Abstract
Alcohol use disorder (AUD) manifests differently in men and women, but little is known about sex differences in the brain’s response to ethanol. It is known that the steroid hormone 17β-estradiol (E2) regulates voluntary ethanol consumption in female rodents. However, the role of E2 as a regulator of ethanol reward has not been investigated. In this study, we tested for the effects of E2 and agonists selective for the classical estrogen receptors, ERα and ERβ, on ethanol reward in ovariectomized (OVX) mice using the conditioned place preference (CPP) test. E2 enhanced ethanol CPP and, while specific activation of either receptor alone had no effect, co-activation of ERα and ERβ also enhanced ethanol CPP, suggesting that E2 enhances ethanol reward in female mice through actions at both ERα and ERβ. These results have implications for sex differences in the development of AUD, suggesting that women may find ethanol more rewarding than men because of higher circulating E2 levels.
Keywords: addiction, alcohol, estrogen, female, reward
1. Introduction
Recent decades have seen dramatic increases in ethanol consumption and the occurrence of alcohol use disorder (AUD) among the female population (Greenfield et al., 2010; Grucza et al., 2008; White et al., 2015). Furthermore, women tend to exhibit a so-called “telescoping” pattern of ethanol abuse, experiencing earlier onset of physical and psychological health complications and progressing more rapidly from first use to treatment entry (Becker, 2016; Hernandez-Avila et al., 2004). AUD is associated with a wide array of health consequences, and in many cases the physiological effects of alcohol abuse are more severe in females than in males. For instance, women develop comparable or more pronounced alcohol-related liver disease at lower levels of alcohol consumption than their male counterparts (Becker et al., 1996; Wilsnack et al., 2013). In addition to increased risk of mouth, throat, esophageal, liver, and colon cancers, the risk of breast cancer in women also rises with increasing ethanol consumption (Wilsnack et al., 2013). Heavy drinking is associated with heightened risk of coronary heart disease and cerebrovascular insult in women compared with men, and women are also more vulnerable to alcoholic brain damage and related cognitive impairment (Hashimoto and Wiren, 2008; Rehm et al., 2003; Sohrabji, 2002; Wilhelm et al., 2016).
Despite the growing risk that AUD poses to women’s health, the majority of studies on ethanol’s biological effects and potential therapeutic treatments for ethanol dependence have been conducted in male animals. Little is known about sex differences in the brain’s response to ethanol or the ways in which sex-specific therapies could be developed to benefit both men and women. Existing research suggests that 17β-estradiol (E2), the main circulating form of estrogen produced by the ovaries in premenopausal females, may increase female vulnerability to AUD. Elevated serum levels of E2 have been associated with higher levels of ethanol consumption in premenopausal women (Martin et al., 1999; Muti et al., 1998). Numerous animal studies demonstrate effects of E2 on drinking behavior. For instance, chronic estradiol replacement has been shown to increase ethanol consumption by OVX animals and ethanol preference in a two-bottle-choice paradigm without increasing water consumption (Ford et al., 2004; Mackie et al., 2013; Rajasingh et al., 2007), and a single injection of the synthetic prodrug estradiol valerate (EV), a slow-release formulation of E2, increases consumption of both sweetened and unsweetened 12% ethanol solution (Reid et al., 2003).
At present, however, the neurobiological mechanisms behind the ability of E2 to increase ethanol consumption are poorly understood. One possibility is that E2 increases the pleasurable or appetitive qualities of the ethanol experience (i.e. reward). The CPP test is a well-established method of measuring drug reward in laboratory animals (Cunningham et al., 2006). This method uses a form of classical conditioning in which the stimulus of interest is paired with a contextually distinct environment in order to form an association between the stimulus-induced state and the environment. Naturally cycling female rats develop stronger preference than males for ethanol-paired environmental cues in the CPP test—a response that can be attenuated by OVX, suggesting that circulating E2 may enhance the pleasurable effects of ethanol in females (Torres et al., 2014). In the present study, we used the CPP test to measure the effects of E2 and two estrogen receptor-selective agonists on ethanol CPP in OVX C57BL/6J mice. Our results suggest that E2 increases ethanol reward in OVX mice through activation of both classical estrogen receptors, ERα and ERβ.
2. Material and methods
2.1. Experimental animals
Experimentally naïve, 8- to 10-week-old female C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were subjected to bilateral ovariectomy (OVX) under anesthesia as described below. Mice were allowed to recover for 2 weeks prior to behavioral testing. All mice were group housed with same-sex cage mates in a temperature- and humidity-controlled environment under a 12-hour light/dark cycle with lights on at 6 am and off at 6 pm. Behavioral testing was conducted during the light phase. All mice had access to food and water ad libitum for the duration of the study and were maintained and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All experimental procedures were approved by the University of Illinois at Chicago (UIC) Institutional Animal Care and Use Committee.
2.2. OVX
Mice were anesthetized with an intraperitoneal (IP) injection of ketamine (100 mg/kg) and xylazine (8 mg/kg). Hair was shaved from the mouse’s back, and small incisions were made through the skin and underlying muscle tissue to expose the ovary. The uterine horn was pulled out of the abdominal cavity, and the ovary was dissected away from the uterine horn using cauterization. The uterine horn was then placed back into the abdominal cavity, and the incisions were closed with sterile sutures (for the muscle tissue) and wound clips (for the skin). The same procedure was repeated on the opposite side of the spine to remove the second ovary. Mice received a subcutaneous (SC) injection of meloxicam (2 mg/kg) immediately after surgery and 24 hours later. Mice were allowed to recover for 2 weeks before behavioral testing. To confirm cessation of the estrous cycle, vaginal smears were taken daily from mice for 4–5 days and analyzed for cell content using bright field microscopy. Cessation was confirmed when cell content resembled diestrus (predominantly leukocytes) for several consecutive days.
