Abstract
Loss of function mutations in the gene ATP13A2 are associated with Kufor-Rakeb Syndrome and Neuronal Ceroid Lipofuscinosis, the former designated as an inherited form of Parkinson’s disease (PD). The function of ATP13A2 is unclear but in vitro studies indicate it is a lysosomal protein and may interact with the presynaptic protein alpha-synuclein (aSyn) and certain heavy metals. Accumulation of aSyn is a major component of lewy bodies, the pathological hallmark of PD. Atp13a2-deficient (13a2) mice develop age-dependent sensorimotor deficits, and accumulation of insoluble aSyn in the brain. To better understand the interaction between ATP13A2 and aSyn, double mutant mice with loss of Atp13a2 function combined with overexpression of human wildtype aSyn were generated. Female and male wildtype (WT), 13a2, aSyn, and 13a2-aSyn mice were tested on a battery of sensorimotor tests including adhesive removal, challenging beam traversal, spontaneous activity, gait, locomotor activity, and nest-building at 2, 4, and 6 months of age. Double mutant mice showed an earlier onset and accelerated alterations in sensorimotor function that were age, sex and test-dependent. Female 13a2-aSyn mice showed early and progressive dysfunction on the beam and in locomotor activity. In males, 13a2-aSyn mice showed more severe impairments in spontaneous activity and adhesive removal. Sex differences were also observed in aSyn and 13a2-aSyn mice on the beam, cylinder, and adhesive removal tests. In other tasks, double mutant mice displayed deficits similar to aSyn mice. These results indicate loss of Atp13a2 function exacerbates the sensorimotor phenotype in aSyn mice in an age and sex-dependent manner.
Keywords: ATP13A2, alpha-synuclein, mice, sensorimotor, phenotype
1. Introduction
ATP13A2 is a P5-ATPase located on lysosomes whose physiological function and substrate specificity are unclear [1–3]. Studies suggest it is involved in multiple cellular functions including heavy metal homeostasis, lysosomal and endo-lysosomal proteostasis, and mitochondrial homeostasis [4–12]. Loss of function mutations in ATP13A2 are associated with several neurodegenerative conditions including Kufor-Rakeb Syndrome (KRS), neuronal ceroid lipofuscinosis (NCL), and most recently complicated hereditary spastic paraplegia [3,13,14]. KRS is classified as an inherited form of Parkinson’s disease (PD; PARK9), the most common neurodegenerative movement disorder [3]. In vitro studies focusing on the link with PD show loss of function of ATP13A2 in fibroblasts and dopamine neurons leads to multiple lysosomal defects [2,5,6]. In conjunction with the lysosomal defects, loss of ATP13A2 expression is shown to increase aSyn accumulation and toxicity, mitochondrial fragmentation, and production of reactive oxygen species [5–9,15,16]. Studies also involve ATP13A2 in the exosomal externalization of aSyn, indicating a potentially important role for ATP13A2 in PD [11,12].
Within the ATP13A2-associated diseases there is substantial heterogeneity in age of onset, severity, and symptom profile [3,13,14]. For example, KRS can include bradykinesia and rigidity similar to PD but also ataxia, dementia, spasticity, and supranuclear gaze palsy [3]. In NCL, common clinical features include cerebellar ataxia, dementia, seizures, and visual impairment [13]. Complicated hereditary spastic paraplegia can also include dementia, ataxia, seizures, and extrapyramidal symptoms in addition to lower limb spasticity [17]. Age of onset can vary from juvenile (age <21 years old) to young onset (age 21–40 years old) [13,18,19]. It has also been suggested that loss or knockdown of ATP13A2 may modify PD onset and risk [20]. Indeed, a recent study showed ATP13A2 variants are common in leucine-rich repeat kinase 2 (LRRK2) PD cases, the most common genetic form of PD, and may hasten disease onset and/or progression [21].
While it is generally considered that the majority of PD cases are caused by gene-environment interactions, gene-gene interactions may also be important in influencing disease onset and progression [21, 22]. Examples of known gene-gene interactions in PD include Parkin and PINK1, LRRK2 and Parkin, VPS35 and EIF4G1, GBA and SNCA (aSyn), ATP13A2 and SNCA, and most recently ATP13A2 and LRRK2 [4,21–27]. In vitro studies establishing a relationship between loss of function of ATP13A2 and aSyn are compelling [4–6]. However, the few in vivo studies to date show inconsistent results regarding ATP13A2 and aSyn accumulation [28–31]. Viral overexpression of ATP13A2 and aSyn did not protect against aSyn toxicity in the substantia nigra in rats [29]. Differential effects are also observed in ATP13A2 null (13a2) mouse lines, as one study found abnormal aSyn accumulation in the brain while the other did not [28,30]. The mouse line with increased abnormal aSyn in the brain also exhibited increased triton-insoluble aSyn in the ventral midbrain in response to systemic manganese administration [31]. In contrast, in the 13a2 mouse line that did not show abnormal accumulation of aSyn in the brain, overexpression of mutant A53T aSyn through crossbreeding did not exacerbate pathology measured [30]. However, the timing (ATP13A2 may need to precede aSyn overexpression) and level of overexpression of ATP13A2 are likely important determinants in viral vector studies and in crossbreeding studies the promoter and type of aSyn being expressed (mutated or wildtype) are known to yield differential phenotypes and pathology [32].
The present study sought to determine the behavioral effect of loss of Atp13a2 function in mice that overexpress human WT aSyn under the thy1 promoter [28,33–37]. Here, 13a2 and aSyn overexpressing mice, both backcrossed onto a C57Bl/6 background, were crossbred to create double mutant 13a2-aSyn mice. Female and male wildtype (WT), 13a2, aSyn, and 13a2-aSyn mice were tested at 2, 4, and 6 months of age on a battery of sensorimotor tests shown to be sensitive in both 13a2 and aSyn mice [28,34]. The effect of the double mutation on sensorimotor performance, aging, and sex was measured.
2. Materials and methods
2.1 Animals
Animal care was conducted in accordance with the United States Public Health Service Guide for the Care and Use of Laboratory Animals, and procedures were pre-approved by the Institutional Animal Care and Use Committee at Northeast Ohio Medical University and University of Cincinnati. Mutant 13a2 mice backcrossed onto a C57Bl/6 background were bred with aSyn mice also backcrossed onto C57Bl/6 to create 13a2 heterozygous and aSyn overexpressing mice (13a2 het-aSyn) [28,33]. Mutant 13a2 het-aSyn mice were then bred with 13a2 het mice to create 13a2-aSyn double mutant mice. Animals were maintained on the C57BL/6-background and littermates were never bred together. The genotype of all WT, 13a2, aSyn, and 13a2-aSyn mice was confirmed by polymerase chain reaction (PCR) amplification analysis of DNA from tail tips at weaning and euthanasia. Female and male mice from 36 litters were included in the study. The n-size for female mice ranged from 13–15 mice per genotype and for males there were 13–14 mice per genotype for a total of 108 mice. All mice were group housed and provided free access to water and standard rodent chow throughout the experiment except during behavioral testing procedures. Animals were maintained on a reverse light/dark cycle with lights off at 10 am and lights on at 10 pm. Behavioral procedures were conducted during the dark cycle under red or low light.
2.2 Sensorimotor Tests
All mice were tested for adhesive removal, motor performance and coordination, spontaneous activity, gait, locomotor activity, and nest building in that order. Potential stress induced by handling and restraint in some tests and the impact of multiple tests was taken into consideration when establishing test order. Mice were run on multiple tests in one day but no more than three tests were performed over the course of the day (ex. beam, cylinder, and gait). Depending on the cohort size, the time between tests was approximately 15–25 minutes. Nest building was always performed last because it requires mice to be individually housed and that could influence performance on some tests such as the activity tests. Mice were tested at 2, 4, and 6 months of age. Body weight and temperature were also measured at each age. Testing was performed over a 4–7 day period.
