Abstract
Oligodendrocyte precursor cells (OPCs) play critical roles in maintaining the number of oligodendrocytes in white matter. Previously, we have shown that oxidative stress dampens oligodendrocyte regeneration after white matter damage, while a clinically proven radical scavenger, edaravone, supports oligodendrocyte repopulation. However, it is not known how edaravone exerts this beneficial effect against oxidative stress. Using in vivo and in vitro experiments, we have examined whether edaravone exhibits direct OPC-protective effects. For in vivo experiments, prolonged cerebral hypoperfusion was induced by bilateral common carotid artery stenosis in mice. OPC damage was observed on day 14 after the onset of cerebral hypoperfusion, and edaravone was demonstrated to decrease OPC death in cerebral white matter. In vitro experiments also confirmed that edaravone reduced oxidative-stress-induced OPC death. Because white matter damage is a major hallmark of many neurological diseases, and OPCs are instrumental in white matter repair after injury, our current study supports the idea that radical scavengers may provide a potential therapeutic approach for white matter related diseases.
Keywords: white matter injury, prolonged cerebral hypoperfusion, oxidative stress, oligodendrocyte precursor cell, edaravone
1. Introduction
During brain injury, reactive oxygen species (ROS) are produced and released into the brain parenchyma, causing further damage to the brain. When the injury involves white matter, the severity of ROS damage may be further amplified to a critical level, because the lipid-rich myelin sheaths in white matter constitute a source of ROS generation. Since white matter damage is an inevitable component of most types of brain injury, strategies that protect white matter during oxidative stress would contribute greatly to brain preservation and recovery. The ideal therapy would provide protection for oligodendrocyte precursor cells (OPC), which comprise one of the major cell types that maintain white matter function. After white matter injury, OPCs proliferate and differentiate into mature oligodendrocytes, which repair damaged myelin sheath as a compensative response [12, 19, 29]. However, OPCs are sensitive to oxidative stress [20], resulting in an inhibit OPC differentiation into oligodendrocytes [8]. As we previously reported in a model of chronic hypoperfusion, prolonged excessive ROS suppresses the regeneration of oligodendrocytes from OPCs, causing further progression of white matter lesions from demyelination [21]. However, no clinically proven agents currently exist that protects OPCs under pathological conditions.
One potential candidate for OPC protection during white matter injury is edaravone (MCI-186, 3-methyl-1-phenyl-2-pyrazolin-5-one), especially because of its many clinically-relevant attributes. Edaravone is a small molecule free radical scavenger that can easily cross the blood-brain barrier. Edaravone has been clinically used in Japan and South Korea for the treatment of acute ischemic stroke [1, 16, 24] and was recently approved in the US for the treatment of amyotrophic lateral sclerosis [2, 25]. In addition, its antioxidant effects have been shown to protect brain cells in animal models of brain ischemia and brain edema [27]. Edaravone also aids in recovery from cerebral infarction [15] and protects against subsequent damage following free radical-related injuries [17].
In a previous study, we showed that radical scavenging with edaravone effectively improved neurologic recovery and accelerated oligodendrocyte regeneration in a mouse model of prolonged cerebral hypoperfusion [21]. We hypothesize that the mechanism of edaravone action involves protection of OPCs, which are the central pool of precursor cells for white matter [19, 29]. However, direct proof of OPC protection by edaravone is so far lacking. Hence, the present study was designed to assess whether edaravone directly protects OPCs against oxidative stress using both in vivo and in vitro systems.
