Abstract
Bupivacaine is a commonly used local anesthetic in postoperative pain management. We evaluated the effects of a prolonged, local delivery of bupivacaine on pain behavior accompanying a chronic compression of the dorsal root ganglion (CCD) – an animal model of radicular pain. Poly(lactide-coglycolide) (PLGA) nanoparticles encapsulating bupivacaine were injected unilaterally into the L3 and L4 DRGs of mice just before producing CCD by implanting a stainless-steel rod in the intervertebral foramen of each ganglion. Behavioral sensitivity to punctate mechanical stimuli (Von Frey filaments) of different forces of indentation, delivered to each hind paw, was measured before and on subsequent days of testing after the CCD. Nanoparticles were spherical in morphology and 150±10 nm in diameter. Bupivacaine was steadily released as measured in vitro over 35 days. A dye that was encapsulated in the nanoparticles was found in the intact DRG after 2 weeks. CCD alone or with injection of blank (control) nanoparticles produced a behavioral hypersensitivity to the punctate stimuli on the ipsilateral paw without affecting sensitivity on the contralateral, over a period of 7 – 14 days. The hypersensitivity was manifested as an increased incidence of paw-withdrawal to indentation forces normally below threshold (allodynia) and an increased shaking to a filament force that always elicited withdrawal prior to CCD (hyperalgesia). In contrast, nanoparticles with bupivacaine prevented any manifestation of allodynia or hyperalgesia on the ipsilateral hind paw while leaving normal nociceptive responses largely intact on both hind paws. CCD induced behavioral hypersensitivity to nociceptive stimuli is known to be associated with a hyperexcitability of sensory neurons originating in the compressed ganglion. We hypothesize that bupivacaine-loaded PLGA nanoparticles may prevent the occurrence of this neuronal hyperexcitability without reducing the nociceptive information normally conducted from the periphery to the central nervous system. The slow, sustained delivery of bupivacaine by nanoparticles may provide a means of preventing the occurrence of postoperative neuronal hyperexcitability that could develop into chronic neuropathic pain.
Keywords: DRG compression, bupivacaine, nanoparticles, allodynia, hyperalgesia
1. Introduction
A chronic compression of the dorsal root ganglion (CCD) in rodents is an animal model of lumbar intraforaminal stenosis and radicular pain for humans [3, 13]. There is a behavioral hypersensitivity to punctate stimuli on the plantar and dorsal hind paw ipsilateral to the compressed DRGs. Specifically, CCD increased the incidence of paw-withdrawal to normally subthreshold forces (≤50% incidence) of punctate indentation (defined as “allodynia”) and increased the incidence of paw-shaking to a normally suprathreshold nociceptive force (“hyperalgesia”) [2, 15]. One or more similar measures of behavioral mechanical hypersensitivity have been observed in the rat after CCD or after the local application of either proinflammatory tissue or inflammatory activators to the lumbar DRG [4, 14, 17].
During CCD, the cell bodies of Intact sensory neurons of the compressed DRG become hyperexcitable as evidenced, for example, by the presence of spontaneous activity originating in the DRG [6, 18], increased responses to electrical, thermal and chemical nociceptive stimuli [5], and an increased density of voltage gated TTX-S and TTX-R current in small and medium sized cell bodies [1, 2]. At 7 days after CCD, cutaneous mechanosensitive C-fiber nociceptors were found more responsive to punctate, mechanical stimuli that elicited a greater incidence of paw-shaking behavior [15].
The present study was designed to test whether a sustained delivery of a local anesthetic to the compressed DRG might act to prevent the mechanical hypersensitivity without impairing the normal capacity to respond to nociceptive stimulation of the skin. To achieve the sustained release of anesthetic, bupivacaine was encapsulated in poly(lactide-coglycolide) (PLGA) nanoparticles that were injected into the DRG at the time of compression.
2. Materials and methods
All experimental procedures with animals were conducted at Yale University, approved by the Institutional Animal Care and Use Committee of Yale University, and were in accordance with the guidelines provided by the International Association for the Study of Pain and National Institutes of Health.
