Abstract
The blood–brain barrier (BBB) is a continuous endothelial membrane within brain microvessels that has sealed cell-to-cell contacts, and is sheathed by mural vascular cells and perivascular astrocyte end-feet. The BBB protects neurons from factors present in the systemic circulation, and maintains the highly regulated CNS internal milieu, which is required for proper synaptic and neuronal functioning. BBB disruption allows influx into the brain of neurotoxic blood-derived debris, cells, and microbial pathogens, and is associated with inflammatory and immune responses, which can initiate multiple pathways of neurodegeneration. This Review discusses neuroimaging studies in the living human brain, post-mortem tissue and biomarker studies demonstrating BBB breakdown in Alzheimer disease, Parkinson disease, Huntington disease, amyotrophic lateral sclerosis, multiple sclerosis, HIV-1-associated dementia and chronic traumatic encephalopathy. The pathogenic mechanisms by which BBB breakdown leads to neuronal injury, synaptic dysfunction, loss of neuronal connectivity and neurodegeneration are described. The importance of a healthy BBB for therapeutic drug delivery, and the adverse effects of disease-initiated, pathological BBB breakdown in relation to brain delivery of neuropharmaceuticals are briefly discussed. Finally, future directions, gaps in the field and opportunities to control the course of neurological diseases by targeting BBB are presented.
Introduction
The human brain contains ~644 km of blood vessels that supply brain cells with oxygen, energy metabolites and nutrients, and remove carbon dioxide and other metabolic waste products from the brain to the systemic circulation1,2. Although representing only 2% of total body mass, the brain consumes ~20% of the body’s glucose and oxygen, and can rapidly increase blood flow and oxygen delivery to its activated regions, a process known as neurovascular coupling2,3. Capillaries are the smallest cerebral blood vessels (FIG. 1); they account for approximately 85% of cerebral vessel length and are a major site of the blood–brain barrier (BBB) (FIG. 1)1. In the human brain, capillaries provide approximately 12 m2 of endothelial cell surface area, which is available for transport of solutes from the blood to the brain, and vice versa. The mean intercapillary distance in the human brain is ~40 μm4; solute equilibration is, therefore, almost instantaneous throughout the brain interstitial space once molecules cross the BBB.
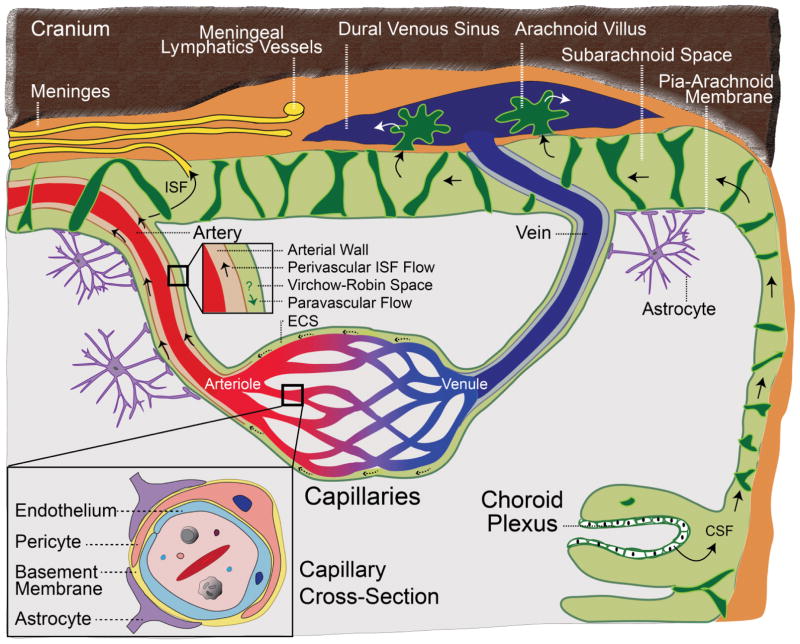
Figure 1. The blood-brain barrier.
Brain capillaries are a key site of the blood–brain barrier (BBB). The capillary cross-section (large inset) shows a tightly sealed endothelium, which shares a common basement membrane with pericytes, and astrocyte end-feet wrapping around the capillary wall. The arterial cross section (small inset) shows perivascular flow of interstitial fluid (ISF) through the arterial wall in the opposite direction to blood flow; paravascular flow might also occur in the same direction as blood flow. CSF is produced by the choroid plexus and flows from brain ventricles into subarachnoid spaces, draining into the meningeal lymphatic system and/or venous blood through the arachnoid villi. ISF can exchange with CSF in the ventricles (not shown) and subarachnoid spaces. ECS, extracellular space.
The endothelial BBB has tightly sealed cell-to-cell contacts that result in high transendothelial electrical resistance and low paracellular and transcellular permeability5 (FIG. 2). The endothelial monolayer is sheathed by mural cells (pericytes in capillaries and vascular smooth muscle cells in arterioles and arteries) and by astrocyte end-feet6,7. In contrast to the highly permeable systemic capillaries8, brain capillaries exhibit a low rate of transendothelial bulk flow by transcytosis, which together with the tightly sealed endothelium restricts the entry of most blood-derived molecules into the brain, unless they have specialized carriers and/or receptors in the brain endothelium that facilitate their transport across the BBB (FIG. 2).
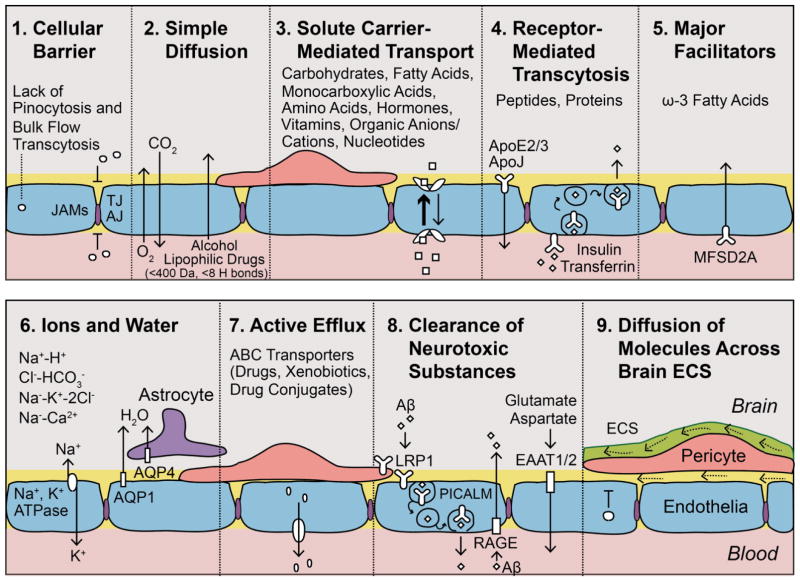
Figure 2. Key transport properties of the capillary endothelium.
a | Tight junctions (TJ), adherens junctions (AJ), and junctional adhesion molecules (JAMs) prevent free paracellular exchanges of solutes. Lack of pinocytosis and bulk flow transcytosis contributes to the endothelial barrier function. b | O2 and CO2 cross the blood–brain barrier (BBB) by simple diffusion, as do small lipophilic molecules (such as ethanol). c | Solute carrier-mediated transport (CMT) of metabolites, nutrients, vitamins, nucleotides and other substrates, according to substrate specificity and concentration gradient. d | Receptor-mediated transcytosis (RMT) of peptides and proteins. e | NLS1 (sodium-dependent lysophosphatidylcholine symporter 1) transports ω3 essential fatty acids into the brain. f | Ion concentrations are regulated by the abluminal sodium pump (Na+,K+ATPase), the luminal sodium-hydrogen exchanger, chloride-bicarbonate exchanger, luminal sodium-potassium-chloride cotransporter, and sodium-calcium exchanger. Water is transported via aquaporin (AQP) receptors: AQP1 on endothelial cells and AQP4 on astrocytic end-feet. g | ATP-binding cassette (ABC) active efflux transporters limit entry of drugs, xenobiotics, and drug conjugates. h | Neurotoxic substances are cleared by phosphatidylinositol binding clathrin assembly protein (PICALM)-mediated transcytosis and LDL receptor-related protein-1 (LRP1), which removes toxic amyloid-β (Aβ) species linked to Alzheimer disease (AD). Excitatory acidic amino acid CMT transporters EAAT1 and EAAT2 clear neurotoxic glutamate and aspartate. However, receptor for advanced glycation end products (RAGE) is upregulated in AD and mediates re-entry of circulating Aβ, which increases brain Aβ levels. i | Solutes diffusing across brain extracellular spaces (ECS) (dotted arrows) are cleared via transvascular transport (c–e, g–i) and by perivascular ISF flow within the arterial wall (solid arrow), in the reverse direction of the blood flow, eventually reaching the CSF-filled subarachnoid space and draining into meningeal lymphatic vessels and cervical lymph nodes.
Maintaining BBB integrity is crucial for tight control of the chemical composition of brain interstitial fluid (ISF), which is critical for proper synaptic functioning, information processing and neuronal connectivity. Loss of BBB integrity results in increased vascular permeability and is associated with reduced cerebral blood flow and impaired haemodynamic responses2,3,5,7,9. Breakdown of the BBB enables toxic blood-derived molecules, cells and microbial agents to enter the brain, and is associated with inflammatory and immune responses, which can initiate multiple pathways of neurodegeneration.
In this Review, we first briefly describe the molecular architecture and transport physiology of the BBB, and then examine vascular pathology, and neuroimaging, post-mortem and biomarker studies demonstrating BBB breakdown in several neurodegenerative diseases: namely, Alzheimer disease (AD), Parkinson disease (PD), Huntington disease (HD), amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS), which is considered to be an autoimmune and neurodegenerative disorder10, as well as in HIV-1-associated dementia and chronic traumatic encephalopathy (CTE). We focus on the pathogenetic mechanisms by which BBB breakdown leads to neurodegeneration, and briefly note the implications of BBB dysfunction for therapeutic drug delivery. Finally, we discuss future directions, gaps in the field, and opportunities to control neurological disease by targeting the BBB. This Review does not examine the role of reduced cerebral blood flow and altered haemodynamic responses in AD and neurodegenerative disorders, nor does it cover BBB disruption in experimental models of AD and neurodegeneration, which have been extensively reviewed elsewhere2,3,9,11.
The BBB molecular architecture
Brain endothelial cells are connected by tight junctions and adherens junctions. Tight junctions involve occludin and claudin-1, claudin-3, claudin-5 and claudin-12, and the membrane-associated guanylate kinases tight junction proteins ZO1, ZO2 and ZO3, whereas adherens junctions involve cadherins, platelet endothelial cell adhesion molecule (PECAM-1), and the junctional adhesion molecules (JAMs) JAMA, JAMB and JAMC6. A paucity of pinocytosis and bulk flow fluid transcytosis contributes to the limited exchange of solutes across the brain endothelium (FIG. 2), although oxygen and carbon dioxide rapidly diffuse across it. Small arterioles12 and capillaries13 are major sources of the brain’s oxygen supply. Additionally, small lipid-soluble molecules and compounds with a molecular weight <400 Da or containing <8 hydrogen bonds (such as ethanol), can cross the BBB by simple transmembrane diffusion4.
Solute carrier-mediated transport (CMT) facilitates the transport of carbohydrates, amino acids, fatty acids, monocarboxylic acids, nucleotides, hormones, vitamins, organic anions and cations across the BBB. Receptor-mediated transcytosis (RMT) enables transendothelial transport of proteins and peptides in both directions: from blood to brain (transferrin and insulin)4 and from brain to blood (apolipoproteins)6. Sodium-dependent lysophosphatidylcholine symporter 1 (NLS1, also known as major facilitator superfamily domain-containing protein 2a), an important transporter, transports essential ω3 fatty acids into the brain14, which is also critical for BBB formation15 (FIG. 2).
The sodium pump (Na+, K+-ATPase) on the abluminal membrane of the BBB regulates sodium influx into the brain ISF, in exchange for potassium16. Other ion transporters regulate the transport of sodium, potassium, chloride and calcium ions, and facilitate the exchange of sodium for hydrogen ions and chloride for bicarbonate ions at the BBB. ATP-binding cassette (ABC) transporters expressed at the luminal side of the BBB prevent brain accumulation of drugs, xenobiotic agents and drug conjugates, via active efflux from endothelium into blood6,17. CMT facilitates CNS-to-blood clearance of excitatory amino acids (such as glutamate and aspartate)18, whereas RMT clearance of amyloid-β (Aβ, some forms of which are associated with AD)6,19–25 across the BBB keeps brain levels of these potentially toxic substances low (FIG. 2). Much more could be said about the molecular architecture of the BBB and its transport physiology, but only a brief overview is given here as these topics have been reviewed in detail elsewhere6,7.
Molecules generated by the brain diffuse across brain extracellular spaces, and are cleared from the brain by two mechanisms: trans-vascular transport across the BBB, via the mechanisms illustrated in FIG. 2 (b–d, f–h)5,6,26; and perivascular transport of ISF, which travels in the reverse direction to the flow of blood within the basement membranes of arterial vessel walls (FIG. 1)26–28. Studies conducted in the 1980s and 1990s showed that solutes carried by the perivascular ISF flow reach the subarachnoid space, which is filled with cerebrospinal fluid (CSF) and drains into deep cervical lymph29,30. In the past 3 years, further studies have confirmed a role of the dural lymphatic vascular system in clearance of ISF and macromolecules by the meningeal lymphatic vessels31, which drain into cervical lymph nodes32–34 (FIG. 1). Under physiological conditions, the perivascular ISF pathway is responsible for 15–20% of the clearance of AD-related forms of Aβ from the mouse brain19,35, whereas 80–85% is removed by transvascular BBB transport. In 1985, solutes were shown to rapidly distribute throughout the brain by paravascular transport from the subarachnoid space through Virchow–Robin spaces, in the same direction to the flow of blood36. Subsequent studies introduced the term ‘glymphatic’ system to describe this paravascular circulation, and suggested that solute transport in the CNS occurs via CSF convective flow through the brain extracellular spaces, in a para-arterial to para-venous direction, regulated by aquaporin-4 (AQP4) water channels on astrocytes37,38. The proposed glymphatic mechanism, however, has not been supported by the latest studies39–42, and the convective, pressure-driven fluid flow of CSF from para-arterial to para-venous extracellular spaces throughout the parenchyma remains unproven43–45,39,40. Furthermore, deletion of Aqp4 in mice and rats does not impair the transport of fluorescent solutes from the subarachnoid space into the brain, which implies that water production by astrocyte end-feet does not have a role in the regulation of solute transport within parenchymal extracellular spaces39. Further experimental work is needed to resolve these controversies.