2. Material and methods
In vivo animal experiments
All experiments were reviewed and approved by a Subcommittee for Research Animal Care of the Massachusetts General Hospital IACUC (Institutional Animal Care and Use Committee) and all these institutionally-approved animal protocols are consistent with the NIH Guide for the Care and Use of Laboratory Animals. For inducing cerebral chronic hypoperfusion, microcoils (0.18 mm diameter, Sawane Spring Co.) were inserted into the common carotid arteries bilaterally. Male C57BL/6 mice (10 weeks old, Charles River Institute) were anesthetized with 4.0% isoflurane and maintained on 1.5% isoflurane in 70% N2O and 30% O2, maintaining the rectal temperature between 36.5°C and 37.5°C. Cerebral blood flow was measured before/after the micro-coil placement as described previously [21, 22]. The radical scavenger edaravone (3 mg/kg ip) or vehicle (saline) was administered starting on day 0 and continued twice per week until the mice were sacrificed. This experimental protocol was the same as our previous study, and in the previous study, we confirmed that the hypoperfused mice showed increased oxidative stress and the edaravone treatment was effective in reducing ROS level in this model [21]. Animals were sacrificed on day 14. All in vivo experiments and measurements were performed in a blinded and randomized fashion. Animal numbers for each experiment are described in the figure legends.
Immunohistochemistry
Mouse brains were removed on day 14, post-fixed for 24 hours at 4°C in 4% paraformaldehyde in phosphate-buffered saline, then bathed in 30% sucrose for 48 hours. Sixteen μm thick coronal sections (3 sections per mouse) were incubated for 24h at 4°C with mouse monoclonal antibody against ssDNA (ssDNA, 1:100, Millipore) and goat polyclonal antibody against PDGF-receptor- α (OPC marker, 1:100, R&D). Images were obtained using a fluorescence microscope (Nikon). Quantitative analysis was conducted by counting cells that were doubly-positive for ssDNA and PDGF-receptor-α. The cell count within a 0.25 mm2 section of the lateral corpus callosum was quantified by an investigator blinded to the experimental groups.
Cell culture
Cultured OPCs were prepared from cerebral cortices of 1–2 day old Sprague-Dawley (SD) rats. Dissociated cortex cells were plated in poly-d-lysine-coated flasks, and cultured in Dulbecco's Modified Eagle's medium (DMEM) containing 20% fetal bovine serum (Thermo Fisher Scientific) and 1% penicillin/streptomycin (P/S, Thermo Fisher Scientific). After the cells were confluent, the flasks were shaken for 1 hour on an orbital shaker (220 rpm) at 37°C to remove microglia. The medium was changed and the cells were again shaken overnight, then collected and plated on non-coated tissue culture dishes for 1 hour at 37°C to remove any contaminating astrocytes and microglia. In doing so, the non-adherent cells were enriched for OPCs. They were collected and cultured in Neurobasal Media containing 2 mM glutamine (Thermo Fisher Scientific), 1% P/S, 10 ng/mL PDGF-AA (Peprotech), 10 ng/mL FGF-2 (Peprotech), and 2% B27 supplement (Thermo Fisher Scientific) onto poly-dl-ornithine-coated plates, and used when 70~80% confluent. More than 97% of the plated cells are positive for the OPC marker PDGF-receptor-α, and can differentiate into mature oligodendrocytes in vitro [6]. For cell culture experiments, the stock solution of edaravone was prepared using 100% dimethylsulfoxide (DMSO, Sigma-Aldrich), and was kept at -80C until use. The final concentration of DMSO in culture media was 0.1%, which had no effects on OPC survival and function [23].
Dichlorofluorescein (DCF) assay
The reactive oxygen species (ROS) level in cultured OPCs was quantified by using CM-H2DCFDA assay kit (Thermo Fisher Scientific) according to the manufacturer’s instruction. Briefly, 10 mM CM-H2DCFDA solution was added into the cell culture wells (final concentration: 10 uM). The cells were then incubated for 1 hour at 37°C, and the wavelengths (excitation: 492nm, emission: 520nm) were measured by plate-reader.
Cytotoxicity/Cell death assay
OPC damage in culture was assessed by two approaches - quantification of intracellular lactate dehydrogenease (LDH) activity and direct cell count. LDH measurement was conducted using Cytotoxicity Detection Kit (Roche) for LDH following manufacturer’s instructions. Briefly, cells were incubated with 0.1% Triton X-100 in phosphate buffered saline. After centrifuging at 10,000 rpm for 5 min, supernatants were mixed with LDH reagent. After 10 min incubation at room temperature, the wavelengths (excitation: 490nm, emission: 630nm) were measured by plate-reader. For the direct cell count method, bright-field images of OPC cultures were taken by microscope, and then the number of OPCs was counted by an investigator blinded to the experimental groups.
