Abstract
AIM
To investigate the risk of end-stage renal disease (ESRD) in hepatitis B virus (HBV)-infected patients with chronic kidney disease (CKD) with and without nucleos(t)ide analogue (NA) therapy.
METHODS
This nationwide cohort study included 103444 Taiwanese CKD adults without hepatitis C virus infection from the Taiwan Longitudinal Health Insurance Database 2005 between 1997 and 2012. We identified 2916 CKD patients who acquired HBV infection and did not receive NAs (untreated cohort), and they were propensity-matched 1:4 with 11664 uninfected counterparts. We also identified 442 CKD patients who acquired HBV infection and received NAs (treated cohort), and they were propensity-matched 1:3 with 1326 untreated counterparts. The association between HBV infection, NA use, and ESRD was analyzed using competing risk analysis.
RESULTS
Multivariable Cox regression analysis showed a 1.67-fold higher risk (P < 0.0001) of ESRD in the untreated cohort (16-year cumulative incidence, 10.1%) than in the matched uninfected cohort (16-year cumulative incidence, 6.6%), which was independent of cirrhosis or diabetes. The treated cohort (16-year cumulative incidence, 2.2%) had an 87% lower ESRD risk (P < 0.0001) compared with the matched untreated cohort (16-year cumulative incidence, 11.9%). The number needed to treat for one fewer ESRD after NA use at 12 years was 12. Multivariable stratified analyses verified these associations in all subgroups.
CONCLUSION
This study suggests that untreated HBV infection and NA therapy are associated with increased and decreased risk of ESRD, respectively, in CKD patients. Identification of HBV status and targeted monitoring for ESRD development are important in CKD patients living in HBV-endemic areas.
Keywords: Hepatitis B virus, Chronic kidney disease, End-stage renal disease, Nucleos(t)ide analogue, Cohort study
Core tip: This nationwide retrospective cohort study used propensity score-matched and competing risk analyses to evaluate the effect of untreated hepatitis B virus (HBV) infection and nucleos(t)ide analogue (NA) therapy on the development of end-stage renal disease (ESRD) in chronic kidney disease (CKD) patients who acquired HBV infection. We found that untreated HBV infection in CKD patients was associated with an increased risk of ESRD, while NA therapy reduced the risk.
INTRODUCTION
Hepatitis B virus (HBV) infection, chronic kidney disease (CKD), and end-stage renal disease (ESRD) are global health challenges which impose a major economic burden[1,2]. Emerging clinical and experimental evidence suggests that in addition to liver inflammation and fibrosis, chronic HBV infection may also play a role in the initiation and progression of renal injury. Untreated chronic HBV infection could lead to an annual decline in the estimated glomerular filtration rate[3], and subsequent development of CKD and ESRD in the general population, even in the absence of cirrhosis[4,5]. Sera of patients with chronic HBV infection, even those negative for the presence of HBV-DNA, could induce apoptosis of cultured human renal tubular cells[6]. Chronic HBV infection has also been shown to progress to ESRD in special populations with diabetic nephropathy[7] and HBV-related glomerulonephritis (HBV-GN)[8]. CKD patients were reported to be at increased risk of acquiring HBV infection[1,9,10], more vulnerable to the cytopathic effects of HBV infection, and prone to becoming chronic carriers. This could be because of increased exposure of these patients to blood products, and the immunosuppressive effects of CKD[1,11]. The risk factors for CKD onset in the general population may differ somewhat from those for progression of established CKD[12]. However, there are presently no cohort studies which have addressed the incidence of chronic HBV infection among CKD patients and their renal outcome regardless of etiology.
HBV infection among CKD patients is associated with higher morbidity and mortality rates, and its management remains challenging for clinicians[1,13]. Nucleos(t)ide analogue (NA) therapy effectively suppresses HBV replication by inhibiting HBV polymerase, and thus decreases the levels of serum HBV-DNA[14] and delays progression of cirrhosis[15]. Although NA therapy is recommended for all patients with chronic HBV infection, regardless of any level of renal dysfunction, there is limited information on NA use in CKD patients[13]. Serum HBV-DNA levels were shown to have a positive correlation with the degree of renal injury[16], and lamivudine treatment resulted in a significant reduction in proteinuria, serum HBV-DNA, and ESRD risk[17] in a small number of adult patients with HBV-GN. However, no randomized clinical trials have been conducted to date to examine the effect of NAs on renal outcome among CKD patients regardless of etiology[1]. This issue assumes greater importance because of the rising global burden of CKD, ESRD, and HBV infection. The high prevalence of these three diseases in Taiwan makes it a particularly suitable location to evaluate this relationship. Our nationwide cohort study analysed reimbursement claims data from the Taiwan Longitudinal Health Insurance Database 2005 (LHID2005) in order to determine the effect of chronic HBV infection and NA use on the renal outcome of CKD patients, and the number needed to treat (NNT) for one less ESRD development.
MATERIALS AND METHODS
Database
This study evaluated data from LHID2005, a subset of the National Health Insurance Research Database (NHIRD) which is derived from the Taiwan National Health Insurance (NHI) program and released by the National Health Research Institutes for academic research. The NHI program is a compulsory and universal program for all residents of Taiwan, had a coverage rate of more than 99% by the end of 2012, and adopts ICD-9 codes to define diseases. All insurance claims are scrutinized by medical reimbursement specialists and undergo peer review under strict audits and heavy penalties in the reimbursement process in order to ensure accuracy of coding. The LHID2005 includes the data of 1 million randomly sampled subjects who were NHI beneficiaries in 2005[18]. There was no significant difference in age, sex, birth year, or average insured payroll-related amount between the subjects enrolled in the NHI program. As all personal information was anonymous in the LHID2005, no informed consent was required and this study was exempt from a full ethical review by the institutional review board of the Dalin Tzu Chi Hospital (B10302011).