Vascular pathology in neurodegeneration
Cerebrovascular dysfunction and vascular pathology contribute to cognitive decline and neuronal loss in AD, in addition to AD-related Aβ and tau pathology5,6,46–53. Many lines of evidence indicate that cerebrovascular dysfunction in AD cannot be simply attributed to comorbid vascular dementia. For instance, in one study of the association between cerebrovascular and neurodegenerative disease, the US National Alzheimer’s Coordinating Centre database was used to identify 5,715 patients with an autopsy-based diagnosis of a single neurodegenerative disease (AD, frontotemporal lobar degeneration, α-synucleinopathy, hippocampal sclerosis, prion disease, and cerebrovascular disease)52. Within the subgroup of 4,629 patients diagnosed as having AD who had no evidence of mixed dementia, 80% had vascular pathology including cerebrovascular disease, lacunae and multiple microinfarcts indicative of small vessel disease, haemorrhage, atherosclerosis, arteriolosclerosis and cerebral amyloid angiopathy (CAA)52. The two subgroups of patients with an autopsy-based diagnosis of either AD or cerebrovascular disease exhibited a remarkably similar prevalence of vascular risk factors, such as coronary disease, hypercholesterolemia and diabetes52.
Cerebral vessel pathology is a major risk factor for AD dementia, and is associated with low scores in most cognitive domains51. CAA, which is an important cause of BBB disruption and one of the three pathological hallmarks of AD54, induces various vascular pathologies that contribute to cognitive decline26. Moreover, in preclinical AD, changes in vascular biomarkers occur before the development of cognitive impairment and before detectable increases in standard AD biomarkers, including amyloid deposition, decreased CSF levels of Aβ42, and increased CSF levels of tau and phosphorylated tau48. Small vessel disease of the brain is prominent in patients with AD, as discussed below, and contributes to ~50% of all dementias worldwide3,55–58.
According to the two-hit vascular hypothesis of AD, damage to blood vessels is the initial insult, causing BBB dysfunction and diminished brain perfusion that, in turn, lead to neuronal injury and Aβ accumulation in the brain5,6,47,50. Cerebrovascular disruption is influenced by lifestyle and might act independently and/or synergistically with Aβ to promote AD pathology, which is accelerated by genetic risk factors, such as carriage of the ε4 allele of apolipoprotein E (APOE*ε4), vascular risk factors (such as hypertension, diabetes and dyslipidemia) and environmental risk factors (such as pollution)47,50.
Vascular pathology also contributes to other neurodegenerative disorders52. For example, cerebrovascular disease plays a part in the pathogenesis of PD52, the second most common neurodegenerative disorder, which is characterized by accumulation of α-synuclein and degeneration of dopaminergic neurons in the substantia nigra. Vascular disease and vascular risk factors aggravate motor dysfunction and cognitive impairment in PD59. Cerebrovascular disease, BBB impairments and neurovascular abnormalities are also found in HD60,61, an autosomal-dominant neurodegenerative disease with motor, cognitive, psychiatric, and metabolic abnormalities caused by the aggregation of mutant huntingtin protein. BBB disruption and trafficking of T cells, B cells and peripheral macrophages across dysfunctional BBB is a pathological hallmark of MS33. BBB disruption has been described in ALS62, is a feature of CTE64 and, in HIV-1-associated dementia, enables HIV-1-infected monocytes and macrophages to enter the brain63.
Neuroimaging evidence of BBB disruption
In this section, we examine recent PET and MRI studies of BBB integrity and function in AD and other neurodegenerative disorders (TABLE 1).
Table 1.
Blood–brain barrier disruption on neuroimaging in neurodegenerative disorders.
| Disease | Region and details | Refs |
|---|---|---|
| Increased leakage of gadolinium ( DCE MRI; Ktrans*) | ||
| MCI | Hippocampus | 46, 49, 74–75, 77 |
| Early AD | Several grey and white matter regions | 46, 74–76 |
| PD | Basal ganglia | 79 |
| HD | Caudate nucleus | 60 |
| MS | Perivascular growth of lesions in white matter regions | 49, 80–84 |
| Microbleeds (T2* and SWI-MRI) | ||
| MCI | 25% of patients | 94, 104 |
| AD | 45–78% of patients | 86–94, 104 |
| PD | Several deep and cortical grey matter regions and white matter | 105, 106 |
| ALS | Deep cortical layers | 107 |
| Diminished glucose transport ( FDG-PET) | ||
| Normal cognition with AD genetic or parental risk | Entorhinal cortex, hippocampus, posterior cingulate cortex, whole brain | 116, 120–121 |
| MCI | Precuneus, cingulate cortex and temporal cortex (prior to conversion to AD) | 115, 117, 118, 122, 123 |
| Early AD | Hippocampus, parietal, temporal and cingulate cortex (prior to development of atrophy and neurodegeneration) | 113–115, 117–119, 122, 123 |
| Diminished P-glycoprotein function ( verapamil PET) | ||
| Mild AD | Parietotemporal, frontal, and posterior cingulate cortices, and hippocampus | 140, 141 |
| AD | Frontal, parietal, temporal and occipital cortices, and posterior and anterior cingulate | 140 |
| PD | Mid-brain | 142 |
| CNS leukocyte infiltration ( MMP inhibitor-PET) | ||
| MS | Leukocyte infiltration (MMP activation) in lesions | 143 |
The regional CNS blood–brain barrier permeability constant. AD, Alzheimer disease; ALS, amyotrophic lateral sclerosis; DCE, dynamic contrast-enhanced; FDG, fluorodeoxyglucose; FLAIR, fluid-attenuated inversion recovery; HD, Huntington disease; MCI, mild cognitive impairment; MMP; matrix metalloproteinase inhibitor MS, multiple sclerosis; PD, Parkinson disease; SW, susceptibility-weighted imaging.
Increased BBB permeability to gadolinium
BBB breakdown in the hippocampus, a centre of memory and learning, has been observed in individuals with mild cognitive impairment (MCI) using dynamic contrast-enhanced (DCE) MRI. In this technique, leakage of gadolinium contrast agent into the brain enables the regional CNS BBB permeability constant, Ktrans, to be quantified using the Patlak analysis method49,65,66. A study that compared BBB breakdown in the hippocampus in individuals with MCI compared to age-matched controls found that the extent of BBB breakdown was not affected by vascular risk factors49, but correlated with increased CSF levels of soluble platelet-derived growth factor receptor-β (PDGFRβ), a marker of pericyte injury49,67. BBB breakdown in the hippocampus occurred prior to hippocampal atrophy49, which is typically seen early in AD68,69, raising the possibility that BBB breakdown might precede neurodegeneration. This concept is supported by data from experimental models of BBB breakdown, which causes neurodegenerative changes over time70–73. Follow-up DCE-MRI studies in patients with early AD confirmed BBB breakdown in several grey matter and white matter regions46,74–76 (TABLE 1). Consistent with these findings, early contrast-enhanced MRI studies in humans showed increased BBB permeability in the hippocampus in individuals with MCI compared to healthy controls77, and suggested that contrast agent accumulates in the brains of individuals with probable AD via a blood-to-brain-to-CSF pathway78.
DCE-MRI studies have detected increased BBB leakage of gadolinium (using the Patlak quantification method49,65,66) in the basal ganglia in patients with PD compared to healthy controls79. In patients with HD, DCE-MRI analysis reveals a positive correlation between increased BBB permeability in the caudate nucleus and increased disease burden score, as well as increased grey matter arterial cerebral blood volume60. DCE-MRI studies have similarly established the presence of increased BBB permeability in white matter in MS49,80–82, particularly in active MS lesions83,84 (TABLE 1). Increased matrix metalloproteinase-9 (MMP-9) activity in the CSF has been suggested to contribute to BBB breakdown in MS82,85. To understand the pathogenetic role of BBB breakdown in the living human brain, future longitudinal DCE-MRI studies are required to investigate the relationships between vascular changes, progression of neurological deficits in AD, PD, HD and MS, and changes in brain structural and functional connectivity. Extending DCE-MRI studies to patients with ALS, HIV-1-associated dementia and CTE will help us to identify whether regional BBB breakdown has a pathogenetic role in neurodegenerative disorders.
Microbleeds
Damage to blood vessels can lead to pronounced BBB breakdown manifested as cerebral microbleeds (microhaemorrhages), which is frequently seen in AD86–93, MCI94, and in APOE*ε4 individuals who have increased genetic risk of AD94. CAA is one of the main causes of vascular degeneration and lobar microbleeds in AD, and contributes to BBB breakdown, infarcts, white matter changes and cognitive impairment26. The microbleed location relates to its aetiology: CAA causes lobar microbleeds and hypertensive vasculopathy causes microbleeds in the basal ganglia, thalamus, cerebellum and brainstem (reviewed elsewhere95). Microbleeds in AD are predominantly lobar88,92,96–99 (similar to CAA-associated microbleeds) and are mainly found in the occipital lobe92,98,99. Amyloid deposition in the brain, as detected by 18F-florbetapir PET, is positively associated with the number of microbleeds in individuals with MCI and AD99. Several studies that reported a high prevalence of microbleeds in patients with AD86–93 or MCI94 did not perform amyloid-PET imaging86–93, however, precluding a direct comparison of microbleeds and CAA severity.
Cortical superficial siderosis (that is, detection of subpial deposits of hemosiderin) has been suggested as an alternative imaging biomarker for CAA100,89,101,102. The extent of cortical superficial siderosis, lobar microbleeds, and amyloid plaque burden is higher in patients with AD than in cognitively normal controls (as shown by MRI and amyloid-PET studies94), and MRI evidence of superficial siderosis was also observed in three individuals with pathologically confirmed CAA. To definitely relate the topography and prevalence of microbleeds and superficial siderosis to CAA in AD, more amyloid-PET and high field strength MRI studies are needed, as discussed below.
Microbleeds are often used as a criterion to define small vessel disease in the brain103. Small hypointense regions on T2*-weighted and susceptibility-weighted imaging (SWI) MRI are thought to representing blood-derived hemosiderin deposits, probably phagocytosed by macrophages in the perivascular spaces, after microbleeding events96. The strength of the MRI magnetic field determines the ability to detect brain microhemorrhages104. For example, 3 T MRI studies indicate that approximately 45% of patients with AD88,90,91,94 and 25% of individuals with MCI94 develop microhemorrhages, whereas a 7 T MRI study found that 78% of patients with AD have microhemorrhages87. Since most current studies involve 1.5T and 3T MRI, the incidence of microhemorrhages in MCI and AD is likely to be underestimated. High-resolution confocal microscopy of brain tissue can detect capillary hemorrhages as small as 20–30 μm in diameter, which are easily missed on 1.5T or 3T MRI62.
Cerebral microbleeds have been detected throughout deep grey matter regions (including the caudate, thalamus, putamen and globus pallidus), cortical regions and white matter in patients with PD by T2*-weighted and SWI-MRI. The incidence of microbleeds is higher in patients with PD dementia than in PD patients without dementia and controls, and is associated with the extent of white matter lesions105,106.
Hypointense areas in the cortex on T2-weighted MRI, suggestive of microbleeds, have also been shown in patients with ALS62. Studies using high-resolution T2-weighted 7T MRI have also reported microbleeds in the brains and spinal cords of patients with ALS107.
Impaired glucose transport
Glucose is a key energy substrate for the brain. Brain uptake of glucose is measured using the radiolabeled glucose analogue, 18F-fluoro-2-deoxyglucose (FDG), as a PET tracer46. FDG enters the brain via solute carrier family 2, facilitated glucose transporter member 1 (also known as glucose transporter-1 (GLUT1)), which is expressed only in the endothelium of the BBB, not in neurons6,108. Besides GLUT1, brain uptake of FDG depends on cerebral blood flow2,5, which is reduced in MCI and early AD, prior to brain atrophic changes2.
Although both glucose and FDG are rapidly transported into the brain via GLUT-1, avidly taken up by brain cells and then phosphorylated by intracellular hexokinase127,128, their subsequent metabolic fate in the brain is completely different129. Glucose-6-phosphate is metabolized rapidly in the glycolytic pathway, whereas FDG-6-phosphate is not a substrate for glucose-6-phosphate isomerase and thus cannot be converted into fructose-6-phosphate, which precludes its further metabolism130,127,131,128. Therefore, approximately 45–90 min after systemic administration of FDG, 90–97% of this compound persists in the mouse128 or rat131,132 brain in the form of FDG-6-phosphate or its epimers; the remainder is FDG. Because brain cells have very low activity of glucose-6-phosphatase, and poor transport of FDG-6-phosphate across cell membranes133,134, FDG-6-phosphate remains trapped within brain cells132,135 and is eliminated only slowly from the brain. Since brain uptake of FDG across the BBB depends on GLUT1not direct neuronal uptake, the diminished uptake of FDG in the AD brain points to a vascular deficit (that is, impaired BBB function). Importantly, GLUT1 levels are substantially reduced in brain microvessels in AD109–112. Diminished BBB transport and brain uptake of FDG precedes neurodegeneration and brain atrophy in patients with MCI who later convert to a diagnosis of AD, as well as in patients with early AD. This vascular deficit should be considered in staging preclinical AD136.
FDG-PET studies also indicate that individuals with MCI have diminished glucose uptake in several brain regions (including the precuneus, posterior cingulate, right angular gyrus and bilateral temporal cortices) prior to any detectable neurodegenerative changes, brain atrophy and/or conversion to AD113. The reductions in FDG uptake in the posterior cingulate gyri and parietotemporal lobes of patients with AD are observed with and without corrections for partial volume effects, confirming that these decreases are not due to brain atrophy 114. Longitudinal FDG-PET findings have additionally suggested that reductions in hippocampal glucose uptake during normal ageing can predict cognitive decline years in advance of a clinical AD diagnosis115. Similarly, asymptomatic carriers of presenilin-1 (PSEN1) mutations associated with early-onset autosomal dominant AD show AD-like reductions in FDG uptake in the absence of brain atrophy116. Diminished glucose uptake in the hippocampus, parieto-temporal cortex and/or posterior cingulate cortex has been repeatedly shown by FDG-PET in early AD117,118, in individuals at genetic risk of AD119,120, with a positive family history of AD121 and/or MCI, as well as in individuals with no cognitive impairment who went on to develop AD122,123. The patterns of FDG brain uptake can also discriminate individuals with normal cognition from those with MCI and AD117. FDG-PET changes preceding neurodegeneration are not only found in humans113–116, but also in transgenic mouse models of AD124, reflecting reductions in glucose transport across the BBB125,126.
Moreover, experimental studies in Slc2a1+/− mice (which express 50% of GLUT1 levels in cerebral blood vessels compared with their wild-type littermates) have shown rapid BBB breakdown followed by secondary neurodegeneration, which is accelerated by Aβ108. No attempts have been made so far to explore whether GLUT1 at the BBB is a therapeutic target in human AD, or whether pharmacological upregulation of this transporter in humans can prevent BBB breakdown, neurodegeneration and cognitive deficits, as it can in animal models. Additionally, the role of glucose transport in other neurodegenerative disorders has not been examined, and should be pursued by future studies.