2.1. Preparation of bupivacaine-loaded PLGA nanoparticles
To produce bupivacaine for nanoparticle synthesis, the hydrochloride group in commercial bupivacaine (Sigma-Aldrich) was removed through titration using NaOH, resulting in bupivacaine soluble in organic solvents. Nanoparticles were synthesized according to the standard, single-emulsion procedure [19]. In brief, 100 mg PLGA, a copolymer of poly-lactic acid and poly-glycolic acid, 40 mg bupivacaine and 1 mg DiI, a red fluorescent dye (dialkylcarbocyanine, Invitrogen), were dissolved in 2 mL of dichloromethane (DCM). The solution was added dropwise to 4 mL 2.5% polyvinyl alcohol (PVA). The emulsion was sonicated for 10 seconds on ice 3 times, poured into a beaker containing aqueous 0.3% (v/v) PVA, and stirred at room temperature for 3 hours to allow the DCM to evaporate and the particles to harden. Particles were collected by centrifugation at 18,000 rpm for 30 minutes, washed twice with water, frozen, and lyophilized. Just before lyophilization, the disaccharide, trehalose, was added to reduce aggregation of the nanoparticles. Thus, the resultant lyophilized material was a mixture of nanoparticles and sugar. Appropriate amounts of material were weighed to achieve 60 mg/ml of nanoparticles in the injectate.
2.2. Characterization of the release of bupivacaine
The release of bupivacaine was monitored over 35 days. Bupivacaine nanoparticles were re-suspended in PBS at 1 mg/ml and rotated in a shaker at 60 rpm and 37°C. At each sampling time, the nanoparticle suspension was centrifuged for 10 min at 15,000 rpm. The supernatant was removed and an equal volume of PBS was replaced for continued monitoring of release.
Using methodology of Schmidt [12], we vortexed all solutions of bupivacaine for 1 minute. The supernatant was transferred to another vial and evaporated to solid residue under a nitrogen stream at 40°C Residues were reconstituted in 200 μL of the mobile phase, i.e. 21:79 (v:v) PBS: acetonitrile, vortexed for 30 seconds, and transferred to an HPLC vial with low volume inserts. Five solutions of known concentrations ranging from 0.188 to 3 mg/L were used to generate a linear calibration curve (R2>0.99). The HPLC analysis was performed on a low-pH reversed phase high-performance liquid chromatography (Waters 2690, Milford, MA, USA) with a Waters Spherisorb C8 column (4.6 mm×250 mm, 5 μm particle size) and an injection volume of 20 μL. The temperature was set at 30°C with a flow rate of 1mL/min for 20 min. A total of 14 fractions were collected for 1, 2, 4, 6, 24, 48, 72, 96, 120,144,168 h, and 21, 28 and 35 days. Aliquots obtained from the solutions of nanoparticle and bupivacaine mixtures were analyzed using a Waters 2487 UV detector optimized at 230 nm. The retention time for bupivacaine was about 7.6 min.
2.3. Preparation of nanoparticles for injection
A special micropipette for DRG injection was constructed [11] (and personal communication, O. A. Samad) to minimize dead space. Glass pipettes (Sutter Instruments, 1.2 mm OD X 0.69 mm ID, 10 cm length) were pulled, resulting in two 5–6 cm micropipettes. The tip of each was beveled (BV-10 micro-pipette beveler, Sutter Instruments) to an opening of 50–100 μm. Micropipettes were fractured 10–15 mm from the tip. PE-10 polyethylene tubing was inserted into the micropipette until it reached the tip. Then, a light cured adhesive (Loctite 3106) was applied to the PE-10 where it entered the micropipette. Through capillary action and by gentle agitation of the PE-10, the glue moved towards the tip. When the glue just reached the end of the PE-10 tubing, a dental curing light was applied to harden the glue, providing a seal between the PE-10 tubing and the glass micropipette tip. The remaining barrel portion of the micropipette was threaded over the back end of the tubing to rejoin the previously fractured end of the other portion. The two pieces of glass were then glued together restoring the 5–6 cm length micropipette. Micropipettes were connected by PE-10 to a 10 μl Hamilton syringe. The syringe, tubing and pipette were filled with sterile water.
