Abstract
Objective
Many high-grade serous carcinomas initiate in fallopian tubes as serous tubal intraepithelial carcinoma (STIC), a microscopic lesion identified with specimen processing according to the Sectioning and Extensive Examination of the Fimbria protocol (SEE-Fim). Given that the tubal origin of these cancers was recently recognized, we conducted a survey of pathology practices to assess processing protocols that are applied to gynecologic surgical pathology specimens in clinical contexts in which finding STIC might have different implications.
Methods
We distributed a survey electronically to the American Society for Clinical Pathology list-serve to determine practice patterns and compared results between practice types by chi-square (χ2) tests for categorical variables. Free text comments were qualitatively reviewed.
Results
Survey responses were received from 159 laboratories (72 academic, 87 non-academic), which reported diverse specimen volumes and percentage of gynecologic samples. Overall, 74.1% of laboratories reported performing SEE-Fim for risk-reducing surgical specimens (82.5% academic versus 65.7% non-academic, p<0.05). In specimens from surgery for benign indications in which initial microscopic sections showed an unanticipated suspicious finding, 75.9% of laboratories reported using SEE-Fim to process the remainder of the specimen (94.8% academic versus 76.4% non-academic, p<0.01), and 84.6% submitted the entire fimbriae.
Conclusions
Changes in the theories of pathogenesis of high-grade serous carcinoma have led to implementation of pathology specimen processing protocols that include detailed analysis of the fallopian tubes. These results have implications for interpreting trends in cancer incidence data and considering the feasibility of developing a bank of gynecologic tissues containing STIC or early cancer precursors.
Keywords: gynecologic tissue, ovarian/tubal cancer, STICs, SEE-Fim, biobank, serous carcinoma
Introduction
Historically, most pelvic high-grade serous carcinomas (HGSCs) were classified as ovarian primary tumors, despite the lack of a defined HGSC precursor in the ovary. Subsequently, increased performance of BRCA1/2 mutation testing and risk reducing surgery (RRS) enabled pathologists to examine adnexal specimens from high-risk women, which resulted in detection of a putative early malignant lesion in the distal fallopian tube, termed serous tubal intraepithelial carcinoma (STIC). Accumulating evidence from histopathologic studies, molecular analyses and preclinical models have strengthened the view that many HGSCs begin as STIC and spread to the ovary and other sites secondarily [1]; however, the frequency with which STIC is reported to be present in surgical tissue varies considerably.
STIC has been reported in 3–8% of RRS specimens [2], concurrently with HGSC in 13%–68% of cases (mean=37% [95% CI 27%–48%]) and in less than 1% of specimens removed for benign indications [2–8]. Although the biologic relationship of STIC to HGSC remains undefined [9], detection of STIC is considered clinically important [10]. Specifically, diagnosis of occult STIC may suggest the need for further staging, post-operative surveillance and genetic testing. In cases of symptomatic HGSC, diagnosis of STIC may affect primary site assignment and staging.
Prior to the description of STIC, pathologists typically evaluated grossly unremarkable fallopian tubes sparingly, mostly to document their removal. However, as recognition of STIC increased, experts began advocating for systematic sampling of the fallopian tube for microscopic diagnosis [11–13]. The Sectioning and Extensive Examination of the Fimbria protocol (SEE-Fim) was developed to optimize pathology processing of the fallopian tube [12] and criteria for the diagnosis of STIC were proposed, including p53 and Ki-67 staining [4]. Nonetheless, interobserver agreement among pathologists on the diagnosis of STIC remains variable, and a gold standard for assessing diagnostic accuracy is needed [4, 14]. Given that STICs are asymptomatic microscopic lesions, diagnosis is highly dependent upon the extent of processing for microscopic examination [15]. However, little is known about how gynecologic specimens are processed in the U.S., especially with regard to sampling of the fallopian tube and application of the SEE-Fim protocol. The dramatic increase in the reported incidence rate of early stage tubal carcinoma suggests that pathology processing protocols are changing and the large increases in reported rates of late stage tubal cancer suggest that pathologists are increasingly classifying HGSC as tubal primary cancer [16, 17]. Further, increased performance of salpingectomy in the U.S. may also be contributing to increasing incidence rates of tubal cancers [18].
Accordingly, we conducted a survey of pathology practices largely in the U.S. to assess methods of routine, diagnostic processing of different types of gynecologic surgical pathology specimens.
Materials and Methods
Survey Participants
The majority of respondents were recruited through an email distributed to 20,535 usable email addresses listed in the American Society for Clinical Pathology (ASCP) list-serve. This list-serve consists of ASCP members and customers (non-ASCP members), of which 18,348 email addresses are associated with pathology laboratories (hospitals, independent reference laboratories, physician office laboratories, and pathology group practices). The majority of the list-serve members are physicians (56.3%) or technologists (39.3%), with 16.4% also serving as staff pathologists and 9.8% also serving as Laboratory Medical Director or Assistant Director. List-serve members included hospital staff (36.6%) and members of independent reference laboratories (8.1%). Other recruitment methods included distribution to the National Cancer Institute (NCI) Community Oncology Research Program (NCORP), through a network of site coordinators and cold calls to NCORP hospitals. Respondents were asked to provide answers for their entire laboratory, and they had the option to submit the survey via the online Google survey, fax, or email.