2.3. Drug treatments
17β-Estradiol-3-benzoate (EB) was purchased from Sigma Aldrich (St. Louis, MO, USA) and prepared in sesame oil vehicle (VEH) to a final concentration of 4 ng/μl. 50 μl was injected SC at a dose of 0.2 μg (~10 μg/kg). We found that this dose of EB results in serum E2 levels four hours after injection that are comparable to levels in mice in proestrus, when E2 levels peak (Nilsson et al., 2015; Vandegrift et al., 2017). To determine the effects of EB on ethanol CPP, OVX mice were treated once daily, beginning on the fourth day after surgery and continuing through preference test day. EB was administered 4 hours before each conditioning session. Two selective estrogen receptor agonists were used in this study: the ERα agonist 4,4′,4″-(4-Propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT) and the ERβ agonist diarylpropionitrile (DPN) (Tocris, Minneapolis, MN, USA). PPT has a 410-fold higher affinity for ERα versus ERβ and DPN has a 70-fold higher affinity for ERβ versus ERα (Meyers et al., 2001; Stauffer et al., 2000). PPT and DPN were prepared in sesame oil with 10% ethanol vehicle (VEH) to a final concentration of 0.5 mg/ml. 50 μl was injected SC for a dose of ~1 mg/kg PPT or DPN (with the final ethanol dose less than 0.2 g/kg). This dose of ethanol does not induce CPP in mice (Groblewski et al., 2008). VEH, PPT, and DPN were administered once daily, one hour prior to each conditioning session. When animals were given both PPT and DPN, the compounds were administered as two separate injections, and controls were given two injections of VEH to account for handling effects. The timing of PPT and DPN injections was performed so that peak plasma levels of these compounds would be achieved during conditioning sessions in the CPP procedure; both are expected to achieve highly selective receptor occupancy at a dose of 1 mg/kg (Sepehr et al., 2012). Ethanol solutions were prepared with 95% ethyl alcohol stock (Decon Laboratories, King of Prussia, PA, USA) diluted to 20% v/v in 0.9% sterile saline. Ethanol was administered IP at a dose of 2.0 g/kg, a moderate dose that is optimal for CPP (Groblewski et al., 2008).
2.4. Behavioral procedures
The CPP apparatus consisted of an open field arena with infrared beams for tracking mouse activity and was 27.3 cm long × 27.3 cm wide × 20.3 cm high (Med Associates, Inc., Fairfax, VT, USA) The arena was divided into two equal sized chambers by an acrylic insert, with each chamber having a different floor texture (Hilderbrand and Lasek, 2014). The timeline and design of each CPP experiment are illustrated in Figs. 1 and 3. For the experiment shown in Fig. 1, we used 18 mice per group. For the experiment shown in Fig. 3B and C, we treated 27 mice with VEH, 32 mice with PPT, and 31 mice with DPN. For the experiment shown in Fig. 3D and E, we treated 16 mice with VEH and 16 mice with both PPT and DPN. The CPP procedure was performed as previously described (Dutton et al. 2016). Briefly, on the first day of the procedure (Test 1), the mouse was placed into the apparatus and allowed 30 minutes of unrestricted access to both sides. Each mouse was then assigned to the initially non-preferred side of the apparatus for ethanol conditioning. Over the next ten days, each mouse was given an injection of ethanol (2 g/kg, IP, given on days 2, 4, 8, and 10) or an equivalent volume of saline (days 3, 5, 9, and 11) immediately before confining it to the appropriate side of the apparatus. After each 5-minute conditioning session, the mouse was promptly removed from the apparatus and returned to its home cage. On the day of the preference test (Test 2), each mouse was allowed to freely explore both chambers of the apparatus for 30 minutes (identical to Test 1). The entire CPP procedure was conducted over a period of 12 days, with a 2-day rest period between days 5 and 8.
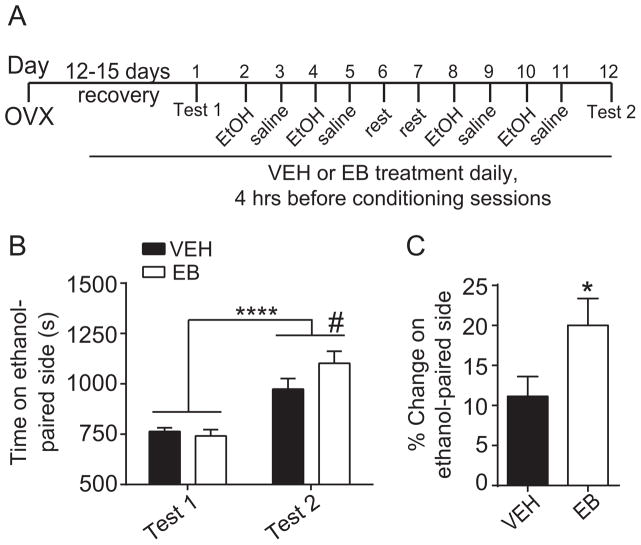
Fig. 1.
