Abstract
Background and Purpose
Oxidative stress is an early response to cerebral ischemia and is likely to play an important role in the pathogenesis of cerebral ischemic injury. We sought to evaluate whether hyperacute plasma concentrations of biomarkers of oxidative stress, inflammation, and tissue damage predict infarct growth (IG).
Methods
We prospectively measured plasma F2-isoP, urinary 8-oxo-7,8-dihydro-2′-deoxyguoanosine, plasma Oxygen Radical Absorbance Capacity assay, high sensitivity-C reactive protein, and matrix metalloproteinase 2 and 9 in consecutive acute ischemic stroke (AIS) patients presenting within 9 h of symptom onset. Patients with baseline DWI MRI and follow-up DWI or CT scan were included to evaluate the final infarct volume (FIV). Baseline DWI volume (DWIV) and FIV were analyzed using semi-automated volumetric method. IG volume (IGV) was defined as the difference between FIV and baseline DWIV.
Results
220 AIS subjects were included in the final analysis. 170 of these had IG. Baseline F2-isoP significantly correlated with IGV (Spearman’s ρ=0.20, P=0.005) and FIV (Spearman’s ρ=0.19, P=0.009). In a multivariate binary logistic regression model, baseline F2-isoP emerged as an independent predictor of the occurrence of IG (OR 2.57, 95% CI 1.37–4.83; P=0.007). In a multivariate linear regression model, baseline F2-isoP was independently associated with IGV (B 0.38, 95% CI 0.04–0.72; P=0.03).
Conclusions
Elevated hyperacute plasma F2-isoP concentrations independently predict the occurrence of IG and IGV in AIS patients. If validated in future studies, measuring plasma F2-isoP might be helpful in the acute setting to stratify AIS patients for relative severity of ischemic injury and expected progression.
Keywords: Oxidative stress, F2-isoprostanes, ischemic stroke, cerebral ischemia, infarct growth
Stroke, with its important individual, social, and economic implications, has a major impact on our entire healthcare system.1 Intravenous (IV) thrombolytic therapy with tissue-type plasminogen activator (t-PA) and endovascular therapies to achieve early recanalization are the only treatment proven to be beneficial in patients with acute ischemic stroke (AIS).2, 3 The narrow therapeutic time window from symptom onset to treatment necessary for these therapies to salvage brain tissue and improve outcome continues to limit their broad application.3 Attempts to extend this therapeutic window require a better decision-making protocol taking into account the pathophysiological aspects of infarct evolution. Therefore, there is a need for practical, time-efficient, and cost-effective measures that might be combined with neuroimaging to predict brain tissue outcome. Such measures would contribute to our understanding of stroke progression in humans and to the development of new therapeutic targets.
Clinical, genetic and imaging variables strongly influence susceptibility to infarct growth (IG).4–6 Experimental studies have demonstrated that free-radical induced oxidative stress is one of the major contributors to the evolution of ischemic lesion and reperfusion-related brain tissue damage.7 The crucial role of oxidative stress in infarct progression has been demonstrated by the reduction of infarct size through the inhibition of neuronal nitric oxide synthase in models of cerebral ischemia.8
Despite this experimental evidence, data on oxidative stress biomarkers in the hyperacute phase of AIS in humans are limited, mainly due to the lack of valid measurable biomarkers.9 F2-isoprostanes (F2-isoPs) are products of non-cyclooxygenase free radical-induced neuronal arachidonic acid peroxidation of membrane phospholipids and lipoproteins.10 F2-isoPs are more stable, sensitive, and specific markers of oxidative stress-induced peroxidation than other biomarkers.11, 12 They are easily quantifiable in plasma, urine, and cerebrospinal fluid in humans.12 A previous study found an increase of plasma levels of F2-isoP in AIS patients in the first 8 hours but not at 24 hours or later time points, suggesting oxidative stress activation early after stroke onset.13 Several measures of “total plasma antioxidant capacity”, such as the Oxidative Radical Absorbance Capacity (ORAC) assay conducted in plasma ex vivo, may offer a complementary approach to the measurement of a broad spectrum of antioxidant micronutrients to assess oxidative stress and antioxidant system.14,15
Therefore, in this study we sought to evaluate the role of hyperacute plasma levels of oxidative stress biomarkers in predicting IG and their association with IG volume (IGV) in AIS patients and compare them to potential biomarkers of inflammation and tissue damage relevant to stroke.
MATERIALS AND METHODS
Study population and clinical data collection
We prospectively enrolled patients presenting to two large academic medical centers with AIS within the first 9 hours of symptom onset. Patients with stroke on awakening and those with unwitnessed stroke onset were included only if the midway point between the last seen well and first seen abnormal times was within 9 hours. Patients with all degrees of stroke severity were included (NIHSS score ≥1). Potential subjects were excluded from the study if they had hemorrhagic stroke; ischemic stroke due to probable vasculitis; endocarditis or venous infarction, transient ischemic attack (TIA) or stroke mimics; active infection (body temperature >38°C or WBC>15 th/mm3); systemic inflammatory disorders; end-stage renal disease; end-stage liver disease; active metastatic malignancy at the time of stroke; a history of stroke within 30 days of the index stroke onset; myocardial infarction, major thromboembolic event or major surgery within 30 days of stroke onset.
Informed consent was obtained from patients or their healthcare proxies. The study was approved by the local institutional review board at both centers.
Demographic (age, gender, race) and clinical data, including functional independence before the index stroke measured by modified Rankin Scale (mRS) score 0–1, vascular risk factors, medications, and vital signs were recorded. Neurological severity at baseline and at 48 hours from symptom onset by using National Institutes of Health Stroke Scale (NIHSS) score and outcome at 3 months by mRS score were assessed by staff certified in administering these scales by in-person or telephone interview of the patient or care giver. Blood samples for routine laboratory exams and biomarkers were performed at admission and at 48 hours (range 36–60 hours). Stroke subtype was determined using the Causative Classification of Stroke System (CCSS).16, 17
Laboratory methods
Blood samples were collected at admission, less than 9 hours after stroke onset. To avoid delays in treatment, all blood samples from patients eligible for IV thrombolytic treatment were obtained after the treatment was initiated. Samples were analyzed by investigators blinded to clinical information.
