Abstract
Objective
Although racial disparities in treatment and outcome for endometrial cancer are well recognized, little work has explored disparities in young women. We performed a population-based analysis to compare survival between black and white women with endometrial cancer at <50 years of age.
Methods
We used the National Cancer Data Base to identify women <50 years of age with endometrial cancer from 1998–2012. Clinical and demographic characteristics were compared between black and white women and survival by race analyzed using Kaplan-Meier curves and multivariable Cox proportional hazards models.
Results
We identified a total of 35,850 women <50 years of age including 31,947 (89.1%) white and 3,903 (10.9%) black patients. Black women were more likely to have advanced stage, poorly differentiated, and non-endometrioid histology neoplasms (P<0.05 for all). In a multivariable model, survival was 19% worse for black patients than white patients (HR=1.19; 95%CI, 1.08–1.32). A similar effect was seen when limited to women with early-stage tumors (HR=1.24; 95%CI, 1.04–1.49), while among patients with advanced stage tumors, no association between race and survival was seen (HR=1.12; 95%CI, 0.89–1.41). Five-year survival rates were 90.6% (95%CI, 88.6–92.3%) for white and 81.5% (95%CI, 73.0–87.5%) for black women with stage IB tumors, and 75.1% (95%CI, 72.5–77.5%) and 63.3% (95%CI, 54.1–71.2%) for white and black women with stage III tumors, respectively.
Conclusions
Young black women are more likely to present with pathologically aggressive, advanced stage tumors. Even after adjusting for these pathologic differences, young black women with endometrial cancer have higher mortality than white women.
Keywords: Endometrial cancer, uterine cancer, disparities, outcomes, hysterectomy
Introduction
There are significant racial disparities in the incidence, treatment and outcomes for women with endometrial cancer.1 While endometrial cancer is more common in white women, black women often present with more advanced stage tumors and often harbor more aggressive histologic subtypes.2–6 Black women are also more likely to die from endometrial cancer; compared to white women, black patients have an 80% higher mortality rate, one of the greatest disparities seen among common solid tumors.7,8
Patients under the age of 45 years account for 5–30% of all endometrial carcinoma cases.9–11 There have been conflicting reports regarding the prognosis of endometrial cancer in younger patients, with some studies reporting favorable outcomes9,10,12 and others reporting no difference when compared to older women.13,14 Endometrial cancer in young women often derives from favorable histologic subtypes and is more commonly diagnosed at earlier stages.10,12,15,16 Hence, young onset endometrial cancer is sufficiently different to be potentially considered as a unique subgroup within endometrial cancer as a whole and merits further study.
Although racial disparities in treatment and outcome for endometrial cancer are well recognized, little work has explored disparities in outcomes for young women with endometrial cancer. We performed a population-based analysis to compare survival between black and white women diagnosed with endometrial cancer at <50 years of age.
Methods
Data was derived from the National Cancer Data Base (NCDB), a clinical oncology database sourced from hospital registries of more than 1,500 Commission on Cancer (CoC)-accredited facilities.17 NCDB data is used to analyze and track patients with malignant neoplastic diseases, their treatments, and outcomes. NCDB includes more than 70% of newly diagnosed cancer cases nationwide and more than 34 million records. The NCDB includes data on clinical and demographic characteristics, pathology, treatment, and survival.
We selected women <50 years of age with endometrial cancer who underwent hysterectomy from 1998–2012. Women who underwent preoperative radiotherapy and those without histologic confirmation were excluded. The cohort was limited to non-Hispanic white and black women.
Age was stratified as <35, 35–39, 40–44, and 45–49 years. Insurance status (private, Medicare, Medicaid, uninsured, and other) and median household income of each patient’s zip code (<$30,000, $30,000–$35,999, $36,000–$45,999, ≥$46,000, unknown) was recorded. Each patient’s area of residence was categorized as urban, rural, or metropolitan. The Deyo classification of the Charlson comorbidity score was used to estimate a patient’s comorbidity (0, 1, ≥2).18,19 The American College of Surgeons Commission on Cancer Accreditation program classification schema was used to classify each reporting facility as a community cancer program, comprehensive community cancer program, academic/research program, or other program.20 Each facility was further classified as academic and non-academic. The geographic region of the treating facility was coded as eastern, southern, midwestern, or western. Tumor characteristics included histology (endometrioid, serous, clear cell, sarcoma and other), grade (well, moderately, poorly differentiated, unknown), and stage based on American Joint Committee on Cancer criteria (IA, IB, I NOS, II, III, IV, and unknown). Therapeutic variables included use of chemotherapy (yes, no, unknown), and what type of radiation (none, external beam, vaginal brachytherapy) was utilized.
The primary outcome of the analysis was survival. The survival time was estimated as the number of months from diagnosis until the date of death from any cause. Survival is reported as all-cause mortality and includes death from cancer as well as other causes.20
Differences in categorical variables between black and white women were compared using χ2 tests with p-values. Kaplan-Meier curves were developed to examine survival by race and compared using the log-rank test and reported with p-values. For each group, estimates of five-year survival with 95% confidence intervals are reported. Marginal multivariable Cox proportional hazards models were developed to determine the association between race and survival while adjusting for the other clinical, demographic and pathologic characteristics of the cohort while accounting for hospital-level clustering. Results from Cox proportional hazards models are reported as hazard ratios (HR) with 95% confidence intervals. A model for all patients and separate models for early-stage (stage I and II) and advanced stage (stage III and IV) are reported. Scaled Schoenfeld residuals for each variable in the Cox model were plotted to visually examine the assumption of proportionality.21,22
To determine whether disparities in survival had changed over time, separate marginal multivariable Cox proportional hazards models were developed for three distinct time periods: 1998–2002, 2003–2007, and 2008–2012. All hypothesis tests were two-sided. A P-value of <0.05 was considered statistically significant. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, North Carolina).
