Abstract
Background
Studies of birth weight associations with ovarian and endometrial cancer risks are limited with inconsistent results, and none has evaluated associations by histologic subtype. We utilized prospectively collected birth weight information to investigate the association with risk of ovarian and endometrial cancers overall and by histologic subtype.
Methods
162,559 girls, born from 1930-1989, from the Copenhagen School Health Records Register (CSHHR) were followed prospectively via linkage with the Danish health registers. Ovarian (n=666) and endometrial (n=694) cancers were identified from 1978-2014. Cox regression was used to estimate hazard ratios (HR) and 95% confidence intervals (CI).
Results
Women with lower (2.0-3.25 vs. 3.26-3.75 kg) and higher (3.75-5.5 vs. 3.26-3.75 kg) birth weights had increased risks of ovarian cancer overall [HR (95% CI): 1.27 (1.06-1.52); 1.51 (1.21-1.87), respectively] and serous ovarian cancers [1.54 (1.19-1.98); 1.98 (1.47-2.67), respectively]. A decreased risk of Type II endometrial tumors was suggested per kilogram increase in birth weight [HR (95% CI): 0.63 (0.40-1.00)].
Conclusions
Our results suggest that both lower and higher birth weights were associated with increased ovarian cancer risk and associations were particularly strong for serous ovarian cancer, the most common subtype. Birth weight was not associated with most types of endometrial cancer.
Keywords: Birth weight, cohort, ovarian cancer, endometrial cancer, histologic subtype
INTRODUCTION
Ovarian cancer is the deadliest gynecological cancer, with 553 new cases and 376 deaths estimated in Denmark per year between 2011 and 2014 [1 ]. Endometrial cancer is the most common and second deadliest gynecological cancer among women in Denmark, with an estimated 769 new cases, and 93 deaths over the same time period [1]. For both tumors many reproductive factors are associated with risk, suggesting an etiological role of hormonal exposure [2]. Early age at menarche, an indicator of early hormonal exposure and increased number of lifetime ovulations, is associated with increased risk of both ovarian and endometrial cancer [2]. It has been suggested that in utero and early life sex-steroid hormone exposures may affect gonadotropin release and subsequent fetal imprinting that could result in earlier ages at menarche [3–5]. Associations with early age at menarche and taller adult height also support that early life may be a critical period for ovarian and/or endometrial cancer initiation [2]. Further, it has been suggested that birth weight may reflect in utero exposures of the individual that may influence later life risk of cardiovascular disease and some cancers [6].
Evidence supporting female cancer risk associations with birth weight only exists for breast cancers, as studies evaluating relations between birth weight and ovarian or endometrial cancers have been limited and showed inconsistent results [7–12]. Further, none of these studies has evaluated etiologic heterogeneity in this context, despite the compelling evidence that adult body size and/or height as well as many other hormonally-related reproductive factors are associated with differing risks for ovarian and endometrial cancer subtypes [13–16]. The aim of this study therefore is to examine birth weight and its association with ovarian and endometrial cancers overall and by histologic subtype.
MATERIALS AND METHODS
Cohort
Data on birth weight were obtained from the Copenhagen School Health Records Register (CSHRR), which has been described previously [17]. Briefly, the CSHRR contains health records for 372,636 children born 1930 through 1989 who attended school in Copenhagen, Denmark. Information on name, sex, and date of birth was systematically recorded on individual health cards along with height and weight measurements collected as part of school-related annual health examinations [17]. From the birth year 1936 onwards birth weight, as reported by parents or guardians, was recorded on each child’s health card [17]. At birth, families are issued an infancy health book with the infants’ birth weight recorded by a visiting health nurse. These books are commonly used as a continuous health record for children and may have been referenced by parents to report birth weight [18]. Correlations above 0.93 have been found between recalled birth weights from the cohort and birth records [18].