Statistical analysis
Statistical significance was evaluated using the unpaired or ratio paired t-test to compare differences between the two groups and a one-way ANOVA with post-hoc test for multiple comparisons. Data are expressed as mean ± S. . A p value of <0.05 was considered statistically significant.
3. Results
Edaravone protected OPCs in vivo
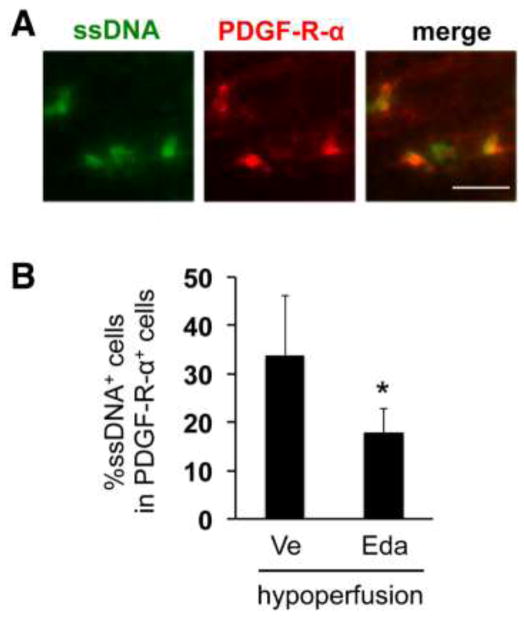
First, we tested the efficacy of edaravone in a mouse model of prolonged hypoperfusion, a clinically-relevant model of white matter pathology in cerebrovascular diseases [11]. To detect damaged cells in cerebral white matter, ssDNA staining was used as a surrogate marker for dead cells. In our previous report, the hypoperfused mice had a larger number of ssDNA positive cells in cerebral white matter 14 days after the onset of cerebral hypoperfusion [21]. In this follow-up study, a similar finding was observed - compared to sham animals, the number of ssDNA-positive cells was larger in the hypoperfused mice (data not shown), and also as reported in our previous study [21], edaravone treatment reduced the number of ssDNA positive cells at day 14 of cerebral hypoperfusion (data not shown). Notably, some OPCs (i.e. PDGF-receptor-α positive cells) in the hypoperfusion group co-localized with ssDNA staining in the corpus callosum (Figure 1A), suggesting that OPCs were indeed damaged in this model. Moreover, our quantitative analysis of ssDNA/PDGF-receptor-α-double positive cells showed that edaravone treatment significantly reduced the number of dead OPCs (Figure 1B).
Figure 1. Edaravone protected OPCs against hypoperfusion stress in vivo.
(A) Representative images of double staining for ssDNA and PDGF-receptor-α in the white matter region (corpus callosum) at day 14 after the bilateral common artery stenosis. ssDNA is a marker for dead/damaged cells. PDGF-receptor-α is a marker for OPCs. Edaravone (3 mg/Kg, i.p.) was treated twice per week for 14 days. Scale bar = 25 μm. (B) Quantitative data for the ratio of ssDNA/PDGF-receptor-α-double positive cells in ssDNA positive cells. Values are mean ± SD from five animals. * <0.05. Ve: vehicle, Eda: edaravone.
Edaravone protected OPCs in vitro
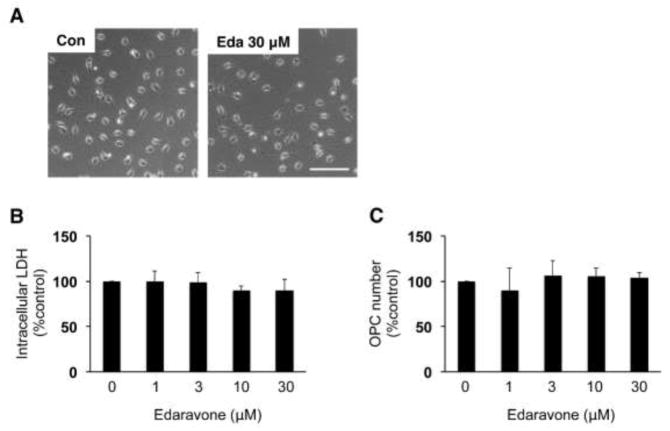
Next, we examined whether edaravone directly suppresses oxidative-stress-induced OPC damage using primary OPC cultures prepared from rat neonatal cortex. In our past studies, we verified that our OPC cultures demonstrate OPC functions, and do not contain significant amounts of other types of cells [5, 6]. Before testing the OPC-protective effects of edaravone in vitro, we checked if edaravone was cytotoxic to OPCs. When the cell density reached 70~80% confluent, the cells were treated with edaravone up to 30 μM. Then 24 hours later, cell toxicity was assessed by measuring intracellular LDH amount or by counting OPC numbers. As expected, edaravone (~ 30 μM) did not induce cytotoxicity in OPC cultures (Figure 2A–C).