2.2.1 Adhesive Removal
Motor response to sensory stimuli was measured using the adhesive removal test [34]. Small adhesive stimuli (Avery adhesive-backed labels, one-quarter inch round) were placed on the snout of the mouse, and the time to remove the stimulus was recorded. Each animal received three trials, and the trials were alternated between mice, so that each mouse had an intertrial interval of at least two min. All testing was performed in the animal’s home-cage, and cage mates and enrichment were temporarily removed during testing because they can interfere with stimulus removal. If the animal did not remove the stimulus within 60 sec, the experimenter removed it, and the trial for the next mouse was initiated. Stimulus removal time was calculated for each animal.
2.2.2 Challenging Beam
Motor performance and coordination was measured with the challenging beam traversal test [28,34]. Briefly, the beam consists of four sections (25 cm each, total length= 1 meter), each section having a different width. The beam starts at a width of 3.5 cm and gradually narrows by one cm increments to a final width of 0.5 cm. Animals were trained to traverse the length of the beam starting at the widest section and ending at the narrowest section. Animals received two days of training prior to testing; on the day of the test a mesh grid (one cm squares) of corresponding width was placed over the beam surface leaving approximately a one cm space between the grid and the beam surface. Animals were then videotaped while traversing the grid-surfaced beam and videos were rated on slow motion by an experimenter blinded to genotype. Errors per step, steps, and time to traverse the beam were measured across five trials and the means of those trials were included in the analysis.
2.2.3 Spontaneous Activity
Spontaneous movements of the mice were measured in a small, transparent cylinder 15.5 cm high and 12.7 cm in diameter [28,34]. The cylinder was placed on a piece of glass with a mirror positioned at an angle beneath the cylinder to allow a clear view of movements along the ground and walls. Spontaneous movements were recorded for 3 minutes. Videotapes were viewed and rated in slow motion by an experimenter blinded to mouse genotype. The number of rears, forelimb and hindlimb steps, and time spent grooming were determined for each mouse.
2.2.4 Gait
To measure gait, animals were trained to walk through a narrow alley leading into their home-cage. Once trained, paper was placed along the alley floor and each animal’s hindlimbs were brushed with non-toxic paint (Crayola©, Easton, PA). Animals were then placed at the beginning of the alley. As they walked into their home-cage they left their paw prints on the paper underneath [28,34,38]. Stride length was determined by measuring the distance between hindlimb prints. Only strides made while continuously walking (no stopping) were included in the analysis. Stride lengths at the beginning and end of the alley were not counted since animals tend to make irregular steps at the beginning and typically stop and make smaller steps just before entering the cage.
2.2.5 Locomotor Activity
Locomotor activity was measured in an open field arena (56×35×19 cm) similar to previous studies in the aSyn mouse line [39,40]. The floor of the open field bin was marked with equal sized grid squares in order to determine the amount of locomotor movement by each mouse (grid or line crosses) in a 15-minute period. The number of line crosses and rears were measured for each mouse.
2.2.6 Nest Building
To measure nest building, pre-weighed cotton was placed into the feeder bin of the cage in individually housed mice [28,34]. Mice then pull the nesting material from the feeder and build a nest. The amount of cotton remaining in the feeder was measured after 24 hours. Percent cotton used was determined for each mouse.
2.3 Statistics
ANOVA and non-parametric statistics were used to compare the effect of genotype and age on behavior. For data that met the assumptions of ANOVA, a 4X3 mixed design ANOVA with genotype (WT, 13a2, aSyn, and 13a2-aSyn) as the between factor and age (2, 4, 6 months of age) as the repeated factor was used. For analyses comparing sex, a 2X3 mixed design ANOVA with sex as the between factor and age as the repeated factor was used for each genotype. Tukey’s Honest Significant Difference (HSD) was used for all post hoc analyses. For data that did not meet the assumptions of ANOVA (homogeneity of variance, normal distribution, and independence of sample), non-parametric tests were used and included Mann-Whitney U for comparisons between groups and Wilcoxon Sign Rank for within groups. The level of significance was set a p<0.05. All statistics were calculated using MATLAB or StatPlus®: Mac 2009.
3. Results
3.1 Body weight and temperature
Body weights were measured at 2, 4, and 6 months of age for both female and male mice (Table 1). For females, all genotypes gained weight as they aged and there were no significant differences between genotypes. Similar to previous studies in male aSyn mice, there were significant differences between genotypes (Genotype main effect: F [3,49]=29.83, p<0.01), with aSyn mice weighing significantly less than WT mice at each age [34,41]. In addition, at the 6m age 13a2-aSyn mice also weighed significantly less than WT mice. Body temperatures did not differ between genotypes or across age in either female or male mice (Table 1).
Table 1.
Body weight and temperature in female and male WT, 13a2, aSyn, ad 13a2-aSyn mice.
| Body Weight | ||||
|---|---|---|---|---|
| Females | ||||
| Age (m) | WT | 13a2 | aSyn | 13a2-aSyn |
| 2 | 19.91 ± 0.44 | 20.08 ± 0.39 | 19.92 ± 0.37 | 19.92 ± 0.44 |
| 4 | 24.38 ± 0.76 | 24.82 ± 0.74 | 26.26 ± 0.86 | 26.16 ± 0.88 |
| 6 | 28.03 ± 1.11 | 27.92 ± 0.99 | 31.43 ± 1.15 | 29.84 ± 1.30 |
| Males | ||||
| Age (m) | WT | 13a2 | aSyn | 13a2-aSyn |
| 2 | 25.38 ± 0.44 | 24.69 ± 0.33 | 22.64 ± 0.44*** | 23.84 ± 0.49 |
| 4 | 31.01 ± 0.55 | 31.83 ± 0.70 | 27.42 ± 0.54*** | 28.75 ± 0.62 |
| 6 | 36.03 ± 0.71 | 36.69 ± 0.85 | 27.25 ± 0.81*** | 26.63 ± 0.96*** |
|
Body Temperature
| ||||
| Females | ||||
| Age (m) | WT | 13a2 | aSyn | 13a2-aSyn |
| 2 | 38.40 ± 0.18 | 38.36 ± 0.18 | 37.18 ± 0.50 | 38.46 ± 0.15 |
| 4 | 38.10 ± 0.16 | 38.23 ± 0.15 | 38.09 ± 0.15 | 38.35 ± 0.16 |
| 6 | 38.31 ± 0.21 | 38.10 ± 0.17 | 38.27 ± 0.14 | 38.13 ± 0.14 |
| Males | ||||
| Age (m) | WT | 13a2 | aSyn | 13a2-aSyn |
| 2 | 37.92 ± 0.26 | 38.27 ± 0.17 | 37.87 ± 0.22 | 37.94 ± 0.30 |
| 4 | 38.08 ± 0.22 | 37.74 ± 0.26 | 38.22 ± 0.24 | 37.61 ± 0.24 |
| 6 | 38.09 ± 0.21 | 37.58 ± 0.41 | 37.81 ± 0.30 | 37.07 ± 0.21 |
Mean ± SEM, 4X3 Mixed design ANOVA and Tukey’s HSD post hoc.
3.2 Sensorimotor tests
Below, the behavioral results are organized according to 1) tests that show an acceleration of behavioral anomalies in females or males, 2) tests where behavioral alterations are similar between aSyn and 13a2-aSyn mice, and 3) tests where male mice performed worse than female mice. Tables S1 and S2 show the results of the parametric analyses and include main and interaction effects, degrees of freedom, F-values, and p-values for analyses in female (Table S1) and male (Table S2) mice.