Linkage
Unique government-issued identification numbers, from the Danish Civil Registration System of vital statistics [19], were recorded on health cards for children who attended school in 1968 or after, and the identification numbers were retrieved for children who left school before this time. Individuals were followed for information in national health registers based on record linkages using these personal identification numbers. Information on vital status was obtained from the Danish Civil Registration System [19]. Hysterectomy and bilateral oophorectomy or salpingo-oophorectomy information was obtained by linkage to the Danish National Patient Register, which contains information on all hospital discharge diagnoses from 1977 onwards [20].
Cancer registration
Ovarian and endometrial cancers were identified through linkage to the Danish Cancer Registry, which has very high validity [19, 21], using ICD-10 codes, which were available from 1 January 1978, due to a Danish recoding project, through 31 December 2014; ovary: C56.0, C56.2–3 and C56.9 and endometrial: C54.0–C54.1, C54.3–C54.6, C54.9 and C55. Using the ICD-O-3 morphology codes (Supplemental Table 1), epithelial ovarian cancers were subdivided into serous, endometrioid, clear cell, mucinous, and other epithelial ovarian cancers. Endometrial cancers were classified as Type I and Type II cancers based on the dualistic model [22], given that it has long been speculated that hormonal exposures are strongly related to Type I tumors, whereas Type II tumors are considered to be unrelated to hormonal exposure. We also evaluated associations with the most common subtype endometrioid adenocarcinoma and other Type I tumors.
Study population
Women eligible for this study were born from 1936 to 1989, had an identification number, were alive and living in Denmark on January 1, 1978 and were 18 years of age or older. Among 184,276 women in the cohort, 161,498 were born 1936-1989. Records from 17,052 women did not have an identification number and were excluded and 3,244 were additionally excluded due to emigration, death or loss to follow-up prior to age 18 years or January 1, 1978 leaving 141,202 women.
For ovarian cancer analyses, we excluded women with an oophorectomy or salpingooophorectomy prior to age 18 years (n=2) or 1978 (n=20), with an ovarian cancer diagnosis prior to 1978 (n=29), or without a date for the ovarian cancer diagnosis (n=2). For endometrial cancer analyses, women with a hysterectomy prior to age 18 years (n=5) or 1978 (n=327), with an endometrial cancer diagnosis prior to 1978 (n=1), or without a date for the endometrial cancer diagnosis (n=1) were excluded. For both outcomes, we further excluded women without information on birth weight (n=19,767 ovarian, 19,717 endometrial) and with birth weight values less than 2 kilograms (n=2117 ovarian, 2112 endometrial) or more than 5.5 kilogram (n=414 ovarian, 411 endometrial). Given these exclusions, 118,851 women were included in ovarian cancer analyses and 118,628 women were included in endometrial cancer analyses.
Each woman was followed from the age of 18 years or from her age in 1978, whichever came later. When investigating ovarian cancer, women were followed up until a diagnosis of ovarian cancer, oophorectomy/salpingo-oophorectomy, death, emigration, loss to follow-up, or December 31, 2014, whichever came first. In the analyses with endometrial cancer as the endpoint, women were followed up until a diagnosis of endometrial cancer, hysterectomy, death, emigration, loss to follow-up, or December 31, 2014, whichever came first.
Statistical analyses
Analyses for ovarian and endometrial cancer outcomes were conducted separately using Cox proportional hazard models. We used age as the underlying time scale and stratified by 5-year birth cohorts to allow the baseline hazard to differ by birth cohorts. We assessed linearity of the birth weight-cancer association using linear splines with 2 knots positioned at 3.25 and 3.75 kilograms (kg) using the likelihood ratio test. When the linear assumptions for the model were not satisfied, birth weight was evaluated with 3 a priori chosen categories (2.0-3.25, 3.26-3.75, 3.76-5.5 kg), with birth weight in the normal range (3.26-3.75 kg) serving as the referent category. We selected these categories to reduce the influence of digit preference [23].
We examined the proportional hazards assumptions underlying the Cox models by testing if associations between birth weight and ovarian or endometrial cancer risks, differed by categories of age at diagnosis using likelihood ratio tests. We assessed heterogeneity in the birth weight association across ovarian and endometrial cancer subtypes via a likelihood ratio test by including an interaction term between birth weight and cancer subtype. Interactions of birth cohort with the associations between birth weight and cancer risk were similarly investigated using likelihood ratio tests. We did not identify violations of the proportional hazards assumption or birth cohort effects in any of these associations (results not shown). Statistical analyses were performed using Stata (version 14.1, StataCorp LP College Station, TX).