17β-Estradiol-3-benzoate (EB) enhances ethanol conditioned place preference (CPP) in ovariectomized (OVX) C57BL/6J mice. Mice were OVX bilaterally and allowed to recover for 12–15 days before the start of behavioral procedures. (A) Timeline and design of the ethanol CPP experiment. (B) Graph shows time spent on the ethanol-paired side (in seconds) before conditioning (Test 1) and after conditioning (Test 2) in VEH- and EB-treated mice (n = 18 per group). Two-way repeated measures ANOVA revealed a significant main effect of time (****p < 0.0001) and a significant time-by-treatment interaction (#p < 0.086, by post hoc Sidak’s tests comparing EB- to VEH-treated within Test 2). (C) Graph shows percent change in preference for the ethanol-paired side in VEH- and EB-treated mice. Student’s t-test revealed a significant increase in preference for the ethanol-paired compartment in EB-treated mice (*p < 0.05). Data are presented as means ± SEM.
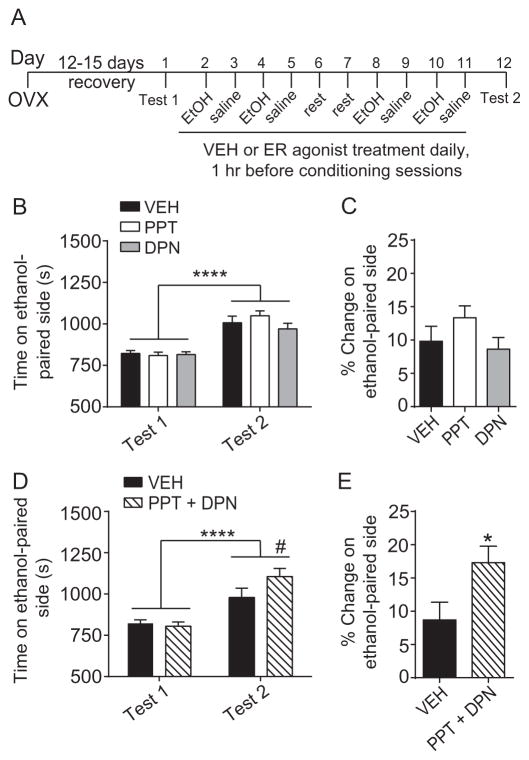
Fig. 3.
Neither PPT (ERα agonist) nor DPN (ERβ agonist) treatment alone is sufficient to enhance ethanol CPP, but combined treatment with both agonists enhances ethanol CPP. Mice were OVX bilaterally and allowed to recover for 12–15 days before the start of behavioral procedures. (A) Timeline and design of the CPP experiments. (B) Graph showing the time spent on the ethanol-paired side (in seconds) before conditioning (Test 1) and after conditioning (Test 2) of vehicle (VEH)-, 4,4′,4″-(4-Propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT)-, and diarylpropionitrile (DPN)-treated mice (n = 27–32 per group). Two-way repeated measures ANOVA revealed a significant main effect of time (****p < 0.0001). (C) Percent change in preference for the ethanol-paired side in VEH-, PPT-, and DPN-treated mice. A one-way ANOVA found no significant difference between treatment groups. (D) Graph shows time spent on the ethanol-paired side before and after conditioning by mice treated with either VEH or a combined treatment of PPT plus DPN (n = 16 per group). Two-way repeated measures ANOVA revealed a significant main effect of time (****p < 0.0001) and a significant time-by-treatment interaction (#p < 0.061, post-hoc Sidak’s test comparing VEH- to PPT+DPN-treated within Test 2). (E) Percent change in preference for the ethanol-paired side in mice treated with either VEH or a combined treatment of PPT plus DPN. Student’s t-test revealed a significant increase in preference for the ethanol-paired compartment in mice treated with PPT plus DPN vs. VEH (*p < 0.05). Data are presented as means ± SEM.
2.5. Ethanol metabolism
Each mouse was given a single SC injection daily of either EB (n = 5) or VEH (n = 5) at 8:00 am for three consecutive days, so that each animal received a total of three treatments. On the third day, four hours after the final EB or VEH treatment, mice received 2.0 g/kg ethanol by IP injection. Five minutes after ethanol injection, the tail was snipped with sterile scissors, and approximately 20 μl of blood was collected from the tail using heparinized capillary tubes. Blood was transferred to 1.5 ml Eppendorf tubes. The time of ethanol injection was staggered so that blood could be collected from all 10 animals at exactly 5, 30, 60, 90, 120, 150, and 180 minutes post-injection. Blood samples were immediately placed on ice and then transferred to a −80°C freezer for storage. Mice were euthanized at the end of the three-hour blood collection procedure. Blood ethanol concentrations (BECs) were determined using a nicotinamide adenine dinucleotide-alcohol dehydrogenase enzymatic assay (Zapata et al., 2006).
