from an interfering background ion detected at m/z 428.2, (0.8 ms separation in drift time) was achieved by integrating MALDI with TWIMS, as well as subsequent fragmentation and identification by collision induced dissociation (CID) (Figure 2, left panel) [25]. Importantly, 2D ion images revealed an increase in stearoylcarnitine levels in hypoxic tumor regions, likely due to the inhibition of β-oxidation pathways. In a different study, MALDI and TWIMS were used to investigate the distribution of nucleotides adenosine triphosphate, adenosine diphosphate and adenosine monophosphate in post-mortem mouse brain tissues, allowing improved selectivity for nucleotide analysis and reduced interference from matrix ions (Figure 2, center panel) [26].
Figure 2.
Examples of small metabolite and drug imaging in biological tissues using MS imaging coupled to IMS. The left panel shows an application of MALDI and TWIMS for separation of a stearoylcarnitine species from an isobaric background ion in breast tumor xenograft model tissue, allowing accurate imaging of the stearoylcarnitine ion. Species were identified by tandem MS analysis following TWIMS separation [25]. The middle panel shows an application of MALDI and TWIMS for imaging adenosine triphosphate, adenosine diphosphate, and adenosine monophosphate in mouse brain tissue, with corresponding ion mobility and mass spectra [26]. The right panel shows an application of LESA and FAIMS to separate isomeric cocaine metabolites BZE and NCOC, in human kidney tissue, with structures shown at the top of the panel. Addition of acetonitrile in the drift gas enables separation between the isomeric species, as shown in the top extracted ion chromatogram [66].
Analysis of the distribution of small synthetic drugs and their metabolites is important in the development of new treatment approaches. Drug imaging has also been explored by MS imaging and IMS techniques to separate the exogenous compounds from endogenous isobaric molecular ions [64]. The anti-cancer drug vinblastine, for example, was imaged directly from whole mouse tissue sections by MALDI-TWIMS to show its distribution prior- and post-dose. Separation of the protonated precursor vinblastine ion at m/z 811.4 from an isobaric endogenous glycerophosphocholine (PC) lipid species at m/z 811.4 as well as other interfering matrix ions was achieved with TWIMS. Identification of vinblastine was then possible by tandem MS analysis, providing increased specificity and higher accuracy determination of its spatial distribution [65]. Enhanced detection of drugs of abuse cocaine, tramadol and morphine and their metabolites in mouse kidney tissue sections was accomplished by coupling LESA to FAIMS. For example, norcocaine (NCOC), a cocaine metabolite indicative of cocaine intake, and benzoylecgonine (BZE), a cocaine metabolite that can result from external contamination, are structural isomers that present very similar fragmentation patterns. FAIMS allowed separation of the compounds (resolution = 3) for improved molecular analysis from kidney tissues (Figure 2, right panel) [66]. These examples clearly demonstrate the value of integrating IMS to MS imaging workflow for improved small molecule analysis.
Imaging of Lipids in Biological Tissues
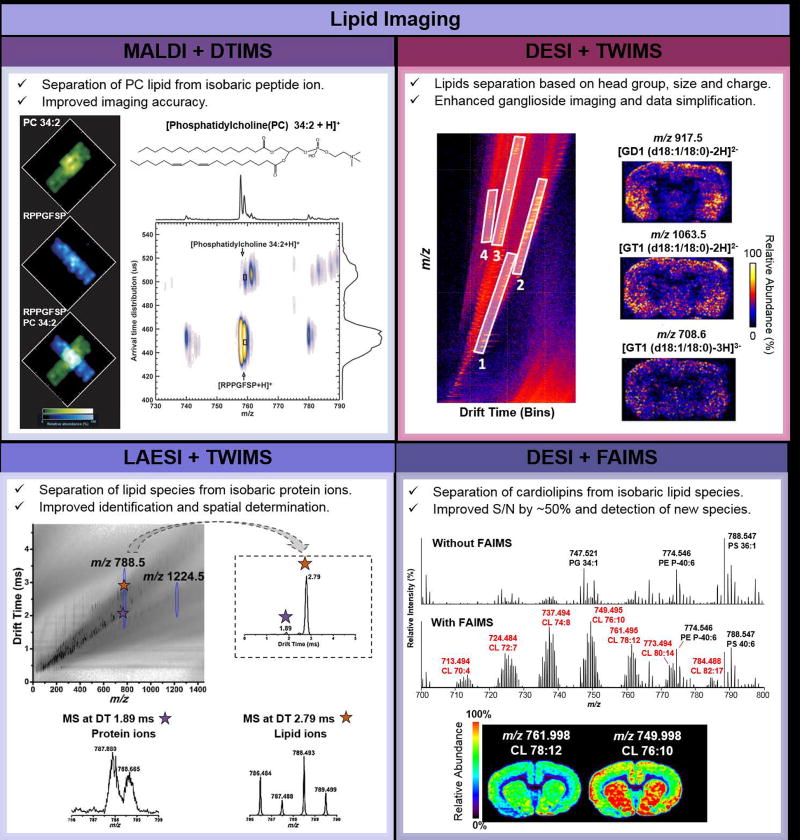
Lipids are integral components of cellular membranes and play important signaling roles in cellular processes. Complex lipids including glycerophospholipids, glycerolipids and sphingolipids present diverse chemical structures, commonly composed of fatty acid (FA) chains and distinct backbone structures, and have been increasingly investigated as disease markers by MS imaging approaches [43,67]. Integration of MS imaging and IMS have been used to reduce isobaric interferences due to isomerism in lipid chemical structures and other interfering molecular ions, which complicate precise structural assignment and analysis of lipid species from tissue samples by MS imaging [32–34,68]. Using MALDI and TWIMS, for example, identification and accurate imaging of a plasmalogen glycerophosphoethanolamine (PE) lipid, PE P-40:6 was achieved by separating the molecular ion from an isobaric lipid, PE 38:0, based on characteristic shifts in drift times experienced by the plasmalogen species compared to the diacyl species [27]. In a different study, the PC(34:2) lipid was separated using TWIMS from a nominally isobaric peptide (RPPGFSP) with a separation in drift-time peak maxima of 0.055 ms, enabling distinct visualization of each molecular ion (Figure 3, top left panel) [34].
