Abstract
U.S. Immigration and Customs Enforcement (ICE) is responsible for detaining unauthorized aliens during immigration proceedings. During 2014–2015, adult ICE detainees at a California facility were invited to complete a survey concerning self-reported varicella history and risk factors. Participants underwent serological testing for varicella-zoster virus (VZV) IgG; susceptible individuals were offered varicella vaccination. Among 400 detainees with available serology results, 48 (12%) were susceptible to varicella. Self-reported varicella history was negatively associated with susceptibility (adjusted odds ratio [aOR]=0.16; 95% CI=0.07, 0.35). Among 196 detainees reporting a positive history, 95% had VZV IgG levels suggestive of varicella immunity. Among 44 susceptible detainees offered vaccination, 86% accepted. Given relatively high varicella susceptibility, targeted screening and vaccination among ICE detainees lacking a positive history might reduce varicella transmission risks.
Keywords: varicella, detention, immigration, vaccination, Immigration and Customs Enforcement
INTRODUCTION
Varicella is a highly contagious infection caused by varicella-zoster virus (VZV), with attack rates of 61%–100% in susceptible close contacts (Heininger & Seward, 2006). Compared with infections contracted during childhood, varicella infections in susceptible adults tend to be more severe, with greater risk for complications and hospitalizations (Marin et al., 2008b). In tropical climates, VZV infection generally occurs at older ages relative to temperate climates, resulting in a greater proportion of susceptible adults (Garnett, Cox, Bundy, Didier, & St Catharine, 1993; Lee, 1998; Liyanage et al., 2007). In one study of adult immigrants in Canada, younger age, recent arrival, and originating from a tropical country were associated with varicella susceptibility (Greenaway et al., 2014). In the United States, the varicella vaccination program, initiated in 1995, has considerably reduced the incidence and severity of VZV infections and associated healthcare utilization (Marin, Meissner, & Seward, 2008a; Marin, Zhang, & Seward, 2011; Zhou, Harpaz, Jumaan, Winston, & Shefer, 2005). Globally, routine varicella vaccination is limited to a few high-income countries (Bonanni et al., 2009).
U.S. Immigration and Customs Enforcement (ICE) is responsible for enforcing federal immigration laws, including the detention of unauthorized aliens during immigration proceedings. ICE admitted 440,557 aliens to detention facilities during fiscal year 2013, of whom 56% were Mexican nationals (Simanski, 2014). Detainees are unlikely to have medical or vaccination records available when brought into ICE custody. Routine varicella serological testing and vaccination are not included in current ICE policies for detainee healthcare.
Varicella outbreaks are an ongoing challenge in detention and correctional facilities (Leung et al., 2014; Levy et al., 2003; Public Health England, 2014; Valdarchi et al., 2008), including facilities that house migrants (de Valliere et al., 2011; Gétaz et al., 2010; Haas, Dukhan, Goldstein, Lyandres, & Gdalevich, 2014). Such outbreaks can be disruptive and costly, requiring isolation of cases, identification, assessment of susceptibility and cohorting contacts, staff furloughs, and restriction in movement, gatherings, visitations, and court appearances (Leung et al., 2014). Outbreaks can also spread to other facilities (Levy et al., 2003), increasing resource expenditures to contain transmission.
We aimed to assess serological varicella susceptibility and associated risk factors among ICE detainees. To inform potential implementation of a varicella vaccination program, we also assessed the validity of self-reported varicella history, vaccination attitudes, and varicella vaccine acceptance among susceptible individuals.
METHODS
Participant recruitment was conducted between August 2014 and February 2015 at a mixed-sex California detention facility housing ICE detainees aged ≥18 years. After initial intake screening, ICE detainees receive a comprehensive health assessment within 14 days of arrival; this assessment includes a physical examination and mental health screening. In the absence of other clinical indications, influenza vaccine is the only routinely offered vaccination at the facility. Screening for other vaccine-preventable diseases is not routinely performed in healthy detainees.
Participants
We reviewed medical records of recently arrived detainees for participation eligibility. Detainees ineligible to receive varicella vaccine due to contraindications for vaccination (including immunocompromising conditions, any known vaccine allergies, currently taking immunosuppressing medications, pregnant, or acutely ill), or those with a known medical condition requiring chronic medications, were excluded. Detainees deemed unable by the attending provider to reliably consent due to mental illness or intellectual disability, or for whom language-specific interpretation was not readily available at the time of interview, were also excluded.
Trained recruitment staff supervised by senior project staff approached eligible detainees to explain project objectives and activities, provided a written information sheet in English or Spanish, and invited detainees to participate. Among detainees with limited English and/or Spanish language proficiency, telephonic interpreter services were used and the written information sheet was verbally translated unless language-specific interpretation was not readily available. Detainees were informed that their participation was voluntary and would not impact their detention, legal claims, or any legal proceedings involving ICE. For all detainees approached, age, sex, language, and nationality were recorded.
