Abstract
Background and Purpose
Mild stroke is the most common cause for thrombolysis exclusion in patients acutely presenting to the hospital. Thrombolysis administration in this subgroup is highly variable amongst different clinicians and institutions. We aim to study the predictors of thrombolysis in patients with mild ischemic stroke in the FL-PR CReSD registry.
Methods
Among 73,712 prospectively enrolled patients with a final diagnosis of ischemic stroke or TIA from January 2010 to April 2015, we identified 7,746 cases with persistent neurological symptoms and NIHSS ≤5 who arrived within 4 hours of symptom onset. Multilevel logistic regression analysis with generalized estimating equations was used to identify independent predictors of thrombolytic administration in the subgroup of patients without contraindications to thrombolysis.
Results
We included 6,826 cases (final diagnosis mild stroke 74.6%, TIA 25.4%). Median age was 72 (IQR=21), 52.7% men, 70.3% white, 12.9% black, 16.8% Hispanic, and median NIHSS=2 (IQR=3). Patients who received thrombolysis (n=1,281, 18.7%) were younger (68 vs. 72 yr.), had less vascular risk factors (HTN, DM, dyslipidemia), lower risk of prior vascular disease (MI, PVD, previous stroke) and had a higher presenting median NIHSS (4 vs. 2). In the multilevel-multivariable model, early hospital arrival (arrive by 0 to 2 hrs. vs. 3.5 hrs. and above) (OR 8.16, 95% CI 4.76–13.98), higher NIHSS (OR 1.87, 95% CI 1.77–1.98), aphasia at presentation (OR 1.35, 95% CI 1.12–1.62), faster door to CT time (OR 1.81, 95% CI 1.53–2.15), presenting to an academic hospital (OR 2.02, 95% CI 1.39–2.95) were independent predictors of thrombolysis administration.
Conclusion
Mild acutely presenting stroke patients are more likely to receive thrombolysis if they are young, White or Hispanic, arrive early to the hospital with more severe neurological presentation. Identification of predictors of thrombolysis is important in design of future studies to assess the use of thrombolysis for mild stroke.
Keywords: Mild Stroke, Thrombolysis, Florida-Puerto Rico
Introduction
The majority of patients with ischemic stroke have either mild or transient neurological symptoms on the initial presentation.1 Despite a seemingly benign presentation, close to one third of these patients are dead or disabled at 3 months follow-up.2–5 The role of thrombolysis in this subgroup of ischemic stroke patients is not well understood and the current practice is widely variable. Patients with rapidly improving or mild neurological symptoms including isolated sensory deficit, ataxia, dysarthria and facial palsy were excluded from randomization in the National Institute of Neurological Disorders and Stroke (NINDS) trial.6 However, these features are not individually considered as a contraindication for thrombolysis, while mild and rapidly improving stroke is listed as a relative thrombolytic contraindication in the current guidelines.7, 8 Furthermore, mild stroke is no longer mentioned as a contraindication for ischemic stroke treatment in the updated package insert of Alteplase by Genetech.9.10, 11
To date, no large-scale study has evaluated the clinical practice and predictors of thrombolysis administration in mild ischemic stroke patients who do not have contradictions to thrombolytic treatment. In a large Florida-Puerto Rico Collaboration to Reduce Stroke Disparities (FL-PR CReSD) study, we aimed to study the clinical and hospital characteristic that are associated with the thrombolytic use in mild stroke ischemic patients who otherwise do not have contraindications to thrombolysis.
Methods
Case Identification and Data Abstraction
The FL-PR Stroke Registry consists of hospitalized patient data collected from participating hospitals in Florida and Puerto Rico and includes patients with the primary diagnosis of ischemic stroke, transient ischemic attack (TIA), subarachnoid hemorrhage, intracerebral hemorrhage and stroke not otherwise specified. Briefly, FL-PR Stroke Registry is a National Institute of Neurological Disorders (NINDS) funded multicenter initiative, as part of the Get with The Guidelines Stroke (GWTG-S) program12, to create high impact, culturally tailored interventions to identify disparities in delivery of stroke care among a diverse population of patients with significant Hispanic representation.
Information collected included patient demographics (age, sex, race/ethnicity (Non-Hispanic (NH) white, NH black, Hispanic in Florida and Hispanic in PR)), clinical characteristics (vascular risk factors and relevant prior medical history), arrival characteristics (mode of hospital arrival (via emergency medical services (EMS) from home/scene, private transport, transfer from other hospital, unknown), presenting National Institutes of Health Stroke Scale (NIHSS) and presenting neurological symptoms (to identify diability producing symptoms not captured by the NIHSS), the onset-to-door time (OTD; time from the stroke onset to arrival to the time of emergency department)), assessment characteristics (time from arrival to the initial head computed tomography (CT) (Door to CT (DTC)), time from hospital arrival to initiation of intravenous thrombolysis (door-to-needle (DTN) time) and hospital-level characteristics (number of beds, academic status, annual stroke volume and number of years in GWTG-S). Stroke severity at presentation was measured by the NIHSS. Case ascertainment for the diagnosis of ischemic stroke was performed by prospective clinical identification and retrospective chart review using International Classification of Diseases, ninth revision, and discharge codes followed by chart review to confirm the final diagnosis.