Oxidative stress biomarkers F2-isoP, urinary 8-OHdG, and plasma ORAC assay were evaluated in this study (total ORAC [ORACTOTAL] and perchloric acid ORAC [ORACPCA]). Inflammatory and tissue damage biomarkers high sensitivity C reactive protein (hs-CRP), matrix metalloproteinase (MMP) 2 and 9 were also evaluated.
For the quantification of F2-isoP, blood samples were collected in 10 mL ethylenediaminetetraacetic acid (EDTA) tubes. Plasma was frozen at −80°C prior to processing. F2-isoP was quantified using an 8-Isoprostane Enzyme Immunoassay Kit (Cayman Chemical, Ann Arbor, MI). Laboratory methods used for the quantification of the other biomarkers are reported in the supplemental material.
Neuroimaging analysis
Computerized tomography (CT) and/or magnetic resonance imaging (MRI) were performed on admission as part of the routine clinical stroke evaluation and based on clinical requirements at follow up. Diffusion-weighted MRI (DWI) performed at admission (within 24 hours of stroke onset) was used to asses baseline infarct volume. Both DWI and CT scans performed at 48 hours (with a range from 24 to 96 hours) from symptom onset were used as the measure of final infarct size; both of these modalities have been shown to define infarct limits reliably after 24 hours from stroke onset.18
For the purpose of analysis, the patient cohort was divided in two groups based on the occurrence of IG (any IGV >0). Infarct growth volume (IGV) was defined as the final infarct volume (FIV) minus the baseline DWI volume (DWIV). Percentage infarct growth (PIG) was calculated by using the formula [(IGV/baseline DWIV)*100]. Data on vessel stenosis/occlusion by CT- or MR-Angiography considered responsible for the index stroke, DWI and perfusion weighted magnetic resonance imaging mismatch volume (calculated as the volume of hypoperfused tissue measured by the mean transit time [MTT] minus baseline DWIV), and symptomatic intracerebral hemorrhage (SICH) (defined as any bleeding plus any neurological deterioration [NIHSS score ≥1] or that leads to death within 7 days) were also evaluated.
All volumetric data were processed using a validated semi-automated protocol.19 The intra-class correlation coefficient for the volumetric lesion analysis was 0.99 for both DWIV and FIV.20 All lesion volumes were corrected for differences in overall brain size using mid-sagittal cross-sectional intracranial area as a surrogate measure of the intracranial volume.21 Imaging analyses were performed by experienced and trained physicians and neurologists blinded to clinical and laboratory data.
Statistical analysis
Results for categorical variables are expressed as percentages, with proportions calculated by dividing the number of events by the total number of patients excluding missing or unknown cases. Results for continuous variables are expressed as medians (IQR, [interquartile range]) or means (±SD [standard deviation]) for non-normal or normal variable distribution, respectively.
As appropriate, χ2 or Fisher’s exact test and Student’s t-test or Mann-Whitney U test were used to compare categorical and continuous variables between two groups. Since biomarkers and lesion volumes had a non-normal distribution, Spearman’s correlation coefficients (Spearman’s ρ) were derived to quantify the association between biomarkers and IGV. Natural logarithm transformation of continuous variables was used if appropriate, in case of non-normal distribution.
Multivariate binary logistic regression and linear regression modeling were used to adjust for the effects of potential confounders and to evaluate whether plasma biomarkers measured within 9 hours of symptom onset independently predicted IG occurrence and more specifically IGV. Covariates with a univariate association with IG or IGV at P-value ≤0.10 were included in the relative multivariate models along with other potential predictors of IG independent of their univariate P value, including age, gender, NIHSS, and IV t-PA. All biomarkers were tested in the resultant multivariate model, independent of the significance of their univariate association with IG; tests were applied one by one to avoid model overfitting and spurious associations. Receiver operating characteristic (ROC) curves and area under the curves (AUC) were calculated to evaluate the predictive power of each of the significant independent predictors for IG.
A two-tailed P value of <0.05 was considered significant. All statistical analyses were performed with SPSS statistical package (SPSS Inc., Chicago, IL).
All supporting data are available within the article and its online supplemental file.
RESULTS
Overall, 525 patients were enrolled, of whom 489 had a final diagnosis of ischemic stroke. Thirty-six patients without ischemic stroke were excluded (20 TIAs, 14 non-stroke alternative diagnoses, 1 unknown diagnosis and 1 patient who withdrew from the study after informed consent). Out of 489 AIS patients, 220 subjects had both baseline DWI and follow-up scans (DWI or CT) and were included in the study (Figure). Compared to these, the remaining 269 patients without both baseline DWI and follow-up scans not included in the analysis differed for a lower functional independence (mRS 0–1) before the index stroke (76.2% vs. 88.8%, P<0.001), lower neurological severity (median NIHSS 5 vs. 8, P=0.001) and smaller baseline DWI lesion volume (median 3.6 vs. 11.0 cm3, P<0.001). They were less likely to be treated with IV t-PA (28.4% vs. 55.7%, P<0.001), more likely to have lower urinary levels of 8-OHdG_cr (median 9.3 vs. 11.8 ng/mL, P=0.04), but higher median plasma levels of F2-isoP (58.2 vs. 52.5 pg/mL, P=0.01), to have a history of congestive heart failure (CHF) (15.4% vs. 8.9%, P=0.03) and hyperlipidemia (53.4% vs. 43.2%, P=0.03), and a higher chance of SICH (30.8% vs. 8.5%, P=0.015) occurrence.
Figure.