CKD population
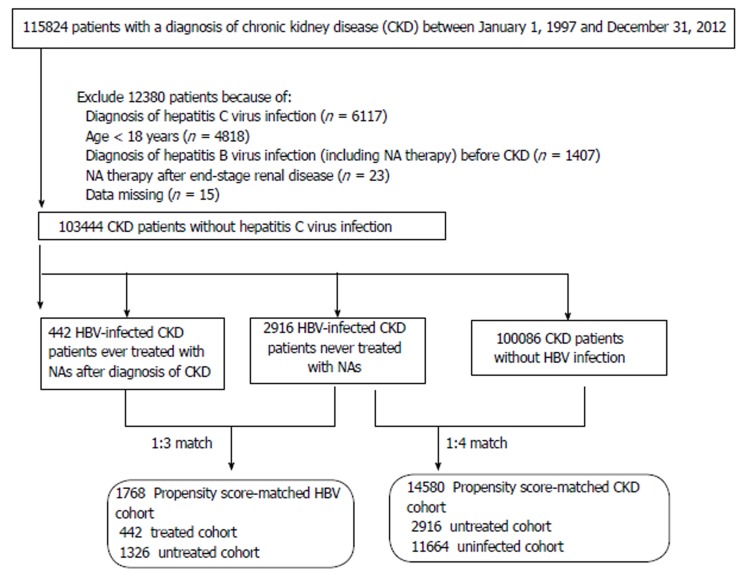
The CKD population comprised patients aged > 18 years who received a primary diagnosis of CKD (ICD-9 codes 250.4*, 274.1*, 283.11, 403.*1, 404.*2, 404.*3, 440.1, 442.1, 447.3, 572.4, 580-588, 642.1*, and 646.2*)[19] between January 1, 1997 and December 31, 2012 and did not have catastrophic illness registration cards for ESRD (indicating the need for long-term renal replacement therapy). We excluded subjects who had claim-based diagnoses of hepatitis C virus infection (ICD-9 codes 070.41, 070.44, 070.51, 070.54, and V02.62) between 1997 and 2012, or of HBV infection (ICD-9 codes 070.22, 070.23, 070.32, 070.33, V02.61) before CKD enrolment. A total of 103,444 CKD patients were eligible for analysis (Figure 1). However, the exact stage of CKD cannot be assessed from the LHID2005.
Figure 1.
Flow diagram of the enrollment process. NAs: Nucleos(t)ide analogues; HBV: Hepatitis B virus; CKD: Chronic kidney disease.
Study cohorts
Based on HBV infection status and NA use including lamivudine, entecavir, telbivudine, and adefovir[20], the eligible CKD patients in this study were divided into three groups: uninfected (n = 100086), untreated (n = 2916), and treated (n = 442) groups. The incidence of CKD patients acquiring HBV infection was 3.2%. The untreated group was defined as the group of CKD patients who did not receive NA therapy over the course of the study period, following the acquisition of HBV infection. The treated group was defined as the group of CKD patients who received NAs throughout the study period. Each treated patient was propensity score (PS)-matched with three untreated patients. The uninfected patients, who were PS-matched 4:1 with their untreated counterparts, comprised CKD patients who never coded for HBV infection. The PS was estimated by logistic regression built on baseline variables including age, sex, comorbidities, urbanization level, enrolee category (EC), number of medical visits, and the Deyo-Charlson comorbidity index (CCI) score. The PS-matched HBV cohort included 1768 CKD patients (442 treated and 1,326 untreated patients). The PS-matched CKD cohort included 14,580 patients (2,916 untreated and 11,664 uninfected patients).
Endpoint and covariates
The index date of the treated cohort was defined as the date of initiation of NA therapy, that of the untreated cohort was defined as the first occurrence of a HBV claim during the entry period, and that of the uninfected cohort was defined as the date of a CKD claim during the entry period. All CKD patients were followed from the index date until the date of ESRD diagnosis, date of death, or the end of 2012, whichever came first. The diagnosis of ESRD was based on the Catastrophic Illness Patient Database, a part of the NHIRD. All Taiwanese patients who develop ESRD and require long-term dialysis are eligible to apply for catastrophic illness registration cards from the NHI Administration, so they have no copayments for healthcare. Censoring resulting from death during CKD progression was regarded as informative and was adjusted by using competing risk methodology[21]. The adjusted covariates comprised sociodemographic and comorbidity factors. The sociodemographic characteristics included age, sex, urbanization level (urban, suburban, and rural), EC [EC1 (highest status)] to EC4 (lowest status), number of medical visits, CCI score, and PS. The comorbidities (ICD-9 codes] investigated in this study were diabetes (250), hypertension (401-405), coronary heart disease (410-414), hyperlipidemia (272-272.4), and cirrhosis (571.2, 571.5, and 571.6).
Statistical analysis
We used a modified Kaplan-Meier method and Gray’s method[22] to calculate and compare the cumulative incidence of ESRD in data with competing risk, and tested differences in the full time-to-event distributions between the study cohorts using the log-rank test. The NNT represented the number of patients needed to be treated to yield one fewer ESRD; the NNT was calculated with the inverse of the absolute risk reduction[23]. After ensuring the assumption of proportional hazards, we applied the modified Cox proportional hazard model with adjustment for all covariates and competing risks[24] to determine whether HBV infection and NA use in CKD patients were associated with increased and reduced risk of ESRD, respectively. We also performed stratified analyses to determine the impact of untreated and treated HBV infection on risk of ESRD among PS-matched CKD patients. All data were analyzed with SAS (version 9.3; SAS Institute, Inc., Cary, NC, United States) and a two-sided P-value less than 0.05 was considered statistically significant. The statistical methods used in this study were reviewed by co-authors Chung-Yi Li and Shiang-Jiun Tsai.
RESULTS
Patient characteristics
The baseline characteristics of the untreated HBV-infected and HBV-uninfected CKD patients as well as the treated and untreated HBV-infected CKD patients are summarized in Tables 1 and 2, respectively. After matching, there were no significant differences in baseline covariates between the untreated and uninfected cohorts, except for EC, as well as between the treated and untreated cohorts.
Table 1.