Data collection
We obtained a convenience sample comprised of the first 400 eligible detainees who consented to participate and for whom varicella serology was performed. Recruitment staff administered a survey to consenting participants in English, Spanish, or via telephonic interpreter services for other languages. The survey included questions about self-reported history of varicella, community of childhood household (defined as the village, town, city, and country where they lived during the majority of their childhood between birth and 12 years of age), and total number of adults and minors (aged <18 years) who lived in their childhood household. Participants were also asked closed-ended questions regarding their general attitudes on vaccination and their trust of medical providers at the facility to recommend vaccines.
Serological testing
Blood was drawn from participants to test for varicella IgG titer (Liaison VZV IgG assay, DiaSorin, Saluggia, Italy) and HIV seropositivity by enzyme-linked immunosorbent assay (ELISA). Varicella IgG titer values were interpreted as positive, equivocal, or negative, according to commercial testing laboratory reference ranges. HIV testing was performed to ascertain eligibility for varicella vaccination. HIV ELISA-positive samples were followed by a confirmatory assay. Participants were informed of their varicella and HIV serological test results. Participants found to be newly HIV-positive were counseled and offered additional testing and treatment in accordance with ICE medical policies.
Vaccination
Detainees with equivocal or negative varicella IgG serology and whom were found to be HIV seronegative were provided the varicella vaccine information sheet (VIS) in English, Spanish, or other languages as available (Centers for Disease Control and Prevention, 2008), informed that vaccination is voluntary, and offered the first of two vaccine doses. Participants who received the first dose and remained in the facility 4–8 weeks after initial vaccine administration were offered the second dose.
Analysis
Interview language was categorized as Spanish, English, or Other. Total childhood household size and number of minors were dichotomized based on the medians. Refusal to answer survey questions or missing responses were excluded. To assess positive and negative predictive value of self-reported varicella history, uncertain and negative histories were combined. Varicella IgG serology results were used to classify participants into susceptible (negative and equivocal results) and immune (positive results). Countries of childhood household were regionally categorized, as described elsewhere (Greenaway et al., 2014), and grouped to ensure adequate participant numbers. To assess climate, latitude, and longitude estimates for the city/village of childhood household were obtained using Google Maps (Google, Palo Alto, CA) and grouped by climate category, according to Köppen-Geiger classification (Greenaway et al., 2014; Kottek, Grieser, Beck, Rudolf, & Rubel, 2006).
Statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC) and EpiInfo 7 (Centers for Disease Control and Prevention (CDC), Atlanta, GA). Bivariate comparisons were performed using Mantel-Haenszel Exact Chi-square tests assessed at α = 0.05. Binomial proportion confidence intervals (CIs) were calculated using the Wilson score method. Univariate and multivariate logistic regression modeling were utilized to test associations between varicella susceptibility and study variables. A backwards elimination approach was used for multivariate logistic regression modeling, retaining sex and age as potential confounders.
Human subjects
As this investigation was conducted to inform the implementation of a public health intervention in a defined population, it was determined to be non-research by CDC and therefore not subject to CDC Institutional Review Board review.
RESULTS
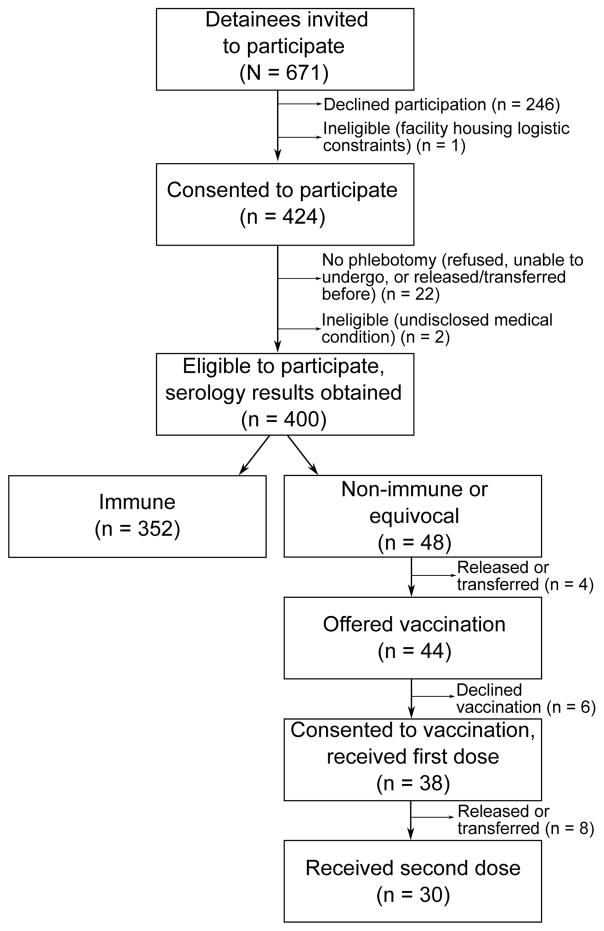
We invited 671 detainees to participate, of whom 424 (63%) consented. After excluding detainees due to previously unidentified ineligibility or inability to obtain blood samples, 400 participants with available serology results were included in analysis (Figure 1). No significant differences by age, sex, or interview language were detected between detainees included in the analysis and those invited but who did not participate (p > .05). Participation rates varied by nationality (p < .0001); detainees born in Europe and Central Asia (6, 26%) had significantly lower participation than those born in East and South Asia, the Pacific, North Africa and the Middle East (30, 60%), Latin America and the Caribbean (307, 61%), or Sub-Saharan Africa (57, 86%).