3.2.1 Exacerbation of sensorimotor deficits
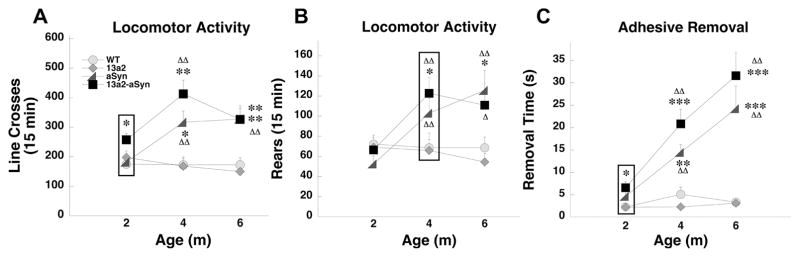
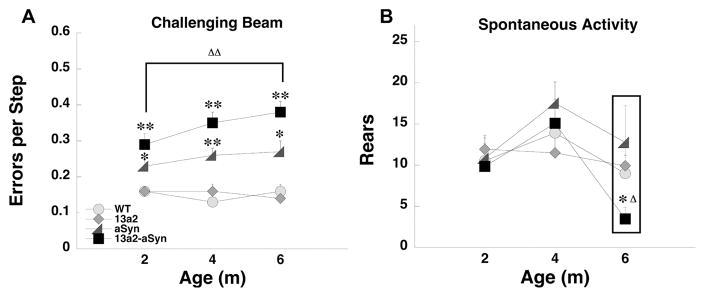
In several tests female and male 13a2-aSyn mice either displayed behavioral anomalies earlier or the alterations progressed more than the other genotypes. For earlier onset, in females in the locomotor activity test, 13a2-aSyn mice made more line crosses compared to WT mice at 2m of age (Figure 1A). Rearing in females in the locomotor activity test was also significantly increased in female 13a2-aSyn mice compared to WT at 4m of age (Figure 1B). In males, adhesive removal latency was significantly longer in 13a2-aSyn compared to WT mice at 2m (Figure 1C). For accelerated progression, in females on the challenging beam, aSyn and 13a2-aSyn mice made significantly more errors per step compared to WT mice at 2m of age; however, the impairment significantly progressed with age only in 13a2-aSyn mice (Figure 2A). In males in the cylinder test of spontaneous activity, rearing did not differ at 2 and 4m of age between male genotypes. However, at 6m, male 13a2-aSyn mice made significantly fewer rears compared to the other genotypes and compared to the earlier 4m testing (Figure 2B).
Figure 1.
Early behavioral anomalies in 13a2-aSyn female and male mice. In the locomotor activity test, female 13a2-aSyn mice made more locomotor movements compared to WT mice at 2m (A) and made more rears compared to WT at 4m (B). In the adhesive removal test (C), male 13a2-aSyn removal latency was increased compared to WT at 2m. Boxes highlight behavioral alterations earlier in 13a2-aSyn mice. *, **, *** represents p<0.05, 0.01,0.001 respectively, compared to WT at same age. ΔΔ represents p<0.01 compared to 2m within the same genotype. 4×3 Mixed design ANOVA, Tukey’s HSD post hoc. N-sizes= 13–15 per genotype.
Figure 2.
Progression of motor alterations in 13a2-aSyn female and male mice. On the challenging beam (A), errors per step increased with age in female 13a2-aSyn mice. For spontaneous activity (B) rears decreased with age in male 13a2-aSyn mice. *, ** represents p<0.05, 0.01, respectively, compared to WT at same age. Δ, ΔΔ represents p< 0.05, 0.01 compared to 2m within the same genotype. 4×3 Mixed design ANOVA, Tukey’s HSD post hoc. N-sizes= 14–15 per genotype.
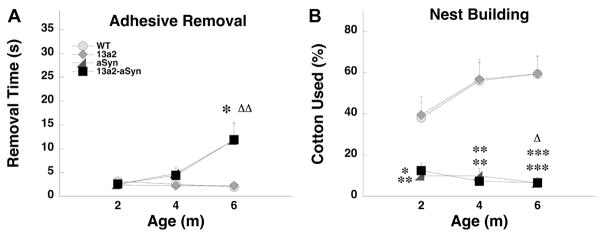
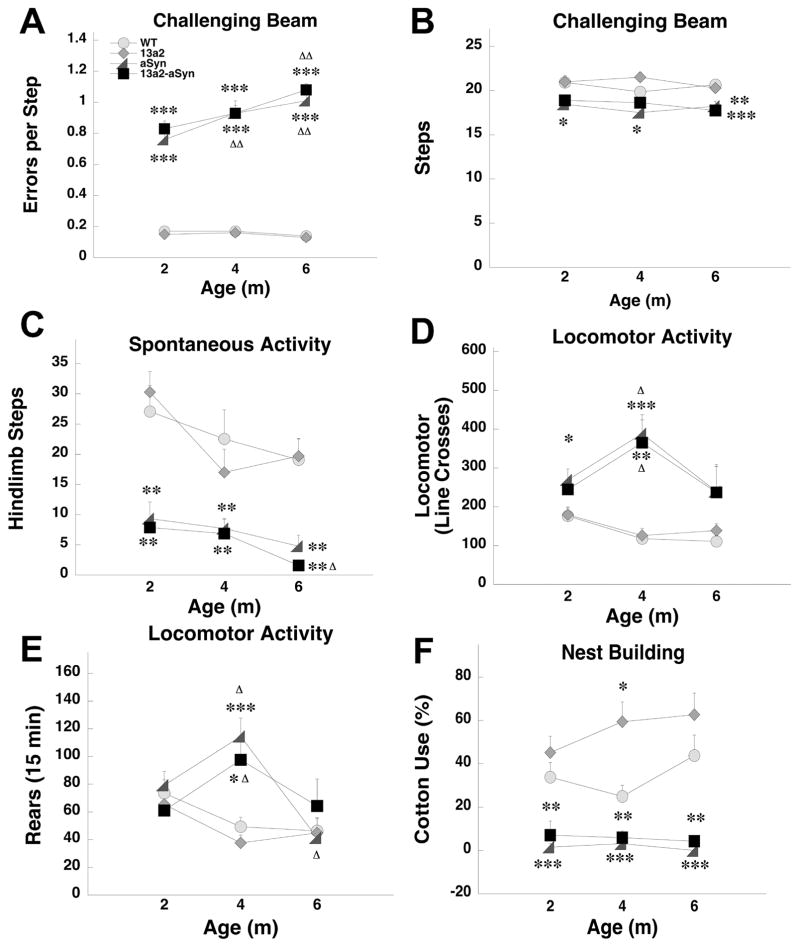
3.2.2 Comparable alterations in aSyn and 13a2-aSyn mice
In several of the behavioral outcomes female or male aSyn and 13a2-aSyn mice showed similar changes in behavior. For females, latency to remove a stimulus was significantly increased in both aSyn and 13a2-aSyn mice compared to WT mice at 6m of age (Figure 3A). In nestbuilding, female aSyn and 13a2-aSyn mice used significantly less cotton compared to WT mice at each age tested (Figures 3B). For males, on the challenging beam, aSyn and 13a2-aSyn mice made significantly more errors per step compared to WT mice at each age tested. Both genotypes also showed significant progression with errors per step significantly increasing at 4m of age in aSyn and 6m of age in 13a2-aSyn mice (Figure 4A). For steps on the beam, male aSyn mice made fewer steps compared to WT at each age tested, while 13a2-aSyn male mice made fewer steps compared to WT at 6m of age (Figure 4B). In hindlimb stepping, male aSyn and 13a2-aSyn made significantly fewer hindlimb steps compared to WT at each age tested. Further, at 6m, hindlimb stepping decreased even more in 13a2-aSyn mice compared to the earlier age (Figure 4C). In open field, male aSyn mice made more line crosses compared to WT at 2 and 4m and 13a2-aSyn mice made more line crosses at 4m. Both male aSyn and 13a2-aSyn mice increased activity at 4m compared to 2m (Figure 4D). Similarly, rearing in the open field was increased in aSyn and 13a2-aSyn at 4m of age compared WT and compared to their 2m testing (Figure 4E). Finally, male aSyn and 13a2-aSyn mice used significantly less cotton compared to WT mice at each age tested similar to females (Figures 4F).