Impaired P-glycoprotein function
P-glycoprotein (encoded by the ABCB1 gene) mediates the active efflux of drugs and xenobiotic compounds from the endothelium to blood, thereby preventing their accumulation in the brain6,17. P-glycoprotein clears Aβ across the BBB, which requires LDL receptor-related protein-1 (LRP1)137–139. P-glycoprotein function is clinically assessed by 11C-verapamil-PET. Verapamil-PET studies in AD have demonstrated increased uptake of verapamil in frontal, parietal, temporal and occipital cortices, and in posterior and anterior cingulate gyri140. Similarly, verapamil-PET studies in patients with mild AD found substantially reduced P-glycoprotein activity in the parietotemporal, frontal, and posterior cingulate cortices and hippocampus141. Furthermore, verapamil-PET studies indicated diminished P-glycoprotein activity, indicating BBB dysfunction, in the mid-brain of patients with PD142. Collectively, these studies suggest that decreased P-glycoprotein function is involved in the pathogenesis of AD — either by enabling xenobiotic compounds to accumulate in the brain (high levels of which can injure neurons and promote inflammation) and/or by reducing Aβ clearance across the BBB. Thus, P-glycoprotein and LRP1 could be important therapeutic targets in AD, and perhaps also in PD.
CNS leukocyte infiltration
Studies using a radiolabelled MMP as a PET tracer showed increased MMP activity in early MS lesions, which is associated with leukocyte infiltration143. Additionally, MS patients show impaired cerebral venous drainage144 and decreased cerebrovascular reactivity of grey matter, which correlates with grey matter atrophy145. As leukocyte infiltration of the CNS also occurs in other neurodegenerative diseases, notably AD146–149, HIV-1-associated dementia150 and CTE151, similar MMP-PET neuroimaging studies would be helpful to identify when in the course of these diseases this cellular infiltration of the CNS takes place. However, as yet, such studies are lacking.
Postmortem evidence of BBB disruption
In this section, we examine the evidence of BBB disruption derived from analyses of post-mortem tissues from patients with AD and other neurodegenerative disorders. In these studies, BBB disruption is demonstrated by brain capillary leakages, degeneration of BBB-associated cells (including pericytes and endothelial cells), brain infiltration of circulating leukocytes and red blood cells, aberrant angiogenesis and molecular changes (TABLE 2).
Table 2.
Blood–brain barrier disruption on post-mortem tissue analysis in neurodegenerative disorders.
| Disease | Details | Refs |
|---|---|---|
| Brain capillary leakages*§ | ||
| AD | Accumulation of blood-derived fibrinogen, thrombin, albumin, IgG, and haemosiderin in the cortex and hippocampus | 146, 147, 152–157 |
| PD | Accumulation of blood-derived proteins in striatum: fibrinogen, IgG, haemosiderin in globus pallidus | 165, 166 |
| HD | Leakage of blood-derived proteins: fibrin in the putamen | 60 |
| ALS | Leakage of blood-derived proteins: fibrinogen, thrombin, IgG, collagen type IV, iron-containing proteins | 62, 107, 168 |
| MS | Leakage of blood-derived proteins: fibrinogen | 170 |
| Chronic traumatic encephalopathy | Perivascular haemosiderin-laden macrophages and histiocytes | 151, 171 |
| Pericyte degeneration | ||
| AD*‡§ | Ultrastructural changes in the cortex: accumulation of osmiophilic material, mitochondrial changes, pinocytosis, loss of pericyte capillary coverage and numbers in the cortex and hippocampus | 155–157, 172, 173 |
| ALS*ठ| Pericyte loss in the medulla, reduced pericyte capillary coverage and number in the cervical spinal cord | 62, 168 |
| HIV-associated dementia* | Loss of pericyte coverage in the frontal cortex | 177 |
| Chronic traumatic encephalopathy* | Mural cell mineralization in deep penetrating vessels | 171 |
| Endothelial degeneration | ||
| AD*‡ | Microvascular reductions, reduced tight junction proteins, capillary basement membrane changes in the cortex and hippocampus | 118, 155, 156, 158, 173, 180 |
| PD‡ | Microvascular degeneration, reduced and disrupted tight junctions, capillary basement membrane changes in the subthalamic nucleus | 166 |
| HD§ | Decreased and disrupted tight junction protein expression in the putamen | 60 |
| ALS*|| | Microvascular degeneration, intracellular vacuolization; reduced or disrupted tight junctions, capillary basement membrane changes, enlarged perivascular spaces in the medulla, cervical spinal cord and lumbar spinal cord | 168, 182, 183 |
| MS* | Decreased and disrupted tight junctions | 170 |
| HIV-associated dementia* | Reduced or disrupted tight junctions | 150, 184 |
| Cellular Infiltration* | ||
| AD | Extravasation of red blood cells, peripheral macrophage infiltration, neutrophils infiltration | 146–149 |
| PD | Red blood cell extravasation in striatum | 165 |
| ALS | Red blood cell extravasation | 62 |
| MS | Leukocyte infiltration | 143 |
| HIV-associated dementia | Peripheral macrophage infiltration | 150 |
| Chronic traumatic encephalopathy | Lymphocyte infiltration in Virchow–Robin spaces | 151 |
| Aberrant angiogenesis* | ||
| PD | increased endothelial cell number in substantia nigra pars compacta; increased angiogenic endothelial integrin-αvβ3 expression in substantia nigra pars compacta, locus coeruleus, putamen | 187–188 |
| HD | Increased vessel density, particularly of capillaries in the cortex, caudate or putamen and substantia nigra | 60, 61 |
| Molecular changes | ||
| AD*§ | Reduced GLUT1 levels (diminished brain glucose uptake); reduced LRP1 levels (diminished Aβ clearance); upregulation of RAGE (increased Aβ re-entry, neurovascular inflammation); activation of proinflammatory cyclophilin A–MMP9 pathway in APOE4 carriers (blood–brain barrier breakdown owing to degradation of tight junctions and basement membrane proteins); increased levels of angiogenic proteins | 19, 20, 109–112, 156, 181, 190, 191, 196 |
| HIV-associated dementia | Reduced P-glycoprotein expression | 204 |
| HD*‡ | Huntingtin protein aggregation in endothelial cells, perivascular macrophages, vascular smooth muscle cells, and vascular basal lamina in the cortex and putamen | 60, 205 |
Detected by immunohistochemistry.
Detected by electron microscopy.
Detected by immunoblotting.
Detected by reverse transcription PCR.
AD, Alzheimer disease; ALS, amyotrophic lateral sclerosis; GLUT1, glucose transporter 1; HD, Huntington disease; LRP1, LDL receptor-related protein 1; MMP, matrix metalloproteinase; MS, multiple sclerosis; PD, Parkinson disease; RAGE, receptor for advanced glycation end products.
Capillary leakages
Several studies of post-mortem brain tissue from patients with AD have found (using various analysis methods: immunohistochemistry, immunoblotting and Prussian blue staining) capillary leakages of blood-derived proteins in the prefrontal and entorhinal cortex and hippocampus, including accumulations of fibrinogen, thrombin, albumin, IgG, and iron-containing proteins such as haemosiderin146,147,152–157. These blood-derived proteins are often found co-localized with deposits of AD-associated Aβ147,153,155. Evidence of BBB breakdown is most pronounced in individuals carrying the APOE*ε4 allele, the major genetic risk factor for AD. By contrast, individuals homozygous for the most common allele, APOE*ε3, have a reduced risk of AD and show a decreased degree of BBB breakdown147,152,156,158. As reviewed elsewhere11, multiple experimental studies have confirmed that BBB breakdown causes capillary leakage in AD models of β-amyloidosis159–162 and in APOE*ε4 transgenic mice71,163,164.
Post-mortem analysis of brain tissue from patients with PD revealed perivascular deposits of fibrinogen or fibrin165, IgG166 and haemosiderin165,167 in the striatum, which is indicative of BBB breakdown. Fibrin(ogen) deposits around capillaries were also found in brain tissue from patients with HD60. Fibrinogen, thrombin, IgG and haemosiderin deposits have been found in brain and spinal cord tissue from patients with sporadic or familial forms of ALS62,107,168 as well as in a transgenic mouse model of ALS, prior to the onset of motor neuron degeneration11,169.
Finally, patients with MS develop capillary leakages of fibrinogen within active and inactive lesions, particularly along vessels with abnormal tight junctions170. The first post-mortem study of a patient with CTE found cerebral oedema and haemosiderin-laden perivascular macrophages in the Virchow–Robin spaces171.
Pericyte degeneration
Electron microscopy studies of brain tissue from patients with AD revealed pericyte degeneration in the cortex associated with large accumulations of osmiophilic material. These changes are suggestive of increased phagocytosis of blood-derived proteins, mitochondrial alterations, and an increased number of pinocytotic vesicles172,173. Immunostaining for the pericyte marker PDGFRβ revealed reduced pericyte coverage and numbers on brain capillaries in brain samples from patients with AD155, which showed evidence of a gene-dose effect linked to the number of APOE*ε4 alleles (compared with homozygosity for APOE*ε3)156. Immunoassay of AD cortical tissue confirmed loss of the pericyte marker PDGFRβ in the precuneus157, a region affected early in the course of AD. Pericytes maintain BBB integrity7,176, and their degeneration leads to BBB breakdown70,174,175. Additionally, pericytes clear Aβ from the brain, and their loss accelerates the onset and progression of Aβ and tau pathology in mouse models of AD159.
Immunohistological analysis of spinal cord or brain tissue from patients with ALS also reveals notable pericyte degeneration62,168. Post-mortem studies of brain tissue from patients with HIV-1-associated dementia or HIV encephalitis have found evidence of microvascular degeneration and BBB breakdown, including reduced pericyte coverage177. Vascular insults, including enlarged perivascular spaces64,151,178 and mineralization of mural cells of deep penetrating blood vessels171, have also been found in brain tissue from patients with CTE64,151,171,178.
Endothelial degeneration
Reductions in capillary length (suggestive of endothelial degeneration), reduced expression of tight junction proteins, and capillary basement membrane changes have been reported in brain tissue from patients with AD5,155,156,158,173,179,180. These changes might reflect aberrant brain angiogenesis, caused by the low brain endothelial cell expression of MEOX2, encoding homeobox protein MEOX2, a regulator of vascular differentiation in AD180. A wide range of proangiogenic factors are also expressed in microvessels isolated from the brains of patients with AD181, which (in the presence of reduced MEOX2 expression) leads to reduced brain capillary density and cell death via increased expression of the AFX1 transcription factor, which regulates apoptosis180. Pericyte-derived soluble factors that maintain a healthy endothelium might also be lacking in the AD brain owing to pericyte degeneration, which could potentially contribute to endothelial degeneration, as shown in animal models70.
Endothelial degeneration with microvascular changes (reductions in endothelial cell thickness, length and density), loss of and abnormalities in tight junction proteins, and basement membrane changes have also been reported in brain tissue from patients with PD166. Immunohistological analysis of spinal cord or brain tissue from patients with ALS revealed endothelial degeneration with reduced tight junctions, capillary basement membrane changes, and enlarged perivascular spaces168,182,183, as well as dissociation of astrocyte end-feet from capillaries107. Reduced expression of endothelial tight junction proteins claudin-5 and occludin has been shown in HD60. Endothelial degeneration with reduced and discontinuous expression of tight junction protein ZO1 has been shown in active and inactive MS lesions, compared with normal-appearing white matter170. Post-mortem studies of brain tissue from patients with HIV-1-associated dementia or HIV encephalitis have found evidence of microvascular degeneration and BBB breakdown, including reduced pericyte coverage177, reduced and disrupted tight junctions150,184, and capillary basement membrane changes150.
Cellular infiltration
Extravasation of red blood cells has been found in AD146, PD165 and ALS62. Infiltration by peripheral macrophages has also been shown in AD149,172 and in HIV encephalitis150. Additionally, neutrophils can cross the BBB in AD148. These findings collectively suggest that BBB breakdown in AD and other neurodegenerative disorders not only enables extravasation of red blood cells, which causes microbleeds and deposition of haemosiderin (derived from the haemoglobin carried by red blood cells), but also activates the innate immune response in the brain. Whether these immune system responses in non-AD neurodegenerative diseases are directed at identifying and eliminating pathogens that would otherwise enter the brain across the disrupted BBB, (and which in models of AD has been shown to accelerate amyloid deposition in exchange for circumscribing the infection process185,186), remains to be determined in future studies.
Aberrant angiogenesis
Increased levels of pro-angiogenic factors have been reported in AD brains181. However, successful renewal of lost capillary networks is compromised in AD brains, which is probably due to ongoing pericyte degeneration7 and low endothelial expression of MEOX2180, as discussed above. Aberrant angiogenesis, as indicated by changes in markers of angiogenesis, has also been found in the substantia nigra, locus coeruleus, and putamen in PD187,188. The effectiveness of deep brain stimulation of the subthalamic nucleus to alleviate motor symptoms in PD might even be attributable to improvements in the microvascular architecture189,166, such as increased capillary length and density, increased endothelial cell thickness, and increased expression of the tight and adherens junction proteins occludin, claudin-5, ZO1 and vascular endothelial (VE)-cadherin, along with reduced perivascular IgG leakage in post-mortem brain samples from deep brain stimulation-treated compared with untreated PD patients166.
Increased density of capillaries (vessels 5–10 μm in diameter) and reduced numbers of larger microvessels (10–20 μm in diameter), suggestive of aberrant angiogenesis, was found in HD60. Abnormal vascularization in HD was also found in the cortex and substantia nigra60,61.
Molecular changes
Several studies have shown that AD brain endothelium expresses low levels of GLUT1, a BBB-specific glucose transporter109–112, which leads to diminished glucose transport across the BBB108. AD brain microvessels also show diminished expression of LRP1, a major Aβ clearance receptor at the BBB19,20,156,190,191 (which change is also present in patients with hereditary cerebral haemorrhage with amyloidosis, Dutch type (HCHWA-D)20). Diminished LRP1 expression leads to reduced Aβ clearance from the brain, promoting its accumulation in the brain20,22,24. Thus, LRP1 is a key target for enhancing transvascular Aβ clearance192. This mechanism could be important for the efficacy of current Aβ clearance therapies based on anti-Aβ antibodies, particularly for therapies with a peripheral Aβ sink mechanism of action, which requires Aβ clearance from brain-to-blood across the BBB193,194,195 (FIG. 2).
Patients with AD develop increased levels of receptor for advanced glycation end products (RAGE) in brain microvessels, both in brain endothelium and mural cells190,191,196. RAGE transports Aβ in the opposite direction to LRP1, mediating the re-entry of circulating Aβ into the brain, which promotes inflammation. Experimental studies have also identified RAGE as a major therapeutic target in AD196–199, which led to initiation of an ongoing phase III trial of a RAGE blocker in patients with AD200.