Figure 1.
Participant recruitment, serological testing, and vaccination among U.S. Immigration and Customs Enforcement detainees at a California detention facility.
Participants were predominantly male (73%) with a median age of 30 years (range: 18–60 years) (Table 1). All global regions were represented in childhood households, although most grew up in Latin America and the Caribbean (71%) or Sub-Saharan Africa (14%). More than half (56%) of participants grew up in a tropical climate. Twenty-four (6%) participants reported a different country of childhood household than their nationality; of these, 17 (71%) were born in Mexico, but reported their childhood household as being in the United States.
Table 1.
Demographic characteristics among participating detainees (N = 400).
| Characteristic | Value |
|---|---|
| Age (years), median (range) | 30 (18–60) |
| Sex, n (%) | |
| Female | 102 (25.5) |
| Male | 298 (74.5) |
| Interview language, n (%) | |
| English | 105 (26.3) |
| Spanish | 254 (63.5) |
| Other | 41 (10.3) |
| Total number of persons in childhood household, median (range)a,b | 7 (2–48) |
| Number of minors aged <18 years in childhood household, median (range)a,c | 4 (1–25) |
| Nationality, n (%)d | |
| Mexico | 215 (53.8) |
| El Salvador | 33 (8.3) |
| Guatemala | 28 (7.0) |
| Honduras | 20 (5.0) |
| Ghana | 15 (3.8) |
| Other | 89 (22.3) |
| Global region of childhood household, n (%)e | |
| Latin America and the Caribbean | 285 (71.3) |
| Sub-Saharan Africa | 57 (14.3) |
| U.S. and Western Europe | 24 (6.0) |
| South Asia | 19 (4.8) |
| North Africa and the Middle East | 6 (1.5) |
| East Asia and the Pacific | 5 (1.3) |
| Eastern Europe and Central Asia | 4 (1.0) |
Footnotes: Rounded figures might not add to 100%.
Refers to self-reported number of persons (adults and/or minors) living in household during majority of childhood between birth and 12 years old.
Responses were missing for two participants.
Responses were missing for four participants.
Refers to country of citizenship based on U.S. Immigration and Customs Enforcement records.
Based on country where detainee spent majority of childhood between birth and 12 years old.
Among the 400 participants, 48 (12%; 95% CI = 9%, 16%) were susceptible to varicella, of whom two (4%) had equivocal serology results (Table 2). One participant was HIV-positive, but he was immune to varicella. Prior varicella history was reported by 196 participants, of whom 187 were immune (positive predictive value = 95%). Among 204 participants denying or unsure of varicella history, 39 were susceptible (negative predictive value = 19%).
Table 2.
Serological varicella susceptibility and logistic regression modeling in relation to demographic and geographic factors among participating detainees.
| Variable | Totaln (%) | Susceptible to varicella% (95% CI) | UnivariateOR (95% CI) | MultivariateaOR (95% CI) |
|---|---|---|---|---|
| Overall | 400 (100) | 12.0 (9.2, 15.6) | ||
| Sex | ||||
| Female | 102 (25.5) | 13.7 (8.4, 21.7) | 1.24 (0.63, 2.41) | 1.52 (0.73, 3.14) |
| Male | 298 (74.5) | 11.4 (8.3, 15.5) | Ref | Ref |
| Age (years) | ||||
| <25 | 107 (26.8) | 10.3 (5.8, 17.5) | 0.80 (0.26, 2.47) | 0.60 (0.18, 1.99) |
| 25–34 | 156 (39.0) | 12.8 (8.5, 19.0) | 1.03 (0.36, 2.94) | 0.74 (0.24, 2.28) |
| 35–44 | 97 (24.3) | 12.4 (7.2, 20.4) | 0.99 (0.32, 3.01) | 0.71 (0.22, 2.29) |
| ≥45 | 40 (10.0) | 12.5 (5.5, 26.1) | Ref | Ref |
| Interview language | ||||
| English | 105 (26.3) | 7.6 (3.9, 14.3) | Ref | - |
| Spanish | 254 (63.5) | 13.4 (9.7, 18.1) | 1.87 (0.84, 4.20) | |
| Other | 41 (10.3) | 14.6 (6.9, 28.4) | 2.08 (0.67, 6.41) | |
| Self-reported history of varicella | ||||
| Yes | 196 (49.0) | 4.6 (2.4, 8.5) | 0.19 (0.09, 0.40) | 0.16 (0.07, 0.35) |
| No | 160 (40.0) | 20.6 (15.1, 27.6) | Ref | Ref |
| Not sure | 44 (11.0) | 13.6 (6.4, 26.7) | 0.61 (0.24, 1.56) | 0.64 (0.24, 1.68) |
| Total number of persons living in childhood householda | ||||
| ≤7 | 238 (59.8) | 13.0 (9.3, 17.9) | 1.35 (0.71, 2.56) | - |
| >7 | 160 (40.2) | 10.0 (6.3, 15.6) | Ref | |
| Number of minors aged <18 years in childhood householda | ||||
| ≤4 | 229 (57.8) | 14.0 (10.1, 19.1) | 1.78 (0.92, 3.44) | |
| >4 | 167 (42.2) | 8.4 (5.1, 13.6) | Ref | - |
| Climate in community of childhood householdb | ||||
