Abstract
AIM
To investigate the signaling pathways involved in the relaxin (RLX) effects on ileal preparations from mice through mechanical and electrophysiological experiments.
METHODS
For mechanical experiments, ileal preparations from female mice were mounted in organ baths containing Krebs-Henseleit solution. The mechanical activity was recorded via force-displacement transducers, which were coupled to a polygraph for continuous recording of isometric tension. Electrophysiological measurements were performed in current- and voltage-clamp conditions by a microelectrode inserted in a single smooth muscle cell (SMC) of the ileal longitudinal layer. Both the membrane passive properties and inward voltage-dependent L-type Ca2+ currents were recorded using suitable solutions and voltage stimulation protocols.
RESULTS
Mechanical experiments showed that RLX induced a decay of the basal tension and a reduction in amplitude of the spontaneous contractions. The effects of RLX were partially reduced by 1H-[1,2,4]oxadiazolo[4,3-a]-quinoxalin-1-one (ODQ) or 9-cyclopentyladenine mesylate (9CPA), inhibitors of guanylate cyclase (GC) and adenylate cyclase (AC), respectively, and were abolished in the concomitant presence of both drugs. Electrophysiological experiments demonstrated that RLX directly influenced the biophysical properties of ileal SMCs, decreasing the membrane conductance, hyperpolarizing the resting membrane potential, reducing the L-type calcium current amplitude and affecting its kinetics. The voltage dependence of the current activation and inactivation time constant was significantly speeded by RLX. Each electrophysiological effect of RLX was reduced by ODQ or 9CPA, and abolished in the concomitant presence of both drugs as observed in mechanical experiments.
CONCLUSION
Our new findings demonstrate that RLX influences ileal muscle through a dual mechanism involving both GC and AC.
Keywords: Relaxin, Gastrointestinal motility, Smooth muscle, AC/cAMP, GC/cGMP
Core tip: Up to now relaxin (RLX) was described to act only through the NO/guanylate cyclase (GC)/cGMP/PKG pathway in different gastrointestinal tracts. The results of the present study, achieved on mice ileal preparations and carried out by a combined mechanical and electrophysiological approach, demonstrate for the first time that both GC and adenylate cyclase are involved in the effects of RLX in this intestinal region. The activation of this dual signaling pathway by RLX might represent a reinforcing (redundant) myorelaxant mechanism in the ileum, underlying the physiological importance of the hormone and leading to speculate translational perspectives in the treatment of intestinal dysmotilities.
INTRODUCTION
It is widely recognized that the effects of the peptide hormone relaxin (RLX) are not limited to reproductive organs[1-3]. Receptors of the RLX family peptides have been indeed identified in both reproductive and non-reproductive tissues[4-6], including human and rodent intestine[7-9]. Concerning on the molecular mechanism of action of RLX on the target cells, multiple intracellular signaling systems appear to be engaged[10,11]. In some cells, such as the smooth muscle of reproductive tissue, the effects of the hormone RLX are due to the activation of adenylate cyclase (AC)[12-16] leading to increase of the cyclic adenosine monophosphate (cAMP) levels. However, in some other different preparations, such as in vascular and intestinal smooth muscle, the mechanism of action activated by RLX occurs through the endogenous nitric oxide (NO) synthesis[17-23] and leads to increased levels of cyclic guanosine monophosphate (cGMP).
Particularly, in the gastrointestinal tract of mice, RLX has been reported to modulate gastric and colonic smooth muscle activity through the L-arginine/NO pathway[9,24-26]. NO, synthesized under the catalytic action of nitric oxide synthases (NOS)[27], is known to cause gastrointestinal relaxation[28] and its altered production has been reported to be involved in different motor disorders[29]. However, Bani et al[30] raised the possibility that in the ileum, at variance with gastric and colonic preparations, the NO signaling pathway could not be the only one activated by the hormone, suggesting the involvement of additional pathways that have not been fully elucidated yet in this preparation. In this regard, the involvement of AC, in addition to guanylate cyclase (GC), in the effects of RLX has been reported in rat and guinea pig airways[31] as well as in human vascular cells[10].
On these grounds, the present study was designed to investigate for the first time, if AC/cAMP, besides the NO/CG/cGMP, could be a further signaling pathway involved in the effects of RLX on ileal smooth muscle. To evaluate this possibility, we performed experiments by a combined mechanical and electrophysiological approach. Particularly, for the first time we investigated the ability of RLX to induce changes of smooth muscle cell (SMC) biophysical properties in ileal preparations through the electrophysiological technique. Moreover, we studied the effects of RLX on the L-type Ca2+ current, ICa,L, that is supposed to be the main voltage-dependent source for Ca2+ entry useful for the activation of the SMC contractile machinery[32]. This knowledge may contribute to a better understanding of the mechanisms engaged by the hormone to modulate intestinal motility and may help in the design of further therapeutic strategies for the treatment of motor disorders.
MATERIALS AND METHODS
Animals
Experiments were performed on 8- to 12-wk-old female mice (CD1 Swiss strain; Envigo, Udine, Italy). The animals were kept under the following conditions: 12-h light/12-h dark photoperiod, constant temperature (21 ± 1 °C), and standard laboratory feed.
After 1 wk of acclimatization, the mice underwent assessment of the phase of the estrous cycle by light microscopic examination of vaginal smears stained with Papanicolaou stain, according to the method of Austin and Rowlands[33]. Only mice in proestrus or estrus (i.e., the estrogen-dominated phases of the ovarian cycle) entered the experiments. This choice was made because estrogen is known to favor RLX responsiveness of several target organs and tissues[34]. The mice were killed by prompt cervical dislocation to minimize animal suffering. According to the procedure reported in previous paper[30] the mouse abdomen was opened, the distal ileum was removed and its content was cleaned with a physiological solution.
Mechanical experiments
For the functional studies, segments of the distal ileum (within 30 mm from the ileocaecal valve) were isolated and placed in Krebs-Henseleit solution, which consisted of 118 mmol/L NaCl, 4.7 mmol/L KCl, 1.2 mmol/L MgSO4, 1.2 mmol/L KH2PO4, 25 mmol/L NaHCO3, 2.5 mmol/L CaCl2, and 10 mmol/L glucose pH 7.4, and bubbled with 95% O2/5% CO2. Two whole full-thickness, transversely cut, ileal segments (10 mm in length) were dissected from each animal and placed in 5-mL organ baths containing Krebs-Henseleit solution gassed with 95% O2/5% CO2, while the temperature was maintained within a range of 37 ± 0.5 °C. The preparations were mounted in the direction of the longitudinal muscle layer and the continuous recording of isometric tension was achieved by a force displacement transducer (FT03; Grass Instrument) coupled to a polygraph (7K; Grass Instrument). The preparations were allowed to equilibrate for at least 1 h under an initial load of 1.5 g. During this period, the preparations underwent repeated and prolonged washes with Krebs-Henseleit solution to avoid accumulation of metabolites in the organ baths.
Electrophysiological experiments
For electrophysiological recording a full-thickness ileal strip was distended and pinned to a Sylgard (Dow Corning, Midland, MI, United States)-coated dissecting Petri dish filled with Krebs-Henseleit solution. First, we pinned the mucosal side up to dissect carefully the mucosa and submucosa away under a dissecting microscope. The residual tissue was re-pinned serosal side up and the connective tissue was removed in order to expose the smooth muscle layer. The obtained tissue was finally pinned, serosal side up, in the recording chamber with a Sylgard floor and a glass microelectrodes was inserted in a cell of the longitudinal smooth muscle layer. The tissue was continuously superfused (Pump 33, Harvard Apparatus) at a rate of 1.8 mL min-1 with the external solution used.
