Abstract
While the epidemiology of adult heart failure has been extensively researched, this systematic review addresses the less well characterized incidence and prevalence of pediatric HF. The search strategy used Cochrane methodology and identified 83 unique studies for inclusion. Studies were categorized according to whether the HF diagnosis was reported as primary (n = 10); associated with other cardiovascular diseases (CVDs) (n = 49); or associated with non-CVDs (n = 24). A narrative synthesis of the evidence is presented. For primary HF, the incidence ranged from 0.87/100,000 (UK and Ireland) to 7.4/100,000 (Taiwan). A prevalence of 83.3/100,000 was reported in one large population-based study from Spain. HF etiology varied across regions with lower respiratory tract infections and severe anemia predominating in lower income countries, and cardiomyopathies and congenital heart disease major causes in higher income countries. Key findings for the other categories included a prevalence of HF associated with cardiomyopathies ranging from 36.1% (Japan) to 79% (US); associated with congenital heart disease from 8% (Norway) to 82.2% (Nigeria); associated with rheumatic heart diseases from 1.5% (Turkey) to 74% (Zimbabwe); associated with renal disorders from 3.8% (India) to 24.1% (Nigeria); and associated with HIV from 1% (US) to 29.3% (Brazil). To our knowledge, this is the first systematic review of the topic and strengthens current knowledge of pediatric HF epidemiology. Although a large body of research was identified, heterogeneity in study design and diagnostic criteria limited the ability to compare regional data. Standardized definitions of pediatric HF are required to facilitate cross-regional comparisons of epidemiological data.
Electronic supplementary material
The online version of this article (10.1007/s00246-017-1787-2) contains supplementary material, which is available to authorized users.
Keywords: Pediatric, Heart failure, Systematic, Prevalence, Incidence, Epidemiology
Introduction
Heart failure (HF) is recognized as a complex clinical syndrome associated with a wide range of abnormalities in cardiac structure or function. Although definitions can vary [1–4], HF can be broadly described as “the failure of the heart to supply blood to either systemic or pulmonary circulation at an appropriate rate of flow, or to receive venous return at an appropriate filling pressure, resulting in adverse effects on the heart, the circulation, and the patient” [4].
While the epidemiology of HF has been extensively researched in the adult population [5], the incidence and prevalence of pediatric HF is not as well characterized. The most common causes of adult HF, which include ischemia, hypertension, and valvular inflammation, rarely occur in children [6]. Furthermore, existing evidence shows that the etiology of pediatric HF varies across regions and this variation affects the inter-regional incidence and prevalence of HF in children and adolescents. According to a 2009 World Health Organization (WHO) report, the main causes for HF in children are congenital malformations, cardiomyopathy and anthracycline toxicity [7]. In lower income countries, many cases of HF are caused or exacerbated by anemia which is often secondary to malaria or malnutrition [7]. Moreover, the WHO report also identifies hypocalcemia and vitamin D deficiency as risk factors for HF among children and adolescents of certain ethnic minorities in developed countries [7]. Etiologies affecting the incidence and prevalence of HF also vary according to age [8]. These factors may explain the current lack of a globally accepted definition of, and standard diagnostic criteria for, pediatric HF [6–9]. In addition, the current understanding of the epidemiology of HF in children and adolescents is poor and this topic has not been assessed in a systematic way.
We report a systematic review and narrative synthesis of the evidence on the incidence and prevalence of HF in children and adolescents (birth to < 18 years of age) over the last 20 years (1996–2016) to strengthen current knowledge on the epidemiology of pediatric HF, which can be helpful in the development of new treatments and guidelines for this patient population.
Methods
The systematic literature review was conducted using standard methodology as published by the Cochrane Collaboration [10] and was reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11].
A full description of the multi-string search strategy is presented in Supplementary Appendix and included a disease term (heart failure/insufficiency or cardiac or myocard*); a population term (pediatric* or paediatric* or neonat* or perinat* or child* or juvenile* or bab* or infant* or toddler* or newborn or new-born or premature* or preterm* or pre-term* preschool* or pre-school* or teen* or adolescen* or minor* or pubescen*); and an outcome term (prevalen* or inciden*).
The review included observational studies. Titles, abstracts, and full-text articles were independently screened for inclusion by two reviewers and any discrepancies were reconciled by a third independent reviewer.
Data on incidence and/or prevalence of HF, and the distribution of HF in various subgroups were extracted by one reviewer, quality checked by the second reviewer, with differences reconciled by a third reviewer. Full-text studies were graded for quality according to the Downs and Black checklist (studies that scored ≤ 14 points were ranked as ‘poor’; 15–19 points as ‘fair’; and 20–25 points as ‘good’) [12]. Conference abstracts inherently lack information on many parameters listed in the checklist and, therefore, were not graded. For uniformity, we have used the term HF for all studies that report the condition as HF, chronic HF (CHF), or congestive HF and used the term acute HF (AHF) for studies that report the condition as decompensated HF or AHF, in the text. The extracted data from all the included studies are presented in Supplementary Appendix. The systematic review protocol is available in Supplementary Appendix.
Results
Study Selection
A final list of 1952 records was generated following the removal of duplicate records, and the application of age limits (< 18 years in EMBASE) and/or definitions for children and adolescents (EMBASE and MEDLINE). From this list, a total of 83 unique records (77 full-text publications and six conference abstracts) were selected for inclusion (see PRISMA flowchart Fig. 1). Study quality was graded as ‘poor’ for 63 and ‘fair’ for 14 of the 77 full-text studies.
Fig. 1.
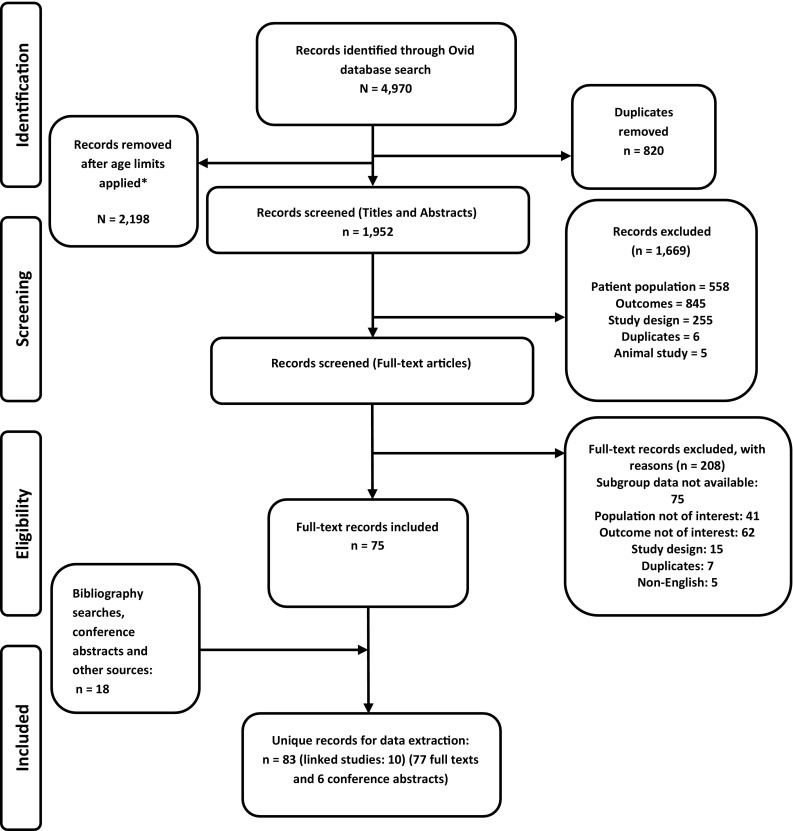
PRISMA flowchart for study selection for the systematic review. * Age-specific limits applied to EMBASE were (infant < to 1 year > or child < unspecified age > or preschool child < 1–6 years > or school child < 7–12 years > or adolescent < 13–17 years >). Age-specific limits applied to MEDLINE were limit 19 to [“all infant (birth to 23 months)” or “all child (0–18 years)” or “newborn infant (birth to 1 month)” or “infant (1–23 months)” or “preschool child (2–5 years)” or “child (6–12 years)” or “adolescent (13–18 years)”]
To account for a lack of disease homogeneity, the included studies were grouped into the following three disease categories: studies in which (1) HF was the primary diagnosis; (2) HF was diagnosed secondary to another cardiovascular disease (CVD); (3) HF was diagnosed secondary to a non-CVD. The results are presented separately for each category.
Summary tables are presented for each category. In addition, tables summarizing all data extracted for each included study, and encompassing data on all subgroups and regional distributions, are presented in Supplementary Appendix.
Primary HF Diagnosis
Incidence
Incidence was reported in 5 studies, 4 of which were multi-center studies [13–16], 2 were prospective [13, 17], and 3 retrospective [14–16]. Incidence data ranged from 0.87 per 100,000 population in a study in the United Kingdom (UK) and Ireland [13] to 7.4 per 100,000 population in a study from Taiwan (Table 1) [16].
Table 1.