| Arid/cold/polar | 66 (16.6) | 12.1 (6.3, 22.1) | 2.05 (0.71, 5.94) | 2.22 (0.74, 6.63) |
| Temperate | 111 (27.9) | 6.3 (3.1, 12.5) | Ref | Ref |
| Tropical | 221 (55.5) | 14.9 (10.8, 20.2) | 2.61 (1.11, 6.10) | 3.04 (1.26, 7.33) |
| Global region of childhood householdc | ||||
| Latin America and the Caribbean | 285 (71.3) | 13.0 (9.6, 17.4) | Ref | - |
| Sub-Saharan Africa | 57 (14.3) | 8.8 (3.8, 19.0) | 0.64 (0.24, 1.72) | |
| U.S./W. Europe/E. Europe & Central Asia | 28 (7.0) | 3.6 (0.6, 17.7) | 0.25 (0.03, 1.87) | |
| S. & E. Asia/Pacific/N. Africa & Middle East | 30 (7.5) | 16.7 (7.3, 33.6) | 1.34 (0.48, 3.72) | |
Footnotes: Rounded figures might not add to 100%. Boldface values indicate statistical significance (p < .05). Abbreviations: CI = confidence interval; OR = odds ratio; aOR = adjusted odds ratio.
Refers to self-reported number of persons (adults and/or minors) living in household during majority of childhood between birth and 12 years old.
Based on the village/town/city where detainee spent majority of childhood between birth and 12 years old.
Based on the country where detainee spent majority of childhood between birth and 12 years old.
Of the 48 susceptible detainees, 44 (91%) were still housed in the facility when serology results became available; of these 44, 38 (86%) consented to varicella vaccination (Figure 1). Among the 38 receiving vaccination, eight (21%) received one dose and 30 (79%) received two doses before being transferred or released from the facility.
In a multivariate logistic regression model (Table 2), controlling for age and sex, childhood household in a tropical climate was positively associated with varicella susceptibility (aOR = 3.04; 95% CI = 1.26, 7.33). Self-reported history of varicella was inversely associated with susceptibility (aOR = 0.16; 95% CI = 0.07, 0.35). No significant differences in susceptibility by any other variables assessed were detected in univariate or multivariate analyses (Table 2).
More than 80% of participants agreed that vaccines can prevent diseases and are safe, and >90% indicated trust in facility medical staff to recommend vaccines (Table 3). Participants aged ≥45 years were more likely to agree that vaccines are safe, compared with those aged 25–34 years (p = .02). No associations between vaccination attitudes and sex or global region of childhood household were detected (p > .05).
Table 3.
Attitudes towards vaccines and vaccination among participating detainees by age, sex, and region of childhood household.
| Characteristic | Attitude statementa,b | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Vaccines can prevent diseases | Vaccines are safe | Vaccines may contain dangerous chemicals | Trust medical staff at detention facility to recommend vaccines | |||||
|
| ||||||||
| % agree (95% CI) | p | % agree (95% CI) | p | % agree (95% CI) | p | % agree (95% CI) | p | |
| Overall | 86.0 (82.3, 89.1) | - | 82.5 (78.5, 85.9) | - | 32.8 (28.3, 37.5) | - | 90.3 (87.0, 92.8) | - |
| Sex | ||||||||
| Female | 88.2 (80.6, 93.1) | 0.51 | 85.3 (77.2, 90.9) | 0.45 | 27.5 (19.7, 36.8) | 0.22 | 92.2 (85.3, 96.0) | 0.56 |
| Malec | 85.2 (80.8, 88.8) | 81.5 (76.8, 85.5) | 34.6 (29.4, 40.1) | 89.6 (85.6, 92.6) | ||||
| Age (years) | ||||||||
| <25 | 84.1 (76.0, 89.8) | 0.11 | 82.2 (73.9, 88.3) | 0.02 | 28.0 (20.4, 37.2) | 0.11 | 86.9 (79.2, 92.0) | 0.06 |
| 25–34 | 84.0 (77.4, 88.9) | 75.6 (68.3, 81.7) | 31.4 (24.7, 39.1) | 89.7 (84.0, 93.6) | ||||
| 35–44 | 87.6 (79.6, 92.8) | 88.7 (80.8, 93.6) | 38.1 (29.1, 48.1) | 91.8 (84.6, 95.8) | ||||
| ≥45 | 95.0 (83.5, 98.6) | 95.0 (83.5, 98.6) | 37.5 (24.2, 53.0) | 97.5 (87.1, 99.6) | ||||
| Global region of childhood householdc | ||||||||
| Latin America and the Caribbean | 87.0 (82.6, 90.4) | 0.15 | 84.6 (79.9, 88.3) | 0.41 | 35.4 (30.1, 41.2) | 0.05 | 89.8 (85.8, 92.8) | 0.14 |
| Sub-Saharan Africa | 87.7 (76.8, 93.9) | 80.7 (68.7, 88.9) | 22.8 (13.8, 35.2) | 96.5 (88.1, 99.0) | ||||
| U.S./W. Europe/E. Europe & Central Asia | 67.9 (49.3, 82.1) | 60.7 (42.4, 76.4) | 35.7 (20.7, 54.2) | 85.7 (68.5, 94.3) | ||||
| S. & E. Asia/Pacific/N. Africa & Middle East | 90.0 (74.4, 96.5) | 86.7 (70.3, 94.7) | 23.3 (11.8, 40.9) | 86.7 (70.3, 94.7) | ||||
Comparing agree responses versus disagree and not sure responses. Refused to answer responses were excluded.