Baseline characteristics and renal outcome of the untreated hepatitis B virus-infected and hepatitis B virus-uninfected chronic kidney disease patients (n = 103002), 1997-2012
| Variable | Overall CKD patients (n = 103002) |
Propensity score-matched CKD patients (n = 14580) |
||||
| Untreated (n = 2916) | Uninfected (n = 100086) | P value | Untreated (n = 2916) | Uninfected (n = 11664) | P value | |
| Sex | < 0.0001 | 0.45 | ||||
| Men | 1713 (58.7) | 51592 (51.6) | 1713 (58.7) | 6762 (58.0) | ||
| Women | 1203 (41.3) | 48494 (48.4) | 1203 (41.3) | 4902 (42.0) | ||
| Age (yr, mean ± SD) | 48.1 ± 14.4 | 56.3 ± 17.4 | < 0.0001 | 48.1 ± 14.4 | 47.7 ± 15.9 | 0.21 |
| Comorbidity | ||||||
| Diabetes | 749 (25.7) | 33023 (33.0) | < 0.0001 | 749 (25.7) | 3049 (26.1) | 0.62 |
| Hypertension | 884 (30.3) | 45139 (45.1) | < 0.0001 | 884 (30.3) | 3420 (29.3) | 0.29 |
| Coronary heart disease | 316 (10.8) | 18668 (18.7) | < 0.0001 | 316 (10.8) | 1152 (9.9) | 0.12 |
| Hyperlipidemia | 695 (23.8) | 29595 (29.6) | < 0.0001 | 695 (23.8) | 2851 (24.4) | 0.49 |
| Cirrhosis | 139 (4.8) | 1528 (1.5) | < 0.0001 | 139 (4.8) | 472 (4.1) | 0.08 |
| Urbanization level | 0.0003 | 0.47 | ||||
| Urban | 892 (30.6) | 27951 (27.9) | 892 (30.6) | 3595 (30.8) | ||
| Suburban | 1351 (46.3) | 46163 (46.1) | 1351 (46.3) | 5498 (47.2) | ||
| Rural | 673 (23.1) | 25972 (26.0) | 673 (23.1) | 2571 (22.0) | ||
| Enrolee category | < 0.0001 | 0.011 | ||||
| 1 + 2 | 1122 (38.5) | 29935 (29.9) | 1122 (38.5) | 4680 (40.1) | ||
| 3 | 1278 (43.8) | 47825 (47.8) | 1278 (43.8) | 4760 (40.8) | ||
| 4 | 516 (17.7) | 22326 (22.3) | 516 (17.7) | 2224 (19.1) | ||
| No. of medical visits (mean ± SD) | 26.1 ± 20.8 | 29.0 ± 22.8 | < 0.0001 | 26.1 ± 20.8 | 25.7 ± 22.5 | 0.31 |
| Charlson comorbidity index score (mean ± SD) | 1.6 ± 1.9 | 1.9 ± 2.3 | < 0.0001 | 1.6 ± 1.9 | 1.5 ± 2.1 | 0.06 |
| Propensity score (mean ± SD) | 0.4 ± 0.2 | 0.3 ± 0.2 | < 0.0001 | 0.4 ± 0.2 | 0.4 ± 0.2 | 1.00 |
| End-stage renal disease | ||||||
| Follow-up year (mean ± SD) | 8.9 ± 3.9 | 6.9 ± 4.5 | < 0.0001 | 8.9 ± 3.9 | 7.4 ± 4.5 | < 0.0001 |
| Total follow-up (person-year) | 26098 | 689592 | < 0.0001 | 26098 | 86780 | < 0.0001 |
| Event | 197 (6.8) | 4076 (4.1) | < 0.0001 | 197 (6.8) | 414 (3.6) | < 0.0001 |
Categorical variables are given as n (%); continuous variable given as mean ± SD. CKD: Chronic kidney disease.
Table 2.
Baseline characteristics and renal outcome of the treated and untreated hepatitis B virus-infected chronic kidney disease patients (n = 3358), 1997-2012
| Variable | Overall HBV patients (n = 3358) |
Propensity score-matched HBV patients (n = 1768) |
||||
| Treated (n = 442) | Untreated (n = 2916) | P value | Treated (n = 442) | Untreated (n = 1326) | P value | |
| Sex | < 0.0001 | 0.76 | ||||
| Men | 319 (72.2) | 1713 (58.7) | 319 (72.2) | 967 (72.9) | ||
| Women | 123 (27.8) | 1203 (41.3) | 123 (27.8) | 359 (27.1) | ||
| Age (yr, mean ± SD) | 51.0 ± 13.9 | 48.1 ± 14.4 | < 0.0001 | 51.0 ± 13.9 | 51.6 ± 14.7 | 0.49 |
| Interval to start nucleos(t)ide analogue therapy (yr, mean ± SD) | 1.2 ± 1.9 | - | 1.2 ± 1.9 | - | ||
| Nucleos(t)ide analogue therapy duration (yr, mean ± SD) | 0.8 ± 0.8 | - | 0.8 ± 0.8 | - | ||
| Comorbidity | ||||||
| Diabetes | 141 (31.9) | 749 (25.7) | 0.006 | 141 (31.9) | 424 (32.0) | 0.98 |
| Hypertension | 150 (33.9) | 884 (30.3) | 0.12 | 150 (33.9) | 457 (34.5) | 0.84 |
| Coronary heart disease | 59 (13.4) | 316 (10.8) | 0.12 | 59 (13.4) | 198 (14.9) | 0.41 |
| Hyperlipidemia | 108 (24.4) | 695 (23.8) | 0.78 | 108 (24.4) | 334 (25.2) | 0.75 |
| Cirrhosis | 59 (13.4) | 139 (4.8) | < 0.0001 | 59 (13.4) | 135 (10.2) | 0.07 |
| Urbanization level | 0.45 | 0.88 | ||||
| Urban | 129 (29.2) | 892 (30.6) | 129 (29.2) | 381 (28.7) | ||
| Suburban | 199 (45.0) | 1351 (46.3) | 199 (45.0) | 615 (46.4) | ||
| Rural | 114 (25.8) | 673 (23.1) | 114 (25.8) | 330 (24.9) | ||
| Enrolee category | 0.32 | 0.61 | ||||
| 1 + 2 | 161 (36.4) | 1122 (38.5) | 161 (36.4) | 460 (34.7) | ||
| 3 | 190 (43.0) | 1278 (43.8) | 190 (43.0) | 606 (45.7) | ||
| 4 | 91 (20.6) | 516 (17.7) | 91 (20.6) | 260 (19.6) | ||
| No. of medical visits (mean ± SD) | 26.5 ± 18.2 | 26.1 ± 20.8 | 0.75 | 26.5 ± 18.2 | 26.3 ± 19.3 | 0.90 |
| Charlson comorbidity index score (mean ± SD) | 1.9 ± 2.1 | 1.6 ± 1.9 | < 0.0001 | 1.9 ± 2.1 | 1.9 ± 2.2 | 0.79 |
| Propensity score (mean ± SD) | 1.6 ± 0.7 | 1.3 ± 0.6 | < 0.0001 | 1.6 ± 0.7 | 1.5 ± 0.7 | 0.51 |
| End-stage renal disease | ||||||
| Follow-up year (mean ± SD) | 7.6 ± 4.3 | 8.9 ± 3.9 | < 0.0001 | 7.6 ± 4.3 | 8.5 ± 4.0 | < 0.0001 |
| Total follow-up (person-year) | 3359 | 26101 | < 0.0001 | 3359 | 22478 | < 0.0001 |
| Event | 5 (1.1) | 197 (6.8) | < 0.0001 | 5 (1.1) | 108 (8.1) | < 0.0001 |
Categorical variables are given as n (%); continuous variable given as mean ± SD. HBV: Hepatitis B virus.