At the time of surgery, the nanoparticle suspension was prepared. Lyophilized nanoparticles were weighed, added to sterile Dulbecco’s PBS to produce a 60 mg/mL solution, vortexed for 20 sec, and placed in an ultrasonic bath for 20 sec to produce a suspension of nanoparticles. Approximately 1 μl of air was drawn into the pipette. The resultant air bubble acted as a barrier between the sterile water filler and the nanoparticle suspension and as an indicator of fluid movement within the micropipette.
2.4. Surgical treatment
Twenty-five male C57BL/6 mice (Charles River, Wilmington, MA), each weighing 25–30 g-wt. were maintained on a 12-hour light/dark cycle. They were housed in groups of 3–5/cage, but singly housed after surgery.
Under 3–4% isoflurane anesthesia, a midline incision was made along the back and the intervertebral foramina of L3 and L4, exposed after separating the paraspinal muscles from the mammillary process and the transverse process [4]. A 1-mm hole was drilled with an HP #1/2 carbide burr (Steribur #100-5860) just above each ganglion. CCD was produced by the insertion of an L-shaped stainless steel rod, (0.3 mm diameter, each arm 2 mm in length), into each foramen to compress the DRGs [2]. Just before rod insertion, two groups of mice received nanoparticles encapsulating either bupivacaine and the fluorescent dye, DiI (“bupivacaine nanoparticles”, n = 10), or DiI without bupivacaine (“blank nanoparticles”, n = 8). A third group received no injection (“CCD only”, n = 7).
Under a dissection microscope, the micropipette was advanced until the tip touched the epineurium over the L3 ganglion. After puncturing the epineurium with a further advancement of 2 μm, the micropipette was retracted and then returned to the same location; 0.5 – 1.0 μl of suspension was injected over a period of 1 minute. The L4 DRG was similarly injected.
The incision was closed in layers and topically treated with ointment containing an antibiotic (TriTop), a local anesthetic and an anti-inflammatory agent. A systemic antibacterial was also administered (Baytril, 10 mg/mL, i.m.).
The midline incision provided no visual clue to the behavioral tester as to the laterality of the CCD. The behavioral tester was also blinded as to experimental treatment (i.e. CCD only vs. CCD with blank- or bupivacaine nanoparticles).
After completion of all behavioral testing, mice were euthanized, and DRGs receiving CCD were microscopically examined to confirm rod placement and, after removal of the epineurium and flushing with saline, that each injected ganglion exhibited the fluorescent dye.
2.5. Immunohistochemistry and microscopy
Fifteen days after CCD surgery, the L3 and L4 DRGs of two mice were removed after transcardial perfusion, first with PBS and second, 4% paraformaldehyde, and post-fixed in the same fixative for 4 h, and cryoprotected in 30% sucrose overnight. Tissue was frozen and sectioned at 12 μm thickness by a cryostat and processed for immunofluorescence labeling [10]. Sections were permeabilized with 0.2% Triton X-100 in PBS for 15 min, then incubated with blocking buffer (3% BSA) for 1 h, incubated at 4°C overnight with primary antibodies (rabbit anti-PGP9.5, 1:100, Invitrogen, CA, USA), and exposed to appropriate secondary antibodies (Alexa Fluor 488-conjugated donkey anti-rabbit, 1:400, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 1 h. Slides were washed in PBS and cover-slipped with VECTASHIELD Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA, USA). Cells were visualized with a C2+ Nikon confocal microscopy imaging system (Nikon, Japan). One field of view (15X) from one section on each ganglion was examined. More than 100 neuronal somata with nucleus profiles for each condition were counted and the percentiles of immunopositive neurons obtained.