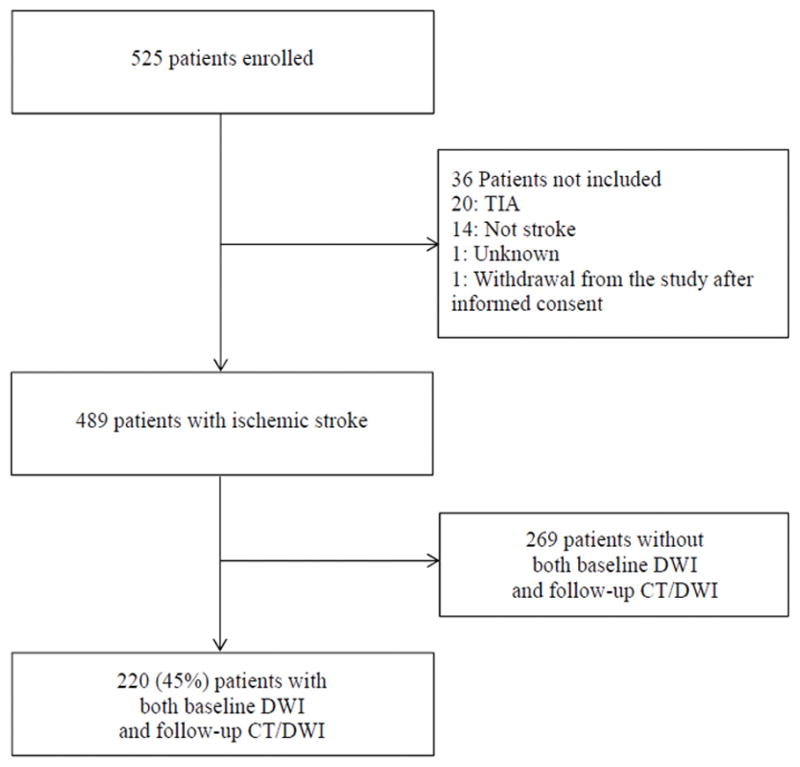
Flowchart of patient selection.
Characteristics of subjects included in the analysis, overall and by IG groups, are reported in Table 1. The mean (±SD) age was 69.4 (±14.7) years with 59.1% (130/220) males; 55.5% (122/220) were treated with IV t-PA. IG was seen in 170 patients and, compared to those without IG, they were more likely to be male (62.9% vs. 46%, P=0.03), to have a history of diabetes mellitus (24.7% vs. 10.0%, P=0.03), were more frequently on statins before the index stroke (49.0% vs. 27.9%, P=0.01), had higher median NIHSS score (9 vs. 6, P=0.01) and blood glucose levels (126 vs. 113.5, P=0.03) at admission, had a larger median baseline DWIV (12.9 vs. 5.2 cm3, P=0.01), a higher proportion of vessel occlusion (41.4% vs. 9.5%, P=0.007), and a larger median FIV (29.7 vs. 2.6, P<0.001). However, they had lower incidence of prior TIA (6.5% vs. 16.0%, P=0.046) and a trend toward a lower use of vitamin supplements (17.6% vs. 30.2%, P=0.07). The presence of IG was also associated with a worse functional outcome at 90-days as per mRS 3–6 (P=0.004).
Table 1.
Characteristics (unadjusted) of the studied patient population as stratified by presence or absence of infarct growth
| Characteristics | All patients (n=220) | No infarct growth (n=50) | Infarct growth (n=170) | P-value |
|---|---|---|---|---|
| Age, years, mean (SD) | 69.4 (±14.7) | 67.9 (±17.2) | 69.9 (±14.0) | 0.40 |
| Male gender (%) | 130/220 (59.1) | 23/50 (46.0) | 107/170 (62.9) | 0.03 |
| Ethnicity, Caucasian (%) | 206/220 (93.6) | 48/50 (96.0) | 158/170 (92.9) | 0.74 |
| Pre-stroke mRS (0–1) (%) | 190/214 (88.8) | 44/49 (89.8) | 146/165 (88.5) | 0.80 |
| Preexisting risk factors (%) | ||||
| Hypertension | 157/220 (71.4) | 34/50 (68.0) | 123/170 (72.4) | 0.55 |
| Diabetes | 47/220 (21.4) | 5/50 (10.0) | 42/170 (24.7) | 0.03 |
| Atrial fibrillation | 71/220 (32.3) | 19/50 (38.0) | 52/170 (30.6) | 0.32 |
| Dyslipidemia | 95/220 (43.2) | 17/50 (34.0) | 78/170 (45.9) | 0.14 |
| Prior stroke | 42/220 (19.1) | 9/50 (18.0) | 33/170 (19.4) | 0.82 |
| Prior TIA | 19/220 (8.6) | 8/50 (16.0) | 11/170 (6.5) | 0.046 |
| Coronary artery disease | 60/220 (27.3) | 10/50 (20.0) | 50/170 (29.4) | 0.19 |
| Congestive heart failure | 19/214 (8.9) | 5/49 (10.2) | 14/165 (8.5) | 0.78 |
| Smoking (current) | 47/217 (21.7) | 9/49 (18.4) | 38/168 (22.6) | 0.53 |
| Alcohol use (current) | 89/213 (41.8) | 22/50 (44.0) | 67/163 (41.1) | 0.72 |
| Carotid Stenosis/occlusion (%) | 24/220 (10.9) | 6/50 (12.0) | 18/170 (10.6) | 0.78 |
| BMI, median (IQR) | 27.4 (24.2–30.5) | 27.8 (23.6–30.5) | 27.4 (24.6–30.6) | 0.58 |
| BMI >30 (%) | 61/200 (30.5) | 14/45 (31.1) | 47/155 (30.3) | 0.92 |
| Preadmission medications (%) | ||||
| Antiplatelets | 92/196 (46.9) | 21/43 (48.8) | 71/153 (46.4) | 0.78 |
| Oral anticoagulation | 23/196 (11.7) | 5/43 (11.6) | 18/153 (11.8) | 0.98 |
| Statin | 87/196 (44.4) | 12/43 (27.9) | 75/153 (49.0) | 0.01 |
| Vitamin supplement | 40/196 (20.4) | 13/43 (30.2) | 27/153 (17.6) | 0.07 |
| SBP, mmHg, mean (SD) | 156 (±28.3) | 152.7 (±25.7) | 157 (±29.1) | 0.36 |
| DBP, mmHg, mean (SD) | 82.7 (±14.9) | 82.5 (±12.8) | 82.7 (±15.5) | 0.93 |
| NIHSS at baseline, median (IQR) | 8 (4–14) | 6 (4–11.3) | 9 (4–16) | 0.01 |
| Serum markers | ||||
| Admission glucose, mg/dL, median (IQR) | 123 (106.3–145.8) | 113.5 (103–131) | 126 (107–152) | 0.03 |