2.6. Statistical analysis
For CPP experiments, the amount of time animals spent on the ethanol-paired side before and after conditioning (Test 1 and Test 2, in seconds) was analyzed using two-way repeated measures analysis of variance (2-way RM ANOVA). Post-hoc multiple comparisons testing using Sidak’s multiple comparisons test was performed if there was a significant interaction. To compare the magnitude of CPP between treatment groups, the percentage of time that each mouse spent on the ethanol-paired side during Tests 1 and 2 was calculated by dividing the time spent on that side of the apparatus by total test time (1800 seconds) and multiplying by 100. Percent change in time spent on the ethanol-paired side was calculated by subtracting the value for Test 1 from the value for Test 2 (Fig. 1C and Fig. 3C and E). Data were analyzed by student’s t-test or one-way ANOVA as appropriate. The ethanol metabolism study was analyzed by two-way RM ANOVA. Effect sizes are reported as Cohen’s d for t-tests and η2 for two-way ANOVA. Error bars represent standard error of the mean (SEM). All data were analyzed using Prism software version 6 (GraphPad, La Jolla, CA). A p value of less than 0.05 was accepted as statistically significant.
3. Results
3.1. E2 enhances ethanol CPP in OVX mice
To determine whether E2 regulates ethanol reward in female mice, we treated OVX mice with either EB or VEH and tested them for ethanol CPP. Fig. 1A illustrates the CPP procedure. Overall, mice developed preference for the ethanol-paired side, as indicated by more time spent on the ethanol-paired side after conditioning (Fig. 1B, time: F1, 34 = 55.84, p < 0.0001, η2 = 0.62). We also observed a significant time-by-treatment interaction (interaction: F1, 34 = 4.56, p = 0.04, η2 = 0.12). Post-hoc Sidak’s multiple comparisons test showed that there was a trend toward more time on the ethanol-paired side in EB-treated mice after conditioning (p = 0.086) relative to VEH-treated mice, whereas there was no difference between EB- and VEH-treated mice pre-conditioning (p = 0.84). When analyzed as the percent change in preference, VEH-treated mice spent 11% and EB-treated mice spent 20% more time on the ethanol-paired side after conditioning, a difference that was statistically significant (Fig. 1C, t = 2.13, df = 34, p = 0.04, Cohen’s d = 0.71). These results demonstrate that E2 enhances ethanol reward in OVX mice.
3.2. E2 does not alter the rate of ethanol metabolism in OVX mice
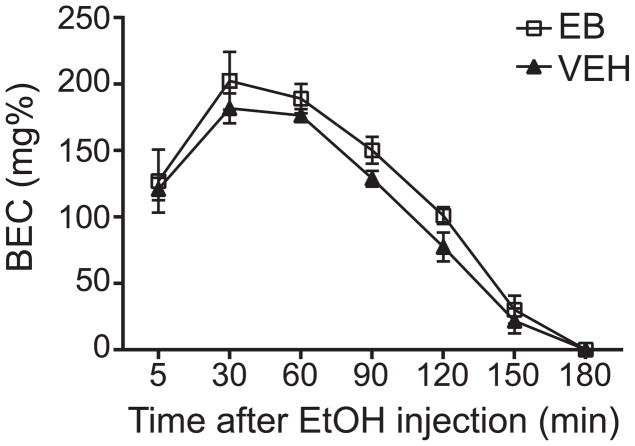
We next tested if E2 can alter the rate of ethanol metabolism, since this might explain the enhancement of ethanol CPP by EB. VEH- and EB-treated mice were injected with 2 g/kg ethanol (the same dose used in the CPP experiments), and blood samples were obtained 5 min to 3 hours after ethanol injection. EB treatment had no effect on the rate of ethanol metabolism in OVX mice compared with VEH-treated mice (Fig. 2, time: F6,48 = 107.4, p < 0.0001; treatment: F1, 8 = 1.85, p = 0.21). These results demonstrate that the effect of EB on ethanol reward is likely not due to a change in ethanol metabolism.
Fig. 2.
EB does not alter the rate of ethanol metabolism in OVX C57BL/6J mice. OVX mice were treated with either VEH or EB (n = 5 per group). Four hours later, 2.0 g/kg ethanol was administered by IP injection. Blood was collected at seven time points post-injection. Graph shows blood ethanol concentration (BEC) in milligrams per 100 milliliters (mg%) over 180 minutes. Two-way ANOVA found no difference in BEC between VEH- and EB-treated animals at any of the seven time points measured. Data are presented as means ± SEM.
3.3. Activation of ERα or ERβ individually is not sufficient to enhance ethanol CPP in OVX mice
E2 binds to two classical estrogen receptors, ERα and ERβ. To determine if ERα or ERβ might be responsible for the ability of E2 to enhance ethanol reward, we performed another ethanol CPP experiment by treating OVX mice with PPT (an ERα-selective agonist) or DPN (an ERβ-selective agonist, Fig. 3A). We observed a significant main effect of time (Fig. 3B, time: F1, 87 = 91.47, p < 0.0001, η2 = 0.51), indicating that mice developed preference for the ethanol-paired side. There were no significant main effects of PPT or DPN treatment and no time-by-treatment interactions. Moreover, there were no differences in the percent change in preference between the three groups (Fig. 3C). These results indicate that selective activation of either ERα or ERβ is not sufficient to enhance ethanol CPP.