This study was approved by the Danish Data Protection Agency. According to Danish law, ethical approval is not required for register-based studies.
RESULTS
Among the approximately 118,000 women included in the ovarian and endometrial cancer analyses, the proportion of women in the lowest (2-3.25 kg), middle (3.26-3.75 kg), and highest (3.76-5.50 kg) birth weight categories varied little over time (Table 1). During follow-up, 666 epithelial ovarian cancers and 694 endometrial cancers were identified. Subtypes of ovarian cancer included 360 serous, 67 endometrioid, 29 clear cell, 75 mucinous, and 135 with other histologic subtypes. Subtypes of endometrial cancer included 624 Type I tumors and 67 Type II tumors. Of the Type I endometrial tumors, 498 were endometrioid adenocarcinomas and 126 other Type I tumors.
Table 1.
Distribution of birth weight by birth year in the ovarian and endometrial cancer analytic cohorts of the Copenhagen School Health Records Register.
| Year of birth | Ovarian Cancer Analytic Cohort (N=118,851) | Endometrial Cancer Analytic Cohort (N=118,628) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Birth weight categories (kilograms (kg)) | ||||||||||||
| 2-3.25 kg | 3.26-3.75 kg | 3.76-5.5 kg | 2-3.25 kg | 3.26-3.75 kg | 3.76-5.5 kg | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
|
|
||||||||||||
| 1936-1940 | 5,804 | 45.0 | 4,676 | 36.2 | 2,433 | 18.8 | 5,772 | 45.0 | 4,642 | 36.2 | 2,417 | 18.8 |
| 1941-1945 | 9,655 | 47.7 | 7,067 | 34.9 | 3,535 | 17.5 | 9,606 | 47.6 | 7,032 | 34.9 | 3,529 | 17.5 |
| 1946-1950 | 9,216 | 48.4 | 6,629 | 34.9 | 3,179 | 16.7 | 9,194 | 48.4 | 6,615 | 34.9 | 3,173 | 16.7 |
| 1951-1955 | 6,902 | 50.0 | 4,750 | 34.4 | 2,167 | 15.7 | 6,899 | 50.0 | 4,747 | 34.4 | 2,167 | 15.7 |
| 1956-1960 | 5,853 | 51.6 | 3,788 | 33.4 | 1,707 | 15.0 | 5,853 | 51.6 | 3,788 | 33.4 | 1,707 | 15.0 |
| 1961-1965 | 4,571 | 48.3 | 3,340 | 35.3 | 1,545 | 16.3 | 4,571 | 48.4 | 3,340 | 35.3 | 1,543 | 16.3 |
| 1966-1970 | 3,954 | 48.7 | 2,866 | 35.3 | 1,298 | 16.0 | 3,954 | 48.7 | 2.866 | 35.3 | 1,298 | 16.0 |
| 1971-1975 | 3,521 | 48.1 | 2,641 | 36.1 | 1,152 | 15.8 | 3,521 | 48.2 | 2,641 | 36.1 | 1,151 | 15.7 |
| 1976-1980 | 2,692 | 47.4 | 2,016 | 35.5 | 971 | 17.1 | 2,691 | 47.4 | 2,017 | 35.5 | 971 | 17.1 |
| 1981-1985 | 2,667 | 48.4 | 1,970 | 35.8 | 869 | 15.8 | 2,667 | 48.5 | 1,970 | 35.8 | 868 | 15.8 |
| 1986-1989 | 2,420 | 44.7 | 1,951 | 36.0 | 1,046 | 19.3 | 2,420 | 44.7 | 1,941 | 36.0 | 1,047 | 19.3 |
kg, kilogram
Ovarian cancer
We found evidence for non-linear birth weight associations with epithelial ovarian cancer overall and serous ovarian cancer (p-values <0.0001), thus birth weight was modelled using three categories with a reference category of 3.26-3.75 kilograms. We observed increased risks of both epithelial ovarian cancer overall and serous ovarian cancer with lower birth weights [2.0-3.25 kg hazard ratio (HR) (95% confidence interval (CI)): 1.27 (1.06-1.52), 1.54 (1.19-1.98), respectively] and higher birth weights [3.76-5.5 kg: 1.51 (1.21-1.87), 1.98 (1.47-2.67), respectively] (Table 2, Figure 1).