Figure 3.
Examples of lipid imaging from biological tissue samples using MS imaging coupled to IMS. The top-left panel shows the application of MALDI and DTIMS to separate and image lipids and protein species. Ion images of PC 34:2 (structure shown at the top of the panel), an isobaric peptide RPPGFSP, and an overlay of the two ion images, representing the result obtained with no IMS are shown. A 2D IMS-MS separation map is shown for the two species [34]. The top-right panel shows the use of DESI and TWIMS for tissue lipid analysis. The 2D plot for m/z vs drift time illustrate the IMS trendlines observed: (1) fatty acids and lysolipids, (2) glycerophospholipids, (3) doubly charged ceramide-based lipids and gangliosides, (4) triply charged gangliosides from mouse brain tissue. Representative ion images for ganglioside species are shown on the right [28]. The bottom-left panel shows the m/z vs drift time plot with a lipid and protein IMS trendline obtained using LAESI and TWIMS from mouse brain tissue. The IMS spectra for a lipid and protein species at m/z 788.5 is shown on the right. Individual mass spectra extracted at 1.89 and 2.79 ms in drift time are shown at the bottom [69]. The bottom-right panel shows an application of DESI and FAIMS to improve detection and imaging of cardiolipins in mouse brain tissue. Mass spectra without IMS (top) and with (bottom) IMS separation by FAIMS from a rat brain tissue sample are shown. Improvements in S/N and number of detected cardiolipin species were observed. Representative ion images for two cardiolipin species are shown at the bottom [56].
Ambient ionization MS imaging techniques have also been coupled to IMS to improve lipid imaging from biological tissue sections [28,69,70]. Gangliosides, for example, are complex lipid species abundant in brain tissue that are not efficiently ionized by DESI, resulting in low relative abundances and S/N. Recently, Skraskova et al. integrated DESI and TWIMS to image mouse brain tissue sections and separate FA and lysolipids, glycerophospholipids, doubly charged ceramide-based lipids and gangliosides, and triply charged gangliosides into distinct mobility trendlines. This workflow resulted in simplification in data analysis and enhanced imaging of ganglioside species (Figure 3, top right panel) [28]. LAESI was also coupled to TWIMS for MS imaging of a sagittal rat brain section [69]. Nominally isobaric lipid and protein molecular ions detected in the narrow m/z 788.493±0.02 range were detected at different drift times (2.79 ms and 1.89 ms, respectively), allowing identification and accurate spatial determination of their specific spatial distributions (Figure 3, bottom left panel). Improvements in the analysis and imaging of multiply charged cardiolipins and gangliosides from rat brain tissue sections have also been accomplished by coupling DESI with a FAIMS device [56]. An overall increase of ~ 50% in S/N, as well as detection of 23 cardiolipins and 7 gangliosides not observed by DESI-MS imaging alone was achieved with the integrated approach (Figure 3, bottom right panel). Altogether, these studies showcase the value of IMS in lipid analysis, allowing for improved specificity, identification and image quality of lipids by MS imaging.