Conventional high resistance microelectrodes were used according to the previously published procedure[9,35]. Microelectrodes were obtained by using a micropipette vertical puller (Narishige PC-10) from borosilicate glass (GC 100-7.5; Clark) and were filled with the following control physiological solution (mmol/L): KCl 130, NaH2PO4 10, CaCl2 0.2, EGTA 1, MgATP 5 and HEPES/KOH 10. Once filled, the pipette resistance measured 60-70 MΩ. The pH was set to 7.4 with NaOH and to 7.2 with tetraethylammonium (TEA) hydroxide for bath and pipette solution, respectively.
To record RMP in current-clamp and the cell membrane passive properties in voltage-clamp mode we used the extracellular Krebs-Henseleit solution. To record only Ca2+ currents we bathed the ileal muscle strip in a Na+- and K+-free high-TEA solution (mmol/L): 10 CaCl2, 145 TEABr and 10 HEPES and the microelectrode was filled by the following solution (mmol/L): 150 CsBr, 5 MgCl2, 10 EGTA, 10 HEPES. Nifedipine (10 μmol/L) was used to block L-type Ca2+ current, ICa,L. Heptanol (1 mmol/L) was consistently used to block gap junctional currents of the functional syncytium and to record membrane passive properties and ionic currents elicited only from the impaled cell[35,36].
The current amplitude was always normalized to cell linear capacitance Cm (in pA/pF) to consent the evaluation of test current recorded from cells of different size; in fact, Cm is usually considered an index of cell-surface area presuming that membrane-specific capacitance has a constant value of 1 μF/cm2.
Pulse protocols of stimulation
By using the current clamp mode of our amplifier and stimulus waveform with I = 0 pA we recorded the SMC resting membrane potential, before and after drug stimulation. Protocol of recording consisted of 8 episodes, having a sampling interval of 20 ms and a total duration of 5.29 min. The inter-episode interval was 1 min.
The membrane passive properties, membrane conductance (Gm) and Cm, were consistently estimated in voltage clamp mode by applying two step voltage pulses 75 ms long to -80 and -60 mV starting from a holding potential (HP) of -70 mV. Their values were calculated as detailed in Squecco et al[37]. Always in this mode, ionic currents were evoked by the following pulse protocols: ICa,L activation was evoked on the SMC held at -80 mV, and 1-s long step pulses were applied in 10-mV increments from -70 to 50 mV. For each episode, the first 100 ms of the trace had a sampling interval of 50 μs, and the remaining of 1 s. The interval between episodes was 20 s to allow recovery. ICa,L inactivation was investigated by a two-pulse protocol with a 1-s pre-pulse to different voltages followed by 1-s test pulse to 10 mV as reported in our previous works[9,35]. When we applied the two-pulse protocol, we used again a 20-s interval between stimulating episodes to allow recovery. Capacitive, linear leak and voltage-independent ionic currents were cancelled on-line using the P/4 procedure.
To evaluate the steady-state ionic current activation through the voltage dependent channels we used the following equation:
Ia(V) = Gmax (V-Vrev)/{1 + exp[(Va-V)/ka]} and the following was used for the steady-state current inactivation: Ih(V) = I/{1 + exp[-(Vh-V)/kh]}, where Gmax represents the maximal conductance for Ia; Vrev is the apparent reversal potential; Va and Vh are the voltages causing the half-maximal activation and inactivation, respectively; ka and kh are steepness factors.
Drugs
We used tetrodotoxin (TTX, 1 μmol/L), nifedipine (10 µmol/L), 1H-[1,2,4] oxadiazolo[4,3-α]-quinoxalin-1-one (ODQ, 1 μmol/L), 9-cyclopentyladenine mesylate (9CPA, 100 μmol/L) and relaxin (RLX, 50 nmol/L). All drugs were obtained from Sigma Chemical (St. Louis, MO, United States), except for highly purified porcine RLX (2500-3000 U/mg) that was generously provided by Dr. O. D. Sherwood (University of Illinois, Urbana, IL, United States). Solutions were prepared on the day of the experiment, except for TTX, for which a stock solution was kept stored at -20 °C. Drugs concentrations are given as final bath concentrations. The concentration of porcine RLX used was in the range of that formerly demonstrated to be effective in murine gastrointestinal preparations[9,30,38-40]. The choice of GC and AC inhibitors was based on previous literature[9,41] and their related concentration was in the range of that previously proven to be effective[9,42].
Data analysis and statistical tests
As previously reported[30,38], in the mechanical experiments, amplitude of spontaneous contractions and variations in basal tension are expressed as grams.
For electrophysiological experiments, mathematical and statistical analysis of data was performed by SigmaPlot (Jandel Scientific) and pClamp9 (Axon Instruments). Statistical analysis was performed by Student’s t-test to compare two experimental groups or one-way ANOVA followed by Newman-Keuls post-test for multiple comparisons. The number of muscle preparations/cells is designated by n in the results. Results are means ± SE. P ≤ 0.05 was considered statistically significant.
RESULTS
Mechanical experiments
Effects of relaxin on the mechanical activity: At basal tension, ileal preparations (n = 24, from 12 mice) showed spontaneous and rhythmic phasic contractions (mean amplitude 0.60 ± 0.03 g) (Figure 1), that were unaffected by 1 μmol/L TTX (mean amplitude 0.61 ± 0.03 g), indicating their myogenic nature, and reduced by 10 μmol/L nifedipine (0.35 ± 0.05 g).
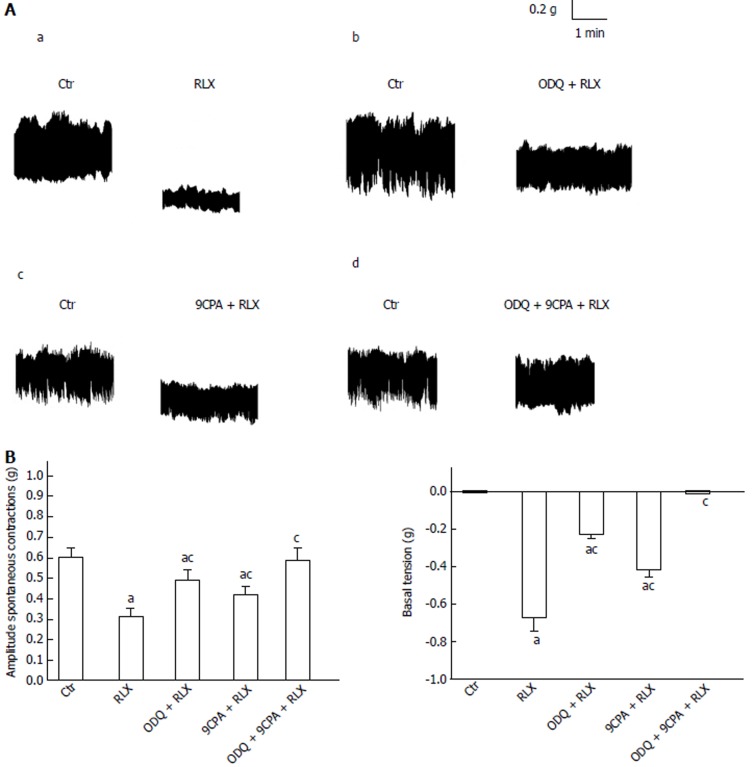
Figure 1.