Study population
From the total of 88,978 patients in the registry from January 2010 through April 2015 from 66 hospitals in Florida and 9 in Puerto Rico, 27,178 patients with ischemic stroke and TIA with documented NIHSS≤5 at presentation were included in the current study. We further excluded those who arrived after 4 hours of symptom onset (n=19,432) and those with other known contraindications to thrombolysis (n=920) (Figure 1). Thrombolysis was defined as any patient who has received intravenous recombinant tissue plasminogen activator (rtPA) - alteplase.
Figure 1.
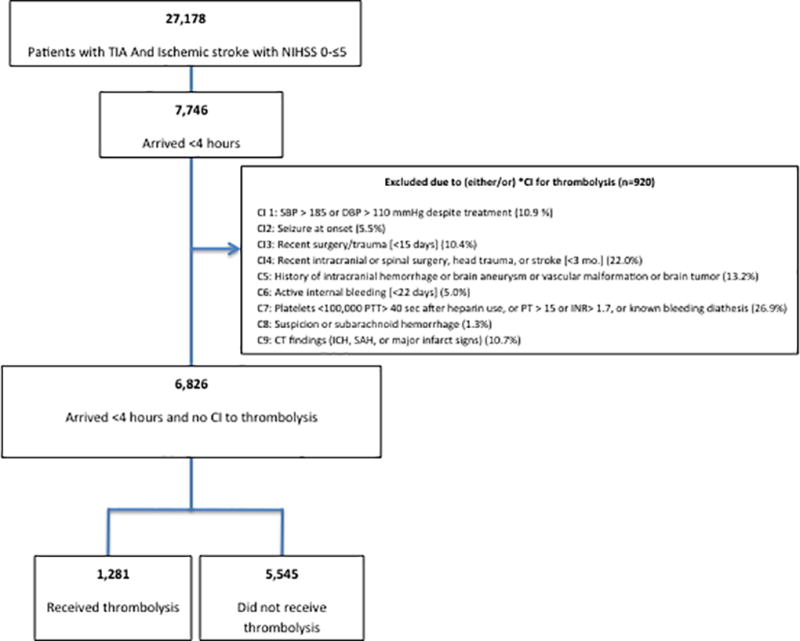
Flowchart of patients included in the study. *CI: Contraindication for thrombolysis
Statistical Analysis
The level of statistical significance was set at p<0.05. The data that support the findings of this study are available from the corresponding author upon reasonable request.
We selected three categories of variables based on our understanding of the disease processes and factors influencing the clinician’s decision to thrombolyze mild stroke patients and thus affecting the therapeutic conduct of the clinicians. These factors included patient’s clinical characteristics (demographics, vascular risk factors, time and mode of arrival and stroke severity), hospital based characteristics (size, experience and academic status of the hospital) as well as geographic characteristics (Four regions in Florida and PR). For patient characteristics, continuous variables were summarized as median with interquartile range (IQR) and categorical variables were presented as frequencies with percentages. For continuous variables, differences were assessed using the Student t test (mean comparison) if normally distributed, or Wilcoxon–Mann–Whitney test (median comparison). For categorical variables, the Pearson chi-square test was used to compare the distributions between groups. Univariate analysis was performed to identify the specific characteristics of mild stroke patients who received thrombolysis. To reduce multicollinearity amongst the correlated factors, we first conducted step-wise logistic regression to select the independent factors. We then conducted multivariable analysis with generalized estimating equations (GEE) to account for clustering effect within each hospital and evaluated the associations between factors and thrombolysis administration. Potential interactions between hospital characteristics and race-ethnicity, region and arrive time were also examined by including their interaction terms in the regression model. The goodness of fit of the regression was assessed by the quasi-likelihood information criterion (QIC), a modification of Akaike’s information criterion (AIC) to apply to models fit by the GEE approach. The final model (parsimonious model, QIC=5041) was close to the full model (QIC=5033) in goodness of fit.
Most variables had missing values in fewer than 5% of cases, except for the mode of arrival and insurance variables (18.9%, 7.2% missing, respectively). The complete case approach and the missing indicator approach were used to include the full sample for variables with a large proportion of missingness as previously described.13 All statistical analyses were performed using SAS Version 9.3 software (SAS Institute).
Results
We included 6,826 cases with acute mild ischemic stroke (documented NIHSS ≤5) within 4 hours of symptom onset and no contraindications to thrombolysis (Figure 1). Median age was 72 (IQR=21), 52.7% were male, 70.3% white, 12.9% black, 16.8% Hispanic and the median presenting NIHSS was 2 (IQR=3). A total of 1,281 (18.7%) patients received thrombolysis.