Survey Instrument
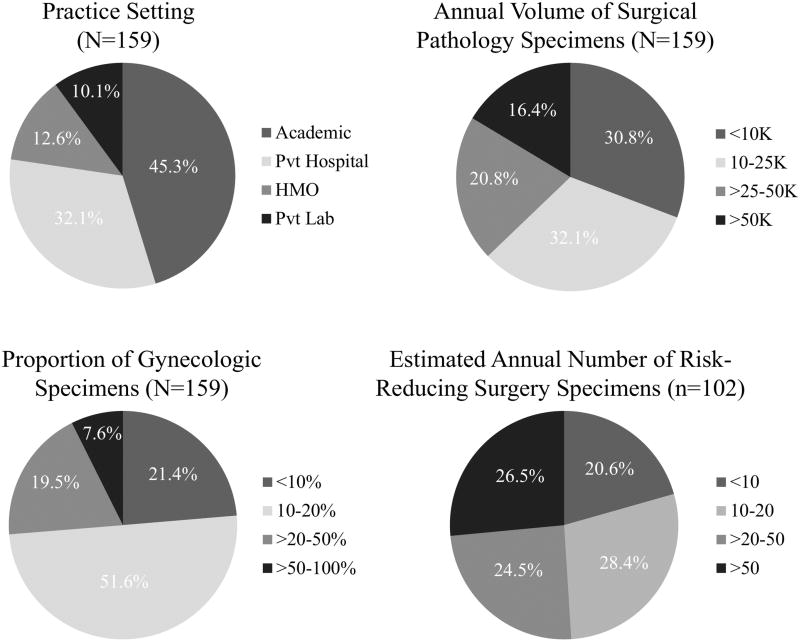
The survey to assess the feasibility of establishing a gynecologic specimen bank for research was developed by the authors (GS, MES, MAD, BT), and was carried out using the Google Survey platform (https://goo.gl/forms/CKiGG7aJczSbclw03). The gynecologic tissue bank survey assessed the practice setting of the laboratory, the volume of surgical pathology specimens processed, including the approximate annual volume, the percentage of gynecologic specimens, and the number of RRS specimens from women at high risk for gynecologic disease or cancer (Figure 1). The survey also included questions about whether the laboratory has a gynecologic pathology sub-specialty sign-out. These queries provided context for responses to questions related to pathology processing of gynecologic surgical specimens removed for different clinical indications. The full survey is presented in the Appendix.
Figure 1.
Distribution of laboratory practices, and across laboratory practices of volume of surgical specimens, proportion of gynecologic specimens and volume of risk-reducing surgical specimens.
Statistical Analysis
Survey responses are presented as raw frequencies and percentages for different sampling protocols by indication and practice setting. Respondents who reported receiving RRS specimens were asked to estimate how many specimens they processed annually, which was converted to a categorical variable: <10 specimens, 10–20 specimens, >20–50 specimens, and >50 specimens. Respondents provided 41 free text comments which were summarized. Results were compared by chi-square (χ2) tests for categorical variables using Stata/IC version 14 (StataCorp, College Station, TX, USA).
Results
Pathology Practices
We received 159 survey responses; 72 (45.3%) from academic practices and 87 (54.7%) from non-academic practices (private hospitals, laboratories affiliated with managed health organizations, or private laboratories) (Figure 1). Pathology laboratories from academic practices were more likely than those from other practice types to report higher annual volumes of surgical pathology specimens and higher percentages of gynecologic specimens (Table 1). The majority of academic practices reported annual surgical pathology specimen volumes of 10,000–50,000 (65.3%), whereas the most frequent volumes among non-academic practices were <10,000 (44.8%). Across practice types, most laboratories reported that 10%–20% of surgical pathology specimens were from gynecologic organs (Figure 1); this category included a higher percentage of academic practices than non-academic practices (61.1% vs. 43.7%) (Table 1).
Table 1.