16-year cumulative incidence of ESRD
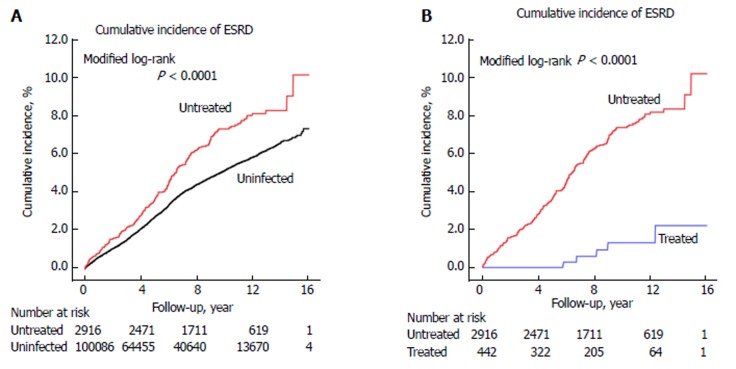
After PS matching, the 16-year cumulative ESRD incidence for the untreated and uninfected cohorts was 10.1% (95%CI: 7.5%-13.2%) and 6.6% (95%CI: 4.7%-9.0%), respectively (P < 0.0001) (Figure 2A). The 16-year cumulative ESRD incidence for the treated and matched untreated cohorts was 2.2% (95%CI: 0.7%-5.2%) and 11.9% (95%CI: 8.5%-15.9%), respectively (P < 0.0001) (Figure 2B). The NNT associated with one fewer ESRD after 4, 8, and 12 years were 28, 14, and 12, respectively (data not shown).
Figure 2.
Cumulative incidence of end-stage renal disease after adjustment for competing mortality among (A) propensity score-matched chronic kidney disease cohort and (B) propensity score-matched hepatitis B virus cohort. ESRD: End-stage renal disease.
ESRD risk in untreated vs uninfected CKD patients
After PS matching, 197 (6.8%) untreated and 414 (3.6%) uninfected patients developed ESRD during the study period (P < 0.0001) (Table 1). Modified multivariable Cox regression indicated that ESRD was independently associated with untreated HBV infection (adjusted hazard ratio: 1.67; 95%CI: 1.40-1.98; P < 0.0001), male gender (1.65; 1.26-2.16; P = 0.0003), diabetes (1.96; 1.63-2.35; P < 0.0001), hypertension (2.51; 1.95-3.22; P < 0.0001), cirrhosis (4.36; 1.56-12.17; P = 0.005), EC3 (1.44; 1.15-1.79; P = 0.001), and EC4 (1.55; 1.21-1.98; P = 0.0006), and inversely associated with advanced age (0.98; 0.96-0.98; P = 0.012) (Table 3). The robustness of these data was tested by sensitivity analyses (Table 5). We excluded diabetic or cirrhotic patients or both from both cohorts at baseline, indicating a significantly positive association of untreated HBV with ESRD risk.
Table 3.
Hazard ratios for end-stage renal disease in the untreated hepatitis B virus-infected and hepatitis B virus-uninfected chronic kidney disease patients, with adjustment for competing mortality
| Variable | Overall CKD patients (n = 103002) |
Propensity score-matched CKD patients (n = 14580) |
||||||
| Crude |
Adjusted1 |
Crude |
Adjusted1 |
|||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| CKD patients | ||||||||
| Uninfected | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | ||||
| Untreated | 1.39 (1.20-1.60) | < 0.0001 | 1.69 (1.47-1.96) | < 0.0001 | 1.64 (1.38-1.94) | < 0.0001 | 1.67 (1.40-1.98) | < 0.0001 |
| Sex (Men/Women) | 0.94 (0.88-0.99) | 0.028 | 1.38 (1.25-1.51) | < 0.0001 | 1.24 (1.05-1.46) | 0.011 | 1.65 (1.26-2.16) | 0.0003 |
| Age (per year) | 1.01 (1.01-1.01) | < 0.0001 | 0.96 (0.96-0.97) | < 0.0001 | 1.03 (1.02-1.03) | < 0.0001 | 0.98 (0.96-0.98) | 0.012 |
| Comorbidity (Yes/No) | ||||||||
| Diabetes | 2.49 (2.35-2.65) | < 0.0001 | 1.96 (1.83-2.10) | < 0.0001 | 2.55 (2.18-2.99) | < 0.0001 | 1.96 (1.63-2.35) | < 0.0001 |
| Hypertension | 2.85 (2.67-3.03) | < 0.0001 | 2.08 (1.88-2.29) | < 0.0001 | 3.59 (3.06-4.20) | < 0.0001 | 2.51 (1.95-3.22) | < 0.0001 |
| Coronary heart disease | 1.26 (1.17-1.35) | < 0.0001 | 0.86 (0.80-1.05) | 0.37 | 1.25 (0.98-1.60) | 0.08 | 0.93 (0.74-1.15) | 0.49 |
| Hyperlipidemia | 1.16 (1.08-1.24) | < 0.0001 | 1.01 (0.93-1.08) | 0.90 | 1.07 (0.89-1.30) | 0.48 | 0.84 (0.68-1.03) | 0.09 |
| Cirrhosis | 0.81 (0.62-1.05) | 0.11 | 8.34 (5.72-12.17) | < 0.0001 | 0.73 (0.46-1.15) | 0.17 | 4.36 (1.56-12.17) | 0.005 |
| Urbanization level | ||||||||
| Urban | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | ||||
| Suburban | 1.01 (0.95-1.08) | 0.69 | 0.93 (0.87-1.00) | 0.06 | 1.10 (0.94-1.28) | 0.26 | 0.99 (0.81-1.19) | 0.87 |
| Rural | 1.03 (0.97-1.11) | 0.35 | 0.84 (0.77-1.03) | 0.07 | 0.99 (0.82-1.20) | 0.94 | 0.80 (0.63-1.01) | 0.06 |
| Enrolee category | ||||||||
| 1 + 2 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | ||||
| 3 | 1.28 (1.21-1.36) | < 0.0001 | 1.34 (1.23-1.47) | < 0.0001 | 1.36 (1.16-1.60) | 0.0001 | 1.44 (1.15-1.79) | 0.001 |
| 4 | 1.26 (1.18-1.35) | < 0.0001 | 1.34 (1.21-1.49) | < 0.0001 | 1.52 (1.27-1.82) | < 0.0001 | 1.55 (1.21-1.98) | 0.0006 |
Adjusted for all covariates (age per year, sex, comorbidities, urbanization level, enrolee category, number of medical visits, Charlson comorbidity index score, and propensity score) and competing mortality. CKD: Chronic kidney disease.