Incidence and prevalence of HF in studies on primary HF diagnosis
| Study name | Study design | Country, period | Setting | Study population (age range) | Subgroups | Type of HF | Sample size | Gender | Cases (n) | (%) | Per 100,000 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female (n) | Female (%) | |||||||||||
| Incidence of HF as a primary diagnosis | ||||||||||||
| Andrews [13] | Prospective | UK and Ireland, 2003 (1 year) | Hospitals (17) | Hospitalized HF cases (0–16 years) | All patients | HF | 11,712,100* | NR | NR | 104 | NR | 0.87 |
| Massin [17] | Prospective | Belgium, 1996–2006 (10 years) | Hospital (1) | Hospitalized cases (0–16 years) | All patients | HF | 1196 | 620 | 51.8* | 124 | 10.4 | – |
| Neumann [14] | Retrospective | Germany, 2000–2006 (7 years) | Country wide hospitals | Hospitalized HF cases (0–< 15 years) | Years 2000–2006 (7 years) | HF | NR | NR | NR | NR | NR | 2.0–3.0 |
| Schmidt [15] | Retrospective | Germany, 1995 and 2009 (2 distinct years) | Country wide hospitals | Hospitalized HF cases | Year 1995 | HF | 1,32,38,000 | NR | NR | 265 | NR | 2 |
| Year 2009 | HF | 1,10,30,000 | NR | NR | 221 | NR | 2 | |||||
| Tseng [16] | Retrospective | Taiwan, 2005 (1 year) | Country wide hospitals | Hospitalized HF cases (0–14 years) | All patients | HF | 190,362* | 90,873 | 47.7 | 14* | NR | 7.4* |
| 0–4 years | HF | 55,262 | 26,319 | 47.6 | 12 | NR | 21.7 | |||||
| 5–9 years | HF | 65,636 | 31,355 | 47.8 | 0 | NR | 0 | |||||
| 10–14 years | HF | 69,464 | 33,199 | 47.8 | 2 | NR | 2.9 | |||||
| Males | 0–14 years | HF | 99,489* | – | – | 6 | NR | 6.0* | ||||
| Females | 0–14 years | HF | 90,873* | 90,873 | 100 | 8* | NR | 8.8* | ||||
| Prevalence of HF as a primary diagnosis | ||||||||||||
| Adekanmbi [18] | Prospective | Nigeria, 2002–2003 (1 year) | Hospital (1) | Hospital admissions and ER | All patients | Congestive HF | 1552 | NR | NR | 109 | 7 | – |
| (1 day–14 years) | ||||||||||||
| Animasahun [19] | Prospective | Nigeria, 2011–2012 (2 years) | Hospital (1) | Hospital admissions | All patients | Congestive HF | 5705 | NR | NR | 156 | 2.7 | – |
| (1 day–12 years) | ||||||||||||
| Jiménez-García [20] | Cross-sectional | Spain, 2012–2013 (1 year) | Community (Madrid) | Influenza vaccination coverage (6 months–14 years) |
HF | 9,81,855 | 4,77,928 | 48.7 | 818 | 0.1 | 83.3* | |
| Rodríguez-Rieiro [21] | Spain, 2009 (point prevalence) | Patients with chronic diseases | HF | 1,17,940 | 48,806 | 41.4 | 689 | 0.6* | 77$ | |||
| Lagunju [22] | Prospective | Nigeria, 2000–2001 (10 months) | Hospital (1) | Hospital admissions (8 days–12 years) | All patients | Congestive HF | 1713 | NR | NR | 100 | 5.8 | – |
| Oyedeji [23] | Prospective | Nigeria, 2007 (6 months) | Hospital (1) | Patients in ER (1 month–12 years) | All patients | Congestive HF | 391 | NR | NR | 35 | 9 | – |
ER emergency room, HF heart failure, NR not reported
*Calculated values from the source article
$Reported as prevalence of 7.7 per 10,000 inhabitants
In the UK and Ireland study undertaken in 2003, the majority of pediatric HF patients (55.8%) had HF associated with familial or idiopathic dilated cardiomyopathies, with 82% of the patients having New York Heart Association (NYHA) class III–IV severity of HF [13]. The incidence varied by regions within the UK and Ireland, with the highest incidence in Scotland and lowest in Ireland (1.27 and 0.11 per 100,000, respectively) (Supplementary Appendix, Table A1) [13].
The incidence of HF was 10.4% in 1196 patients aged 0–16 years (60% of whom were infants) primarily diagnosed with congenital or acquired heart disease and prospectively indexed at a single center in Belgium over a 10-year period (Table 1) [17]. Congenital heart disease was the HF etiology in 52% of patients, cardiomyopathies in 19.4%, and acquired heart disease in 18.5% (Supplementary Appendix, Table A1).
Two German studies reported on the nationwide incidence of HF hospitalizations [14, 15]. According to the first study, the incidence of hospitalized HF ranged from 2 to 3 per 100,000 population among children and adolescents (aged 0 to < 15 years; period covered from 2000 to 2006) [14]. A similar incidence of hospitalized HF of 2 per 100,000 population was reported in the second German study over two distinct 1-year periods in 1995 and 2009 [15].
A 2005 study from Taiwan reported an incidence of hospitalized HF of 7.4 per 100,000 pediatric patients aged 0–14 years. The incidence was slightly higher among girls versus boys (8.8 vs. 6 per 100,000, respectively) and was highest in the 0–4 year age group (21.7 per 100,000 population) [16] (Table 1).
Prevalence
Prevalence data were obtained from 5 unique studies comprising one large population-based study from Spain [20, 21] and 4 smaller studies from different university hospitals in Nigeria [18, 19, 22, 23].
In a 2009 study conducted to determine the extent of influenza vaccine coverage in chronically ill patients in Madrid, the prevalence of HF in 117,940 pediatric patients was 0.6% (77 per 100,000 inhabitants) (Table 1) [21]. In a subsequent 2012–2013 publication using the same computerized immunization registry, but not restricted to chronically ill patients, a HF prevalence of 0.1% (83.3 per 100,000) was reported among 981,855 children aged 6 months–14 years [20].
The four hospital-based studies from Nigeria reported a pediatric HF prevalence ranging from 2.7 to 9% in studies of children presenting at emergency rooms or admitted to pediatric hospital wards (Table 1) [18, 19, 22, 23]. The highest prevalence was observed in the youngest age group (1 month–5 years) (Supplementary Appendix, Table A2). The most common HF etiologies in these studies were anemia and respiratory tract infections (Supplementary Appendix, Table A3) [18, 19, 22, 23].
Secondary HF Diagnosis in CVDs
HF as a diagnosis secondary to other CVDs was reported in 49 of 83 identified studies. Five studies reported HF incidence alone, 42 studies reported HF prevalence only, and 2 studies had both incidence and prevalence data (Table 2).
Table 2.
Incidence of HF secondary to other CVDs
| Study name | Study design | Country, period | Setting | Study population (age range) | Subgroups | Type of HF | Sample size | Gender | HF incidence | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Female (n) | Female (%) | Cases (n) | Incidence (%) | ||||||||
| Congenital heart defects/disease | |||||||||||
| Hong [24] | Retrospective | South Korea, 2000–2010 (11 years) | Hospital (1) | TGA (1–108 days) | All patients | HF | 28 | 11 | 39.3 | 5 | 17.9 |
| Najm [25] | Retrospective | Canada, 1975–1985 (21 years) | Hospital (1) | Scimitar syndrome (1–335 days) | – | HF | 19 | 14 | 73.7* | 11 | 57.9* |
| Tomlinson [26]# | Retrospective | Jamaica, 1995–2004 (10 years) | Hospital (1) | Trisomy 21 with congenital heart disease and cardiac lesions (0–12 years) | – | Congestive HF | 46 | NR | NR | 11* | 23.9* |
| Vascular malformations | |||||||||||
| Rialon [27] | Retrospective | US, 1995–2012 (18 years) | Hospital (1) | Hepatic hemangiomas (0 to < 1 year) | All patients | Congestive HF | 72 | NR | NR | 16* | 22.2* |
| Patients who underwent initial screening for hemangiomas | Congestive HF | 43 | NR | NR | 2 | 5 | |||||
| Unscreened patients | Congestive HF | 29 | NR | NR | 14 | 48 | |||||
| Post-OHT | |||||||||||
| LaPage [28]$ | Retrospective | US, 1991–2006 (16 years) | Hospital (1) | Tachyarrhythmia (0–17 years) | Acute congestive HF | 19 | NR | NR | 2* | 10.5* | |
| Murtuza [29] | Retrospective | UK, 2000–2011 (8 years) | Hospital (1) | DCM and RCM (0.1–17.1 years) | All patients | VHF (right) | 159* | 83* | 52.2* | 30* | 18.9 |
| Patients with DCM | VHF (right) | 136 | 74* | 54.4* | 20 | 14.7 | |||||
| Patients with RCM | VHF (right) | 23 | 9* | 39.1* | 10 | 43.5 | |||||
| IE | |||||||||||
| Marom [30]$ | Retrospective | Israel, 1992–2004 (12.5 years) | Hospital (1) | IE (0 to < 18 years) | Children with no predisposing factors for IE | HF | 9 | NR | NR | 7 | 77.8 |
DCM dilated cardiomyopathy, HF heart failure, IE infective endocarditis, NR not reported, OHT orthotopic heart transplantation, RCM restrictive cardiomyopathy, VHF ventricular heart failure
*Calculated from source article
#In Tomlinson et al., 30 of the 76 children had congestive HF at presentation and this is captured in “Prevalence,” and in 11 of the remaining 46 children congestive HF developed during the study. A total of 41 patients (30 + 11) had congestive HF in this study
$In Marom et al., 9 of a total of 51 patients with IE had no predisposing cardiac anomalies (HF cases are new). Of these, 7 cases had HF and have contributed to incidence data, whereas 42 patients had predisposing cardiac anomalies (unclear if HF cases are new) and the data are captured in “Prevalence”
Incidence
Congenital Heart Disease
Three retrospective studies reported the incidence of HF in pediatric patients diagnosed with congenital heart disease [24–26]. A Canadian study reported a HF incidence of 57.9% among 19 infants with Scimitar Syndrome (Table 2) [25]. In a Jamaican study, HF developed in 23.9% of 46 patients with trisomy 21 and congenital heart disease and/or cardiac lesions [26]. A study from South Korea reported that, overall, HF developed in 17.9% of 28 patients presenting with transposition of the great arteries (TGA), and the rate was 41.7% in patients who also had ventricular septal defects (VSDs) (Table 2) [24].
Vascular Malformations
In a retrospective study from the US covering 1995–2012, HF developed in more than 20% of the 72 infants with multiple cutaneous and hepatic hemangiomas (Table 2) [27]. The incidence of HF was lower among patients identified through screening for hemangiomas (5% of 43 vs. 48% of 29 not screened) [27].
Post-orthotopic Heart Transplantation
Two retrospective studies (one UK- and one US-based) reported the incidence of HF in post-orthotopic heart transplantation (OHT) pediatric recipients [28, 29]. In the UK-based study, 18.9% of 159 patients developed right ventricular heart failure (VHF) during the perioperative period. Complex congenital heart disease, restrictive cardiomyopathy (RCM), and dilated cardiomyopathy (DCM) were the main reasons for OHT in these populations. The incidence of HF was 43.5% in 23 RCM patients and 14.7% in 136 DCM patients (Table 2) [29]. The US study reported that acute congestive HF developed in 10.5% of 19 patients (0–17 years) who presented with tachyarrhythmia beyond the first 2 weeks post-OHT (Table 2) [28].
Infective Endocarditis
HF is one of the many complications of infective endocarditis (IE). A retrospective study from Israel reported incident cases of HF occurring in 77.8% of 9 children with IE, without any predisposing factors [30].
Prevalence
Due to the large number of studies included for the prevalence of HF secondary to other CVDs, only those that ranked ‘fair’ or ‘good’ on the Downs and Black checklist and/or had a sample size > 50 and/or report acute HF are summarized in the text below and listed in Table 3. However, a consolidated table of all included studies is presented in Supplementary Appendix, Table B2.
Table 3.