P-value corresponds to chi-square test; boldface values indicate statistical significance (p < .05).
Based on the country where detainee spent majority of childhood between birth and 12 years old.
DISCUSSION
We documented 12% serological susceptibility to varicella among ICE detainees at a large facility in California, approximately six times that of U.S. adults aged 20–49 years (Reynolds, Kruszon-Moran, Jumaan, Schmid, & McQuillan, 2010). Participants from tropical climates were more likely to be susceptible, while no significant differences by age, sex, interview language, household size, or global region were detected. Our report is the first assessing infectious disease susceptibility among ICE detainees and is useful to inform measures to prevent varicella infections in this population.
As a confined population, ICE detainees have increased risk of varicella exposure given congregate housing and frequent movement of persons (Bick, 2007; Breuer, 2004). Furthermore, being foreign-born adults, ICE detainees have greater likelihood of varicella susceptibility than the U.S.-born (Meyer, Seward, Jumaan, & Wharton, 2000), and are unlikely to have received childhood varicella vaccination (Bonanni et al., 2009). The detected level of susceptibility exceeded prior reports of 3%–6% susceptibility among adult migrants in Canada and U.S.-bound refugees (Greenaway et al., 2014; Leung et al., 2015), but was comparable to 13% susceptibility among migrant inmates at a Swiss prison where a varicella outbreak occurred (Gétaz et al., 2010). Given herd immunity threshold estimates of 86%–91% to prevent varicella transmission (Plans-Rubio, 2012), and documented varicella outbreaks in correctional facilities where VZV seroprevalence exceeded 85% (Centers for Disease Control and Prevention, 1989; Gétaz et al., 2010; Valdarchi et al., 2008), our findings suggest that VZV transmission and outbreaks would likely occur at this facility if an infectious person was introduced.
More than half of participating ICE detainees originated in a tropical climate, and were more likely to be susceptible than detainees from temperate climates. Contributing factors to susceptibility in tropical climates include reduced transmission efficiency in hot, humid environments (Garnett et al., 1993; Lolekha et al., 2001) and being more likely to live in a rural setting, a proxy for low population density and frequency and closeness of interactions (Mandal, Mukherjee, Murphy, Mukherjee, & Naik, 1998). Since climate can be deduced from household location, obtaining childhood household information during intake procedures (in addition to self-reported varicella history) and systematizing climate determination might help identify potentially susceptible detainees for further testing and vaccination.
In our sample, more than one-third of detainees were aged ≥35 years. Compared with a prior study of migrants in Canada, where nearly all persons in this demographic were immune (Greenaway et al., 2014), 12% of detainees in this group were susceptible. Primary VZV infection in older age groups is associated with higher consequences in severe morbidity and mortality (Marin et al., 2008b). Hence, transmission of varicella within this facility could result in more severe cases requiring hospitalization, presenting additional challenges in terms of patient transport and population movement in a confined setting (Leung et al., 2014).
Vaccination programs reduce the risk of disease transmission within correctional facilities and in the wider community following release (Sequera, Garcia-Basteiro, & Bayas, 2013). Strategies to prevent varicella transmission in adults include: (i) post-exposure vaccination; (ii) routine vaccination; and (iii) routine or targeted serological testing with subsequent vaccination of susceptible individuals. Post-exposure vaccination can considerably reduce varicella infections and hospitalizations (Souty et al., 2015), but timely implementation of response measures and identification of exposed detainees and staff can be challenging (Leung et al., 2014). Routine varicella vaccination increases coverage at elevated cost, given unnecessary vaccine administration among immune individuals. In one migrant detention facility in Switzerland, general vaccination was more effective than targeted testing in preventing varicella outbreaks, but incurred more than double the costs (de Valliere et al., 2011). Serological testing and subsequent vaccination can provide a more cost-effective approach for varicella prevention, including for recent migrants (Merrett, Schwartzman, Rivest, & Greenaway, 2007; Smith & Roberts, 2000). However, the transient nature of ICE detainees presents challenges in obtaining test results and administering vaccine prior to release.
