Abstract
Male parental care is an important social behavior for several mammalian species. Psychosocial stress is usually found to inhibit maternal behavior, but effects on paternal behavior have been less consistent. We tested the effects of social defeat stress on pair bond formation and paternal behavior in the monogamous California mouse (Peromyscus californicus). Social defeat reduced time spent in a chamber with a stranger female during a partner preference test conducted 24 h after pairing, but increased latency to the first litter. In 10 minute partner preference tests conducted after the birth of pups, both control and stressed males exhibited selective aggression towards stranger females. Unlike prairie voles, side by side contact was not observed in either partner preference test. Stressed male California mice engaged in more paternal behavior than controls and had reduced anxiety-like responses in the open-field test. Defeat stress enhanced prodynorphin and KOR expression in the medial preoptic area (MPOA) but not PVN. Increased KOR signaling has been linked to increased selective aggression in prairie voles. Together the results show that defeat stress enhances behaviors related to parental care and pair bonding in male California mice.
Introduction
Raising offspring requires a substantial investment of energy and time. In many cases, adverse environmental conditions are associated with reduced investment in parental effort. This is most frequently observed in females (Ivy et al., 2008; Roth et al., 2009; Roth and Sullivan, 2005), which typically make a greater investment in offspring than males. For example, increased corticosterone levels can reduce the quality of maternal care toward pups (Pereira et al., 2015; Workman et al., 2016). Less attention has been devoted to how adverse conditions affect male parental care. In the few studies that have examined this question, conflicting results have been observed. In the biparental prairie vole (Microtus ochrogaster), acute stress exposure through forced swim increased parental care in males, but not females (Bales et al., 2006). In contrast, a chronic variable stress study reported modest decreases in paternal behavior and proximity to pups by California mouse (Peromyscus californicus) fathers (Harris et al., 2013). This study used multiple stressors including wet bedding, a shaker, an injection of hypertonic saline, cold exposure, restraint, forced swim, and predator urine. In these studies, parental behavior was examined within minutes of stressor exposure. What is less clear is whether stressful experiences exert longer lasting effects on paternal care. In humans, combat stress is associated increased rates of domestic violence years after returning home (Jordan et al., 1992). This suggests that psychological stress could induce changes in brain function that have long lasting changes on behavior.
Social defeat stress is a useful paradigm to address this question, as it exerts long-lasting behavioral and molecular effects after cessation of social defeat (Golden et al., 2011; Huhman, 2006; Steinman and Trainor, 2017; Wood, 2014). Social defeat is an ethologically relevant form of stress that is based on aversive social interactions that can occur in naturalistic states (Howerton et al., 2008; Ribble and Salvioni, 1990; Williamson et al., 2017). However, the majority of social defeat studies have been conducted in rodent species in which males do not normally provide parental care to their offspring. An exception is the California mouse, a monogamous species. Male California mice exposed to defeat stress exhibit increased freezing behavior in the resident-intruder test (Steinman et al. 2015), decreased behavioral flexibility (Laredo et al., 2015), sucrose anhedonia (Williams et al., in review) and increased sensitivity to other stressors (Duque-Wilckens et al., 2016; Laredo et al., 2015).
Here we examine how exposure to social defeat affects paternal behavior and the formation of pair bonds. In many monogamous species pair bonds are closely associated with male parental care (Carter et al., 1995; Diaz-Munoz and Bales, 2016). We also considered the effects of defeat stress on aggression because male California mice exhibit enhanced aggression following the birth of pups (Trainor et al., 2008). Finally, we examined the expression of neuropeptide- and kappa opioid receptor (KOR)-related transcripts in the medial preoptic area (MPOA) and paraventricular nucleus (PVN). Oxytocin and vasopressin signaling within the MPOA and PVN have been linked to facilitation of parental behavior in other rodent species (Bosch and Neumann, 2012; Champagne et al., 2001; Pedersen et al., 1994) while galanin acting in the MPOA has been reported to promote parental care (Wu et al., 2014). We examined kappa opioid receptor related genes based on previous studies linking KOR function with aggressive behavior in pair bonded prairie voles (Resendez et al., 2012, 2016). Our results suggest that defeat stress enhances pair bond formation and parental behavior in male California mice.
Methods
Animals
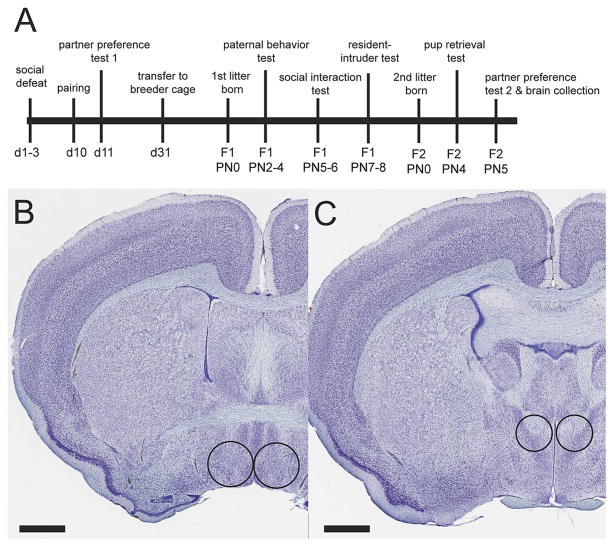
Male California mice (Peromyscus californicus) were raised in a vivarium at UC Davis while females were obtained from the Peromyscus Genetic Stock Center (Columbia, S. C.) or from vivarium breeding colony (Table 1). Mice were ear punched for identification purposes and maintained in polypropylene cages with Sani-Chip bedding, cotton nestlets, and Enviro-Dri enrichment nesting material. Food (Harlan Teklad 2016) and water was provided ad libitum. Mice were kept on a 16 h light/8 h dark cycle (lights off at 1500 PST). All behavior testing was performed during the dark phase. All mice were 3–6 months old at the beginning of the study and housed with same-sex individuals. Mated pairs were kept in standard shoebox cages for three weeks and then moved to larger cages (15x25x46 cm) for the rest of the study. One set of observations was conducted using experienced colony breeders (see below). All experiments were approved by the UC Davis Institutional Animal Care and Use Committee, and animals were maintained according to the recommendations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. A timeline of experimental analyses is listed in Fig. 1A.
Table 1.