Prevalence of HF in CVD studies
| Study name | Study design | Country, period | Setting | Study population (age range) | Subgroups | Type of HF | Sample size | Gender | HF prevalence and distribution in study subgroups | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female (n) | Female (%) | Cases (n) | Prevalence (%) | Distribution of prevalent cases of HF in study subgroups (%) | ||||||||
| Congenital heart defects/disease | ||||||||||||
| Azhari [31] | Retrospective | Saudi Arabia, 1990–2003 (14 years and 1 month) | Hospital (1) | ASD (1 day–11 years) | All patients | Congestive HF | 121 | 74 | 61.2 | 14 | 11.6 | – |
| Small defects | Congestive HF | 22 | 9* | 41 | 0 | 0 | – | |||||
| Medium defects | Congestive HF | 27 | NR | NR | 1 | 3.7* | – | |||||
| Large defects | Congestive HF | 72 | NR | NR | 13 | 18.1* | – | |||||
| Pulmonary arterial hypertension@ | Congestive HF | 8 | 2 | 25 | 8 | 100* | – | |||||
| Meberg [32] | Longitudinal (prospective and retrospective) | Norway, 1982–1996 (15 years) | Hospitals (NR) | Congenital heart disease (2 weeks–11 years) | Detected subsequent to discharge from hospital after birth | Decompensation | 84 | NR | NR | 7 | 8.0 | – |
| Miyake [33] | Prospective | Japan, 1986–1996 (11 years) | Hospital (1) | VSD (1–88 days) | All patients | Congestive HF | 225 | 109 | 48.4* | 104 | 46.0 | – |
| Subpulmonary VSD | Congestive HF | 104 | NR | NR | 18* | – | 17.3* | |||||
| Perimembranous VSD | Congestive HF | 104 | NR | NR | 85 | – | 81.7* | |||||
| Muscular | Congestive HF | 104 | NR | NR | 1 | – | 1* | |||||
| Spontaneous closure | Congestive HF | 104 | NR | NR | 20 | – | 19.2* | |||||
| Small open | Congestive HF | 104 | NR | NR | 31 | – | 29.8* | |||||
| Surgical closure | Congestive HF | 104 | NR | NR | 53 | – | 51* | |||||
| Najm [34] | Retrospective | Canada, 1982–1996 (14 years and 5 months) | Hospital (1) | ASD (1 month–16.4 years) | – | Congestive HF | 180 | 97 | 53.9 | 35 | 20 | – |
| Okoromah [35] | Case–control | Nigeria, 2006–2008 (2 years) | Cases; hospital (1) | Cases: malnutrition and congenital heart disease (3–192 months) | All cases | Congestive HF | 73 | NR | NR | 60 | 82.2 | – |
| Controls; community (primary school) | Controls: malnutrition with no congenital heart disease (3–192 months) | All controls | Congestive HF | 76 | NR | NR | 0 | 0 | – | |||
| Sadoh [36] | Prospective | Nigeria, 2006–2009 (2 years and 5 months) | Hospital (1) | VSD (2–24 months) | All patients | Congestive HF | 61 | 35 | 57.4 | 15 | 24.6 | – |
| Spontaneous closure | Congestive HF | 15 | NR | NR | 3 | – | 20 | |||||
| Sadoh [37] | Prospective | Nigeria, 2011–2012 (1 year) | Hospital (1) | Pneumonia with and without congenital heart disease (1–48 months) | All patients | Congestive HF | 121 | 60 | 49.6 | 49 | 40.5 | – |
| Pneumonia and congenital heart disease | Congestive HF | 14 | 9 | 64.3 | – | |||||||
| Pneumonia without congenital heart disease | Congestive HF | 107 | 40 | 37.4 | – | |||||||
| Shah [38] | Retrospective | Nepal, 2006 (1 year) | Hospital (1) | Congenital heart disease (0 to < 15 years) | – | Congestive HF | 84 | 33 | 39.3 | 46 | 54.8 | – |
| Tomlinson [26] | Retrospective | Jamaica, 1995–2004 (10 years) | Hospital (1) | Trisomy 21 with congenital heart disease (0–12 years) | – | Congestive HF | 76 | 46 | 60 | 30 | 39.5* | – |
| Vaidyanathan [39] | Prospective | India, 2005–2006 (1 year) | Hospital (1) | Malnutrition with congenital heart disease (0 to < 5 years) | – | Congestive HF | 476 | 243* | 51.5* | 194 | 40.8 | – |
| Cardiomyopathies | ||||||||||||
| Alvarez [40], Colan [41], Everitt [42], Towbin [43], Webber [44], Wilkinson [45] (PCMR studies) | Longitudinal (prospective and retrospective cohorts) | US, Canada, 1990 (ongoing) | Hospitals (98 centers for the prospective cohort and 39 centers for the retrospective cohort) | Cardiomyopathies (0 to < 18 years) | All patients | Congestive HF | 3549± | NR | NR | NR | NR | – |
| All HCM patients | Congestive HF | 849 | NR | NR | 115* | 13.5* | – | |||||
| Inborn errors of metabolism | Congestive HF | 74 | NR | NR | 30* | 40.3 | – | |||||
| Malformation syndromes | Congestive HF | 77 | NR | NR | 18* | 23.4 | – | |||||
| Neuromuscular disorders | Congestive HF | 64 | NR | NR | 4* | 6.4 | – | |||||
| Infantile/ idiopathic | Congestive HF | 634 | NR | NR | 63* | 9.9 | – | |||||
| US, Canada, 1990–2007 (18 years) | DCM (0 to < 18 years) | All DCM patients | Congestive HF | 1682 | 777* | 46.2* | 1,205* | 71.6* | – | |||
| Idiopathic DCM | Congestive HF | 1192 | 599* | 50.2 | 894 | 75 | – | |||||
| Neuromuscular disease | Congestive HF | 139 | 5* | 3.6* | 40 | 28.8 | – | |||||
| Familial isolated DCM | Congestive HF | 79 | 35* | 44.3* | 44 | 55.7 | – | |||||
| Myocarditis | Congestive HF | 272 | 138* | 51* | 227 | 83.4 | – | |||||
| US, Canada, 1990–2008 (19 years) | RCM (0 to < 18 years) | All RCM patients | Congestive HF | 152 | 79* | 52* | 56* | 37 | – | |||
| Pure RCM | Congestive HF | 101 | 51* | 51* | 42* | 42 | – | |||||
| RCM/HCM | Congestive HF | 51 | 27* | 53* | 13* | 26 | – | |||||
| Nugent [46] | Retrospective | Australia, 1987–1996 (10 years) | Hospitals (21) | Cardiomyopathies (0 to < 10 years) | All patients | Congestive HF | 314 | 148* | 47.1* | 206* | 65.6* | – |
| DCM | Congestive HF | 184 | 103 | 56 | 165 | 89.7 | – | |||||
| HCM | Congestive HF | 80 | 25 | 31.2 | 6 | 7.5 | – | |||||
| RCM | Congestive HF | 8 | 4 | 50 | 4 | 50 | – | |||||
| Unclassified cardiomyopathy | Congestive HF | 42 | 16 | 38.1 | 31 | 73.8 | – | |||||
| Saji [47] | Retrospective | Japan, 1997–2002 (6 years) | Hospitals (65) | Myocarditis (1 month–17 years) | All patients | HF | 169 | NR | NR | 61 | 36.1 | – |
| Fulminant myocarditis | HF | 64 | NR | NR | 34 | 53.1 | – | |||||
| Acute myocarditis | HF | 89 | NR | NR | 27 | 30.3 | – | |||||
| Chronic myocarditis | HF | 8 | NR | NR | NR | NR | ||||||
| Myocarditis of unknown type | HF | 8 | NR | NR | NR | NR | ||||||
| Soongwang [48] | Retrospective | Thailand, 1996–2000 (5 years) | Hospitals (5) | Myocardial diseases (0.1–14.5 years) | All Patients | Congestive HF | 209 | 117* | 56.0 | 151* | 72.0 | – |
| DCM | Congestive HF | 94 | 51 | 54.3 | 79 | 84.1 | – | |||||
| Acute myocarditis | Congestive HF | 57 | 38 | 66.7 | 45 | 78.9 | – | |||||
| HCM | Congestive HF | 38* | 18 | 47.4 | 17 | 44.7 | – | |||||
| Hypertrophic obstructive cardiomyopathy | Congestive HF | 17* | 8 | 47.1 | 8 | 47.1 | – | |||||
| RCM | Congestive HF | 3 | 2 | 66.7 | 2 | 66.6 | – | |||||
| Tsirka [49] | Retrospective | US, 1990–1999 (10 years) | Hospitals (2) | DCM (0–17.8 years) | – | Congestive HF | 91 | 33* | 36.3* | 72 | 79 | – |
| Rheumatic fever/rheumatic heart disease | ||||||||||||
| Bitar [50] | Retrospective | Lebanon, 1980–1995 (16 years) | Hospital (1) | RF (3–17 years) | – | Acute congestive HF | 91 | 38* | 42* | 40* | 44 | – |
| da Silva [51] | Retrospective | Brazil, 1989–1994 (6 years) | Hospitals (7) | RF (3–17 years) | – | HF | 786 | 382 | 48.7 | 119 | 15.1* | – |
| Gapu [52] | Cross-sectional | Zimbabwe, 2012–2013 (11 months) | Hospitals (2) | Acute RF and/or RHD (1–12 years) | All patients | Any HF | 50 | 32 | 64.0 | 37* | 74* | – |
| Outpatients | Chronic HF | 19 | NR | NR | 15 | 78.9 | – | |||||
| Hospitalized children with acute RF and/or RHD | Congestive HF | 31 | NR | NR | 22 | 71.0* | – | |||||
| Hospitalized with RHD only | AHF | 22 | NR | NR | 20 | – | 90.9 | |||||
| Hospitalized with acute RF only | AHF | 9 | NR | NR | 2 | – | 22.2 | |||||
| Karlassan [53] | Retrospective | Turkey, 1993–1998 (5 years) | Hospital (1) | Acute RF (5–17 years) | – | Congestive HF | 274 | 147 | 53.6 | 4 | 1.5* | – |
| Örün [54] | Retrospective | Turkey, 1980–2009 (30 years) | Hospital (1) | Acute RF (2–15 years) | – | HF | 1115 | 510 | 45.8 | 100 | 9.0* | – |
| Qurashi [55] | Longitudinal (retrospective and prospective) | Saudi Arabia, 1994–2003 (10 years) | Hospital (1) | Acute RF (4–12 years) | – | HF | 83 | NR | NR | 14 | 16.9* | – |
| Rayamajhi [56] | Prospective | Nepal, 2003–2005 (2 years) | Hospital (1) | Acute RF (5–14 years) | – | HF | 51 | NR | NR | 14 | 28 | – |
| IE | ||||||||||||
| Lertsapcharoen [57] | Retrospective | Thailand, 1987–2004 (18 years) | Hospital (1) | IE (2 months–15 years) | – | Congestive HF | 57 | 28 | 49.1* | 15 | 26 | – |
| Marom [30] | Retrospective | Israel, 1992–2004 (12.5 years) | Hospital (1) | IE (0 to < 18 years) | Children with predisposing factors for IE€ | HF | 42 | NR | NR | 10 | 23.8 | – |
| Sadiq [58] | Prospective | Pakistan, 1997–2000 (4 years) | Hospital (1) | IE (4 months–16 years) | All patients | HF | 45 | 15 | 33.3* | 18 | 40 | – |
| Rheumatic heart disease | HF | 24 | 10 | 42 | – | |||||||
| Congenital heart disease | HF | 20 | 8 | 40 | ||||||||
| Myocarditis | HF | 1 | – | – | 0 | 0 | ||||||
| Rhythm and conduction disturbances | ||||||||||||
| Massin [59] | Retrospective | Belgium, 1995–2006 (11 years) | Hospitals (3) | Tachyarrhythmia (0 to < 16 years) | All patients | HF | 250 | 92* | 36.8* | 49 | 19.6* | |
| Infants | HF | 109 | 33 | 30.3* | ||||||||
| Others | ||||||||||||
| Borzouee [60] | Retrospective | Iran, 2001–2003 (2 years) | Hospital (1) | Cardiac problems (1 day–16 years) | HF | 1817 | NR | NR | 25 | 1.4 | ||
ASD atrial septal defect, CVD cardiovascular disease, DCM dilated cardiomyopathy, IE infective endocarditis, HCM hypertrophic cardiomyopathy, HF heart failure, NR not reported, RCM restrictive cardiomyopathy, RF rheumatic fever, RHD rheumatic heart disease, VSD ventricular septal defect
*Calculated data from source article
@In Azhari et al. [31], the patients with pulmonary arterial hypertension is inclusive pf patients with small, medium, or large defects and so is not a stand-alone group
±3549 is the most recent number of total patients with different cardiomyopathies (HCM, DCM, RCM) from PCMR registry studies. However, the total of HCM, DCM, and RCM does not add up to this number (Wilkinson et al. [45]). The data for HF in HCM, DCM, and RCM are taken from different PCMR publications
€Data on 9 children without predisposing factors in Marom et al. [30] are present in “Incidence,” so the total does not add up to 50
Congenital Heart Disease
The prevalence of HF in various congenital heart diseases was reported and summarized from 17 studies, and ranged from 8% of 84 patients in a study from Norway [32] to 82.2% of 73 patients from a study in Nigeria [35] (Supplementary Appendix, Table B2).