Results
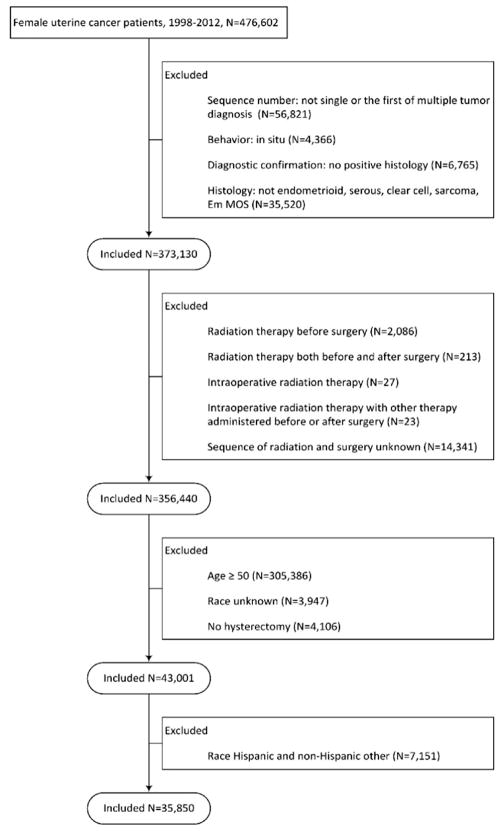
A total of 35,850 women <50 years of age were identified (Figure I). The cohort consisted of 31,947 (89.1%) white and 3,903 (10.9%) black patients (Table 1). Among white women, 7.8% were <35 years of age and 13.6% were 35–39 years of age compared to 13.6% and 15.6% of black women, respectively (P<0.001). Among white women, 79.9% were privately insured compared to 64.9% of black patients (P<0.001). Black women were more likely to have advanced stage, poorly differentiated, and non-endometrioid histology neoplasms. Stage IA tumors were noted in 49.3% of white women versus 39.9% of black women (P<0.01). Likewise, endometrioid neoplasms accounted for 68.7% of the cancers in white women compared to 54.9% in black women (P<0.001), while grade 1 malignancies were seen in 51.8% and 40.0% (P<0.001) of the groups, respectively.
Figure 1.
Flowchart of cohort selection.
Table 1.
Demographical and clinical characteristics of the cohort.
| White | Black | P-value | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| All | 31,947 | (89.1) | 3,903 | (10.9) | |
| Age | <0.001 | ||||
| <35 | 2,497 | (7.8) | 531 | (13.6) | |
| 35–39 | 4,330 | (13.6) | 609 | (15.6) | |
| 40–44 | 8,865 | (27.7) | 1,125 | (28.8) | |
| 45–49 | 16,255 | (50.9) | 1,638 | (42.0) | |
| Year of diagnosis | <0.001 | ||||
| 1998 | 1,750 | (5.5) | 150 | (3.8) | |
| 1999 | 1,781 | (5.6) | 142 | (3.6) | |
| 2000 | 1,839 | (5.8) | 173 | (4.4) | |
| 2001 | 2,001 | (6.3) | 229 | (5.9) | |
| 2002 | 1,977 | (6.2) | 234 | (6.0) | |
| 2003 | 2,139 | (6.7) | 238 | (6.1) | |
| 2004 | 2,120 | (6.6) | 253 | (6.5) | |
| 2005 | 2,253 | (7.1) | 262 | (6.7) | |
| 2006 | 2,314 | (7.2) | 266 | (6.8) | |
| 2007 | 2,181 | (6.8) | 257 | (6.6) | |
| 2008 | 2,240 | (7.0) | 310 | (7.9) | |
| 2009 | 2,330 | (7.3) | 346 | (8.9) | |
| 2010 | 2,384 | (7.5) | 324 | (8.3) | |
| 2011 | 2,315 | (7.2) | 375 | (9.6) | |
| 2012 | 2,323 | (7.3) | 344 | (8.8) | |
| Insurance status | <0.001 | ||||
| Private | 25,527 | (79.9) | 2,534 | (64.9) | |
| Medicare | 1,508 | (4.7) | 255 | (6.5) | |
| Medicaid | 2,199 | (6.9) | 549 | (14.1) | |
| Other government/unknown | 1,042 | (3.3) | 144 | (3.7) | |
| Uninsured | 1,671 | (5.2) | 421 | (10.8) | |
| Income | <0.001 | ||||
| <$38,000 | 4,543 | (14.2) | 1,566 | (40.1) | |
| $38,000–$47,999 | 7,640 | (23.9) | 860 | (22.0) | |
| $48,000–$62,999 | 8,928 | (27.9) | 789 | (20.2) | |
| $63,000+ | 10,106 | (31.6) | 571 | (14.6) | |
| Unknown | 730 | (2.3) | 117 | (3.0) | |
| Location | <0.001 | ||||
| Metropolitan | 25,162 | (78.8) | 3,405 | (87.2) | |
| Urban | 4,890 | (15.3) | 292 | (7.5) | |
| Rural | 522 | (1.6) | 46 | (1.2) | |
| Unknown | 1,373 | (4.3) | 160 | (4.1) | |
| Charlson/Deyo comorbidity score | <0.001 | ||||
| 0 | 18,127 | (56.7) | 2,314 | (59.3) | |
| 1 | 3,801 | (11.9) | 539 | (13.8) | |
| ≥2 | 671 | (2.1) | 122 | (3.1) | |
| Unknown | 9,348 | (29.3) | 928 | (23.8) | |
| Facility type | <0.001 | ||||
| Community cancer | 2,689 | (8.4) | 272 | (7.0) | |
| Comprehensive community cancer | 16,872 | (52.8) | 1,652 | (42.3) | |