Figure 2. Edaravone did not induce cell death in primary rat OPC cultures.
(A) Representative images of OPCs at 24 hours after vehicle or 30 μM edaravone treatment. Scale bar = 100 um. Eda: edaravone. (B, C) Edaravone did not induce OPC damage assessed by intracellular LDH amount (B) and direct cell count (C). Values are mean ± SD from five independent experiments.
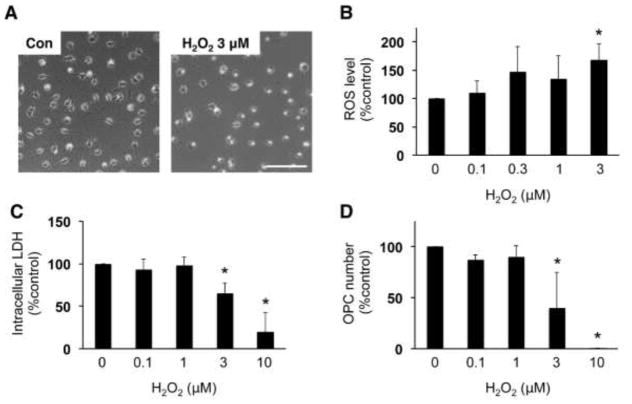
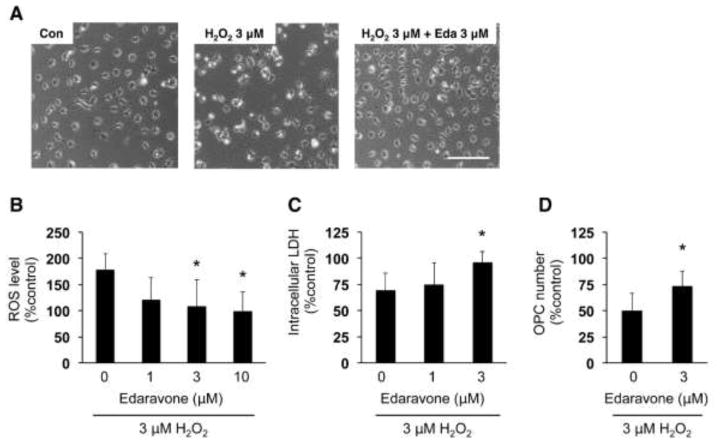
We then examined whether edaravone protected OPCs against oxidative stress. OPCs were exposed to hydrogen peroxide (H2O2), a known inducer of oxidative stress. As expected, H2O2 exposure increased ROS level in the cells (Figure 3B). Correspondingly, H2O2 led to cell damage in OPC cultures in a dose-dependent manner (Figure 3C, D). However, co-treatment with edaravone attenuated the H2O2-induced increase in ROS (Figure 4B) and increased OPC survival (Figure 4C, D).
Figure 3. Hydrogen peroxide (H2O2) increased ROS level and caused cell damage in OPC cultures.