Table 5.
Sensitivity analysis in the propensity-matched chronic kidney disease patients (n = 14580) n (%)
| Model 1: CKD patients without diabetes at baseline (n =10782) | ||
| Uninfected cohort ( = 8615) | Untreated cohort (n = 2167) | |
| No. of ESRD | 222 (2.58) | 118 (5.45) |
| Adjusted HR (95%CI)1 | 1.00 (reference) | 1.82 (1.45-2.28) |
| Model 2: CKD patients without cirrhosis at baseline (n = 13969) | ||
| Uninfected cohort (n = 11192) | Untreated cohort (n = 2777) | |
| No. of ESRD | 401 (3.58) | 191 (6.88) |
| Adjusted HR (95%CI)1 | 1.00 (reference) | 1.67 (1.40-1.99) |
| Model 3: CKD patients without diabetes and cirrhosis at baseline (n = 10420) | ||
| Uninfected cohort (n = 8327) | Untreated cohort (n = 2093) | |
| No. of ESRD | 218 (2.62) | 116 (5.54) |
| Adjusted HR (95%CI)1 | 1.00 (reference) | 1.81 (1.44-2.27) |
Adjusted for all covariates (age per year, sex, comorbidities, urbanization level, enrolee category, number of medical visits, Charlson comorbidity index score, and propensity score) and competing mortality. ESRD: End-stage renal disease; CKD: Chronic kidney disease.
ESRD risk in treated vs untreated CKD patients
After PS matching, 5 (1.1%) treated and 108 (8.1%) untreated patients developed ESRD during the study period (P < 0.0001) (Table 2). The treated cohort had a significantly lower risk of ESRD than the untreated cohort (0.13; 0.05-0.31; P < 0.0001) (Table 4). As expected, diabetes (1.77; 1.08-2.91; P = 0.024), hypertension (3.52; 2.09-5.95; P < 0.0001), and EC4 (1.89; 1.10-3.26; P = 0.022) were associated with a significant risk of ESRD.
Table 4.
Hazard ratios for end-stage renal disease in the untreated hepatitis B virus-infected and hepatitis B virus-treated chronic kidney disease patients, with adjustment for competing mortality
| Variable | Overall HBV patients (n = 3358) |
Propensity score-matched HBV patients (n = 1768) |
||||||
| Crude |
Adjusted1 |
Crude |
Adjusted1 |
|||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| HBV cohort | ||||||||
| Untreated | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | ||||
| Treated | 0.17 (0.07-0.41) | < 0.0001 | 0.15 (0.06-0.36) | < 0.0001 | 0.14 (0.06-0.34) | < 0.0001 | 0.13 (0.05-0.31) | < 0.0001 |
| Sex (Men/Women) | 1.12 (0.84-1.49) | 0.43 | 0.91 (0.46-1.79) | 0.78 | 0.87 (0.58-1.30) | 0.49 | 0.63 (0.24-1.63) | 0.34 |
| Age (per year) | 1.02 (1.01-1.02) | 0.0002 | 1.00 (0.98-1.02) | 0.86 | 1.01 (1.00-1.02) | 0.20 | 0.99 (0.96-1.01) | 0.34 |
| Comorbidity (Yes/No) | ||||||||
| Diabetes | 2.01 (1.52-2.67) | < 0.0001 | 1.92 (1.33-2.77) | 0.0005 | 1.89 (1.31-2.74) | 0.0007 | 1.77 (1.08-2.91) | 0.024 |
| Hypertension | 3.09 (2.35-4.06) | < 0.0001 | 3.90 (2.66-5.72) | < 0.0001 | 2.59 (1.79-3.74) | < 0.0001 | 3.52 (2.09-5.95) | < 0.0001 |
| Coronary heart disease | 0.85 (0.53-1.36) | 0.49 | 0.51 (0.31-0.84) | 0.008 | 0.93 (0.54-1.60) | 0.80 | 0.59 (0.32-1.09) | 0.09 |
| Hyperlipidemia | 1.12 (0.81-1.54) | 0.51 | 0.84 (0.58-1.20) | 0.33 | 1.23 (0.81-1.86) | 0.33 | 0.98 (0.61-1.56) | 0.91 |
| Cirrhosis | 0.57 (0.27-1.22) | 0.15 | 0.35 (0.05-2.61) | 0.30 | 0.53 (0.25-1.14) | 0.10 | 0.30 (0.03-3.32) | 0.33 |
| Urbanization level | ||||||||
| Urban | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | ||||
| Suburban | 1.18 (0.90-1.56) | 0.24 | 1.18 (0.85-1.65) | 0.33 | 1.27 (0.88-1.84) | 0.20 | 1.06 (0.68-1.64) | 0.80 |
| Rural | 1.03 (0.75-1.43) | 0.85 | 1.09 (0.73-1.64) | 0.68 | 0.79 (0.50-1.23) | 0.30 | 0.91 (0.52-1.59) | 0.73 |
| Enrolee category | ||||||||
| 1 + 2 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | ||||
| 3 | 0.96 (0.73-1.27) | 0.78 | 1.05 (0.74-1.50) | 0.78 | 0.79 (0.54-1.14) | 0.21 | 0.99 (0.60-1.62) | 0.97 |
| 4 | 1.57 (1.14-2.16) | 0.006 | 1.60 (1.06-2.40) | 0.024 | 1.78 (1.19-2.67) | 0.005 | 1.89 (1.10-3.26) | 0.022 |
Adjusted for all covariates (age per year, sex, comorbidities, urbanization level, enrolee category, number of medical visits, Charlson comorbidity index score, and propensity score). HBV: Hepatitis B virus.