2.6. Behavioral Measurements
Each day for one week before testing mice were habituated to handling, to the test chamber, and to stimulation of their paws with Von-Frey filaments. Testing began within 1 day before, surgery. The mouse was placed in a transparent, plastic chamber (8×7×3.5 cm) with a mesh floor mounted on a platform 7 cm above the table. After 30 min of acclimation, the incidence of nociceptive responses was obtained to Von-Frey filaments, applied for 1 second, every 45–50 seconds, 10 times alternately to the plantar surface of each hind paw. Tests were administered before and on days 1, 3, 5, 8, 11, and 14 after CCD surgery. Incidence was expressed as the percentage of 10 trials that resulted in a response.
Two tests were given on each day of testing. Firstly, the incidence of paw-withdrawal was obtained in response to Von-Frey filaments, each having the same tip diameter of 200 μm but delivering different bending forces (2, 6 or 20 mN). These forces elicited a preoperative incidence of paw-withdrawal near, or below, a threshold criterion of 50%.
Secondly, the incidence of paw-shaking was measured in response to a normally aversive stimulus, i.e., one that preoperatively always elicited paw-withdrawal. This stimulus was produced by a filament with a tip diameter of 100 μm that delivered a bending force of 40 mN. Hyperalgesia was defined as a significant postoperative increase over preoperative in the incidence of shaking the stimulated paw [15]. Conversely, we defined analgesia as a decrease in postoperative vs. preoperative incidence of paw-withdrawal or shaking.
2.7. Statistical analyses
Separate analyses were made of the withdrawal and shaking incidences for each paw. For each hind paw, the incidence of response to a particular stimulus delivered preoperatively was subtracted from the incidence of response to the same stimulus delivered on each postoperative day of testing to obtain the primary outcome, an incidence-difference score.
The statistical comparison of this repeatedly measured outcome was performed using the mixed-effects regression model method, with an unstructured covariance matrix specified to account for the within animal correlation of repeated measures. Adjusted covariates in the regression model included baseline preoperative incidence score, treatment (CCD only vs. CCD with bupivacaine nanoparticles vs. CCD with blank nanoparticles), time (1,3,5,8,11,14 postoperative days), and time-by-treatment interaction. The least square mean difference and 95% confidence intervals between different groups (at different days and overall) and/or between different days (within the same group) were obtained.
The robust standard errors and test statistics involving the fixed effects were computed by invoking the EMPIRICAL option within the SAS Proc Mixed procedure. Separate regression models were fitted under different forces (2, 6, and 20 mN) at each paw, respectively. All analyses were performed using SAS, version 9.4 (Cary, NC). A two-sided p-value of less than 0.05 was selected as statistically significant.
3. Results
3.1. Characteristics and release profile of bupivacaine-loaded nanoparticles
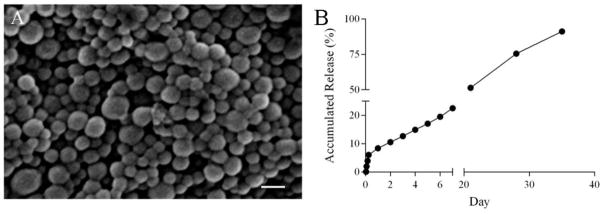
Scanning electron microscopy revealed that bupivacaine-loaded nanoparticles were spherical with a mean diameter of 150±10 nm (Fig. 1A). We measured the release of bupivacaine from nanoparticles using HPLC. When totally dissolved in DMSO, the weight of bupivacaine in 2 mg of nanoparticles was 0.48 mg. This amount of bupivacaine was taken as 100%. using an equivalent weight of nanoparticles, in a subsequent HPLC analysis of how much bupivacaine was released (normalized to the total) in a series of aliquots taken over a period of 35 days (Fig. 1B).
Figure 1.
Characterization of bupivacaine-encapsulated PLGA nanoparticles. (A) Morphology of bupivacaine nanoparticles captured by scanning electron microscopy showing a diameter of 150±10 nm. (Scale bar=500 nm). (B) Release profile of bupivacaine from nanoparticles over 35 days.