| Academic/research | 12,358 | (38.7) | 1,974 | (50.6) | |
| Other | 28 | (0.1) | 5 | (0.1) | |
| Facility region | <0.001 | ||||
| Eastern | 6,845 | (21.4) | 660 | (16.9) | |
| Midwest | 11,740 | (36.7) | 1,234 | (31.6) | |
| South | 8,402 | (26.3) | 1,787 | (45.8) | |
| West | 4,960 | (15.5) | 222 | (5.7) | |
| Stage | <0.001 | ||||
| IA | 15,764 | (49.3) | 1,559 | (39.9) | |
| IB | 1,418 | (4.4) | 203 | (5.2) | |
| INOS | 1,470 | (4.6) | 156 | (4.0) | |
| II | 1,788 | (5.6) | 267 | (6.8) | |
| III | 1,639 | (5.1) | 208 | (5.3) | |
| IV | 747 | (2.3) | 141 | (3.6) | |
| Unknown | 9,121 | (28.6) | 1,369 | (35.1) | |
| Histology | <0.001 | ||||
| Endometrioid | 21,934 | (68.7) | 2,143 | (54.9) | |
| Serous | 374 | (1.2) | 55 | (1.4) | |
| Clear Cell | 198 | (0.6) | 26 | (0.7) | |
| Sarcoma | 3,593 | (11.2) | 1,082 | (27.7) | |
| Other/not otherwise specified | 5,848 | (18.3) | 597 | (15.3) | |
| Grade | <0.001 | ||||
| Well | 16,542 | (51.8) | 1,562 | (40.0) | |
| Moderate | 7,928 | (24.8) | 919 | (23.5) | |
| Poorly | 3,903 | (12.2) | 731 | (18.7) | |
| Unknown | 3,574 | (11.2) | 691 | (17.7) | |
| Regional nodes examined | 0.99 | ||||
| No | 14,444 | (45.2) | 1,767 | (45.3) | |
| Yes | 17,068 | (53.4) | 2,082 | (53.3) | |
| Unknown | 435 | (1.4) | 54 | (1.4) | |
| Radiation | <0.001 | ||||
| None | 26,122 | (81.8) | 3,112 | (79.7) | |
| External beam | 3,698 | (11.6) | 551 | (14.1) | |
| Brachytherapy | 2,030 | (6.4) | 234 | (6.0) | |
| Unknown | 97 | (0.3) | 6 | (0.2) | |
| Chemotherapy | <0.001 | ||||
| Yes | 3,865 | (12.1) | 657 | (16.8) | |
| No | 27,523 | (86.2) | 3,157 | (80.9) | |
| Unknown | 559 | (1.7) | 89 | (2.3) | |
P-values were from Chi-square tests.
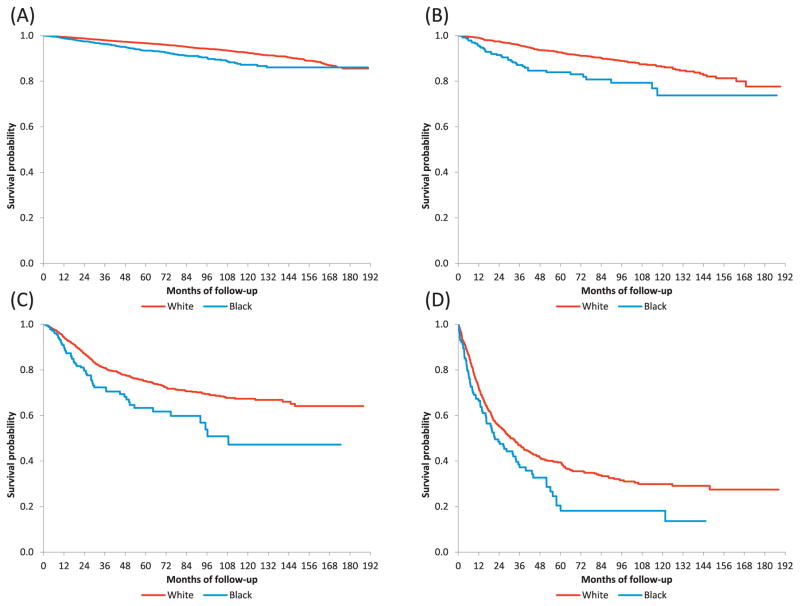
Mean 5-year overall survival rates were lower among black patients (Table 2). For women with stage IA neoplasms, 5-year survival was 97.2% (95% CI, 96.9–97.5%) among white women compared to 94.5% (95% CI, 93.0–95.8%) among black patients. Corresponding 5-year survival rates were 90.6% (95% CI, 88.6–92.3%) and 81.5% (95% CI, 73.0–87.5%), respectively, for those with stage IB neoplasms. The results were similar for advanced stage tumors. Among women with stage III cancers, 5-year survival was 75.1% (95% CI, 72.5–77.5%) in white women versus 63.3% (95% CI, 54.1–71.2%) in black patients. Figure 2 displays Kaplan-Meier survival curves stratified by stage, for each stage, survival was lower for black compared to white women (P<0.05 for all).
Table 2.
Five-year survival rates by race.
| White (95% CI) | Black (95% CI) | |
|---|---|---|
| Stage | ||
| IA | 97.2 (96.9–97.5) | 94.5 (93.0–95.8) |
| IB | 90.6 (88.6–92.3) | 81.5 (73.0–87.5) |
| INOS | 96.8 (95.5–97.8) | 93.8 (80.1–98.2) |
| II | 92.7 (91.2–93.9) | 83.9 (77.9–88.4) |
| III | 75.1 (72.5–77.5) | 63.3 (54.1–71.2) |
| IV | 39.4 (35.5–43.3) | 20.4 (11.7–30.9) |
| Unknown | 85.0 (84.2–85.8) | 71.8 (69.1–74.3) |
2,669 patients were not included because of missing follow-up time or vital status.
NOS: not otherwise specified.
Figure 2.