Table 2.
Birth weight associations with ovarian cancer, overall and by subtype, Copenhagen School Health Records Register, follow-up from 1978-2014.
| Per kg increase in BW | ||||||||
|---|---|---|---|---|---|---|---|---|
| Case group | Birth weight categories | N Cases | Woman-years | HRa | 95% CI | p -valueb | HRa | 95% CI |
|
|
||||||||
| Epithelial ovarian (n=666) |
2-3.25 kg 3.26-3.75 kg 3.76-5.5 kg |
331 193 142 |
1,707,386 1,240,235 590,186 |
1.27 1.00 1.51 |
(1.06-1.52) (ref) (1.21-1.87) |
0.0002 | – | – |
| Serous (n=360) |
2-3.25 kg 3.26-3.75 kg 3.76-5.5 kg |
185 89 86 |
1,707,386 1,240,235 590,186 |
1.54 1.00 1.98 |
(1.19-1.98) (ref) (1.47-2.67) |
0.0001 | – | – |
| Endometrioid (n=67) |
2-3.25 kg 3.26-3.75 kg 3.76-5.5 kg |
28 26 13 |
1,707,386 1,240,235 590,186 |
0.79 1.00 1.03 |
(0.46-1.34) (ref) (0.53-2.01) |
0.56 | 1.11 | (0.71-1.74) |
| Clear cell (n=29) |
2-3.25 kg 3.26-3.75 kg 3.76-5.5 kg |
11 15 3 |
1,707,386 1,240,235 590,186 |
0.54 1.00 0.41 |
(0.25-1.18) (ref) (0.12-1.41) |
0.32 | 1.25 | (0.64-2.46) |
| Mucinous (n=75) |
2-3.25 kg 3.26-3.75 kg 3.76-5.5 kg |
35 21 19 |
1,707,386 1,240,235 590,186 |
1.23 1.00 1.87 |
(0.71-2.10) (ref) (1.00-3.48) |
0.75 | 1.31 | (0.87-2.00) |
| Other epithelial (n=135) |
2-3.25 kg 3.26-3.75 kg 3.76-5.5 kg |
72 42 21 |
1,707,386 1,240,235 590,186 |
1.29 1.00 1.01 |
(0.88-1.89) (ref) (0.60-1.71) |
0.45 | 0.81 | (0.59-1.12) |
HR, hazards ratio; CI, confidence interval; kg, kilogram
HR and 95% CI estimated from Cox proportional hazards regression stratified by birth cohort.
p-value for linearity using the likelihood ratio test for linear splines with 2 knots positioned at 3.25 and 3.75 kg
p-value for heterogeneity of birth weight-ovarian cancer associations across subtype=0.37
Figure 1.