Baseline characteristics of patients based on thrombolysis treatment are summarized in Table 1. Patients who were thrombolyzed were younger, more likely to be male, less likely to have pre-existing vascular risk factors including hypertension (HTN), diabetes (DM) and dyslipidemia as compared to those who did not receive thrombolysis. Similarly they were less likely to have had a prior history of stoke/TIA or coronary artery disease (CAD)/Myocardial Infarction (MI).
Table 1.
Patient and hospital level characteristics of mild ischemic stroke patients (NIHSS≤5) in the FL-PR Stroke Registry stratified by thrombolysis treatment
| Clinical Characteristics | All (n=6,826) |
Thrombolysis (n=1,281) |
No Thrombolysis (n=5,545) |
p value |
|---|---|---|---|---|
|
| ||||
| Age (yrs), median (IQR) | 72 (21) | 68 (20) | 72 (21) | <.0001 |
|
| ||||
| Sex (male), % | 3,598 (52.7) | 718 (56.1) | 2880 (51.9) | <.0001 |
|
| ||||
| Vascular Risk Factor, % | ||||
|
| ||||
| Current smoker | 1,048 (15.4) | 248 (19.4) | 800 (14.4) | <.0001 |
|
| ||||
| Hypertension | 4,713 (69.0) | 745 (58.2) | 3,968 (71.6) | <.0001 |
|
| ||||
| Diabetes mellitus | 1,839 (26.9) | 304 (23.7) | 1,535 (27.7) | 0.004 |
|
| ||||
| Dyslipidemia | 2,990 (43.8) | 428 (33.4) | 2,562 (46.2) | < .0001 |
|
| ||||
| Medical History, % | ||||
|
| ||||
| AF | 1,067 (15.6) | 187 (14.6) | 880 (15.9) | 0.26 |
|
| ||||
| CAD/prior MI | 1,733 (25.4) | 260 (20.3) | 1,473 (26.6) | <.0001 |
|
| ||||
| Previous stroke/TIA | 1,911 (28.0) | 233 (18.2) | 1,678 (30.3) | <.0001 |
|
| ||||
| Ethnicity, % | ||||
| NH- white | 4,798(70.3) | 855 (66.7) | 3,943 (71.1) | <.0001 |
| NH- black | 881 (12.9) | 174 (13.6) | 707 (12.8) | |
| FL-Hispanic | 889 (13.0) | 173 (13.5) | 716 (12.9) | |
| PR-Hispanic | 258 (3.8) | 79 (6.2) | 179 (3.2) | |
|
| ||||
| Medical Insurance, % | ||||
| Private* | 2,777 (40.7) | 498 (38.9) | 2,279 (41.1) | <.0001 |
| Medicare | 2,112 (30.9) | 311 (24.3) | 1,801 (32.5) | |
| Medicaid/No Insurance** | 645 (9.5) | 147 (11.5) | 498 (9.0) | |
| Unknown | 1,292 (18.9) | 325 (25.4) | 967 (17.4) | |
|
| ||||
| Arrival Time (%) | ||||
| On –hours | 3,241 (47.5) | 629 (49.1) | 2,612 (47.1) | 0.20 |
| Off- hours | 3,585 (52.5) | 652 (50.9) | 2,933 (52.9) | |
|
| ||||
| Arrival Time from Onset Minutes (median, IQR) | 76 (85) | 63 (64) | 81 (92) | 0.001 |
|
| ||||
| Arrival Time from Onset Minutes | ||||
| 0 to 2 hour | 4861 (71.2) | 1061 (82.8) | 3800 (68.5) | <.0001 |
| 2 to 3.5 hour | 1600 (23.4) | 206 (16.1) | 1394 (25.1) | |
| 3.5 hour and above | 365 (5.4) | 14 (1.1) | 351 (6.3) | |
|
| ||||
| Final Diagnosis TIA | 1,735 (25.4) | 32 (2.5) | 1,703 (30.7) | <.0001 |
|
| ||||
| NIHSS (median), IQR | 2 (3) | 4 (2) | 2 (2) | <.0001 |
|
| ||||
| Clinical Signs/symptoms, % | ||||
|
| ||||
| Weakness | 3,420 (50.1) | 738 (57.6) | 2,682 (48.4) | <.0001 |
|
| ||||
| Aphasia | 2,253 (33.0) | 533 (41.6) | 1,720 (31.0) | <.0001 |
|
| ||||
| Altered level of Consciousness | 494 (7.2) | 67 (5.2) | 427 (7.7) | 0.002 |
|
| ||||
| Other Neurological Signs/symptoms | 1,120 (16.4) | 188 (14.7) | 932 (16.8) | 0.06 |
|
| ||||
| No Neurological Signs/symptoms | 57 (0.8) | 2 (0.2) | 55 (1.0) | 0.003 |
|
| ||||
| Mode of Arrival EMS | ||||
| Yes | 3,748 (54.9) | 821 (64.1) | 2,927 (52.8) | <.0001 |
| No | 2585 (37.9) | 346 (27.0) | 2239 (40.4) | |