Compared to controls, patients with AD have increased levels of both cyclophilin A (a proinflammatory cytokine) and matrix metalloproteinase 9 (MMP9) in the brain endothelium and pericytes. These increases are particularly pronounced in APOE*ε4 carriers156, findings comparable to those in transgenic APOE*ε4 mice71, which suggests that these increases represent activation of a BBB-degrading pathway involving cyclophilin A and MMP9. Activation of this cyclophilin A–MMP9 pathway has been confirmed by CSF analysis in non-symptomatic APOE*ε4 carriers, in whom it is associated with BBB breakdown201, and by analysis of cyclophilin A mRNA levels in brain tissue202. As the cyclophilin A inhibitor alisporivir has shown promise in a phase III clinical trial as an add-on treatment for hepatitis C203, these studies raise a possibility that these agents might also be useful in stabilizing the BBB in APOE*ε4 AD carriers. Whether inhibition of the BBB cyclophilin A–MMP9 pathway can influence the neurodegenerative process in human APOE*ε4 carriers with AD (as it does in humanized APOE*ε4 transgenic mice71), is an interesting topic for future studies.
Post-mortem studies of patients with HIV-1-associated dementia or HIV encephalitis also report reduced brain endothelial expression of P-glycoprotein204. Additionally, in patients with HD, mutant huntingtin aggregates accumulate in brain endothelial cells, perivascular macrophages, vascular smooth muscle cells, and vascular basal lamina60, and in genetically unrelated fetal neural allografts in the brains of patients with advanced HD205. These data suggest that the cerebral vasculature and immune system contribute to the spread of mutant huntingtin as well as the ability of non-neuronal cells including vascular cells to contribute to spreading of HTT mutant.
CSF evidence of BBB disruption
Here we examine CSF biomarkers of BBB breakdown in AD and other neurodegenerative disorders (TABLE 3). Other CSF biomarkers of aberrant angiogenesis, endothelial dysfunction, mural cell injury, inflammatory cytokines and chemokines in AD and other neurodegenerative disorders have been reviewed in detail elsewhere50,206,207, and are not examined here.
Table 3.
Blood–brain barrier disruption on CSF ELISA in neurodegenerative disorders.
| CSF ELISA analysis method | Disease | Details | Refs |
|---|---|---|---|
| Albumin | Preclinical AD or MCI | Increased Qalb | 49, 50, 201 |
| Albumin | AD | Increased or no change in Qalb | 208–210, 212 |
| Albumin | AD with vascular risk factors: | Increased Qalb | 211, 213, 214 |
| Albumin | PD | Increased Qalb | 106, 209, 222, 223 |
| Albumin | ALS | Increased Qalb in 40% of patients | 62, 224 |
| Albumin | MS | Increased Qalb | 80, 218 |
| Albumin | HIV-associated dementia | Increased Qalb | 225 |
| Plasminogen | Preclinical AD or MCI | Increased CSF levels of blood-derived proteins (plasminogen) | 221 |
| Fibrinogen | Preclinical AD or MCI | Increased CSF levels of blood-derived proteins (fibrinogen) | 220 |
| IgG | PD | Increased CSF:serum IgG ratio | 222 |
| IgG | ALS | Increased CSF levels of blood-derived proteins (IgG) | 62 |
AD, Alzheimer disease; ALS, amyotrophic lateral sclerosis; CSF, cerebrospinal fluid; ELISA, enzyme-linked immunosorbent assay; HD, Huntington disease; MCI, mild cognitive impairment; MMP, matrix metalloproteinase; MS, multiple sclerosis; PD, Parkinson disease; Qalb, CSF:serum albumin ratio.
Since albumin is a blood-derived protein, an increase in the ratio of CSF albumin to serum albumin levels, which is known as the albumin quotient (Qalb), is frequently used as a measure of BBB breakdown50. Several studies report that Qalb is elevated in individuals with preclinical AD201, MCI49, and AD208–210. However, other studies did not find any increase in Qalb in patients with AD211 unless vascular risk factors211–214 including mild arterial hypertension, diabetes mellitus, ischemic heart disease211,213 or dyslipidemia214 were also present. However, the majority of patients with AD have vascular risk factors: 65% of patients aged 65 years and 80% of patients aged 85 years3,56,57. Although some studies have not specifically examined the relationship between vascular risk factors and Qalb201,208, vascular risk factors did not worsen the extent of BBB breakdown, as measured by gadolinium efflux from blood into the brain extravascular-extracellular space on DCE-MRI Ktrans permeability analysis49. These observations support the view that BBB breakdown is associated with AD independently from vascular risk factors. Future studies should examine carefully whether ischemic vascular damage from comorbidities and vascular risk factors3,47,215 can augment BBB breakdown in AD.
However, CSF albumin levels could be influenced by proteolytic cleavage, as well as albumin uptake by brain macrophages, microglia, astrocytes, neurons, and oligodendrocytes (cells that express chondroitin sulfate proteoglycan 4 (also known as NG2))216–218. Therefore, Qalb might underestimate the degree of BBB breakdown. On the other hand, decreased CSF reabsorption and/or production could elevate Qalb, leading to false-positive results that might not reflect BBB breakdown206. In support of this notion, one study in seven patients with AD found that these individuals had a considerably reduced rate of CSF production219. To determine definitively whether diminished CSF turnover underlies increased Qalb in some patients with AD, detailed human studies of CSF dynamics are needed. Sensitive tests of BBB integrity, including DCE-MRI49, microbleed T2*-weighted MRI87, and/or measurement of alternative CSF blood-derived biomarkers (such as fibrinogen220 and plasminogen221, which have previously been used to detect BBB breakdown in patients with MCI and early AD, respectively) should additionally be considered.
Several important CSF biomarker studies have reported increased Qalb106,209,222,223 as well as an increased CSF:serum IgG ratio222 in non-demented patients with early stage PD compared to controls. Four independent studies reported increased Qalb in 55 of 138 (~40%) of patients with ALS, reviewed elsewhere62. Increased CSF levels of albumin, IgG and other blood-derived proteins have also been reported in patients with ALS62,224. Qalb is also elevated in patients with MS218, and this change correlates with increased white matter BBB permeability detected by DCE-MRI80. In patients with HIV-1-associated dementia, increased Qalb was associated with axonal injury as measured by CSF levels of neurofilament light chain225. Finally, increased MMP9 activity in CSF in patients with MS82,85 and APOE*ε4 carriers prior to cognitive decline201 is associated with BBB breakdown.
BBB breakdown and neurodegeneration
The neurodegenerative disorders discussed above share pathological alterations of the vessel wall resulting in BBB disruption. Endothelial degeneration leads to loss of tight junction proteins and/or increased transendothelial bulk flow via transcytosis5,6. The associated pericyte degeneration causes BBB breakdown7,70,174–176 and initiates multiple pathways of neurodegeneration (FIG. 3) owing to the entry of several neurotoxic blood-derived proteins, including plasminogen, thrombin and fibrinogen, which enter different areas of the CNS in different neurodegenerative disorders (TABLES 1–3).
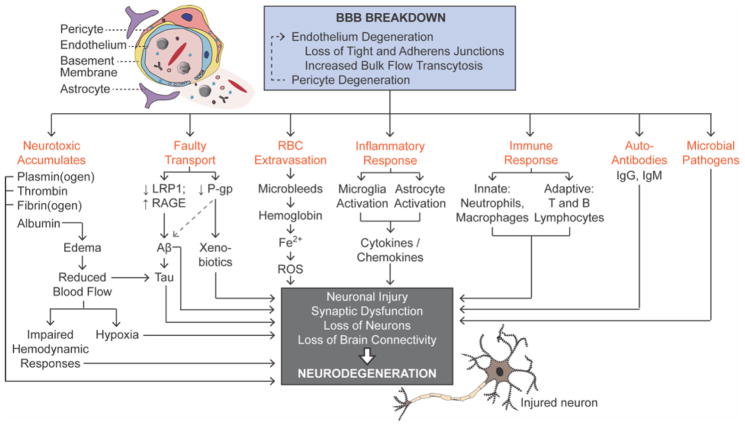
Figure 3. Blood-brain barrier (BBB) breakdown promotes neurodegeneration.
BBB breakdown is characterized by pericyte and endothelial degeneration, with loss of tight and adherens junctions and increased bulk flow transcytosis. BBB breakdown leads to the entry of microbial pathogens, accumulation of neurotoxic material faulty BBB transport, red blood cell extravasation and the release of neurotoxic free iron (Fe2+), which generates reactive oxygen species and oxidative stress. Inflammatory and immune responses lead to the generation of autoantibodies. CMT, solute carrier-mediated transport; ECS, extracellular spaces, L-DOPA, L-3,4-dihydroxyphenylalanine; RMT, receptor-mediated transcytosis.
Plasmin, which is generated from circulating plasminogen, degrades the neuronal matrix protein laminin, thereby promoting neuronal injury226. High concentrations of thrombin mediate neurotoxicity and memory impairment227 and accelerate BBB disruption228. Fibrinogen leads to axonal retraction229 and BBB damage that promotes neuroinflammation230. Additionally, fibrin depletion delays the onset of neuroinflammation and demyelination in transgenic mouse models of MS231, and treatment with fibrin induced M1-type activation and induction of antigen-presenting genes in both primary microglia and bone marrow-derived macrophages232. The role of coagulation and fibrinolysis proteins on development of brain pathology in MS has been reviewed elsewhere233.
Influx of albumin leads to perivascular oedema that obstructs the brain microcirculation and blood flow. In turn, these hypoxic conditions lead to neuronal injury and impaired haemodynamic responses that contribute to neurodegeneration2,13. Extravasation of red blood cells (microbleeds) are seen in almost all neurodegenerative disorders92,104, and lead to the perivascular accumulation of toxic, iron-containing proteins (such as haemoglobin) that release of free iron (Fe2+) as they are broken down107,62,73, generating reactive oxygen species (ROS) and subjecting neurons to oxidative stress234.
In neurodegenerative diseases such as AD, PD, and HIV-1-associated dementia140–142,204, dysfunction of P-glycoprotein-mediated active efflux transport at the BBB leads to the accumulation of toxic xenobiotic agents (such as environmental pollutants, food additives, pesticides and drugs) in brain. Reduced levels of P-glycoprotein and LRP1 at the BBB20,25,137, and increased levels of RAGE in brain microvessels6,190,191,196,199, lead to faulty clearance of toxic Aβ species linked to AD and their accumulation the in brain. Reduced blood flow and increased Aβ levels can both promote tau pathology, another key pathological hallmark of AD2. Whether faulty clearance of other proteins — tau in AD and CTE, α-synuclein in PD and/or huntingtin in HD — can also contribute to their respective accumulations in CNS is not clear at present.
Interestingly, experimental studies suggest that α-synuclein is transported into and out of the brain across the BBB as a free peptide235. Moreover, systemically administered α-synuclein oligomers, ribbons and fibrils cause distinct synucleinopathies, implying that they can all cross the BBB236. In humans, extracellular vesicles containing α-synuclein have been found in the CSF and blood, suggesting bidirectional transport of this protein between the blood and CSF237,238. As red blood cells are a source of α-synuclein-containing extracellular vesicles237, and extravasation of red blood cells into the striatum has been detected in patients with PD165,166, extravasated red blood cells might contribute to the development of α-synucleinopathy in humans. Since levels of α-synuclein are two orders of magnitude higher in the circulation than in the CNS238, α-synuclein transport across the BBB might be implicated in the pathogenesis of PD, and could be a novel therapeutic target.
Accumulates of neurotoxic material and reduced blood flow can activate microglia and astrocytes, leading to an inflammatory response with secretion of neurotoxic cytokines and chemokines47. Additionally, in some diseases (such as AD) brain infiltration of peripheral macrophages147,149 and neutrophils148 suggests activation of an innate immune response. Besides peripheral macrophage infiltration, influx of T and B lymphocytes across the BBB was found in patients with MS, indicating an adaptive immune response33. Altogether, these studies suggest that breakdown of the BBB enables the entry of circulating leukocytes into the brain.
BBB breakdown leads to the generation of several anti-CNS autoantibodies in humans239, but their roles in the pathogenesis of neurodegenerative disorders have not been fully explored. Additionally, BBB breakdown can enable circulating pathogens to enter the brain and injure neurons, and/or provoke an amyloid response that aggravates β-amyloidosis, as shown in animal models of AD185,186.
How BBB breakdown affects drug delivery
Successful delivery of therapeutic agents across the BBB requires functionally and structurally healthy blood vessels, normal vascularization, adequate blood flow, and recruitment of solute carrier-mediated transport (CMT) or receptor-mediated transcytosis (RMT) systems to facilitate drug delivery to the CNS (FIG. 4). Strategies that use existing CMT and RMT BBB systems have been explored to increase brain penetration and potency of neurotherapeutic agents (FIG. 2). For example, the large neutral amino acid CMT transporter delivers L-3,4-dihydroxyphenylalanine (L-DOPA) to the brain in PD240, and the transferrin RMT can deliver therapeutic antibodies to the brain in various neurological conditions4,241–243. Other approaches, such as using nanoparticles244 and/or opening the BBB by focused ultrasound245,246 to improve the delivery of therapeutic agents to CNS, have been attempted.
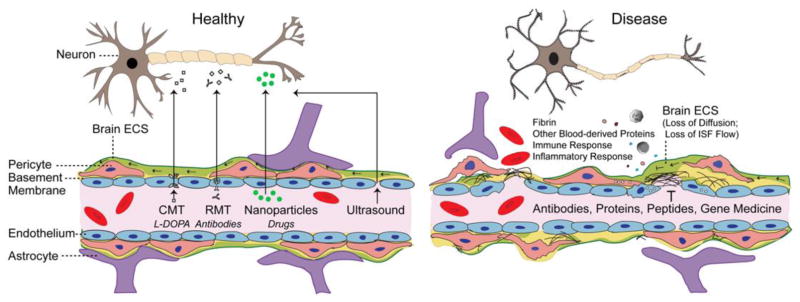
Figure 4. Blood-brain barrier (BBB) dysfunction – implications for drug delivery.
In a healthy BBB (left), strategies to breach the barrier and deliver neuropharmaceuticals to brain rely on carrier-mediated transporters (CMT), receptor-mediated transporters (RMT), nanoparticles, and/or transient opening of BBB as for example by focused ultrasound. Under pathological conditions (right), the disrupted BBB leads to accumulation of blood-derived debris and cells into enlarged perivascular spaces. This blocks normal distribution of molecules throughout the CNS by concentration gradient-driven diffusion across brain extracellular spaces (ECS) and interrupts regionally formation of interstitial fluid (ISF) and ISF flow preventing the therapeutic antibodies, proteins, peptides, gene medicine and other drugs to efficiently reach their neuronal targets. See main text for details.