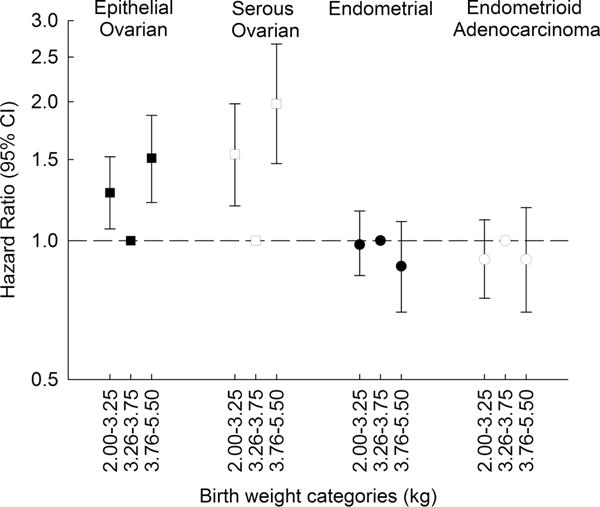
We did not detect indications of non-linearity in the association with endometrioid, clear cell, mucinous and other epithelial ovarian cancers (all p-values for non-linearity ≥ 0.66). Risks of these ovarian cancer subtypes were not associated with birth weight [HR (95% CI) per one kilogram increase: endometrioid 1.11 (0.71-1.74), clear cell 1.25 (0.64-2.46), mucinous 1.31 (0.87-2.00), other 0.81 (0.59-1.12), p-heterogeneity (p-het)=0.37] (Table 2). Small numbers for the rarer subtypes could have limited our ability to detect non-linear associations; thus, results are also presented by birth weight categories for comparison (Table 2).
Endometrial cancer
We did not identify non-linearity in the associations with endometrial cancer (all p-values ≥ 0.15), thus, birth weight was modelled linearly per kilogram increase. Associations were also reported by birth weight categories for comparison with ovarian cancer risk estimates. Birth weight was not associated with overall endometrial cancer risk, with risk of Type I tumors, or with risk of endometrioid adenocarcinomas or other Type I tumors (Table 3, Figure 1). A decreased risk of Type II tumors was suggested per kilogram increase in birth weight [HR (95% CI): 0.63 (0.40-1.00); p-het=0.03].
Table 3.
Birth weight associations with endometrial cancer, overall and by subtype, Copenhagen School Health Records Register, follow-up from 1978-2014.
| Per kg increase in birth weight | ||||||||
|---|---|---|---|---|---|---|---|---|
| Case group | Birth weight | N Cases | Woman-years | HRa | 95% CI | p-valueb | HRa | 95% CI |
|
|
||||||||
| Endometrial (n=694) |
2.0-3.25 kg 3.26-3.75 kg 3.76-5.5 kg |
334 252 108 |
1,624,649 1,178,348 559,216 |
0.98 1.00 0.88 |
(0.84-1.16) (ref) (0.70-1.10) |
0.39 | 1.01 | (0.88-1.16) |
| Type I (n=624) |
2.0-3.25 kg 3.26-3.75 kg 3.76-5.5 kg |
293 231 100 |
1,624,649 1,178,348 559,216 |
0.94 1.00 0.89 |
(0.79-1.12) (ref) (0.70-1.12) |
0.52 | 1.06 | (0.91-1.23) |
| Endometrioid Adenocarcinoma (n=498) |
2.0-3.25 kg 3.26-3.75 kg 3.76-5.5 kg |
230 186 82 |
1,624,649 1,178,348 559,216 |
0.91 1.00 0.91 |
(0.75-1.11) (ref) (0.70-1.18) |
0.31 | 1.11 | (0.94-1.31) |
| Other Type I (n=126) |
2.0-3.25 kg 3.26-3.75 kg 3.76-5.5 kg |
63 45 18 |
1,624,649 1,178,348 559,216 |
1.05 1.00 0.80 |
(0.72-1.54) (ref) (0.46-1.38) |
0.90 | 0.89 | (0.64-1.23) |
| Type II (n=67) |
2.0-3.25 kg 3.26-3.75 kg 3.76-5.5 kg |
40 19 8 |
1,624,649 1,178,348 559,216 |
1.58 1.00 0.85 |
(0.92-2.73) (ref) (0.37-1.95) |
0.56 | 0.63 | (0.40-1.00) |
HR, hazards ratio; CI, confidence interval; kg, kilogram
HR and 95% CI estimated from Cox proportional hazards regression stratified by birth cohort
p-value for linearity using the likelihood ratio test for linear splines with 2 knots positioned at 3.25 and 3.75 kg
p-value for heterogeneity of birth weight-endometrial cancer associations by Type I/Type II tumors=0.03
DISCUSSION
In this analysis of women in the CSHRR, we find evidence for a positive non-linear (u-shaped) association between birth weight and ovarian cancer. Specifically, both lower and higher birth weights were associated with increased risks of epithelial ovarian cancer overall and serous ovarian cancer, the most common subtype but also the subtype with the fewest risk factor associations to date [16]. Birth weight was not associated with endometrial cancer risk overall or the most common endometrial cancer subtype; however, there was the suggestion of an inverse association between birth weight and Type II endometrial cancers, which include serous cancers of the endometrium.