Kaplan-Meier curves by race, stratified by stage. (A) stage I, p-value < 0.001; (B) stage II, p-value < 0.001; (C) stage III, p-value < 0.001; (D) stage IV, p-value = 0.02. Patients with missing follow-up time or vital status were excluded, including 1,691 stage I, 130 stage II, 218 stage III, and 87 stage IV patients. P-values were from log-rank tests.
In a multivariable model (Table 3), black women were 19% more likely to die than white women (HR=1.19; 95% CI, 1.08–1.32). Older age, an increased number of comorbidities, advanced stage, non-endometrioid histology, and higher grade were associated with increased mortality (P<0.05 for all). In contrast, private insurance coverage, higher zip code income, and use of chemotherapy were associated with decreased mortality. In a model limited to women with early stage tumors, black women were 24% more likely to die than white women (HR=1.24; 95% CI, 1.04–1.49). Among patients with advanced stage tumors, there was no statistically significant association between race and survival (HR=1.12; 95% CI, 0.89–1.41).
Table 3.
Multivariable model for predictors of mortality.
| All | Early Stage | Advanced Stage | |
|---|---|---|---|
| aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | |
| Race | |||
| White | Referent | Referent | Referent |
| Black | 1.19 (1.08–1.32)* | 1.24 (1.04–1.49)* | 1.12 (0.89–1.41) |
| Age | |||
| <35 | Referent | Referent | Referent |
| 35–39 | 1.07 (0.91–1.26) | 1.01 (0.74–1.37) | 1.12 (0.80–1.55) |
| 40–44 | 1.18 (1.02–1.37)* | 1.25 (0.96–1.62) | 1.02 (0.76–1.39) |
| 45–49 | 1.34 (1.16–1.54)* | 1.44 (1.13–1.84)* | 1.18 (0.89–1.58) |
| Year of diagnosis | 0.98 (0.96–0.99)* | 0.99 (0.96–1.02) | 1.00 (0.97–1.03) |
| Insurance status | |||
| Private | Referent | Referent | Referent |
| Medicare | 2.34 (2.05–2.68)* | 2.68 (2.17–3.31)* | 1.67 (1.27–2.19)* |
| Medicaid | 1.59 (1.42–1.77)* | 2.09 (1.74–2.51)* | 1.15 (0.95–1.40) |
| Other government/unknown | 1.08 (0.88–1.32) | 1.32 (0.98–1.78) | 0.87 (0.57–1.33) |
| Uninsured | 1.53 (1.35–1.73)* | 1.55 (1.21–1.98)* | 1.17 (0.89–1.54) |
| Income | |||
| <$38,000 | Referent | Referent | Referent |
| $38,000–$47,999 | 0.87 (0.78–0.96)* | 0.87 (0.73–1.03) | 0.92 (0.75–1.13) |
| $48,000–$62,999 | 0.80 (0.72–0.90)* | 0.71 (0.60–0.86)* | 0.78 (0.62–0.97)* |
| $63,000+ | 0.67 (0.60–0.76)* | 0.61 (0.49–0.75)* | 0.66 (0.53–0.83)* |
| Unknown | 1.30 (0.99–1.70) | 1.38 (0.87–2.19) | 1.26 (0.52–3.04) |
| Location | |||
| Metropolitan | Referent | Referent | Referent |
| Urban | 0.96 (0.86–1.06) | 0.93 (0.77–1.12) | 0.97 (0.79–1.20) |
| Rural | 1.10 (0.86–1.42) | 1.50 (1.07–2.10)* | 0.90 (0.55–1.49) |
| Unknown | 1.02 (0.79–1.31) | 1.13 (0.80–1.59) | 0.97 (0.42–2.21) |
| Charlson/Deyo comorbidity score | |||
| 0 | Referent | Referent | Referent |
| 1 | 1.30 (1.15–1.47)* | 1.20 (0.95–1.51) | 1.13 (0.90–1.42) |
| ≥2 | 1.91 (1.52–2.39)* | 2.86 (2.13–3.83)* | 1.33 (0.82–2.16) |
| Unknown | 1.04 (0.92–1.19) | 1.04 (0.83–1.30) | 0.99 (0.75–1.30) |
| Facility type | |||
| Academic/research | Referent | Referent | Referent |
| Community cancer | 1.02 (0.89–1.16) | 0.93 (0.74–1.17) | 1.10 (0.85–1.44) |
| Comprehensive community cancer | 0.99 (0.92–1.07) | 0.97 (0.84–1.12) | 1.04 (0.90–1.21) |
| Other | 0.94 (0.85–1.04) | 1.74 (1.45–2.08)* | 0.70 (0.53–0.92)* |
| Facility region | |||
| Eastern | Referent | Referent | Referent |
| Midwest | 1.00 (0.90–1.11) | 1.16 (0.98–1.38) | 0.88 (0.71–1.08) |
| South | 1.10 (0.996–1.22) | 1.29 (1.06–1.56)* | 0.89 (0.72–1.09) |
| West | 0.93 (0.82–1.05) | 1.19 (0.94–1.50) | 0.79 (0.62–1.01) |
| Stage | |||
| IA | Referent | Referent | ─ |
| IB | 1.71 (1.43–2.04)* | 2.02 (1.65–2.48)* | ─ |
| INOS | 0.84 (0.62–1.14) | 0.90 (0.66–1.21) | ─ |
| II | 1.54 (1.30–1.83)* | 1.76 (1.44–2.14)* | ─ |
| III | 2.60 (2.22–3.04)* | ─ | Referent |
| IV | 6.94 (5.93–8.13)* | ─ | 2.74 (2.34–3.21)* |
| Unknown | 1.91 (1.72–2.11)* | ─ | ─ |
| Histology | |||
| Endometrioid | Referent | Referent | Referent |
| Serous | 1.11 (0.92–1.35) | 0.62 (0.32–1.21) | 1.30 (1.04–1.63)* |
| Clear Cell | 1.14 (0.85–1.52) | 1.12 (0.63–2.00) | 1.24 (0.84–1.83) |
| Sarcoma | 2.67 (2.41–2.95)* | 2.63 (2.00–3.47)* | 1.93 (1.53–2.44)* |
| Other/not otherwise specified | 1.13 (1.02–1.24)* | 1.00 (0.86–1.17) | 1.26 (1.04–1.52)* |
| Grade | |||
| Well | Referent | Referent | Referent |