| Missing | 493 (7.2) | 114 (8.9) | 379 (6.8) | |
|
| ||||
| Door to CT time minutes (median, IQR) | 27(35) | 20 (20) | 30(40) | <.0001 |
|
| ||||
| Door to CT time (% <25 min) | 3,029 (46.7) | 792 (65.0) | 2,237 (42.5) | <.0001 |
|
| ||||
| Hospital Characteristics | ||||
|
| ||||
| Hospital Size | ||||
| Median bed (IQR) | 468 (387) | 466 (462) | 468 (396) | <.0001 |
| Small (<250beds) | 1355(19.8) | 201 (15.7) | 1154 (20.8) | |
| Mid (250–450 beds) | 1658 (24.3) | 367 (28.7) | 1291 (23.3) | |
| Large (>450) | 3813 (55.9) | 713 (55.7) | 3100 (55.9) | |
|
| ||||
| Academic hospital, % | ||||
| Yes | 1207 (17.7) | 398 (31.1) | 809 (14.6) | <.0001 |
| No | 5619 (82.3) | 883 (68.9) | 4736 (85.4) | |
|
| ||||
| Years in GWTG, median (IQR) | 8 (2) | 7 (3) | 8 (2) | <.0001 |
|
| ||||
| Number of tPA treated patients peryear | <.0001 | |||
| Low volume (<100) | 3291 (48.2) | 503 (39.3) | 2788 (50.3) | |
| High volume (≥100) | 3535 (51.8 | 778 (60.7) | 2757 (49.7) | |
|
| ||||
| State | ||||
| Florida | 6568 (96.2) | 1202 (93.8) | 5366 (96.8) | <.0001 |
| Puerto Rico | 258 (3.8) | 79 (6.2) | 179 (3.2) | |
|
| ||||
| Stroke Center Type | <.0001 | |||
| Primary Stroke Center | 3054 (44.7) | 477 (37.2) | 2577 (46.5) | |
| Comprehensive Stroke Center | 3611 (52.9) | 749 (58.5) | 2862 (51.6) | |
| Not Primary/Comprehensive | 161 (2.4) | 55 (4.3) | 106 (1.9) | |
|
| ||||
| Region in Florida | ||||
| South | 2402 (36.6) | 517 (43.0) | 1885 (35.1) | <.0001 |
| East Central | 918 (14.0) | 205 (17.1) | 713 (13.3) | |
| West Central | 2286 (34.8) | 232 (19.3) | 2054 (38.3) | |
| North and Panhandle | 962 (14.7) | 248 (20.6) | 714 (13.3) | |
AF=atrial fibrillation, CAD= Coronary Artery Disease, MI=Myocardial Infarction. NH-White= Non-Hispanic White, NH-black= Non-Hispanic black. EMS= Emergency Medical Services.
Includes Private insurance, VA and other.
Includes Medicaid, self-pay and No insurance.
As described in Table 1, thrombolyzed patients had a higher NIHSS (4 vs. 2), and more likely to have weakness (57.6% vs. 48.4%) and aphasia (41.6% vs. 31.0%) compared to those who did not receive thrombolysis. Presenting with altered level of consciousness (5.2% vs. 7.7%) or other neurological symptoms (14.7% vs.16.8%) were inversely related to thrombolysis utilization.
Thrombolyzed patients were more likely to arrive early to the hospital after symptom onset than non-thrombolyzed patients (63 min vs. 81 min) and were more likely to have arrived via EMS (64.1% vs. 52.8%). The rate of thrombolysis administration decreased from 22% for those presenting between 0–2 hours from symptom onset to 4% in those presenting between 3.5 to 4 hours. The median DTC time was shorter in patients who received thrombolysis relative to those who did not (20 min vs. 30 min). Compared to non-thrombolyzed mild stroke patients, thrombolyzed patients were more likely assessed in an academic hospital (31.1% vs. 14.6%), hospitals with greater annual thrombolysis volumes (≥100) (60.7% vs. 49.7%), and Comprehensive Stroke Centers versus Primary Stroke Centers (58.5% vs. 51.6%) and were less likely treated at smaller hospitals (<250 beds) (15.7% vs. 20.8%). Regional analysis of thrombolysis utilization showed a disproportionately higher rate of thrombolysis use in South Florida relative to all other regions in Florida.