(A) Representative images of OPCs at 24 hours after vehicle or 3 μM H2O2 treatment. Scale bar = 100 um. (B) ROS levels in cultured OPCs were detected by the DCF assay. OPCs were subjected to vehicle, 0.1, 0.3, 1, or 3 μM H2O2. Three hours later, the OPCs were used for DCF assay to measure ROS levels. Values are mean ± SD from five independent experiments. *P<0.05 vs control. (C, D) OPC damage was assessed by measuring intracellular LDH amount (C) and counting OPC numbers (D). OPCs were subjected to vehicle, 0.1, 1, 3, or 10 μM H2O2. Twenty-four hours later, OPCs images were taken for cell counting and then cell culture lysates wereused for LDH assay. Values are mean ± SD from three independent experiments. *P<0.05 vs control.
Figure 4. Edaravone protected OPCs against H2O2-induced oxidative stress.
(A) Representative images of OPCs at 24 hours after H2O2 treatment. Scale bar = 100 um. (B) ROS levels in cultured OPCs were detected by the DCF assay. OPCs were subjected to 3 μM H2O2 with or without edaravone. Three hours later, the OPCs were used for DCF assay to measure ROS levels. The percent of ROS level was calculated based on the control group (e.g. cells without drug treatment). Values are mean ± SD from five independent experiments. *P<0.05 vs H2O2 alone. (C, D) OPC damage was assessed by measuring intracellular LDH amount (C) and counting OPC numbers (D). OPCs were subjected to 3 μM H2O2 with or without edaravone. Twenty-four hours later, OPCs images were taken for cell counting and cell culture lysates were used for LDH assay. The percent of intracellular LDH or OPC number was calculated based on the control group (e.g. cells without drug treatment). Values are mean ± SD from six independent experiments. *P<0.05 vs H2O2 alone.
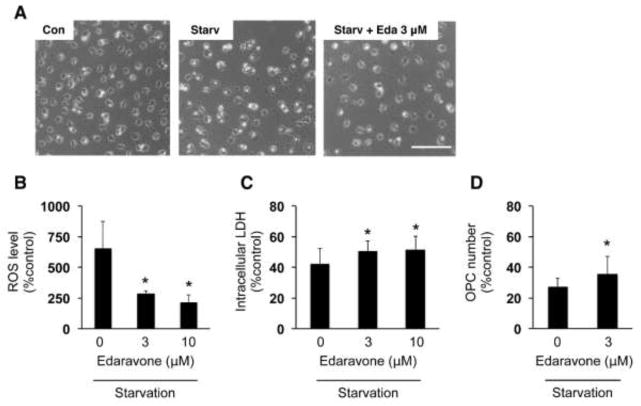
Finally, we also tested whether edaravone protects OPCs against starvation conditions. When growth factors were removed from OPC culture media, cultured OPCs no longer survive. In fact, at 48 hours after the starvation, OPCs exhibited significant cell damage (Figure 5A, C, D). In addition, ROS level of cell culture was also increased under the starvation conditions (Figure 5B). However, co-treatment with edaravone suppressed both OPC damage (Figure 5A, C, D) and ROS accumulation (Figure 5B), confirming again that edaravone protects OPCs from ROS-related cellular damage. These data suggest that edaravone is protective for OPCs against oxidative stress in the setting of different types of oxidative stress inducers.
Figure 5. Edaravone protected OPCs against starvation stress.
(A) Representative images of OPCs at 48 hours after starvation. Scale bar = 100um. (B) ROS levels in cultured OPCs were detected by the DCF assay. OPCs were maintained in growth factor-free culture media with or without edaravone. Forty-eight hours later, t the OPCs were used for DCF assay to measure ROS levels. The percent of ROS level was calculated based on the control group (e.g. cells without drug treatment). Values are mean ± SD from three independent experiments. *P<0.05 vs starvation without edaravone. (C, D) OPC damage was assessed by measuring intracellular LDH amount (C) and counting OPC numbers (D). OPCs were maintained in growth factor-free culture media with or without edaravone. Forty-eight hours later, OPC images were taken for cell counting and cell culture lysates were used for LDH assay. The percent of intracellular LDH or OPC number was calculated based on the control group (e.g. cells without drug treatment). Values are mean ± SD from five (for LDH) or four (for cell counting) independent experiments. *P<0.05 vs starvation without edaravone.