Figure 3.
Double mutant 13a2-aSyn female mice have sensorimotor deficits comparable to aSyn mice in some tests. In the adhesive removal test (A) both aSyn and 13a2-aSyn female mice had increased removal latencies at 6m. Similarly, in nestbuilding (B), aSyn and 13a2-aSyn female mice used less cotton for nesting. *, **, *** represents p<0.05, 0.01, 0.001, respectively, compared to WT at same age. Δ, ΔΔ represents p< 0.05, 0.01, respectively compared to 2m within the same genotype. 4×3 Mixed design ANOVA, Tukey’s HSD post hoc or Mann-Whitney U and Wilcoxon Sign Rank. N-sizes= 13–15 per genotype.
Figure 4.
Double mutant 13a2-aSyn male mice also have sensorimotor deficits comparable to aSyn mice in some tests. These include errors per step and steps on the challenging beam (A and B), hindlimb stepping in the cylinder (C), locomotor activity (D and E), and nestbuilding (F). *, **, *** represents p<0.05, 0.01, 0.001 respectively, compared to WT at same age. Δ, ΔΔ represents p< 0.05, 0.01, respectively compared to 2m within the same genotype. 4×3 Mixed design ANOVA, Tukey’s HSD post hoc or Mann-Whitney U and Wilcoxon Sign Rank. N-sizes= 14–15 per genotype.
3.2.3 Sex differences
Sex differences have been shown in the thy1-aSyn (Line 61) mouse line previously and were detected in the present study [42]. On the challenging beam, male aSyn and 13a2-aSyn mice made significantly more errors per step at each age compared to female aSyn and 13a2-aSyn mice (Table 2). There were no sex differences in beam performance in WT or 13a2 mice. In the cylinder test of spontaneous activity, hindlimb stepping values were significantly lower in male aSyn and 13a2-asyn mice compared to females of the same genotype (Table 2). Lastly, in the adhesive removal test, removal latencies were longer in male 13a2-aSyn mice compared to female 13a2-aSyn mice at each age tested and male aSyn mice displayed longer removal latencies compared to female aSyn mice at 4m of age (Table 2).
Table 2.
Sex differences in aSyn and 13a2-aSyn mice.
| Test | Age (m) | Females | Males | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| WT | 13a2 | aSyn | 13a2-aSyn | WT | 13a2 | aSyn | 13a2-aSyn | ||
|
|
|||||||||
| Beam: Errors per Step | 2 | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.23 ± 0.02 | 0.29 ± 0.03 | 0.17 ± 0.01 | 0.15 ± 0.02 | 0.76 ± 0.05*** | 0.83 ± 0.05*** |
| 4 | 0.13 ± 0.02 | 0.16 ± 0.02 | 0.26 ± 0.02 | 0.35 ± 0.03 | 0.17 ± 0.02 | 0.16 ± 0.02 | 0.93 ± 0.05*** | 0.93 ± 0.08*** | |
| 6 | 0.16 ± 0.02 | 0.14 ± 0.02 | 0.27 ± 0.03 | 0.38 ± 0.03 | 0.14 ± 0.01 | 0.13 ± 0.01 | 1.01 ± 0.08*** | 1.08 ± 0.04*** | |
|
| |||||||||
| Cylinder: Hindlimb Steps | 2 | 33.67 ± 5.65 | 33.77 ± 4.74 | 17.69 ± 1.93 | 26.07 ± 6.66 | 27.08 ± 4.31 | 30.29 ± 3.38 | 9.31 ± 2.79* | 7.85 ± 1.63* |
| 4 | 28.80 ± 5.70 | 25.31 ± 4.22 | 15.08 ± 2.25 | 17.79 ± 1.97 | 22.54 ± 4.82 | 17.00 ± 3.86 | 7.69 ± 1.51* | 6.85 ± 2.46*** | |
| 6 | 25.33 ± 4.04 | 28.15 ± 5.47 | 25.31 ± 5.96 | 22.00 ± 4.39 | 19.08 ± 3.52 | 19.71 ± 2.82 | 4.77 ± 1.80** | 1.58 ± 0.65*** | |
|
| |||||||||
| Adhesive Removal | 2 | 3.13 ± 0.66 | 2.36 ± 0.42 | 2.46 ± 0.41 | 2.57 ± 0.71 | 2.26 ± 0.27 | 2.24 ± 0.43 | 4.64 ± 0.98 | 6.56 ± 1.36* |
| 4 | 2.51 ± 0.60 | 2.20 ± 0.33 | 4.80 ± 1.15 | 4.40 ± 0.69 | 5.10 ± 1.60 | 2.29 ± 0.41 | 14.4 ± 1.74*** | 20.85 ± 3.27*** | |
| 6 | 1.93 ± 0.20 | 2.20 ± 0.30 | 11.80 ± 3.63 | 11.88 ± 3.29 | 3.36 ± 0.53 | 3.12 ± 0.60 | 24.23 ± 5.10 | 31.59 ± 5.21** | |
Mean ± SEM shown. WT= wildtype, 13a2= ATP13A2 knockout mice, aSyn= alpha-synuclein overexpressing, and 13a2-aSyn= double mutant ATP13A2 knockout with overexpression of alpha-synuclein.
represents p<0.05, 0.01, 0.001 respectively compared to Females of the same genotype and age. 2X3 Mixed Design ANOVA, Tukey’s HSD post hoc.
3.2.4 Additional Outcomes
For spontaneous activity in the cylinder in females, there were no significant differences between genotypes or age in rears, hindlimb steps, and groom time (Tables S1 and S3). In forelimb stepping, there was a main effect of genotype and interaction in females, with most mice showing a decrease in forelimb stepping with repeated testing due to habituation except for the aSyn mice (Table S3). In males, a similar habituation effect in forelimb stepping was observed in each genotype (Table S4). In addition, 13a2-aSyn male mice made fewer forelimb steps at 6m compared to WT at the same age (Tables S2 and S4). A decrease in grooming was detected in male aSyn and 13a2-aSyn mice at 2m and at 6m only aSyn mice differed from WT mice (Table S4).
In the challenging beam test, time to traverse on the beam did not differ between genotypes or with age in females (Table S5). For males on the beam, aSyn mice at 6m of age took longer to traverse the beam than WT mice (Table S5). For number of steps on the beam in females, there were main effects of genotype and age and WT mice showed a decrease in steps at 6m of age (Table S5).
Gait was measured in all mice. Stride length in females and males did not differ between genotypes or age (Table S6). For maximum stride difference, there was a significant interaction in females but no significant differences between WT and the other genotypes. Female 13a2 mice did show a significant increase in stride difference at 6m of age (Table S6). There were no effects on stride difference in male mice.
Tables 3 and 4 summarize all the behavioral outcomes in female (Table 3) and male (Table 4) mice.
Table 3.