Neurologists commonly assume that disease-initiated BBB breakdown might present an opportunity to deliver therapeutic antibodies, proteins, peptides, small molecules, and/or genetic medicines to affected neurons, without further need to manipulate the BBB. However, brain regions affected by neurodegeneration develop a pathological BBB breakdown characterized by functional and structural changes in the blood vessels, which often develop before neurodegeneration and persist as the disease progresses. These vascular changes include endothelial degeneration, reduced expression of tight junctions and adherens junctions at the BBB, increased endothelial bulk flow transcytosis, disrupted BBB transporter expression, pericyte degeneration, perivascular accumulation of toxic products, inflammation, and immune responses (FIG. 3), which all hinder the delivery of therapeutic agents to the brain. Under pathological conditions, blood-derived products, water and electrolytes accumulate in the enlarged perivascular spaces, which interferes with the normal diffusion of solutes across brain extracellular spaces, ISF formation and ISF flow, resulting in impaired distribution of solutes throughout the CNS (FIG. 4). As a consequence of disease-driven BBB disruption, impaired solute transport across parenchymal extracellular spaces and diminished ISF regional flow, therapeutic agents (including antibodies, proteins, peptides and small molecules) are likely to get trapped in pathologically altered brain tissue within the enlarged perivascular spaces, along with other blood-derived debris, preventing them from reaching their neuronal targets. Decreased function of CMT and RMT systems in neurodegenerative diseases additionally complicates their use for therapeutic drug delivery. Therefore, brain regions with healthy blood vessels and/or stabilization of the damaged vasculature in disease-affected brain regions are needed to improve cerebrovascular integrity and re-establish diffusion across extracellular spaces and ISF circulation, factors that are important for the successful delivery of neurotherapeutic agents to disease-affected brain.
Insight from genetic studies
In this Review, we position BBB breakdown as a key pathogenic feature of neurodegenerative diseases (TABLES 1–3). Importantly, BBB breakdown is also found in (rare), inherited monogenic neurological disorders involving a primary genetic deficit in brain endothelial cells and/or mural cells of the vascular wall6. The known genetic aetiology of these diseases offers valuable insights into the shared causal mechanisms underpinning BBB genetic defects and neurodegeneration. For example, mutations in the SLC2A1 gene (encoding solute carrier family 2, facilitated glucose transporter member 1 (GLUT1)) lead to GLUT1 deficiency syndrome, which is characterized by onset of seizures in infancy, microcephaly, mild movement disorder, and developmental delay247 associated with early onset of BBB breakdown108. Mutations in the MFSD2A gene (encoding sodium-dependent lysophosphatidylcholine symporter 1 (NLS1), the brain endothelial transporter of essential ω3 fatty acids), leads to lethal or nonlethal microcephaly, cognitive disorder, spasticity and absent speech248,249 and early BBB breakdown15. Genetic defects in endothelial monocarboxylate transporter-8 (MCT8; encoded by SLC16A2), which transports thyroid hormones into the CNS, leads to Allan–Herndon–Dudley syndrome with severe psychomotor retardation250. Mutations in OCLN (encoding the tight junction BBB protein occludin) lead to early-onset seizures, microcephaly, and grey matter calcification, whereas mutations in the JAM3 gene (encoding another tight junction BBB protein, JAMC) lead to brain haemorrhages and subependymal calcifications due to leakage from the BBB251,252. Mutations in the PDGFRβ gene in pericytes lead to BBB breakdown and cause a primary familial brain calcification characterized by early-onset microvascular calcification in basal ganglia, which induces seizures and motor and cognitive problems253,254.
Conclusions
By contrast, we have limited knowledge about the molecular mechanisms underlying BBB breakdown in non-monogenic human neurodegenerative disorders (TABLES 1–3). Most mechanistic insights have been gained from animal models of these disorders6,11. However, the development of advanced neuroimaging techniques that are capable of interrogating changes in BBB integrity in humans in regions as small as hippocampal subfields46,49,65,66, and improved imaging techniques for determining regional cerebral blood flow and haemodynamic responses2, enlarged perivascular spaces55, and incidence and distribution of microbleeds, using high strength 7T magnets to increase the detectability of these vascular changes87,104, hold considerable promise for future neurovascular research in humans. The development of new molecular ligands (in addition to the presently used amyloid and tau PET ligands for AD255 and FDG-PET113–123 and verapamil-PET140–142) for use in other neurodegenerative disorders — for example, ligands to visualize MMP activity at the BBB in vivo143 and/or the activity of other BBB transporters, receptors and/or junctional proteins affected by disease processes), is expected to provide important mechanistic insights into the role of the brain vascular system in neurodegeneration. Development of new biomarkers of vascular injury and/or repair in CSF and blood, and studies to determine how they relate to other systemic and cell-specific biomarkers of the neurovascular unit (including astrocytes, neuronal, oligodendrocytes, microglia and inflammatory biomarkers, and/or standard disease biomarkers, such as Aβ and tau in AD50), would also advance our understanding of vascular contributions to neurodegeneration and cognitive decline.
Besides the key question — what exactly is the role of the vascular system in the pathogenesis of neurodegenerative disorders, dementia and motor CNS changes — emerging questions relate to the prognostic and diagnostic value of neurovascular imaging and molecular biomarkers in predicting neurodegenerative processes and cognitive decline. If CNS vascular changes drive the initial pathogenic events that contribute to onset and progression of neurodegeneration, loss of brain connectivity, and neuronal injury and loss in complex neurodegenerative disorders, as they do in human monogenic neurological BBB diseases6, the question persists will therapeutic targeting of BBB arrest and reverse the course of neurological disorder in humans in a way as shown in some animal models71,73,192,196,199? How genetics, vascular risk factors, environment and lifestyle influence the BBB functions during normal aging and disease, and how this relates to neurological disorder, is another important focus for future studies.
Findings from this review suggest that healthy brain needs healthy vascular system for its normal functioning. As studies in animal models have begun developing advanced RNA-seq molecular atlas of the BBB and its associated cells6,256, similar studies in humans should be pursued to understand at the molecular level functions of human BBB. Using stem cell technology to develop in vitro human BBB models derived from induced pluripotent stem cells (iPSCs) from patients with different neurodegenerative disorders carrying genetic risk and from those with sporadic form of disease will advance drug discovery to stabilize vascular function in neurodegenerative disorders and/or develop new drug delivery approaches targeting BBB. Going forward, the BBB should be regarded as an important therapeutic opportunity, in combination with other approaches, to prevent, arrest and ultimately reverse neurodegenerative process and clinical deficits.
Key points.
The blood-brain barrier protects neurons from factors present in the systemic circulation, and maintains the highly regulated brain internal milieu, which is required for proper synaptic and neuronal functioning.
Blood-brain barrier breakdown allows entry into the brain of neurotoxic blood-derived products, cells, and pathogens, and is associated with inflammatory and immune responses, which can initiate multiple pathways of neurodegeneration.
Neuroimaging studies have demonstrated early blood-brain barrier dysfunction in Alzheimer’s disease and other neurodegenerative disorders, also supported by the biomarkers biofluid data, and consistently observed by post-mortem tissue analysis.
Blood-brain barrier dysfunction in neurodegenerative disorders includes increased blood-brain barrier permeability, microbleeds, impaired glucose transport, impaired P-glycoprotein function, perivascular deposits of blood-derived products, cellular infiltration, pericyte and endothelial cell degeneration.
Acknowledgments
The work of B.V.Z. is supported by the National Institutes of Health grants R01AG023084, R01NS090904, R01NS034467, R01AG039452, R01NS100459, and 5P01AG052350, in addition to the Cure Alzheimer’s Fund, Alzheimer’s Association and the Foundation Leducq Transatlantic Network of Excellence for the Study of Perivascular Spaces in Small Vessel Disease reference no. 16 CVD 05.
Glossary items
- Blood-brain barrier (BBB)
a continuous endothelial membrane of the brain vasculature with sealed cell-to-cell contacts that is sheathed by vascular mural cells and perivascular astrocyte end-feet; functions to separate the circulating blood and brain compartments and strictly regulates blood-to-brain and brain-to-blood transport of solutes
- Pericytes
mural cells that wrap brain capillary endothelium and are important for the formation and maintenance of the blood-brain barrier
- Neurodegeneration
progressive neuronal dysfunction causing neuronal degenerative changes and loss of neurons in various regions of the central nervous system in different neurodegenerative diseases
- Tight junctions (TJ)
endothelial proteins that tightly connect brain endothelial cells forming the anatomical blood-brain barrier with low paracellular permeability and high transendothelial electrical resistance
- Transmembrane diffusion
a type of passive transport across a cellular membrane where the net movement of molecules occurs down their respective concentration gradients
- Carrier-mediated transport (CMT)
transport of molecules across the blood-brain barrier down the concentration gradient via specific membrane carrier proteins
- Receptor-mediated transcytosis (RMT)
transport of molecules across the blood-brain barrier in a highly specific fashion via membrane receptors that become internalized with the ligand during trans-endothelial transcytosis
- Cerebrospinal fluid (CSF)
a fluid continually produced in the choroid plexus that flows throughout the brain’s ventricular system; functions as a clearance pathway, maintains intraventricular intracranial pressure in the brain and is often used to measure brain-derived biomarkers of disease
- Cerebral amyloid angiopathy (CAA)
amyloid deposition in the vascular wall of small brain arteries and capillaries causing vascular degeneration and lobar microbleeds in Alzheimer’s disease, which contributes to blood-brain barrier breakdown, infarcts, white matter changes and cognitive impairment
- Two-hit vascular hypothesis of AD
blood vessel damage is an initial insult through which BBB dysfunction and/or diminished brain perfusion lead directly to secondary neuronal injury in an Alzheimer’s amyloid-β (Aβ) independent manner, from one hand (hit 1), and Aβ accumulation in the brain due to faulty Aβ clearance and increased Ab production, from the other hand (hit 2)
- Apolipoprotein E epsilon 4 (APOE*ε4)
the major genetic risk factor for sporadic late-onset Alzheimer’s disease
- Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI)
dynamic MRI sequence used to quantify regional BBB permeability to a gadolinium contrast agent
- T2*-weighted susceptibility-weighted imaging (SWI) MRI
a MRI sequence where haemosiderin deposits yield hypointense signal; allows for the regional in vivo measurement of cerebral microbleeds in the living human brain
- 18F-fluoro-2-deoxyglucose (FDG)
a 18F (F) radiolabeled analog of glucose, 2-deoxyglucose (2DG) is not metabolized in brain in contrast to glucose; FDG is used in clinic as a surrogate PET ligand for glucose to provide an estimate of glucose uptake by the brain across the blood-brain barrier via GLUT1 glucose transporter
- Low density lipoprotein receptor-related protein-1 (LRP1)
major efflux transporter for Alzheimer’s amyloid-β at the blood-brain barrier responsible for clearance of Aβ from brain-to-blood
- Verapamil-positron emission tomography (PET)
a radioactive 14C-labeled PET ligand allows for the in vivo detection of P-glycoprotein function at the blood-brain barrier in the living human brain
- Receptor for advanced glycation end products (RAGE)
major influx transporter of Alzheimer’s amyloid-β (Aβ) at the blood-brain barrier contributing to Aβ accumulation in brain, inflammatory response, suppression of blood flow and blood-brain barrier breakdown
- RNA-sequencing
transcriptomic approach to reveal the presence and quantity of RNA transcripts in a biological sample
- Induced pluripotent stem cells (iPSCs)
an adult cell reprogrammed back into an embryonic-like pluripotent state for the purposes of differentiating to a cell type of interest for research studies and/or potential therapeutic efforts
Footnotes
Author contributions
All authors contributed to the literature search and writing the review. BVZ worked closely with MDS and APS to write the review and design figures and tables.