3.4. Activation of both ERα and ERβ enhances ethanol CPP
We next reasoned that activation of both receptors might be necessary to increase ethanol CPP. To test this, we treated OVX mice with both PPT and DPN and tested them for ethanol CPP (Fig. 3). There was a significant main effect of time (Fig. 3D, time: F1, 30 = 50.73, p < 0.0001, η2 = 0.63) and a significant time-by-treatment interaction (interaction: F1, 30 = 5.55, p = 0.025, η2 = 0.16). Post-hoc Sidak’s multiple comparisons tests demonstrated that mice treated concurrently with PPT and DPN exhibited a trend towards more time on the ethanol-paired side after conditioning compared with VEH-treated mice (p = 0.061), whereas no difference was observed before conditioning (p = 0.87). Consistent with this observation, the percent change in preference was 17% in PPT- and DPN-treated mice and only 9% in VEH-treated mice (Fig. 3E, t = 2.36, df = 30, p = 0.025, Cohen’s d = 0.83). These results demonstrate that activation of both ERα and ERβ is needed to enhance ethanol reward.
4. Discussion
The main conclusion from this study is that E2 enhances ethanol CPP in OVX mice, likely through activation of both ERα and ERβ. To our knowledge, this is the first study to directly demonstrate an effect of E2 on a behavioral test of ethanol reward in rodents. Our findings complement previous research by Torres et al., who reported that gonadally intact female rats develop stronger preference for an ethanol-paired compartment than either males or OVX females at a dose of 1 g/kg ethanol (Torres et al., 2014). In that study, only intact females developed ethanol CPP, suggesting that ovarian steroids are necessary for the acquisition and/or expression of ethanol CPP in female rats. In contrast, we found that OVX C57BL/6J mice develop ethanol CPP despite the absence of ovarian hormones. This could be due to a difference in species or the dose of ethanol used. Our results suggest that ovarian hormones are not required for the development of, but instead appear to enhance, ethanol CPP in mice. Since we found no effect of E2 on ethanol metabolism, we conclude that E2 enhances ethanol reward through a neural mechanism.
E2 was previously demonstrated to increase ethanol consumption by female rats and mice (Ford et al., 2002, 2004; Marinelli et al., 2003; Quirarte et al., 2007; Rajasingh et al., 2007; Reid et al., 2002; Reid et al., 2003), although E2 administration has also been shown to decrease ethanol intake (Almeida et al., 1998; Hilakivi-Clarke, 1996; Sandberg et al., 1982; Sandberg and Stewart, 1982). These contradictory results may be due to differences in the timing or doses of E2 used and/or to differences in the strain of mouse or rat tested. We recently observed that E2 administration to OVX C57BL6/J mice increases ethanol consumption in the “drinking in the dark” procedure, which is a measure of binge-like ethanol consumption (Satta et al., 2017b). In general, it appears that E2 plays a modulatory role in ethanol consumption in female rodents, with most studies finding that E2 increases ethanol consumption. The results presented here indicate that E2 also enhances the rewarding properties of ethanol in the CPP test.
The ability of E2 to increase ethanol reward may contribute to higher levels of ethanol intake by female rodents. Although both male and female rodents will drink ethanol solutions, females tend to consume more ethanol proportional to body weight and obtain higher blood ethanol concentrations (BECs) than males in a number of different ethanol consumption tests (Becker and Koob, 2016; Hwa et al., 2011; Jury et al., 2017; Middaugh et al., 1999; Priddy et al., 2017). Many of the studies that have examined this sex difference suggest that it is due to the presence of ovarian steroids in females. For example, Lancaster et al. have demonstrated that voluntary ethanol intake in gonadally-intact female rats increases after puberty (Lancaster et al., 1996). Further evidence comes from the four core genotypes (FCG) mouse model, which dissociates gonadal phenotype (ovaries or testes) from sex chromosome complement (XX or XY) by moving the sex-determining region (Sry) of the Y chromosome to an autosomal chromosome (Arnold and Chen, 2009; De Vries et al., 2002). In the FCG model, the presence of female-typical gonads predicts alcohol drinking, with gonadal females drinking more than gonadal males, regardless of sex chromosome complement (Barker et al., 2010). Studies have also reported decreased ethanol consumption in OVX rats and mice compared to naturally cycling controls (Becker et al., 1985; Ford et al., 2002), although this observation is not consistent throughout the literature (Almeida et al., 1998; Vetter-O’Hagen and Spear, 2011).
Previous work has demonstrated that E2 also facilitates the development of CPP for other drugs of abuse. For example, others have reported heightened methamphetamine and morphine CPP in OVX mice that were treated with E2 (Chen et al., 2003; Mirbaha et al., 2009) and increased amphetamine and cocaine CPP in E2-treated OVX rats (Segarra et al., 2014; Silverman and Koenig, 2007). Silverman and Koenig also investigated effects of the selective estrogen receptor agonists PPT and DPN on amphetamine CPP. While activation of ERα by PPT had no effect, DPN treatment increased amphetamine CPP to the same degree as E2, suggesting that estrogenic enhancement of amphetamine reward is mediated by ERβ. We have discovered similar effects on cocaine reward in OVX mice: both E2 and DPN facilitate the development of cocaine CPP, but the behavior of PPT-treated animals is not significantly different from controls (Satta et al., 2017a). This is interesting in light of the findings we report here: that treatment with PPT or DPN alone was not sufficient to enhance ethanol CPP but activation of both ERα and ERβ was needed to increase ethanol CPP. Taken together, these studies indicate that although E2 can increase the rewarding properties of multiple drugs of abuse, there are likely different molecular mechansims regulated by E2 that affect ethanol and psychostimulant reward in females.