Imaging of Peptides and Proteins
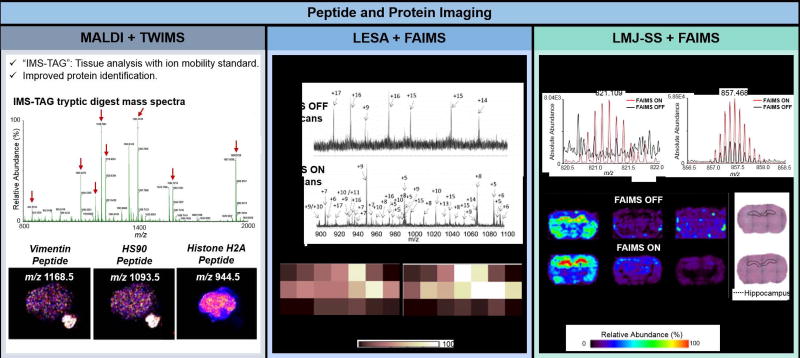
Proteins are critical to the structure, function, and regulation of cells. Investigating the dysregulation of protein expression and distribution within diseased tissues is important for biomarker discovery and identification of novel therapeutic targets. The addition of ion mobility separations into MS imaging workflows has allowed increased specificity and sensitivity for protein analysis, further advancing MS imaging into proteomics applications. Integration of MALDI with TWIMS and DTIMS has been explored for tissue analysis after on-tissue enzymatic digestions of proteins into peptides [29–31,36,71], enabling mass spectra deconvolution, as well as increased species detection, identification, and determination of their spatial distribution. For example, Hart et al. were unable to identify a tryptic peptide at m/z 1118.5 from a human skin tissue section by MALDI-TOF alone due to the presence of fragment ions from interfering isobaric glycerophospholipid and matrix ions in the CID mass spectra. Precise isolation for CID and further identification as a keratin 1 peptide was possible by separating the peptide ion from interfering isobaric species by TWIMS [71]. More recently, Cole et al. implemented the “IMS TAG” technique to improve protein identification by MALDI using TWIMS. The technique uses synthetic recombinant proteins that when digested yield peptides identical to those from a protein of interest within a biological tissue sample (Figure 4, left panel) [35]. The recombinant protein is analyzed concomitantly to the tissue sample of interest after on-tissue digestion, yielding tryptic peptides with characteristic ion mobility drift times and mass spectra that are used as a standard for comparison with peptides from the tissue and accurate protein identification.
Figure 4.
Examples of peptide and protein imaging from biological tissue samples using MS imaging coupled to IMS. The left panel shows the application of MALDI and TWIMS in the “IMS-TAG” method, which employs an internal standard protein that, when digested, yields peptides of interest (denoted with red arrows) within a tissue sample. The images show the distribution of three peptides within the ion images, a vimentin peptide, a HS90 peptide, and a histone H2A peptide, where only vimentin and HS90 are within the tissue [35]. The middle panel shows the use of LESA and FAIMS to image proteins from mouse brain tissue. An increase in the S/N and number of proteins detected with LESA was observed when FAIMS was used. The spectra when FAIMS voltage are applied shows greater variation in the species within a mouse brain tissue section observed within the same analysis time, and the images show a higher intensity of the calmodulin protein when FAIMS is used. Similarly, left panel shows the use of the LMJ-SS and FAIMS to image proteins in rat brain tissue. Increased ion intensity of protein species from rat brain tissue and improved spatial correlation of the molecular ions within the tissue section are observed, with the thymosin β-4 located majorly in the hippocampus of the rat brain [56].
Ambient ionization MS imaging of proteins have been notoriously challenging due to the low desorption and ionization efficiencies of proteins, interfering chemical noise, and ion loss inherent of analysis at atmospheric pressure conditions. Recent success implementing ambient ionization MS for protein imaging was propelled through the integration of FAIMS into the analytical workflow. Griffiths et. al coupled LESA to a chip-based FAIMS device to image the spatial distribution of proteins within mouse brain and liver tissue sections, enabling detection of 26 and 29 total proteins respectively, including 7 proteins in brain and 13 proteins in liver that were not observed with LESA alone (Figure 4, central panel) [54,72]. Feider et. al coupled the same FAIMS system to an LMJ-SSP source to image proteins in rat brain and human ovarian tissues [56]. In rat brain tissue, 66 protein ions were exclusively observed when utilizing FAIMS separation, and not detected using LMJ-SS alone (Figure 4, right panel). Improved correlation between the spatial distribution of protein ions and histologic structures of tissues was also achieved, such as a clear definition of thymosin β-4 within the brain hippocampus. In high grade serous carcinomas (HGSC) tissues, clear visualization of the protein calcyclin was achieved in cancer tissue regions, while high abundance of hemoglobin α was observed in regions with necrosis.