3.2. Confocal imaging of DRG
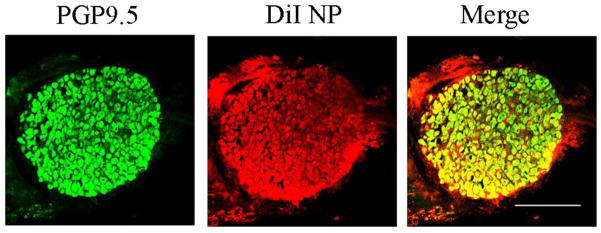
To obtain an indirect indication of whether bupivacaine nanoparticles had entered into DRG neurons, we examined the distribution of DiI-labeled neuronal cell bodies in the DRG after CCD. Dil emits red-orange fluorescence when excited. Co-encapsulation of DiI allowed detection of nanoparticles using florescence microscopy. A total of 12 cryosections from four DRGs were examined with confocal microscopy. There were no signs of any necrosis. DiI from nanoparticles in neurons was confirmed via immunostaining (Fig. 2). From a total of 569 neurons, 295 (51.85%) contained DiI.
Figure 2.
Fluorescent images of neurons with DiI (red) and immunostained for PGP9.5 (green) in a 12 μm thick cryosection from the L4 DRG 15 days after CCD surgery. Scale bar=500 μm.
3.3. Effects of bupivacaine on the incidence of mechanically evoked paw-withdrawal and-shaking after CCD
All animals exhibited no obvious signs of motor impairment from CCD. Furthermore, neither spontaneous behaviors, such as paw lifting or shaking, nor abnormal behaviors directed toward the hind limb, such as biting or licking, were observed.
For each of the three filaments, there were no significant differences in the preoperative mean incidence of withdrawal between the three treatment groups and between values obtained for each paw (supplementary Table 1). The forces of 2 and 6 mN were useful, then, for our measurements of CCD-induced allodynia because they normally did not elicit a nociceptive response on most applications.
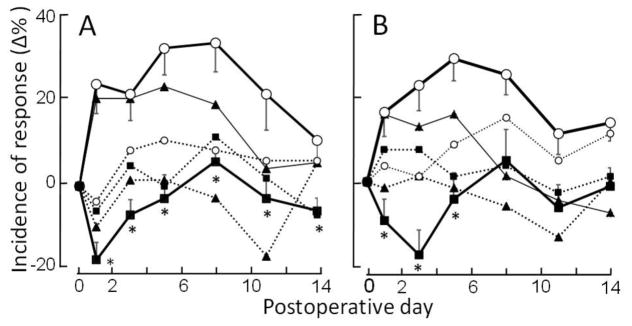
For the contralateral paw, there were no significant changes in withdrawal incidences to any of the filaments for the three groups except for a significant increase to the 2mN filament on one or two postoperative days after CCD alone or CCD with blank nanoparticles (Fig. 3A and supplementary Fig. 4A).
Figure 3.
Effects of bupivacaine on the incidences of paw withdrawal and paw shaking in response to Von Frey filaments delivered to each hind paw on different days postoperative to CCD surgery. Each data point is the mean percentage change (±SEM) in incidence from preoperative values obtained on day zero (supplementary Tables 1 and 2). (A) Mean changes over time in the incidence of paw withdrawal evoked by a filament with a tip-diameter of 200 μm and exerting a bending force of 6 mN. (B) Mean changes over time in the incidence of paw shaking in response to a filament with a 100μm tip and bending force of 40mN. Mice receiving either CCD only (solid triangles), CCD and bupivacaine nanoparticles (solid squares) or CCD with blank nanoparticles (no drug) (open circles) were tested on each paw, one ipsilateral (solid lines) and the other contralateral (dotted lines) to the CCD. The asterisks indicate significant differences (P<0.05) between mice receiving bupivacaine- vs. blank-nanoparticles tested on the paw ipsilateral to the CCD (thicker solid lines). SEMs not shown were similar to those displayed.