Source of females paired with males and distribution of pup births and infertile pairs. There was no effect of stress or source of female on reproduction for the first or second litter.
| Male group | Female Source | First litter | Second litter | ||
|---|---|---|---|---|---|
| Had pups | No pups | Had pups | No pups | ||
| Control | Stock Center | 4 | 3 | 2 | 2 |
| Colony | 6 | 2 | 4 | 2 | |
| Total | 10 | 5 | 6 | 4 | |
| Stress | Stock Center | 6 | 1 | 6 | 0 |
| Colony | 5 | 2 | 4 | 1 | |
| Total | 11 | 3 | 10 | 1 | |
|
| |||||
| Total | 21 | 8 | 16 | 5 | |
Figure 1.
Experimental timeline of behavioral analyses (A) and diagram of brain punch samples. A 17 gauge needle was used to collect the MPOA (B) and an 18 gauge needle was used to collect the PVN (C). Nissl stained images are from the online California mouse brain atlas (brainmaps.org).
Experimental Design
Males were initially housed in same-sex groups and randomly assigned to social defeat or control groups. Males assigned to defeat were placed in the home cage of a vasectomized resident male for either 7 min or until the male received 7 bites (Trainor et al., 2013, Greenberg et al., 2014). Resident males were vasectomized to prevent these pairs from having litters, and resident female mates were removed from the home cage before episodes of social defeat. In the control condition, males were placed into an empty cage for 7 min. One week following the last day of social defeat or control conditions, each male was paired with a female. Twenty-four hours after pairing, each male underwent a 3 hour partner preference test (Bales et al., 2013; DeVries and Carter, 1999). Paired males and females remained together for the duration of the entire experiment. Parental behavior, social interaction, and resident intruder tests began after the birth of the first litter (Fig. 1A). A second partner preference was conducted following the birth of the 2nd litter, when pair bonds were expected to be stronger.
Twenty-one of the 29 pairs gave birth to litters within 60 days after pairing (See Table 1). Those that had not given birth by that interval of time were removed from further analyses. There were no effects of source of female (χ2 = 0.013, p = 0.909) or stress treatment (χ2 = 0.514, p = 0.474) on whether or not the pair had pups. Following the birth of the first litter, we documented pup number, weight, and latency to give birth to the first litter.
Partner Preference Test
Each male was tested twice in the partner preference test (Bales et al., 2013; Williams et al., 1992). Testing occurred in three shoebox-size polypropylene cages connected with 2 Plexiglas tubes, with cages situated with one in the back (neutral cage) and two in the front (stimulus cages). One side cage contained their cohabited female (partner) and the other side cage contained a tethered unfamiliar, age-matched cycling unfamiliar female (stranger, randomly assigned). The center cage was empty. Cages were covered with wire cage tops, food pellets placed into each chamber, and water was available in each chamber. The test began when males were placed in the neutral chamber. Time spent in each chamber with either partner or a randomly assigned stranger were scored. Side-by-side contact, typically observed in prairie vole tests, was rarely observed. We also recorded time engaged in aggression with the partner versus the stranger.
In the first test, behavior was digitally recorded for 3 hours. A second test was performed following the birth of the pair’s second litter, but intense aggression towards stranger females was observed. The duration of this test was thus shortened to 10 minutes. The first 10 minutes of the first partner preference test were rescored to code for aggressive behaviors as well. Approximately 1 h following the second partner preference test, pairs and pups were euthanized. Brains were flash-frozen and then stored at −40°C.
Paternal Behavior Testing
For the first litter, spontaneous paternal behavior was recorded in the home cage between PN 2–4. The home cage was transferred to a testing room and mice were allowed to habituate for 30 min. Next, the wire cage lid was removed and replaced with a lid that did not slope down in to the cage to restrict visual access. Behavior was recorded for 20 min. Males’ flanks were shaved several days prior to the test for identification purposes. Time spent huddling and grooming pups were recorded. Behavior from one control mouse was excluded because the nest interfered with observations. A 2nd paternal behavior test was performed on PN 4 after the second litter was born using a paradigm previously described (Gleason and Marler, 2010). For this test the wire cage lid was replaced during the test as with the first litter. The dam and pup(s) were removed from the home cage for approximately 90 s and then the pup(s) were returned to the home cage outside of the nest. An advantage of this test is that it can induce retrieval behavior. Behavior was recorded for 10 min. While these are relatively brief tests, previous studies demonstrate that these 10 minute tests produce repeatable results in California mice. For example, decreased grooming behavior induced by castration detected in a single 10 minute pup retrieval test (Trainor and Marler, 2001, 2002) was replicated in a series of eight 10 minute observations (Frazier et al., 2006). Also, in previous analyses of California mouse paternal behavior, 20 min observations in the home cage and pup retrieval tests have yielded similar results (Trainor and Marler, 2001).
After these experiments were concluded, we conducted a small study on colony breeders to determine the possible effect of removing the wire cage lid on behavior. The breeder males used for this study had multiple litters and were older (1+ years in colony). For this study, 10 minute recordings of breeder mice were made with the wire cage lid in place. Next, the wire cage lid was replaced with the same lid used in the stress study and behavior was recorded for an additional 10 minutes.
Open Field and Social Interaction
During PN 5–6, males were tested in a social interaction test as previously described (Trainor et al., 2013). The focal mouse was placed in a large open field (89×63×60cm) for three min (open field), which was then followed by the placement of an empty wire cage at one end of the field, and behavior recorded for 3 min (acclimation). This was then followed by the introduction of an unfamiliar same-sex conspecific into the wire cage, during which time spent within 8 cm of the wire cage was recorded for 3 min. Locomotor behavior (distance traveled), time spent in center portion, sides, and cage zone was scored using AnyMaze (San Diego Instruments).
Resident-Intruder Aggression Tests
During PN 7–8 males were tested in a resident-intruder test. The female partner and pup(s) were removed from the home cage during the test. A novel, male intruder was then placed into the home cage of the focal male for 5 min. Boxing, chasing, biting, freezing, and attack latency were recorded (Steinman et al., 2015).
Punch sample collection
Flash-frozen brains were cut on a cryostat at 500 μm coronal sections and stored in RNAlater™ (ThermoFisher Scientific, AM7020) overnight at 4° C. Punch samples from the MPOA (17 gauge, Fig. 1B) and PVN (18 gauge, Fig. 1C) were collected. Punch samples were immediately stored in 2.0 ml cylindrical tubes over dry ice, and then stored in a −40°C freezer until RNA was to be extracted.