| Moderate | 1.73 (1.56–1.93)* | 1.44 (1.26–1.66)* | 1.52 (1.12–2.08)* |
| Poorly | 4.01 (3.60–4.46)* | 2.37 (1.96–2.87)* | 3.04 (2.25–4.11)* |
| Unknown | 2.19 (1.95–2.46)* | 1.83 (1.43–2.34)* | 2.46 (1.73–3.49)* |
| Radiation | |||
| None | Referent | Referent | Referent |
| Brachytherapy | 0.74 (0.62–0.88)* | 0.87 (0.67–1.13) | 0.53 (0.38–0.74)* |
| External beam | 1.02 (0.93–1.12) | 1.18 (0.96–1.45) | 0.74 (0.64–0.86)* |
| Unknown | 0.78 (0.48–1.27) | 0.58 (0.20–1.67) | 0.77 (0.32–1.89) |
| Chemotherapy | |||
| Yes | Referent | ─ | Referent |
| No | 0.53 (0.48–0.59)* | ─ | 1.23 (1.05–1.45)* |
| Unknown | 0.45 (0.35–0.59)* | ─ | 0.72 (0.45–1.15) |
Marginal Cox proportional hazard model included race, age, year of diagnosis, insurance status, income, location, comorbidity, facility type, region, stage, histology, grade and radiation in early stage patients (stage IA, IB, INOS and II) accounting for facility-level clustering. Chemotherapy was also adjusted for in the models for advanced stage (stage III and IV) and for all patients. In the model for all patients, 2,669 patients were excluded because of missing follow-up time or vital status, including 1,821 being excluded in the model for early stage, and 305 for advanced stage.
NOS: not otherwise specified. aHR: adjusted hazard ratio. CI: confidence interval.
P-value < 0.05.
When stratified by year of diagnosis, black women were 34% more likely to die than white women when diagnosed between 1998–2002 (HR=1.34; 95%CI, 1.14–1.58) (Table 4). Between 2003 and 2007, there was 22% increased risk of death for black compared to white women. (HR=1.22; 95%CI, 1.04–1.43) In contrast, there was no increased risk of mortality for black women diagnosed between 2008–2012 (HR=0.96; 95% CI 0.77–1.19) but it did not reach statistical significance.
Table 4.
Multivariable model for predictors of mortality stratified by year of diagnosis.
| 1998–2002 | 2003–2007 | 2008–2012 | |
|---|---|---|---|
|
| |||
| aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | |
| Race | |||
| White | Referent | Referent | Referent |
| Black | 1.34 (1.14–1.58)* | 1.22 (1.04–1.43)* | 0.96 (0.77–1.19) |
| Age | |||
| <35 | Referent | Referent | Referent |
| 35–39 | 1.16 (0.90–1.50) | 0.92 (0.70–1.21) | 1.19 (0.85–1.66) |
| 40–44 | 1.38 (1.09–1.75)* | 1.08 (0.85–1.37) | 0.98 (0.72–1.33) |
| 45–49 | 1.55 (1.23–1.96)* | 1.19 (0.96–1.49) | 1.17 (0.87–1.56) |
| Year of diagnosis | 1.00 (0.97–1.04) | 0.99 (0.95–1.03) | 0.99 (0.92–1.07) |
| Insurance status | |||
| Private | Referent | Referent | Referent |
| Medicare | 2.56 (2.03–3.22)* | 2.27 (1.84–2.80)* | 2.07 (1.51–2.82)* |
| Medicaid | 1.58 (1.30–1.92)* | 1.67 (1.40–1.99)* | 1.52 (1.22–1.90)* |
| Other government/unknown | 1.09 (0.85–1.40) | 0.89 (0.61–1.30) | 1.65 (1.08–2.54)* |
| Uninsured | 1.50 (1.21–1.85)* | 1.61 (1.32–1.96)* | 1.46 (1.08–1.98)* |
| Income | |||
| <$38,000 | Referent | Referent | Referent |
| $38,000–$47,999 | 0.83 (0.71–0.97)* | 0.83 (0.71–0.98)* | 1.03 (0.81–1.30) |
| $48,000–$62,999 | 0.75 (0.63–0.88)* | 0.84 (0.71–0.997)* | 0.84 (0.66–1.07) |
| $63,000+ | 0.67 (0.56–0.79)* | 0.63 (0.53–0.75)* | 0.78 (0.60–1.003) |
| Unknown | 1.09 (0.75–1.59) | 1.44 (0.95–2.17) | 1.70 (0.87–3.33) |
| Location | |||
| Metropolitan | Referent | Referent | Referent |
| Urban | 0.98 (0.82–1.16) | 0.90 (0.75–1.07) | 1.02 (0.83–1.26) |
| Rural | 1.03 (0.68–1.57) | 1.24 (0.85–1.83) | 1.00 (0.56–1.79) |
| Unknown | 1.00 (0.71–1.40) | 0.94 (0.65–1.35) | 1.33 (0.77–2.31) |
| Charlson/Deyo comorbidity score | |||
| 0 | – | Referent | Referent |
| 1 | – | 1.24 (1.06–1.45)* | 1.48 (1.20–1.83)* |
| ≥2 | – | 2.34 (1.78–3.08)* | 1.56 (1.06–2.28)* |
| Facility type | |||
| Academic/research | Referent | Referent | Referent |
| Community cancer | 1.01 (0.83–1.23) | 1.00 (0.81–1.24) | 1.05 (0.79–1.40) |
| Comprehensive community cancer | 1.01 (0.89–1.14) | 0.93 (0.83–1.04) | 1.06 (0.91–1.24) |
| Other | 2.20 (1.79–2.72)* | –‡ | –‡ |
| Facility region | |||
| Eastern | Referent | Referent | Referent |
| Midwest | 1.06 (0.91–1.24) | 0.94 (0.81–1.10) | 0.97 (0.78–1.21) |
| South | 1.13 (0.96–1.33) | 1.06 (0.91–1.24) | 1.06 (0.85–1.33) |
| West | 0.80 (0.65–0.97)* | 1.06 (0.88–1.28) | 0.93 (0.72–1.21) |
| Stage | |||
| IA | Referent | Referent | Referent |
| IB | 1.67 (1.29–2.17)* | 1.60 (1.18–2.19)* | 2.39 (1.63–3.52)* |