Volume of Gynecologic Tissue Samples by Practice Type
| Academic N (%) |
Non-Academic N (%) |
|
|---|---|---|
| Annual Surgical Pathology Specimen Volume*** (Academic=72, Non-Academic=87) | ||
| <10K | 10 (13.9) | 39 (44.8) |
| 10–25K | 25 (34.7) | 26 (29.9) |
| >25–50K | 22 (30.6) | 11 (12.6) |
| >50K | 15 (20.8) | 11 (12.6) |
|
| ||
| Proportion of Gynecologic Specimens** (Academic=72, Non-Academic=87) | ||
| <10% | 7 (9.7) | 27 (31.0) |
| 10%–20% | 44 (61.1) | 38 (43.7) |
| >20%–50% | 16 (22.2) | 15 (17.2) |
| >50%–100% | 5 (6.9) | 7 (8.1) |
|
| ||
| Sub-Specialty Sign-Out*** (Academic=71, Non-Academic=86) | ||
| No | 26 (36.6) | 69 (80.2) |
| Yes | 45 (63.4) | 17 (19.8) |
|
| ||
| Receive Risk-Reducing Surgery Specimens*** (Academic=72, Non-Academic=87) | ||
| No | 9 (12.5) | 35 (40.2) |
| Yes | 63 (87.5) | 52 (59.8) |
|
| ||
| Estimated Annual Number of Risk-Reducing Surgery Specimens (Academic=57, Non-Academic=45) | ||
| <10 | 8 (14.0) | 13 (28.9) |
| 10–20 | 14 (24.6) | 15 (33.3) |
| >20–50 | 19 (33.3) | 6 (13.3) |
| >50 | 16 (28.1) | 11 (24.4) |
p<0.01
p<0.001
Laboratories from academic practices were significantly more likely than non-academic practices to report having a gynecologic pathology sub-specialty sign-out [χ2 (degrees of freedom (df)=2, N=157) = 30.96, p<0.001]. Although RRS specimens were handled by 102 of 159 of practices (72.3%), these cases were more common in academic practices (87.5%) vs. non-academic settings (59.8%). Laboratories reported estimated annual numbers of RRS specimens ranging from 1 to >50. Laboratories from academic practices were somewhat more likely to report higher estimated annual numbers of RRS specimens than non-academic practice types.
Processing Gynecologic Tissue
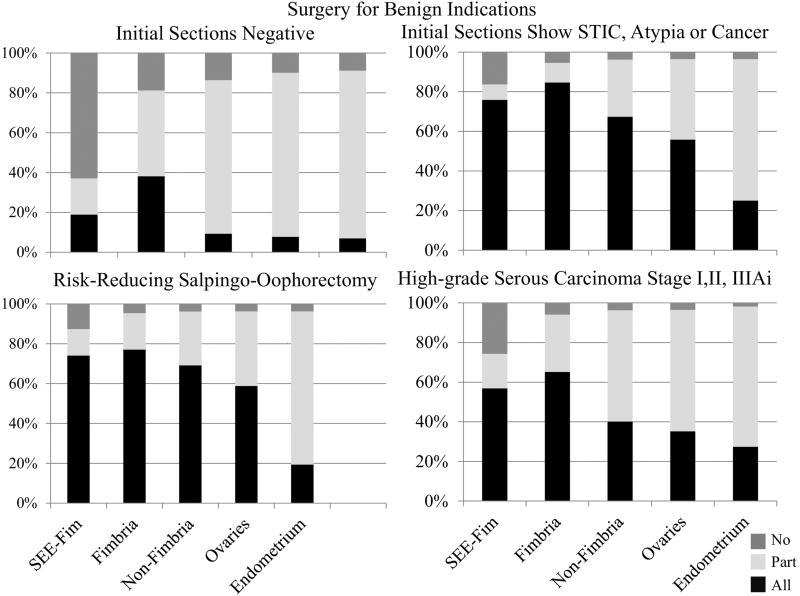
Laboratories were asked about how their laboratories processed gynecologic specimens in different clinical contexts (Figure 2 and Table 2). Overall, 74.1% of laboratories reported using SEE-Fim to process RRS specimens and 56.9% reported using SEE-Fim to process HGSC (stages I, II, IIIAi) (Figure 2). In the context of surgery for benign indications, final specimen processing was related to microscopic findings in the initial sections submitted; when first sections demonstrated STIC, epithelial atypia or occult cancer, 75.9% of laboratories reported use of SEE-FIM to process the remainder of the specimen and 84.6% submitted the entire fimbriae. Further, the non-fimbriated tube and ovaries were also extensively sampled. In contrast, when suspicious or malignant lesions were not found microscopically in initial sections examined from surgeries performed for benign indications, only 18.9% of laboratories reported performing SEE-Fim, and 38.2% reported microscopically examining the fimbriae in its entirety; generally, the remaining tissues were less extensively sampled (Figure 2).
Figure 2.
Distribution across laboratory practices of gynecologic tissue processing (SEE-Fim, fimbria, non-fimbria, ovary, and endometrium) by surgery (benign, risk-reducing or carcinoma).
Table 2.