Conclusion
The addition of IMS into a MS imaging workflow alleviates challenges inherent to direct analysis of complex biological tissues, including reducing chemical noise interference, improving separation and chemical identification of isobaric species, and visualization of ions at low S/N. When applied to tissue imaging, more accurate and clear spatial determination of metabolites, lipids, and proteins within histologic features are achieved. These analytical workflows have expanded the molecular information acquirable by MS imaging, making these techniques more attractive for biomedical research. Several improvements are envisioned to further expand and advance the use of these approaches in biological tissue imaging. IMS separation is inherently associated with ion loss due to the increased ion travel path, often resulting in lower sensitivity despite improvements in S/N. This decrease in sensitivity has been particularly detrimental when used with ambient ionization MS techniques, which intrinsically suffers from low ion transmission at atmospheric pressure. Developments in hardware to integrate these systems and improve ion transmission are needed to increase sensitivity for MS semi-targeted imaging applications. Further, there has been limited success using IMS to separate isomeric lipids and metabolites in biological MS tissue imaging. Improvements in ion mobility separation resolution would further expand the molecular information acquirable by MS imaging and improve identification of molecules for biomarker discovery. As MS imaging techniques have been integrated to high resolving power mass analyzers, the molecular information acquired from tissue samples have become increasingly large and complex, which complicates data analysis and interpretation. Data complexity becomes even more intricate when the dimension of separation from IMS analysis is added to MS imaging workflows. Thus, software solutions that provide functionalities to process and interpret the spatial, separation and molecular information obtained are necessary to advance research. Lastly, inclusion of CCS information into molecular databases obtained from MS imaging experiments could significantly improve identification of molecular ions to further propel the use of IMS for biological tissue imaging research.
Highlights.
IMS improves analytical performance of MS imaging of biological tissue samples.
Improvements in specificity and imaging accuracy are achieved for small molecules.
IMS reduces background and isobaric interferences for small molecule MS imaging.
Improved imaging and identification of proteins are achieved using IMS separation.
Acknowledgments
This work was supported by the AAAS Marion Mason Award for Women in Chemical Sciences, The Welch Foundation (grant F-1895), and the National Cancer Institute (NCI) of the National Institutes of Health (grant R00CA190783).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Kherlopian AR, Song T, Duan Q, Neimark MA, Po MJ, Gohagan JK, Laine AF. A review of imaging techniques for systems biology. Bmc Systems Biology. 2008;2 doi: 10.1186/1752-0509-2-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckingham M, Bajard L, Chang T, Daubas P, Hadchouel J, Meilhac S, Montarras D, Rocancourt D, Relaix F. The formation of skeletal muscle: from somite to limb. Journal of Anatomy. 2003;202:59–68. doi: 10.1046/j.1469-7580.2003.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–322. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- 4.Vandoorne K, Sapoznik S, Raz T, Biton I, Neeman M. Imaging in Developmental Biology. In: Kiessling F, Pichler BJ, editors. Small Animal Imaging: Basics and Practical Guide. Springer Berlin Heidelberg; 2011. pp. 417–436. [Google Scholar]
- 5.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 6.McDonnell LA, Heeren RMA. Imaging mass spectrometry. Mass Spectrometry Reviews. 2007;26:606–643. doi: 10.1002/mas.20124. [DOI] [PubMed] [Google Scholar]
- 7.Weaver EM, Hummon AB. Imaging mass spectrometry: From tissue sections to cell cultures. Advanced Drug Delivery Reviews. 2013;65:1039–1055. doi: 10.1016/j.addr.2013.03.006. [DOI] [PubMed] [Google Scholar]
- *8.Schwamborn K, Caprioli RM. INNOVATION Molecular imaging by mass spectrometry - looking beyond classical histology. Nature Reviews Cancer. 2010;10:639–646. doi: 10.1038/nrc2917. Review article on the use of MALDI for molecular iamging of tissue samples. [DOI] [PubMed] [Google Scholar]