Stratified analysis
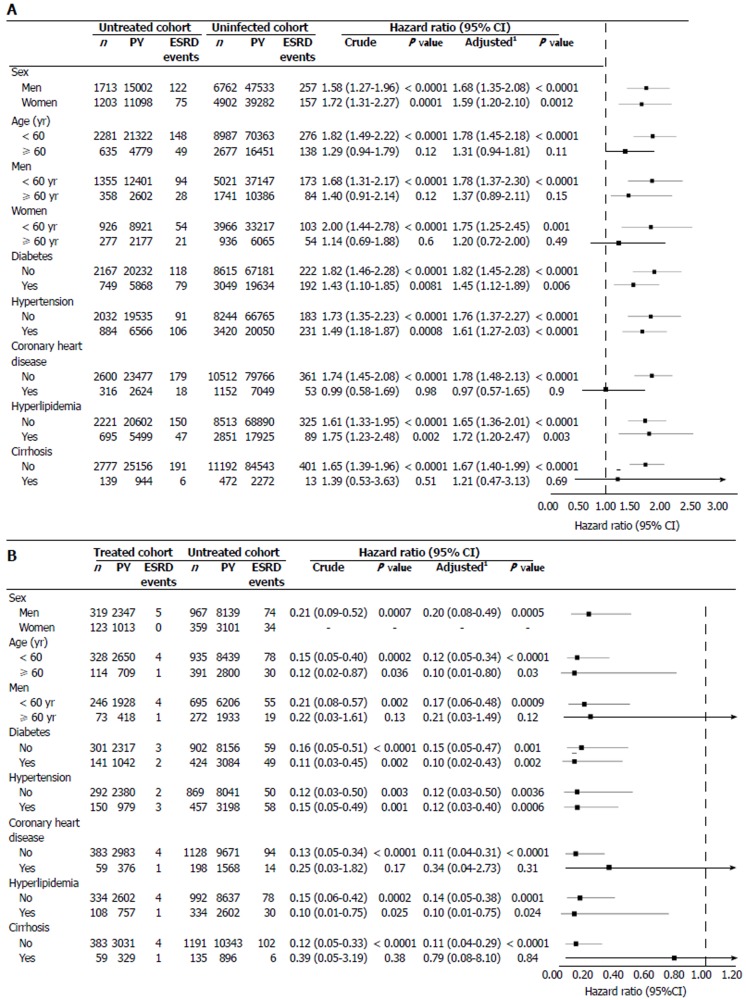
The untreated cohort exhibited an increased risk of ESRD on all stratified analyses (Figure 3A), except for those with coronary heart disease. This association was more pronounced in patients younger than 60 years (1.78; 1.45-2.18; P < 0.0001), men younger than 60 years (1.78; 1.37-2.30; P < 0.0001), women younger than 60 years (1.75; 1.25-2.45; P = 0.001), and patients without coronary heart disease (1.78; 1.48-2.13; P < 0.0001) and cirrhosis (1.67; 1.40-1.99; P < 0.0001). The treated cohort exhibited a reduced risk of ESRD on all stratified analyses (Figure 3B). This association was more pronounced in men younger than 60 years (0.17; 0.06-0.48; P = 0.0009) and patients without coronary heart disease (0.11; 0.04-0.31; P < 0.0001) and cirrhosis (0.11; 0.04-0.29; P < 0.0001).
Figure 3.
Stratified analyses after adjustment for competing mortality in the (A) propensity score-matched chronic kidney disease cohort and (B) propensity score-matched hepatitis B virus cohort. PY: person-year; ESRD: end-stage renal disease; HBV: Hepatitis B virus; CI: confidence interval. 1Adjusted for all covariates (age per year, sex, comorbidities, geographic region, urbanization level, enrolee category, number of medical visits, Charlson comorbidity index score, and propensity score) and competing mortality, minus the covariate on which stratified.
DISCUSSION
After employing PS-matching and competing mortality, this 16-year nationwide cohort study provided three novel insights. First, the incidence of CKD patients acquiring HBV infection was 3.2%. Second, the risk of ESRD was 1.67-fold higher in the untreated cohort than the uninfected CKD cohort, was independent of cirrhosis or diabetes, and was reduced by 87% in the treated cohort compared to the untreated cohort. These associations were consistent in stratified subgroup analyses. Third, the NNT in association with one patient free of ESRD at 12 years after NA use was 12. These findings implied that HBV infection may have a role in the pathogenesis of renal injury, and that HBV suppression may improve renal outcome among CKD patients. Given the increased risk of exposure to HBV in CKD patients, this information has important clinical implications for the design of surveillance programs that assess HBV status in CKD patients.
To the best of our knowledge, this is the first study to investigate the incidence and renal outcome in CKD patients acquiring chronic HBV infection. This has long been a neglected issue because there is no recommendation in the K/DOQI guidelines[25] on whether HBV serological evaluation should be carried out in CKD patients. The majority (≥ 60%) of CKD patients acquiring chronic HBV infection are known to become chronic carriers because of a deficiency in CD8+ cytotoxic and CD4+ helper T lymphocytes[1,11]; however, the incidence was unknown. Only two hospital-based studies and one community-based study reported that the prevalence of HBV infection was 10.5% in Turkish patients with CKD stages 4-5[26], 3.5% in Turkish patients with CKD stages 3-5[10], and 9.9% in Taiwanese patients with CKD stages 1-5[27]. Only two case-series studies and one population-based study from China reported the potential risk of ESRD in target CKD subpopulations such as patients with primary GN[28] or HBV-GN[8], and those with diabetic nephropathy[7]. However, these studies did not clarify the difference between HBV-untreated vs uninfected CKD patients or perform subgroup analyses. The present study indicated that untreated chronic HBV infection was an independent predictor of ESRD risk in CKD patients, suggesting its role in CKD progression. Given the fact that there are 50 million new cases of HBV diagnosed annually[29] and the burden of CKD continues to increase[1], it is conceivable that the burden of ESRD in CKD patients following chronic HBV infection is increasing.