Summary of sensorimotor alterations in female 13a2, aSyn, ad 13a2-aSyn mice.
| Sensorimotor Test | 2m | 4m | 6m | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 13a2 | aSyn | 13a2 aSyn | 13a2 | aSyn | 13a2 aSyn | 13a2 | aSyn | 13a2 aSyn | |
| Adhesive Removal | |||||||||
| Removal latency | = | = | = | = | = | = | = | ↑ | ↑ |
| ⇑ | ⇑ | ||||||||
| Challenging Beam | |||||||||
| Errors per | = | ↑ | ↑↑ | = | ↑↑ | ↑↑ | = | ↑ | ↑↑ |
| Step | ⇑ | ||||||||
| Time | = | = | = | = | = | = | = | = | = |
| Steps | = | = | = | = | = | = | = | = | = |
| Spontaneous Activity (Cylinder) | |||||||||
| Rears | = | = | = | = | = | = | = | = | = |
| FLSteps | = | = | = | = | = | = | = | = | = |
| HLSteps | = | = | = | = | = | = | = | = | = |
| Grooming | = | = | = | = | = | = | = | = | = |
| Gait | |||||||||
| Stride Length | = | = | = | = | = | = | = | = | = |
| Maximum difference | = | = | = | = | = | = | ⇑ | = | = |
| Locomotor Activity (Open Field) | |||||||||
| Line Crosses | = | = | ↑ | = | ↑↑ | ↑↑ | = | ↑↑ | ↑↑ |
| ⇑⇑ | ⇑⇑ | ⇑ | |||||||
| Rears | = | = | = | = | = | ↑ | = | ↑ | |
| ⇑⇑ | ⇑⇑ | ⇑⇑ | ⇑ | ||||||
| Nestbuilding | |||||||||
| % Cotton Used | = | ↓↓ | ↓ | = | ↓↓ | ↓↓ | = | ↓↓↓ | ↓↓↓ |
| ⇓ | |||||||||
↑, ↑↑, ↑↑↑ represents significant increase at p<0.05, 0.01, 0.001, respectively, compared to WT at the same age. ↓, ↓↓, ↓↓↓ represents significant decrease at p<0.05, 0.01, 0.001, respectively, compared to WT at the same age. ⇑, ⇑⇑ represents significant increase at p<0.05, 0.01, respectively, compared to same genotype at 2m. ⇓, ⇓⇓ represents significant decrease at p<0.05, 0.01, respectively, compared to same genotype at 2m.
Table 4.
Summary of sensorimotor alterations in male 13a2, aSyn, ad 13a2-aSyn mice.
| Sensorimotor Test | 2m | 4m | 6m | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 13a2 | aSyn | 13a2 aSyn | 13a2 | aSyn | 13a2 aSyn | 13a2 | aSyn | 13a2 aSyn | |
| Adhesive Removal | |||||||||
| Removal latency | = | = | ↑ | = | ↑↑ | ↑↑↑ | = | ↑↑↑ | ↑↑↑ |
| ⇑⇑ | ⇑⇑ | ⇑⇑ | ⇑⇑ | ||||||
| Challenging Beam | |||||||||
| Errors per Step | = | ↑↑↑ | ↑↑↑ | = | ↑↑↑ | ↑↑↑ | = | ↑↑↑ | ↑↑↑ |
| ⇑⇑ | ⇑⇑ | ⇑⇑ | |||||||
| Time | = | = | = | = | = | = | = | ⇑⇑ | = |
| Steps | = | ↓ | = | = | ↓ | = | = | ↓↓ | ↓↓↓ |
| Spontaneous Activity (Cylinder) | |||||||||
| Rears | = | = | = | = | = | = | = | = | ↓ |
| ⇓ | |||||||||
| FLSteps | = | = | = | = | = | = | ⇓ | ⇓⇓ | ↓ |
| HLSteps | = | ↓↓ | ↓↓ | = | ↓↓ | ↓↓ | = | ↓↓ | ↓↓ |
| ⇓ | |||||||||
| Grooming | = | ↓↓ | ↓ | = | = | = | ↓ | ↓↓ | = |
| Gait | |||||||||
| Stride Length | = | = | = | = | = | = | = | = | = |
| Maximum difference | = | = | = | = | = | = | = | = | = |
| Locomotor Activity (Open Field) | |||||||||
| Line Crosses | = | ↑ | = | = | ↑↑↑ | ↑↑ | = | = | = |
| ⇑ | ⇑ | ||||||||
| Rears | = | = | = | = | ↑↑↑ | ↑ | = | = | = |
| ⇑ | ⇑ | ||||||||
| Nestbuilding | |||||||||
| % Cotton Used | = | ↓↓↓ | ↓↓ | = | ↓↓↓ | ↓↓ | = | ↓↓↓ | ↓↓ |
↑, ↑↑, ↑↑↑ represents significant increase at p<0.05, 0.01, 0.001, respectively, compared to WT at the same age. ↓, ↓↓, ↓↓↓ represents significant decrease at p<0.05, 0.01, 0.001, respectively, compared to WT at the same age. ⇑, ⇑⇑ represents significant increase at p<0.05, 0.01, respectively, compared to same genotype at 2m. ⇓, ⇓⇓ represents significant decrease at p<0.05, 0.01, respectively, compared to same genotype at 2m.
4. Discussion
Gene-gene interactions in PD may modulate disease risk, onset, and severity. In the present study, the effect of the interaction between loss of function of the lysosomal ATPase ATP13A2 and increased aSyn burden on sensorimotor function was determined in vivo. Double mutant 13a2-aSyn mice displayed an acceleration of sensorimotor deficits compared to WT, 13a2, and aSyn mice. Depending on the test, age, and sex, double mutant 13a2-aSyn mice either displayed differences in behavior earlier as in locomotor activity and adhesive removal or behavior deficits were more progressive as in the challenging beam and cylinder tests. These results provide in vivo support for the earlier in vitro findings of a potentially detrimental interaction between loss of ATP13A2 and aSyn [4–6,43]. This suggests that reduced ATP13A2 levels may be able to modulate aSyn pathology, such that it leads to an earlier onset of motor dysfunction and/or faster progression.
The sensorimotor phenotypes of aSyn and 13a2 mice have been previously characterized [28,34,42]. Although both genotypes show alterations in motor function, the aSyn line develops a more pronounced and earlier phenotype compared to 13a2 mice including increased errors per step on the beam, reduced hindlimb stepping in the cylinder, increased locomotor activity in the open field, and increased adhesive removal time [28,34,42]. Whereas 13a2 mice do not display deficits in beam performance, spontaneous activity, or nestbuilding until much older at ~20–27 m of age [28]. In the double mutant 13a2-aSyn line, mice displayed alterations in locomotor activity and adhesive removal at a younger age compared to the other genotypes. Female 13a2-aSyn mice showed an increase in locomotor activity compared to WT at two months of age, whereas, aSyn mice did not show an increase in activity until four months of age. Similarly, male 13a2-aSyn mice showed a slight but significant increase in time to remove sensory stimuli compared to WT at two months of age. Adhesive removal time in aSyn mice did not differ from WT until four months of age. Progression of deficits also differed in 13a2-aSyn compared to the other genotypes. Female 13a2-aSyn mice made significantly more errors per step on the beam at six months compared to their two-month testing and although aSyn mice displayed an increase in errors per step, the deficit did not progress. In males, rearing in the cylinder was similar between all genotypes at two and four months of age but sharply decreased in 13a2-aSyn mice at six months. Comparable decreases were not observed in the other genotypes. These results highlight the effect of aging, the most significant risk factor in PD.