After step-wise logistic regression, age, race, medical history of stroke/TIA, medical history of hypertension, medical history of peripheral vascular disease, final diagnosis stroke type, onset to arrival time, arrival time of day, NIHSS, aphasia language disturbance, door to CT time, academic status and region were retained for inclusion in multivariable analysis (supplemental table I). In the final multilevel-multivariable model, early hospital arrival (arrive by 0 to 2 hrs vs. 3.5 hrs and above)(OR 8.16, 95% CI 4.76–13.98), higher NIHSS (OR 1.87, 95% CI 1.77–1.98), aphasia at presentation (OR 1.35, 95% CI 1.12–1.62), faster door to CT time (OR 1.81, 95% CI 1.53–2.15), presenting to an academic hospital (OR 2.02, 95% CI 1.39–2.95) were independent predictors of thrombolysis administration. In contrast, the odds of thrombolysis administration were lower in older patients (OR 0.98, CI 0.97–0.98), NH-blacks vs. NH-white (OR 0.80, 95% CI 0.69–0.93), those with a prior history of stroke/TIA (OR 0.61, 95% CI 0.52–0.72), prior history of hypertension (OR 0.74, 95% CI 0.64–0.87)), prior history of peripheral vascular disease (OR 0.67, CI 0.46–0.97), arriving during off hours vs. on hours (OR 0.87, CI (0.77–0.99), hospitals in west central vs. south (OR 0.38, CI 0.24–0.61). No significant interactions between hospital characteristics and race-ethnicity, region and arrive time were found.
The overall rate of thrombolysis in mild stroke patients presenting within the thrombolytic window increased over time from 15% in 2010 (10%) to 25% in 2015 (p value for trend <0.0001) (Figure 2).
Figure 2.
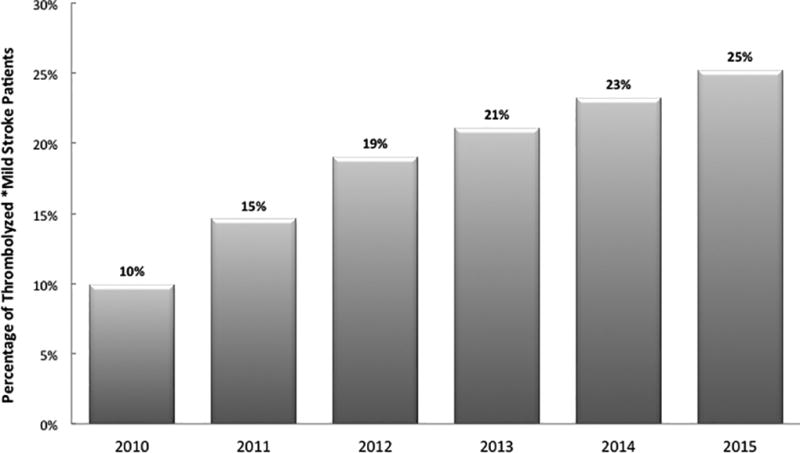
Trends of thrombolysis Administration in Patients with Mild Ischemic Stroke by year (2010–2015). *Among mild stroke patients who presented within 4 hours of symptoms onset and did not otherwise have contraindications to thrombolysis.
In patients who received thrombolysis, the rate of symptomatic intracerebral hemorrhage within 36 hours of treatment was 2.5% and that of life threatening, serious systemic hemorrhage was 0.3%.
Predictors of Fast Thrombolysis (DTN ≤ 60 min) in Patients with Mild Ischemic Stroke
The median door to needle (DTN) time was 71 min (IQR=43). Over time, DTN decreased from median 89 min (IQR= 44) in 2010 to 62 min (IQR=46) in 2015 (P<. 0001). A total of 461 (39%) patients had fast thrombolysis, defined as a DTN of ≤ 60 min. The characteristics of thrombolyzed patients based on fast vs. slow DTN time are shown in in Supplemental Table II. Accounting for all significant variables, presenting with aphasia (OR=1.36, 95% CI 1.01– 1.84, p=0.04), arrival via EMS (OR=1.89, 95% CI 1.41– 2.54, P<. 0001), to an academic institution (OR=1.93, 95% CI 1.28–2.91, p=0.0016) were independently associated with an increased chance of receiving fast thrombolysis. In contrast, a history of prior ischemic stroke was associated with reduced odds of fast thrombolysis treatment (OR=0.64, 95% CI 0.47–0.88, P=.0067).
Similarly, OTD time was inversely associated with faster DTN time, which decreased from a median of 72 min (IQR=45) in those presenting within 0–2 hours of symptoms to the hospital to 36 min (IQR=12) in those presenting between 3.5 to 4 hours from symptom onset. The median symptom onset to arrival time was longer in patients with fast DTN (67 min, IQR=68) relative to those with delayed DTN (60 min, IQR=53). In multivariable analysis, delay in hospital presentation was associated with higher odds of receiving fast thrombolysis (OR=1.08 per every 10 mins delay, 95% CI 1.05–1.11, P<. 001). Patients with fast DTN times, had also rapid Door to CT times relative to those with delayed DTNs (15 min vs. 23 min).
Discussion
In this large cohort of patients with acute mild ischemic stroke without contraindications to thrombolysis, we identified multiple demographic, clinical and hospital characteristics associated with administration of thrombolytic therapy. Treatment patterns in mild stroke differ by age, race and neurological symptoms. Patients were more likely to receive thrombolysis if they arrived early, via EMS, to a large academic hospital with more thrombolysis experience.