Influence of oxadiazolo[4,3-a]-quinoxalin-1-one and 9-cyclopentyladenine mesylate on relaxin effects on the mechanical activity of the mouse ileum. A: Typical tracings showing the spontaneous activity of the ileal segments (Ctr, left hand recordings of each panel). RLX (50 nmol/L), 30 min from its application to the bath medium, causes a decrease in amplitude of the spontaneous contractions and a decay of the basal tension (a, right trace) compared to its control (a, left trace). Both these effects are reduced when RLX is added to the bath medium in the presence of 1 µmol/L ODQ (b, right trace) or 100 µmol/L 9CPA (c, right trace) and abolished in the presence of ODQ plus 9CPA (d, right trace). Data of each panel (a-d) are obtained from different preparations; B: Bar charts showing the influence of ODQ and 9CPA on RLX effects on the mean amplitude of spontaneous contractions (left hand histogram) and basal tension (right hand histogram) with respect to the control. All values are means ± SE of 6 preparations from 3 mice. aP < 0.05 vs Ctr; cP < 0.05 vs RLX (one-way ANOVA and Newman-Keuls post-test); RLX: Relaxin; ODQ: Oxadiazolo[4,3-a]-quinoxalin-1-one; 9CPA: 9-cyclopentyladenine.
Addition of RLX (50 nmol/L) to the bath medium (n = 18, from 9 mice) caused a clear-cut decay of the basal muscle tension (62.3% ± 2.2%) and a reduction in amplitude (36.1% ± 1.8%) of the spontaneous contractions (Figure 1). The action of the hormone on basal tension and on spontaneous activity was just evident 10 min and 20 min, respectively, after its addition to the bath medium. The influence of RLX on ileal motility was long lasting, because its effects could still be revealed 1 h after the peptide addition to the organ baths (longer time not observed). The effects of the hormone on basal muscle tension and spontaneous contractions were unaffected by TTX (60% ± 3.1% and 37.2% ± 1.5%, respectively) whereas they were no longer detectable 10 min after the addition of nifedipine (P > 0.05 in respect to nifedipine alone) to the bath medium.
Addition of the GC inhibitor ODQ (1 μmol/L) to the bath medium did not induce significant effects (P > 0.05). In the presence of ODQ (1 μmol/L) both spontaneous contractions and muscle basal tension were only reduced by 50 nmol/L RLX (Figure 1), thus suggesting the contribution of other signaling paths in the hormone action. We therefore tested the possible involvement of the AC pathway, employing the AC inhibitor 9CPA (100 μmol/L): 9CPA had not “per se” significant effects (P > 0.05) but reduced the actions of RLX although it was unable to completely restore the control mechanical activity (Figure 1). RLX added to the bath medium in the presence of ODQ plus 9CPA had no longer effects on both muscle tension and spontaneous contractions (Figure 1).
Electrophysiological experiments
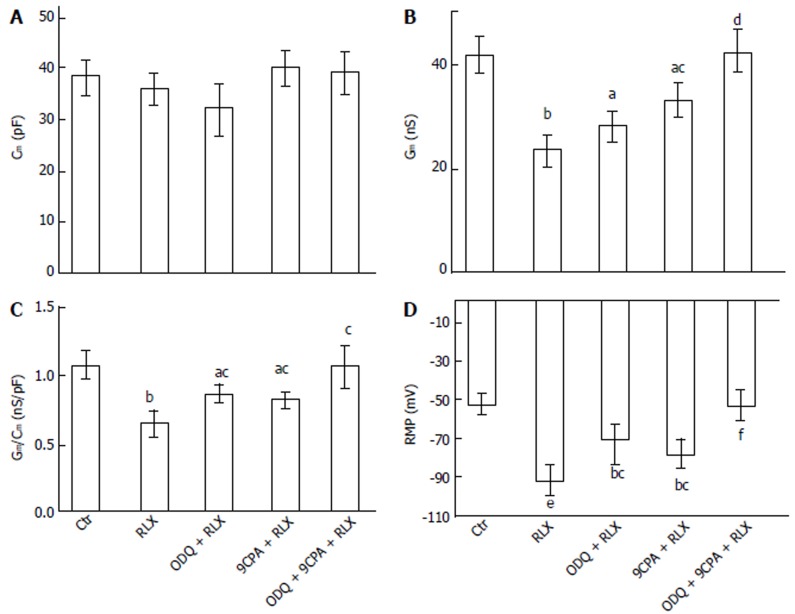
Influence of relaxin on the smooth muscle cell membrane passive properties: To further test the possible direct action of RLX on ileal SMC, we investigated the effects of the hormone on the SMC resting membrane properties. RLX did not alter significantly Cm (Figure 2A) but decreased Gm and the specific conductance (Gm/Cm) values (Figure 2B-C). Addition of ODQ (1 μmol/L) or 9CPA (100 μmol/L) to the external recording medium did not affect significantly the membrane passive properties compared to the untreated control (P > 0.05). The effects of RLX on Gm and Gm/Cm were partially inhibited when was added in the presence of ODQ or 9CPA, and were completely abolished in their concomitant presence, reaching values similar to those observed in control condition (Figure 2B and C).
Figure 2.
Effect of relaxin on smooth muscle cell membrane passive properties and resting membrane potential (RMP) of the mouse ileum. Influence of relaxin on Cm (A), Gm (B), Gm/Cm (C) and RMP (D) alone (RLX) or in the presence of ODQ (ODQ + RLX), 9CPA (9CPA + RLX) or both of them (ODQ + 9CPA + RLX). aP < 0.05, bP < 0.01, eP < 0.001 vs control SMC (Ctr); cP < 0.05, dP < 0.01, fP < 0.001 vs RLX. In each experimental condition, data are mean ± SE from n = 22 cells (11 strips from 5 mice) (one-way ANOVA and Newman-Keuls post-test), RLX: Relaxin; ODQ: Oxadiazolo[4,3-a]-quinoxalin-1-one; 9CPA: 9-cyclopentyladenine.
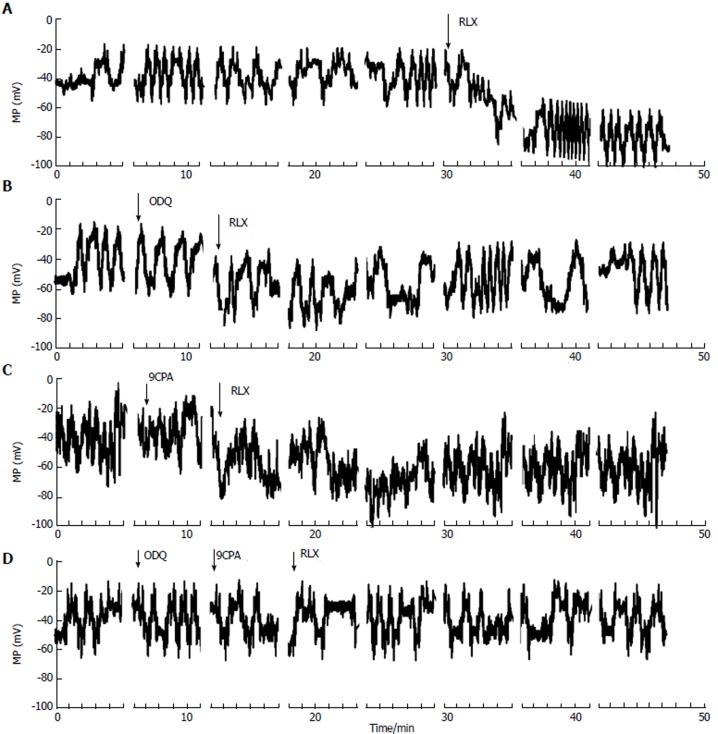
Effects of relaxin on smooth muscle cell resting membrane potential: We then evaluated the RLX effects on resting membrane potential (RMP) of SMCs, to test its role on cell excitability. In control condition, the membrane potential showed spontaneous irregular slow waves (n = 22 cells; 11 strips from 5 mice) (Figure 3). These waves of the membrane potential lasted 0.4-1.1 min and showed alternating periods of very low (0.5 ± 0.1 wave/min) and higher (1.1 ± 0.2 wave/min) frequencies lasting 5-6 and 15 min, respectively. The maximal hyperpolarization value of the waves was considered as the RMP. The mean amplitude of the irregular waves was 37.5 ± 8 mV.