Of note is that the rearing response differed between the cylinder and locomotor activity tests in both the females and males. Rearing either did not change (as in the females) or it decreased in the cylinder (as in the 6m males), while in locomotor activity when rearing was altered it was increased. However, the criteria for scoring rearing behavior did not differ between the cylinder and locomotor activity tests, the mouse must go from all four limbs on the floor to standing upright on only the hindlimbs. This indicates that the environmental context had a differential effect on the frequency of rearing. The open field is likely a stronger environmental stimulus than the cylinder that interacts with the underlying pathology in mutants. Contributing test factors may include the size of the cylinder and that it restricts locomotion and that the open field allows for more goal-directed behaviors compared to the cylinder. In addition to widespread neuronal overexpression of aSyn in the brain, corticostriatal abnormalities and elevated levels of tonic extracellular dopamine in the striatum have been shown in aSyn mice compared to WT [39,44]. Taken together though, the behavioral findings support an exacerbation of the sensorimotor phenotype with the combination of loss of Atp13a2 and increased aSyn burden.
Overall, the double mutant 13a2-aSyn phenotype more closely resembled that of the aSyn rather than 13a2 mice, suggesting aSyn levels and pathology are a strong driver of the sensorimotor deficits observed [28,34]. Indeed, in several tests both 13a2-aSyn and aSyn mice showed similar changes in behavior compared to WT mice. In females, 13a2-aSyn and aSyn mice had deficits in adhesive removal and nest building that were comparable in their magnitude and rate of progression. In males, errors per step on the challenging beam, hindlimb stepping in the cylinder, locomotor activity, and nest building were also comparable between 13a2-aSyn and aSyn mice and replicate behavioral anomalies previously shown in aSyn mice [34,37,39,40].
Differences between female and male mice in this aSyn line have been reported previously and were observed in the present study [40,45]. In the aSyn overexpressing mice the transgene is inserted in the X chromosome and random inactivation of the X chromosome may account for differences between the sexes [33,42]. Indeed, recent work shows female aSyn mice express less human aSyn compared to males [45]. Given this, we powered the present study large enough to detect sex differences (females=13–15 mice per genotype, males=13–14 mice per genotype). Sex differences in several of the tests were detected in the aSyn and 13a2-aSyn mice with male mice showing increased sensorimotor alterations compared to females. In hindlimb stepping in the cylinder, female aSyn and 13a2-aSyn mice did not statistically differ between genotypes at any age. In contrast, male aSyn and 13a2-aSyn showed a robust decrease in hindlimb stepping at each age tested. For errors per step on the beam, both female aSyn and 13a2-aSyn mice displayed significant deficits; however, in male aSyn and 13a2-aSyn mice the impairments were much worse across all three ages. Similarly, impairments in adhesive removal were observed earlier in male mice compared to female. Given the more profound phenotype of males on hindlimb stepping in the cylinder and errors on the beam, it is likely a “ceiling effect” in males prevented determination of the effects of Atp13a2 deficiency. Collectively in females, locomotor activity and motor performance on the beam were most sensitive to the double mutation. While in males, response to sensory stimuli and spontaneous activity were most affected. In both sexes, gait was essentially unaltered by each mutation alone or in combination. This suggests the severity of the pathology in the brain likely varies across the brain regions important in each aspect of sensorimotor function (e.g. substantia nigra, basal ganglia, and cortex). Taken together, the double mutant mice show effects of aging and sex similar what is observed in PD.
Sex differences in PD have been reported previously with a higher prevalence in males [46–48]. Previous studies in rodent models of PD also show sex differences both in pathology and sensorimotor function [49–52]. In the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of PD, male mice have higher levels of dopamine depletion than females, increases in dopamine turnover were more pronounced in females than males, increases in inducible nitric oxide synthase (iNOS) occurred faster in males than females, and gonadectomized male mice treated with MPTP perform better on the rotarod than females [49,50,53]. In the lipopolysaccharide (LPS) model, female mice are more resistant to the endotoxin than male mice [50]. Further, in the 6-hydroxydopamine model, male mice have higher levels of dopamine depletion than females and males make fewer hindpaw movements during rearing in the cylinder than females [52,54]. These studies support a wide variety of behavioral and pathological differences between sexes in PD models.
In PD mouse models, some gene-gene interactions have been found to modify in vivo phenotypes and pathology while others have not [30,55,56]. For example, the Gaucher mutation (L444P GBA) worsens motor and gastrointestinal phenotypes in an A53T mouse model of PD [56]. In contrast, a lack of gene-gene interaction effects was found in parkin knockout with A53T aSyn overexpression and in triple transgenic Parkin/DJ-1/PINK1 knockout mice [55,57]. The present study shows an exacerbation of the behavioral phenotype with the double 13a2-aSyn mutation; however, in a different line of Atp13a2-deficient mice crossed with mice overexpressing the mutated A53T form of aSyn under the prion promoter, no exacerbation of gliosis or lysosomal pathology was observed in the cortex or cerebellum in double mutants [30]. The behavioral phenotype was not measured in this double mutant line but compared to the present study there are several notable differences between models, including the type of aSyn overexpressed (WT vs A53T) and promoter used (thy1 vs prion). These factors likely account for any differences between the mice and highlight the rigor required to understand these complex interactions in vivo.
Loss of function of ATP13A2 may interact with aSyn overexpression through various mechanisms. Although the molecular function and substrate specificity of ATP13A2 remain unclear, in vitro studies involve it in lysosomal function, autophagy, exosome release, mitochondrial function, and heavy metal homeostasis [4–10]. Recent biochemical studies show that ATP13A2 activity depends on the signaling lipids phosphatidic acid and phosphatidylinositol (3,5) bisphosphate and their interaction with the N-terminus of ATP13A2 [7,58]. Both signaling lipids are associated with endo-/lysosomal membranes and are involved in vesicular trafficking, membrane fission and fusion, and autophagy [59–63]. This lipid-dependent ATP13A2 activity has also been shown to protect against the mitochondrial toxin rotenone in cells [7]. Based on these findings, loss of function of Atp13a2 could then potentially exacerbate lysosomal and mitochondrial dysfunction already present in aSyn overexpressing mice [64,65]. Current studies are now examining the effect of the double mutation (13a2-aSyn) on aSyn accumulation, lysosomal and mitochondrial function, and neuronal integrity in the brain in these mice. In conclusion, this is the first study to show in vivo that loss of function of Atp13a2 exacerbates the behavioral phenotype in aSyn overexpressing mice indicating a pathological interaction between two PD-related genetic loci. Further, this study reveals aging, the highest risk factor for PD, and sex impacted performance in several tests similar to what is observed in PD.
Supplementary Material
Highlights.
Sensorimotor function is significantly worse in 13a2-aSyn mice compared to controls.
Double mutant behavior was similar to alpha-synuclein mice in some test parameters.
Some sensorimotor deficits were more pronounced in males compared to females.