In clinical practice, the majority of patients with low NIHSS do not receive thrombolysis therapy and physicians are inclined to withhold therapy, as symptoms are perceived as non-disabling.2 However, a low score on the NIHSS can still be associated with disabling symptomatology.14 A sub-analysis of the patients with baseline NIHSS ≤6 in the Trial of ORG 10172 in Acute Stroke, showed neither the individual NIHSS items nor the type of neurological syndrome to be independent predictors of disability and long-term outcome.15 More recent studies suggest that the majority of persistent neurological deficits, however mild, do affect functional outcomes and is therefore disability producing.14 Indeed, recent scientific statements have emphasized that mild but disabling symptoms should be treated with thrombolytic therapy.16 Despite this, our results show that over 80% of patients with mild stroke do not receive thrombolysis, even if they present within the time window and have no other contraindications for treatment. Uncontrolled hypertension and a prior history of stroke in combination with diabetes are considered as relative thrombolysis contraindications in certain patients with all severity strokes.8 In our study, the lower rates of thrombolysis in those with a prior history of stroke and HTN may represent the clinician’s uncertainty regarding thrombolysis utilization in mild stroke patients with concurrent other relative thrombolysis contraindications. Our results suggest that many factors beyond the clinical characteristics play a role in the clinician’s decision to use thrombolysis therapy in a mild stroke patient, including time of presentations, mode of hospital arrival and the type and the size of the hospital.
In recent years, an increase in the proportion of mild stroke patients treated with thrombolysis has been reported.17–19 Our study supports these trends, with the frequency of thrombolysis more than doubling in our study population over a span of five years. This further emphasizes the importance of completing studies to look at the safety and efficacy of thrombolysis in mild stroke. Two randomized controlled trials have aimed to answer these question, although the eligibility criteria differ between the studies,10, 11 and one recently stopped enrollment due to slow recruitment.10
The efficacy of thrombolytic therapy in ischemic stroke is highly time dependent and guidelines recommend a DTN time of 60 minutes or less.20 Early treatment is associated with reduced mortality and lower complication rates.21, 22 In prior studies, greater stroke severity, arrival by ambulance, and arrival during regular hours have been shown to be factors most strongly associated with fast DTN times.21 In our study, the overall rate of thrombolytic administration for mild stroke patients significantly dropped with later presentation to the hospital. Patients arriving at the ED within the first 2 hours of symptoms had a 5.5 fold higher rate of IV thrombolytic therapy than did patients arriving between 3.5 to 4.5 hours. Of concern, DTN was significantly slower in those arriving earlier. The absolute DTN time was 2 fold faster in patients arriving between 3.5–4.5 hours compared to those arriving within the first 2 hours. Other groups have reported a similar inverse relationship between OTD and DTN in patients with all severity strokes.23, 24 One possible explanation is selection bias, where the majority of cases in whom treatment could take too long to be initiated within the determined timelines were excluded from receiving the therapy. Alternatively, the shorter treatment times could also reflect a more rapid response time on the part of emergency and stroke teams to implement a therapy when the available time is limited. The fact that DTN times were shorter in comprehensive stroke centers is a testament to the latter, where systems of care have been modified to improve the delivery of care in a more expedited manner.
There is a natural tendency on the part of the patients with mild symptoms to “wait and watch” rather than to immediately present to the emergency department. Similarly, physicians are inclined to obtain additional information and advanced neuroimaging to improve their diagnostic certainty. The perception that these patients have good outcomes and the fear of intracranial hemorrhage among other side effects of treatment may all play a role in withholding or delaying thrombolysis. In this study, we identified a 2.5% symptomatic intracranial hemorrhage rate, which compares favorably with the 4.7% reported for all thrombolyzed patients within GWTG-S.25 Efficacy of thrombolysis in this population is difficult to ascertain from retrospective data given the many confounders and bias by indication. One ongoing prospective study of patients with mild and rapidly improving stroke26 and randomized clinical trials10,11 will shed light on the impact of thrombolysis in mild stroke.
Our study has several limitations. The FL-PR Stroke Registry is a voluntary program including only GWTG participating hospitals, which are generally larger teaching hospitals with higher than average thrombolytic volumes as compared to non-participating hospitals. It is therefore likely that our data over represent the percentage of mild stroke patients treated with thrombolysis. Furthermore, mild stroke was defined using NIHSS and not level of disability and we excluded those with missing NIHSS value at presentation. It is plausible that patients with mild deficits are more likely to have missing NIHSS values, as compared to those with more severe symptoms, which may decrease the estimated rate of thrombolysis in this population. Brain imaging information was inconsistently documented in our registry and not analyzed as part of this report. Advanced imaging findings predict outcomes in TIA and minor stroke27, 28 and therefore can affect the decision to administer thrombolysis treatment. Finally, we do not report on the outcomes of mild stroke with thrombolysis as this paper focuses on identifying characteristics that are associated with thrombolysis utilization, not thrombolysis outcome. Despite these limitations, our study is the largest cohort to report the current practice pertaining to acute thrombolytic treatment of patients with mild stroke.