Figure 3.
Effects of relaxin, in the absence or in the presence of oxadiazolo[4,3-a]-quinoxalin-1-one and 9-cyclopentyladenine, on membrane potential recorded from smooth muscle cell of the mouse ileum. Typical tracings of membrane potential from four different single ileal SMCs. A-D: Irregular waves of MP recorded in control condition. RLX addition (arrow) induced a persistent hyperpolarization occurring right after about 2 min from its application (A); The hyperpolarization caused by RLX was reduced in the presence of ODQ (B), 9CPA (C) and was abolished when both blockers were concomitantly present in the bath solution (D). Breaks in X-axis (and consequently in the MP traces) indicate the end of each episode, RLX: Relaxin; ODQ: Oxadiazolo[4,3-a]-quinoxalin-1-one; 9CPA: 9-cyclopentyladenine.
After RLX addition to the bath solution, the irregular waves were still recorded but the membrane potential showed a hyperpolarization trend that was already appreciable 1-3 min after its application (Figure 3A). The irregular waves were always recorded during the hyperpolarization due to RLX, and maintained a similar amplitude, duration and frequency.
The statistical analysis of the RMP values is reported as histograms in Figure 2D for each experimental condition. Noteworthy, the mean value of control RMP was -53.4 ± 7 mV and it reached -91.1 ± 12 mV in the presence of RLX, clearly indicating a statistically significant hyperpolarization. Furthermore, being the size of the irregular wave similar to the control value (41.5 ± 8 mV), the positive peak of the waves was different in RLX compared to control (Figure 3): it reached a mean value of -20 ± 5 (range from -30 to -12) mV in control and -49.6 ± 16 (range from -75 to -25 mV) mV after RLX addition. This observation indicates that RLX does not influence the amplitude and the frequency of the slow potentials and suggests that RLX may hamper the probability to reach a depolarized voltage threshold suitable for ion channels activation. Consequently, since the slow voltage waves arise from a voltage value that is under threshold for the voltage-dependent L-type Ca2+ current activation (see below) and their amplitude in this hyperpolarized state is not altered, we could deduce that they are not due to voltage-dependent channels opening.
In order to investigate the possible effectors involved in these RLX effects, we used the GC and AC inhibitors also in this set of experiments. Again, addition of ODQ or 9CPA alone to the external solution did not modify significantly the wave amplitude, duration, frequency and the resting membrane potential compared to the untreated control (Figure 3) (P > 0.05). RLX added at least 5 min after ODQ or 9CPA, hyperpolarized the RMP to a lesser extent (Figure 3B and C) compared to RLX alone, suggesting the involvement of both GC and AC pathways. Once again, in agreement with the mechanical results, RLX added in the presence of both inhibitors was no more able to induce hyperpolarization (Figures 2D and 3D).
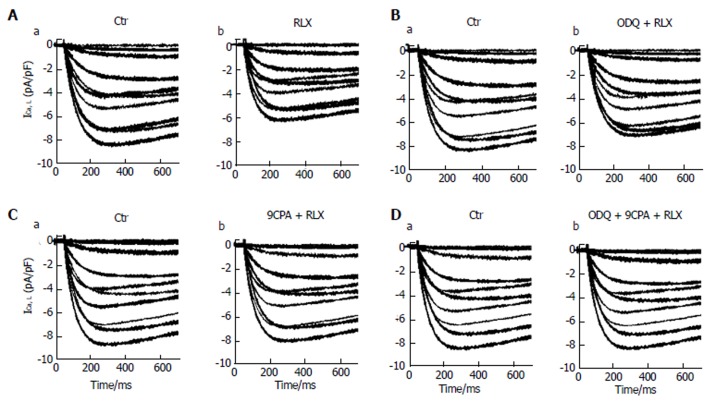
Influence of relaxin on the voltage-dependent Ca2+ current: The effects of the hormone were then evaluated on the inward voltage-dependent Ca2+ currents, supposed to be the chief path for Ca2+ entry useful to trigger the contractile machinery in intestinal SMCs. Notably, in our experiments we did not consistently record TTX-sensitive Na+ current. Accordingly, to better put in evidence the appearance of voltage-dependent Ca2+ currents, we used appropriate filling pipette solution and a bath recording high TEA-Ca2+ solution (see Materials and Methods section). The time course of typical current traces recorded from a control cell and after RLX addition is shown in Figure 4 (Aa and Ab, respectively). Since this kind of current had a high voltage threshold (about -30 mV), a slow activation and inactivation, and was blocked by nifedipine we assumed that it was an L-type Ca2+ current (ICa,L). No T-type Ca2+ current was revealed in our preparations. It can be clearly seen (Table 1, Figures 4A and 5A) that RLX reduced the maximal peak current amplitude (P < 0.05) and the time to peak (tp) (P < 0.01). Moreover, the hormone shifted the mean voltage eliciting the maximal peak current size (from 17.5 ± 2 to 20.1 ± 2 mV).
Figure 4.
Relaxin affects L-type Ca2+ currents in smooth muscle cell of the mouse ileum. A: Representative time course of L-type Ca2+ currents (ICa,L) recorded in the high TEA-Ca2+ solution from a control cell (a) and after RLX addition (b); B-D: ICa,L tracings in the control (a) and in the presence of RLX (b) added 15 min after ODQ (B) or 9CPA(C) or both ODQ + 9CPA (D). Current records shown in the different panels (A-D) are obtained from four different SMCs. The first 700 ms of the current response are displayed.
Table 1.