Gynecologic Tissue Processing by Surgery and Practice Type
| High-Grade Serous Cancer Stage I, II, IIIAi |
Risk-Reducing Salpingo- Oophorectomy or Salpingectomy |
Surgery for Benign Indications, First Sections STIC, Epithelial Atypia in Ovary or Occult Cancer |
Surgery for Benign Indications, First Sections Negative |
|||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Acad. N (%) |
Non- Acad. N (%) |
Acad. N (%) |
Non- Acad. N (%) |
Acad. N (%) |
Non- Acad. N (%) |
Acad. N (%) |
Non- Acad. N (%) |
|
| See-Fim | ||||||||
|
| ||||||||
| No | 15 (22.4) | 22 (28.6) | 4 (6.2)* | 13 (18.6)* | 7 (10.3) | 16 (21.9) | 38 (63.3) | 45 (62.5) |
| Yes, Partial | 9 (13.4) | 16 (20.8) | 7 (10.7)* | 11 (15.7)* | 5 (7.4) | 6 (8.2) | 10 (16.7) | 14 (19.4) |
| Yes, Total | 43 (64.2) | 39 (50.7) | 54 (83.1)* | 46 (65.7)* | 56 (82.4) | 51 (69.9) | 12 (20.0) | 13 (18.1) |
| Total: | 67 | 77 | 65 | 70 | 68 | 73 | 60 | 72 |
|
| ||||||||
| Submit Fimbria | ||||||||
|
| ||||||||
| No | 2 (3.3) | 6 (8.1) | 2 (3.3) | 4 (5.6) | 2 (3.5)** | 5 (6.9)** | 9 (13.4) | 18 (23.4) |
| Yes, Partial | 15 (24.6) | 24 (32.4) | 8 (13.3) | 16 (22.5) | 1 (1.7)** | 12 (16.7)** | 28 (41.8) | 34 (44.2) |
| Yes, Total | 44 (72.1) | 44 (59.5) | 50 (83.3) | 51 (71.8) | 55 (94.8)** | 55 (76.4)** | 30 (44.8) | 25 (32.5) |
| Total: | 61 | 74 | 60 | 71 | 58 | 72 | 67 | 77 |
|
| ||||||||
| Submit Non-Fimbriated Tube | ||||||||
|
| ||||||||
| No | 2 (3.2) | 3 (4.1) | 1 (1.6) | 4 (5.6) | 1 (1.7) | 4 (5.6) | 7 (11.1) | 12 (15.6) |
| Yes, Partial | 34 (54.0) | 43 (58.1) | 14 (22.6) | 22 (31.0) | 15 (25.0) | 23 (31.9) | 51 (81.0) | 57 (74.0) |
| Yes, Total | 27 (42.9) | 28 (37.8) | 47 (75.8) | 45 (63.4) | 44 (73.3) | 45 (62.5) | 5 (7.9) | 8 (10.4) |
| Total: | 63 | 74 | 62 | 71 | 60 | 72 | 63 | 77 |
|
| ||||||||
| Submit Ovaries | ||||||||
|
| ||||||||
| No | 1 (1.5) | 4 (5.2) | 1 (1.5)** | 4 (5.6)** | 0 (0.0)* | 5 (6.7)* | 5 (7.8) | 9 (11.5) |
| Yes, Partial | 39 (60.0) | 48 (62.3) | 17 (26.2)** | 34 (47.9)** | 22 (34.4)* | 34 (46.0)* | 53 (82.8) | 64 (82.1) |
| Yes, Total | 25 (38.5) | 25 (32.5) | 47 (72.3)** | 33 (46.5)** | 42 (65.6)* | 35 (47.3)* | 6 (9.8) | 5 (6.1) |
| Total: | 65 | 77 | 65 | 71 | 64 | 74 | 64 | 78 |
|
| ||||||||
| Submit Endometrium | ||||||||
|
| ||||||||
| No | 0 (0.0) | 2 (3.5) | 2 (3.6) | 2 (3.8) | 1 (1.8) | 3 (5.3) | 3 (5.5) | 7 (11.9) |
| Yes, Partial | 38 (69.1) | 42 (72.4) | 40 (72.7) | 43 (81.1) | 37 (67.3) | 43 (75.4) | 47 (85.5) | 49 (83.1) |
| Yes, Total | 17 (30.9) | 14 (24.1) | 13 (23.6) | 8 (15.1) | 17 (30.9) | 11 (19.3) | 5 (9.1) | 3 (5.1) |
| Total: | 55 | 58 | 55 | 53 | 55 | 57 | 55 | 59 |
p<0.05
p<0.01
Processing of RRS specimens varied by practice setting (Table 2): 82.5% of academic laboratories reported processing these specimens using SEE-Fim, whereas that protocol was followed by only 65.7% of non-academic practices (χ2 (df=2, N=135) = 6.12, p<0.05), Similarly, in the context of surgery for benign indications in which initial microscopic sections showed STIC, epithelial atypia in ovary or occult cancer, academic centers were more likely to submit the entire fimbriae for microscopy (94.8% vs. 76.4%, χ2 (df=2, N=130) = 9.19, p<0.01), and to submit ovaries and tubes entirely from RRS and surgery for benign indications in which potential abnormalities were identified (p<0.01, p<0.05, respectively) (Table 2).