While randomized controlled trials are needed to provide robust clinical evidence for use of thrombolysis in mild stroke, data from large multi-center registries such as ours offer an insight to the practice patterns in the real world and can guide design and implement successful educational interventions and future studies to improve the care of those with mild strokes.
Supplementary Material
Acknowledgments
Funding sources
The Florida Puerto Rico Collaboration to Reduce Stroke Disparities Study is supported by the National Institute of Health (NIH)/National Institute of Neurological Disorders (NINDs) and Stroke Prevention and Intervention Research Program (SPIRP) cooperative grant (Grant Number: U54NS081763).
Dr. Sacco is the recipient and the primary investigator of the SPIRP cooperative grant from the NIH/NINDs (Grant Number: U54NS081763). Dr. Rundek is the recipient of the women’s supplement from the NIH, Office of Research on Women’s Health (Grant Number: 3U54NS081763-01S1). Dr. Romano receives research salary support from the SPIRP cooperative grant from the NIH/NINDS (Grant Number: U54NS081763). Dr. Koch receives research salary support from the SPIRP cooperative grant from the NIH/NINDS (Grant Number: U54NS081763).
References
- 1.Reeves M, Khoury J, Alwell K, Moomaw C, Flaherty M, Woo D, et al. Distribution of national institutes of health stroke scale in the cincinnati/northern kentucky stroke study. Stroke; a journal of cerebral circulation. 2013;44:3211–3213. doi: 10.1161/STROKEAHA.113.002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith EE, Fonarow GC, Reeves MJ, Cox M, Olson DM, Hernandez AF, et al. Outcomes in mild or rapidly improving stroke not treated with intravenous recombinant tissue-type plasminogen activator: Findings from get with the guidelines-stroke. Stroke; a journal of cerebral circulation. 2011;42:3110–3115. doi: 10.1161/STROKEAHA.111.613208. [DOI] [PubMed] [Google Scholar]
- 3.Nedeltchev K, Schwegler B, Haefeli T, Brekenfeld C, Gralla J, Fischer U, et al. Outcome of stroke with mild or rapidly improving symptoms. Stroke; a journal of cerebral circulation. 2007;38:2531–2535. doi: 10.1161/STROKEAHA.107.482554. [DOI] [PubMed] [Google Scholar]
- 4.Smith EE, Abdullah AR, Petkovska I, Rosenthal E, Koroshetz WJ, Schwamm LH. Poor outcomes in patients who do not receive intravenous tissue plasminogen activator because of mild or improving ischemic stroke. Stroke; a journal of cerebral circulation. 2005;36:2497–2499. doi: 10.1161/01.STR.0000185798.78817.f3. [DOI] [PubMed] [Google Scholar]
- 5.Romano JG, Smith EE, Liang L, Gardener H, Campo-Bustillo I, Khatri P, et al. Distinct short-term outcomes in patients with mild versus rapidly improving stroke not treated with thrombolytics. Stroke; a journal of cerebral circulation. 2016;47:1278–1285. doi: 10.1161/STROKEAHA.115.011528. [DOI] [PubMed] [Google Scholar]
- 6.National Institute of Neurological Disorders Stroke rt PASSG. Recombinant tissue plasminogen activator for minor strokes: The national institute of neurological disorders and stroke rt-pa stroke study experience. Annals of emergency medicine. 2005;46:243–252. doi: 10.1016/j.annemergmed.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 8.Demaerschalk BM, Kleindorfer DO, Adeoye OM, Demchuk AM, Fugate JE, Grotta JC, et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: A statement for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2016;47:581–641. doi: 10.1161/STR.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 9.Activase (Alteplase) for Acute Ischemic Stroke. Genentech USA IA, Genentech I. [Accessed 2015];Activase (alteplase) for acute ischemic stroke. Https://www.Activase.Com/iscstroke.
- 10.PRISMS. A study of the efficacy and safety of alteplase in participants with mild stroke (prisms) [Accessed 2015]; https://clinicaltrials.Gov/ct2/show/record/nct02072226.
- 11.TEMPO-2. Tnk-tpa evaluation for minor ischemic stroke with proven occlusion) [Accessed 2015]; http://www.Ucalgary.Ca/quicr/research - tempo-2.