Acknowledgments
This work was supported by the National Institutes of Health NS077022 (S.F.). We also gratefully acknowledge assistance in video rating by Ruhi Gulati, Emily Devine, and Nick Santiago.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schultheis PJ, Hagen TT, O’Toole KK, Tachibana A, Burke CR, McGill DL, et al. Characterization of the P5 subfamily of P-type transport ATPases in mice. Biochem Biophys Res Commun. 2004;323:731–738. doi: 10.1016/j.bbrc.2004.08.156. [DOI] [PubMed] [Google Scholar]
- 2.Ramonet D, Podhajska A, Stafa K, Sonnay S, Trancikova A, Tsika E, et al. PARK9-associated ATP13A2 localizes to intracellular acidic vesicles and regulates cation homeostasis and neuronal integrity. Hum Mol Genet. 2012;21:1725–1743. doi: 10.1093/hmg/ddr606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramirez A, Heimbach A, Gründemann J, Stiller B, Hampshire D, Cid LP, et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type 13A2ase. Nat Genet. 2006;38:1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- 4.Gitler AD, Chesi A, Geddie ML, Strathearn KE, Hamamichi S, Hill KJ, et al. Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet. 2009;41:308–315. doi: 10.1038/ng.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dehay B, Ramirez A, Martinez-Vicente M, Perier C, Canron MH, Doudnikoff E, et al. Loss of P-type ATPase ATP13A2/PARK9 function induces general lysosomal deficiency and leads to Parkinson disease neurodegeneration. Proc Natl Acad Sci U S A. 2012;109:9611–9616. doi: 10.1073/pnas.1112368109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Usenovic M, Tresse E, Mazzulli JR, Taylor JP, Krainc D. Deficiency of ATP13A2 leads to lysosomal dysfunction, α-synuclein accumulation, and neurotoxicity. J Neurosci. 2012;32:4240–4246. doi: 10.1523/JNEUROSCI.5575-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holemans T, Sørensen DM, van Veen S, Martin S, Hermans D, Kemmer GC, et al. A lipid switch unlocks Parkinson’s disease-associated ATP13A2. Proc Natl Acad Sci U S A. 2015;112:9040–9045. doi: 10.1073/pnas.1508220112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grünewald A, Arns B, Seibler P, Rakovic A, Münchau A, Ramirez A, et al. ATP13A2 mutations impair mitochondrial function in fibroblasts from patients with Kufor-Rakeb syndrome. Neurobiol Aging. 2012;33:e1–7. doi: 10.1016/j.neurobiolaging.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 9.Gusdon AM, Zhu J, Van Houten B, Chu CT. ATP13A2 regulates mitochondrial bioenergetics through macroautophagy. Neurobiol Dis. 2012;45:962–972. doi: 10.1016/j.nbd.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JS, Koentjoro B, Veivers D, Mackay-Sim A, Sue CM. Parkinson’s disease-associated human ATP13A2 (PARK9) deficiency causes zinc dyshomeostasis and mitochondrial dysfunction. Hum Mol Genet. 2014;23:2802–2815. doi: 10.1093/hmg/ddt623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong SM, Chan BK, Park JS, Hill KJ, Aitken JB, Cottle L, et al. Parkinson’s disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes α-Synuclein externalization via exosomes. Hum Mol Genet. 2014;23(11):2816–2833. doi: 10.1093/hmg/ddu099. [DOI] [PubMed] [Google Scholar]
- 12.Tsunemi T, Krainc D. Zn2+ dyshomeostasis caused by loss of ATP13A2/PARK9 leads to lysosomal dysfunction and alpha-synuclein accumulation. Hum Mol Genet. 2014;23:2791–2801. doi: 10.1093/hmg/ddt572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bras J, Verloes A, Schneider SA, Mole SE, Guerreiro RJ. Mutation of the parkinsonism gene ATP13A2 causes neuronal ceroid-lipofuscinosis. Hum Mol Genet. 2012;21:2646–2650. doi: 10.1093/hmg/dds089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estrada-Cuzcano A, Martin S, Chamova T, Synofzik M, Timmann D, Holemans T, et al. Loss-of-function mutations in the ATP13A2/PARK9 gene cause complicated hereditary spastic paraplegia (SPG78) Brain. 2017;140:287–305. doi: 10.1093/brain/aww307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Usenovic M, Knight AL, Ray A, Wong V, Brown KR, Caldwell GA, et al. Identification of novel ATP13A2 interactors and their role in α-synuclein misfolding and toxicity. Hum Mol Genet. 2012;21:3785–3794. doi: 10.1093/hmg/dds206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Usenovic M, Krainc D. Lysosomal dysfunction in neurodegeneration: the role of ATP13A2/PARK9. Autophagy. 2012;8:987–988. doi: 10.4161/auto.20256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kara E, Tucci A, Manzoni C, Lynch DS, Elpidorou M, Bettencourt C, et al. Genetic and phenotypic characterization of complex hereditary spastic paraplegia. Brain. 2016;139:1904–1918. doi: 10.1093/brain/aww111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Fonzo A, Chien HF, Socal M, Giraudo S, Tassorelli C, Iliceto G, et al. ATP13A2 missense mutations in juvenile parkinsonism and young onset Parkinson disease. Neurology. 2007;68:1557–1562. doi: 10.1212/01.wnl.0000260963.08711.08. [DOI] [PubMed] [Google Scholar]
- 19.Lin CH, Tan EK, Chen ML, Tan LC, Lim HQ, Chen GS, et al. Novel ATP13A2 variant associated with Parkinson disease in Taiwan and Singapore. Neurology. 2008;71:1727–1732. doi: 10.1212/01.wnl.0000335167.72412.68. [DOI] [PubMed] [Google Scholar]
- 20.Park JS, Blair NF, Sue CM. The role of ATP13A2 in Parkinson’s disease: Clinical phenotypes and molecular mechanisms. Mov Disord. 2015;30:770–779. doi: 10.1002/mds.26243. [DOI] [PubMed] [Google Scholar]
- 21.Lubbe SJ, Escott-Price V, Gibbs JR, Nalls MA, Bras J, Price TR, et al. Additional rare variant analysis in Parkinson’s disease cases with and without known pathogenic mutations: evidence for oligogenic inheritance. Hum Mol Genet. 2016;25:5483–5489. doi: 10.1093/hmg/ddw348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleming SM. Mechanisms of gene-environment interactions in Parkinson’s disease. Curr Environ Health Rep. 2017;4:192–199. doi: 10.1007/s40572-017-0143-2. [DOI] [PubMed] [Google Scholar]
- 23.Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 24.Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 25.Smith WW, Pei Z, Jiang H, Moore DJ, Liang Y, West AB, et al. Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration. Proc Natl Acad Sci U S A. 2005;102:18676–18681. doi: 10.1073/pnas.0508052102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, et al. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146:37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhungel N, Eleuteri S, Li LB, Kramer NJ, Chartron JW, Spencer B, et al. Parkinson’s disease genes VPS35 and EIF4G1 interact genetically and converge on α-synuclein. Neuron. 2015;85:76–87. doi: 10.1016/j.neuron.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schultheis PJ, Fleming SM, Clippinger AK, Lewis J, Tsunemi T, Giasson B, et al. Atp13a2-deficient mice exhibit neuronal ceroid lipofuscinosis, limited α-synuclein accumulation and age-dependent sensorimotor deficits. Hum Mol Genet. 2013;22:2067–2082. doi: 10.1093/hmg/ddt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daniel G, Musso A, Tsika E, Fiser A, Glauser L, Pletnikova O, et al. α-Synuclein-induced dopaminergic neurodegeneration in a rat model of Parkinson’s disease occurs independent of ATP13A2 (PARK9) Neurobiol Dis. 2015;73C:229–243. doi: 10.1016/j.nbd.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Kett LR, Stiller B, Bernath MM, Tasset I, Blesa J, Jackson-Lewis V, et al. α-synuclein-Independent histopathological and motor deficits in mice lacking the endolysosomal Parkinsonism protein Atp13a2. J Neurosci. 2015;5:5724–5742. doi: 10.1523/JNEUROSCI.0632-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fleming SM, Santiago NA, Mullin EJ, Pamphile S, Lemkuhl A, Karkare S, et al. The effect of manganese exposure on sensorimotor function and alpha-synuclein accumulation in Atp13a2-deficient mice. Neurotoxicology. 2017;17:30101–30108. doi: 10.1016/j.neuro.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleming SM, Fernagut PO, Chesselet MF. Genetic mouse models of Parkinsonism: Strengths and limitations. NeuroRx. 2005;2:495–503. doi: 10.1602/neurorx.2.