Use of Immunohistochemistry to Examine Fimbriae
Most laboratories reported that the decision to perform immunostains for Ki67, p53 and other markers to assess the fimbriae was based on the initial morphologic impression based on examination of hematoxylin and eosin stains (N=92, 61.7%). Compared with non-academic practices, academic practices were more likely to apply stains (73.2% vs. 51.3%, χ2 (df=2, N=149) = 7.59, p<0.01) (data not shown).
Contributing Tissue and Pathology Reports
The majority (63.8%) of laboratories indicated a potential willingness to provide de-identified blocks and matched pathology reports to support a national specimen bank. Laboratories from academic practices more often indicated willingness than those from non-academic practices (73.2% vs. 55.6%, χ2 (df=2, N=152) = 5.12, p<0.05) (data not shown). Potential barriers to providing de-identified blocks include lack of resources, funding or personnel, and concerns related to laboratory management, patient consent, and institutional review board approval.
Discussion
Survey responses from 159 pathology practices indicate that approximately 75% of laboratories apply SEE-Fim to RRS specimens and specimens removed for benign indications when STIC, tubal atypia or cancer is identified. Slightly over 50% of laboratories reported applying SEE-Fim to HGSC specimens (stage IIIi or earlier). Few laboratories submit entire ovaries or endometrium for microscopy, irrespective of clinical setting. Academic laboratories are more likely to perform extensive sampling than non-academic laboratories, although differences between academic and non-academic laboratories could be confounded by other features that we did not analyze. While our results are based on responses from a limited portion of U.S. pathology practices, they reflect the diversity of U.S. pathology laboratories. The results suggest that pathologists give considerable attention to microscopic examination of the fallopian tubes when a diagnosis of STIC or early tubal carcinoma is present, and a small but considerable percentage of laboratories also sample the tube extensively in cases of clinically symptomatic HGSC. These findings mirror results of a previous international survey of 173 pathologists and 101 clinicians, which showed that many physicians have embraced a tubal origin of HGSC and that clinicians in particular are concerned with defining the primary site of tumor origin [10].
Clinical studies assessing the prognosis of isolated STIC are limited in size and follow-up time, which precludes formulation of evidence-based management guidelines. A literature review through 2016 identified 103 reported cases of isolated STIC [19], mostly found in the context of RRS for BRCA1/2 mutation. Suggested management of STIC ranges from observation to performance of surgical staging and treatment [20, 21]. In a small clinical series, positive peritoneal cytology was reported among 12% of patients with isolated STIC and cases have been described in which surgical staging prompted by detection of STIC led to identification of occult cancer (reviewed in [19]). These findings indicate the possibility of tumor spread beyond the fallopian tube when STIC is found, which may affect management. Some studies suggest that development of primary peritoneal carcinoma after reportedly benign gynecologic surgery may represent delayed recurrences of occult cancers derived from undetected STIC [22, 23]. While risk of short-term recurrence among women with isolated STIC is probably low, detection of STIC likely remains important as an indication for genetic counseling and testing, which could have implications for prevention of cancer at other sites and for family members.
The American College of Obstetricians and Gynecologists suggests that gynecologists counsel average risk patients about salpingectomy when planning gynecologic surgery for benign disease or tubal ligation in order to lower ovarian/tubal cancer risk [24]. Clinical trials to assess whether salpingectomy with delayed oophorectomy among BRCA1/2 carriers can reduce HGSC risks, while avoiding adverse consequences of early menopause have been initiated [25]. Increased use of salpingectomy for risk reduction or in the context of benign gynecologic surgery has important implications for cancer surveillance because registries do not adjust at-risk populations for removal of target organs, leading to an under-estimate of population-based incidence rates. However, performance of salpingectomy with SEE-Fim may increase detection of early stage tubal cancers and STIC, thereby elevating incidence rates among younger women and potentially leading to “over-diagnosis” (detection of lesions without clinical significance). Additionally, given that many pathologists classify HGSC associated with STIC as tubal cancer [10, 13], the incidence rates of tubal cancers are increasing [16].