4. Discussion
Our current study demonstrated that a free radical scavenger edaravone protected OPCs against oxidative stress both in vivo and in vitro. Edaravone is a radical scavenging drug that has been clinically used in Japan and South Korea for acute ischemic stroke [1, 16] and was recently approved in the US for use in amyotrophic lateral sclerosis [2, 25]. Thus far, several studies in basic research have reported the neuroprotective effects of edaravone in stroke or other CNS disease models both in vivo [3, 4, 9, 13] and in vitro [7, 14, 18, 27, 28]. Our findings further add to the existing literature documenting the efficacy of edaravone in protecting OPCs against oxidative stress.
OPCs are active during development for generating mature oligodendrocytes. However, some population of OPCs remains in adult brains. These residual OPCs may monitor neighboring environments and proliferate/differentiate as needed for repairing white matter damage [10, 19, 29]. But like neurons, OPCs are also highly susceptible to oxidative stress [8, 20] and easily die under pathological conditions, resulting in poor oligodendrocyte regeneration. Therefore, injury to OPCs would significantly interfere with their ability to mediate the endogenous compensatory responses of proliferating and differentiating into mature oligodendrocytes. In a previous study, we had used the same model of prolonged cerebral hypoperfusion to show that edaravone successfully promoted oligodendrocyte regeneration [21]. Our follow-up study here shows that edaravone protected OPCs against oxidative stress in vitro and in vivo, supporting our hypothesis that the mechanism of action of edaravone in promoting oligodendrogenesis (e.g. proliferation and differentiation) after white matter injury involves the protection of OPCs. Hence, the use of edaravone or other radical scavengers offers a rational therapeutic approach for white matter injury in CNS diseases through enhancing endogenous repairing mechanisms, including OPC proliferation and differentiation.
Although our study provides proof-of-concept that edaravone is protective for OPCs, there are some important caveats and limitations that should be addressed. First, for in vivo experiments, we used the cerebral hypoperfusion model. A mouse model of prolonged cerebral hypoperfusion is clinically relevant, as patients with vascular dementia also suffer from cerebral hypoperfusion [26], and the mice subjected to cerebral hypoperfusion with micro-coils show a similar pattern of white matter dysfunction and cognitive decline [11]. However, whether edaravone can protect OPCs in other disease models, such as stroke, trauma, and intracranial hemorrhage, should be tested in future studies to further evaluate the protective effects of edaravone on OPCs in these diseases. Second, in our in vivo mouse model experiments, we tested the efficacy of edaravone only at one time point (e.g. day 14). Our previous reports (as well as our current study) showed that damaged cells appeared in corpus callosum at least 14 days after the onset of cerebral hypoperfusion [21, 22]. But OPC damage may also occur at earlier or later time points, and it would be ideal to check if edaravone protects OPCs at other time points in future studies. Especially, for the purpose of identifying the edaravone effects on compensatory oligodendrogenesis in this cerebral hypoperfusion model, OPC survival/damage needs to be carefully assessed in longer periods. And finally, our experimental scheme for in vitro cell culture experiments may also have some limitations. Exposure of ROS inducers to a culture system consisting only of OPCs without other types of cells, or removal of supplements from culture media of OPCs, may be an overly artificial and simplistic model for the in vivo situation. Therefore, future studies will be needed to provide utilize more complex cell culture models by using a co-culture system of OPCs together with other neurovascular unit components, or by subjecting OPC cultures to injury conditions that represent additional white matter pathology.
In summary, this proof-of-concept study demonstrates that a radical scavenger edaravone protects OPCs against oxidative stress both in vitro and in vivo models of white matter injury. OPCs play pivotal roles in white matter homeostasis, and cerebral hypoperfusion would lead to white matter damage in stroke and vascular dementia patients. Therefore, anti-oxidant radical scavengers such as edaravone may provide a broad therapeutic approach for white matter related diseases. Acknowledgements: Supported in part by National Institutes of Health, Research Abroad from the Japan Brain Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research, and the Rotary Foundation Global Scholarship Grants (GG1759314).
Highlights.
Edaravone protected OPCs in a mouse model of prolonged cerebral hypoperfusion.