- 9.Rathahao-Paris E, Alves S, Junot C, Tabet J-C. High resolution mass spectrometry for structural identification of metabolites in metabolomics. Metabolomics. 2015;12:10. [Google Scholar]
- 10.Sokolowska I, Wetie AGN, Woods AG, Darie CC. Applications of Mass Spectrometry in Proteomics. Australian Journal of Chemistry. 2013;66:721–733. [Google Scholar]
- 11.Takats Z, Strittmatter N, McKenzie JS. Ambient Mass Spectrometry in Cancer Research. In: Drake RR, McDonnell LA, editors. Applications of Mass Spectrometry Imaging to Cancer. Elsevier Academic Press Inc; 2017. pp. 231–256. Advances in Cancer Research, vol 134.] [DOI] [PubMed] [Google Scholar]
- 12.Gessel MM, Norris JL, Caprioli RM. MALDI imaging mass spectrometry: Spatial molecular analysis to enable a new age of discovery. Journal of Proteomics. 2014;107:71–82. doi: 10.1016/j.jprot.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spengler B. Mass Spectrometry Imaging of Biomolecular Information. Analytical Chemistry. 2015;87:64–82. doi: 10.1021/ac504543v. [DOI] [PubMed] [Google Scholar]
- 14.Liebl H. Ion Microprobe Mass Analyzer. Journal of Applied Physics. 1967;38:5277-&. [Google Scholar]
- 15.Benninghoven A, Jaspers D, Sichtermann W. Secondary-Ion Emission of Amino-Acids. Applied Physics. 1976;11:35–39. [Google Scholar]
- 16.Winograd N. The magic of cluster SIMS. Analytical Chemistry. 2005;77:142a–149a. [Google Scholar]
- 17.Karas M, Bachmann D, Bahr U, Hillenkamp F. Matrix-Assisted Ultraviolet-Laser Desorption of Nonvolatile Compounds. International Journal of Mass Spectrometry and Ion Processes. 1987;78:53–68. [Google Scholar]
- 18.Seeley EH, Caprioli RM. Molecular imaging of proteins in tissues by mass spectrometry. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18126–18131. doi: 10.1073/pnas.0801374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ide Y, Waki M, Matsunuma R, Hosokawa Y, Ogura H, Shiiya N, Setou M. Human Breast Cancer Tissues Contain Abundant Pc(36:1) with High Scd1 Expression. Breast. 2013;22:S30–S31. doi: 10.1371/journal.pone.0061204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balluff B, Frese CK, Maier SK, Schone C, Kuster B, Schmitt M, Aubele M, Hofler H, Deelder AM, Heck AJR, et al. De novo discovery of phenotypic intratumour heterogeneity using imaging mass spectrometry. Journal of Pathology. 2015;235:3–13. doi: 10.1002/path.4436. [DOI] [PubMed] [Google Scholar]
- 21.Everest-Dass AV, Briggs MT, Kaur G, Oehler MK, Hoffmann P, Packer NH. N-glycan MALDI Imaging Mass Spectrometry on Formalin-Fixed Paraffin-Embedded Tissue Enables the Delineation of Ovarian Cancer Tissues. Molecular & Cellular Proteomics. 2016;15:3003–3016. doi: 10.1074/mcp.M116.059816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zavalin A, Yang J, Caprioli R. Laser Beam Filtration for High Spatial Resolution MALDI Imaging Mass Spectrometry. Journal of The American Society for Mass Spectrometry. 2013;24:1153–1156. doi: 10.1007/s13361-013-0638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feenstra AD, Duenas ME, Lee YJ. Five Micron High Resolution MALDI Mass Spectrometry Imaging with Simple, Interchangeable, Multi-Resolution Optical System. Journal of the American Society for Mass Spectrometry. 2017;28:434–442. doi: 10.1007/s13361-016-1577-8. [DOI] [PubMed] [Google Scholar]
- 24.Kompauer M, Heiles S, Spengler B. Atmospheric pressure MALDI mass spectrometry imaging of tissues and cells at 1.4-mu m lateral resolution. Nature Methods. 2017;14:90–96. doi: 10.1038/nmeth.4071. [DOI] [PubMed] [Google Scholar]
- **25.Chughtai K, Jiang L, Greenwood TR, Glunde K, Heeren RMA. Mass spectrometry images acylcarnitines, phosphatidylcholines, and sphingomyelin in MDA-MB-231 breast tumor models. Journal of Lipid Research. 2013;54:333–344. doi: 10.1194/jlr.M027961. Application of MALDI imaging and TWIMS for detection of acyl carnitines and carnitines in breast cancer xenografts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *26.Blatherwick EQ, Svensson CI, Frenguelli BG, Scrivens JH. Localisation of adenine nucleotides in heat-stabilised mouse brains using ion mobility enabled MALDI imaging. International Journal of Mass Spectrometry. 2013;345:19–27. Application of MALDI imaging and TWIMS for separating nucleotides from interferring matix ions in murine brain tissue. [Google Scholar]