Few studies in this disease category focused on specific congenital defects, such as atrial septal defects (ASDs) or VSDs. A prospective study from India reported a prevalence of HF of 40.8% in 476 malnourished children with congenital heart disease aged < 5 years [39], demonstrating the importance of the association between malnutrition and congenital heart disease and consequent sequelae such as HF. Similarly, a Nigerian case–control study reported a prevalence of HF of 82.2% among 73 children with congenital heart disease (90.4% of these 73 children were malnourished) compared with none among 76 children without congenital heart disease (21.1% of these 76 children were malnourished) (Table 3) [35]. Another prospective study, from Nigeria, reported a 64.3% prevalence of HF among 14 children with congenital heart disease and pneumonia compared with 37.4% among 107 children without congenital heart disease, but with pneumonia (Table 3) [37].
In a retrospective, hospital-based study from Jamaica, a HF prevalence of 39.5% was found in 76 patients with trisomy 21 and congenital heart disease [26]. A Nepalese study reported a HF prevalence of 54.8% of 84 pediatric patients aged < 15 years with congenital heart disease (Table 3) [38].
A Norwegian study reported acute heart failure (AHF) as the presenting symptom in 8% of 84 pediatric patients aged 2 weeks–11 years with congenital heart disease (Table 3) [32]. Four of these patients had VSDs, one had an atrioventricular septal defect, and another had coarctation of the aorta. There was one case of endocardial fibroelastosis (Supplementary Appendix Table B2) [32].
Two prospective studies that reported on the prevalence of comorbidities, including HF, in patients with VSDs are summarized in Table 3. In a Japanese prospective study, the prevalence of HF was 46% among 225 Japanese infants < 3 months of age diagnosed with VSDs over a period of 11 years (1986–1996) [33]. HF was most prevalent in patients with perimembranous VSDs and least prevalent among patients with a defect in the muscular septum (81.7 and 1%, respectively) (Table 3). Spontaneous closure of the VSDs occurred in 19 versus 72% of the patients with and without HF, respectively, and surgical closure was required 51 versus 5% of these respective patients [33]. HF was the presenting symptom in 24.6% of the 61 Nigerian children with VSD aged 2–24 months (Table 3), of whom only 20% had spontaneous closure of the VSDs [36].
Two retrospective studies focused on pediatric patients with ASDs are summarized in Table 3. In a hospital-based study from Canada, HF was the presenting symptom in 20% of the 180 ASD patients aged 1 month–16.4 years [34] (Table 3). Another hospital-based study from Saudi Arabia reported that HF was prevalent in 11.6% of 121 ASD patients aged 1 day–11 years [31]. In the Saudi Arabian study, HF prevalence was 18.1% among patients with large defects (≥ 8 mm), 3.7% with medium defects (5–8 mm), and 0% in patients with small defects (3–5 mm) (Table 3) [31].
Cardiomyopathies/Myocarditis
Seven unique studies reported the prevalence of HF in myocardial diseases (cardiomyopathies and myocarditis; Supplementary Appendix, Table B2) and five are summarized below. As shown in Table 3, studies from the Pediatric Cardiomyopathy Registry (PCMR) had the largest population base regarding prevalence of pediatric HF in cardiomyopathies and contains data from multiple centers in the US and Canada. In this registry, the prevalence of HF was 71.6% among 1682 DCM patients, 37% among 152 RCM patients, and 13.5% among 849 hypertrophic cardiomyopathy (HCM) patients [40, 44, 45]. Idiopathic DCM was the most common cause of DCM, and 75% of these patients presented with HF. For HCM, the highest proportion of HF was among those with inborn errors of metabolism (40.3%). The most common etiology for HCM was idiopathic (unknown) (Table 3) [40, 45].
An overall prevalence of HF of 65.6% was reported in an Australian 21-center retrospective study of children < 10 years with different cardiomyopathies [46]. In this study, a prevalence of 89.7% was reported for 184 DCM patients, a prevalence of 50% among 8 RCM patients, and 7.5% among 80 HCM patients (Table 3) [46]. Similarly, a high prevalence of HF (79%) was also observed in 91 DCM patients, in a US-based retrospective study [49]. In another study from 5 hospitals in Thailand that included cardiomyopathy patients aged 0.1–14.5 years, HF was reported in 84.1% of 94 patients with DCM, 66.6% of 3 RCM patients, 47.1% of 17 patients with hypertrophic obstructive cardiomyopathy, and 44.7% of 38 HCM patients [48]. Additionally, HF was present in almost 80% of 57 patients with acute myocarditis [48], which contrasts with a smaller percentage reported in a Japanese study [47]. The Japanese study reported that 53.1% of the 64 patients with fulminant myocarditis had HF at admission, whereas HF was present at admission in only 30.3% of 89 patients with acute myocarditis (Table 3). In this Japanese study, the authors stated that “fulminant myocarditis represents approximately 20–30% of myocarditis cases, and can be clinically differentiated from acute myocarditis by the presence of severe hemodynamic deterioration, cardiogenic shock, severe ventricular dysfunction, and/or refractory life-threatening arrhythmias requiring inotropic support or mechanical cardiopulmonary assist devices” [47]. It is thus unclear why HF was “present” in only 53.1% of patients with fulminant myocarditis [47]. Myocarditis is often associated with viral infection and in this Japanese study, 25% (22 of 89) and 19% (12 of 64) of the total number of acute and fulminant cases were associated with viral pathogens, respectively. Coxsackie A/B and influenza were the most commonly reported infections.
Rheumatic Fever/Rheumatic Heart Disease
Ten studies reported the prevalence of HF in rheumatic fever (RF) and rheumatic heart disease (RHD) ranging from 1.5% in Turkey to 74% in Zimbabwe (Supplementary Appendix, Table B2) [50–56, 61–63].
The retrospective Turkish study had the largest sample size of 1115 acute RF and comprised patients admitted to a single hospital, aged 2–15 years. HF was detected in 9% of the included patients (and in 13.8% of those diagnosed with carditis), over a 30 year period (Table 3) [54]. Another retrospective study from Turkey showed that HF was the presenting symptom in only 1.5% of 274 patients with acute RF (Table 3) [53].
Among all the included studies, the cross-sectional study from Zimbabwe reported the highest proportion of patients with any HF (74% of 50 included patients) among patients with acute RF or RHD. In this study, AHF was present in 71% of the 31 hospitalized patients, and HF was detected in 78.9% of the 19 children seen in outpatient clinics (Table 3) [52]. AHF was reported in 44% of the 91 RF patients at initial presentation, in a retrospective study from Lebanon [50].
Infective Endocarditis
HF is one of the many complications of IE. Three studies reported the incidence of HF in the pediatric population with IE, ranging from 23.8% in Israel to 40% in Pakistan (Table 3) [30, 57, 58]. The retrospective study from Israel reported HF in 23.8% of 42 IE patients who had at least one predisposing factor such as the presence of congenital or acquired heart disease, intravenous therapy within 4 weeks before the onset of endocarditis, and previous invasive procedures (Table 3) [57].
Other Studies
Details of a Belgian study on the prevalence of HF patients admitted for arrhythmias and an Iranian study on the prevalence of HF patients with cardiac problems are also listed in Table 3 [59, 60].
Secondary HF Diagnosis in Non-CVD
Of the 83 identified studies, 24 studies reported HF as secondary diagnosis in non-CVDs.
Incidence of HF Associated with Anthracycline Treatment, HIV/AIDS, and Pneumonia
Three retrospective studies reported an incidence of HF between 1 and 5%, following anthracycline treatment of various childhood cancers (Table 4) [64–66]. In a cohort of 808 children from the Netherlands (aged 0‒16 years), 94% of 17 cases occurred during or within the first year of anthracycline therapy [66]. In a US study, HF developed in 1% of 97 doxorubicin-treated patients aged 7 months–17 years. The one patient who developed HF received a cumulative dose of 450 mg/m2 doxorubicin [64]. The highest rate of 5% was reported in a Japanese study, in which 6 of patients on anthracycline developed HF. In the Japanese study, the mean total anthracycline dose received by these patients was 383 mg/m2 (range: 180–520) [65].
Table 4.