Given that the term STIC is not included as a specific diagnosis in the Surveillance, Epidemiology, and End Results Program (SEER) [16, 26], and that pathologists are using variable procedures for processing of gynecologic specimens, incidence rates for gynecologic cancers may become increasingly difficult to interpret. Immunohistochemistry for p53 and Ki-67 may improve reproducibility of the diagnosis of STIC [4, 27], and it has been proposed that staining for laminin γ1 (LAMC1) and high-mobility group AT-hook 2 (HMGA2) may also have value [28, 29]. Primary tumor assignment is further complicated by molecular analyses, suggesting that when STIC and cancer are found in the same woman, the two lesions are not always related [8, 30–32]. Improving management of STIC relies on a better understanding of : 1) the percentage of HGSC that originate as STIC; 2) the clinical implications of STIC, especially without further treatment and 3) age-specific incidence of STIC by population demographics. Further, it is unclear whether prior chemotherapy for a preceding cancer (e.g. breast cancer) or neoadjuvant therapy for HGSC can induce regression of STIC; while one report suggests that STIC remains detectable after these treatments, additional studies are needed [33]. Nonetheless, the increased use of SEE-Fim suggests that a biobank could be established by pooling selected specimens from multiple laboratories that routinely apply standardized rigorous sampling protocols, thus creating a resource to study these issues [34]. A network comprised of pathology laboratories that routinely perform SEE-Fim could enable retrospective collection of blocks of STIC and benign tubes for molecular analysis.
Given that STIC and occult tubal HGSCs are primarily microscopic diagnoses, the extent and meticulousness of histopathologic processing has a direct impact on detection of these lesions, which has potential implications for clinical management, cancer surveillance and research. Previous studies have recommended slicing of RRS specimens into thin (2mm) tissue sections with microscopic examination of each section, coupled with peritoneal washes, to optimize detection of occult early tubal carcinoma in high-risk women [15, 35]. A study applying systematic processing of fimbriae from 522 average-risk women undergoing salpingectomy for benign indications revealed 4 cases of STIC, 3 of which would have otherwise gone undetected using routine sampling, which historically involved a single representative tissue section from the non-fimbriated portion of the fallopian tube [36]. In a study applying SEE-Fim to 300 salpingectomy specimens from surgeries for gynecologic conditions, 12 cases of serous carcinoma were found to have STIC, while 56 additional serous carcinoma cases were originally negative for STIC [33]; however, a follow-up study in which fallopian tube tissue of the negative cases underwent additional sectioning at a level of 100 µm deeper in the block identified an additional 4 STICs [6]. Together, these studies indicate that more extensive fallopian tube sampling (concentrating on the fimbriae and distal tube) for microscopy may increase the detection of STIC, as these lesions may occur in an irregular or sparse distribution.
A recent report found remarkable molecular similarities between HGSCs associated with STIC and those in which STIC was not found [9], but whether this reflects a failure to detect STIC or pathogenesis through a pathway that does not involve STIC is an important unresolved question with implications for early detection and prevention. More thorough understanding of these critical topics would likely require research infrastructure that leverages existing practices that trend towards routine performance of SEE-Fim and more extensive specimen processing, which will enable more comprehensive molecular pathologic analysis and increased detection of small early lesions, including tubal lesions of uncertain biological potential.
In summary, our analyses of gynecologic specimen processing in a diverse set of 159 pathology laboratories suggest that a substantial percentage of laboratories use SEE-Fim or extensive processing of the tubal fimbriae for RRS specimens and when incidental suspicious or neoplastic lesions are found unexpectedly. However, fewer laboratories perform SEE-Fim to process HGSC cases, despite guidelines that support this approach, presumably because management would not be affected currently. National efforts are needed to encourage implementation of standardized processing protocols for gynecologic specimens to improve monitoring of population-based incidence and mortality rates by registries and to facilitate organized specimen biobanking to facilitate research to improve management of HGSC.
Supplementary Material
Highlights.
Survey to assess processing protocols of gynecologic surgical pathology specimens
Majority of pathology labs perform SEE-Fim on risk-reducing specimens
Most labs perform SEE-Fim on benign specimens if first sections are suspicious
Results suggest detailed processing of fallopian tubes pathology specimens
Acknowledgments
Conflict of Interest Statement
JLR reports grants from National Cancer Institute during the conduct of the study. KIC reports other from National Cancer Institute during the conduct of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kurman RJ, Shih IeM. The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded. Am J Pathol. 2016;186:733–47. doi: 10.1016/j.ajpath.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–9. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 3.Schneider S, Heikaus S, Harter P, Heitz F, Grimm C, Ataseven B, et al. Serous Tubal Intraepithelial Carcinoma Associated With Extraovarian Metastases. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2017;27:444–51. doi: 10.1097/IGC.0000000000000920. [DOI] [PubMed] [Google Scholar]