- 27.Jackson SN, Barbacci D, Egan T, Lewis EK, Schultz JA, Woods AS. MALDI-ion mobility mass spectrometry of lipids in negative ion mode. Analytical Methods. 2014;6:5001–5007. doi: 10.1039/C4AY00320A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **28.Skraskova K, Claude E, Jones EA, Towers M, Ellis SR, Heeren RMA. Enhanced capabilities for imaging gangliosides in murine brain with matrix-assisted laser desorption/ionization and desorption electrospray ionization mass spectrometry coupled to ion mobility separation. Methods. 2016;104:69–78. doi: 10.1016/j.ymeth.2016.02.014. Application of DESI imaging and TWIMS for improved detection of doubly and triply charged gangliosides in murine brain tissue. [DOI] [PubMed] [Google Scholar]
- 29.Djidja MC, Claude E, Snel MF, Francese S, Scriven P, Carolan V, Clench MR. Novel molecular tumour classification using MALDI-mass spectrometry imaging of tissue micro-array. Analytical and Bioanalytical Chemistry. 2010;397:587–601. doi: 10.1007/s00216-010-3554-6. [DOI] [PubMed] [Google Scholar]
- 30.Djidja MC, Claude E, Snel MF, Scriven P, Francese S, Carolan V, Clench MR. MALDI-Ion Mobility Separation-Mass Spectrometry Imaging of Glucose-Regulated Protein 78 kDa (Grp78) in Human Formalin-Fixed, Paraffin-Embedded Pancreatic Adenocarcinoma Tissue Sections. Journal of Proteome Research. 2009;8:4876–4884. doi: 10.1021/pr900522m. [DOI] [PubMed] [Google Scholar]
- 31.Djidja MC, Francese S, Claude E, Loadman P, Sutton C, Shynder S, Cooper P, Patterson LH, Carolan VA, Clench MR. Targeting of Hypoxia in AQ4N-treated Tumour Xenografts by MALDI-Ion Mobility Separation-Mass Spectrometry Imaging. Current Analytical Chemistry. 2013;9:212–225. [Google Scholar]
- 32.Jackson SN, Ugarov M, Egan T, Post JD, Langlais D, Schultz JA, Woods AS. MALDI-ion mobility-TOFMS imaging of lipids in rat brain tissue. Journal of Mass Spectrometry. 2007;42:1093–1098. doi: 10.1002/jms.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matusch A, Fenn LS, Depboylu C, Klietz M, Strohmer S, McLean JA, Becker JS. Combined Elemental and Biomolecular Mass Spectrometry Imaging for Probing the Inventory of Tissue at a Micrometer Scale. Analytical Chemistry. 2012;84:3170–3178. doi: 10.1021/ac203112c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *34.McLean JA, Ridenour WB, Caprioli RM. Profiling and imaging of tissues by imaging ion mobility-mass spectrometry. Journal of Mass Spectrometry. 2007;42:1099–1105. doi: 10.1002/jms.1254. Application of MALDI imaging and TWIMS for separation of lipids from interferring background and peptide ions. [DOI] [PubMed] [Google Scholar]
- **35.Cole LM, Mahmoud K, Haywood-Small S, Tozer GM, Smith DP, Clench MR. Recombinant "IMS TAG" proteins - A new method for validating bottom-up matrix-assisted laser desorption/ionisation ion mobility separation mass spectrometry imaging. Rapid Communications in Mass Spectrometry. 2013;27:2355–2362. doi: 10.1002/rcm.6693. Introduction of the IMS TAG method employing recombinant proteins and on-tissue digestion to detect and identify proteins from tissues using MALDI imaging and TWIMS. [DOI] [PubMed] [Google Scholar]
- 36.Stauber J, MacAleese L, Franck J, Claude E, Snel M, Kaletas BK, Wiel I, Wisztorski M, Fournier I, Heeren RMA. On-Tissue Protein Identification and Imaging by MALDI-Ion Mobility Mass Spectrometry. Journal of the American Society for Mass Spectrometry. 2010;21:338–347. doi: 10.1016/j.jasms.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 37.Touboul D, Halgand F, Brunelle A, Kersting R, Tallarek E, Hagenhoff B, Laprevote O. Tissue molecular ion imaging by gold cluster ion bombardment. Analytical Chemistry. 2004;76:1550–1559. doi: 10.1021/ac035243z. [DOI] [PubMed] [Google Scholar]
- 38.Sjovall P, Lausmaa J, Johansson B. Mass spectrometric imaging of lipids in brain tissue. Analytical Chemistry. 2004;76:4271–4278. doi: 10.1021/ac049389p. [DOI] [PubMed] [Google Scholar]
- 39.Klitzing HA, Weber PK, Kraft ML. Secondary ion mass spectrometry imaging of biological membranes at high spatial resolution. Methods Mol Biol. 2013;950:483–501. doi: 10.1007/978-1-62703-137-0_26. [DOI] [PubMed] [Google Scholar]
- 40.Takats Z, Wiseman JM, Gologan B, Cooks RG. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science. 2004;306:471–473. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- 41.Cooks RG, Ouyang Z, Takats Z, Wiseman JM. Ambient mass spectrometry. Science. 2006;311:1566–1570. doi: 10.1126/science.1119426. [DOI] [PubMed] [Google Scholar]
- 42.Wiseman JM, Ifa DR, Song QY, Cooks RG. Tissue imaging at atmospheric pressure using desorption electrospray ionization (DESI) mass spectrometry. Angewandte Chemie-International Edition. 2006;45:7188–7192. doi: 10.1002/anie.200602449. [DOI] [PubMed] [Google Scholar]