To our knowledge, we are the first to report an effect of E2 on addiction-related behavior mediated by both ERα and ERβ. This novel finding is particularly intriguing in the larger context of estrogen-regulated behaviors. In cases when both of the classical estrogen receptors have been shown to regulate a given behavior or class of behaviors—for instance, when measuring effects of PPT and DPN on anxiety-like behavior—the actions of ERα and ERβ have generally been found to oppose one another (Weiser et al., 2008). This is often true with regard to the molecular actions of estrogen receptors as well (Heldring et al., 2007; Matthews and Gustafsson, 2003). Nonetheless, ERα and ERβ are also known to form functional heterodimers, both as regulators of gene expression and when signaling from the cell membrane (Levin, 2009). It is possible that ERα and ERβ heterodimer formation is involved in the regulation of ethanol CPP in females, or that, alternatively, activation of each of these receptors in different brain regions is necessary to increase ethanol CPP. One caveat to our conclusion that activation of both receptors by the combination of PPT and DPN mimics the effect of E2 is that the timing of the EB treatments was different from the timing of PPT and DPN treatments. Treatment with EB was initiated a few days after OVX and continued throughout conditioning and test days, whereas we only treated with the combination of PPT and DPN on conditioning days. These differences in exposure history could affect perceived ethanol reward. Optimally, it would be good to know if treatment with EB only on conditioning days has the same effect as the longer treatment with EB and whether a longer treatment with each agonist alone might mimic the effect of E2. However, it does appear as if the activation of both ERα and ERα is needed to enhance ethanol CPP when the agonists are administered only on conditioning days.
It is also important to note that we only used one dose of PPT and DPN in these studies. We chose this dose because we wanted to achieve a balance between high receptor occupancy and selectivity. It is possible that increasing the doses of these agonists might reveal that activation of either ERα or ERβ is sufficient to enhance ethanol CPP. However, these agonists lose their selectivity at higher doses, making it difficult to discern if the effects of the agonists are due to high activation of the target receptor or non-selective activation of the other estrogen receptor. It will be useful in future studies to use more specific genetic manipulations of ERα and ERβ in gonadally intact females to determine the contribution of these receptors to ethanol reward under more physiological conditions.
Sex differences exist at all phases of the addiction cycle: preoccupation/anticipation (i.e. craving), binge/intoxication, and withdrawal/negative affect (Becker et al., 2017). From a treatment standpoint, understanding the ways in which sex hormones modulate the brain’s response to drugs of abuse is undeniably valuable. The present study adds important new information to our understanding of how ovarian steroids and estrogen receptors specifically may act to regulate ethanol reward, which may contribute to high levels of binge drinking, in females. In particular, the findings that E2 enhances ethanol reward and that ERα and ERβ are both involved in this process poses an interesting contrast to what we know about the role of estrogen receptors in other types of drug reward (i.e. cocaine and other psychomotor stimulants). In future studies, this information will help us discover more about sex differences in general and about the mechanisms by which different drugs of abuse can increase the vulnerability of females to become addicted to these substances.
Highlights.
Estradiol treatment enhances ethanol CPP in ovariectomized female C57BL/6J mice.
Estradiol does not alter the rate of ethanol metabolism in ovariectomized C57BL/6J mice.
Activation of both ERα and ERβ is needed to enhance ethanol CPP.
Acknowledgments
This work was supported by the National Institutes of Health (grant numbers P50 AA022538, U01 AA016654, U01 AA020912, and R01 DA033429 to A.W.L and F31 AA024344 to E.R.H.). All authors declare no potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida OF, Shoaib M, Deicke J, Fischer D, Darwish MH, Patchev VK. Gender differences in ethanol preference and ingestion in rats. The role of the gonadal steroid environment. J Clin Invest. 1998;101:2677–2685. doi: 10.1172/JCI1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Torregrossa MM, Arnold AP, Taylor JR. Dissociation of genetic and hormonal influences on sex differences in alcoholism-related behaviors. J Neurosci. 2010;30:9140–9144. doi: 10.1523/JNEUROSCI.0548-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Anton RF, De Trana C, Randall CL. Sensitivity to ethanol in female mice: effects of ovariectomy and strain. Life Sci. 1985;37:1293–1300. doi: 10.1016/0024-3205(85)90244-9. [DOI] [PubMed] [Google Scholar]
- Becker JB. Sex differences in addiction. Dialogues in clinical neuroscience. 2016;18:395–402. doi: 10.31887/DCNS.2016.18.4/jbecker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF. Sex Differences in Animal Models: Focus on Addiction. Pharmacological reviews. 2016;68:242–263. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, McClellan ML, Reed BG. Sex differences, gender and addiction. J Neurosci Res. 2017;95:136–147. doi: 10.1002/jnr.23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker U, Deis A, Sorensen TI, Gronbaek M, Borch-Johnsen K, Muller CF, Schnohr P, Jensen G. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23:1025–1029. doi: 10.1002/hep.510230513. [DOI] [PubMed] [Google Scholar]