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, et al. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68:568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- 34.Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, et al. Early and progressive motor abnormalities in mice overexpressing wild-type human alpha-synuclein. J Neurosci. 2004;24:9434–9440. doi: 10.1523/JNEUROSCI.3080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fleming SM, Salcedo J, Hutson CB, Rockenstein E, Masliah E, Levine MS, et al. Behavioral effects of dopaminergic agonists in transgenic mice overexpressing human wildtype α-synuclein. Neuroscience. 2006;142:1245–1253. doi: 10.1016/j.neuroscience.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleming SM, Tetreault NA, Mulligan CK, Hutson CB, Masliah E, Chesselet MF. Olfactory dysfunction in mice overexpressing human wildtype alpha-synuclein. Eur J Neurosci. 2008;28:247–256. doi: 10.1111/j.1460-9568.2008.06346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleming SM, Mulligan CK, Richter F, Zhu C, Lemesre V, Frias C, et al. (2011) The microtubule assembly promoting protein (NAP) improves motor function and reduces alpha synuclein inclusions in mice overexpressing alpha synuclein. Mol Cell Neurosci. 2011;46:597–606. doi: 10.1016/j.mcn.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schallert T, Whishaw IQ, Ramirez VD, Teitelbaum P. 6-hydroxydopamine and anticholinergic drugs. Science. 1978;202:1216–1217. doi: 10.1126/science.202.4373.1216. [DOI] [PubMed] [Google Scholar]
- 39.Lam HA, Wu N, Cely I, Kelly RL, Hean S, Richter F, et al. Elevated tonic extracellular dopamine concentration and altered dopamine modulation of synaptic activity precede dopamine loss in the striatum of mice overexpressing human α-synuclein. J Neurosci Res. 2011;89:1091–102. doi: 10.1002/jnr.22611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magen I, Ostritsky R, Richter F, Zhu C, Fleming SM, Lemesre V, et al. Intranasal NAP (davunetide) decreases tau hyperphosphorylation and moderately improves behavioral deficits in mice overexpressing α-synuclein. Pharmacol Res Perspect. 2014;2:e00065. doi: 10.1002/prp2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Fleming SM, Chesselet MF, Taché Y. Abnormal colonic motility in mice overexpressing human wildtype alpha-synuclein. Neuroreport. 2008;19:873–876. doi: 10.1097/WNR.0b013e3282ffda5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chesselet MF, Richter F, Zhu C, Magen I, Watson MB, Subramaniam SR. A progressive mouse model of Parkinson’s disease: the Thy1-aSyn (“Line 61”) mice. Neurotherapeutics. 2012;9:297–314. doi: 10.1007/s13311-012-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopes da Fonseca T, Pinho R, Outeiro TF. A familial ATP13A2 mutation enhances alpha-synuclein aggregation and promotes cell death. Hum Mol Genet. 2016;25:2959–2971. doi: 10.1093/hmg/ddw147. [DOI] [PubMed] [Google Scholar]
- 44.Wu N, Joshi PR, Cepeda C, Masliah E, Levine MS. Alpha-synuclein overexpression in mice alters synaptic communication in the corticostriatal pathway. J Neurosci Res. 2010;88:1764–76. doi: 10.1002/jnr.22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerstenberger J, Bauer A, Helmschrodt C, Richter A, Richter F. The novel adaptive rotating beam test unmasks sensorimotor impairments in a transgenic mouse model of Parkinson’s disease. Behav Brain Res. 2016;304:102–10. doi: 10.1016/j.bbr.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 46.Twelves D, Perkins KS, Counsell C. Systematic review of incidence studies of Parkinson’s disease. Mov Disord. 2003;18:19–31. doi: 10.1002/mds.10305. [DOI] [PubMed] [Google Scholar]
- 47.Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leenders KL, Eshuis S, et al. Gender differences in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:819–24. doi: 10.1136/jnnp.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2014;29:1583–90. doi: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- 49.Antzoulatos E, Jakowec MW, Petzinger GM, Wood RI. Sex differences in motor behavior in the MPTP mouse model of Parkinson’s disease. Pharmacol Biochem Behav. 2010;95:466–72. doi: 10.1016/j.pbb.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ookubo M, Yokoyama H, Kato H, Araki T. Gender differences on MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) neurotoxicity in C57BL/6 mice. Mol Cell Endocrinol. 2009;311:62–8. doi: 10.1016/j.mce.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Qin L, Wilson B, Wu X, Qian L, Granholm AC, et al. Endotoxin induces a delayed loss of TH-IR neurons in substantia nigra and motor behavioral deficits. Neurotoxicology. 2008;29:864–70. doi: 10.1016/j.neuro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Field EF, Metz GA, Pellis SM, Whishaw IQ. Sexually dimorphic postural adjustments during vertical behaviour are altered in a unilateral 6-OHDA rat model of Parkinson’s disease. Behav Brain Res. 2006;174:39–48. doi: 10.1016/j.bbr.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Joniec I, Ciesielska A, Kurkowska-Jastrzebska I, Przybylkowski A, Czlonkowska A, Czlonkowski A. Age- and sex-differences in the nitric oxide synthase expression and dopamine concentration in the murine model of Parkinson’s disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Brain Res. 2009;1261:7–19. doi: 10.1016/j.brainres.2008.12.081. [DOI] [PubMed] [Google Scholar]
- 54.Tamás A, Lubics A, Lengvári I, Reglodi D. Effects of age, gender, and gonadectomy on neurochemistry and behavior in animal models of Parkinson’s disease. Endocrine. 2006;29:275–87. doi: 10.1385/ENDO:29:2:275. [DOI] [PubMed] [Google Scholar]
- 55.Kitada T, Tong Y, Gautier CA, Shen J. Absence of nigral degeneration in aged parkin/DJ-1/PINK1 triple knockout mice. J Neurochem. 2009;111:696–702. doi: 10.1111/j.1471-4159.2009.06350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fishbein I, Kuo YM, Giasson BI, Nussbaum RL. Augmentation of phenotype in a transgenic Parkinson mouse heterozygous for a Gaucher mutation. Brain. 2014;137:3235–3247. doi: 10.1093/brain/awu291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.von Coelln R, Thomas B, Andrabi SA, Lim KL, Savitt JM, Saffary R, et al. Inclusion body formation and neurodegeneration are parkin independent in a mouse model of alpha-synucleinopathy. J Neurosci. 2006;26:3685–3696. doi: 10.1523/JNEUROSCI.0414-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin S, Holemans T, Vangheluwe P. Unlocking ATP13A2/PARK9 activity. Cell Cycle. 2015;14:3341–2. doi: 10.1080/15384101.2015.1093420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ho CY, Alghamdi TA, Botelho RJ. Phosphatidylinositol-3,5-bisphosphate: no longer the poor. PIP2 Traffic. 2012;13:1–8. doi: 10.1111/j.1600-0854.2011.01246.x. [DOI] [PubMed] [Google Scholar]
- 60.Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, et al. PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat Commun. 2010;1:38. doi: 10.1038/ncomms1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weigert R, Silletta MG, Spanò S, Turacchio G, Cericola C, Colanzi A, et al. CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature. 1999;402:429–433. doi: 10.1038/46587. [DOI] [PubMed] [Google Scholar]
- 62.Blackwood RA, Smolen JE, Transue A, Hessler RJ, Harsh DM, Brower RC, et al. Phospholipase D activity facilitates Ca2+-induced aggregation and fusion of complex liposomes. Am J Physiol. 1997;272:C1279–C1285. doi: 10.1152/ajpcell.1997.272.4.C1279. [DOI] [PubMed] [Google Scholar]
- 63.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942–1945. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 64.Subramaniam SR, Vergnes L, Franich NR, Reue K, Chesselet MF. Region specific mitochondrial impairment in mice with widespread overexpression of alpha-synuclein. Neurobiol Dis. 2014;70:204–213. doi: 10.1016/j.nbd.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mak SK, McCormack AL, Manning-Bog AB, Cuervo AM, Di Monte DA. Lysosomal degradation of alpha-synuclein in vivo. J Biol Chem. 2010;285:13621–13629. doi: 10.1074/jbc.M109.074617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.