Prior studies evaluating the relation between birth weight and ovarian cancer risk are limited and none of them have reported associations by histologic subtype. Consistent with our study, the Million Women’s Study (n=2009 ovarian cancers) [12], reported a non-linear association demonstrating increased risk of ovarian cancer overall with both low (<2.5 kg) and high (≥4.0 vs. 3.0-<3.5 kg) birth weight categories. However, they did not evaluate associations by histologic subtype. In contrast to our findings and those of the Million Women’s Study, a combined analysis of Nurses’ Health Study and Nurses’ Health Study II data (n=872 cases) [8], based on participants recall of birth weight, reported that neither lower (<2.5, 2.5-3.1 kg) nor higher (≥3.8 vs. 3.2-3.8 kg) birth weight categories were associated with ovarian cancer risk. Further, a one kilogram increase in birth weight was not associated with ovarian cancer risk in the Uppsala Birth Cohort Study (n=89 ovarian cancers) [10] or in a prior analysis of CSHRR data (n=427 ovarian cancers) with follow-up through 2003 [7]. Differences in results for the prior studies and our current study may be due to differences in exposure measurement (parental recall vs. self-report) [8] or the study did not specifically test for non-linearity [8, 10], thus limiting the comparability with the current results.
It has been suggested that altered patterns of gonadotropin release established in utero when the fetal hypothalamus is imprinted may contribute to subsequent risk of ovarian cancer [5]. Gonadotropin secretion remains high during infancy; it is thought that resulting estrogen release could directly or indirectly promote infant weight gain. Gonadotropin secretion falls after infancy until it increases again at puberty, where an altered pattern could result in early menarche and/or subsequent infertility that are both associated with increased ovarian cancer risk [2, 24]. It is plausible that the U-shaped associations suggest multiple pathways may be affecting cancer risk in opposite directions so that the increased risk with low birth weight could be driven by a different mechanism than the increased risk with higher birth weights.
Prior studies evaluating the birth weight-endometrial cancer association have also been limited and did not evaluate associations by histologic subtype. The Million Women’s Study (n=2623 cases) [12], reported no association with endometrial cancer per kilogram increase in birth weight. A similar null association was reported in a prior analysis of the CSHRR data (n=296 cases) through 2003 [7]. A 38% decreased risk of endometrial cancer was reported per 1 standard deviation increase in birth weight in the Uppsala Birth Cohort [10], albeit based on a limited number of cases (n=112). Evaluating birth weight in categories, a linkage study in Sweden (n=73 cases) [9] and a combined analysis of Nurses’ Health Study and Nurses’ Health Study II data (n=676 cases) [9] reported no association between low or high birth weight and endometrial cancer risk overall. Our findings are thus in-line with most of the previous reports supporting no association between birth weight and endometrial cancer and no evidence of non-linearity in the association. We extend the findings of these prior studies and report a lack of association with the classic Type I tumors. However, we do report an inverse relationship between birth weight and risk of Type II tumors, although this is based on limited number and requires confirmation in further studies.
Given the strong association between child and adult BMI and endometrial cancer risk [25–27], it could be expected that birth weight would be associated with an increased risk of endometrial cancer via the correlation between birth weight and child and adult BMI, however this correlation is modest at best. Babies with a high birth weight have a greater risk of being overweight in childhood, and both low and high birth weight babies have a greater likelihood of being obese in childhood and adulthood [28–30]. However, we did not detect any evidence of non-linearity in the endometrial cancer analyses.