- Chen HH, Yang YK, Yeh TL, Cherng CF, Hsu HC, Hsiao SY, Yu L. Methamphetamine-induced conditioned place preference is facilitated by estradiol pretreatment in female mice. The Chinese journal of physiology. 2003;46:169–174. [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, Samson HH. Ethanol consumption in the female Long-Evans rat: a modulatory role of estradiol. Alcohol. 2002;26:103–113. doi: 10.1016/s0741-8329(01)00203-8. [DOI] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, Samson HH. Determination of an estradiol dose-response relationship in the modulation of ethanol intake. Alcohol Clin Exp Res. 2004;28:20–28. doi: 10.1097/01.ALC.0000108647.62718.5A. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Back SE, Lawson K, Brady KT. Substance abuse in women. Psychiatr Clin North Am. 2010;33:339–355. doi: 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groblewski PA, Bax LS, Cunningham CL. Reference-dose place conditioning with ethanol in mice: empirical and theoretical analysis. Psychopharmacology (Berl) 2008;201:97–106. doi: 10.1007/s00213-008-1251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grucza RA, Bucholz KK, Rice JP, Bierut LJ. Secular trends in the lifetime prevalence of alcohol dependence in the United States: a re-evaluation. Alcohol Clin Exp Res. 2008;32:763–770. doi: 10.1111/j.1530-0277.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto JG, Wiren KM. Neurotoxic consequences of chronic alcohol withdrawal: expression profiling reveals importance of gender over withdrawal severity. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;33:1084–1096. doi: 10.1038/sj.npp.1301494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiological reviews. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74:265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke L. Role of estradiol in alcohol intake and alcohol-related behaviors. Journal of studies on alcohol. 1996;57:162–170. doi: 10.15288/jsa.1996.57.162. [DOI] [PubMed] [Google Scholar]
- Hilderbrand ER, Lasek AW. Sex differences in cocaine conditioned place preference in C57BL/6J mice. Neuroreport. 2014;25:105–109. doi: 10.1097/WNR.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res. 2011;35:1938–1947. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jury NJ, DiBerto JF, Kash TL, Holmes A. Sex differences in the behavioral sequelae of chronic ethanol exposure. Alcohol. 2017;58:53–60. doi: 10.1016/j.alcohol.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Levin ER. Plasma membrane estrogen receptors. Trends in endocrinology and metabolism: TEM. 2009;20:477–482. doi: 10.1016/j.tem.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie AR, Krishnamurthy P, Verma SK, Thorne T, Ramirez V, Qin G, Abramova T, Hamada H, Losordo DW, Kishore R. Alcohol consumption negates estrogen-mediated myocardial repair in ovariectomized mice by inhibiting endothelial progenitor cell mobilization and function. The Journal of biological chemistry. 2013;288:18022–18034. doi: 10.1074/jbc.M113.468009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Quirion R, Gianoulakis C. Estradiol valerate and alcohol intake: a comparison between Wistar and Lewis rats and the putative role of endorphins. Behav Brain Res. 2003;139:59–67. doi: 10.1016/s0166-4328(02)00057-8. [DOI] [PubMed] [Google Scholar]
- Martin CA, Mainous AG, 3rd, Curry T, Martin D. Alcohol use in adolescent females: correlates with estradiol and testosterone. The American journal on addictions/American Academy of Psychiatrists in Alcoholism and Addictions. 1999;8:9–14. doi: 10.1080/105504999306036. [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Molecular interventions. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM, Bandy AL, McGroarty KK. Ethanol consumption by C57BL/6 mice: influence of gender and procedural variables. Alcohol. 1999;17:175–183. doi: 10.1016/s0741-8329(98)00055-x. [DOI] [PubMed] [Google Scholar]
- Mirbaha H, Tabaeizadeh M, Shaterian-Mohammadi H, Tahsili-Fahadan P, Dehpour AR. Estrogen pretreatment modulates morphine-induced conditioned place preference in ovariectomized mice. Pharmacology, biochemistry, and behavior. 2009;92:399–403. doi: 10.1016/j.pbb.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Muti P, Trevisan M, Micheli A, Krogh V, Bolelli G, Sciajno R, Schunemann HJ, Berrino F. Alcohol consumption and total estradiol in premenopausal women. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1998;7:189–193. [PubMed] [Google Scholar]
- Nilsson ME, Vandenput L, Tivesten A, Norlen AK, Lagerquist MK, Windahl SH, Borjesson AE, Farman HH, Poutanen M, Benrick A, Maliqueo M, Stener-Victorin E, Ryberg H, Ohlsson C. Measurement of a Comprehensive Sex Steroid Profile in Rodent Serum by High-Sensitive Gas Chromatography-Tandem Mass Spectrometry. Endocrinology. 2015;156:2492–2502. doi: 10.1210/en.2014-1890. [DOI] [PubMed] [Google Scholar]