| Admission white blood cells (x103/μL) | 9.5 (±3.0) | 9.4 (±2.7) | 9.6 (±3.0) | 0.71 |
| IV t-PA (%) | 122/220 (55.5) | 31/50 (62.0) | 91/170 (53.5) | 0.31 |
| Imaging Data | ||||
| DWIV, cm3, median (IQR) | 11.0 (2.4–33.0) | 5.2 (1.3–16.2) | 12.9 (2.7–42.0) | 0.01 |
| FIV, cm3, median (IQR) | 18.9 (3.5–55.6) | 2.6 (0.4–11.2) | 29.7 (7.4–78.8) | <0.001 |
| Mismatch Volume, cm3, median (IQR) | 23.3 (0.5–63.1) | 4.4 (–0.3, 61.5) | 27.2 (1.3–64.4) | 0.28 |
| Vessel occlusion at baseline (%) | 31/91 (34.1) | 2/21 (9.5) | 29/70 (41.4) | 0.007 |
| Onset-to-blood draw time, min, median (IQR) | 400 (290–490) | 408 (335–509) | 392.5 (289.5–485) | 0.33 |
| Onset-to-imaging time, median (IQR) | 300 (199–379) | 300.5 (217.3–421.8) | 300 (196–364) | 0.39 |
| Imaging-to-blood draw time, min, median (IQR) | 96 (40–159) | 95 (30–149.5) | 96 (42–159.3) | 0.73 |
| Stroke subtype (%) | ||||
| Large-artery atherosclerosis | 44/220 (20.0) | 6/50 (12.0) | 38/170 (22.4) | 0.11 |
| Cardioembolic | 111/220 (50.5) | 27/50 (54.0) | 84/170 (49.4) | 0.57 |
| Small Vessel | 13/220 (5.9) | 4/50 (8.0) | 9/170 (5.3) | 0.50 |
| Stroke of other cause | 15/220 (16.8) | 4/50 (8.0) | 11/170 (6.5) | 0.75 |
| Stroke of undetermined cause | 37/220 (16.8) | 9/50 (18.0) | 28/170 (16.5) | 0.80 |
| Any ICH (%) | 47/220 (21.4) | 5/50 (10.0) | 42/170 (24.7) | 0.03 |
| SICH (%) | 4/220 (1.8) | 1/50 (2.0) | 3/170 (1.8) | 0.37 |
| mRS 2–6 at 90 days (%) | 113/192 (58.9) | 20/43 (46.5) | 93/149 (62.4) | 0.06 |
| mRS 3–6 at 90 days (%) | 86/192 (44.8) | 11/43 (25.6) | 75/149 (50.3) | 0.004 |
| Death at 90 days (%) | 25/192 (13.0) | 4/43 (9.3) | 21/149 (14.1) | 0.41 |
DBP=diastolic blood pressure; DWIV=diffusion weighted imaging volume; FIV=final infarct volume; ICH=intracerebral hemorrhage; IQR=interquartile range; IV=intravenous; mRS=modified Rankin Scale; NIHSS=National Institutes of Health Stroke Scale; t-PA=tissue plasminogen activator; SBP=systolic blood pressure; SD=standard deviation; SICH=symptomatic intracerebral hemorrhage; TIA=transient ischemic attack; WBC=white blood cells.
Results of baseline biomarker assays are shown in Table 2. Higher median (IQR) baseline levels of F2-isoP were found in patients with IG compared to those without IG (56.2 [36.4–75.3] vs. 42.0 [27.0–61.8] pg/mL, P=0.009). Levels of other biomarkers tested did not correlate with the occurrence of infarct growth.
Table 2.
Baseline plasma biomarkers and univariate associations for patients with and without infarct growth
| All patients (n=220) | No infarct growth (n=50) | Infarct growth (n=170) | P-value | |
|---|---|---|---|---|
| F2-isoP, pg/mL | 52.5 (34.3–73.1) | 42.0 (27.0–61.8) | 56.2 (36.4–75.3) | 0.009 |
| ORACTOTAL, μmol TE/L | 18040.5 (13516.1–24428.0) | 15050.4 (12804.0–24752.1) | 18126.9 (14288.3–24314.4) | 0.38 |
| ORACPCA, μmol TE/L | 1546.6 (1193.0–2100.9) | 1564.5 (1240.0–2436.0) | 1530.7 (1182.9–2029.2) | 0.37 |
| 8-OHdG, ng/mL | 4.9 (2.6–9.6) | 3.4 (2.0–8.1) | 5.6 (2.7–9.8) | 0.18 |
| 8-OHdG_cr, ng/mL | 11.8 (7.0–21.6) | 13.5 (6.7–27.9) | 11.6 (7.1–21.6) | 0.73 |
| MMP-2, ng/mL | 302.3 (239.9–386.3) | 287.4 (238.0–387.7) | 313.6 (239.4–386.4) | 0.62 |
| MMP-9, ng/mL | 179.1 (97.6–318.6) | 165.3 (86.3–331.0) | 181.8 (100.2–319.2) | 0.65 |
| hs-CRP, mg/L | 3.6 (1.7–7.9) | 3.8 (1.7–9.3) | 3.5 (1.7–7.7) | 0.93 |
Values are medians (interquartile ranges, IQR).