- 4.Visvanathan K, Vang R, Shaw P, Gross A, Soslow R, Parkash V, et al. Diagnosis of serous tubal intraepithelial carcinoma based on morphologic and immunohistochemical features: a reproducibility study. Am J Surg Pathol. 2011;35:1766–75. doi: 10.1097/PAS.0b013e31822f58bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roh MH, Kindelberger D, Crum CP. Serous tubal intraepithelial carcinoma and the dominant ovarian mass: clues to serous tumor origin? Am J Surg Pathol. 2009;33:376–83. doi: 10.1097/PAS.0b013e3181868904. [DOI] [PubMed] [Google Scholar]
- 6.Tang SG, Onuma K, Deb P, Wang E, Lytwyn A, Sur M, et al. Frequency of Serous Tubal Intraepithelial Carcinoma in Various Gynecologic Malignancies: A Study of 300 Consecutive Cases. International Journal of Gynecological Pathology. 2012;31:103–10. doi: 10.1097/PGP.0b013e31822ea955. [DOI] [PubMed] [Google Scholar]
- 7.Powell CB. Risk reducing salpingo-oophorectomy for BRCA mutation carriers: twenty years later. Gynecol Oncol. 2014;132:261–3. doi: 10.1016/j.ygyno.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Chen F, Gaitskell K, Garcia MJ, Albukhari A, Tsaltas J, Ahmed AA. Serous tubal intraepithelial carcinomas associated with high-grade serous ovarian carcinomas: a systematic review. BJOG : an international journal of obstetrics and gynaecology. 2017;124:872–8. doi: 10.1111/1471-0528.14543. [DOI] [PubMed] [Google Scholar]
- 9.Ducie J, Dao F, Considine M, Olvera N, Shaw PA, Kurman RJ, et al. Molecular analysis of high-grade serous ovarian carcinoma with and without associated serous tubal intra-epithelial carcinoma. Nat Commun. 2017;8:990. doi: 10.1038/s41467-017-01217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCluggage WG, Hirschowitz L, Gilks CB, Wilkinson N, Singh N. The Fallopian Tube Origin and Primary Site Assignment in Extrauterine High-grade Serous Carcinoma: Findings of a Survey of Pathologists and Clinicians. Int J Gynecol Pathol. 2017;36:230–9. doi: 10.1097/PGP.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 11.College of American Pathologists. http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution%20Folders/WebContent/pdf/fallopiantube-15protocol-3102.pdf.
- 12.Medeiros F, Muto MG, Lee Y, Elvin JA, Callahan MJ, Feltmate C, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30:230–6. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 13.McCluggage WG, Judge MJ, Clarke BA, Davidson B, Gilks CB, Hollema H, et al. Data set for reporting of ovary, fallopian tube and primary peritoneal carcinoma: recommendations from the International Collaboration on Cancer Reporting (ICCR) Mod Pathol. 2015;28:1101–22. doi: 10.1038/modpathol.2015.77. [DOI] [PubMed] [Google Scholar]
- 14.Carlson JW, Miron A, Jarboe EA, Parast MM, Hirsch MS, Lee Y, et al. Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J Clin Oncol. 2008;26:4160–5. doi: 10.1200/JCO.2008.16.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabban JT, Krasik E, Chen LM, Powell CB, Crawford B, Zaloudek CJ. Multistep level sections to detect occult fallopian tube carcinoma in risk-reducing salpingo-oophorectomies from women with BRCA mutations: implications for defining an optimal specimen dissection protocol. Am J Surg Pathol. 2009;33:1878–85. doi: 10.1097/PAS.0b013e3181bc6059. [DOI] [PubMed] [Google Scholar]
- 16.Trabert B, Coburn SB, Mariani A, Yang HP, Rosenberg PS, Gierach GL, et al. Reported Incidence and Survival of Fallopian Tube Carcinomas: A Population-Based Analysis From the North American Association of Central Cancer Registries. J Natl Cancer Inst. 2017 doi: 10.1093/jnci/djx263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visvanathan K, Wang TL, Shih IM. Precancerous Lesions of Ovarian Cancer-A US Perspective. J Natl Cancer Inst. 2017 doi: 10.1093/jnci/djx269. [DOI] [PubMed] [Google Scholar]
- 18.Hicks-Courant KD. Growth in salpingectomy rates in the United States since 2000. Am J Obstet Gynecol. 2016;215:666–7. doi: 10.1016/j.ajog.2016.07.055. [DOI] [PubMed] [Google Scholar]
- 19.Patrono MG, Corzo C, Iniesta M, Ramirez PT. Management of Preinvasive Lesions. Clin Obstet Gynecol. 2017;60:771–9. doi: 10.1097/GRF.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 20.Vaughan MH, Modesitt SC, Mo Y, Trowbridge ER. Serous tubal intraepithelial carcinoma: an incidental finding at the time of prophylactic bilateral salpingo-oophorectomy. Case Rep Obstet Gynecol. 2015;2015:760429. doi: 10.1155/2015/760429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinberger V, Bednarikova M, Cibula D, Zikan M. Serous tubal intraepithelial carcinoma (STIC) - clinical impact and management. Expert Rev Anticancer Ther. 2016;16:1311–21. doi: 10.1080/14737140.2016.1247699. [DOI] [PubMed] [Google Scholar]
- 22.Seidman JD, Zhao P, Yemelyanova A. "Primary peritoneal" high-grade serous carcinoma is very likely metastatic from serous tubal intraepithelial carcinoma: assessing the new paradigm of ovarian and pelvic serous carcinogenesis and its implications for screening for ovarian cancer. Gynecol Oncol. 2011;120:470–3. doi: 10.1016/j.ygyno.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 23.Przybycin CG, Kurman RJ, Ronnett BM, Shih IeM, Vang R. Are all pelvic (nonuterine) serous carcinomas of tubal origin? Am J Surg Pathol. 2010;34:1407–16. doi: 10.1097/PAS.0b013e3181ef7b16. [DOI] [PubMed] [Google Scholar]
- 24.American College of Obstetricians and Gynecologists. https://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/Salpingectomy-for-Ovarian-Cancer-Prevention.