Incidence and prevalence of HF in non-CVD studies
| Incidence of HF secondary to non-CVDs | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study name | Study design | Country, period | Setting | Study population (age range) | Subgroups | Type of HF | Sample size | Gender | HF incidence | |||
| Female (n) | Female (%) | Cases (n) | Incidence (%) | |||||||||
| Hematology/oncology | ||||||||||||
| Berrak [64] | Retrospective | US, 1988–1998 (10 years) | Hospital (1) | Doxorubicin for childhood cancer (7 months–17 years) | – | Congestive HF | 97 | 38 | 39.2* | 1 | 1.0* | |
| Godoy [65] | Retrospective | Japan, 1985–1994 (10 years) | Hospital (1) | Anthracyclines for childhood cancer (5 months–17 years) | – | Congestive HF | 120 | 51 | 42.5* | 6 | 5.0* | |
| van Dalen [66] | Retrospective | Netherlands, 1976–2001 (26 years) | Hospital (1) | Anthracyclines for childhood cancer (< 2 to > 16 years) | Age < 2 to 16 years | Congestive HF | 808* | NR | NR | 17* | 2.1* | |
| HIV/AIDS | ||||||||||||
| Starc [67] | Prospective | US, 1990–1997 (6 years) | Hospitals (10) | Children of HIV-infected mothers (0–14 years) | Infected children with echocardiographic evaluation available (5 years of follow-up) | Congestive HF | 199# | NR | NR | 14 | 14 (cumulative incidence) | |
| Fisher [68] | 7.0 (incidence) | |||||||||||
| Lipshultz [69] | Infants of HIV-infected mothers (0 to < 28 days) | Infected infants (5 years of follow-up) | Congestive HF | 93 | NR | NR | 4 | 5.1(cumulative incidence) | ||||
| Starc [70] | 4.3 (incidence) | |||||||||||
| Uninfected infants(5 years of follow-up) | Congestive HF | 463 | NR | NR | 1 | 0.2(cumulative incidence) | ||||||
| 0.2 (incidence) | ||||||||||||
| Pneumonia | ||||||||||||
| llten [71] | Prospective | Turkey, NR | Hospital (1) | Acute pneumonia (2–24 months) | Congestive HF | 50 | 14 | 28 | 7 | 14 | ||
| Prevalence of HF secondary to non-CVDs | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study name | Study design | Country, period | Setting | Study population (age range) | Subgroups | Type of HF | Sample size | Gender | HF prevalence and distribution in study subgroups | |||
| Female (n) | Female (%) | Cases (n) | Prevalence (%) | Distribution of prevalent cases of HF in study subgroups (%) | ||||||||
| Renal disorders | ||||||||||||
| Duzova [72] | Prospective | Turkey, 2006–2007 (1 year) | Hospitals (17) | AKI (1–18 years) | Newborn (< 1 month) | HF | 154 | NR | NR | 15 | 9.7 | – |
| Gunasekaran [73] | Prospective | India, 2013–2014 (1 year and 6 months) | Hospital (1) | ANS (1–13 years) | PIGN | Congestive HF | 72 | 32* | 44.4* | 8 | 11.1 | – |
| PIGN | PSGN | Congestive HF | 65 | 30* | 46.1* | 8 | 12.3 | – | ||||
| Krishnamurthy [74] | Prospective | India, 2010–2011 (10 months) | Hospital (1) | AKI (1–144 months) | Congestive HF | 54 | 25 | 46.3 | 2 | 3.8 | – | |
| Sarkissian [75] | Prospective | Armenia, 1992–1996 (5 years) | Hospital (1) | Acute PIGN (1 to < 16 years) | Congestive HF | 474 | 166* | 35* | 45 | 10 | – | |
| Vachvanichsanong [76] | Retrospective | Thailand, 1984–2007 (26 years) | Hospital (1) | AKI (0–30 days) | All patients | Congestive HF | 139 | 51 | 36.7* | 17* | 12.2* | – |
| Vachvanichsanong [77] | Retrospective | Thailand, 1982–2004 (22 years and 10 months) | Hospital (1) | Acute renal failure (1 month–16.7 years) | HF | 311 | NR | NR | 26 | 8.4 | – | |
| Wong [78] | Prospective | New Zealand, 2007–2009 (2 years) | Hospitals (country wide) | Acute PSGN (definite/probable) (1.4–14.7 years) | Congestive HF | 176 | 62 | 35.2* | 8 | 4.5* | – | |
| HIV/AIDS | ||||||||||||
| Cunha [79] | Retrospective | Brazil, 1990–2002 (13 years) | Hospital (1) | AIDS (0 to < 13 years) | Congestive HF | 93 | 47 | 50.5 | 12 | 12.9* | – | |
| Diogenes [80] | Prospective | Brazil, 1996–2004 (8 years) | Hospital (NR) | HIV-1 (13 days–13 years) | HIV infected | Congestive HF | 41 | NR | NR | 12 | 29.3* | – |
| HIV seroconverted | Congestive HF | 43 | NR | NR | 0 | 0 | – | |||||
| Dilated cardiomyopathy (as etiology for congestive HF in HIV) | Congestive HF | 12 | 5 | 41.7* | ||||||||
| Okoromah [81] | Case–control | Nigeria, 2004–2007 (3 years) | Hospital (1) | HIV positive (18–144 months) | Congestive HF | 83 | NR | NR | 10 | 12 | – | |
| Community and hospital | HIV negative (18–144 months) | Congestive HF | 83 | NR | NR | 0 | 0 | – | ||||
| Starc [67] | Prospective | US, 1990 to Jan 1997 (6 years) | Hospitals (10) | Children of HIV-infected mothers (0–14 years) | Congestive HF | 201 | NR | NR | 2 | 1 | – | |
| Fisher [68] | ||||||||||||
| Lipshultz [69] | ||||||||||||
| Starc [70] | ||||||||||||
| Hematology/oncology | ||||||||||||
| Karimi [82] | Cross-sectional | Iran, 2007–2010 (3 years) | Hospital (1) | BTM (1–15 years) | All patients | Congestive HF | 328 | NR | NR | 47* | 14.3* | – |
| Other conditions | ||||||||||||
| Ahmed [83] | Retrospective | Scotland, 2002–2008 (6 years) | Hospital (1) | Vitamin D deficiency (2 weeks–14 years) | HF | 160 | 77 | 48.1 | 1 | 0.6 | – | |
| Camilla [84] | Cross-sectional (Pt prevalence) | Italy | Community | Organ failure (0 to < 18 years) | All inhabitants | CHF | 6,47,727 | NR | NR | 21 | 0.0032* | – |
| DCM (as etiology) | 21 | NR | NR | 13 | 62* | |||||||
| Lagunju [85] | Retrospective | Nigeria, 2000–2004 (5 years) | Hospital (1) | Measles (4 months–10 years) | HF | 666 | 319 | 47.9 | 2 | 0.3 | – | |
ANS acute nephrotic syndrome, AIDS acquired immunodeficiency syndrome, AKI acute kidney injury, APGN acute post-infectious glomerulonephritis, APSGN acute post-streptococcal glomerulonephritis, BTM β-thalassemia major, CHF chronic heart disease, CVD cardiovascular disease, DCM dilated cardiomyopathy, HF heart failure, HIV human immunodeficiency virus, NR not reported, PIGN post-infectious glomerulonephritis, PSGN post-streptococcal glomerulonephritis
*Calculated data from source article
#In Starc et al., 2 of the 201 children had congestive HF at presentation and this is captured in “Prevalence,” and in the remaining 199 children congestive HF developed during the study
Multiple publications from the US-based P2C2 HIV study reported the incidence of HF in children of HIV-infected mothers (Table 4) [67–70]. The study categorized children into two groups: group 1 included 199 vertically infected children aged 0.1–14 years with echocardiographic evaluations and group 2 included newborns (93 HIV-infected and 463 uninfected). In group 1, a 5-year cumulative HF incidence of 14% was reported during the 5-year follow-up. In group 2, a 5-year cumulative HF incidence of 5.1 versus 0.2% was reported among the infected and uninfected infants, respectively (Table 4) [67].
In a further prospective Turkish study, 14% of 50 children aged 2–24 months with pneumonia developed HF (Table 4) [71].
Prevalence of HF Associated with Renal Disorders, HIV/AIDS, and Other Conditions
Nine studies reported the HF prevalence in pediatric patients with renal disorders (Supplementary Appendix, Table C2) [72–78, 86, 87]. The prevalence of HF ranged from 3.8% among patients with acute kidney injury (AKI) [74] to 24.1% among those with a primary diagnosis of acute glomerulonephritis (AGN) [87].
HF was diagnosed in 4.5‒11.1% of pediatric patients with acute post-infectious glomerulonephritis (PIGN) [73, 75, 78] and was the most common extra-renal diagnosis in a prospective study from Armenia (10% of 474 pediatric patients (Table 4)) [75]. A large prospective multi-center study from Turkey reported HF as prevalent in 9.7% of 154 children with AKI aged < 1 month old [72], while a prospective study from a hospital in India reported that 3.8% of 54 AKI patients had underlying HF [74]. Two studies from Thailand reported HF as a cause of AKI and acute renal failure in pediatric patients. The first study reported HF as present 12.2% of 139 AKI patients aged ≤ 30 days [76], whereas the second study reported HF in 8.4% of 311 acute renal failure patients aged 1 month–16.7 years (Table 4) [77].
The prevalence of HIV/AIDS patients presenting with HF ranged from 1% in the US [67] to 29.3% in Brazil (Supplementary Appendix, Table C2, Table 4) [80]. Of note, a Brazilian study reported that more than 25% of 41 HIV-infected pediatric patients had HF versus none in 43 HIV-negative patients and that DCM was the main etiology in 41.7% of these HF patients [80].
One study from Iran reported that HF accounted for 14.3% of 328 hospital admissions in β-thalassemia major patients (Table 4) [82]. In other studies, a HF prevalence of 0.3% of 666 patients was reported from a study of the complications of measles [85], 0.6% of 160 (one patient) with vitamin D deficiencies [83], and 5.3% of 38 with foreign body aspiration [88]. Of note, one population-based cross-sectional study carried out to determine the epidemiology of childhood chronic organ failure reported a prevalence of chronic HF of 0.0032%, for 647,727 inhabitants aged < 18 years. Furthermore, DCM was the main cause of HF, being the etiology in 62% of these patients (Table 4) [84].
Discussion
This systematic review and narrative synthesis collates the existing evidence on the incidence and prevalence of HF in the pediatric population (< 18 years) and strengthens the current knowledge on the epidemiology of pediatric HF.
In studies reporting HF as a primary diagnosis, there appears to be a relatively higher incidence of HF in Taiwan (7.4 per 100,000 population) [16] compared with the European (0.87–3 per 100,000 population) pediatric population [13–15]. Possible reasons for the variation in the reported incidence rates include different definitions of HF used across studies, statistical methods (crude incidence [16] versus adjusted incidence [14, 15] rates reported), definitions of the study populations (e.g., defined population such as children with ‘heart muscle disease’ (cardiomyopathy/myocarditis, etc.) [13] versus overall HF diagnosis rates [14–16]). Furthermore, as the Asian data were from one single Taiwanese study, the results may not be generalizable to other regions of the Asian continent.
Variation within the same geographic regions was also apparent. The slight difference in incidence reported from Germany [14, 15] and the UK and Ireland study [13] may be due to differences in HF etiology, with the German studies not specifying etiology, but the UK and Ireland study including cases mainly due to heart muscle diseases. However, even within the UK and Ireland, the incidence varied, with rates from Ireland and Scotland ranging from 0.11 to 1.27 per 100,000, respectively [13].
A wider variation was observed in Nigerian studies, which showed HF prevalence ranging from 2.7 to 9% in children presenting to the emergency room or admitted into pediatric wards [18, 19, 22, 23]. The differences in HF prevalence from different Nigerian centers could be due to differences in the study designs, patient selection, diagnosis and definition of HF, and the different time periods in which the studies were conducted. Similar differences in diagnosis and definition may underlie the differences in the rate of HF prevalence associated with RF reported in two Turkish studies (9% [54] and 1.5% [53]).
Overall, comparisons between studies and countries need to be interpreted with caution as the studies were highly heterogeneous and reported diverse etiologies across countries.