Self-reported varicella history offers a rapid approach to identify potentially susceptible individuals. Among ICE detainees, a positive self-reported history of varicella was generally reliable; our calculated positive predictive value (95%) exceeded prior estimates based on migrant prisoners in Switzerland (Gétaz et al., 2010) and refugees in the United States (Christiansen & Barnett, 2004). However, a negative history was not accurate; fewer than 20% of participants who denied history or were unsure were susceptible. Hence, detainees denying or unsure of their history might be targeted for serological testing. Among immunocompromised, pregnant, or other high-risk detainees, serological testing might be performed regardless of varicella history. For the general population in the United States, the Advisory Committee on Immunization Practices recommends a healthcare provider’s documentation or verification of a history of varicella or herpes zoster as evidence of immunity, since this is a more specific definition than self-reported history and minimizes the possibility of false-positive results (Marin, Guris, Chaves, Schmid, & Seward, 2007). However, given lack of access to historical medical records and potential challenges among ICE detainees of accurately describing the disease presentation, the feasibility of implementing this definition in this population is low.
Barriers to varicella vaccination implementation in facilities housing ICE detainees include the requirement to administer two doses; a single dose of varicella vaccine does not provide sufficient population immunity to prevent transmission, especially in high-contact settings (Marin et al., 2008a), and the minimum interval between the doses is 4–8 weeks (Marin et al., 2007). Frequent movement among ICE detainees can interfere with medical follow-up (Schneider & Lobato, 2007). In the present study, more than one-fifth of susceptible detainees who received one dose of varicella vaccine were transferred or released before the second dose could be administered. Susceptible detainees were advised to seek medical consultation to receive a second dose if released before series completion. However, vaccine access may be limited if the detainee is removed from the United States, or if they are uninsured or without financial means after release. Distrust of facility and medical staff is another potential challenge to vaccination implementation. Although ICE detainees generally reported positive attitudes toward vaccination and trust for medical providers, 14% of susceptible detainees declined to receive varicella vaccination, comparable to refusal rates in an immigrant detention facility in Israel (15.6%) (Haas et al., 2014). As detainees who agreed to participate might have greater trust concerning vaccination and medical providers, the refusal rate among all ICE detainees might exceed that observed.
This study has several limitations. We obtained a convenience sample of detainees from one facility. The top four countries represented (Mexico, El Salvador, Guatemala, and Honduras) were consistent with broader ICE population demographics (Simanski, 2014). However, overrepresentation of participants from Latin America and the Caribbean might have obscured susceptibility comparisons by global region. Low participation rates among European and Central Asian detainees were observed, although these represented a minority of individuals approached. Attitude and provider trust assessments among participants might overestimate those of the wider detainee population. Given the migration history of ICE detainees, childhood household was used in lieu of nationality when assessing varicella susceptibility. Only 6% of detainees had a different country of birth than their childhood household. However, as the age of probable VZV exposure can vary between climates, childhood household might not be the primary exposure site for all detainees. We did not ask participants about their vaccination history, given their nationalities and limited global implementation of varicella vaccination (Bonanni et al., 2009), although a few spent their childhood in high-income countries where varicella vaccine is routinely administered. We did not assess population density in community of childhood household; reliable population size and geographic area estimates for communities represented in the sample were unavailable. Variability in the specificity of participants’ village/town/city of childhood household may have reduced overall geocoding accuracy for climate assessments. We also did not ask about participant occupation or contact with children, which may be relevant to VZV exposure. Finally, given their detention status, participants may have provided inaccurate interview responses due to perceived benefit. However, no participation incentives were provided, and project staff emphasized that participation would have no impact on their detention or legal proceedings.
CONCLUSION
Our study represents the first assessment of a preventive health initiative in an ICE detention facility. We detected 12% varicella susceptibility among adult ICE detainees and 95% positive predictive value of a reported history of varicella. Given the high level of population susceptibility, targeted screening and vaccination among detainees who deny or are unsure of their varicella history, had a childhood household in a tropical climate, or are at elevated risk for complications from varicella might be considered. While this approach does not eliminate the possibility of VZV transmission, it could provide an effective strategy for minimizing disruptions caused by VZV exposures in ICE facilities.
Acknowledgments
We thank Denise Borntrager and CDR William Waldron for funding management assistance, Tess Jocson for phlebotomy assistance, Deborah Lee for consultation on specimen management, Jennie Tomilson for laboratory assistance, and CAPT Deborah Schneider for administrative support. This work was supported in part by an appointment to the Applied Epidemiology Fellowship Program administered by the Council of State and Territorial Epidemiologists (CSTE) and funded by the Centers for Disease Control and Prevention (CDC) [cooperative agreement number 1U380T000143-01]. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of CDC, Department of Homeland Security, or U.S. Immigration and Customs Enforcement.
FUNDING
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by an appointment to the Applied Epidemiology Fellowship Program administered by the Council of State and Territorial Epidemiologists (CSTE) and funded by the Centers for Disease Control and Prevention (CDC) [cooperative agreement number 1U380T000143-01].