This is also the first study to evaluate the renal effect of NA therapy and its NNT for one fewer ESRD in HBV-infected CKD patients. Previous studies demonstrated the benefit of NA therapy in reducing proteinuria[16,17,30,31], serum HBV-DNA[16,17,31], and risk of ESRD[17] in HBV-GN patients. However, these studies followed small numbers of patients for short periods, did not provide the NNT after NA therapy, and failed to guide NA therapy in HBV-infected CKD patients. In the present study, we did not have information on baseline HBV-DNA or alanine aminotransferase levels. However, based on the fact that reimbursement for NAs in Taiwan’s NHI program requires twice-elevated alanine aminotransferase and higher HBV-DNA levels (> 2000 IU/mL)[20,32], it is reasonable to assume that the treated cohort had higher baseline HBV-DNA and alanine aminotransferase levels, or a higher prevalence of liver decompensation than the untreated cohort to be eligible for reimbursement. Our present data showed a higher prevalence of cirrhosis in the treated cohort compared to the untreated cohort. Since higher HBV-DNA levels are associated with a higher risk of renal injury, the higher baseline HBV-DNA levels in the treated cohort may have led to a more conservative estimation of the association of the renoprotective effect of NAs[20,32] and more favorable renal response to NAs[33]. This may explain why NA treatment for HBV-infected CKD patients in our study was associated with an 87% lower risk of ESRD compared to NA-naive HBV-infected CKD patients in a 16-year follow-up. Moreover, we used a large nationwide dataset, which afforded considerable statistical power and allowed long-term tracking of incident ESRD events.
The exact mechanism of how HBV infection and NA therapy are associated with increased and decreased ESRD risk, respectively, is unclear. Our data suggested that this association can be explained by NA-mediated HBV suppression and reduction in serum HBV-DNA levels. Previous studies[6,16,34] have addressed the association between serum HBV-DNA and renal injury. HBV-DNA was identified in renal tissue from 95.3% of HBV-GN patients[34], and the level of HBV-DNA correlated well with the duration of proteinuria. The degree of proteinuria and the extent of pathological injury, which was defined by the glomerular deposition of HBV antigens, also correlated well with the level of serum HBV-DNA in HBV-GN patients[16]. The same study also showed that patients with higher levels of serum HBV-DNA had lower level of serum complement, suggesting a correlation between serum HBV-DNA levels and the deposition of immune complexes, and the existence of a mechanism involving HBV-related immune complexes formed by passive trapping or in situ formation. Du et al[35] demonstrated that high levels of serum HBV-DNA correlated with the expression of inflammatory factors in renal biopsy specimens from HBV-GN patients. Deposition of immune complexes in the kidney is perceived to play a pivotal role in the pathogenesis of HBV-related nephropathy. Reducing the quantity of viral antigens and thereby reducing immune complexes in the kidney might ameliorate kidney damage. Other researchers postulated other mechanisms for HBV-related renal injury. Sera from adult patients with mild chronic HBV infection, regardless of the presence of HBV-DNA, induced apoptosis of cultured human renal tubular cells via up-regulation of Fas gene expression, and the induction of apoptosis correlated well with the level of serum HBV-DNA[6]. Furthermore, sera from patients with chronic HBV infection also had higher levels of transforming growth factor-beta, a growth factor implicated in the development of apoptosis and renal fibrosis. This may explain why the untreated cohort, a proxy for lower serum HBV-DNA levels in the present study, had a higher risk of ESRD compared to the uninfected cohort. Further research is warranted to better understand the mechanism.
In the present study, we used many methods to prevent potential confounders. PS matching was used to select comparable controls to imitate a randomized clinical trial and competing risk analysis was used to prevent overestimation of nonfatal outcomes in the untreated cohort[36] and during CKD progression[21]. Another strength of our study was that it was designed to reduce selection bias (through the use of a large nationwide and highly representative sample with random sampling)[5,36,37]; reduce environmental effects (because of the availability of socioeconomic indicators for all subjects)[5,36,37]; avoid detection bias (because of the universal availability of medical services)[5,36,37]; and avoid immortal time bias (because the time when patients received NA was chosen as the entry of observation)[9,20]. In addition, the study population was well defined and follow-up was complete because our design relied on the universal coverage of Taiwan’s NHI, which fully reimbursed NA therapy for HBV infection and thus minimized disparity in healthcare accessibility or financial status as a determinant for receiving NA therapy. Although unmeasured confounders may still exist, as with any observational study, we believe that the methodology used in the present study is solid and robust.
It is important to note the limitations of this study. First, coding errors are possible in a database. We could not check the accuracy of NA use in the NHIRD. However, the information regarding insurance-paid NAs was accurate because NA prescription was under strict NHI regulations. Second, some patients may have used self-paid NAs and may have therefore been misclassified into the untreated cohort. Some of our controls may have had sub-clinical HBV infection. These potential misclassifications may have led to an underestimation of the association. Third, information about adverse events of NAs was not available from the NHIRD. However, NAs are generally well-tolerated, relatively safe after dosage adjustment, and are the best options for HBV-positive CKD patients[13]. Fourth, the NHIRD lacks information on family history of kidney diseases, lifestyle, and laboratory data (e.g., levels of serum HBV DNA and HBV genotype), which may contribute to ESRD risk. Thus, we could not adjust for these variables in the PS-matching. Nevertheless, it has been shown that HBV genotype is not related to extrahepatic manifestations[38] and does not influence treatment outcome of NAs[15]. Moreover, we added CCI scores into the PS analysis and included the CCI score and PS in the regression analysis to control confounding in healthcare administrative databases[5,36]. This method had been used in previous NHIRD-based research on patients with chronic HBV infection[5,32]. Finally, the PS matching cannot fit well in all treated, untreated, and uninfected groups. The cumulative ESRD incidence of the PS-matched three cohorts warrants further prospective research.
In conclusion, this large cohort study provides evidence that untreated HBV infection and NA therapy are associated with increased and decreased risk of ESRD, respectively, in CKD patients. Our data suggest that clinicians should consider HBV serological evaluation in CKD patients, especially in areas of high HBV endemicity, so that these patients can be closely monitored for development of ESRD. Further research is warranted to explore the pathological mechanism underlying this association.
ARTICLE HIGHLIGHTS
Research background
The evidence on whether HBV infection affects renal outcome in patients with chronic kidney disease (CKD) is limited. Here we retrospectively explored the association between HBV infection with and without nucleos(t)ide analogue use and the risk of end-stage renal disease (ESRD) in patients with CKD.