Boltzmann parameters of ICa,L activation and inactivation in a smooth muscle cell in control (Ctr), in the presence of relaxin (RLX) alone or RLX with ODQ (ODQ + RLX), 9CPA (9CPA + RLX) and ODQ + 9CPA (ODQ + 9CPA + RLX)
| Parameters | Ctr | RLX | ODQ + RLX | 9CPA + RLX | ODQ + 9CPA + RLX |
| ICa,L/Cm (pA/pF) | 8.8 ± 0.5 | 7.2 ± 0.7a | 7.9 ± 0.6 | 8.2 ± 0.6 | 8.7 ± 0.6c |
| Vp (mV) | 17.5 ± 2 | 20.1 ± 2 | 17.9 ± 2 | 18.1 ± 1 | 17.5 ± 1.9 |
| tp (ms) | 270.8 ± 22 | 194.1 ± 19b | 230.8 ± 15ac | 244.8 ± 22c | 259.8 ± 24c |
| Va (mV) | 4.0 ± 0.3 | 8.9 ± 0.6e | 6.0 ± 0.6bc | 4.9 ± 0.5ad | 4.3 ± 0.4d |
| ka (mV) | 6.5 ± 0.4 | 6.0 ± 0.5 | 6.2 ± 0.4 | 6.4 ± 0.4 | 6.4 ± 0.4 |
| Vrev (mV) | 67.2 ± 4 | 68.8 ± 4 | 68.1 ± 5 | 67.5 ± 4 | 67.8 ± 4 |
| Vh (mV) | -14.4 ± 2 | -10.9 ± 1a | -12.4 ± 1 | -12.6 ± 2 | -14.0 ± 1c |
| kh (mV) | 7.6 ± 0.5 | 7.5 ± 0.5 | 7.6 ± 0.6 | 7.6 ± 0.7 | 7.6 ± 0.6 |
| I2h (Norm) | 0.09 ± 0.02 | 0.23 ± 0.02b | 0.14 ± 0.01ad | 0.13 ± 0.03d | 0.09 ± 0.03f |
| V2h (mV) | 22.0 ± 2 | 22.3 ± 1.9 | 21.8 ± 2.1 | 21.5 ± 2 | 21.9 ± 1.8 |
| k2h (mV) | 8.0 ± 0.7 | 6.3 ± 0.6a | 7.1 ± 0.7 | 7.5 ± 0.3c | 7.9 ± 0.2c |
In the U shaped inactivation curves (h) the best fit to the data is obtained by the sum of two Boltzmann terms whose parameters are indicated with the subscript h for the term at negative potential, and 2h for the term at positive potential; the amplitude of the normalized second term, I2h (Norm), was evaluated at 50 mV.
P < 0.05,
P < 0.01 and
P < 0.001 RLX compared to the related control;
P < 0.05,
P < 0.01 and
P < 0.001 compared to RLX. Data for Ctr ( = 67 cells, 25 strips from 12 mice), for RLX (n = 20 cells, 10 strips from 5 mice), for ODQ + RLX (n = 18 cells, 9 strips from 5 mice), for 9CPA + RLX (n = 15 cells, 8 strips from 5 mice) and for ODQ + 9CPA + RLX (n = 16 cells, 9 strips from 5 mice), RLX: Relaxin; ODQ: Oxadiazolo[4,3-a]-quinoxalin-1-one; 9CPA: 9-cyclopentyladenine.
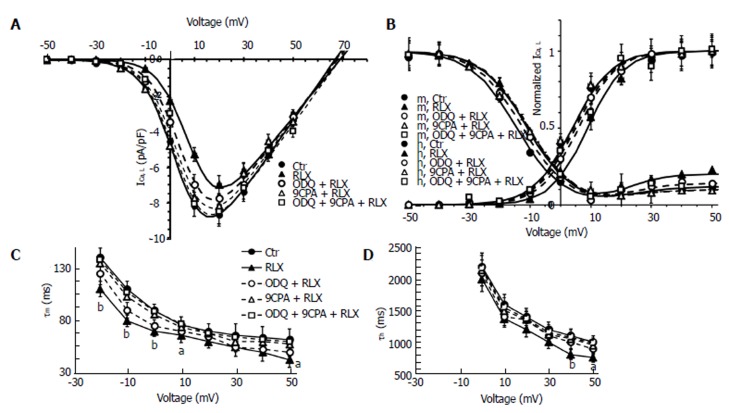
Figure 5.
Relaxin affects ICa,L voltage dependence and kinetics in smooth muscle cell of the mouse ileum. A: I-V plots of L-type Ca2+ currents (ICa,L) recorded in Ctr (filled circles), RLX (filled up triangles), ODQ + RLX (open circles), 9CPA + RLX (open up triangles) and ODQ+9CPA + RLX (open squares) from experiments as in Figure 4. Statistical significance not shown in the plot for clarity; B: Steady-state ICa,L activation (m) and inactivation curves (h) in all the conditions tested; the best fit to the inactivation data is obtained by the sum of two Boltzmann terms. A and B: The Boltzmann fits for Ctr and RLX are superimposed to the experimental data as continuous lines, those related to ODQ, 9CPA and ODQ + 9CPA as dashed lines. Data are mean ± SE. Boltzmann parameters, statistical analysis and number of analyzed cells are listed in Table 1. Current values are normalized to cell capacitance (pA/pF). C and D: Voltage dependence of the time constant of ICa,L activation (τm) and inactivation (τ h). aP < 0.05 and bP < 0.01 RLX vs control SMC (Ctr). P < 0.05 for 9CPA + RLX and for 9CPA + ODQ + RLX vs RLX alone for the -20, -10 and 0 mV (τm data); P < 0.05 for 9CPA + RLX, ODQ + RLX and 9CPA + ODQ + RLX vs RLX alone for +40 and +50 mV (τ h data). Statistical significance for the inhibitors not shown in the panels for clarity (one-way ANOVA and Newman-Keuls post-test), RLX: Relaxin; ODQ: Oxadiazolo[4,3-a]-quinoxalin-1-one; 9CPA: 9-cyclopentyladenine.
To evaluate the general behavior of the phenomenon, we plotted our experimental data related to all the experiments done in the I-V relationship, reporting the normalized mean ICa,L maximal amplitude for any voltage step applied (Figure 5A). The analysis of the I-V plot confirmed that RLX caused the decrease of current amplitude and a shift of the Va and Vh values towards more positive potential (Table 1). The ODQ or 9CPA pre-treatment did not completely abolish the effect of RLX, but slightly reduced the size decrease (Figure 4) and the alteration of the Va and Vh values. These parameters completely recovered the control values in the presence of ODQ plus 9CPA (Table 1, Figure 5C and D).
The voltage dependence of the current activation time constant, τm, (Figure 5C) as well as that of inactivation, τh, (Figure 5D) was significantly speeded by RLX. Particularly, the ODQ pre-treatment did not entirely eliminate the effects of RLX on τm,, that did not result significantly different to that recorded in the presence of RLX alone, whereas 9CPA resulted more effective in counteracting RLX effects (Figure 5C) which were abolished in the concomitant presence of the inhibitors. Instead, the action of RLX on τh was similarly prevented by each of the inhibitors (Figure 5D).
The effects of RLX and those of the effector inhibitors on ICa,L voltage dependence were analyzed by the steady-state activation and inactivation curves (Figure 5B): RLX shifted the activation and inactivation curves towards more positive potentials making this latter more U-shaped for depolarized voltages. All the Boltzmann parameters were only partially reverted to control values when RLX was added after ODQ or 9CPA and recovered when the hormone was applied in the concomitant presence of both the inhibitors. ODQ or 9CPA added alone to the bath solution did not cause appreciable differences in current records compared to control records (P > 0.05). The best fit parameters, statistical analysis and number of analyzed cells are reported in Table 1.
DISCUSSION
The present mechanical and electrophysiological results indicate, for the first time, that the hormone RLX is able to influence the ileal smooth muscle activity from female mice acting through a dual pathway, where both CG and AC activity are involved.