Quantitative real-time PCR analysis
MPOA and PVN mRNA was extracted from these punch samples using RNeasy® Plus Micro Kit (Qiagen) and reverse transcribed using iScript (BioRad). All sequences were amplified using SYBR green chemistry on an Applied Biosystems ViiA7 instrument. Previously designed primers (Steinman et al., 2016; Steinman et al., 2015) for oxytocin (oxt), arginine vasopressin (avp), and β-2 micoglobulin (b2m) were used to quantify relative gene expression (Table 2). We designed and validated the following primers, including vasopressin V1a receptor (avpr1a), galanin (gmap), oxytocin receptor (otr), prodynorphin (pdyn), and kappa-opioid receptor (oprk1) (Table 2). For each transcript, expression was normalized to b2m and there were no differences in mean cycle threshold for b2m between control and stressed mice.
Table 2.
Accession numbers for transcripts and primer sequences used for pPCR.
| Transcript | Accession # | Forward Primer | Reverse Primer |
|---|---|---|---|
|
| |||
| oxt | NW_006501268.1 | CTGCGACCCTGAGTCTGC | GGAGTGAAGGTGAGCTCTAAA |
| otr | NW_006501727.1 | GCCCTTGACGCCTTTCTTCT | TTCCTTGGGCGCATTGAC |
| avp | NW_006501268.1 | AGTGTCGCGAGGGTTTTC | GGGCTTGGCAGAATCCAC |
| avpr1a | NW_006501066.1 | GAACAGCACAGGGATGTGGA | GCTCTTATGATCTCTAGCCGGA |
| gmap | NW_006501181.1 | GGGATGCCAGCAAAGGAGAA | TGTGCACGATGTTGCTCTCA |
| pdyn | NW_006501268.1 | ACAGAGTGGAGCCTTAAAACGA | GGTCATAATCCCTGCCCA |
| oprk1 | NW_006501700.1 | GGGGACATTGGAATTGAGCC | GCTCTGGATCCCTTGCTTCC |
| b2m | N\V_006501899.1 | TCTAGTGGGAGGTCCTGTGG | TGCGTTAGACCAGCAGAAGG |
Data Analysis
All data were checked for normality and homogeneity of variance using SPSS. Most variables were not normally distributed so non-parametric analyses were used. Group differences between stressed versus control groups were performed using Mann-Whitney U to assess litter outcomes, parental care, partner preference, aggression during resident intruder, social interaction, and gene expression. Paired-sample analysis was assessed using Wilcoxon tests for related samples. Analyses of qPCR data were not corrected for multiple comparisons because previous studies linked these transcripts to pair bonding and/or parental behavior.
Results
Partner Preference Tests
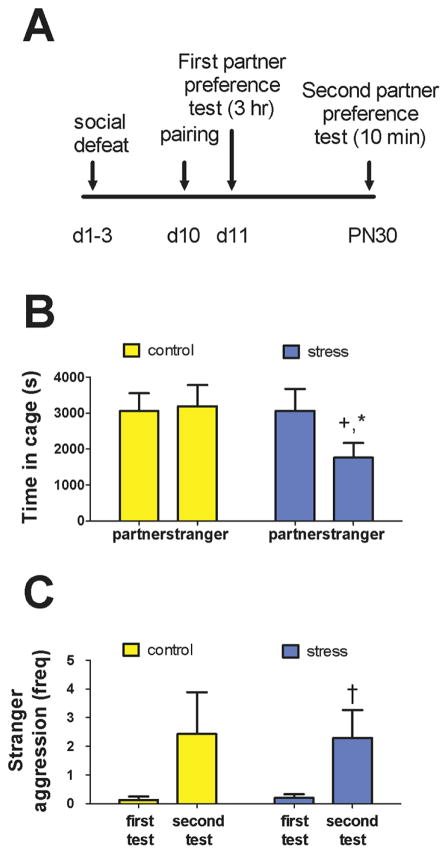
One day after pairing (Fig. 2A), neither control nor stressed males exhibited a significant preference for their mate over a stranger (Fig. 2B). However, stressed males tended to spend more time in partner’s cage over the stranger’s cage (Wilcoxon Z = 1.867, p = 0.06; Cohen’s d: 0.69). In contrast, control males spent significantly more time in the stranger cage compared to stressed males (Fig. 2B, Mann-Whitney U = 19.0, p = 0.04; Cohen’s d: 1.08). There were no effects of stress on time spent in the neutral chamber or on time in contact with partner.
Figure 2.
Effects of social defeat stress on behavior in partner preference tests. Timeline of procedures (A). Males exposed to social stress spent less time in the stranger female cage (B). High levels of aggression directed towards the stranger female were observed in the second partner preference test (C), which was shortened to 10 minutes. * p < 0.05 vs. control male, + p = 0.06 vs. partner cage, †p < 0.05 vs first partner preference test
In the second partner preference test conducted after pups were born (Fig. 2A), males were very aggressive towards stranger females. This test was only 10 minutes due to high aggression levels. The first 10 minutes of the first partner preference test was rescored so that the first and second tests could be compared. Across both groups, male aggression toward a stranger female increased following the birth of pups (Fig 2C, Wilcoxon Z = −2.51, p = 0.01; Cohen’s d: 0.83). This effect was driven primarily by stressed males, which displayed significantly increased attack frequency (Fig 2C, Wilcoxon Z = −2.51, p = 0.01; Cohen’s d: 1.03). Control males also showed increased aggression, but there was more variability and the increase in aggression was not statistically significant (Fig. 2C, Wilcoxon Z = −1.51, p = 0.13; Cohen’s d: 0.68).