Competing interests statement
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18:419–434. doi: 10.1038/nrn.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardridge WM. Targeted delivery of protein and gene medicines through the blood-brain barrier. Clin Pharmacol Ther. 2015;97:347–361. doi: 10.1002/cpt.18. [DOI] [PubMed] [Google Scholar]
- 5.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and Dysfunction of the Blood-Brain Barrier. Cell. 2015;163:1064–1078. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci. 2016;19:771–783. doi: 10.1038/nn.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mann GE, Zlokovic BV, Yudilevich DL. Evidence for a lactate transport system in the sarcolemmal membrane of the perfused rabbit heart: kinetics of unidirectional influx, carrier specificity and effects of glucagon. Biochim Biophys Acta. 1985;819:241–248. doi: 10.1016/0005-2736(85)90179-8. [DOI] [PubMed] [Google Scholar]
- 9.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 10.Friese MA, Schattling B, Fugger L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat Rev Neurol. 2014;10:225–238. doi: 10.1038/nrneurol.2014.37. [DOI] [PubMed] [Google Scholar]
- 11.Montagne A, Zhao Z, Zlokovic B. Alzheimer’s disease: a matter of blood-brain barrier dysfunction? J Exp Med. doi: 10.1084/jem.20171406. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakadžić S, et al. Large arteriolar component of oxygen delivery implies a safe margin of oxygen supply to cerebral tissue. Nat Commun. 2014;5:5734. doi: 10.1038/ncomms6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kisler K, et al. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat Neurosci. 2017;20:406–416. doi: 10.1038/nn.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen LN, et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509:503–506. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Zvi A, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mokgokong R, Wang S, Taylor CJ, Barrand MA, Hladky SB. Ion transporters in brain endothelial cells that contribute to formation of brain interstitial fluid. Pflugers Arch. 2014;466:887–901. doi: 10.1007/s00424-013-1342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 18.Vazana U, et al. Glutamate-Mediated Blood-Brain Barrier Opening: Implications for Neuroprotection and Drug Delivery. J Neurosci Off J Soc Neurosci. 2016;36:7727–7739. doi: 10.1523/JNEUROSCI.0587-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibata M, et al. Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deane R, et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Bell RD, et al. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2007;27:909–918. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deane R, et al. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zlokovic BV. Neurodegeneration and the neurovascular unit. Nat Med. 2010;16:1370–1371. doi: 10.1038/nm1210-1370. [DOI] [PubMed] [Google Scholar]
- 24.Storck SE, et al. Endothelial LRP1 transports amyloid-β1-42 across the blood-brain barrier. J Clin Invest. 2015 doi: 10.1172/JCI81108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Z, et al. Central role for PICALM in amyloid-β blood-brain barrier transcytosis and clearance. Nat Neurosci. 2015;18:978–987. doi: 10.1038/nn.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saito S, Ihara M. Interaction between cerebrovascular disease and Alzheimer pathology. Curr Opin Psychiatry. 2016;29:168–173. doi: 10.1097/YCO.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 27.Tarasoff-Conway JM, et al. Clearance systems in the brain--implications for Alzheimer diseaser. Nat Rev Neurol. 2016;12:248. doi: 10.1038/nrneurol.2016.36. [DOI] [PubMed] [Google Scholar]
- 28.Bakker ENTP, et al. Lymphatic Clearance of the Brain: Perivascular, Paravascular and Significance for Neurodegenerative Diseases. Cell Mol Neurobiol. 2016;36:181–194. doi: 10.1007/s10571-015-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradbury MW, Cserr HF, Westrop RJ. Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. Am J Physiol. 1981;240:F329–336. doi: 10.1152/ajprenal.1981.240.4.F329. [DOI] [PubMed] [Google Scholar]
- 30.Ichimura T, Fraser PA, Cserr HF. Distribution of extracellular tracers in perivascular spaces of the rat brain. Brain Res. 1991;545:103–113. doi: 10.1016/0006-8993(91)91275-6. [DOI] [PubMed] [Google Scholar]
- 31.Aspelund A, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Louveau A, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engelhardt B, et al. Vascular, glial, and lymphatic immune gateways of the central nervous system. Acta Neuropathol (Berl) 2016;132:317–338. doi: 10.1007/s00401-016-1606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol. 2017;18:123–131. doi: 10.1038/ni.3666. [DOI] [PubMed] [Google Scholar]
- 35.Xie L, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985;326:47–63. doi: 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- 37.Iliff JJ, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jessen NA, Munk ASF, Lundgaard I, Nedergaard M. The Glymphatic System: A Beginner’s Guide. Neurochem Res. 2015;40:2583–2599. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith AJ, Yao X, Dix JA, Jin B-J, Verkman AS. Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. eLife. 2017;6 doi: 10.7554/eLife.27679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holter KE, et al. Interstitial solute transport in 3D reconstructed neuropil occurs by diffusion rather than bulk flow. Proc Natl Acad Sci U S A. 2017;114:9894–9899. doi: 10.1073/pnas.1706942114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hladky SB, Barrand MA. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS. 2014;11:26. doi: 10.1186/2045-8118-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spector R, Robert Snodgrass S, Johanson CE. A balanced view of the cerebrospinal fluid composition and functions: Focus on adult humans. Exp Neurol. 2015;273:57–68. doi: 10.1016/j.expneurol.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 43.Asgari N, Berg CT, Mørch MT, Khorooshi R, Owens T. Cerebrospinal fluid aquaporin-4-immunoglobulin G disrupts blood brain barrier. Ann Clin Transl Neurol. 2015;2:857–863. doi: 10.1002/acn3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asgari M, de Zélicourt D, Kurtcuoglu V. Glymphatic solute transport does not require bulk flow. Sci Rep. 2016;6:38635. doi: 10.1038/srep38635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin BJ, Smith AJ, Verkman AS. Spatial model of convective solute transport in brain extracellular space does not support a ‘glymphatic’ mechanism. J Gen Physiol. 2016;148:489–501. doi: 10.1085/jgp.201611684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montagne A, et al. Brain imaging of neurovascular dysfunction in Alzheimer’s disease. Acta Neuropathol (Berl) 2016;131:687–707. doi: 10.1007/s00401-016-1570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson AR, Sweeney MD, Sagare AP, Zlokovic BV. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim Biophys Acta. 2016;1862:887–900. doi: 10.1016/j.bbadis.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iturria-Medina Y, et al. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun. 2016;7:11934. doi: 10.1038/ncomms11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montagne A, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sweeney MD, Sagare AP, Zlokovic BV. Cerebrospinal fluid biomarkers of neurovascular dysfunction in mild dementia and Alzheimer’s disease. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2015 doi: 10.1038/jcbfm.2015.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol. 2016;15:934–943. doi: 10.1016/S1474-4422(16)30029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toledo JB, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain J Neurol. 2013;136:2697–2706. doi: 10.1093/brain/awt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenberg GA. Blood-Brain Barrier Permeability in Aging and Alzheimer’s Disease. J Prev Alzheimers Dis. 2014;1:138–139. doi: 10.14283/jpad.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- 55.Wardlaw JM, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montine TJ, et al. Recommendations of the Alzheimer’s Disease-Related Dementias Conference. Neurology. 2014;83:851–860. doi: 10.1212/WNL.0000000000000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Snyder HM, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2014 doi: 10.1016/j.jalz.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hachinski V World Stroke Organization. Stroke and Potentially Preventable Dementias Proclamation: Updated World Stroke Day Proclamation. Stroke. 2015;46:3039–3040. doi: 10.1161/STROKEAHA.115.011237. [DOI] [PubMed] [Google Scholar]
- 59.Malek N, et al. Vascular disease and vascular risk factors in relation to motor features and cognition in early Parkinson’s disease. Mov Disord Off J Mov Disord Soc. 2016;31:1518–1526. doi: 10.1002/mds.26698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drouin-Ouellet J, et al. Cerebrovascular and blood-brain barrier impairments in Huntington’s disease: Potential implications for its pathophysiology. Ann Neurol. 2015;78:160–177. doi: 10.1002/ana.24406. [DOI] [PubMed] [Google Scholar]
- 61.Lin CY, et al. Neurovascular abnormalities in humans and mice with Huntington’s disease. Exp Neurol. 2013;250:20–30. doi: 10.1016/j.expneurol.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 62.Winkler EA, et al. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol (Berl) 2013;125:111–120. doi: 10.1007/s00401-012-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Strazza M, Pirrone V, Wigdahl B, Nonnemacher MR. Breaking down the barrier: the effects of HIV-1 on the blood-brain barrier. Brain Res. 2011;1399:96–115. doi: 10.1016/j.brainres.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Doherty CP, et al. Blood-Brain Barrier Dysfunction as a Hallmark Pathology in Chronic Traumatic Encephalopathy. J Neuropathol Exp Neurol. 2016;75:656–662. doi: 10.1093/jnen/nlw036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barnes SR, et al. ROCKETSHIP: a flexible and modular software tool for the planning, processing and analysis of dynamic MRI studies. BMC Med Imaging. 2015;15:19. doi: 10.1186/s12880-015-0062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barnes SR, et al. Optimal acquisition and modeling parameters for accurate assessment of low Ktrans blood-brain barrier permeability using dynamic contrast-enhanced MRI. Magn Reson Med. 2016;75:1967–1977. doi: 10.1002/mrm.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sagare AP, Sweeney MD, Makshanoff J, Zlokovic BV. Shedding of soluble platelet-derived growth factor receptor-β from human brain pericytes. Neurosci Lett. 2015;607:97–101. doi: 10.1016/j.neulet.2015.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whitwell JL, et al. Neuroimaging correlates of pathologically defined subtypes of Alzheimer’s disease: a case-control study. Lancet Neurol. 2012;11:868–877. doi: 10.1016/S1474-4422(12)70200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Apostolova LG, et al. Subregional hippocampal atrophy predicts Alzheimer’s dementia in the cognitively normal. Neurobiol Aging. 2010;31:1077–1088. doi: 10.1016/j.neurobiolaging.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bell RD, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bell RD, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Winkler EA, Sengillo JD, Bell RD, Wang J, Zlokovic BV. Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2012;32:1841–1852. doi: 10.1038/jcbfm.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Winkler EA, et al. Blood-spinal cord barrier disruption contributes to early motor-neuron degeneration in ALS-model mice. Proc Natl Acad Sci U S A. 2014;111:E1035–1042. doi: 10.1073/pnas.1401595111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van de Haar HJ, et al. Blood-Brain Barrier Leakage in Patients with Early Alzheimer Disease. Radiology. 2016;281:527–535. doi: 10.1148/radiol.2016152244. [DOI] [PubMed] [Google Scholar]
- 75.van de Haar HJ, et al. Neurovascular unit impairment in early Alzheimer’s disease measured with magnetic resonance imaging. Neurobiol Aging. 2016;45:190–196. doi: 10.1016/j.neurobiolaging.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 76.van de Haar HJ, et al. Subtle blood-brain barrier leakage rate and spatial extent: considerations for dynamic contrast-enhanced MRI. Med Phys. 2017 doi: 10.1002/mp.12328. [DOI] [PubMed] [Google Scholar]
- 77.Wang H, Golob EJ, Su MY. Vascular volume and blood-brain barrier permeability measured by dynamic contrast enhanced MRI in hippocampus and cerebellum of patients with MCI and normal controls. J Magn Reson Imaging JMRI. 2006;24:695–700. doi: 10.1002/jmri.20669. [DOI] [PubMed] [Google Scholar]
- 78.Starr JM, Farrall AJ, Armitage P, McGurn B, Wardlaw J. Blood-brain barrier permeability in Alzheimer’s disease: a case-control MRI study. Psychiatry Res. 2009;171:232–241. doi: 10.1016/j.pscychresns.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 79.Al-Bachari S. PhD Thesis. University of Manchester; 2016. MRI assessment of neurovascular changes in idiopathic Parkinson’s disease; p. 201. [Google Scholar]
- 80.Taheri S, Gasparovic C, Shah NJ, Rosenberg GA. Quantitative measurement of blood-brain barrier permeability in human using dynamic contrast-enhanced MRI with fast T1 mapping. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2011;65:1036–1042. doi: 10.1002/mrm.22686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cramer SP, Simonsen H, Frederiksen JL, Rostrup E, Larsson HBW. Abnormal blood-brain barrier permeability in normal appearing white matter in multiple sclerosis investigated by MRI. NeuroImage Clin. 2014;4:182–189. doi: 10.1016/j.nicl.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cramer SP, Modvig S, Simonsen HJ, Frederiksen JL, Larsson HBW. Permeability of the blood-brain barrier predicts conversion from optic neuritis to multiple sclerosis. Brain J Neurol. 2015;138:2571–2583. doi: 10.1093/brain/awv203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gaitán MI, et al. Evolution of the blood-brain barrier in newly forming multiple sclerosis lesions. Ann Neurol. 2011;70:22–29. doi: 10.1002/ana.22472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ingrisch M, et al. Quantification of perfusion and permeability in multiple sclerosis: dynamic contrast-enhanced MRI in 3D at 3T. Invest Radiol. 2012;47:252–258. doi: 10.1097/RLI.0b013e31823bfc97. [DOI] [PubMed] [Google Scholar]
- 85.Fainardi E, et al. Cerebrospinal fluid and serum levels and intrathecal production of active matrix metalloproteinase-9 (MMP-9) as markers of disease activity in patients with multiple sclerosis. Mult Scler Houndmills Basingstoke Engl. 2006;12:294–301. doi: 10.1191/135248506ms1274oa. [DOI] [PubMed] [Google Scholar]
- 86.Goos JDC, et al. Patients with Alzheimer disease with multiple microbleeds: relation with cerebrospinal fluid biomarkers and cognition. Stroke J Cereb Circ. 2009;40:3455–3460. doi: 10.1161/STROKEAHA.109.558197. [DOI] [PubMed] [Google Scholar]
- 87.Brundel M, et al. High prevalence of cerebral microbleeds at 7Tesla MRI in patients with early Alzheimer’s disease. J Alzheimers Dis JAD. 2012;31:259–263. doi: 10.3233/JAD-2012-120364. [DOI] [PubMed] [Google Scholar]
- 88.Uetani H, et al. Prevalence and topography of small hypointense foci suggesting microbleeds on 3T susceptibility-weighted imaging in various types of dementia. AJNR Am J Neuroradiol. 2013;34:984–989. doi: 10.3174/ajnr.A3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zonneveld HI, et al. Prevalence of cortical superficial siderosis in a memory clinic population. Neurology. 2014;82:698–704. doi: 10.1212/WNL.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 90.Olazarán J, et al. Pattern of and risk factors for brain microbleeds in neurodegenerative dementia. Am J Alzheimers Dis Other Demen. 2014;29:263–269. doi: 10.1177/1533317513517043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heringa SM, et al. Multiple microbleeds are related to cerebral network disruptions in patients with early Alzheimer’s disease. J Alzheimers Dis JAD. 2014;38:211–221. doi: 10.3233/JAD-130542. [DOI] [PubMed] [Google Scholar]
- 92.Shams S, et al. Cerebral microbleeds: different prevalence, topography, and risk factors depending on dementia diagnosis—the Karolinska Imaging Dementia Study. AJNR Am J Neuroradiol. 2015;36:661–666. doi: 10.3174/ajnr.A4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Poliakova T, Levin O, Arablinskiy A, Vasenina E, Zerr I. Cerebral microbleeds in early Alzheimer’s disease. J Neurol. 2016;263:1961–1968. doi: 10.1007/s00415-016-8220-2. [DOI] [PubMed] [Google Scholar]
- 94.Yates PA, et al. Incidence of cerebral microbleeds in preclinical Alzheimer disease. Neurology. 2014;82:1266–1273. doi: 10.1212/WNL.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Greenberg SM, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Ann Neurol. 2011;70:871–880. doi: 10.1002/ana.22516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hanyu H, Tanaka Y, Shimizu S, Takasaki M, Abe K. Cerebral microbleeds in Alzheimer’s disease. J Neurol. 2003;250:1496–1497. doi: 10.1007/s00415-003-0245-7. [DOI] [PubMed] [Google Scholar]
- 98.Pettersen JA, et al. Microbleed topography, leukoaraiosis, and cognition in probable Alzheimer disease from the Sunnybrook dementia study. Arch Neurol. 2008;65:790–795. doi: 10.1001/archneur.65.6.790. [DOI] [PubMed] [Google Scholar]
- 99.Kantarci K, et al. Focal hemosiderin deposits and β-amyloid load in the ADNI cohort. Alzheimers Dement J Alzheimers Assoc. 2013;9:S116–123. doi: 10.1016/j.jalz.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feldman HH, et al. Superficial siderosis: a potential diagnostic marker of cerebral amyloid angiopathy in Alzheimer disease. Stroke. 2008;39:2894–2897. doi: 10.1161/STROKEAHA.107.510826. [DOI] [PubMed] [Google Scholar]
- 101.Charidimou A, et al. Cortical Superficial Siderosis in Memory Clinic Patients: Further Evidence for Underlying Cerebral Amyloid Angiopathy. Cerebrovasc Dis Basel Switz. 2016;41:156–162. doi: 10.1159/000442299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shams S, et al. Cortical superficial siderosis: Prevalence and biomarker profile in a memory clinic population. Neurology. 2016;87:1110–1117. doi: 10.1212/WNL.0000000000003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blair GW, Hernandez MV, Thrippleton MJ, Doubal FN, Wardlaw JM. Advanced Neuroimaging of Cerebral Small Vessel Disease. Curr Treat Options Cardiovasc Med. 2017;19:56. doi: 10.1007/s11936-017-0555-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shams S, Wahlund LO. Cerebral microbleeds as a biomarker in Alzheimer’s disease? A review in the field. Biomark Med. 2016;10:9–18. doi: 10.2217/bmm.15.101. [DOI] [PubMed] [Google Scholar]