- **43.Ifa DR, Eberlin LS. Ambient Ionization Mass Spectrometry for Cancer Diagnosis and Surgical Margin Evaluation. Clin Chem. 2016;62:111–123. doi: 10.1373/clinchem.2014.237172. Comprehensive review article on ambient ionization MS imaging techniques and their use for human cancer diagnosis and clinical applications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eberlin LS, Norton I, Dill AL, Golby AJ, Ligon KL, Santagata S, Cooks RG, Agar NY. Classifying human brain tumors by lipid imaging with mass spectrometry. Cancer Res. 2012;72:645–654. doi: 10.1158/0008-5472.CAN-11-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dill AL, Eberlin LS, Costa AB, Zheng C, Ifa DR, Cheng LA, Masterson TA, Koch MO, Vitek O, Cooks RG. Multivariate Statistical Identification of Human Bladder Carcinomas Using Ambient Ionization Imaging Mass Spectrometry. Chemistry-a European Journal. 2011;17:2897–2902. doi: 10.1002/chem.201001692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sans M, Gharpure K, Tibshirani R, Zhang J, Liang L, Liu J, Young JH, Dood RL, Sood AK, Eberlin LS. Metabolic Markers and Statistical Prediction of Serous Ovarian Cancer Aggressiveness by Ambient Ionization Mass Spectrometry Imaging. Cancer Res. 2017;77:2903–2913. doi: 10.1158/0008-5472.CAN-16-3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guenther S, Muirhead LJ, Speller AV, Golf O, Strittmatter N, Ramakrishnan R, Goldin RD, Jones E, Veselkov K, Nicholson J, et al. Spatially resolved metabolic phenotyping of breast cancer by desorption electrospray ionization mass spectrometry. Cancer Res. 2015;75:1828–1837. doi: 10.1158/0008-5472.CAN-14-2258. [DOI] [PubMed] [Google Scholar]
- 48.Calligaris D, Caragacianu D, Liu X, Norton I, Thompson CJ, Richardson AL, Golshan M, Easterling ML, Santagata S, Dillon DA, et al. Application of desorption electrospray ionization mass spectrometry imaging in breast cancer margin analysis. Proc Natl Acad Sci U S A. 2014;111:15184–15189. doi: 10.1073/pnas.1408129111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu CP, Dill AL, Eberlin LS, Cooks RG, Ifa DR. Mass spectrometry imaging under ambient conditions. Mass Spectrometry Reviews. 2013;32:218–243. doi: 10.1002/mas.21360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kertesz V, Van Berkel GJ. Fully automated liquid extraction-based surface sampling and ionization using a chip-based robotic nanoelectrospray platform. Journal of Mass Spectrometry. 2010;45:252–260. doi: 10.1002/jms.1709. [DOI] [PubMed] [Google Scholar]
- 51.Van Berkel GJ, Sanchez AD, Quirke JME. Thin-layer chromatography and electrospray mass spectrometry coupled using a surface sampling probe. Analytical Chemistry. 2002;74:6216–6223. doi: 10.1021/ac020540+. [DOI] [PubMed] [Google Scholar]
- 52.Roach PJ, Laskin J, Laskin A. Nanospray desorption electrospray ionization: an ambient method for liquid-extraction surface sampling in mass spectrometry. Analyst. 2010;135:2233–2236. doi: 10.1039/c0an00312c. [DOI] [PubMed] [Google Scholar]
- *53.Laskin J, Lanekoff I. Ambient Mass Spectrometry Imaging Using Direct Liquid Extraction Techniques. Analytical Chemistry. 2016;88:52–73. doi: 10.1021/acs.analchem.5b04188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **54.Griffiths RL, Creese AJ, Race AM, Bunch J, Cooper HJ. LESA FAIMS Mass Spectrometry for the Spatial Profiling of Proteins from Tissue. Analytical Chemistry. 2016;88:6758–6766. doi: 10.1021/acs.analchem.6b01060. Application of LESA profiling and FAIMS for improved detection and top-down imaging of proteins in murine brain and liver tissues. [DOI] [PubMed] [Google Scholar]
- 55.Laskin J, Heath BS, Roach PJ, Cazares L, Semmes OJ. Tissue Imaging Using Nanospray Desorption Electrospray Ionization Mass Spectrometry. Analytical Chemistry. 2012;84:141–148. doi: 10.1021/ac2021322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **56.Feider CL, Elizondo N, Eberlin LS. Ambient Ionization and FAIMS Mass Spectrometry for Enhanced Imaging of Multiply Charged Molecular Ions in Biological Tissues. Anal. Chem. 2016 doi: 10.1021/acs.analchem.6b02798. Application of DESI imaging and FAIMS for improved imaging of doubly charged cardiolipins and gangliosides wihtin rat brain and human thyroid tissue. Application of LMJ-SSP and FAIMS for top-down imaging of proteins in rat brain and human ovarian tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nemes P, Vertes A. Laser ablation electrospray ionization for atmospheric pressure, in vivo, and imaging mass spectrometry. Analytical Chemistry. 2007;79:8098–8106. doi: 10.1021/ac071181r. [DOI] [PubMed] [Google Scholar]