Breeding
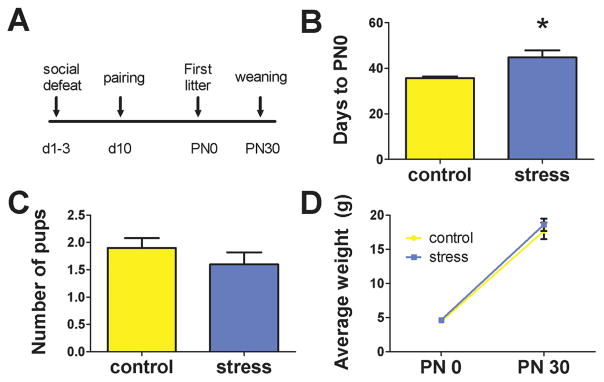
The typical gestation period for established California mouse breeder pairs is approximately 30 days. Latency to parturition for the first litter was significantly longer for females paired with defeated males compared to females paired to control males (Fig. 3B, Mann-Whitney U = 20.5, p = 0.014; Cohen’s d: 1.25). There were no significant stress-related differences in the number of pups per litter (Fig. 3C) or average pup weight (Fig. 3D).
Figure 3.
Effects of social defeat stress in males on reproduction. Timeline of procedures and measurements (A). Males exposed to stress had longer latency to first litter than controls (B). There was no difference in the total number of pups (C) or the average weight of pups (D) between control and stressed males. * p < 0.05.
Paternal Behavior Testing
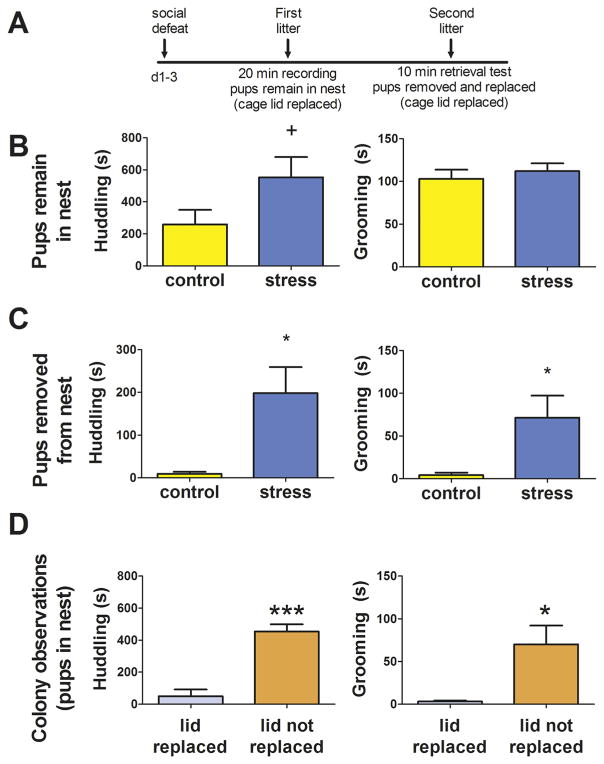
During observations of the first litter (Fig. 4A), there was no significant effect of stress on huddling (Mann-Whitney U = 68, p = 0.06; Cohen’s d: 0.86; Fig. 4B) or grooming (Mann-Whitney U = 48.5, p = 0.48; Cohen’s d: 0.08; Fig. 4B). However, there was a large effect size for stressed males to spend more time huddling with pups. Pup retrieval was not observed during these tests. Differences between control and stressed males were more pronounced during the pup retrieval test with the second litter (Fig 4A). Unexpectedly, control males spent little time huddling or grooming pups. In contrast, stressed males spent significantly more time huddling with (Fig. 4C, Mann-Whitney U = 15.00, p = 0.048; Cohen’s d: 0.89) and grooming pups (Fig. 4C, Mann-Whitney U = 41.5, p = 0.02; Cohen’s d: 1.30) than controls. Stressed males were also more likely to retrieve pups than controls (mean ± s.e. control 0±0, stress 1.1±0.3; Mann-Whitney U = 12.00, p = 0.02; Cohen’s d: 1.12). Although the higher levels of paternal behavior in stressed males were consistent with results in the first parental behavior test, prior studies have observed more extensive paternal behavior in pup retrieval tests (Trainor and Marler, 2001, 2002). Using colony breeders, we tested whether removal of the wire cage lid inhibited male parental behavior (Fig. 4D). During observations when the cage lid was not removed, huddling and grooming rates were relatively high. However, when the cage lid was replaced with chicken-wire top (as used in the stress experiment), both huddling and pup grooming behavior was significantly lower. These data indicate that while removing the wire cage lid provides better visual access for behavioral observations, it induces a disruption of normal parental behavior.
Figure 4.
Effects of social defeat stress on paternal behavior. Timeline of procedures and measurements (A). For observations of the first litter, pups were not removed from the nest (B). For observations of the second litter, pups were removed from the nest and replaced outside of the nest (C). Observations of colony breeders showed that huddling and grooming behavior was reduced when the wire cage lid was replaced with a lid that did not visually restrict access to the cage (D). * p < 0.05. *** p < 0.001.
Open Field and Social Interaction
Defeated males showed increased time spent in the center portion of the open field phase compared to controls (Table 3, Mann-Whitney U = 24.0, p = .049; Cohen’s d: 0.98). There were no effects of stress on total distance traveled during the open field phase or time spent in the interaction zone during the acclimation or interaction phases of the test (Table 3).
Table 3.
Results from 3-stage social interaction test and resident-intruder test.
| Social interaction test | Control | Stress |
|---|---|---|
| Open field:time in center (s) | 36.2±4.4 | 49.1±5.3* |
| Open field:total distance (m) | 35.9±5.9 | 27.8±3.4 |
| Acclimation: time in cage zone (s) | 87.6±7.4 | 94.1±10.9 |
| Interaction: time in cage zone (s) | 107.2±10.3 | 100.3±13.3 |
| Resident intruder test | Control | Stress |
|---|---|---|
| Boxing (freq) | 0.8±0.4 | 2.4±0.6* |
| Biting (freq) | 8.1±2.2 | 10.1±2.6 |
| Anogenital sniffing (s) | 13.4±5.8 | 11.1±3.9 |
| Attack latency (s) | 121.9±45.1 | 79.7±40.9 |
p < 0.05 vs. control
Resident Intruder
In the resident-intruder test, stressed males engaged in more boxing than control males (Table 3; Mann-Whitney U = 51, p = 0.04 Cohen’s d: 1.25). However, the most frequent aggressive behavior was biting and there was no difference between control and stressed males (Table 3). There was also no difference in attack latency or anogenital sniffing.