F2-isoP=F2-isoprostane; hs-CRP=high sensitivity C Reactive Protein; μmol TE/L=micromoles of Trolox equivalents per liter; MMP-2=metalloproteinase 2; MMP-9=metalloproteinase 9; ng/mL=nanograms per millilitre; 8-OHdg=8-hydro-2′-deoxyguoanosine; 8-OHdg_cr=8-hydro-2′-deoxyguoanosine adjusted by creatinine (cr) levels; ORACPCA =Oxygen Radical Absorbance Capcity (PCA perchloric acid); pg/mL=picograms per milliliter.
Median (IQR) IGV was 4.84 cm3 (0.21–27.34). IGV was significantly associated with diabetes mellitus (P=0.044) and vessel occlusion (P<0.001), higher NIHSS at baseline (Spearman’s ρ=0.47, P<0.001) and 48 h (Spearman’s rho=0.52, P<0.001), with changes of NIHSS at 48 h from baseline (Spearman’s rho=0.17, P=0.018), baseline blood glucose levels (Spearman’s ρ=0.17, P=0.01) and DWIV (Spearman’s ρ=0.50, P<0.001) and mismatch volume (Spearman’s ρ=0.17, P=0.041). IGV resulted to be significantly associated also with a worse functional outcome (mRS 2–6, P<0.001; mRS 3–6, P<0.001) and death (P<0.001) at 90 days. Univariate metrics with a trend (P<0.10) toward association with IGV included less frequent prior history of TIA (P=0.09) and age (Spearman’s ρ=0.12, P=0.08). Baseline F2-isoP was significantly correlated with current smoking (P=0.02), IGV (Spearman’s ρ=0.20, P= 0.005), PIG (Spearman’s ρ= 0.17, P=0.02), FIV (Spearman’s ρ=0.19, P=0.009), and was also associated with the presence of mismatch (P=0.03) and mismatch >20% (P=0.037).
Multivariate binary logistic regression model including all variables associated with IG with univariate P value ≤0.10, as well as age and IV t-PA, indicated that baseline lnF2-isoP is an independent predictor of IG (OR 2.66, 95% CI 1.30–5.44; P=0.007) (Table 3). The AUC for F2-isoP (AUC 0.64, 95% CI 0.55–0.74; P=0.007) was found to be superior to the AUC of the other significant predictors (NIHSS: 0.60, 95% CI 0.50–0.69, P=0.07; prior TIA: 0.46, 95% CI 0.35–0.57, P=0.46; prior statin use: 0.62, 95% CI 0.52–0.72, P=0.023). A cut-off level of 54.9 pg/mL had a specificity of 74% and sensitivity of 51%. lnF2-isoP remained a statistically significant independent predictor of IG after inclusion of current smoking status (OR 2.51, 95% CI 1.22–5.16; P= 0.01) or time interval stroke onset-to-blood draw (OR 3.31, 95% CI 1.49–7.34; P=0.003) or both time intervals stroke-to-imaging and imaging-to-blood draw (OR 3.54, 95% CI 1.56–8.04; P=0.003) or any ICH (OR 2.79, 95% CI 1.35–5.79; P=0.006) or new brain infarct occurring during the hospital stay (OR 2.72, 95% CI 1.33–5.58; P=0.006) in the multivariate model as potential confounders (see Supplemental Tables I and III and Supplemental Tables V and VII). Moreover, inclusion of other biomarkers (hs-CRP, MMP-2, MMP-9, ORACTOT, ORACPCA) one by one, to avoid spurious associations and model overfitting, did not change the significance (P≤0.01, in each of these models) of F2-isoP in predicting IG.
Table 3.
Multivariable binary logistic regression analysis for infarct growth (IG)
| OR (95% CI) | P-value | |
|---|---|---|
| lnF2-isoP | 2.66 (1.30–5.44) | 0.007 |
| Age | 0.99 (0.96–1.02) | 0.52 |
| Gender | 0.45 (0.19–1.02) | 0.08 |
| NIHSS | 1.12 (1.01–1.23) | 0.03 |
| IV t-PA | 0.42 (0.18–1.00) | 0.05 |
| Prior TIA | 0.19 (0.05–0.72) | 0.02 |
| Diabetes mellitus | 0.87 (0.22–3.44) | 0.84 |
| Statin | 4.03 (1.41–11.52) | 0.009 |
| Vitamin supplement | 0.50 (0.17–1.46) | 0.21 |
| Glucose | 1.01 (0.10–1.02) | 0.10 |
| lnDWIV | 1.16 (0.80–1.67) | 0.44 |
IV=intravenous; t-PA=tissue plasminogen activator; lnDWIV=natural logarithm of diffusion weighted imaging volume; lnF2-isoP=natural logarithm of F2-isoprostane; NIHSS=National Institutes of Health Stroke Scale; TIA=transient ischemic attack
Similarly, by the addition of other imaging characteristic, such as vessel occlusion, to a further multivariate model including only relevant variables such as baseline lnF2-isoP, NIHSS, prior TIA, statin therapy, and lnDWIV, in order to avoid model overfitting, F2-isoP was confirmed as a significant independent predictor of IG occurrence (OR 2.76, 95% CI 1.01–7.53; P=0.048).
Baseline F2-isoP was also independently associated with IGV handled as a continuous dependent variable in a linear regression model (B 0.38, 95% CI 0.04–0.72; P=0.03) (Table 4). The r2 and the adjusted r2 of the model were respectively 0.52 and 0.47. The inclusion of time interval stroke onset-to-blood draw or both time intervals stroke-to-imaging and imaging-to-blood draw in the multivariate model did not modify the results (B 0.44, 95% CI 0.43–0.85, P=0.031 and B 0.45, 95% CI 0.04–0.86; P=0.034, respectively) (see Supplemental Tables II and IV). Results were similar when any ICH or new brain infarct occurring during the hospital stay were included in the model (B 0.45, 95% CI 0.05–0.86; P=0.03 and (B 0.43, 95% CI 0.28–0.84; P=0.036, respectively) (see Supplemental Tables VI and VIII).