| INOS | 0.80 (0.45–1.42) | 0.99 (0.65–1.51) | 0.95 (0.49–1.86) |
| II | 1.31 (1.02–1.68)* | 1.67 (1.27–2.20)* | 2.00 (1.32–3.04)* |
| III | 1.97 (1.51–2.56)* | 2.98 (2.31–3.83)* | 3.87 (2.75–5.44)* |
| IV | 5.36 (4.03–7.14)* | 7.75 (6.13–9.80)* | 11.03 (7.75–15.69)* |
| Unknown | 1.68 (1.44–1.96)* | 1.91 (1.62–2.26)* | 2.88 (2.18–3.82)* |
| Histology | |||
| Endometrioid | Referent | Referent | Referent |
| Serous | 0.77 (0.54–1.09) | 1.31 (0.99–1.74) | 1.54 (1.08–2.21)* |
| Clear Cell | 1.02 (0.66–1.58) | 1.07 (0.63–1.84) | 1.57 (0.83–2.98) |
| Sarcoma | 2.28 (1.91–2.72)* | 3.00 (2.55–3.54)* | 2.90 (2.38–3.52)* |
| Other/not otherwise specified | 0.97 (0.85–1.11) | 1.25 (1.06–1.48)* | 1.44 (1.06–1.94)* |
| Grade | |||
| Well | Referent | Referent | Referent |
| Moderate | 1.64 (1.41–1.90)* | 1.81 (1.51–2.17)* | 1.93 (1.46–2.54)* |
| Poorly | 3.63 (3.07–4.29)* | 3.84 (3.23–4.56)* | 5.64 (4.27–7.44)* |
| Unknown | 2.23 (1.88–2.65)* | 2.06 (1.70–2.50)* | 2.66 (2.02–3.52)* |
| Radiation | |||
| None | Referent | Referent | Referent |
| Brahytherapy | 0.84 (0.61–1.16) | 0.73 (0.55–0.95)* | 0.66 (0.49–0.88)* |
| External beam | 1.22 (1.06–1.40)* | 0.90 (0.77–1.04) | 0.91 (0.73–1.13) |
| Unknown | –‡ | 0.95 (0.55–1.64) | 0.63 (0.10–4.02) |
| Chemotherapy | |||
| Yes | Referent | Referent | Referent |
| No | 0.47 (0.40–0.56)* | 0.56 (0.48–0.65)* | 0.60 (0.48–0.75)* |
| Unknown | 0.39 (0.26–0.59)* | 0.45 (0.31–0.65)* | 0.53 (0.27–1.02) |
Marginal Cox proportional hazard models included race, age, year of diagnosis, insurance status, income, location, comorbidity, facility type, region, stage, histology, grade, radiation and chemotherapy accounting for facility-level clustering. In the stratum of 1998–2002, comorbidity was not adjusted for because all patients were unknown. In the stratum of 2008–2012, 2 patients in 2009 and 2,667 patients in 2012 were excluded because of missing follow-up time or vital status.
NOS: not otherwise specified. aHR: adjusted hazard ratio. CI: confidence interval.
P-value < 0.05.
Non-estimable.
Discussion
We noted that young black women are more likely to present with pathologically aggressive, advanced stage tumors than their white counterparts. Even after adjusting for these pathologic differences, young black women with endometrial cancer are 19% more likely to die than white women. The survival disparity is most pronounced in women with early-stage neoplasms.
Although, endometrial cancer is predominantly a disease of postmenopausal women, 5–10% of tumors occur in women less than 40 years of age.23 The overall probability of developing endometrial cancer from birth to 39 years of age is approximately 0.05%.24 Importantly, the incidence of endometrial cancer in young women has been rising steadily since the 1990’s.25 Endometrial cancer in young women is often associated with obesity and nulliparity and is typically associated with a more favorable prognosis than in older women.26,27
Black women are disproportionately affected by pathologically aggressive uterine tumors. Prior studies have consistently demonstrated that black women more frequently present with non-endometrioid, aggressive histologic subtypes, have higher grade tumors, and more commonly have more advanced stage disease.2,3,5,7,28–31 One population-based study noted that black women were 50% more likely to have uterine sarcomas, 85% more likely to have serous and clear cell tumors, and over twice as likely to have carcinosarcomas as their white counterparts.2 We noted similar findings among young women with endometrial cancer; 69% of the tumors in white women were endometrioid histologies compared to only 55% in black patients. Likewise, 49% of white patients had stage IA tumors compared to 40% of black women.
Black women with endometrial cancer are significantly more likely to die from their tumors than white women.2,5,30,32,33 An analysis of over 80,000 women found that even after adjusting for prognostic factors, black women were 60% more likely to die from their tumors than white women.30 We identified similar disparate outcomes in young women with endometrial cancer. After adjustment for clinical and demographic factors, black women were 19% more likely to die than white women. The survival disadvantage was most pronounced for women with early-stage tumors.