Edaravone did not induce cell death in OPC culture
H2O2 exposure or starvation stress caused ROS accumulation in OPCs
Edaravone protected OPCs against oxidative stress in vitro
Footnotes
Disclosures: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis. 2003;15:222–229. doi: 10.1159/000069318. [DOI] [PubMed] [Google Scholar]
- 2.Abe K, Itoyama Y, Sobue G, Tsuji S, Aoki M, Doyu M, Hamada C, Kondo K, Yoneoka T, Akimoto M, Yoshino H, Edaravone ALSSG. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:610–617. doi: 10.3109/21678421.2014.959024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abe K, Yuki S, Kogure K. Strong attenuation of ischemic and postischemic brain edema in rats by a novel free radical scavenger. Stroke. 1988;19:480–485. doi: 10.1161/01.str.19.4.480. [DOI] [PubMed] [Google Scholar]
- 4.Amemiya S, Kamiya T, Nito C, Inaba T, Kato K, Ueda M, Shimazaki K, Katayama Y. Anti-apoptotic and neuroprotective effects of edaravone following transient focal ischemia in rats. Eur J Pharmacol. 2005;516:125–130. doi: 10.1016/j.ejphar.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 5.Arai K, Lo E. Astrocytes protect oligodendrocyte precursor cells via MEK/ERK and PI3K/Akt signaling. Journal of neuroscience research. 2010;88:758–821. doi: 10.1002/jnr.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arai K, Lo E. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:4351–4356. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Wang S, Ding JH, Hu G. Edaravone protects against MPP+-induced cytotoxicity in rat primary cultured astrocytes via inhibition of mitochondrial apoptotic pathway. J Neurochem. 2008;106:2345–2352. doi: 10.1111/j.1471-4159.2008.05573.x. [DOI] [PubMed] [Google Scholar]
- 8.French HM, Reid M, Mamontov P, Simmons RA, Grinspan JB. Oxidative stress disrupts oligodendrocyte maturation. J Neurosci Res. 2009;87:3076–3087. doi: 10.1002/jnr.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujiwara N, Som AT, Pham LD, Lee BJ, Mandeville ET, Lo EH, Arai K. A free radical scavenger edaravone suppresses systemic inflammatory responses in a rat transient focal ischemia model. Neurosci Lett. 2016 doi: 10.1016/j.neulet.2016.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16:668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ihara M, Taguchi A, Maki T, Washida K, Tomimoto H. A mouse model of chronic cerebral hypoperfusion characterizing features of vascular cognitive impairment. Methods Mol Biol. 2014;1135:95–102. doi: 10.1007/978-1-4939-0320-7_8. [DOI] [PubMed] [Google Scholar]
- 12.Joseph MJ, Caliaperumal J, Schlichter LC. After Intracerebral Hemorrhage, Oligodendrocyte Precursors Proliferate and Differentiate Inside White-Matter Tracts in the Rat Striatum. Transl Stroke Res. 2016;7:192–208. doi: 10.1007/s12975-015-0445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawai H, Nakai H, Suga M, Yuki S, Watanabe T, Saito KI. Effects of a novel free radical scavenger, MCl-186, on ischemic brain damage in the rat distal middle cerebral artery occlusion model. J Pharmacol Exp Ther. 1997;281:921–927. [PubMed] [Google Scholar]
- 14.Kawasaki T, Kitao T, Nakagawa K, Fujisaki H, Takegawa Y, Koda K, Ago Y, Baba A, Matsuda T. Nitric oxide-induced apoptosis in cultured rat astrocytes: protection by edaravone, a radical scavenger. Glia. 2007;55:1325–1333. doi: 10.1002/glia.20541. [DOI] [PubMed] [Google Scholar]
- 15.Kikuchi K, Kawahara K, Tancharoen S, Matsuda F, Morimoto Y, Ito T, Biswas KK, Takenouchi K, Miura N, Oyama Y, Nawa Y, Arimura N, Iwata M, Tajima Y, Kuramoto T, Nakayama K, Shigemori M, Yoshida Y, Hashiguchi T, Maruyama I. The free radical scavenger edaravone rescues rats from cerebral infarction by attenuating the release of high-mobility group box-1 in neuronal cells. J Pharmacol Exp Ther. 2009;329:865–874. doi: 10.1124/jpet.108.149484. [DOI] [PubMed] [Google Scholar]