Quantitative real-time PCR
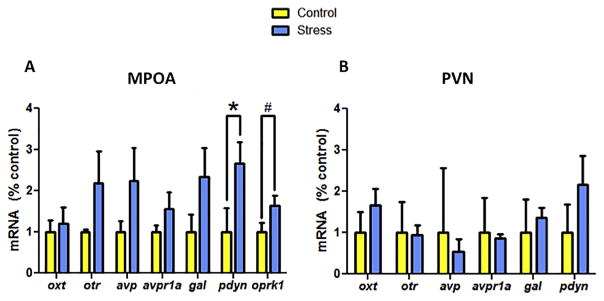
In the MPOA, stressed males had significantly more pdyn mRNA expression compared to controls (Mann-Whitney U = 4.00, p = .045; Cohen’s d: 1.753; Fig. 5A). There was a large effect size for increased Oprk1 mRNA in stressed males but this difference was not significant (Mann-Whitney U = 13.00, p = .050; Cohen’s d: 1.123; Fig. 4A). There were no differences in the other transcripts quantified (oxt, avp, avpr1a, otr, gal) in the MPOA and no differences were observed in the PVN (Fig. 5B). In the PVN amplification for oprk1 was not detectable.
Figure 5.
Gene expression analysis in MPOA (A) and PVN (B) samples. In MPOA, prodynorphin and kappa opioid receptor show enhanced expression in stressed compared to control males. There were no effects of stress on expression of these transcripts in PVN. *p < .05; # p = .05.
Discussion
Our results demonstrate that social defeat stress facilitates parental behavior in males as well as behavior that may affect pair bond formation. Interestingly, these effects are observed almost 2 months after the last episode of defeat, indicating that the effects of social stress endure. Social defeat had anatomically specific effects on transcription, enhancing the expression of transcripts related to the kappa opioid receptor (KOR) pathway in the MPOA but not the PVN. These results are intriguing in light of prior reports of the importance of KOR signaling on sexual behavior and selective aggression. Together our results show that effects of social stress on brain function and behavior are persistent, even after males are paired with a female and reproduce.
Effects of Stress in the Partner Preference Test
The behavior of California mice in the partner preference tests differed from prairie voles in that the side-by-side contact normally observed in voles (Bales et al., 2007; Carter et al., 1988; Cho et al., 1999; Young et al., 2011) was not observed in California mice. Despite this species difference in behavior, there were signs that prior stress exposure affected behavior related to pair bonding. One day after pairing with a female, stressed males spent less time in the chamber with a stranger female compared to controls. The avoidance of stranger females by stressed males could be an initial step in pair bond formation. On balance though, it did not appear that strong pair bonds were present 24 hr after pairing. Mating likely takes longer to occur in California mice after pairing than in prairie voles. Prairie voles are induced ovulators (Carter et al., 1987; Hasler & Conaway, 1973), while California mice are spontaneous ovulators with significant individual variation in cycle length (Davis and Marler, 2003; Gubernick, 1988). It is likely that not all male California mice had mated with females before the first partner preference test, especially considering that the mean latency for the first litter for control males was 36 days and the typical gestation length is about 30 days. This may contribute to the increased time associating with stranger females by control males in the first test. In the second test, selective aggression towards unfamiliar females by both control and stressed males was intense, consistent with previous reports (Gubernick and Addington, 1994; Gubernick and Nordby, 1993). Selective aggression is a key sign of pair bond formation (Carter et al., 1995), suggesting that strong pair bonds are in place after California mice have pups. At this time point, aggression was not significantly different between control and stressed males. This suggests that while stress may affect behavior that contributes to pair bond formation, effects of stress on pair bond maintenance are less significant. Based on our results, we can’t determine what cues males use to distinguish between the partner versus the stranger in the second test. While it’s likely that males can recognize their partner, in the second test the partner was lactating but the stranger female was not. Regardless, male aggression directed towards virgin females is an unusual phenotype that is rarely observed in non-monogamous rodents.
In the first partner preference test, the effects of stress were observed primarily in the context of avoiding stranger females. A recent study showed that unpaired male California mice produce more ultrasonic vocalizations (USVs) in response to novel females than pair bonded males (Pultorak et al., 2015). These data suggest that reduced stranger contact by stressed male California mice in the first test could be indicative of facilitated pair bond formation. In prairie voles, acute stress (forced swim) or corticosterone injections facilitate pair bonding in males (DeVries et al., 1996). Our results extend these findings by showing that exposure to brief stressors can impact behavior weeks later. Curiously, while defeat stress appeared to enhance components of pair bond formation, the latency to first litter was longer in stressed males compared to controls. Previous studies have reported that defeat stress can inhibit sexual behavior in male mice (Kahn, 1961) and tree shrews (Van Kampen et al., 2002). Additionally, three weeks of social defeat stress with sensory contact induced deficits in spermatogenesis in male C57Bl/6J (Wang et al., 2017). However, our protocol uses only 3 days of stress with no sensory contact, and we observed no effect of stress on the total number or weight of pups. Alternatively, stressed males may be less attractive to females. Male California mice use urine to produce scent marks (Williams et al., 2013) and in other rodents, dominant males produce more major urinary proteins (Lee et al., 2017), which can influence sexual attraction in females (Roberts et al., 2010). Female California mice paired with preferred males had shorter latencies to first litters and more pups than females paired to an unpreferred male (Gleason, Holschbach, & Marler 2012). Further study is needed to resolve how defeat affects male sexual behavior and female mating preferences.
Stress and Paternal Behavior
Previous studies examining effects of stress on paternal behavior applied psychosocial stress after males had pups (Bales et al., 2006, Harris et al., 2013). This may be an important distinction, as behavioral responses to an acute stressor are reduced in male California mice as they gain parental experience (Bardi et al., 2011). In our study, exposure to social defeat two weeks before pairing facilitated later paternal behavior. However, this effect may be context dependent. Parental responsiveness in control males was much lower than expected, especially in the pup retrieval test. Analyses of paternal behavior in colony breeders suggests that replacing the cage lid with a screen top during testing had a strong inhibitory effect on parental behavior. When the wire cage lid was not replaced, paternal responsiveness was much higher in colony breeders and similar to previous reports (Bester-Meredith et al., 1999; Trainor and Marler, 2001). Novel environments can have strong inhibitory effects on parental behavior (Stern and Mackinnon, 1976; Stolzenberg et al., 2012), and our results suggest that changing the cage top is a significant alteration to the environment. Interestingly, male California mouse parents with prior exposure to defeat appear to be less sensitive to novel environments. Stressed males spent more time in the center of the open field test compared to controls. Although this phenotype has not been observed in virgin male California mice exposed to defeat, other evidence suggests that the effects of defeat stress are weaker in novel environments for males. For example, effects of social defeat on social interaction behavior in a novel environment is weaker in male California mice than in females (Greenberg et al., 2014). In contrast, when confronted with an intruder in the home cage stressed virgin males exhibit freezing and escape behavior (Steinman et al., 2015). These behaviors resemble the “conditioned defeat” phenotype in Syrian hamsters exposed to defeat stress (Gray et al., 2015). Interestingly, freezing behavior was not observed in stressed male parents in the resident-intruder test. Our results suggest that social defeat has anxiolytic effects in male California mouse parents, which facilitates paternal behavior in novel environments. Future studies of paternal behavior should carefully consider the potential impact of recording conditions.