- Priddy BM, Carmack SA, Thomas LC, Vendruscolo JC, Koob GF, Vendruscolo LF. Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacology, biochemistry, and behavior. 2017;152:61–67. doi: 10.1016/j.pbb.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirarte GL, Reid LD, de la Teja IS, Reid ML, Sanchez MA, Diaz-Trujillo A, Aguilar-Vazquez A, Prado-Alcala RA. Estradiol valerate and alcohol intake: dose-response assessments. BMC pharmacology. 2007;7:3. doi: 10.1186/1471-2210-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasingh J, Bord E, Qin G, Ii M, Silver M, Hamada H, Ahluwalia D, Goukassian D, Zhu Y, Losordo DW, Kishore R. Enhanced voluntary alcohol consumption after estrogen supplementation negates estrogen-mediated vascular repair in ovariectomized mice. Endocrinology. 2007;148:3618–3624. doi: 10.1210/en.2006-1357. [DOI] [PubMed] [Google Scholar]
- Rehm J, Gmel G, Sempos CT, Trevisan M. Alcohol-related morbidity and mortality. Alcohol research & health: the journal of the National Institute on Alcohol Abuse and Alcoholism. 2003;27:39–51. [PMC free article] [PubMed] [Google Scholar]
- Reid LD, Marinelli PW, Bennett SM, Fiscale LT, Narciso SP, Oparowski CJ, Reid ML, Merrigan BA, Moricone J, Hubbell CL, Gianoulakis C. One injection of estradiol valerate induces dramatic changes in rats’ intake of alcoholic beverages. Pharmacology, biochemistry, and behavior. 2002;72:601–616. doi: 10.1016/s0091-3057(02)00732-3. [DOI] [PubMed] [Google Scholar]
- Reid ML, Hubbell CL, Reid LD. A pharmacological dose of estradiol can enhance appetites for alcoholic beverages. Pharmacology, biochemistry, and behavior. 2003;74:381–388. doi: 10.1016/s0091-3057(02)01008-0. [DOI] [PubMed] [Google Scholar]
- Sandberg D, David S, Stewart J. Effects of estradiol benzoate on the pattern of eating and ethanol consumption. Physiol Behav. 1982;29:61–65. doi: 10.1016/0031-9384(82)90366-3. [DOI] [PubMed] [Google Scholar]
- Sandberg D, Stewart J. Effects of estradiol benzoate and MER-25 on ethanol consumption in the ovariectomized rat. J Comp Physiol Psychol. 1982;96:635–648. doi: 10.1037/h0077913. [DOI] [PubMed] [Google Scholar]
- Satta R, Certa B, He D, Lasek AW. Estrogen Receptor beta in the nucleus accumbens regulates the rewarding properties of cocaine in female mice. Int J Neuropsychopharm. 2017a doi: 10.1093/ijnp/pyx118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta R, Hilderbrand ER, Lasek AW. Ovarian Hormones Contribute to High Levels of Binge-Like Drinking by Female Mice. Alcohol Clin Exp Res. 2017b doi: 10.1111/acer.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra AC, Torres-Diaz YM, Silva RD, Puig-Ramos A, Menendez-Delmestre R, Rivera-Bermudez JG, Amadeo W, Agosto-Rivera JL. Estrogen receptors mediate estradiol’s effect on sensitization and CPP to cocaine in female rats: role of contextual cues. Hormones and behavior. 2014;65:77–87. doi: 10.1016/j.yhbeh.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehr E, Lebl-Rinnova M, Mann MK, Pisani SL, Churchwell MI, Korol DL, Katzenellenbogen JA, Doerge DR. Pharmacokinetics of the estrogen receptor subtype-selective ligands, PPT and DPN: quantification using UPLC-ES/MS/MS. Journal of pharmaceutical and biomedical analysis. 2012;71:119–126. doi: 10.1016/j.jpba.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Koenig JI. Evidence for the involvement of ERbeta and RGS9-2 in 17-beta estradiol enhancement of amphetamine-induced place preference behavior. Hormones and behavior. 2007;52:146–155. doi: 10.1016/j.yhbeh.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F. Neurodegeneration in women. Alcohol research & health: the journal of the National Institute on Alcohol Abuse and Alcoholism. 2002;26:316–318. [PMC free article] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Torres OV, Walker EM, Beas BS, O’Dell LE. Female rats display enhanced rewarding effects of ethanol that are hormone dependent. Alcohol Clin Exp Res. 2014;38:108–115. doi: 10.1111/acer.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandegrift BJ, You C, Satta R, Brodie MS, Lasek AW. Estradiol increases the sensitivity of ventral tegmental area dopamine neurons to dopamine and ethanol. PLoS One. 2017;12:e0187698. doi: 10.1371/journal.pone.0187698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP. The effects of gonadectomy on age- and sex-typical patterns of ethanol consumption in Sprague-Dawley rats. Alcohol Clin Exp Res. 2011;35:2039–2049. doi: 10.1111/j.1530-0277.2011.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta in the brain: from form to function. Brain Res Rev. 2008;57:309–320. doi: 10.1016/j.brainresrev.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A, Castle IJ, Chen CM, Shirley M, Roach D, Hingson R. Converging Patterns of Alcohol Use and Related Outcomes Among Females and Males in the United States, 2002 to 2012. Alcohol Clin Exp Res. 2015;39:1712–1726. doi: 10.1111/acer.12815. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Hashimoto JG, Roberts ML, Bloom SH, Andrew MR, Wiren KM. Astrocyte Dysfunction Induced by Alcohol in Females but Not Males. Brain pathology (Zurich, Switzerland) 2016;26:433–451. doi: 10.1111/bpa.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsnack SC, Wilsnack RW, Kantor LW. Focus on: women and the costs of alcohol use. Alcohol research: current reviews. 2013;35:219–228. doi: 10.35946/arcr.v35.2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata A, Gonzales RA, Shippenberg TS. Repeated ethanol intoxication induces behavioral sensitization in the absence of a sensitized accumbens dopamine response in C57BL/6J and DBA/2J mice. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2006;31:396–405. doi: 10.1038/sj.npp.1300833. [DOI] [PMC free article] [PubMed] [Google Scholar]