Leading causes of pediatric HF reported from lower income countries were lower respiratory tract infections and severe anemia [18, 19, 22, 23]. Inadequate treatment for conditions such as malaria, which can cause severe anemia and associated HF, may be a reason for the above finding [18, 19, 22, 23]. In comparison, studies from the developed world reported congenital heart disease and cardiomyopathies as two leading causes of HF in the pediatric population, with other major causes including rhythm and conduction disturbances and acquired heart diseases [13, 17].
More than half of the studies included in the review summarized evidence of HF incidence/prevalence diagnosed secondary to another CVD. Only three studies on the incidence of HF secondary to CHD were identified in this review, including two studies with rare etiology (secondary to Scimitar syndrome [25] and trisomy 21 with congenital heart disease [26]). It is widely recognized that many infants with left heart obstructive lesions and large VSDs will present with HF [89], but data on the incidence are lacking. Most reports of HF prevalence were in the context of congenital heart disease, particularly VSD and ASD [31–34, 36, 90]. Similarly, this is likely due to a reporting bias, as some of the other congenital heart diseases that are associated with HF may be under-reported.
A high HF prevalence was observed when congenital heart disease co-existed with conditions such as malnutrition, pneumonia, and trisomy 21 [26, 35, 37, 39, 91]. Findings from these studies also suggest that spontaneous closure of ASDs/VSDs was less common in young children with co-existing HF than in those without HF [33, 36, 55].
Evidence suggests that approximately 40% of children with symptomatic cardiomyopathy develop HF of such severity that it leads to transplantation or death [92]. This review provides information on the incidence and prevalence of HF in different types of cardiomyopathies, including DCM, HCM, and RCM and myocarditis [13, 40, 44–49]. We found that the proportion of HF was highest among patients with DCM, followed by patients with RCM and then HCM [13, 40, 44–46, 48, 49]. Additionally, we found that HF is a major complication in conditions such as acute rheumatic fever, rheumatic heart disease, and IE [30, 53, 56].
The third disease category summarized evidence of pediatric HF incidence/prevalence diagnosed secondary to non-CVDs. Anthracyclines are used widely for the treatment of numerous childhood malignancies and have known cardiac toxicity. The data indicate that the risk of developing HF is related to the treatment dose or mode of delivery (pulsatile versus continuous). Many patients developed HF within the first year of treatment [64–66], and that younger children were more vulnerable to anthracycline cardiotoxicity [64–66].
The close relationship between HF and renal disorders is reflected in our findings. The studies on renal disorders included patients with AKI, acute renal failure, or with AGN due to PIGN. While HF was a presenting symptom in patients with PIGN, it was reported as an etiology for AKI or acute renal failure, along with other conditions [72–78, 86, 87]. Another major area in which HF was reported was among pediatric HIV/AIDS patients. The studies reported a wide range of prevalence from different geographic locations owing to the fact that the included patients were in different stages of HIV, across different pediatric ages, and it was noted that the rate of cardiac complications increases as these patients progress to AIDS [67, 79–81, 93, 94].
Limitations
In all three disease categories, a lack of large population-based studies and the heterogeneity of study design limit the scope for generalizations and comparisons. Therefore, differences between studies and countries need to be interpreted with caution. Furthermore, much of the evidence was derived from hospital-based studies, introducing a greater potential for selection bias compared with population-based studies.
The large proportion of full-text studies (63 of 77) that were graded as ‘poor’ according to the Downs and Black checklist suggests the need for studies with improved design and methodology. Furthermore, the development of standardized definitions of pediatric HF would help in reducing heterogeneity, facilitating higher quality comparisons of outcomes between studies.
The search strategy did not include the various comorbid conditions as dedicated search terms. Therefore, relevant articles could have been missed. Nevertheless, we believe that the comprehensive nature of our methodology ensured that the prevalence/incidence of HF in all major CVDs and non-CVDs in the pediatric population is captured.
Conclusion
In summary, this systematic review provides valuable information and insights into the incidence and prevalence of HF in children and adolescents over the last 20 years (1996–2016) and strengthens the current knowledge on the epidemiology of pediatric HF. While a substantial number of studies were identified, more large population-based studies are needed to consolidate the evidence base. Moreover, there is a need to use standard definitions for HF in future pediatric epidemiological studies, to assess the true differences in incidence and prevalence among various studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Paul Coyle and Laoighse Mulrane (employees of Novartis) for providing writing/editorial assistance. All authors reviewed and critically revised the manuscript for content and approved the final version of the manuscript for submission.
Funding
This study, and the Open Access fee, was funded by Novartis.
Compliance with Ethical Standards
Conflict of interest
Robert Shaddy, Joseph Rossano, and Michael Burch are consultants of Novartis; Aneesh Thomas George, Eimear Nic Lochlainn, Lalit Thakur, Rumjhum Agrawal, Susan Solar-Yohay, Fabian Chen, and Thomas Severin are employees of Novartis; Thomas Jaecklin is an employee of Shire International GmbH; Robert Shaddy received grants/research support from NIH/NHLBI.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s00246-017-1787-2) contains supplementary material, which is available to authorized users.
References
- 1.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, ESC Committee for Practice Guidelines ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Heart Failure Society of America. Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WH, Teerlink JR, Walsh MN. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1–e194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Kantor PF, Lougheed J, Dancea A, McGillion M, Barbosa N, Chan C, Dillenburg R, Atallah J, Buchholz H, Chant-Gambacort C, Conway J, Gardin L, George K, Greenway S, Human DG, Jeewa A, Price JF, Ross RD, Roche SL, Ryerson L, Soni R, Wilson J, Wong K, Children. ’. s Heart Failure Study Group Presentation, diagnosis, and medical management of heart failure in children: Canadian Cardiovascular Society guidelines. Can J Cardiol. 2013;29:1535–1552. doi: 10.1016/j.cjca.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13:368–378. doi: 10.1038/nrcardio.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaturvedi V, Saxena A. Heart failure in children: clinical aspect and management. Indian J Pediatr. 2009;76:195–205. doi: 10.1007/s12098-009-0050-0. [DOI] [PubMed] [Google Scholar]
- 7.WHO . Cardiac failure in children. 17th expert committee on the selection and use of essential medicines. Geneva: WHO; 2008. [Google Scholar]
- 8.Madriago E, Silberbach M. Heart failure in infants and children. Pediatr Rev. 2010;31:4–11. doi: 10.1542/pir.31-1-4. [DOI] [PubMed] [Google Scholar]
- 9.Kay JD, Colan SD, Graham TP., Jr Congestive heart failure in pediatric patients. Am Heart J. 2001;142:923–928. doi: 10.1067/mhj.2001.119423. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JPT, Green S (eds) (2011) Cochrane handbook for systematic reviews of interventions. 5.1.0 (updated March 2011)
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Commun Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrews RE, Fenton MJ, Ridout DA, Burch M. New-onset heart failure due to heart muscle disease in childhood: a prospective study in the United kingdom and Ireland. Circulation. 2008;117:79–84. doi: 10.1161/CIRCULATIONAHA.106.671735. [DOI] [PubMed] [Google Scholar]
- 14.Neumann T, Biermann J, Erbel R, Neumann A, Wasem J, Ertl G, Dietz R. Heart failure: the commonest reason for hospital admission in Germany: medical and economic perspectives. Dtsch Arztebl Int. 2009;106:269–275. doi: 10.3238/arztebl.2009.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt S, Hendricks V, Griebenow R, Riedel R. Demographic change and its impact on the health-care budget for heart failure inpatients in Germany during 1995–2025. Herz. 2013;38:862–867. doi: 10.1007/s00059-013-3955-3. [DOI] [PubMed] [Google Scholar]
- 16.Tseng CH. The age- and sex-specific incidence and medical expenses of heart failure hospitalization in 2005 in Taiwan: a study using data from the National Health Insurance. J Am Geriatr Soc. 2010;58:611–613. doi: 10.1111/j.1532-5415.2010.02755.x. [DOI] [PubMed] [Google Scholar]
- 17.Massin MM, Astadicko I, Dessy H. Epidemiology of heart failure in a tertiary pediatric center. Clin Cardiol. 2008;31:388–391. doi: 10.1002/clc.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adekanmbi AF, Ogunlesi TA, Olowu AO, Fetuga MB. Current trends in the prevalence and aetiology of childhood congestive cardiac failure in Sagamu. J Trop Pediatr. 2007;53:103–106. doi: 10.1093/tropej/fml064. [DOI] [PubMed] [Google Scholar]
- 19.Animasahun A, Itiola J, Falase B. Congestive cardiac failure among Nigerian children; pattern and outcome. Int Cardiovasc Res J. 2015;9:164–168. [Google Scholar]