- 25.Harmsen MG, Arts-de Jong M, Hoogerbrugge N, Maas AH, Prins JB, Bulten J, et al. Early salpingectomy (TUbectomy) with delayed oophorectomy to improve quality of life as alternative for risk-reducing salpingo-oophorectomy in BRCA1/2 mutation carriers (TUBA study): a prospective non-randomised multicentre study. BMC Cancer. 2015;15:593. doi: 10.1186/s12885-015-1597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adamo MB, Johnson CH, Ruhl JL, Dickie LA, editors. SEER Program Coding and Staging Manual. Bethesda, MD: National Cancer Institute, NIH; 2012. [Google Scholar]
- 27.Malpica A, Deavers MT, Lu K, Bodurka DC, Atkinson EN, Gershenson DM, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28:496–504. doi: 10.1097/00000478-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn E, Kurman RJ, Soslow RA, Han G, Sehdev AS, Morin PJ, et al. The diagnostic and biological implications of laminin expression in serous tubal intraepithelial carcinoma. Am J Surg Pathol. 2012;36:1826–34. doi: 10.1097/PAS.0b013e31825ec07a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei JJ, Wu J, Luan C, Yeldandi A, Lee P, Keh P, et al. HMGA2: a potential biomarker complement to P53 for detection of early-stage high-grade papillary serous carcinoma in fallopian tubes. Am J Surg Pathol. 2010;34:18–26. doi: 10.1097/PAS.0b013e3181be5d72. [DOI] [PubMed] [Google Scholar]
- 30.Singh R, Cho KR. Serous Tubal Intraepithelial Carcinoma or Not? Metastases to Fallopian Tube Mucosa Can Masquerade as In Situ Lesions. Arch Pathol Lab Med. 2017;141:1313–5. doi: 10.5858/arpa.2017-0231-RA. [DOI] [PubMed] [Google Scholar]
- 31.McDaniel AS, Stall JN, Hovelson DH, Cani AK, Liu CJ, Tomlins SA, et al. Next-Generation Sequencing of Tubal Intraepithelial Carcinomas. JAMA oncology. 2015 doi: 10.1001/jamaoncol.2015.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eckert MA, Pan S, Hernandez KM, Loth RM, Andrade J, Volchenboum SL, et al. Genomics of Ovarian Cancer Progression Reveals Diverse Metastatic Trajectories Including Intraepithelial Metastasis to the Fallopian Tube. Cancer Discov. 2016;6:1342–51. doi: 10.1158/2159-8290.CD-16-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahe E, Tang S, Deb P, Sur M, Lytwyn A, Daya D. Do deeper sections increase the frequency of detection of serous tubal intraepithelial carcinoma (STIC) in the "sectioning and extensively examining the FIMbriated end" (SEE-FIM) protocol? Int J Gynecol Pathol. 2013;32:353–7. doi: 10.1097/PGP.0b013e318264ae09. [DOI] [PubMed] [Google Scholar]
- 34.Sherman ME, Drapkin RI, Horowitz NS, Crum CP, Friedman S, Kwon JS, et al. Rationale for Developing a Specimen Bank to Study the Pathogenesis of High-Grade Serous Carcinoma: A Review of the Evidence. Cancer Prev Res (Phila) 2016;9:713–20. doi: 10.1158/1940-6207.CAPR-15-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powell CB, Chen LM, McLennan J, Crawford B, Zaloudek C, Rabban JT, et al. Risk-reducing salpingo-oophorectomy (RRSO) in BRCA mutation carriers: experience with a consecutive series of 111 patients using a standardized surgical-pathological protocol. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2011;21:846–51. doi: 10.1097/IGC.0b013e31821bc7e3. [DOI] [PubMed] [Google Scholar]
- 36.Rabban JT, Garg K, Crawford B, Chen LM, Zaloudek CJ. Early detection of high-grade tubal serous carcinoma in women at low risk for hereditary breast and ovarian cancer syndrome by systematic examination of fallopian tubes incidentally removed during benign surgery. Am J Surg Pathol. 2014;38:729–42. doi: 10.1097/PAS.0000000000000199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.