We previously observed in the same kind of preparation[30] that RLX increased the expression of both inducible and endothelial nitric oxide synthases but its depressant effects were not completely abolished by the NO synthesis inhibitor L-NNA. These observations indicated that the inhibitory effects of the hormone mainly occurred through the activation of intrinsic NO biosynthesis but, at the same time, suggested the involvement of additional signaling pathways. Accordingly, the mechanical results of the present study show that the decay of the muscular tension and the decrease in amplitude of the spontaneous contractions caused by RLX are reduced not only by ODQ but also by 9CPA leading to the novel finding that, other than GC, AC too is involved in the effects of the hormone on ileal motility. The involvement of other signaling pathways, besides the NO/cGMP, may account for the site-related actions of RLX in the modulation of the activity of the different GI tract in mice[26]. In this view, regional differences among mouse small intestine tracts concerning the nitrergic mechanisms mediating inhibitory control of longitudinal SMC contraction have been reported: the effects of NO donors were indeed completely abolished by ODQ in jejunum but only partially eliminated in ileum, suggesting the involvement of an additional pathway[43].
Another novel aspect of this research is represented by the electrophysiological results demonstrating, for the first time in ileal preparations, that RLX was able to act directly on the SMC, by influencing its biophysical membrane features and the voltage-dependent L-type Ca2+ channels properties. Regarding to the biophysical properties, the observations that RLX decreases the total and specific membrane conductance and hyperpolarizes the membrane potential definitely strengthen each other, since the decrease of the resting membrane conductance can indicate the reduction of aspecific cationic influxes that, in turn, hampering the entrance of positive charges may favor RMP hyperpolarization. They are also in good agreement with the depressant mechanical effects observed in this study, since changes of the RMP value lead to the reduction of the contractile activity that strictly depends on membrane potential (electro-mechanical coupling)[44]. However, the signaling cascade associated to membrane hyperpolarization is complicated and still debated; the GC/cGMP/PKG- and AC/cAMP/PKA-mediated smooth muscle hyperpolarization is also controversial[44-46]. In the present experiments, the observation that RLX was able to induce hyperpolarization and that both ODQ and 9CPA prevented this effect actually supports the involvement of GC and AC paths on the RMP modulation.
A remarkable consequence of changes in RMP can be relayed on L-type Ca2+ channels activation, since a greater depolarization is necessary to reach their activation voltage threshold. In this view, it could be hypothesized that the rhythmic spontaneous slow waves of the membrane potential, mainly due to voltage independent channels, only allow a slight number of SMC to reach the voltage threshold for L-type Ca2+ current, useful for contractile activation whereas most of the cells remain relaxed because their RMP is too negative for the achievement of the electro-mechanical coupling. In fact, the mean positive membrane value of the waves observed in the presence of RLX resulted significantly more negative than that recorded in control condition. Hence, this RLX effect could also have a role in the control of RMP when the SMC reaches depolarized potentials at which L-type Ca2+ channels can be activated: in this case, any reduction of Ca2+ influx through membrane channels can facilitate the setting of negative charges in the inner side of the membrane and thus the hyperpolarization. In addition, whenever the channel can be activated, its activation would be reduced by RLX, since it also modified its voltage-dependence. The consequent reduction of Ca2+ influx caused by RLX fits well with its depressant actions on the mechanical activity, since L-type Ca2+ channels are a chief path through which Ca2+ can enter into the cell and trigger the contractile machinery in intestinal SMCs. Therefore, their modulation represents an effective mechanism playing a role in SMC relaxation. Noteworthy, these voltage-gated Ca2+ channels can be modulated by several signaling systems such as cyclic nucleotides cascades and, even if data about this item are sometimes contradictory[47], in general it is accepted that cAMP and cGMP inhibit L-type Ca2+ channels. Our results actually support this clue in the ileum: L-type Ca2+ channels resulted inhibited by RLX treatment and either ODQ or 9CPA reduced the effect of RLX, thus indicating that RLX exerts its effects through the activation of both NO/GC/cGMP and AC/cAMP signaling pathways. Since we observed that the blockade of the synthesis of each cyclic nucleotide seems to prevent a full RLX action also through the other pathway, we also suggest a sort of cross-talk mechanism between the two pathways, as previously proposed in vascular[44] and uterine smooth muscle[48].
In conclusion, the bulk of the above results indicate that RLX can be considered an efficient intestinal motility modulator since it is able to induce significant changes in smooth muscle mechanical and electrical activity of ileal preparations. In particular, we found that RLX reduces the basal tension and the amplitude of the spontaneous contractions. These effects fit well with the observed decrease of the resting conductance, the membrane hyperpolarization and the diminished Ca2+ influx through L-type channels. All of these actions are exerted by RLX through a dual signaling pathway involving both GC and AC, as reported in other tissues[10,31].
From a physiological point of view, it could be speculated that the dual signaling pathway may represent a reinforcing and cross-talking (redundant) mechanism for RLX aimed to guarantee its actions and to prolong its modulatory effects in the small intestine. Furthermore, the observation that two pathways are engaged by RLX to cause myorelaxation may create the basis for identifying new therapeutic targets, offering stimulating translational perspectives in the treatment of those intestinal dysmotilities characterized by hypermotility.
In conclusion, this study shows for the first time that RLX exerts its relaxant effects on ileal smooth muscle through the involvement of both GC and AC and suggests a cross-talk between GC/cGMP and AC/cAMP pathways.
ARTICLE HIGHLIGHTS
Background
Relaxin (RLX) has been reported to modulate gastrointestinal smooth muscle activity in mice through the L-arginine/NO pathway. However, the possibility that the depressant effects of RLX in ileal preparations could involve additional pathways, not fully elucidated yet, was raised. On these grounds, the present study was designed to investigate the signaling pathways involved in the effects of RLX on ileal preparations. To this aim, we performed experiments using a combined mechanical and electrophysiological approach.
Research frontiers
The actions of RLX occur through a dual signaling pathway that, from a physiological point of view, might represent a reinforcing and cross-talking mechanism for the hormone aimed to guarantee and to prolong its myorelaxant effects in the small intestine.
Innovations and breakthroughs
This study shows, for the first time in ileal preparations, that RLX is able to influence the smooth muscle mechanical and electrophysiological activity through a dual signaling pathway.
Applications
The activation of both adenylate cyclase and guanylate cyclase pathways by RLX underlines the physiological importance of the hormone to relax ileal smooth muscle. In this view, it could be speculated that RLX may represent a potential therapeutic tool in those intestinal dysfunctions characterized by hypermotility states.
Terminology
The modulation of gastrointestinal smooth muscle activity by hormones may be investigated “in vitro” by recording either the mechanical responses or the electrophysiological properties. In ileal preparations, the hormone RLX has been shown to exert a modulatory role by depressing spontaneous contractions and by influencing the electrophysiological activity.
ACKNOWLEDGMENTS
The Authors thank Adrio Vannucchi for preparation of Figure 1.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Supported by University of Florence (ex 60%) ROBERTASQUECCORICATEN15 (to Squecco R).
Institutional animal care and use committee statement: The experimental protocol was designed in compliance with the guidelines of the European Communities Council Directive 2010/63/UE and the recommendations for the care and use of laboratory animals approved by the Animal Care Committee of the University of Florence, Italy, with authorization from the Italian Ministry of Health nr. 787/2016-PR.
Conflict-of-interest statement: No conflicts of interest, financial or otherwise, are declared by the authors.
Data sharing statement: No additional data are available.