Footnotes
Conflict of interest: Aiden K. Varan declares that he has no conflict of interest. Edith R. Lederman declares that she has no conflict of interest. Shanon S. Stous declares that she has no conflict of interest. Diana Elson declares that she has no conflict of interest. Jennifer L. Freiman declares that she has no conflict of interest. Mona Marin declares that she has no conflict of interest. Adriana S. Lopez declares that she has no conflict of interest. William M. Stauffer declares that he has no conflict of interest. Rachael H. Joseph declares that she has no conflict of interest. Stephen H. Waterman declares that he has no conflict of interest.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Bick JA. Infection control in jails and prisons. Clinical Infectious Diseases. 2007;45(8):1047–1055. doi: 10.1086/521910. [DOI] [PubMed] [Google Scholar]
- Bonanni P, Breuer J, Gershon A, Gershon M, Hryniewicz W, Papaevangelou V, … Wutzler P. Varicella vaccination in Europe – taking the practical approach. BMC Medicine. 2009;7(1):26. doi: 10.1186/1741-7015-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer J. Varicella immunisation for staff and inmates of institutions: is this the next step in the UK? Communicable Disease and Public Health. 2004;7(3):162–163. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Epidemiologic Notes and Reports Varicella Outbreak in a Women’s Prison -- Kentucky. MMWR. 1989;38(37):635–636. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Vaccine Information Statement: Chickenpox Vaccine. 2008 Retrieved from http://www.cdc.gov/vaccines/hcp/vis/vis-statements/varicella.html.
- Christiansen D, Barnett ED. Comparison of varicella history with presence of varicella antibody in refugees. Vaccine. 2004;22(31–32):4233–4237. doi: 10.1016/j.vaccine.2004.04.024. [DOI] [PubMed] [Google Scholar]
- de Valliere S, Cani N, Grossenbacher M, Puig F, Masserey E, Bodenmann P. Comparison of two strategies to prevent varicella outbreaks in housing facilities for asylum seekers. International Journal of Infectious Diseases. 2011;15(10):e716–e721. doi: 10.1016/j.ijid.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Garnett GP, Cox MJ, Bundy DAP, Didier JM, St Catharine J. The age of infection with varicella-zoster virus in St Lucia, West Indies. Epidemiology & Infection. 1993;110(02):361–372. doi: 10.1017/s0950268800068308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gétaz L, Siegrist CA, Stoll B, Humair JP, Scherrer Y, Franziskakis C, … Wolff H. Chickenpox in a Swiss prison: Susceptibility, post-exposure vaccination and control measures. Scandinavian Journal of Infectious Diseases. 2010;42(11–12):936–940. doi: 10.3109/00365548.2010.511259. [DOI] [PubMed] [Google Scholar]
- Greenaway C, Boivin JF, Cnossen S, Rossi C, Tapiero B, Schwartzman K, … Miller M. Risk factors for susceptibility to varicella in newly arrived adult migrants in Canada. Epidemiology & Infection. 2014;142(8):1695–1707. doi: 10.1017/S0950268813002768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas EJ, Dukhan L, Goldstein L, Lyandres M, Gdalevich M. Use of vaccination in a large outbreak of primary varicella in a detention setting for African immigrants. International Health. 2014;6(3):203–207. doi: 10.1093/inthealth/ihu017. [DOI] [PubMed] [Google Scholar]
- Heininger U, Seward JF. Varicella. Lancet. 2006;368(9544):1365–1376. doi: 10.1016/S0140-6736(06)69561-5. [DOI] [PubMed] [Google Scholar]
- Kottek M, Grieser J, Beck C, Rudolf B, Rubel F. World map of the Köppen-Geiger climate classification updated. Meteorologische Zeitschrift. 2006;15(3):259–263. [Google Scholar]
- Lee BW. Review of varicella zoster seroepidemiology in India and South-east Asia. Tropical Medicine and International Health. 1998;3(11):886–890. doi: 10.1046/j.1365-3156.1998.00316.x. [DOI] [PubMed] [Google Scholar]
- Leung J, Lopez A, Mitchell T, Weinberg M, Lee D, Thieme M, … Bialek SR. Seroprevalence of Varicella-Zoster Virus in Five US-Bound Refugee Populations. Journal of Immigrant and Minority Health. 2015;17(1):310–313. doi: 10.1007/s10903-013-9946-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J, Lopez AS, Tootell E, Baumrind N, Mohle-Boetani J, Leistikow B, … Marin M. Challenges With Controlling Varicella in Prison Settings Experience of California, 2010 to 2011. Journal of Correctional Health Care. 2014;20(4):292–301. doi: 10.1177/1078345814541535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy MH, Quilty S, Young LC, Hunt W, Matthews R, Robertson PW. Pox in the docks: varicella outbreak in an Australian prison system. Public Health. 2003;117(6):446–451. doi: 10.1016/S0033-3506(03)00138-0. [DOI] [PubMed] [Google Scholar]