Stress and Kappa Opioid Receptors
Gene expression analyses detected increases in KOR-related transcripts (opkr1 and pdyn) in the MPOA but not PVN. Several lines of evidence suggest that KOR acting in the MPOA inhibits male sexual motivation. In starlings, males that successfully compete for nest boxes had lower opkr1 expression in the MPOA, and opkr1 expression was negatively associated with sexually motivated singing behavior (Riters et al., 2017). Furthermore, infusion of dynorphin in to the MPOA inhibited motivation to engage in sexual behavior in male rats (Leyton and Stewart, 1992). These findings suggest that increased KOR activity in stressed males could be a contributing factor to the longer latency for the first litter. Increased KOR activity might also affect pair bonding. In pair-bonded male and female prairie voles, KOR activation in the nucleus accumbens facilitates aggression towards same-sex intruders (Resendez et al., 2012). In addition pair-bonding increases both pdyn and opkr1 mRNA in the NAc (Resendez et al., 2016). Currently, it is unclear whether KOR in the MPOA has a similar role. While the activation of KOR can induce aversion and depression-like behaviors, stressed male California mice were less sensitive to novelty-stress in the open field test and parental behavior tests. Studies in both males (Al-Hasani et al., 2013; Kudryavtseva et al., 2006) and females (Laman-Maharg et al., 2017) suggest that the aversive properties of KOR become weaker after defeat stress. Overall, our results suggest that further study of the behavioral effects of KOR in the MPOA could produce interesting results.
We observed no differences in transcripts related to vasopressin or oxytocin signaling pathways, similar to a previous qPCR study that compared virgin and parental male California mice (Perea-Rodriguez et al., 2015). These results suggest there is less plasticity in these systems within the MPOA. In the PVN, there were no differences in Avp gene expression between control and stress males. A recent report showed that Avp expression in the hypothalamus contributes to species and individual variability in nest-building behavior but not parental care (Bendesky et al., 2017). Our results are consistent with the hypothesis that Avp gene expression in the PVN does not contribute to individual differences in parental care.
Conclusions
Our study demonstrates that social stress facilitates stranger aversion during initial partner preference tests and enhances the robustness of parental behavior in male California mice. While these behavior changes are associated with increased expression of KOR-related transcripts in the MPOA, further study is needed to determine the functional consequences of these changes in transcription. The enhanced paternal behavior observed in stressed males suggests that there are important sex differences in how mechanisms of paternal behavior respond to psychosocial stressors.
Highlights.
Effects of defeat stress on behavior were examined in paired male California mice
Defeat stress increased avoidance of stranger females in a partner preference test
Defeat stress had anxiolytic effects in the open field test
Defeat stress increased male parental behavior in a novel environment
In the medial preoptic area stress increased expression of prodynorphin
Acknowledgments
Special thanks to Karen Bales and Danielle Stolzenberg for project suggestions, and to Natalia Duque-Wilckens, Rebecca Hao, Abigail Laman-Maharg, and Alexia Williams for helping with procedures and methods. Supported by R01 MH103322 to BCT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Hasani R, McCall JG, Bruchas MR. Exposure to chronic mild stress prevents kappa opioid-mediated reinstatement of cocaine and nicotine place preference. Frontiers in Behavioral Neuroscience. 2013;4:1–10. doi: 10.3389/fphar.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Kramer KM, Lewis-Reese AD, Carter CS. Effects of stress on parental care are sexually dimorphic in prairie voles. Physiology & Behavior. 2006;87:424–429. doi: 10.1016/j.physbeh.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Bales KL, Lewis-Reese AD, Pfeifer LA, Kramer KM, Carter CS. Early experience affects the traits of monogamy in a sexually dimorphic manner. Dev Psychobiol. 2007;49:335–342. doi: 10.1002/dev.20216. [DOI] [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM, Conley OG, Lee MH, Guoynes CD, Downing GM, Yun CR, Solomon M, Jacob S, Mendoza SP. Chronic Intranasal Oxytocin Causes Long-Term Impairments in Partner Preference Formation in Male Prairie Voles. Biological Psychiatry. 2013;74:180–188. doi: 10.1016/j.biopsych.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardi M, Franssen CL, Hampton JE, Shea EA, Fanean AP, Lambert KG. Paternal Experience and Stress Responses in California Mice (Peromyscus californicus) Comparative Medicine. 2011;61:20–30. [PMC free article] [PubMed] [Google Scholar]
- Bendesky A, Kwon YM, Lassance JM, Lewarch CL, Yao S, Peterson BK, He MX, Dulac C, Hoekstra HE. The genetic basis of parental care evolution in monogamous mice. Nature. 2017;544:434–439. doi: 10.1038/nature22074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bester-Meredith JK, Young LJ, Marler CA. Species differences in paternal behavior and aggression in Peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm Behav. 1999;36:25–38. doi: 10.1006/hbeh.1999.1522. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopiressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm Behav. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Carter CS, Witt DM, Thompson EG, Carlstead K. Effects of hormonal, sexual, and social history on mating and pair bonding in prairie voles. Physiology and Behavior. 1988;44:691–697. doi: 10.1016/0031-9384(88)90049-2. [DOI] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Davis ES, Marler CA. The progesterone challenge: steroid hormone changes following a simulated territorial intrusion in female Peromyscus californicus. Horm Behav. 2003;44:189–198. doi: 10.1016/s0018-506x(03)00128-4. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Carter CS. Sex differences in temporal parameters of partner preference in prairie voles (Microtus ochrogaster) Canadian Journal of Zoology. 1999;77:885–889. [Google Scholar]