While the survival difference between black and white women with endometrial cancer is well described, the factors that underlie the survival disparity have been harder to define. Decreased access to care and less aggressive treatment may explain a portion of the disparity in outcomes.3–5,34,35 A previous study of the National Cancer Data Base found that black women were treated less often than white women at every stage. Among women who were treated, black women were less likely to undergo hysterectomy and less likely to receive adjuvant radiation therapy than white women.3 In addition to access to care and treatment, a number of studies have suggested that biologic differences also account for a portion of the differences in outcomes between black and white women.29 Mutations in the PTEN tumor suppressor gene, an abnormality associated with a favorable prognosis, are less common in tumors in black women.36 In contrast, p53 mutations, often associated with aggressive tumors, occur more frequently in black women.37–39
While our study benefits from the inclusion of a large number of young women with endometrial cancer, we recognize a number of important limitations. First, NCDB lacks data on cause of death and we are only able to examine overall survival. Second, we lack detailed data on some important clinical details such as body mass index, follow-up care, as well as treatment of recurrent disease. Disparities in downstream care may have contributed to some of the differences in outcomes we noted. Third, given that there is no central pathology review, there may have been misclassification of a small number of women. Lastly, as with any study of observational data, we cannot account for individual patient and physician preferences that likely influenced treatment and outcomes.
Despite these limitations, our study suggests that there are pronounced differences in outcomes based on race for young women with endometrial cancer. Even after adjusting for the presence of more pathologically aggressive and advanced stage tumors in black women, we found that black women are nearly 20% more likely to die than their white counterparts. Given the rising incidence of endometrial cancer in young women, further studies to examine the individual contributions of barriers to access to care, differential treatment, and biologic differences between young black and white women are needed.
Research Highlights.
Young black women are more likely to present with aggressive, advanced stage tumors.
Young black women with endometrial cancer have higher mortality than white women.
Disparities in outcomes are most pronounced for early-stage disease.
Acknowledgments
Dr. Wright (NCI R01CA169121-01A1) and Dr. Hershman (NCI R01 CA166084) are recipients of grants from the National Cancer Institute.
Dr. Wright has served as a consultant for Tesaro and Clovis Oncology. Dr. Neugut has served as a consultant to Pfizer, Teva, Otsuka, and United Biosource Corporation. He is on the medical advisory board of EHE, Intl. No other authors have any conflicts of interest or disclosures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edwards BK, Noone A-M, Mariotto AB, et al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–314. doi: 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sherman ME, Devesa SS. Analysis of racial differences in incidence, survival, and mortality for malignant tumors of the uterine corpus. Cancer. 2003;98:176–86. doi: 10.1002/cncr.11484. [DOI] [PubMed] [Google Scholar]
- 3.Hicks ML, Phillips JL, Parham G, et al. The National Cancer Data Base report on endometrial carcinoma in African-American women. Cancer. 1998;83:2629–37. doi: 10.1002/(SICI)1097-0142(19981215)83:12<2629::AID-CNCR30>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Maxwell GL, Tian C, Risinger J, et al. Racial disparity in survival among patients with advanced/recurrent endometrial adenocarcinoma: a Gynecologic Oncology Group study. Cancer. 2006;107:2197–205. doi: 10.1002/cncr.22232. [DOI] [PubMed] [Google Scholar]
- 5.Randall TC, Armstrong K. Differences in treatment and outcome between African-American and white women with endometrial cancer. J Clin Oncol. 2003;21:4200–6. doi: 10.1200/JCO.2003.01.218. [DOI] [PubMed] [Google Scholar]
- 6.Wright JD, Fiorelli J, Kansler AL, et al. Optimizing the management of stage II endometrial cancer: the role of radical hysterectomy and radiation. Am J Obstet Gynecol. 2009;200:419, e1–7. doi: 10.1016/j.ajog.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 7.DeSantis C, Naishadham D, Jemal A. Cancer statistics for African Americans, 2013. CA: a cancer journal for clinicians. 2013;63:151–66. doi: 10.3322/caac.21173. [DOI] [PubMed] [Google Scholar]
- 8.Farley J, Risinger JI, Rose GS, Maxwell GL. Racial disparities in blacks with gynecologic cancers. Cancer. 2007;110:234–43. doi: 10.1002/cncr.22797. [DOI] [PubMed] [Google Scholar]
- 9.Quinn MA, Kneale BJ, Fortune DW. Endometrial carcinoma in premenopausal women: a clinicopathological study. Gynecologic Oncology. 1985;20:298–306. doi: 10.1016/0090-8258(85)90211-2. [DOI] [PubMed] [Google Scholar]
- 10.Jeffery JD, Taylor R, Robertson DI, Stuart GC. Endometrial carcinoma occurring in patients under the age of 45 years. American Journal of Obstetrics and Gynecology. 1987;156:366–70. doi: 10.1016/0002-9378(87)90285-7. [DOI] [PubMed] [Google Scholar]
- 11.Uharcek P, Mlyncek M, Ravinger J, Matejka M. Prognostic factors in women 45 years of age or younger with endometrial cancer. International Journal of Gynecological Cancer: Official Journal of the International Gynecological Cancer Society. 2008;18:324–8. doi: 10.1111/j.1525-1438.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- 12.Yamazawa K, Seki K, Matsui H, Kihara M, Sekiya S. Prognostic factors in young women with endometrial carcinoma: a report of 20 cases and review of literature. International Journal of Gynecological Cancer: Official Journal of the International Gynecological Cancer Society. 2000;10:212–22. doi: 10.1046/j.1525-1438.2000.010003212.x. [DOI] [PubMed] [Google Scholar]
- 13.Evans-Metcalf ER, Brooks SE, Reale FR, Baker SP. Profile of women 45 years of age and younger with endometrial cancer. Obstetrics and Gynecology. 1998;91:349–54. doi: 10.1016/s0029-7844(97)00668-6. [DOI] [PubMed] [Google Scholar]
- 14.Tran BN, Connell PP, Waggoner S, Rotmensch J, Mundt AJ. Characteristics and outcome of endometrial carcinoma patients age 45 years and younger. American Journal of Clinical Oncology. 2000;23:476–80. doi: 10.1097/00000421-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Pellerin GP, Finan MA. Endometrial cancer in women 45 years of age or younger: a clinicopathological analysis. American Journal of Obstetrics and Gynecology. 2005;193:1640–4. doi: 10.1016/j.ajog.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Leo L, Arduino S, Febo G, et al. Endometrial carcinoma in women 45 years of age or younger. European Journal of Gynaecological Oncology. 1996;17:403–5. [PubMed] [Google Scholar]
- 17.The National Cancer Data Base. at https://www.facs.org/qualityprograms/cancer/ncdb.