Table 4.
Multivariable linear logistic regression analysis for infarct growth volume (IGV)
| B (95% CI) | P-value | |
|---|---|---|
| lnF2-isoP | 0.38 (0.04, 0.72) | 0.03 |
| Age | −0.003 (−0.018, 0.013) | 0.73 |
| Gender | 0.08 (−0.37, 0.53) | 0.72 |
| NIHSS | 0.04 (−0.01, 0.09) | 0.13 |
| IV t-PA | −0.08 (−0.51, 0.36) | 0.73 |
| Prior TIA | −0.01 (−1.04, 1.03) | 0.99 |
| Diabetes mellitus | 0.29 (−0.33, 0.91) | 0.35 |
| Glucose | −0.002 (−0.007, 0.002) | 0.33 |
| lnDWIV | 0.52 (0.29, 0.75) | <0.001 |
| ln_Mismatch V | 0.10 (−0.11, 0.31) | 0.36 |
IV=Intravenous; lnDWIV=natural logarithm of diffusion weighted imaging volume; lnF2-isoP=natural logarithm of F2-isoprostane; NIHSS=National Institutes of Health Stroke Scale; TIA=transient ischemic attack; t-PA=tissue plasminogen activator.
DISCUSSION
In this study we found that the hyperacute plasma levels of F2-isoP, a biomarker of oxidative stress-induced lipid peroxidation, is an independent predictor of the occurrence of IG and IGV in patients with AIS evaluated within 9 hours of symptom onset.
Several variables have been evaluated as correlates of susceptibility to IG in AIS patients,4–6 but no specific peripheral blood biomarker has been validated so far. An extraordinarily dynamic series of complex biochemical processes occurs at stroke onset. Oxidative stress, triggered in the early phase of ischemic brain injury, plays an important role in pathogenesis and evolution of the vascular lesions.7,13 In experimental models, high levels of free radicals and lipid peroxidation products have been associated with larger infarct volumes and more severe neurological impairment.22 The reduction of infarct size induced through antioxidants in experimental studies has confirmed the fundamental role of oxidative stress in infarct progression.7,8,23
Despite these experimental models, evidence is limited on oxidative stress biomarkers in the hyperacute phase of AIS in humans. Previous studies investigating biomarkers of oxidative stress, observed higher levels of malondialdehyde and myeloperoxidase in stroke patients compared to controls.24–27 Few studies focused on F2-isoPs, which are stable, sensitive and specific markers of oxidative stress as compared to other biomarkers.11,25,27 Moreover, they are easily measurable in plasma, urine, and cerebrospinal fluid.12 Plasma levels of esterified and free F2-isoP increase within the first 8 hours of stroke onset13,28 suggesting an early oxidative stress activation in AIS patients. Therefore, F2-isoP can represent a good predictor of IG.
To our knowledge, this is the first study reporting F2-isoP levels as predictors of IG and IGV in patients with AIS. In our cohort, patients with IG had more severe disease. They had a larger baseline DWIV, a higher incidence of large vessel occlusion and diabetes, and higher baseline blood glucose levels. These factors may play a role in the infarct evolution by promoting the progression of ischemia through complex mechanisms.
F2-isoP levels and their influence on the progression of ischemic brain damage could be affected by confounders. Interestingly, women were less likely to have IG, although they usually tend to have worse outcome measures when we consider the natural stroke evolution.29 This difference has to be viewed in context of mean (SD) age of our cohort patients which was relatively low (69.4±14.7), with men aged 68.1 (±13.6) and women 71.3 (±16.1). It seems that younger women have a lower likelihood of IG as compared to their age matched male counterparts,30 confirming a differential age-dependent worsening in tissue outcome in the two genders. Moreover, older patients and patients with vessel occlusion were more likely to have a higher volume of IG. Premorbid history of TIA correlated with lower incidence of IG, suggesting a potential role of these transient events in ischemic preconditioning. Unfortunately we do not have data on whether prior TIA occurred less or more than 30 days of the index stroke onset. Furthermore, intake of vitamin supplements prior to the index stroke had a protective effect leading to lower FIV suggesting a role of these supplements as antioxidants. However, it should be taken into account that many variables can influence the extraordinary complexity of the antioxidant system including lifestyle habits, such as nutrition. Unfortunately we did not have specific data on nutrition for this analysis.
In order to account for potential confounders, adjusted models in multivariate analyses were used. F2-isoP levels retained their ability to predict IG in these models emphasizing the fact that it is an independent predictor of IG.
Strengths of this study were the prospective design and the use of specific, well defined, and precise exclusion criteria to minimize the impact of confounders. Blood samples were obtained in the early phase of stroke onset at the time when oxidative stress is likely to peak, providing valuable information about the pathophysiological aspects of ischemia progression. Finally, the large sample size allowed adjustment for variables that could potentially have an influence on the plasma levels of biomarkers and their association with IG.