Peer-review started: October 23, 2017
First decision: November 8, 2017
Article in press: November 28, 2017
P- Reviewer: Chiba T, Grizzi F S- Editor: Chen K L- Editor: A E- Editor: Ma YJ
Contributor Information
Eglantina Idrizaj, Department of Experimental and Clinical Medicine, Section of Physiological Sciences, University of Florence, Florence 50134, Italy.
Rachele Garella, Department of Experimental and Clinical Medicine, Section of Physiological Sciences, University of Florence, Florence 50134, Italy.
Fabio Francini, Department of Experimental and Clinical Medicine, Section of Physiological Sciences, University of Florence, Florence 50134, Italy.
Roberta Squecco, Department of Experimental and Clinical Medicine, Section of Physiological Sciences, University of Florence, Florence 50134, Italy. roberta.squecco@unifi.it.
Maria Caterina Baccari, Department of Experimental and Clinical Medicine, Section of Physiological Sciences, University of Florence, Florence 50134, Italy.
References
- 1.Bani D. Relaxin: a pleiotropic hormone. Gen Pharmacol. 1997;28:13–22. doi: 10.1016/s0306-3623(96)00171-1. [DOI] [PubMed] [Google Scholar]
- 2.Baccari MC, Calamai F. Relaxin: new functions for an old peptide. Curr Protein Pept Sci. 2004;5:9–18. doi: 10.2174/1389203043486928. [DOI] [PubMed] [Google Scholar]
- 3.Sherwood OD. Relaxin’s physiological roles and other diverse actions. Endocr Rev. 2004;25:205–234. doi: 10.1210/er.2003-0013. [DOI] [PubMed] [Google Scholar]
- 4.Bathgate RA, Halls ML, van der Westhuizen ET, Callander GE, Kocan M, Summers RJ. Relaxin family peptides and their receptors. Physiol Rev. 2013;93:405–480. doi: 10.1152/physrev.00001.2012. [DOI] [PubMed] [Google Scholar]
- 5.Halls ML, Bathgate RA, Sutton SW, Dschietzig TB, Summers RJ. International Union of Basic and Clinical Pharmacology. XCV. Recent advances in the understanding of the pharmacology and biological roles of relaxin family peptide receptors 1-4, the receptors for relaxin family peptides. Pharmacol Rev. 2015;67:389–440. doi: 10.1124/pr.114.009472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamasato K, Tsai PS, Davis J, Yamamoto SY, Bryant-Greenwood GD. Human relaxins (RLNH1, RLNH2), their receptor (RXFP1) and fetoplacental growth. Reproduction. 2017;154:67–77. doi: 10.1530/REP-17-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu SY, Kudo M, Chen T, Nakabayashi K, Bhalla A, van der Spek PJ, van Duin M, Hsueh AJ. The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Mol Endocrinol. 2000;14:1257–1271. doi: 10.1210/mend.14.8.0510. [DOI] [PubMed] [Google Scholar]
- 8.Scott DJ, Layfield S, Riesewijk A, Morita H, Tregear GW, Bathgate RA. Identification and characterization of the mouse and rat relaxin receptors as the novel orthologues of human leucine-rich repeat-containing G-protein-coupled receptor 7. Clin Exp Pharmacol Physiol. 2004;31:828–832. doi: 10.1111/j.1440-1681.2004.04075.x. [DOI] [PubMed] [Google Scholar]
- 9.Squecco R, Garella R, Idrizaj E, Nistri S, Francini F, Baccari MC. Relaxin Affects Smooth Muscle Biophysical Properties and Mechanical Activity of the Female Mouse Colon. Endocrinology. 2015;156:4398–4410. doi: 10.1210/en.2015-1428. [DOI] [PubMed] [Google Scholar]
- 10.Sarwar M, Samuel CS, Bathgate RA, Stewart DR, Summers RJ. Serelaxin-mediated signal transduction in human vascular cells: bell-shaped concentration-response curves reflect differential coupling to G proteins. Br J Pharmacol. 2015;172:1005–1019. doi: 10.1111/bph.12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Summers RJ. Recent progress in the understanding of relaxin family peptides and their receptors. Br J Pharmacol. 2017;174:915–920. doi: 10.1111/bph.13778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braddon SA. Relaxin-dependent adenosine 6’,5’-monophosphate concentration changes in the mouse pubic symphysis. Endocrinology. 1978;102:1292–1299. doi: 10.1210/endo-102-4-1292. [DOI] [PubMed] [Google Scholar]
- 13.Cheah SH, Sherwood OD. Target tissues for relaxin in the rat: tissue distribution of injected 125I-labeled relaxin and tissue changes in adenosine 3’,5’-monophosphate levels after in vitro relaxin incubation. Endocrinology. 1980;106:1203–1209. doi: 10.1210/endo-106-4-1203. [DOI] [PubMed] [Google Scholar]
- 14.Sanborn BM, Kuo HS, Weisbrodt NW, Sherwood OD. The interaction of relaxin with the rat uterus. I. Effect on cyclic nucleotide levels and spontaneous contractile activity. Endocrinology. 1980;106:1210–1215. doi: 10.1210/endo-106-4-1210. [DOI] [PubMed] [Google Scholar]
- 15.Sanborn BM, Sherwood OD. Effect of relaxin on bound cAMP in rat uterus. Endocr Res Commun. 1981;8:179–192. doi: 10.3109/07435808109045738. [DOI] [PubMed] [Google Scholar]
- 16.Hsu CJ, McCormack SM, Sanborn BM. The effect of relaxin on cyclic adenosine 3’,5’-monophosphate concentrations in rat myometrial cells in culture. Endocrinology. 1985;116:2029–2035. doi: 10.1210/endo-116-5-2029. [DOI] [PubMed] [Google Scholar]
- 17.Bani D, Baccari MC, Nistri S, Calamai F, Bigazzi M, Sacchi TB. Relaxin up-regulates the nitric oxide biosynthetic pathway in the mouse uterus: involvement in the inhibition of myometrial contractility. Endocrinology. 1999;140:4434–4441. doi: 10.1210/endo.140.10.7055. [DOI] [PubMed] [Google Scholar]
- 18.Novak J, Ramirez RJ, Gandley RE, Sherwood OD, Conrad KP. Myogenic reactivity is reduced in small renal arteries isolated from relaxin-treated rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R349–R355. doi: 10.1152/ajpregu.00635.2001. [DOI] [PubMed] [Google Scholar]
- 19.Schlossmann J, Feil R, Hofmann F. Signaling through NO and cGMP-dependent protein kinases. Ann Med. 2003;35:21–27. doi: 10.1080/07853890310004093. [DOI] [PubMed] [Google Scholar]
- 20.Conrad KP, Novak J. Emerging role of relaxin in renal and cardiovascular function. Am J Physiol Regul Integr Comp Physiol. 2004;287:R250–R261. doi: 10.1152/ajpregu.00672.2003. [DOI] [PubMed] [Google Scholar]
- 21.Samuel CS, Du XJ, Bathgate RA, Summers RJ. ‘Relaxin’ the stiffened heart and arteries: the therapeutic potential for relaxin in the treatment of cardiovascular disease. Pharmacol Ther. 2006;112:529–552. doi: 10.1016/j.pharmthera.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Baccari MC, Bani D. Relaxin and nitric oxide signalling. Curr Protein Pept Sci. 2008;9:638–645. doi: 10.2174/138920308786733921. [DOI] [PubMed] [Google Scholar]