- DeVries AC, DeVries MB, Taymans SE, Carter CS. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proceedings of the National Academy of Sciences. 1996;93:11980–11984. doi: 10.1073/pnas.93.21.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Munoz SL, Bales KL. “Monogamy” in primates: variability, trends, and synthesis: introduction to special issu on primate monogamy. Am J Primatol. 2016;78:283–287. doi: 10.1002/ajp.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Wilckens N, Steinman M, Laredo S, Hao R, Perkeybile A, Bales K, Trainor B. Anxiolytic effects of vasopressin V1a receptor in the medioventral bed nucleus of the stria terminalis: sex specific effects in social and nonsocial contexts. Neuropharmacology. 2016 doi: 10.1016/j.neuropharm.2016.07.018. (in revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CRM, Trainor BC, Cravens CJ, Whitney TK, Marler CA. Paternal behavior influences development of aggression and vasopressin expression in male California mouse offspring. Horm Behav. 2006;50:699–707. doi: 10.1016/j.yhbeh.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Gleason ED, Holschbach MA, Marler CA. Compatibility drives female preference and reproductive success in the monogamous California mouse (Peromyscus californicus) more strongly than male testosterone measures. Horm Behav. 2012 Jan;61(1):100–7. doi: 10.1016/j.yhbeh.2011.10.009. Epub 2011 Nov 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason ED, Marler CA. Testosterone response to courtship predicts future paternal behavior in the California mouse, Peromyscus californicus. Hormones and Behavior. 2010;57:147–154. doi: 10.1016/j.yhbeh.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Covington HE, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protocols. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CL, Krebs-Kraft DL, Solomon MB, Norvelle A, Parent MB, Huhman KL. Immediate post-defeat infusions of the noradrenergic receptor antagonist propranolol impair the consolidation of conditioned defeat in male Syrian hamsters. Physiology & Behavior. 2015;152(Part A):56–61. doi: 10.1016/j.physbeh.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg GD, Laman-Maharg A, Campi KL, Voigt H, Orr VN, Schaal L, Trainor BC. Sex differences in stress-induced social withdrawal: role of brain derived neurotrophic factor in the bed nucleus of the stria terminalis. Front Behav Neurosci. 2014;7:223. doi: 10.3389/fnbeh.2013.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubernick DJ. Reproduction in the California mouse Peromyscus californicus. J Mammal. 1988;69:857–860. [Google Scholar]
- Gubernick DJ, Addington RL. The stability of female social and mating preferences in the monogamous California mouse, Peromyscus californicus. Animal Behaviour. 1994;47:559–567. [Google Scholar]
- Gubernick DJ, Nordby JC. Mechanisms of sexual fidelity in the monogamous California mouse, Peromyscus californicus. Behavioral Ecology and Sociobiology. 1993;32:211–219. [Google Scholar]
- Harris BN, de Jong TR, Yang V, Saltzman W. Chronic variable stress in fathers alters paternal and social behavior but not pup development in the biparental California mouse (Peromyscus californicus) Hormones and Behavior. 2013;64:799–811. doi: 10.1016/j.yhbeh.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howerton CL, Garner JP, Mench JA. Effects of a running wheel-igloo enrichment on aggression, hierarchy linearity, and stereotypy in group-housed male CD-1 (ICR) mice. Appl Anim Behav Sci. 2008;115:90–103. [Google Scholar]
- Huhman KL. Social conflict models: Can they inform us about human psychopathology? Hormones and Behavior. 2006;50:640–646. doi: 10.1016/j.yhbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: A clinically relevant model for early-life stress. Neuroscience. 2008;154:1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn MW. The effect of socially learned aggression on submission on the mating behavior of C57 mice. J Genet Psychol. 1961;98:211–217. doi: 10.1080/00221325.1961.10534371. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva N, Gerrits MAFM, Avgustinovich DF, Tenditnik MV, Van Ree JM. Anxiety and ethanol consumption in victorious and defeated mice; effect of κ-opioid receptor activation. European Neuropsychopharmacology. 2006;16:504–511. doi: 10.1016/j.euroneuro.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Laman-Maharg A, Copeland T, Ordoñes Sanchez E, Campi KL, Trainor BC. The long-term effects of stress and kappa opioid receptor activation on conditioned place aversion in male and female California mice. Behav Brain Res. 2017;332:299–307. doi: 10.1016/j.bbr.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laredo SA, Steinman MQ, Robles CF, Ferrer E, Ragen BJ, Trainor BC. Effects of defeat stress on behavioral flexibility in males and females: modulation by the mu-opioid receptor. European Journal of Neuroscience. 2015;41:434–441. doi: 10.1111/ejn.12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Khan A, Curley JP. Major urinary protein levels are associated with social status and context in mouse social hierarchies. Proc R Soc B. 2017;284:20171570. doi: 10.1098/rspb.2017.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M, Stewart J. The stimulation of central k opioid receptors decreases male sexual behavior and locomotor activity. Brain Res. 1992;594:56–74. doi: 10.1016/0006-8993(92)91029-e. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci. 1994;108:1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- Perea-Rodriguez JP, Takahashi EY, Amador TM, Hao RC, Saltzman W, Trainor BC. Effects of Reproductive Experience on Central Expression of Progesterone, Oestrogen α, Oxytocin and Vasopressin Receptor mRNA in Male California Mice (Peromyscus californicus) Journal of Neuroendocrinology. 2015;27:245–252. doi: 10.1111/jne.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AS, Giusti-Paiva A, Vilela FC. Central corticosterone disrupts behavioral and neuroendocrine responses during lactation. Neuroscience Letters. 2015;606:88–93. doi: 10.1016/j.neulet.2015.08.018. [DOI] [PubMed] [Google Scholar]