Limitations of this study include retrospective analysis of the neuroimaging variables, which were not pre-specified in the protocol. Advanced neuroimaging was obtained at the discretion of the treating stroke neurologist which might have led to patient selection bias. Moreover, baseline and follow-up images were obtained at different time points (within 24 h and from 24 to 96 h of symptom onset, respectively) based on clinical requirements which could lead to over- or underestimation of final infarct volume. However, we did not find any significant differences in the time intervals from stroke onset or imaging to blood draw and from stroke onset to imaging between patients with and those without IG. Stroke severity in our cohort was moderate as measured by NIHSS which limits the generalizability of these findings to patients with severe strokes. In this analysis on infarct growth, we had a wide range of missing values for the biomarkers investigated and measured at baseline: hs-CRP, 1.8% (4/220); MMP-2 and MMP-9, 6.4% (14/220); F2-isoP, 13% (29/220); ORACTOTAL and ORACPCA 17.7% (39/220 each); 8-OHdG and 8-OHdG_cr, 58.6% (129/220 each). However, we think that most of these percentages can be considered acceptable for an analysis on biomarkers in the acute phase of stroke like this -particularly for those biomarkers that have been less investigated compared to others in the acute stroke in humans, such as F2-isoP. Moreover, no difference in the baseline characteristics was found between patients with missing values of F2-isoP at admission and those with no such missing values, except for a lower proportion of females (P=0.049) and prior stroke (P=0.034), a higher proportion of undetermined stroke (P=0.021), and a higher time interval stroke-to-imaging (median 366 vs 293; P=0.002) in the group of patients with missing admission F2-isoP levels. Therefore, we are in the opinion that the percentage of missing values for F2-isoP did not impact the main results of our study. It is acknowledged that the percentage of missing values for 8-OHdG and 8-OHdG_cr is high, however, we think that the number of measurements for this biomarker that, to our knowledge, has never been studied in the acute phase of stroke in humans, can still be considered valuable in order to evaluate in an exploratory manner the presence of an association with IG. Specificity and sensitivity of F2-isoP are not optimal, therefore further specific studies with larger sample size and without the above mentioned limitations are needed in order to confirm the promise of this test for acute stroke.
Conclusions
Elevated hyperacute plasma F2-isoP concentrations independently predict the occurrence of IG and IGV in patients with AIS, suggesting a potential role of oxidative stress in promoting brain tissue injury and cell death. If validated in future studies, this biomarker could be helpful in understanding the pathophysiological aspects of ischemia and stroke progression in humans. This understanding will enable us to develop novel therapeutic targets. Moreover, these molecular markers could be adopted in clinical trials for patient selection to undergo acute stroke treatments as well as predicting outcomes.
Supplementary Material
Acknowledgments
We thank all the patients who participated in this SPOTRIAS project. We thank the NIH/NINDS SPOTRIAS (Specialized Program of Transitional Research in Acute Stroke) program, the Massachusetts General Hospital, and the Brigham and Women’s Hospital. We thank Harvard Catalyst, the Harvard Clinical and Translational Science Center, Harvard University.
Source of funding
This study was supported by the NIH/NINDS Specialized Program of Transitional Research in Acute Stroke (SPOTRIAS) grant P50-NS051343.
Footnotes
Disclosures
Dr. Svetlana Lorenzano was supported through the NIH/NINDS Specialized Program of Transitional Research in Acute Stroke (SPOTRIAS) grant P50-NS051343; she served as expert consultant for Boehringer Ingelheim from 2013 to 2014; she received two travel grants from Boehringer Ingelheim (one in 2016 one in 2017), one travel grant from Bayer (2014), Quintiles IMS (2017), Daichii Sankyo (in 2017).
Dr. Natalia S. Rost was supported by NIH-NINDS K23NS064052 & R01 NS082285.
Dr. Ona Wu was supported in part by NIH-NINDS P50NS051343, R01NS059775 and R01NS063925. She is the co-inventor of a patent on “Delay-compensated calculation of tissue blood flow,” US Patent 7,512,435. March 31, 2009, and the patent has been licensed to General Electric, Siemens, Imaging Biometrics and Olea Medical.
Dr. Muhib Khan, Dr. Hua Li, Dr. Fabricio O. Lima, Dr. Matthew B. Maas, Rebecca E. Green, Tijy K. Thankachan, Allison J. Dipietro, Dr. Ken Arai, Angel T. Som, Loc-Duyen D. Pham, Dr. Gordon J. Harris, Dr. Eng H. Lo, Dr. Jeffrey B. Blumberg, Dr. Paul E. Milbury, Dr. Steven K. Feske, and Dr. Karen L. Furie have no disclosure.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: A report from the american heart association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley R, et al. Recombinant tissue plasminogen activator for acute ischemic stroke: an updated systematic review and meta-analysis. Lancet. 2012;379:2364–2372. doi: 10.1016/S0140-6736(12)60738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Touma L, Filion B, Sterling, Atallah R, Windle SB, Eisenberg MJ. Stent retrievers for the treatment of acute ischemic stroke. A systematic review and meta-analysis of randomized clinical trials. JAMA Neurol. 2016;73:1155–1156. doi: 10.1001/jamaneurol.2015.4441. [DOI] [PubMed] [Google Scholar]
- 4.Ay H, Koroshetz WJ, Vangel M, Benner T, Melinosky C, Zhu M, et al. Conversion of ischemic brain tissue into infarction increases with age. Stroke. 2005;36:2632–2636. doi: 10.1161/01.STR.0000189991.23918.01. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Nuutinen J, Laakso MP, Karonen JO, Soimakallio S, Aronen HJ, et al. Apoe polymorphism and acute stroke: A study with diffusion- and perfusion-weighted mri and mr angiography. Acta Neurol Scand. 2006;114:323–328. doi: 10.1111/j.1600-0404.2006.00654.x. [DOI] [PubMed] [Google Scholar]