- 58.Harvey SR, MacPhee CE, Barran PE. Ion mobility mass spectrometry for peptide analysis. Methods. 2011;54:454–461. doi: 10.1016/j.ymeth.2011.05.004. [DOI] [PubMed] [Google Scholar]
- *59.Cumeras R, Figueras E, Davis CE, Baumbach JI, Gràcia I. Review on Ion Mobility Spectrometry. Part 1: Current Instrumentation. The Analyst. 2015;140:1376–1390. doi: 10.1039/c4an01100g. Review article on ion mobility spectrometry technologies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng XY, Wojcik R, Zhang X, Ibrahim YM, Burnum-Johnson KE, Orton DJ, Monroe ME, Moore RJ, Smith RD, Baker ES. Coupling Front-End Separations, Ion Mobility Spectrometry, and Mass Spectrometry For Enhanced Multidimensional Biological and Environmental Analyses. In: Cooks RG, Pemberton JE, editors. Annual Review of Analytical Chemistry, Vol 10. 2017. pp. 71–92. Annual Review of Analytical Chemistry, vol 10.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kolakowski BM, Mester Z. Review of applications of high-field asymmetric waveform ion mobility spectrometry (FAIMS) and differential mobility spectrometry (DMS) Analyst. 2007;132:842–864. doi: 10.1039/b706039d. [DOI] [PubMed] [Google Scholar]
- 62.Shvartsburg AA, Li F, Tang K, Smith RD. High-Resolution FAIMS Using New Planar Geometry Analyzers. Analytical chemistry. 2006;78:3706–3714. doi: 10.1021/ac052020v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernie AR, Trethewey RN, Krotzky AJ, Willmitzer L. Innovation - Metabolite profiling: from diagnostics to systems biology. Nature Reviews Molecular Cell Biology. 2004;5:763–769. doi: 10.1038/nrm1451. [DOI] [PubMed] [Google Scholar]
- 64.Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS. Molecular imaging in drug development. Nature Reviews Drug Discovery. 2008;7:591–607. doi: 10.1038/nrd2290. [DOI] [PubMed] [Google Scholar]
- 65.Trim PJ, Henson CM, Avery JL, McEwen A, Snel MF, Claude E, Marshall PS, West A, Princivalle AP, Clench MR. Matrix-Assisted Laser Desorption/Ionization-Ion Mobility Separation-Mass Spectrometry Imaging of Vinblastine in Whole Body Tissue Sections. Analytical Chemistry. 2008;80:8628–8634. doi: 10.1021/ac8015467. [DOI] [PubMed] [Google Scholar]
- *66.Porta T, Varesio E, Hopfgartner G. Gas-Phase Separation of Drugs and Metabolites Using Modifier-Assisted Differential Ion Mobility Spectrometry Hyphenated to Liquid Extraction Surface Analysis and Mass Spectrometry. Analytical Chemistry. 2013;85:11771–11779. doi: 10.1021/ac4020353. Application of LESA and FAIMS to separate and image drugs of abuse and their isomeric metabolites in mouse kidney tissue. [DOI] [PubMed] [Google Scholar]
- 67.Wenk MR. The emerging field of lipidomics. Nature Reviews Drug Discovery. 2005;4:594–610. doi: 10.1038/nrd1776. [DOI] [PubMed] [Google Scholar]
- 68.Snel MF, Fuller M. High-Spatial Resolution Matrix-Assisted Laser Desorption Ionization Imaging Analysis of Glucosylceramide in Spleen Sections from a Mouse Model of Gaucher Disease. Analytical Chemistry. 2010;82:3664–3670. doi: 10.1021/ac902939k. [DOI] [PubMed] [Google Scholar]
- *69.Li H, Smith BK, Mark L, Nemes P, Nazarian J, Vertes A. Ambient molecular imaging by laser ablation electrospray ionization mass spectrometry with ion mobility separation. International Journal of Mass Spectrometry. 2015;377:681–689. Application of LAESI and TWIMS for improved detection and imaging of lipids in murine brain tissue. [Google Scholar]
- *70.Bennett RV, Gamage CM, Galhena AS, Fernandez FM. Contrast-Enhanced Differential Mobility-Desorption Electrospray Ionization-Mass Spectrometry Imaging of Biological Tissues. Analytical Chemistry. 2014;86:3756–3763. doi: 10.1021/ac5007816. Application of DESI imaging and FAIMS for improved imaging of lipids in mouse brain tissue. [DOI] [PubMed] [Google Scholar]
- 71.Hart PJ, Francese S, Woodroofe MN, Clench MR. Matrix assisted laser desorption ionisation ion mobility separation mass spectrometry imaging of ex-vivo human skin. International Journal for Ion Mobility Spectrometry. 2013;16:71–83. [Google Scholar]
- 72.Sarsby J, Griffiths RL, Race AM, Bunch J, Randall EC, Creese AJ, Cooper HJ. Liquid Extraction Surface Analysis Mass Spectrometry Coupled with Field Asymmetric Waveform Ion Mobility Spectrometry for Analysis of Intact Proteins from Biological Substrates. Analytical Chemistry. 2015;87:6794–6800. doi: 10.1021/acs.analchem.5b01151. [DOI] [PubMed] [Google Scholar]