- Pultorak JD, Fuxjager MJ, Kalcounis-Rueppell MC, Marler CA. Male fidelity expressed through rapid testosterone suppression of ultrasonic vocalizations to novel females in the monogamous California mouse. Horm Behav. 2015;70:47–56. doi: 10.1016/j.yhbeh.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Resendez SL, Keyes PC, Day JJ, Hambro C, Austin CJ, Maina FK, Eidson LN, Porter-Stransky KA, Nevárez N, McLean JW, Kuhnmuench MA, Murphy AZ, Mathews TA, Aragona BJ. Dopamine and opioid systems interact within the nucleus accumbens to maintain monogamous pair bonds. eLife. 2016;5:e15325. doi: 10.7554/eLife.15325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Kuhnmuench M, Krzywosinski T, Aragona BJ. κ-Opioid Receptors within the Nucleus Accumbens Shell Mediate Pair Bond Maintenance. The Journal of Neuroscience. 2012;32:6771–6784. doi: 10.1523/JNEUROSCI.5779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribble DO, Salvioni M. Social organization and nest coocupancy in Peromyscus californicus, a monogamous rodent. Behav Ecol Sociobiol. 1990;26:9–15. [Google Scholar]
- Riters LV, Cordes MA, Stevenson SA. Prodynorphin and kappa opioid recetpor mRNA expression in the brain relates to social status and behavior in male European starlings. Behav Brain Res. 2017;320:37–47. doi: 10.1016/j.bbr.2016.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SA, Simpson DM, Armstrong SD, Davidson AJ, Robertson DH, McLean L, Beynon RJ, Hurst JL. Darcin: a male pheremone that stimulates female memory and sexual attraction to an individual male’s odour. BMC Biology. 2010;8:75. doi: 10.1186/1741-7007-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting Epigenetic Influence of Early-Life Adversity on the BDNF Gene. Biological Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: Neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biological Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Steinman MQ, Duque-Wilckens N, Greenberg GD, Hao R, Campi KL, Laredo SA, Laman-Maharg A, Manning CE, Doig IE, Lopez EL, Walch K, Bales KL, Trainor BC. Sex-specific effects of stress on oxytocin neurons correspond with responses to intranasal oxytocin. Biol Psychiatry. 2016;80:406–414. doi: 10.1016/j.biopsych.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MQ, Laredo SA, Lopez EM, Manning CE, Hao RC, Doig IE, Campi KL, Flowers AE, Knight JK, Trainor BC. Hypothalamic vasopressin systems are more sensitive to social defeat in males versus females. Psychoneuroendocrinology. 2015;51:122–134. doi: 10.1016/j.psyneuen.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MQ, Trainor BC. Sex differences in the effects of social defeat on brain and behavior in the California mouse: Insights from a monogamous rodent. Seminars in Cell & Developmental Biology. 2017;61:92–98. doi: 10.1016/j.semcdb.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JM, Mackinnon DA. Postpartum, hormonal, and nonhormonal induction of maternal behavior in rats: effects on T-maze retrieval of pups. Horm Behav. 1976;7:305–316. doi: 10.1016/0018-506x(76)90036-2. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, Stevens JS, Rissman EF. Experience-facilitated improvements in pup retrieval: evidence for an epigenetic effect. Horm Behav. 2012;62:128–135. doi: 10.1016/j.yhbeh.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Finy MS, Nelson RJ. Paternal aggression in a biparental mouse: parallels with maternal aggression. Horm Behav. 2008;53:200–207. doi: 10.1016/j.yhbeh.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Marler CA. Testosterone, paternal behavior, and aggression in the monogamous California mouse (Peromyscus californicus) Horm Behav. 2001;40:32–42. doi: 10.1006/hbeh.2001.1652. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Marler CA. Testosterone promotes paternal behaviour in a monogamous mammal via conversion to oestrogen. Proc R Soc Lond B Biol Sci. 2002;269:823–829. doi: 10.1098/rspb.2001.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Takahashi EY, Campi KL, Florez SA, Greenberg GD, Laman-Maharg A, Laredo SA, Orr VN, Silva AL, Steinman MQ. Sex differences in stress-induced social withdrawal: independence from adult gonadal hormones and inhibition of female phenotype by corncob bedding. Hormones and Behavior. 2013;63:543–550. doi: 10.1016/j.yhbeh.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kampen M, Kramer M, Hiemke C, Flugge G, Fuchs E. The chronic psychosocial stress paradigm in male tree shrews: evaluation of a novel animal model for depressive disorders. Stress. 2002;5:37–46. doi: 10.1080/102538902900012396. [DOI] [PubMed] [Google Scholar]
- Wang B, Zhou J, Zhuang YY, Wang LL, Pu JX, Huang YH, Xia F, Lv JX. The non-peptide vasopressin V1b receptor antagonist, SSR149415, ameliorates spermatogenesis function in a mouse model of chronic social defeats tress. J Cell Biochem. 2017;118:3891–3898. doi: 10.1002/jcb.26040. [DOI] [PubMed] [Google Scholar]
- Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus ochrogaster): The role of social and sexual experience. Hormones and Behavior. 1992;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- Williams SA, Jasarevic E, Vandas GM, Warzak DA, Geary DC, Ellersieck MR, Roberts RM, Rosenfeld CS. Effects of Developmental Bisphenol A Exposure on Reproductive-Related Behaviors in California Mice (Peromyscus californicus): A Monogamous Animal Model. PloS One. 2013;8:e55698. doi: 10.1371/journal.pone.0055698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson CM, Romeo RD, Curley JP. Dynamic changes in social dominance and mPOA GnRH expression in male mice following social opportunity. Horm Behav. 2017;87:80–88. doi: 10.1016/j.yhbeh.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Wood SK. Individual Differences in the Neurobiology of Social Stress: Implications for Depression-Cardiovascular Disease Comorbidity. Current Neuropharmacology. 2014;12:205–211. doi: 10.2174/1570159X11666131120224413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman JL, Gobinath AR, Kitay NF, Chow C, Brummelte S, Galea LAM. Parity modifies the effects of fluoxetine and corticosterone on behavior, stress reactivity, and hippocampal neurogenesis. Neuropharmacology. 2016;105:443–453. doi: 10.1016/j.neuropharm.2015.11.027. [DOI] [PubMed] [Google Scholar]
- Young KA, Liu Y, Gobrogge KL, Dietz DM, Wang H, Kabbaj M, Wang ZX. Amphetamine alters behavior and mesocorticolimbic dopamine receptor expression in the monogamous female prairie vole. Brain Research. 2011;1367:213–222. doi: 10.1016/j.brainres.2010.09.109. [DOI] [PMC free article] [PubMed] [Google Scholar]