- 20.Jimenez-Garcia R, Esteban-Vasallo MD, Rodriguez-Rieiro C, Hernandez-Barrera V, Dominguez-Berjon MA, Carrasco Garrido P, Lopez de Andres A, Cameno Heras M, Iniesta Fornies D, Astray-Mochales J. Coverage and predictors of vaccination against 2012/13 seasonal influenza in Madrid, Spain: analysis of population-based computerized immunization registries and clinical records. Hum Vaccin Immunother. 2014;10:449–455. doi: 10.4161/hv.27152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Rieiro C, Dominguez-Berjon MF, Esteban-Vasallo MD, Sanchez-Perruca L, Astray-Mochales J, Fornies DI, Ordonez DB, Jimenez-Garcia R. Vaccination coverage against 2009 seasonal influenza in chronically ill children and adults: analysis of population registries in primary care in Madrid (Spain) Vaccine. 2010;28:6203–6209. doi: 10.1016/j.vaccine.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Lagunju IA, Omokhodion SI. Childhood heart failure in Ibadan. West Afr J Med. 2003;22:42–45. doi: 10.4314/wajm.v22i1.27978. [DOI] [PubMed] [Google Scholar]
- 23.Oyedeji OA, Oluwayemi IO, Oyedeji AT. Heart failure in Nigerian children. Cardiology. 2010;5:18–22. doi: 10.3923/tcard.2010.18.22. [DOI] [Google Scholar]
- 24.Hong SJ, Choi HJ, Kim YH, Hyun MC, Lee SB, Cho JY. Clinical features and surgical outcomes of complete transposition of the great arteries. Korean J Pediatr. 2012;55:377–382. doi: 10.3345/kjp.2012.55.10.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Najm HK, Williams WG, Coles JG, Rebeyka IM, Freedom RM. Scimitar syndrome: twenty years’ experience and results of repair. J Thorac Cardiovasc Surg. 1996;112:1161–1168. doi: 10.1016/S0022-5223(96)70129-0. [DOI] [PubMed] [Google Scholar]
- 26.Tomlinson TW, Scott CH, Trotman HL. Congenital cardiovascular lesions in children with trisomy 21 at the Bustamante Hospital for Children. Cardiol Young. 2010;20:327–331. doi: 10.1017/S1047951110000417. [DOI] [PubMed] [Google Scholar]
- 27.Rialon KL, Murillo R, Fevurly RD, Kulungowski AM, Zurakowski D, Liang M, Kozakewich HP, Alomari AI, Fishman SJ. Impact of screening for hepatic hemangiomas in patients with multiple cutaneous infantile hemangiomas. Pediatr Dermatol. 2015;32:808–812. doi: 10.1111/pde.12656. [DOI] [PubMed] [Google Scholar]
- 28.LaPage M, Rhee EK, Canter CE. Tachyarrhythmias after pediatric heart transplantation. J Heart Lung Transplant. 2010;29:273–277. doi: 10.1016/j.healun.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Murtuza B, Fenton M, Burch M, Gupta A, Muthialu N, Elliott MJ, Hsia TY, Tsang VT, Kostolny M. Pediatric heart transplantation for congenital and restrictive cardiomyopathy. Ann Thorac Surg. 2013;95:1675–1684. doi: 10.1016/j.athoracsur.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Marom D, Ashkenazi S, Samra Z, Birk E. Infective endocarditis in previously healthy children with structurally normal hearts. Pediatr Cardiol. 2013;34:1415–1421. doi: 10.1007/s00246-013-0665-9. [DOI] [PubMed] [Google Scholar]
- 31.Azhari N, Shihata MS, Al-Fatani A. Spontaneous closure of atrial septal defects within the oval fossa. Cardiol Young. 2004;14:148–155. doi: 10.1017/S1047951104002069. [DOI] [PubMed] [Google Scholar]
- 32.Meberg A, Otterstad JE, Froland G, Hals J, Sorland SJ. Early clinical screening of neonates for congenital heart defects: the cases we miss. Cardiol Young. 1999;9:169–174. doi: 10.1017/S1047951100008398. [DOI] [PubMed] [Google Scholar]
- 33.Miyake T, Shinohara T, Nakamura Y, Fukuda T, Tasato H, Toyohara K, Tanihira Y. Spontaneous closure of ventricular septal defects followed up from < 3 months of age. Pediatr Int. 2004;46:135–140. doi: 10.1046/j.1442-200x.2004.01858.x. [DOI] [PubMed] [Google Scholar]
- 34.Najm HK, Williams WG, Chuaratanaphong S, Watzka SB, Coles JG, Freedom RM. Primum atrial septal defect in children: early results, risk factors, and freedom from reoperation. Ann Thorac Surg. 1998;66:829–835. doi: 10.1016/S0003-4975(98)00607-9. [DOI] [PubMed] [Google Scholar]
- 35.Okoromah CA, Ekure EN, Lesi FE, Okunowo WO, Tijani BO, Okeiyi JC. Prevalence, profile and predictors of malnutrition in children with congenital heart defects: a case-control observational study. Arch Dis Child. 2011;96:354–360. doi: 10.1136/adc.2009.176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sadoh WE. Natural history of ventricular septal defects in Nigerian children. S Afr J Child Health. 2010;4:16–19. [Google Scholar]
- 37.Sadoh WE, Osarogiagbon WO. Underlying congenital heart disease in Nigerian children with pneumonia. Afr Health Sci. 2013;13:607–612. doi: 10.4314/ahs.v13i3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah GS, Singh MK, Pandey TR, Kalakheti BK, Bhandari GP. Incidence of congenital heart disease in tertiary care hospital. Kathmandu Univ Med J (KUMJ) 2008;6:33–36. [PubMed] [Google Scholar]
- 39.Vaidyanathan B, Nair SB, Sundaram KR, Babu UK, Shivaprakasha K, Rao SG, Kumar RK. Malnutrition in children with congenital heart disease (CHD) determinants and short term impact of corrective intervention. Indian Pediatr. 2008;45:541–546. [PubMed] [Google Scholar]
- 40.Alvarez JA, Orav EJ, Wilkinson JD, Fleming LE, Lee DJ, Sleeper LA, Rusconi PG, Colan SD, Hsu DT, Canter CE, Webber SA, Cox GF, Jefferies JL, Towbin JA, Lipshultz SE. Competing risks for death and cardiac transplantation in children with dilated cardiomyopathy: results from the pediatric cardiomyopathy registry. Circulation. 2011;124:814–823. doi: 10.1161/CIRCULATIONAHA.110.973826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colan SD, Lipshultz SE, Lowe AM, Sleeper LA, Messere J, Cox GF, Lurie PR, Orav EJ, Towbin JA. Epidemiology and cause-specific outcome of hypertrophic cardiomyopathy in children: findings from the Pediatric Cardiomyopathy Registry. Circulation. 2007;115:773–781. doi: 10.1161/CIRCULATIONAHA.106.621185. [DOI] [PubMed] [Google Scholar]
- 42.Everitt MD, Sleeper LA, Lu M, Canter CE, Pahl E, Wilkinson JD, Addonizio LJ, Towbin JA, Rossano J, Singh RK, Lamour J, Webber SA, Colan SD, Margossian R, Kantor PF, Jefferies JL, Lipshultz SE. Recovery of echocardiographic function in children with idiopathic dilated cardiomyopathy: results from the pediatric cardiomyopathy registry. J Am Coll Cardiol. 2014;63:1405–1413. doi: 10.1016/j.jacc.2013.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, Messere J, Cox GF, Lurie PR, Hsu D, Canter C, Wilkinson JD, Lipshultz SE. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867–1876. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 44.Webber SA, Lipshultz SE, Sleeper LA, Lu M, Wilkinson JD, Addonizio LJ, Canter CE, Colan SD, Everitt MD, Jefferies JL, Kantor PF, Lamour JM, Margossian R, Pahl E, Rusconi PG, Towbin JA. Outcomes of restrictive cardiomyopathy in childhood and the influence of phenotype: a report from the Pediatric Cardiomyopathy Registry. Circulation. 2012;126:1237–1244. doi: 10.1161/CIRCULATIONAHA.112.104638. [DOI] [PubMed] [Google Scholar]
- 45.Wilkinson JD, Westphal JA, Bansal N, Czachor JD, Razoky H, Lipshultz SE. Lessons learned from the Pediatric Cardiomyopathy Registry (PCMR) Study Group. Cardiol Young. 2015;25(Suppl 2):140–153. doi: 10.1017/S1047951115000943. [DOI] [PubMed] [Google Scholar]
- 46.Nugent AW, Daubeney PE, Chondros P, Carlin JB, Cheung M, Wilkinson LC, Davis AM, Kahler SG, Chow CW, Wilkinson JL, Weintraub RG, National Australian Childhood Cardiomyopathy Study The epidemiology of childhood cardiomyopathy in Australia. N Engl J Med. 2003;348:1639–1646. doi: 10.1056/NEJMoa021737. [DOI] [PubMed] [Google Scholar]
- 47.Saji T, Matsuura H, Hasegawa K, Nishikawa T, Yamamoto E, Ohki H, Yasukochi S, Arakaki Y, Joo K, Nakazawa M. Comparison of the clinical presentation, treatment, and outcome of fulminant and acute myocarditis in children. Circ J. 2012;76:1222–1228. doi: 10.1253/circj.CJ-11-1032. [DOI] [PubMed] [Google Scholar]
- 48.Soongswang J, Sangtawesin C, Sittiwangkul R, Wanitkun S, Muangmingsuk S, Sopontammarak S, Klungratana C, Kangkagate C. Myocardial diseases in Thai children. J Med Assoc Thai. 2002;85(Suppl 2):S648–S657. [PubMed] [Google Scholar]
- 49.Tsirka AE, Trinkaus K, Chen SC, Lipshultz SE, Towbin JA, Colan SD, Exil V, Strauss AW, Canter CE. Improved outcomes of pediatric dilated cardiomyopathy with utilization of heart transplantation. J Am Coll Cardiol. 2004;44:391–397. doi: 10.1016/j.jacc.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 50.Bitar FF, Hayek P, Obeid M, Gharzeddine W, Mikati M, Dbaibo GS. Rheumatic fever in children: a 15-year experience in a developing country. Pediatr Cardiol. 2000;21:119–122. doi: 10.1007/s002469910017. [DOI] [PubMed] [Google Scholar]
- 51.da Silva CH. Rheumatic fever: a multicenter study in the state of Sao Paulo. Pediatric Committee–Sao Paulo Pediatric Rheumatology Society. Rev Hosp Clin Fac Med Sao Paulo. 1999;54:85–90. doi: 10.1590/S0041-87811999000300004. [DOI] [PubMed] [Google Scholar]
- 52.Gapu P, Bwakura-Dangarembizi M, Kandawasvika G, Kao D, Bannerman C, Hakim J, Matenga JA. Rheumatic fever and rheumatic heart disease among children presenting to two referral hospitals in Harare, Zimbabwe. S Afr Med J. 2015;105:384–388. doi: 10.7196/SAMJ.9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karaaslan S, Oran B, Reisli I, Erkul I. Acute rheumatic fever in Konya, Turkey. Pediatr Int. 2000;42:71–75. doi: 10.1046/j.1442-200x.2000.01180.x. [DOI] [PubMed] [Google Scholar]
- 54.Orun UA, Ceylan O, Bilici M, Karademir S, Ocal B, Senocak F, Ozgur S, Dogan V, Yilmaz O, Keskin M. Acute rheumatic fever in the Central Anatolia Region of Turkey: a 30-year experience in a single center. Eur J Pediatr. 2012;171:361–368. doi: 10.1007/s00431-011-1555-x. [DOI] [PubMed] [Google Scholar]
- 55.Qurashi MA. The pattern of acute rheumatic fever in children: experience at the children’s hospital, Riyadh, Saudi Arabia. J Saudi Heart Assoc. 2009;21:215–220. doi: 10.1016/j.jsha.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rayamajhi A, Sharma D, Shakya U. Clinical, laboratory and echocardiographic profile of acute rheumatic fever in Nepali children. Ann Trop Paediatr. 2007;27:169–177. doi: 10.1179/146532807X220271. [DOI] [PubMed] [Google Scholar]
- 57.Lertsapcharoen P, Khongphatthanayothin A, Chotivittayatarakorn P, Thisyakorn C, Pathmanand C, Sueblinvong V. Infective endocarditis in pediatric patients: an eighteen-year experience from King Chulalongkorn Memorial Hospital. J Med Assoc Thai. 2005;88(Suppl 4):S12–S16. [PubMed] [Google Scholar]