Strengths of this analysis include: a large, unselected study population; ability to evaluate histologic subtypes of both ovarian and endometrial cancers; record linkage via unique identification numbers that enabled accurate passive follow up; and careful evaluation of and control for potential birth cohort effects. Limitations of our analysis include: the lack of information on gestational age at birth and birth length, allowing the calculation of Ponderal Index, which may provide more information than birth weight alone and the inability to adjust our estimates for reproductive or lifestyle factors that may be associated with ovarian or endometrial cancer risk. However, these latter factors may be highly correlated with birth weight or they may serve as mediators of the association between birth weight and subsequent cancer risk, as factors like age at menarche or adult BMI could be on the causal pathway between birth weight and cancer risk.
In conclusion, using CSHRR data we have shown that both lower and higher birth weights are associated with increased ovarian cancer risk and specifically increased risk of serous ovarian cancer. Birth weight was not associated with the predominant subtypes of endometrial cancer. Our results suggest that in utero exposures may be relevant to the etiology of serous ovarian and endometrial cancers that requires replication in an additional study population with well documented information on infant birth weight and other characteristics of the newborns such as birth length and gestational age.
Supplementary Material
Highlights.
Low and high birthweights increase risks of ovarian cancer
Birthweight was not associated with most types of endometrial cancer
In utero exposures may be relevant for ovarian & endometrial cancers
Acknowledgments
Data were combined as a collaboration between the Department of Clinical Epidemiology and the Copenhagen City Archives. We would like to thank the school health doctors and nurses from the Copenhagen Municipality School Health Service for the collection of data that form the basis of the CSHRR.
Funding
JA and JLB were funded by the European Research Council under the European Union’s Seventh Framework Programme (FP/2007-2013)/ERC Grant Agreement no. 281419, childgrowth2cancer and The Danish Council for Independent Research (DFF)|FSS Grant Agreement no. 1331-00218. JA received additional support from the Johannes Clemmesens Fund, Bispebjerg and Frederiksberg Hospital, and Else and Mogens Wedell-Wedellsborgs Fund. JA and JLB were also funded by the Danish Council for Independent Research (DFF)|FSS 1331-00218, and JLB by the European Union’s Horizon 2020 research and innovation programme DynaHEALTH, 633595. BT and NW were funded by the Intramural Research Program of the US National Cancer Institute. The funding sources were not involved in any parts of the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors have no conflicts of interest to declare.
Authorship contribution
Study concept and design: BT, JA, JLB
Data acquisition: TIAS, JLB
Data analysis: JA
Interpretation of data: BT, JA, JLB, LGU, TIAS, NW
Manuscript preparation: BT, JA, JLB
Manuscript review: BT, JA, LGU, NW, TIAS, JLB
References
- 1.Engholm G, et al. Association of the Nordic Cancer Registries. Danish Cancer Society; 2016. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 7.3 (08.07.2016) [Google Scholar]
- 2.Brinton L, Sahasrabuddhe VV, Trabert B. Epidemiology of Gynecologic Cancers. In: Barakat R, et al., editors. Principles and Practive of Gynecologic Oncology. Lippincott Williams & Wilkins; Philadelphia, PA: 2013. pp. 1–29. [Google Scholar]
- 3.Ruder EH, et al. Birth characteristics and age at menarche: results from the dietary intervention study in children (DISC) Cancer Causes & Control. 2010;21(9):1379–1386. doi: 10.1007/s10552-010-9565-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perry JRB, et al. Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche. Nature. 2014;514(7520):92-+. doi: 10.1038/nature13545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker DJ, et al. Weight gain in infancy and cancer of the ovary. Lancet. 1995;345(8957):1087–8. doi: 10.1016/s0140-6736(95)90821-8. [DOI] [PubMed] [Google Scholar]
- 6.Barker DJ, et al. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298(6673):564–7. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahlgren M, et al. Birth weight and risk of cancer. Cancer. 2007;110(2):412–9. doi: 10.1002/cncr.22773. [DOI] [PubMed] [Google Scholar]