After blank nanoparticles, the incidences of ipsilateral paw-withdrawal to each force were significantly higher on each postoperative day than they were before CCD. These increases to the 6 mN filament and to the other two filaments, reveal CCD-induced mechanical allodynia (Fig. 3A and supplementary Fig. 4A). Blank nanoparticles with CCD did not diminish allodynia on the ipsilateral paw compared to CCD alone. For both groups, postoperative increases in withdrawal incidence were the same on each postoperative day for the 2 mN filament but statistically greater for the blank- nanoparticle group than the CCD-only group one or two days for the other two filaments (Fig. 3A and supplementary Fig. 4).
Mice receiving bupivacaine nanoparticles exhibited no significant increases in the incidences of withdrawal over their preoperative values. Furthermore, the changes in withdrawal incidence were significantly less at each postoperative day for the bupivacaine group compared to the blank nanoparticle group in response to each filament. The bupivacaine group exhibited a significant decrease in the incidence of withdrawal (analgesia) on the first day of testing in response to the 6 mN filament and to the 20 mN filament on the first and third days (Fig. 3A and supplementary. Fig. 4A). There were no changes in the response to the 2 mN filament. That is, after the first few postoperative days the mice continued to detect and withdraw to these filaments as they did prior to CCD. Thus, the bupivacaine prevented manifestation of CCD-induced allodynia without producing a sustained analgesia.
The postoperative incidence of shaking the paw in response to the 40mN-filament did not increase over preoperative values for the paw contralateral to CCD except on days 8 and 11 after CCD with blank nanoparticles (Fig. 3B and supplementary Table 2). But there was a significant increase in shaking (indicating hyperalgesia) for the paw ipsilateral to CCD that persisted for two weeks for CCD with blank nanoparticles and one week for CCD alone.
By contrast, mice receiving bupivacaine exhibited no significant postoperative increases in shaking (no hyperalgesia) ipsilateral to the CCD and, on day 3, a significant decrease (analgesia). The contralateral paw exhibited no significant change in shaking. The magnitude of postoperative changes in shaking incidence were significantly greater for the blank nanoparticle group than for the bupivacaine group for up to a week after CCD (Fig. 3B). Because the bupivacaine group shook the paw at the same incidence as it did preoperatively (except for a decrease on the third day), the effects of bupivacaine were to prevent any manifestation of CCD-induced hyperalgesia without producing sustained analgesia.
4. Discussion
CCD with no nanoparticles or with blank nanoparticles without bupivacaine produced persistent allodynia and hyperalgesia on the ipsilateral plantar hind paw as previously demonstrated in rodents [2, 3, 13, 15]. However, nanoparticles containing bupivacaine prevented manifestation of both the allodynia and the hyperalgesia produced by CCD and brought the mechanical sensitivity to within the range of values normally obtained for naïve animals.
Early spontaneous afferent activity is associated with the onset of neuropathic pain behavior in rodent models of neuropathic pain produced by an injury of the peripheral nerve or dorsal root ganglion. Powdered bupivacaine applied proximal to an injured peripheral nerve prevented the onset of spontaneous action potentials from the injury site and the development of mechanical allodynia [16]. And, the expression of Kir2.1 by an inducible adenoviral vector reduced the neuronal hyperexcitability and hyperalgesia induced by CCD [7]. The interventions in each study were effective if given at the time of injury but not after neuronal hyperexcitability and mechanical hypersensitivity became fully developed. We hypothesize that in the present study, the bupivacaine nanoparticles prevented the development of CCD-induced neuronal hyperexcitability and the allodynia and hyperalgesia. Future experiments are needed to determine the time course of bupivacaine release in vivo, and whether it has an antinociceptive effect if delivered to the uninjured DRG or delivered to the injured DRG after allodynia and hyperalgesia are fully developed. Parallel electrophysiological recordings from nerve fibers and cell bodies of DRG neurons are needed to determine whether bupivacaine blocks axonal conduction and/or acts mainly to reduce the membrane excitability of cell bodies in the DRG.