- 58.Sadiq M, Nazir M, Sheikh SA. Infective endocarditis in children–incidence, pattern, diagnosis and management in a developing country. Int J Cardiol. 2001;78:175–182. doi: 10.1016/S0167-5273(01)00374-6. [DOI] [PubMed] [Google Scholar]
- 59.Massin MM, Benatar A, Rondia G. Epidemiology and outcome of tachyarrhythmias in tertiary pediatric cardiac centers. Cardiology. 2008;111:191–196. doi: 10.1159/000121603. [DOI] [PubMed] [Google Scholar]
- 60.Borzouee M, Jannati M. Distribution and Characteristics of the Heart Disease in Pediatric Age Group in Southern Iran. Int Cardivasc Res J. 2008;2:48–51. [Google Scholar]
- 61.Bejiqi R, Retkoceri R, Bejiqi H, Zeka N, Gerguri A, Kelmendi M. OP-017 valvular heart lesion after attack of the rheumatic fever disease 11 years experience in single centre. Int J Cardiol. 2012;155(Suppl 1):S3. doi: 10.1016/S0167-5273(12)70011-6. [DOI] [Google Scholar]
- 62.Prakoso R, Roebiono PS, Lilyasar IO. Incidence and pattern of rheumatic heart disease among children at National Cardiovascular Center Harapan Kita, Jakarta. Ann Pediatr Cardiol Conf. 2014;7:S42–S43. [Google Scholar]
- 63.Thakur JS, Negi PC, Ahluwalia SK, Vaidya NK. Epidemiological survey of rheumatic heart disease among school children in the Shimla Hills of northern India: prevalence and risk factors. J Epidemiol Commun Health. 1996;50:62–67. doi: 10.1136/jech.50.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berrak SG, Ewer MS, Jaffe N, Pearson P, Ried H, Zietz HA, Benjamin RS. Doxorubicin cardiotoxicity in children: reduced incidence of cardiac dysfunction associated with continuous-infusion schedules. Oncol Rep. 2001;8:611–614. doi: 10.3892/or.8.3.611. [DOI] [PubMed] [Google Scholar]
- 65.Godoy LY, Fukushige J, Igarashi H, Matsuzaki A, Ueda K. Anthracycline-induced cardiotoxicity in children with malignancies. Acta Paediatr Jpn. 1997;39:188–193. doi: 10.1111/j.1442-200X.1997.tb03579.x. [DOI] [PubMed] [Google Scholar]
- 66.van Dalen EC, van der Pal HJ, Kok WE, Caron HN, Kremer LC. Clinical heart failure in a cohort of children treated with anthracyclines: a long-term follow-up study. Eur J Cancer. 2006;42:3191–3198. doi: 10.1016/j.ejca.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 67.Starc TJ, Lipshultz SE, Easley KA, Kaplan S, Bricker JT, Colan SD, Lai WW, Gersony WM, Sopko G, Moodie DS, Schluchter MD. Incidence of cardiac abnormalities in children with human immunodeficiency virus infection: the prospective P2C2 HIV study. J Pediatr. 2002;141:327–334. doi: 10.1067/mpd.2002.126301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fisher SD, Easley KA, Orav EJ, Colan SD, Kaplan S, Starc TJ, Bricker JT, Lai WW, Moodie DS, Sopko G, Lipshultz SE. Mild dilated cardiomyopathy and increased left ventricular mass predict mortality: the prospective P2C2 HIV Multicenter Study. Am Heart J. 2005;150:439–447. doi: 10.1016/j.ahj.2005.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lipshultz SE, Easley KA, Orav EJ, Kaplan S, Starc TJ, Bricker JT, Lai WW, Moodie DS, McIntosh K, Schluchter MD, Colan SD. Left ventricular structure and function in children infected with human immunodeficiency virus: the prospective P2C2 HIV Multicenter Study. Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection (P2C2 HIV) Study Group. Circulation. 1998;97:1246–1256. doi: 10.1161/01.CIR.97.13.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Starc TJ, Lipshultz SE, Kaplan S, Easley KA, Bricker JT, Colan SD, Lai WW, Gersony WM, Sopko G, Moodie DS, Schluchter MD. Cardiac complications in children with human immunodeficiency virus infection. Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection (P2C2 HIV) Study Group, National Heart, Lung, and Blood Institute. Pediatrics. 1999;104:e14. doi: 10.1542/peds.104.2.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ilten F, Senocak F, Zorlu P, Tezic T. Cardiovascular changes in children with pneumonia. Turk J Pediatr. 2003;45:306–310. [PubMed] [Google Scholar]
- 72.Duzova A, Bakkaloglu A, Kalyoncu M, Poyrazoglu H, Delibas A, Ozkaya O, Peru H, Alpay H, Soylemezoglu O, Gur-Guven A, Bak M, Bircan Z, Cengiz N, Akil I, Ozcakar B, Uncu N, Karabay-Bayazit A, Sonmez F. Etiology and outcome of acute kidney injury in children. Pediatr Nephrol. 2010;25:1453–1461. doi: 10.1007/s00467-010-1541-y. [DOI] [PubMed] [Google Scholar]
- 73.Gunasekaran K, Krishnamurthy S, Mahadevan S, Harish BN, Kumar AP. Clinical characteristics and outcome of post-infectious glomerulonephritis in children in Southern India: a prospective study. Indian J Pediatr. 2015;82:896–903. doi: 10.1007/s12098-015-1752-0. [DOI] [PubMed] [Google Scholar]
- 74.Krishnamurthy S, Narayanan P, Prabha S, Mondal N, Mahadevan S, Biswal N, Srinivasan S. Clinical profile of acute kidney injury in a pediatric intensive care unit from Southern India: a prospective observational study. Indian J Crit Care Med. 2013;17:207–213. doi: 10.4103/0972-5229.118412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sarkissian A, Papazian M, Azatian G, Arikiants N, Babloyan A, Leumann E. An epidemic of acute postinfectious glomerulonephritis in Armenia. Arch Dis Child. 1997;77:342–344. doi: 10.1136/adc.77.4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vachvanichsanong P, McNeil E, Dissaneevate S, Dissaneewate P, Chanvitan P, Janjindamai W. Neonatal acute kidney injury in a tertiary center in a developing country. Nephrol Dial Transplant. 2012;27:973–977. doi: 10.1093/ndt/gfr477. [DOI] [PubMed] [Google Scholar]
- 77.Vachvanichsanong P, Dissaneewate P, Lim A, McNeil E. Childhood acute renal failure: 22-year experience in a university hospital in southern Thailand. Pediatrics. 2006;118:e786–e791. doi: 10.1542/peds.2006-0557. [DOI] [PubMed] [Google Scholar]
- 78.Wong W, Lennon DR, Crone S, Neutze JM, Reed PW. Prospective population-based study on the burden of disease from post-streptococcal glomerulonephritis of hospitalised children in New Zealand: epidemiology, clinical features and complications. J Paediatr Child Health. 2013;49:850–855. doi: 10.1111/jpc.12295. [DOI] [PubMed] [Google Scholar]
- 79.Cunha Mdo C, Siqueira Filho AG, Santos SR, Abreu TF, Oliveira RH, Baptista DM, Dantas MC, Carvalho MF, Guedes LG. AIDS in childhood: cardiac involvement with and without triple combination antiretroviral therapy. Arq Bras Cardiol. 2008;90:11–17. doi: 10.1590/s0066-782x2008000100003. [DOI] [PubMed] [Google Scholar]
- 80.Diogenes MS, Succi RC, Machado DM, Moises VA, Novo NF, Carvalho AC. [Cardiac longitudinal study of children perinatally exposed to human immunodeficiency virus type 1] Arq Bras Cardiol. 2005;85:233–240. doi: 10.1590/S0066-782X2005001700002. [DOI] [PubMed] [Google Scholar]
- 81.Okoromah CAN, Ojo OO, Ogunkunle OO. Cardiovascular dysfunction in human immunodeficiency virus (HIV)-infected children in a sub-saharan african country: comparative cross-sectional observational Study. J Trop Pediatr. 2012;58:3–11. doi: 10.1093/tropej/fmr009. [DOI] [PubMed] [Google Scholar]
- 82.Karimi M, Emadmarvasti V, Hoseini J, Shoja L. Major causes of hospital admission in Beta thalassemia major patients in southern iran. Iran J Pediatr. 2011;21:509–513. [PMC free article] [PubMed] [Google Scholar]
- 83.Ahmed SF, Franey C, McDevitt H, Somerville L, Butler S, Galloway P, Reynolds L, Shaikh MG, Wallace AM. Recent trends and clinical features of childhood vitamin D deficiency presenting to a children’s hospital in Glasgow. Arch Dis Child. 2011;96:694–696. doi: 10.1136/adc.2009.173195. [DOI] [PubMed] [Google Scholar]
- 84.Camilla R, Magnetti F, Barbera C, Bignamini E, Riggi C, Coppo R. Children with chronic organ failure possibly ending in organ transplantation: a survey in an Italian region of 5,000,000 inhabitants. Acta Paediatr. 2008;97:1285–1291. doi: 10.1111/j.1651-2227.2008.00854.x. [DOI] [PubMed] [Google Scholar]
- 85.Lagunju IA, Orimadegun AE, Oyedemi DG. Measles in Ibadan: a continuous scourge. Afr J Med Med Sci. 2005;34:383–387. [PubMed] [Google Scholar]
- 86.Becquet O, Pasche J, Gatti H, Chenel C, Abely M, Morville P, Pietrement C. Acute post-streptococcal glomerulonephritis in children of French Polynesia: a 3-year retrospective study. Pediatr Nephrol. 2010;25:275–280. doi: 10.1007/s00467-009-1325-4. [DOI] [PubMed] [Google Scholar]
- 87.Olowu WA. Systemic complications of acute glomerulonephritis in Nigerian children. Niger Postgrad Med J. 2002;9:83–87. [PubMed] [Google Scholar]
- 88.Li Y, Wu W, Yang X, Li J. Treatment of 38 cases of foreign body aspiration in children causing life-threatening complications. Int J Pediatr Otorhinolaryngol. 2009;73:1624–1629. doi: 10.1016/j.ijporl.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 89.Odland HH, Thaulow EM. Heart failure therapy in children. Expert Rev Cardiovasc Ther. 2006;4:33–40. doi: 10.1586/14779072.4.1.33. [DOI] [PubMed] [Google Scholar]
- 90.Harshangi SV, Itagi LN, Patil V. Clinical study of congenital heart disease in infants in tertiary care hospital. J Pharm Sci Innov. 2013;2:15–18. [Google Scholar]
- 91.Parvathy U, Balakrishnan KR, Ranjith MS, Saldanha R, Sai S, Vakamudi M. Surgical experience with congenital heart disease in Down’s syndrome. Indian Heart J. 2000;52:438–441. [PubMed] [Google Scholar]
- 92.Madriago E, Silberbach M. Heart failure in infants and children. Pediatr Rev. 2010;31:4–12. doi: 10.1542/pir.31-1-4. [DOI] [PubMed] [Google Scholar]
- 93.Herdy GV, Pinto CA, Lopes VG, Ribeiro RP, Gomes IM, Tchou HY, Melo R, Kurdian B, Junior Tavares Pde A. Study of the cardiac alterations in HIV-infected children consequent to the antiretroviral therapy. Prospective study of 47 cases. Arq Bras Cardiol. 2003;80:311–320. doi: 10.1590/S0066-782X2003000300007. [DOI] [PubMed] [Google Scholar]
- 94.Dimitriu AG, Jitareanu C, Dimitriu L. Cardiac involvement, major problem in human immunodeficiency virus infection (HIV) in children. 5th Congress of the European Academy of Paediatric Societies, EAPS 2014 Barcelona Spain. Arch Dis Child. 2014;99:A320. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