- 12.Smaha LA American Heart A. The american heart association get with the guidelines program. American heart journal. 2004;148:S46–48. doi: 10.1016/j.ahj.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Shen C, Li X, Robins JM. On weighting approaches for missing data. Stat Methods Med Res. 2013;22:14–30. doi: 10.1177/0962280211403597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coutts SB, Modi J, Patel SK, Aram H, Demchuk AM, Goyal M, et al. What causes disability after transient ischemic attack and minor stroke?: Results from the ct and mri in the triage of tia and minor cerebrovascular events to identify high risk patients (catch) study. Stroke; a journal of cerebral circulation. 2012;43:3018–3022. doi: 10.1161/STROKEAHA.112.665141. [DOI] [PubMed] [Google Scholar]
- 15.Leira EC, Ludwig BR, Gurol ME, Torner JC, Adams HP., Jr The types of neurological deficits might not justify withholding treatment in patients with low total national institutes of health stroke scale scores. Stroke; a journal of cerebral circulation. 2012;43:782–786. doi: 10.1161/STROKEAHA.111.620674. [DOI] [PubMed] [Google Scholar]
- 16.Re-examining Acute Eligibility for Thrombolysis Task F. Levine SR, Khatri P, Broderick JP, Grotta JC, Kasner SE, et al. Review, historical context, and clarifications of the ninds rt-pa stroke trials exclusion criteria: Part 1: Rapidly improving stroke symptoms. Stroke; a journal of cerebral circulation. 2013;44:2500–2505. doi: 10.1161/STROKEAHA.113.000878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stecksen A, Asplund K, Appelros P, Glader EL, Norrving B, Eriksson M, et al. Thrombolytic therapy rates and stroke severity: An analysis of data from the swedish stroke register (riks-stroke) 2007–2010. Stroke; a journal of cerebral circulation. 2012;43:536–538. doi: 10.1161/STROKEAHA.111.630590. [DOI] [PubMed] [Google Scholar]
- 18.Romano JG, Smith EE, Liang L, Gardener H, Camp S, Shuey L, et al. Outcomes in mild acute ischemic stroke treated with intravenous thrombolysis: A retrospective analysis of the get with the guidelines-stroke registry. JAMA Neurol. 2015;72:423–431. doi: 10.1001/jamaneurol.2014.4354. [DOI] [PubMed] [Google Scholar]
- 19.Schwamm LH, Ali SF, Reeves MJ, Smith EE, Saver JL, Messe S, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at get with the guidelines-stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6:543–549. doi: 10.1161/CIRCOUTCOMES.111.000303. [DOI] [PubMed] [Google Scholar]
- 20.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: An updated pooled analysis of ecass, atlantis, ninds, and epithet trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 21.Saver JL, Fonarow GC, Smith EE, Reeves MJ, Grau-Sepulveda MV, Pan W, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309:2480–2488. doi: 10.1001/jama.2013.6959. [DOI] [PubMed] [Google Scholar]
- 22.Fonarow GC, Zhao X, Smith EE, Saver JL, Reeves MJ, Bhatt DL, et al. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA. 2014;311:1632–1640. doi: 10.1001/jama.2014.3203. [DOI] [PubMed] [Google Scholar]
- 23.Albers GW, Bates VE, Clark WM, Bell R, Verro P, Hamilton SA. Intravenous tissue-type plasminogen activator for treatment of acute stroke: The standard treatment with alteplase to reverse stroke (stars) study. JAMA. 2000;283:1145–1150. doi: 10.1001/jama.283.9.1145. [DOI] [PubMed] [Google Scholar]
- 24.Saver JL, Smith EE, Fonarow GC, Reeves MJ, Zhao X, Olson DM, et al. The "golden hour" and acute brain ischemia: Presenting features and lytic therapy in >30,000 patients arriving within 60 minutes of stroke onset. Stroke; a journal of cerebral circulation. 2010;41:1431–1439. doi: 10.1161/STROKEAHA.110.583815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta RH, Cox M, Smith EE, Xian Y, Bhatt DL, Fonarow GC, et al. Race/ethnic differences in the risk of hemorrhagic complications among patients with ischemic stroke receiving thrombolytic therapy. Stroke; a journal of cerebral circulation. 2014;45:2263–2269. doi: 10.1161/STROKEAHA.114.005019. [DOI] [PubMed] [Google Scholar]
- 26.(MaRISS). Mild and rapidly improving stroke study. [Accessed 2017]; https://clinicaltrials.Gov/ct2/show/nct02072681.
- 27.Coutts SB, Modi J, Patel SK, Demchuk AM, Goyal M, Hill MD, et al. Ct/ct angiography and mri findings predict recurrent stroke after transient ischemic attack and minor stroke: Results of the prospective catch study. Stroke; a journal of cerebral circulation. 2012;43:1013–1017. doi: 10.1161/STROKEAHA.111.637421. [DOI] [PubMed] [Google Scholar]
- 28.Asdaghi N, Hill MD, Coulter JI, Butcher KS, Modi J, Qazi A, et al. Perfusion mr predicts outcome in high-risk transient ischemic attack/minor stroke: A derivation-validation study. Stroke; a journal of cerebral circulation. 2013;44:2486–2492. doi: 10.1161/STROKEAHA.111.000208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