- 6.Darby DG, Barber PA, Gerraty RP, Desmond PM, Yang Q, Parsons M, et al. Pathophysiological topography of acute ischemia by combined diffusion-weighted and perfusion mri. Stroke. 1999;30:2043–2052. doi: 10.1161/01.str.30.10.2043. [DOI] [PubMed] [Google Scholar]
- 7.Kontos HA. Oxygen radicals in cerebral ischemia: The 2001 willis lecture. Stroke. 2001;32:2712–2716. doi: 10.1161/hs1101.098653. [DOI] [PubMed] [Google Scholar]
- 8.Samdani AF, Dawson TM, Dawson VL. Nitric oxide synthase in models of focal ischemia. Stroke. 1997;28:1283–1288. doi: 10.1161/01.str.28.6.1283. [DOI] [PubMed] [Google Scholar]
- 9.Cherubini A, Ruggiero C, Polidori MC, Mecocci P. Potential markers of oxidative stress in stroke. Free Radic Biol Med. 2005;39:841–852. doi: 10.1016/j.freeradbiomed.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol. 2005;25:279–286. doi: 10.1161/01.ATV.0000152605.64964.c0. [DOI] [PubMed] [Google Scholar]
- 11.Polidori MC, Frei B, Cherubini A, Nelles G, Rordorf G, Keaney JF, Jr, et al. Increased plasma levels of lipid hydroperoxides in patients with ischemic stroke. Free Radic Biol Med. 1998;25:561–567. doi: 10.1016/s0891-5849(98)00085-9. [DOI] [PubMed] [Google Scholar]
- 12.Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, et al. Biomarkers of oxidative stress study ii: Are oxidation products of lipids, proteins, and DNA markers of ccl4 poisoning? Free Radic Biol Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Kelly PJ, Morrow JD, Ning M, Koroshetz W, Lo EH, Terry E, et al. Oxidative stress and matrix metalloproteinase-9 in acute ischemic stroke: The biomarker evaluation for antioxidant therapies in stroke (beat-stroke) study. Stroke. 2008;39:100–104. doi: 10.1161/STROKEAHA.107.488189. [DOI] [PubMed] [Google Scholar]
- 14.Cao G, Giovanoni M, Prior RL. Antioxidant capacity in different tissues of young and old rats. Proc Soc Exp Biol Med. 1996;211:359–365. doi: 10.3181/00379727-211-43981. [DOI] [PubMed] [Google Scholar]
- 15.Cao G, Prior RL. Measurement of oxygen radical absorbance capacity in biological samples. Methods Enzymol. 1999;299:50–62. doi: 10.1016/s0076-6879(99)99008-0. [DOI] [PubMed] [Google Scholar]
- 16.Arsava EM, Ballabio E, Benner T, Cole JW, Delgado-Martinez MP, Dichgans M, et al. The causative classification of stroke system: An international reliability and optimization study. Neurology. 2010;75:1277–1284. doi: 10.1212/WNL.0b013e3181f612ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol. 2005;58:688–697. doi: 10.1002/ana.20617. [DOI] [PubMed] [Google Scholar]
- 18.Mohr JP, Biller J, Hilal SK, Yuh WT, Tatemichi TK, Hedges S, et al. Magnetic resonance versus computed tomographic imaging in acute stroke. Stroke. 1995;26:807–812. doi: 10.1161/01.str.26.5.807. [DOI] [PubMed] [Google Scholar]
- 19.Gurol ME, Irizarry MC, Smith EE, Raju S, Diaz-Arrastia R, Bottiglieri T, et al. Plasma beta-amyloid and white matter lesions in ad, mci, and cerebral amyloid angiopathy. Neurology. 2006;66:23–29. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- 20.Ay H, Arsava EM, Vangel M, Oner B, Zhu M, Wu O, et al. Interexaminer difference in infarct volume measurements on mri: A source of variance in stroke research. Stroke. 2008;39:1171–1176. doi: 10.1161/STROKEAHA.107.502104. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson KJ, Wardlaw JM, Edmond CL, Deary IJ, Maclullich AM. Intracranial area: A validated method for estimating intracranial volume. J Neuroimaging. 2005;15:76–78. doi: 10.1177/1051228404270243. [DOI] [PubMed] [Google Scholar]
- 22.Leinonen JS, Ahonen JP, Lonnrot K, Jehkonen M, Dastidar P, Molnar G, et al. Low plasma antioxidant activity is associated with high lesion volume and neurological impairment in stroke. Stroke. 2000;31:33–39. doi: 10.1161/01.str.31.1.33. [DOI] [PubMed] [Google Scholar]
- 23.Liu TH, Beckman JS, Freeman BA, Hogan EL, Hsu CY. Polyethylene glycol-conjugated superoxide dismutase and catalase reduce ischemic brain injury. Am J Physiol. 1989;256:H589–593. doi: 10.1152/ajpheart.1989.256.2.H589. [DOI] [PubMed] [Google Scholar]
- 24.Dominguez C, Delgado P, Vilches A, Martin-Gallan P, Ribo M, Santamarina E, et al. Oxidative stress after thrombolysis-induced reperfusion in human stroke. Stroke. 2010;41:653–660. doi: 10.1161/STROKEAHA.109.571935. [DOI] [PubMed] [Google Scholar]
- 25.El Kossi MM, Zakhary MM. Oxidative stress in the context of acute cerebrovascular stroke. Stroke. 2000;31:1889–1892. doi: 10.1161/01.str.31.8.1889. [DOI] [PubMed] [Google Scholar]
- 26.Sharpe PC, Mulholland C, Trinick T. Ascorbate and malondialdehyde in stroke patients. Ir J Med Sci. 1994;163:488–491. doi: 10.1007/BF02967089. [DOI] [PubMed] [Google Scholar]
- 27.Chang CY, Lai YC, Cheng TJ, Lau MT, Hu ML. Plasma levels of antioxidant vitamins, selenium, total sulfhydryl groups and oxidative products in ischemic-stroke patients as compared to matched controls in taiwan. Free Radic Res. 1998;28:15–24. doi: 10.3109/10715769809097872. [DOI] [PubMed] [Google Scholar]
- 28.Seet RC, Lee CY, Chan BP, Sharma VK, Teoh HL, Venketasubramanian N, et al. Oxidative damage in ischemic stroke revealed using multiple biomarkers. Stroke. 2011;42:2326–2329. doi: 10.1161/STROKEAHA.111.618835. [DOI] [PubMed] [Google Scholar]
- 29.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, et al. Sex differences in stroke: Epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gokcay F, Arsava EM, Baykaner T, Vangel M, Garg P, Wu O, et al. Age-dependent susceptibility to infarct growth in women. Stroke. 2011;42:947–951. doi: 10.1161/STROKEAHA.110.603902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