Local anesthetics acting on the peripheral nervous system are often limited due to their short duration of action. Liposomal bupivacaine is an extended-release formulation of the local anesthetic intended to provide postsurgical analgesia for up to 72 h when administrated into surgical wounds. In addition to its use in wound infiltration, liposomal bupivacaine has been administered in either the intracisternal or epidural space[8, 9]. Treating peripheral pain directly with local DRG injection may advance the goal of selective pain medicine while reducing unwanted side effects, potentially offering a significant improvement on current strategies for controlling severe pain. A highly localized method of delivery, such as what we used here, may help to decrease unintended systemic consequences. Advantages of using polymeric nanoparticles over the liposomal approach include better control of drug release for persistent efficacy and a lower toxicity [19]. PLGA is fully degraded by hydrolysis to the endogenous metabolite monomers, lactic acid and glycolic acid each of which is a normal by-product of various metabolic pathways and easily metabolized in the Krebs cycle to CO2 and water. PLGA is a polymer that has been safely used in clinical applications since 1969 [19]. The approach developed in the present study is safe and highly translatable. Bupivacaine in PLGA nanoparticles might not have to be injected into the DRG but could be deposited by transforaminal injection outside the epineurium where they could diffuse into the ganglion
Treating peripheral pain directly with local application to the DRG may advance the goal of selective pain medicine while reducing unwanted side effects, potentially offering a significant improvement on current strategies for controlling or preventing persistent pain.
Supplementary Material
Highlights.
We made PLGA nanoparticles that released bupivacaine steadily for 35 days, in vitro.
DRG chronic compression produced sustained ipsilateral cutaneous hypersensitivity
Prior DRG injection of bupivacaine nanoparticles prevented the hypersensitivity
Bupivacaine PLGA nanoparticles provide a way to prevent or reduce neuropathic pain
Acknowledgments
Funding provided by National Institutes of Health [NS014624(RHL), NS095817(JZ), and NS095147(JZ)]; and National Natural Science Foundation of China [NSFC #81271239(CM), #91632113(CM)]; and the CAMS Innovation Fund for Medical Sciences [CIFMS #2017-I2M-4-005(TW), #2017-12M-3-008(CM)].
Footnotes
Poly(lactide-coglycolide)
Author contributions:
TW performed the surgery, nanoparticle injections, and the HPLC and immunochemical analyses. DT and JZ produced the nanoparticles and SGS prepared them for injection. OH collected the behavioral data and FD performed the statistical analyses. TW and RHL wrote the manuscript with assistance from CM, FD, JZ, OH, and SGS. The project was supervised by CM and RHL.
Conflict of interest statement
Authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fan N, Donnelly DF, LaMotte RH. Chronic compression of mouse dorsal root ganglion alters voltage-gated sodium and potassium currents in medium-sized dorsal root ganglion neurons. Journal of neurophysiology. 2011;106:3067–3072. doi: 10.1152/jn.00752.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan N, Sikand P, Donnelly DF, Ma C, Lamotte RH. Increased Na+ and K+ currents in small mouse dorsal root ganglion neurons after ganglion compression. Journal of neurophysiology. 2011;106:211–218. doi: 10.1152/jn.00065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu SJ, Xing JL. An experimental model for chronic compression of dorsal root ganglion produced by intervertebral foramen stenosis in the rat. Pain. 1998;77:15–23. doi: 10.1016/S0304-3959(98)00067-0. [DOI] [PubMed] [Google Scholar]
- 4.Kawakami M, Tamaki T, Weinstein JN, Hashizume H, Nishi H, Meller ST. Pathomechanism of pain-related behavior produced by allografts of intervertebral disc in the rat. Spine. 1996;21:2101–2107. doi: 10.1097/00007632-199609150-00009. [DOI] [PubMed] [Google Scholar]