- 16.Lapchak PA. A critical assessment of edaravone acute ischemic stroke efficacy trials: is edaravone an effective neuroprotective therapy? Expert Opin Pharmacother. 2010;11:1753–1763. doi: 10.1517/14656566.2010.493558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lapchak PA, Araujo DM. Development of the nitrone-based spin trap agent NXY-059 to treat acute ischemic stroke. CNS Drug Rev. 2003;9:253–262. doi: 10.1111/j.1527-3458.2003.tb00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee BJ, Egi Y, van Leyen K, Lo EH, Arai K. Edaravone, a free radical scavenger, protects components of the neurovascular unit against oxidative stress in vitro. Brain research. 1307:22–27. doi: 10.1016/j.brainres.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maki T, Liang AC, Miyamoto N, Lo EH, Arai K. Mechanisms of oligodendrocyte regeneration from ventricular-subventricular zone-derived progenitor cells in white matter diseases. Front Cell Neurosci. 2013;7:275. doi: 10.3389/fncel.2013.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto N, Maki T, Pham LD, Hayakawa K, Seo JH, Mandeville ET, Mandeville JB, Kim KW, Lo EH, Arai K. Oxidative stress interferes with white matter renewal after prolonged cerebral hypoperfusion in mice. Stroke. 2013;44:3516–3521. doi: 10.1161/STROKEAHA.113.002813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyamoto N, Pham LD, Hayakawa K, Matsuzaki T, Seo JH, Magnain C, Ayata C, Kim KW, Boas D, Lo EH, Arai K. Age-related decline in oligodendrogenesis retards white matter repair in mice. Stroke. 2013;44:2573–2578. doi: 10.1161/STROKEAHA.113.001530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyamoto N, Pham LD, Maki T, Liang AC, Arai K. A radical scavenger edaravone inhibits matrix metalloproteinase-9 upregulation and blood-brain barrier breakdown in a mouse model of prolonged cerebral hypoperfusion. Neurosci Lett. 2014;573:40–45. doi: 10.1016/j.neulet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morihara R, Kono S, Sato K, Hishikawa N, Ohta Y, Yamashita T, Deguchi K, Manabe Y, Takao Y, Kashihara K, Inoue S, Kiriyama H, Abe K. Thrombolysis with Low-Dose Tissue PlasminogenActivator 3-4.5 hAfterAcute Ischemic Stroke in Five Hospital Groups in Japan. Transl Stroke Res. 2016;7:111–119. doi: 10.1007/s12975-016-0448-8. [DOI] [PubMed] [Google Scholar]
- 25.Nagase M, Yamamoto Y, Miyazaki Y, Yoshino H. Increased oxidative stress in patients with amyotrophic lateral sclerosis and the effect of edaravone administration. Redox Rep. 2016;21:104–112. doi: 10.1179/1351000215Y.0000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao H, Sadoshima S, Kuwabara Y, Ichiya Y, Fujishima M. Cerebral blood flow and oxygen metabolism in patients with vascular dementia of the Binswanger type. Stroke. 1990;21:1694–1699. doi: 10.1161/01.str.21.12.1694. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida H, Yanai H, Namiki Y, Fukatsu-Sasaki K, Furutani N, Tada N. Neuroprotective effects of edaravone: a novel free radical scavenger in cerebrovascular injury. CNS Drug Rev. 2006;12:9–20. doi: 10.1111/j.1527-3458.2006.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan WJ, Yasuhara T, Shingo T, Muraoka K, Agari T, Kameda M, Uozumi T, Tajiri N, Morimoto T, Jing M, Baba T, Wang F, Leung H, Matsui T, Miyoshi Y, Date I. Neuroprotective effects of edaravone-administration on 6-OHDA-treated dopaminergic neurons. BMC Neurosci. 2008;9:75. doi: 10.1186/1471-2202-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang R, Chopp M, Zhang ZG. Oligodendrogenesis after cerebral ischemia. Front Cell Neurosci. 2013;7:201. doi: 10.3389/fncel.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]