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 20.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–90. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thabut G, Christie JD, Kremers WK, Fournier M, Halpern SD. Survival differences following lung transplantation among us transplant centers. JAMA. 2010;304:53–60. doi: 10.1001/jama.2010.885. [DOI] [PubMed] [Google Scholar]
- 22.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26. [Google Scholar]
- 23.Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstetrics and Gynecology. 1984;64:417–20. [PubMed] [Google Scholar]
- 24.Azim A, Oktay K. Letrozole for ovulation induction and fertility preservation by embryo cryopreservation in young women with endometrial carcinoma. Fertility and Sterility. 2007;88:657–64. doi: 10.1016/j.fertnstert.2006.12.068. [DOI] [PubMed] [Google Scholar]
- 25.Semaan A, Ali-Fehmi R, Munkarah AR, et al. Clinical/pathologic features and patient outcome in early onset endometrial carcinoma: a population based analysis and an institutional perspective from the Detroit metropolitan area, Michigan. Gynecol Oncol. 2012;124:265–9. doi: 10.1016/j.ygyno.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 26.Uharcek P, Mlyncek M, Ravinger J, Matejka M. Prognostic factors in women 45 years of age or younger with endometrial cancer. Int J Gynecol Cancer. 2008;18:324–8. doi: 10.1111/j.1525-1438.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- 27.Soliman PT, Oh JC, Schmeler KM, et al. Risk factors for young premenopausal women with endometrial cancer. Obstet Gynecol. 2005;105:575–80. doi: 10.1097/01.AOG.0000154151.14516.f7. [DOI] [PubMed] [Google Scholar]
- 28.Oliver KE, Enewold LR, Zhu K, et al. Racial disparities in histopathologic characteristics of uterine cancer are present in older, not younger blacks in an equal-access environment. Gynecologic Oncology. 2011;123:76–81. doi: 10.1016/j.ygyno.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 29.Long B, Liu FW, Bristow RE. Disparities in uterine cancer epidemiology, treatment, and survival among African Americans in the United States. Gynecologic Oncology. 2013;130:652–9. doi: 10.1016/j.ygyno.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright JD, Fiorelli J, Schiff PB, et al. Racial disparities for uterine corpus tumors: changes in clinical characteristics and treatment over time. Cancer. 2009;115:1276–85. doi: 10.1002/cncr.24160. [DOI] [PubMed] [Google Scholar]
- 31.Babatunde OA, Adams SA, Eberth JM, Wirth MD, Choi SK, Hebert JR. Racial disparities in endometrial cancer mortality-to-incidence ratios among Blacks and Whites in South Carolina. Cancer causes & control: CCC. 2016;27:503–11. doi: 10.1007/s10552-016-0724-7. [DOI] [PubMed] [Google Scholar]
- 32.Madison T, Schottenfeld D, Baker V. Cancer of the corpus uteri in white and black women in Michigan, 1985–1994: an analysis of trends in incidence and mortality and their relation to histologic subtype and stage. Cancer. 1998;83:1546–54. doi: 10.1002/(sici)1097-0142(19981015)83:8<1546::aid-cncr9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 33.Bain RP, Greenberg RS, Chung KC. Racial differences in survival of women with endometrial cancer. Am J Obstet Gynecol. 1987;157:914–23. doi: 10.1016/s0002-9378(87)80089-3. [DOI] [PubMed] [Google Scholar]
- 34.Madison T, Schottenfeld D, James SA, Schwartz AG, Gruber SB. Endometrial cancer: socioeconomic status and racial/ethnic differences in stage at diagnosis, treatment, and survival. Am J Public Health. 2004;94:2104–11. doi: 10.2105/ajph.94.12.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trimble EL, Harlan LC, Clegg LX, Stevens JL. Pre-operative imaging, surgery and adjuvant therapy for women diagnosed with cancer of the corpus uteri in community practice in the United States. Gynecol Oncol. 2005;96:741–8. doi: 10.1016/j.ygyno.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 36.Maxwell GL, Risinger JI, Hayes KA, et al. Racial disparity in the frequency of PTEN mutations, but not microsatellite instability, in advanced endometrial cancers. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2000;6:2999–3005. [PubMed] [Google Scholar]
- 37.Kohler MF, Carney P, Dodge R, et al. p53 overexpression in advanced-stage endometrial adenocarcinoma. American Journal of Obstetrics and Gynecology. 1996;175:1246–52. doi: 10.1016/s0002-9378(96)70036-4. [DOI] [PubMed] [Google Scholar]
- 38.Clifford SL, Kaminetsky CP, Cirisano FD, et al. Racial disparity in overexpression of the p53 tumor suppressor gene in stage I endometrial cancer. American Journal of Obstetrics and Gynecology. 1997;176:S229–32. doi: 10.1016/s0002-9378(97)70380-6. [DOI] [PubMed] [Google Scholar]
- 39.Alkushi A, Lim P, Coldman A, Huntsman D, Miller D, Gilks CB. Interpretation of p53 immunoreactivity in endometrial carcinoma: establishing a clinically relevant cut-off level. International Journal of Gynecological Pathology: Official Journal of the International Society of Gynecological Pathologists. 2004;23:129–37. doi: 10.1097/00004347-200404000-00007. [DOI] [PubMed] [Google Scholar]