- 23.Leo CH, Jelinic M, Ng HH, Marshall SA, Novak J, Tare M, Conrad KP, Parry LJ. Vascular actions of relaxin: nitric oxide and beyond. Br J Pharmacol. 2017;174:1002–1014. doi: 10.1111/bph.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baccari MC, Bani D, Bigazzi M, Calamai F. Influence of relaxin on the neurally induced relaxant responses of the mouse gastric fundus. Biol Reprod. 2004;71:1325–1329. doi: 10.1095/biolreprod.104.029579. [DOI] [PubMed] [Google Scholar]
- 25.Baccari MC, Nistri S, Quattrone S, Bigazzi M, Bani Sacchi T, Calamai F, Bani D. Depression by relaxin of neurally induced contractile responses in the mouse gastric fundus. Biol Reprod. 2004;70:222–228. doi: 10.1095/biolreprod.103.018374. [DOI] [PubMed] [Google Scholar]
- 26.Garella R, Squecco R, Baccari MC. Site-related Effects of Relaxin in the Gastrointestinal Tract Through Nitric Oxide Signalling: An Updated Report. Curr Protein Pept Sci. 2017;18:1254–1262. doi: 10.2174/1389203718666170612104719. [DOI] [PubMed] [Google Scholar]
- 27.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 28.Groneberg D, Voussen B, Friebe A. Integrative Control of Gastrointestinal Motility by Nitric Oxide. Curr Med Chem. 2016;23:2715–2735. doi: 10.2174/0929867323666160812150907. [DOI] [PubMed] [Google Scholar]
- 29.Vallance P. Nitric oxide: therapeutic opportunities. Fundam Clin Pharmacol. 2003;17:1–10. doi: 10.1046/j.1472-8206.2003.00124.x. [DOI] [PubMed] [Google Scholar]
- 30.Bani D, Baccari MC, Quattrone S, Nistri S, Calamai F, Bigazzi M, Bani Sacchi T. Relaxin depresses small bowel motility through a nitric oxide-mediated mechanism. Studies in mice. Biol Reprod. 2002;66:778–784. doi: 10.1095/biolreprod66.3.778. [DOI] [PubMed] [Google Scholar]
- 31.Lam M, Royce SG, Donovan C, Jelinic M, Parry LJ, Samuel CS, Bourke JE. Serelaxin Elicits Bronchodilation and Enhances β-Adrenoceptor-Mediated Airway Relaxation. Front Pharmacol. 2016;7:406. doi: 10.3389/fphar.2016.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolton TB, Prestwich SA, Zholos AV, Gordienko DV. Excitation-contraction coupling in gastrointestinal and other smooth muscles. Annu Rev Physiol. 1999;61:85–115. doi: 10.1146/annurev.physiol.61.1.85. [DOI] [PubMed] [Google Scholar]
- 33.Austin CR, Rowlands JW. Mammalian reproduction. The IAT Manual of Laboratory Animal Practice and Technique. 2nd edn, ed. Short DJ Woodnott DP. Bungay, UK: Lockwood; 1969. pp. 340–349. [Google Scholar]
- 34.Mercado-Simmen RC, Bryant-Greenwood GD, Greenwood FC. Relaxin receptor in the rat myometrium: regulation by estrogen and relaxin. Endocrinology. 1982;110:220–226. doi: 10.1210/endo-110-1-220. [DOI] [PubMed] [Google Scholar]
- 35.Squecco R, Garella R, Luciani G, Francini F, Baccari MC. Muscular effects of orexin A on the mouse duodenum: mechanical and electrophysiological studies. J Physiol. 2011;589:5231–5246. doi: 10.1113/jphysiol.2011.214940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Squecco R, Garella R, Francini F, Baccari MC. Influence of obestatin on the gastric longitudinal smooth muscle from mice: mechanical and electrophysiological studies. Am J Physiol Gastrointest Liver Physiol. 2013;305:G628–G637. doi: 10.1152/ajpgi.00059.2013. [DOI] [PubMed] [Google Scholar]
- 37.Squecco R, Sassoli C, Garella R, Chellini F, Idrizaj E, Nistri S, Formigli L, Bani D, Francini F. Inhibitory effects of relaxin on cardiac fibroblast-to-myofibroblast transition: an electrophysiological study. Exp Physiol. 2015;100:652–666. doi: 10.1113/EP085178. [DOI] [PubMed] [Google Scholar]
- 38.Baccari MC, Nistri S, Vannucchi MG, Calamai F, Bani D. Reversal by relaxin of altered ileal spontaneous contractions in dystrophic (mdx) mice through a nitric oxide-mediated mechanism. Am J Physiol Regul Integr Comp Physiol. 2007;293:R662–R668. doi: 10.1152/ajpregu.00214.2007. [DOI] [PubMed] [Google Scholar]
- 39.Vannucchi MG, Garella R, Cipriani G, Baccari MC. Relaxin counteracts the altered gastric motility of dystrophic (mdx) mice: functional and immunohistochemical evidence for the involvement of nitric oxide. Am J Physiol Endocrinol Metab. 2011;300:E380–E391. doi: 10.1152/ajpendo.00375.2010. [DOI] [PubMed] [Google Scholar]
- 40.Baccari MC, Traini C, Garella R, Cipriani G, Vannucchi MG. Relaxin exerts two opposite effects on mechanical activity and nitric oxide synthase expression in the mouse colon. Am J Physiol Endocrinol Metab. 2012;303:E1142–E1150. doi: 10.1152/ajpendo.00260.2012. [DOI] [PubMed] [Google Scholar]
- 41.Ibrahimi A, Abumrad N, Maghareie H, Golia M, Shoshani I, Désaubry L, Johnson RA. Adenylyl cyclase P-site ligands accelerate differentiation in Ob1771 preadipocytes. Am J Physiol. 1999;276:C487–C496. doi: 10.1152/ajpcell.1999.276.2.C487. [DOI] [PubMed] [Google Scholar]
- 42.Boland BB, Alarcón C, Ali A, Rhodes CJ. Monomethylated-adenines potentiate glucose-induced insulin production and secretion via inhibition of phosphodiesterase activity in rat pancreatic islets. Islets. 2015;7:e1073435. doi: 10.1080/19382014.2015.1073435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ueno T, Duenes JA, Zarroug AE, Sarr MG. Nitrergic mechanisms mediating inhibitory control of longitudinal smooth muscle contraction in mouse small intestine. J Gastrointest Surg. 2004;8:831–841. doi: 10.1016/j.gassur.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Morgado M, Cairrão E, Santos-Silva AJ, Verde I. Cyclic nucleotide-dependent relaxation pathways in vascular smooth muscle. Cell Mol Life Sci. 2012;69:247–266. doi: 10.1007/s00018-011-0815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res. 2007;100:309–327. doi: 10.1161/01.RES.0000256354.95791.f1. [DOI] [PubMed] [Google Scholar]
- 46.He XD, Goyal RK. CaMKII inhibition hyperpolarizes membrane and blocks nitrergic IJP by closing a Cl(-) conductance in intestinal smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2012;303:G240–G246. doi: 10.1152/ajpgi.00102.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- 48.Sanborn BM. Hormones and calcium: mechanisms controlling uterine smooth muscle contractile activity. The Litchfield Lecture. Exp Physiol. 2001;86:223–237. doi: 10.1113/eph8602179. [DOI] [PubMed] [Google Scholar]