- 5.Ma C, Greenquist KW, Lamotte RH. Inflammatory mediators enhance the excitability of chronically compressed dorsal root ganglion neurons. Journal of neurophysiology. 2006;95:2098–2107. doi: 10.1152/jn.00748.2005. [DOI] [PubMed] [Google Scholar]
- 6.Ma C, LaMotte RH. Multiple sites for generation of ectopic spontaneous activity in neurons of the chronically compressed dorsal root ganglion. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:14059–14068. doi: 10.1523/JNEUROSCI.3699-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma C, Rosenzweig J, Zhang P, Johns DC, LaMotte RH. Expression of inwardly rectifying potassium channels by an inducible adenoviral vector reduced the neuronal hyperexcitability and hyperalgesia produced by chronic compression of the spinal ganglion. Molecular pain. 2010;6:65. doi: 10.1186/1744-8069-6-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malinovsky JM, Benhamou D, Alafandy M, Mussini JM, Coussaert C, Couarraze G, Pinaud M, Legros FJ. Neurotoxicological assessment after intracisternal injection of liposomal bupivacaine in rabbits. Anesthesia and analgesia. 1997;85:1331–1336. doi: 10.1097/00000539-199712000-00027. [DOI] [PubMed] [Google Scholar]
- 9.Malinovsky JM, Le Corre P, Meunier JF, Chevanne F, Pinaud M, Leverge R, Legros F. A dose-response study of epidural liposomal bupivacaine in rabbits. Journal of controlled release: official journal of the Controlled Release Society. 1999;60:111–119. doi: 10.1016/s0168-3659(99)00062-0. [DOI] [PubMed] [Google Scholar]
- 10.Qu L, Zhang P, LaMotte RH, Ma C. Neuronal Fc-gamma receptor I mediated excitatory effects of IgG immune complex on rat dorsal root ganglion neurons. Brain, behavior and immunity. 2011;25:1399–1407. doi: 10.1016/j.bbi.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samad OA, Tan AM, Cheng X, Foster E, Dib-Hajj SD, Waxman SG. Virus-mediated shRNA Knockdown of Nav1.3 in Rat Dorsal Root Ganglion Attenuates Nerve Injury-induced Neuropathic Pain. Molecular therapy: the journal of the American Society of Gene Therapy. 2013;21:49–56. doi: 10.1038/mt.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt B, Ohri R, Wang JC, Blaskovich P, Kesselring A, Scarborough N, Herman C, Strichartz G. Local pathology and systemic serum bupivacaine after subcutaneous delivery of slow-releasing bupivacaine microspheres. Anesthesia and analgesia. 2015;120:36–44. doi: 10.1213/ANE.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 13.Song XJ, Hu SJ, Greenquist KW, Zhang JM, LaMotte RH. Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. Journal of neurophysiology. 1999;82:3347–3358. doi: 10.1152/jn.1999.82.6.3347. [DOI] [PubMed] [Google Scholar]
- 14.Strong JA, Xie W, Bataille FJ, Zhang JM. Preclinical studies of low back pain. Molecular pain. 2013;9:17. doi: 10.1186/1744-8069-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang T, Hurwitz O, Shimada SG, Qu L, Fu K, Zhang P, Ma C, LaMotte RH. Chronic Compression of the Dorsal Root Ganglion Enhances Mechanically Evoked Pain Behavior and the Activity of Cutaneous Nociceptors in Mice. PloS one. 2015;10:e0137512. doi: 10.1371/journal.pone.0137512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie W, Strong JA, Meij JT, Zhang JM, Yu L. Neuropathic pain: early spontaneous afferent activity is the trigger. Pain. 2005;116:243–256. doi: 10.1016/j.pain.2005.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie WR, Deng H, Li H, Bowen TL, Strong JA, Zhang JM. Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience. 2006;142:809–822. doi: 10.1016/j.neuroscience.2006.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang JM, Song XJ, LaMotte RH. Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglion. Journal of neurophysiology. 1999;82:3359–3366. doi: 10.1152/jn.1999.82.6.3359. [DOI] [PubMed] [Google Scholar]
- 19.Zhou J, Patel TR, Sirianni RW, Strohbehn G, Zheng MQ, Duong N, Schafbauer T, Huttner AJ, Huang Y, Carson RE, Zhang Y, Sullivan DJ, Jr, Piepmeier JM, Saltzman WM. Highly penetrative, drug-loaded nanocarriers improve treatment of glioblastoma. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:11751–11756. doi: 10.1073/pnas.1304504110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.