- 105.Ham JH, et al. Cerebral microbleeds in patients with Parkinson’s disease. J Neurol. 2014;261:1628–1635. doi: 10.1007/s00415-014-7403-y. [DOI] [PubMed] [Google Scholar]
- 106.Janelidze S, et al. Increased CSF biomarkers of angiogenesis in Parkinson disease. Neurology. 2015;85:1834–1842. doi: 10.1212/WNL.0000000000002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kwan JY, et al. Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: correlating 7 tesla MRI and pathology. PloS One. 2012;7:e35241. doi: 10.1371/journal.pone.0035241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Winkler EA, et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci. 2015;18:521–530. doi: 10.1038/nn.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Simpson IA, Chundu KR, Davies-Hill T, Honer WG, Davies P. Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer’s disease. Ann Neurol. 1994;35:546–551. doi: 10.1002/ana.410350507. [DOI] [PubMed] [Google Scholar]
- 110.Mooradian AD, Chung HC, Shah GN. GLUT-1 expression in the cerebra of patients with Alzheimer’s disease. Neurobiol Aging. 1997;18:469–474. doi: 10.1016/s0197-4580(97)00111-5. [DOI] [PubMed] [Google Scholar]
- 111.Kalaria RN, Harik SI. Reduced glucose transporter at the blood-brain barrier and in cerebral cortex in Alzheimer disease. J Neurochem. 1989;53:1083–1088. doi: 10.1111/j.1471-4159.1989.tb07399.x. [DOI] [PubMed] [Google Scholar]
- 112.Horwood N, Davies DC. Immunolabelling of hippocampal microvessel glucose transporter protein is reduced in Alzheimer’s disease. Virchows Arch Int J Pathol. 1994;425:69–72. doi: 10.1007/BF00193951. [DOI] [PubMed] [Google Scholar]
- 113.Hunt A, et al. Reduced cerebral glucose metabolism in patients at risk for Alzheimer’s disease. Psychiatry Res. 2007;155:147–154. doi: 10.1016/j.pscychresns.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 114.Samuraki M, et al. Partial volume effect-corrected FDG PET and grey matter volume loss in patients with mild Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2007;34:1658–1669. doi: 10.1007/s00259-007-0454-x. [DOI] [PubMed] [Google Scholar]
- 115.Mosconi L, et al. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J Nucl Med Off Publ Soc Nucl Med. 2008;49:390–398. doi: 10.2967/jnumed.107.045385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mosconi L, et al. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer’s disease. J Nucl Med Off Publ Soc Nucl Med. 2006;47:1778–1786. [PubMed] [Google Scholar]
- 117.Landau SM, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32:1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bailly M, et al. Precuneus and Cingulate Cortex Atrophy and Hypometabolism in Patients with Alzheimer’s Disease and Mild Cognitive Impairment: MRI and (18)F-FDG PET Quantitative Analysis Using FreeSurfer. BioMed Res Int. 2015;2015:583931. doi: 10.1155/2015/583931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ossenkoppele R, et al. Differential effect of APOE genotype on amyloid load and glucose metabolism in AD dementia. Neurology. 2013;80:359–365. doi: 10.1212/WNL.0b013e31827f0889. [DOI] [PubMed] [Google Scholar]
- 120.Protas HD, et al. Posterior cingulate glucose metabolism, hippocampal glucose metabolism, and hippocampal volume in cognitively normal, late-middle-aged persons at 3 levels of genetic risk for Alzheimer disease. JAMA Neurol. 2013;70:320–325. doi: 10.1001/2013.jamaneurol.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mosconi L, et al. Amyloid and metabolic positron emission tomography imaging of cognitively normal adults with Alzheimer’s parents. Neurobiol Aging. 2013;34:22–34. doi: 10.1016/j.neurobiolaging.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Landau SM, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mosconi L, et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2009;36:811–822. doi: 10.1007/s00259-008-1039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Niwa K, Kazama K, Younkin SG, Carlson GA, Iadecola C. Alterations in cerebral blood flow and glucose utilization in mice overexpressing the amyloid precursor protein. Neurobiol Dis. 2002;9:61–68. doi: 10.1006/nbdi.2001.0460. [DOI] [PubMed] [Google Scholar]
- 125.Jagust WJ, et al. Diminished glucose transport in Alzheimer’s disease: dynamic PET studies. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 1991;11:323–330. doi: 10.1038/jcbfm.1991.65. [DOI] [PubMed] [Google Scholar]
- 126.Piert M, Koeppe RA, Giordani B, Berent S, Kuhl DE. Diminished glucose transport and phosphorylation in Alzheimer’s disease determined by dynamic FDG-PET. J Nucl Med Off Publ Soc Nucl Med. 1996;37:201–208. [PubMed] [Google Scholar]
- 127.Sokoloff L, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 128.McDougal DB, et al. Use of nonradioactive 2-deoxyglucose to study compartmentation of brain glucose metabolism and rapid regional changes in rate. Proc Natl Acad Sci U S A. 1990;87:1357–1361. doi: 10.1073/pnas.87.4.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cunnane S, et al. Brain fuel metabolism, aging, and Alzheimer’s disease. Nutr Burbank Los Angel Cty Calif. 2011;27:3–20. doi: 10.1016/j.nut.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Crane RK, Sols A. The non-competitive inhibition of brain hexokinase by glucose-6-phosphate and related compounds. J Biol Chem. 1954;210:597–606. [PubMed] [Google Scholar]
- 131.Rokka J, Grönroos TJ, Viljanen T, Solin O, Haaparanta-Solin M. HPLC and TLC methods for analysis of [(18)F]FDG and its metabolites from biological samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1048:140–149. doi: 10.1016/j.jchromb.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 132.Southworth R, Parry CR, Parkes HG, Medina RA, Garlick PB. Tissue-specific differences in 2-fluoro-2-deoxyglucose metabolism beyond FDG-6-P: a 19F NMR spectroscopy study in the rat. NMR Biomed. 2003;16:494–502. doi: 10.1002/nbm.856. [DOI] [PubMed] [Google Scholar]
- 133.Hers HG, De Duve C. The hexosephosphatase system; partition of activity of glucose-6-phosphatase in the tissues. Bull Soc Chim Biol (Paris) 1950;32:20–29. [PubMed] [Google Scholar]
- 134.Sokoloff L. Measurement of local cerebral glucose utilization and its relation to local functional activity in the brain. Adv Exp Med Biol. 1991;291:21–42. doi: 10.1007/978-1-4684-5931-9_4. [DOI] [PubMed] [Google Scholar]
- 135.Huang MT, Veech RL. Metabolic fluxes between [14C]2-deoxy-D-glucose and [14C]2-deoxy-D-glucose-6-phosphate in brain in vivo. J Neurochem. 1985;44:567–573. doi: 10.1111/j.1471-4159.1985.tb05450.x. [DOI] [PubMed] [Google Scholar]
- 136.Sperling RA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cirrito JR, et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115:3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang W, Bodles-Brakhop AM, Barger SW. A Role for P-Glycoprotein in Clearance of Alzheimer Amyloid β-Peptide from the Brain. Curr Alzheimer Res. 2016;13:615–620. doi: 10.2174/1567205013666160314151012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McInerney MP, Short JL, Nicolazzo JA. Neurovascular Alterations in Alzheimer’s Disease: Transporter Expression Profiles and CNS Drug Access. AAPS J. 2017;19:940–956. doi: 10.1208/s12248-017-0077-5. [DOI] [PubMed] [Google Scholar]
- 140.van Assema DME, et al. Blood-brain barrier P-glycoprotein function in Alzheimer’s disease. Brain J Neurol. 2012;135:181–189. doi: 10.1093/brain/awr298. [DOI] [PubMed] [Google Scholar]
- 141.Deo AK, et al. Activity of P-Glycoprotein, a β-Amyloid Transporter at the Blood-Brain Barrier, Is Compromised in Patients with Mild Alzheimer Disease. J Nucl Med Off Publ Soc Nucl Med. 2014;55:1106–1111. doi: 10.2967/jnumed.113.130161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kortekaas R, et al. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol. 2005;57:176–179. doi: 10.1002/ana.20369. [DOI] [PubMed] [Google Scholar]
- 143.Gerwien H, et al. Imaging matrix metalloproteinase activity in multiple sclerosis as a specific marker of leukocyte penetration of the blood-brain barrier. Sci Transl Med. 2016;8:364ra152. doi: 10.1126/scitranslmed.aaf8020. [DOI] [PubMed] [Google Scholar]
- 144.Zamboni P, et al. The value of cerebral Doppler venous haemodynamics in the assessment of multiple sclerosis. J Neurol Sci. 2009;282:21–27. doi: 10.1016/j.jns.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 145.Marshall O, et al. Impaired cerebrovascular reactivity in multiple sclerosis. JAMA Neurol. 2014;71:1275–1281. doi: 10.1001/jamaneurol.2014.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cullen KM, Kócsi Z, Stone J. Pericapillary haem-rich deposits: evidence for microhaemorrhages in aging human cerebral cortex. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2005;25:1656–1667. doi: 10.1038/sj.jcbfm.9600155. [DOI] [PubMed] [Google Scholar]
- 147.Hultman K, Strickland S, Norris EH. The APOE ε4/ε4 genotype potentiates vascular fibrin(ogen) deposition in amyloid-laden vessels in the brains of Alzheimer’s disease patients. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2013;33:1251–1258. doi: 10.1038/jcbfm.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zenaro E, et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med. 2015;21:880–886. doi: 10.1038/nm.3913. [DOI] [PubMed] [Google Scholar]
- 149.Fiala M, et al. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer’s disease brain and damage the blood-brain barrier. Eur J Clin Invest. 2002;32:360–371. doi: 10.1046/j.1365-2362.2002.00994.x. [DOI] [PubMed] [Google Scholar]
- 150.Persidsky Y, et al. Rho-mediated regulation of tight junctions during monocyte migration across the blood-brain barrier in HIV-1 encephalitis (HIVE) Blood. 2006;107:4770–4780. doi: 10.1182/blood-2005-11-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Omalu BI, Fitzsimmons RP, Hammers J, Bailes J. Chronic traumatic encephalopathy in a professional American wrestler. J Forensic Nurs. 2010;6:130–136. doi: 10.1111/j.1939-3938.2010.01078.x. [DOI] [PubMed] [Google Scholar]
- 152.Zipser BD, et al. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol Aging. 2007;28:977–986. doi: 10.1016/j.neurobiolaging.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 153.Ryu JK, McLarnon JG. A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer’s disease brain. J Cell Mol Med. 2009;13:2911–2925. doi: 10.1111/j.1582-4934.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cortes-Canteli M, et al. Fibrinogen and beta-amyloid association alters thrombosis and fibrinolysis: a possible contributing factor to Alzheimer’s disease. Neuron. 2010;66:695–709. doi: 10.1016/j.neuron.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sengillo JD, et al. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer’s disease. Brain Pathol Zurich Switz. 2013;23:303–310. doi: 10.1111/bpa.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Halliday MR, et al. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2016;36:216–227. doi: 10.1038/jcbfm.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Miners JS, Schulz I, Love S. Differing associations between Aβ accumulation, hypoperfusion, blood-brain barrier dysfunction and loss of PDGFRB pericyte marker in the precuneus and parietal white matter in Alzheimer’s disease. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2017 doi: 10.1177/0271678X17690761. 271678X17690761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Salloway S, et al. Effect of APOE genotype on microvascular basement membrane in Alzheimer’s disease. J Neurol Sci. 2002;203–204:183–187. doi: 10.1016/s0022-510x(02)00288-5. [DOI] [PubMed] [Google Scholar]
- 159.Sagare AP, et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun. 2013;4:2932. doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 160.Park L, et al. Innate immunity receptor CD36 promotes cerebral amyloid angiopathy. Proc Natl Acad Sci U S A. 2013;110:3089–3094. doi: 10.1073/pnas.1300021110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kelly P, et al. Restoration of cerebral and systemic microvascular architecture in APP/PS1 transgenic mice following treatment with LiraglutideTM. Microcirc N Y N 1994. 2015;22:133–145. doi: 10.1111/micc.12186. [DOI] [PubMed] [Google Scholar]
- 162.Park JC, et al. Annexin A1 restores Aβ1-42-induced blood-brain barrier disruption through the inhibition of RhoA-ROCK signaling pathway. Aging Cell. 2017;16:149–161. doi: 10.1111/acel.12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Alata W, Ye Y, St-Amour I, Vandal M, Calon F. Human apolipoprotein E ε4 expression impairs cerebral vascularization and blood-brain barrier function in mice. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2015;35:86–94. doi: 10.1038/jcbfm.2014.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nishitsuji K, Hosono T, Nakamura T, Bu G, Michikawa M. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. J Biol Chem. 2011;286:17536–17542. doi: 10.1074/jbc.M111.225532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gray MT, Woulfe JM. Striatal blood-brain barrier permeability in Parkinson’s disease. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2015;35:747–750. doi: 10.1038/jcbfm.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pienaar IS, et al. Deep-brain stimulation associates with improved microvascular integrity in the subthalamic nucleus in Parkinson’s disease. Neurobiol Dis. 2015;74:392–405. doi: 10.1016/j.nbd.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 167.Loeffler DA, et al. Transferrin and iron in normal, Alzheimer’s disease, and Parkinson’s disease brain regions. J Neurochem. 1995;65:710–724. doi: 10.1046/j.1471-4159.1995.65020710.x. [DOI] [PubMed] [Google Scholar]
- 168.Garbuzova-Davis S, et al. Impaired blood-brain/spinal cord barrier in ALS patients. Brain Res. 2012;1469:114–128. doi: 10.1016/j.brainres.2012.05.056. [DOI] [PubMed] [Google Scholar]
- 169.Zhong Z, et al. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat Neurosci. 2008;11:420–422. doi: 10.1038/nn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kirk J, Plumb J, Mirakhur M, McQuaid S. Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood-brain barrier leakage and active demyelination. J Pathol. 2003;201:319–327. doi: 10.1002/path.1434. [DOI] [PubMed] [Google Scholar]
- 171.Omalu BI, et al. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57:128–134. doi: 10.1227/01.neu.0000163407.92769.ed. discussion 128–134. [DOI] [PubMed] [Google Scholar]
- 172.Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- 173.Baloyannis SJ, Baloyannis IS. The vascular factor in Alzheimer’s disease: a study in Golgi technique and electron microscopy. J Neurol Sci. 2012;322:117–121. doi: 10.1016/j.jns.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 174.Armulik A, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 175.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Niu F, Yao H, Zhang W, Sutliff RL, Buch S. Tat 101-mediated enhancement of brain pericyte migration involves platelet-derived growth factor subunit B homodimer: implications for human immunodeficiency virus-associated neurocognitive disorders. J Neurosci Off J Soc Neurosci. 2014;34:11812–11825. doi: 10.1523/JNEUROSCI.1139-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kokjohn TA, et al. Neurochemical profile of dementia pugilistica. J Neurotrauma. 2013;30:981–997. doi: 10.1089/neu.2012.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bailey TL, Rivara CB, Rocher AB, Hof PR. The nature and effects of cortical microvascular pathology in aging and Alzheimer’s disease. Neurol Res. 2004;26:573–578. doi: 10.1179/016164104225016272. [DOI] [PubMed] [Google Scholar]
- 180.Wu Z, et al. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat Med. 2005;11:959–965. doi: 10.1038/nm1287. [DOI] [PubMed] [Google Scholar]
- 181.Grammas P, Tripathy D, Sanchez A, Yin X, Luo J. Brain microvasculature and hypoxia-related proteins in Alzheimer’s disease. Int J Clin Exp Pathol. 2011;4:616–627. [PMC free article] [PubMed] [Google Scholar]
- 182.Henkel JS, Beers DR, Wen S, Bowser R, Appel SH. Decreased mRNA expression of tight junction proteins in lumbar spinal cords of patients with ALS. Neurology. 2009;72:1614–1616. doi: 10.1212/WNL.0b013e3181a41228. [DOI] [PubMed] [Google Scholar]
- 183.Miyazaki K, et al. Disruption of neurovascular unit prior to motor neuron degeneration in amyotrophic lateral sclerosis. J Neurosci Res. 2011;89:718–728. doi: 10.1002/jnr.22594. [DOI] [PubMed] [Google Scholar]
- 184.Yamamoto M, et al. Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am J Pathol. 2008;172:521–533. doi: 10.2353/ajpath.2008.070076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kumar DKV, et al. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Transl Med. 2016;8:340ra72. doi: 10.1126/scitranslmed.aaf1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Soscia SJ, et al. The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PloS One. 2010;5:e9505. doi: 10.1371/journal.pone.0009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wada K, et al. Expression levels of vascular endothelial growth factor and its receptors in Parkinson’s disease. Neuroreport. 2006;17:705–709. doi: 10.1097/01.wnr.0000215769.71657.65. [DOI] [PubMed] [Google Scholar]
- 188.Desai Bradaric B, Patel A, Schneider JA, Carvey PM, Hendey B. Evidence for angiogenesis in Parkinson’s disease, incidental Lewy body disease, and progressive supranuclear palsy. J Neural Transm Vienna Austria 1996. 2012;119:59–71. doi: 10.1007/s00702-011-0684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hill KK, et al. Cerebral blood flow responses to dorsal and ventral STN DBS correlate with gait and balance responses in Parkinson’s disease. Exp Neurol. 2013;241:105–112. doi: 10.1016/j.expneurol.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Donahue JE, et al. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol (Berl) 2006;112:405–415. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- 191.Miller MC, et al. Hippocampal RAGE immunoreactivity in early and advanced Alzheimer’s disease. Brain Res. 2008;1230:273–280. doi: 10.1016/j.brainres.2008.06.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sagare AP, Deane R, Zlokovic BV. Low-density lipoprotein receptor-related protein 1: a physiological Aβ homeostatic mechanism with multiple therapeutic opportunities. Pharmacol Ther. 2012;136:94–105. doi: 10.1016/j.pharmthera.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM. Brain to plasma amyloid-beta efflux: a measure of brain amyloid burden in a mouse model of Alzheimer’s disease. Science. 2002;295:2264–2267. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- 194.DeMattos RB, et al. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.US National Library of Medicine. clinicaltrials.gov. 2017 https://clinicaltrials.gov/ct2/show/NCT02008357.