- 8.Baer HJ, Hankinson SE, Tworoger SS. Body size in early life and risk of epithelial ovarian cancer: results from the Nurses’ Health Studies. Br J Cancer. 2008;99(11):1916–22. doi: 10.1038/sj.bjc.6604742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lof M, et al. Birth weight in relation to endometrial and breast cancer risks in Swedish women. Br J Cancer. 2007;96(1):134–6. doi: 10.1038/sj.bjc.6603504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCormack VA, et al. Birth characteristics and adult cancer incidence: Swedish cohort of over 11,000 men and women. Int J Cancer. 2005;115(4):611–7. doi: 10.1002/ijc.20915. [DOI] [PubMed] [Google Scholar]
- 11.Xue F, et al. Longitudinal study on birthweight and the incidence of endometrial cancer. Br J Cancer. 2008;98(7):1288–91. doi: 10.1038/sj.bjc.6604304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang TO, et al. Birth weight and adult cancer incidence: large prospective study and meta-analysis. Ann Oncol. 2014;25(9):1836–43. doi: 10.1093/annonc/mdu214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang HP, et al. Ovarian cancer risk factors by histologic subtypes in the NIH-AARP diet and health study. Int J Cancer. 2011 doi: 10.1002/ijc.26469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang HP, et al. Endometrial cancer risk factors by 2 main histologic subtypes: the NIH-AARP Diet and Health Study. Am J Epidemiol. 2013;177(2):142–51. doi: 10.1093/aje/kws200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Setiawan VW, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31(20):2607–18. doi: 10.1200/JCO.2012.48.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wentzensen N, et al. Ovarian Cancer Risk Factors by Histologic Subtype: An Analysis From the Ovarian Cancer Cohort Consortium. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.66.8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker JL, et al. Cohort profile: the Copenhagen School Health Records Register. Int J Epidemiol. 2009;38(3):656–62. doi: 10.1093/ije/dyn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen CB, et al. Comparison of birth weight between school health records and medical birth records in Denmark: determinants of discrepancies. BMJ Open. 2015;5(11):e008628. doi: 10.1136/bmjopen-2015-008628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 Suppl):22–5. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 20.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7 Suppl):30–3. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 21.Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39(7 Suppl):42–5. doi: 10.1177/1403494810393562. [DOI] [PubMed] [Google Scholar]
- 22.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10–7. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 23.Baker JL, Olsen LW, Sorensen TI. Weight at birth and all-cause mortality in adulthood. Epidemiology. 2008;19(2):197–203. doi: 10.1097/EDE.0b013e31816339c6. [DOI] [PubMed] [Google Scholar]
- 24.Louis GMB, Cooney MA, Peterson CM. The ovarian dysgenesis syndrome. Journal of Developmental Origins of Health and Disease. 2011;2(1):25–35. [Google Scholar]
- 25.Schmandt RE, et al. Understanding obesity and endometrial cancer risk: opportunities for prevention. Am J Obstet Gynecol. 2011;205(6):518–25. doi: 10.1016/j.ajog.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedenreich C, et al. Anthropometric factors and risk of endometrial cancer: the European prospective investigation into cancer and nutrition. Cancer Causes Control. 2007;18(4):399–413. doi: 10.1007/s10552-006-0113-8. [DOI] [PubMed] [Google Scholar]
- 27.Aarestrup J, et al. Childhood body mass index and height and risk of histologic subtypes of endometrial cancer. Int J Obes (Lond) 2016;40(7):1096–102. doi: 10.1038/ijo.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leong NM, et al. Early life risk factors in cancer: the relation of birth weight to adult obesity. Int J Cancer. 2003;103(6):789–91. doi: 10.1002/ijc.10886. [DOI] [PubMed] [Google Scholar]
- 29.Rugholm S, et al. Stability of the association between birth weight and childhood overweight during the development of the obesity epidemic. Obes Res. 2005;13(12):2187–94. doi: 10.1038/oby.2005.271. [DOI] [PubMed] [Google Scholar]
- 30.Sorensen HT, et al. Relation between weight and length at birth and body mass index in young adulthood: cohort study. BMJ. 1997;315(7116):1137. doi: 10.1136/bmj.315.7116.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