- Liyanage NPM, Fernando S, Malavige GN, Mallikahewa R, Sivayogan S, Jiffry MTM, Vitarana T. Seroprevalence of varicella zoster virus infections in Colombo district, Sri Lanka. Indian Journal of Medical Sciences. 2007;61(3):128–134. [PubMed] [Google Scholar]
- Lolekha S, Tanthiphabha W, Sornchai P, Kosuwan P, Sutra S, Warachit B, … Bock HL. Effect of climatic factors and population density on varicella zoster virus epidemiology within a tropical country. The American Journal of Tropical Medicine and Hygiene. 2001;64(3–4):131–136. doi: 10.4269/ajtmh.2001.64.131. [DOI] [PubMed] [Google Scholar]
- Mandal BK, Mukherjee PP, Murphy C, Mukherjee R, Naik T. Adult Susceptibility to Varicella in the Tropics Is a Rural Phenomenon Due to the Lack of Previous Exposure. The Journal of Infectious Diseases. 1998;178(Suppl 1):S52–S54. doi: 10.1086/514262. [DOI] [PubMed] [Google Scholar]
- Marin M, Guris D, Chaves SS, Schmid S, Seward JF. Prevention of Varicella: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recommendations and Reports. 2007;56(RR-4):1–40. [PubMed] [Google Scholar]
- Marin M, Meissner HC, Seward JF. Varicella Prevention in the United States: A Review of Successes and Challenges. Pediatrics. 2008a;122(3):e744–e751. doi: 10.1542/peds.2008-0567. [DOI] [PubMed] [Google Scholar]
- Marin M, Watson TL, Chaves SS, Civen R, Watson BM, Zhang JX, … Seward JF. Varicella among Adults: Data from an Active Surveillance Project, 1995–2005. The Journal of Infectious Diseases. 2008b;197(Suppl 2):S94–S100. doi: 10.1086/522155. [DOI] [PubMed] [Google Scholar]
- Marin M, Zhang JX, Seward JF. Near Elimination of Varicella Deaths in the US After Implementation of the Vaccination Program. Pediatrics. 2011;128(2):214–220. doi: 10.1542/peds.2010-3385. [DOI] [PubMed] [Google Scholar]
- Merrett P, Schwartzman K, Rivest P, Greenaway C. Strategies to Prevent Varicella among Newly Arrived Adult Immigrants and Refugees: A Cost-Effectiveness Analysis. Clinical Infectious Diseases. 2007;44(8):1040–1048. doi: 10.1086/512673. [DOI] [PubMed] [Google Scholar]
- Meyer PA, Seward JF, Jumaan AO, Wharton M. Varicella Mortality: Trends before Vaccine Licensure in the United States, 1970–1994. Journal of Infectious Diseases. 2000;182(2):383–390. doi: 10.1086/315714. [DOI] [PubMed] [Google Scholar]
- Plans-Rubio P. Evaluation of the establishment of herd immunity in the population by means of serological surveys and vaccination coverage. Human Vaccines & Immunotherapeutics. 2012;8(2):184–188. doi: 10.4161/hv.18444. [DOI] [PubMed] [Google Scholar]
- Public Health England. Guidance on Infection Control for Chickenpox and Shingles in Prisons, Immigration Removal Centres and other Prescribed Places of Detention. (3) 2014 Retrieved from https://www.gov.uk/government/publications/chickenpox-and-shingles-infection-control-in-prisons-and-other-places-of-detention.
- Reynolds MA, Kruszon-Moran D, Jumaan A, Schmid DS, McQuillan GM. Varicella seroprevalence in the US: Data from the National Health and Nutrition Examination Survey, 1999–2004. Public Health Reports. 2010;125(6):860–869. doi: 10.1177/003335491012500613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DL, Lobato MN. Tuberculosis control among people in US Immigration and Customs Enforcement custody. American Journal of Preventive Medicine. 2007;33(1):9–14. doi: 10.1016/j.amepre.2007.02.044. [DOI] [PubMed] [Google Scholar]
- Sequera VG, Garcia-Basteiro AL, Bayas JM. The role of vaccination in prisoners’ health. Expert Review of Vaccines. 2013;12(5):469–471. doi: 10.1586/erv.13.28. [DOI] [PubMed] [Google Scholar]
- Simanski JF. Immigration Enforcement Actions: 2013. 2014 Retrieved from https://www.dhs.gov/sites/default/files/publications/ois_enforcement_ar_2013.pdf.
- Smith KJ, Roberts MS. Cost effectiveness of vaccination strategies in adults without a history of chickenpox. The American Journal of Medicine. 2000;108(9):723–729. doi: 10.1016/s0002-9343(00)00445-9. [DOI] [PubMed] [Google Scholar]
- Souty C, Boos E, Turbelin C, Blanchon T, Hanslik T, Pierre-Yves B. Vaccination against varicella as post-exposure prophylaxis in adults: A quantitative assessment. Vaccine. 2015;33(3):446–450. doi: 10.1016/j.vaccine.2014.11.045. [DOI] [PubMed] [Google Scholar]
- Valdarchi C, Farchi F, Dorrucci M, De Michetti F, Paparella C, Babudieri S, … Rezza G. Epidemiological investigation of a varicella outbreak in an Italian prison. Scandinavian Journal of Infectious Diseases. 2008;40(11–12):943–945. doi: 10.1080/00365540802308449. [DOI] [PubMed] [Google Scholar]
- Zhou F, Harpaz R, Jumaan AO, Winston CA, Shefer A. Impact of varicella vaccination on health care utilization. The Journal of the American Medical Association. 2005;294(7):797–802. doi: 10.1001/jama.294.7.797. [DOI] [PubMed] [Google Scholar]