- 196.Deane R, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 197.Yan SD, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 198.Mackic JB, et al. Human blood-brain barrier receptors for Alzheimer’s amyloid-beta 1-40. Asymmetrical binding, endocytosis, and transcytosis at the apical side of brain microvascular endothelial cell monolayer. J Clin Invest. 1998;102:734–743. doi: 10.1172/JCI2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Deane R, et al. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122:1377–1392. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.US National Library of Medicine. clinicaltrials.gov. 2017 https://clinicaltrials.gov/ct2/show/NCT02916056.
- 201.Halliday MR, et al. Relationship between cyclophilin a levels and matrix metalloproteinase 9 activity in cerebrospinal fluid of cognitively normal apolipoprotein e4 carriers and blood-brain barrier breakdown. JAMA Neurol. 2013;70:1198–1200. doi: 10.1001/jamaneurol.2013.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Conejero-Goldberg C, et al. APOE2 enhances neuroprotection against Alzheimer’s disease through multiple molecular mechanisms. Mol Psychiatry. 2014;19:1243–1250. doi: 10.1038/mp.2013.194. [DOI] [PubMed] [Google Scholar]
- 203.Zeuzem S, et al. Aliment Pharmacol Ther. 2015;42:829–844. doi: 10.1111/apt.13342. [DOI] [PubMed] [Google Scholar]
- 204.Langford D, et al. Altered P-glycoprotein expression in AIDS patients with HIV encephalitis. J Neuropathol Exp Neurol. 2004;63:1038–1047. doi: 10.1093/jnen/63.10.1038. [DOI] [PubMed] [Google Scholar]
- 205.Cicchetti F, et al. Mutant huntingtin is present in neuronal grafts in Huntington disease patients. Ann Neurol. 2014;76:31–42. doi: 10.1002/ana.24174. [DOI] [PubMed] [Google Scholar]
- 206.Erickson MA, Banks WA. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2013;33:1500–1513. doi: 10.1038/jcbfm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Nuzzo D, et al. Inflammatory mediators as biomarkers in brain disorders. Inflammation. 2014;37:639–648. doi: 10.1007/s10753-013-9780-2. [DOI] [PubMed] [Google Scholar]
- 208.Skoog I, et al. A population study on blood-brain barrier function in 85-year-olds: relation to Alzheimer’s disease and vascular dementia. Neurology. 1998;50:966–971. doi: 10.1212/wnl.50.4.966. [DOI] [PubMed] [Google Scholar]
- 209.Janelidze S, et al. Increased blood-brain barrier permeability is associated with dementia and diabetes but not amyloid pathology or APOE genotype. Neurobiol Aging. 2017;51:104–112. doi: 10.1016/j.neurobiolaging.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Skillback T, et al. CSF/serum albumin ratio in dementias: a cross-sectional study on 1861 patients. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2017.06.028. (Accepted) [DOI] [PubMed] [Google Scholar]
- 211.Blennow K, et al. Blood-brain barrier disturbance in patients with Alzheimer’s disease is related to vascular factors. Acta Neurol Scand. 1990;81:323–326. doi: 10.1111/j.1600-0404.1990.tb01563.x. [DOI] [PubMed] [Google Scholar]
- 212.Wallin A, Blennow K, Rosengren L. Cerebrospinal fluid markers of pathogenetic processes in vascular dementia, with special reference to the subcortical subtype. Alzheimer Dis Assoc Disord. 1999;13(Suppl 3):S102–105. [PubMed] [Google Scholar]
- 213.Blennow K, Wallin A, Uhlemann C, Gottfries CG. White-matter lesions on CT in Alzheimer patients: relation to clinical symptomatology and vascular factors. Acta Neurol Scand. 1991;83:187–193. doi: 10.1111/j.1600-0404.1991.tb04675.x. [DOI] [PubMed] [Google Scholar]
- 214.Bowman GL, Kaye JA, Quinn JF. Dyslipidemia and blood-brain barrier integrity in Alzheimer’s disease. Curr Gerontol Geriatr Res. 2012;2012:184042. doi: 10.1155/2012/184042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertens Dallas Tex 1979. 2013;62:810–817. doi: 10.1161/HYPERTENSIONAHA.113.01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ivens S, et al. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain J Neurol. 2007;130:535–547. doi: 10.1093/brain/awl317. [DOI] [PubMed] [Google Scholar]
- 217.Braganza O, et al. Albumin is taken up by hippocampal NG2 cells and astrocytes and decreases gap junction coupling. Epilepsia. 2012;53:1898–1906. doi: 10.1111/j.1528-1167.2012.03665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.LeVine SM. Albumin and multiple sclerosis. BMC Neurol. 2016;16:47. doi: 10.1186/s12883-016-0564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Silverberg GD, et al. The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer’s type. Neurology. 2001;57:1763–1766. doi: 10.1212/wnl.57.10.1763. [DOI] [PubMed] [Google Scholar]
- 220.Craig-Schapiro R, et al. Multiplexed immunoassay panel identifies novel CSF biomarkers for Alzheimer’s disease diagnosis and prognosis. PloS One. 2011;6:e18850. doi: 10.1371/journal.pone.0018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hanzel CE, et al. Analysis of matrix metallo-proteases and the plasminogen system in mild cognitive impairment and Alzheimer’s disease cerebrospinal fluid. J Alzheimers Dis JAD. 2014;40:667–678. doi: 10.3233/JAD-132282. [DOI] [PubMed] [Google Scholar]
- 222.Pisani V, et al. Increased blood-cerebrospinal fluid transfer of albumin in advanced Parkinson’s disease. J Neuroinflammation. 2012;9:188. doi: 10.1186/1742-2094-9-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Liguori C, et al. Cerebrospinal-fluid Alzheimer’s Disease Biomarkers and Blood-Brain Barrier Integrity in a natural population of cognitive intact Parkinson’s Disease patients. CNS Neurol Disord Drug Targets. 2016 doi: 10.2174/1871527316666161205123123. [DOI] [PubMed] [Google Scholar]
- 224.Brettschneider J, Petzold A, Süssmuth SD, Ludolph AC, Tumani H. Axonal damage markers in cerebrospinal fluid are increased in ALS. Neurology. 2006;66:852–856. doi: 10.1212/01.wnl.0000203120.85850.54. [DOI] [PubMed] [Google Scholar]
- 225.Jessen Krut J, et al. Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PloS One. 2014;9:e88591. doi: 10.1371/journal.pone.0088591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chen ZL, Strickland S. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell. 1997;91:917–925. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- 227.Mhatre M, et al. Thrombin, a mediator of neurotoxicity and memory impairment. Neurobiol Aging. 2004;25:783–793. doi: 10.1016/j.neurobiolaging.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 228.Chen B, Cheng Q, Yang K, Lyden PD. Thrombin mediates severe neurovascular injury during ischemia. Stroke. 2010;41:2348–2352. doi: 10.1161/STROKEAHA.110.584920. [DOI] [PubMed] [Google Scholar]
- 229.Schachtrup C, et al. Fibrinogen inhibits neurite outgrowth via beta 3 integrin-mediated phosphorylation of the EGF receptor. Proc Natl Acad Sci U S A. 2007;104:11814–11819. doi: 10.1073/pnas.0704045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Paul J, Strickland S, Melchor JP. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer’s disease. J Exp Med. 2007;204:1999–2008. doi: 10.1084/jem.20070304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Akassoglou K, et al. Fibrin depletion decreases inflammation and delays the onset of demyelination in a tumor necrosis factor transgenic mouse model for multiple sclerosis. Proc Natl Acad Sci U S A. 2004;101:6698–6703. doi: 10.1073/pnas.0303859101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ryu JK, et al. Blood coagulation protein fibrinogen promotes autoimmunity and demyelination via chemokine release and antigen presentation. Nat Commun. 2015;6:8164. doi: 10.1038/ncomms9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bardehle S, Rafalski VA, Akassoglou K. Breaking boundaries-coagulation and fibrinolysis at the neurovascular interface. Front Cell Neurosci. 2015;9:354. doi: 10.3389/fncel.2015.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhong Z, et al. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J Clin Invest. 2009;119:3437–3449. doi: 10.1172/JCI38476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sui YT, Bullock KM, Erickson MA, Zhang J, Banks WA. Alpha synuclein is transported into and out of the brain by the blood-brain barrier. Peptides. 2014;62:197–202. doi: 10.1016/j.peptides.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Peelaerts W, et al. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015;522:340–344. doi: 10.1038/nature14547. [DOI] [PubMed] [Google Scholar]
- 237.Matsumoto J, et al. Transmission of α-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: another mechanism for initiation and progression of Parkinson’s disease? Acta Neuropathol Commun. 2017;5:71. doi: 10.1186/s40478-017-0470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Shi M, et al. Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol (Berl) 2014;128:639–650. doi: 10.1007/s00401-014-1314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Calderón-Garcidueñas L, et al. Air pollution and children: neural and tight junction antibodies and combustion metals, the role of barrier breakdown and brain immunity in neurodegeneration. J Alzheimers Dis JAD. 2015;43:1039–1058. doi: 10.3233/JAD-141365. [DOI] [PubMed] [Google Scholar]
- 240.Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2012;32:1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Bray N. Biologics: Transferrin’ bispecific antibodies across the blood-brain barrier. Nat Rev Drug Discov. 2015;14:14–15. doi: 10.1038/nrd4522. [DOI] [PubMed] [Google Scholar]
- 242.Niewoehner J, et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron. 2014;81:49–60. doi: 10.1016/j.neuron.2013.10.061. [DOI] [PubMed] [Google Scholar]
- 243.Yu YJ, et al. Therapeutic bispecific antibodies cross the blood-brain barrier in nonhuman primates. Sci Transl Med. 2014;6:261ra154. doi: 10.1126/scitranslmed.3009835. [DOI] [PubMed] [Google Scholar]
- 244.Yemisci M, et al. Systemically administered brain-targeted nanoparticles transport peptides across the blood-brain barrier and provide neuroprotection. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2015;35:469–475. doi: 10.1038/jcbfm.2014.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Burgess A, Hynynen K. Microbubble-Assisted Ultrasound for Drug Delivery in the Brain and Central Nervous System. Adv Exp Med Biol. 2016;880:293–308. doi: 10.1007/978-3-319-22536-4_16. [DOI] [PubMed] [Google Scholar]
- 246.Poon C, McMahon D, Hynynen K. Noninvasive and targeted delivery of therapeutics to the brain using focused ultrasound. Neuropharmacology. 2017;120:20–37. doi: 10.1016/j.neuropharm.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wang D, Kranz-Eble P, De Vivo DC. Mutational analysis of GLUT1 (SLC2A1) in Glut-1 deficiency syndrome. Hum Mutat. 2000;16:224–231. doi: 10.1002/1098-1004(200009)16:3<224::AID-HUMU5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 248.Alakbarzade V, et al. A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat Genet. 2015;47:814–817. doi: 10.1038/ng.3313. [DOI] [PubMed] [Google Scholar]
- 249.Guemez-Gamboa A, et al. Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat Genet. 2015;47:809–813. doi: 10.1038/ng.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Novara F, et al. Clinical and Molecular Characteristics of SLC16A2 (MCT8) Mutations in Three Families with the Allan-Herndon-Dudley Syndrome. Hum Mutat. 2017;38:260–264. doi: 10.1002/humu.23140. [DOI] [PubMed] [Google Scholar]
- 251.Abdel-Hamid MS, Abdel-Salam GMH, Issa MY, Emam BA, Zaki MS. Band-like calcification with simplified gyration and polymicrogyria: report of 10 new families and identification of five novel OCLN mutations. J Hum Genet. 2017;62:553–559. doi: 10.1038/jhg.2017.4. [DOI] [PubMed] [Google Scholar]
- 252.Akawi NA, et al. Delineation of the clinical, molecular and cellular aspects of novel JAM3 mutations underlying the autosomal recessive hemorrhagic destruction of the brain, subependymal calcification, and congenital cataracts. Hum Mutat. 2013;34:498–505. doi: 10.1002/humu.22263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Keller A, et al. Mutations in the gene encoding PDGF-B cause brain calcifications in humans and mice. Nat Genet. 2013;45:1077–1082. doi: 10.1038/ng.2723. [DOI] [PubMed] [Google Scholar]
- 254.Nicolas G, et al. Mutation of the PDGFRB gene as a cause of idiopathic basal ganglia calcification. Neurology. 2013;80:181–187. doi: 10.1212/WNL.0b013e31827ccf34. [DOI] [PubMed] [Google Scholar]
- 255.Vemuri P, Schöll M. Linking Amyloid-β and Tau Deposition in Alzheimer Disease. JAMA Neurol. 2017;74:766–768. doi: 10.1001/jamaneurol.2017.0323. [DOI] [PubMed] [Google Scholar]
- 256.He L, et al. Analysis of the brain mural cell transcriptome. Sci Rep. 2016;6:35108. doi: 10.1038/srep35108. [DOI] [PMC free article] [PubMed] [Google Scholar]