Research motivation
There is a significant and increasing burden of CKD, ESRD, and HBV infection in Taiwan and worldwide. CKD patients have an increased risk of acquiring chronic HBV infection. However, the effect of HBV infection and nucleos(t)ide analogue use on the risk of ESRD in CKD patients remains unclear. Taiwan provides an ideal setting for studying this relationship because it has a high prevalence of these three conditions.
Research objectives
To investigate the effect of HBV infection with and without nucleos(t)ide analogue use on the ESRD risk in Taiwanese CKD patients.
Research methods
By analyzing the Taiwan Longitudinal Health Insurance Database 2005, the authors used propensity score-matched and competing risk analyses to evaluate the effect of HBV infection with and without nucleos(t)ide analogue therapy on the development of ESRD in CKD patients. The authors used a modified Kaplan-Meier method and Gray’s method to calculate and compare the cumulative incidence of ESRD, and a modified Cox proportional hazard model in the presence of competing risk and stratified analyses to determine the ESRD risk between propensity-matched untreated HBV-infected and HBV-uninfected CKD patients as well as propensity-matched untreated and treated HBV-infected CKD patients. The authors also calculated the number of patients needed to be treated to yield one fewer ESRD.
Research results
In the propensity-matched untreated HBV-infected and HBV-uninfected CKD patients, the risk of ESRD was significantly higher in the untreated cohort (16-year cumulative incidence, 10.1%) than in the uninfected cohort (16-year cumulative incidence, 6.6%) with a significant adjusted hazard ratio of 1.67. In the propensity-matched HBV-treated and HBV-untreated CKD patients, the treated cohort (16-year cumulative incidence, 2.2%) had a significantly 87% reduced ESRD risk compared with the untreated cohort (16-year cumulative incidence, 11.9%). The number needed to treat for one fewer ESRD after NA use at 12 years was 12. Multivariable stratified analyses verified these associations in all subgroups. However, the cumulative ESRD incidence of the propensity-matched three cohorts warrants further prospective research because the propensity score matching cannot fit well in all treated, untreated, and uninfected CKD patients.
Research conclusions
To the best of our knowledge, this is the first study to investigate the incidence and renal outcome in CKD patients acquiring chronic HBV infection and to evaluate the renal effect of nucleos(t)ide analogue therapy and the number needed to treat for one fewer ESRD in HBV-infected CKD patients. This has long been a neglected issue because there is no recommendation in the K/DOQI guidelines on whether HBV serological evaluation should be carried out in CKD patients. This large retrospective cohort study suggests that the incidence of CKD patients acquiring HBV infection is 3.2% and that untreated HBV infection and nucleos(t)ide analogue therapy are associated with increased and decreased risk of ESRD, respectively, in CKD patients. These findings imply that HBV infection may have a role in the pathogenesis of renal injury, and that HBV suppression may improve renal outcome among CKD patients. Our findings will be helpful in the future CKD prevention care program that assesses HBV status in CKD patients.
Research perspectives
Future prospective study is warranted to confirm our findings and better understand the pathological mechanism underlying this association.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Supported by Dalin Tzu Chi Hospital, No. DTCRD 104-I-16.
Institutional review board statement: This study was approved by the institutional review board of the Dalin Tzu Chi Hospital (B10302011).
Informed consent statement: All patients’ information was de-identified in the database (LHID2005) and no informed consent was required. This study was exempt from a full ethical review by the institutional review board of the Dalin Tzu Chi Hospital (B10302011).
Conflict-of-interest statement: All authors have no conflict of interests.
Data sharing statement: No additional data are available.
Peer-review started: November 4, 2017
First decision: November 22, 2017
Article in press: December 20, 2017
P- Reviewer: Kao JT, Kute VB, Nemcsik J, Robles NR S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Ma YJ
Contributor Information
Yi-Chun Chen, Division of Nephrology, Department of Internal Medicine, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Chiayi County 622, Taiwan; School of Medicine, Tzu Chi University, Hualien 970, Taiwan.
Chung-Yi Li, Department and Graduate Institute of Public Health, College of Medicine, National Cheng Hung University, Tainan 701, Taiwan; Department of Public Health, College of Public Health, China Medical University, Taichung 404, Taiwan.
Shiang-Jiun Tsai, Department of Medical Research, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Chiayi County 622, Taiwan.
Yen-Chun Chen, Division of Hepato-Gastroenterology, Department of Internal Medicine, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Chiayi County 622, Taiwan。 chenyichun0320@yahoo.com.tw.
References
- 1.Chacko EC, Surrun SK, Mubarack Sani TP, Pappachan JM. Chronic viral hepatitis and chronic kidney disease. Postgrad Med J. 2010;86:486–492. doi: 10.1136/pgmj.2009.092775. [DOI] [PubMed] [Google Scholar]
- 2.Cooke GS, Lemoine M, Thursz M, Gore C, Swan T, Kamarulzaman A, DuCros P, Ford N. Viral hepatitis and the Global Burden of Disease: a need to regroup. J Viral Hepat. 2013;20:600–601. doi: 10.1111/jvh.12123. [DOI] [PubMed] [Google Scholar]
- 3.Mauss S, Berger F, Filmann N, Hueppe D, Henke J, Hegener P, Athmann C, Schmutz G, Herrmann E. Effect of HBV polymerase inhibitors on renal function in patients with chronic hepatitis B. J Hepatol. 2011;55:1235–1240. doi: 10.1016/j.jhep.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 4.Chen YC, Su YC, Li CY, Hung SK. 13-year nationwide cohort study of chronic kidney disease risk among treatment-naïve patients with chronic hepatitis B in Taiwan. BMC Nephrol. 2015;16:110. doi: 10.1186/s12882-015-0106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen YC, Su YC, Li CY, Wu CP, Lee MS. A nationwide cohort study suggests chronic hepatitis B virus infection increases the risk of end-stage renal disease among patients in Taiwan. Kidney Int. 2015;87:1030–1038. doi: 10.1038/ki.2014.363. [DOI] [PubMed] [Google Scholar]
- 6.Deng CL, Song XW, Liang HJ, Feng C, Sheng YJ, Wang MY. Chronic hepatitis B serum promotes apoptotic damage in human renal tubular cells. World J Gastroenterol. 2006;12:1752–1756. doi: 10.3748/wjg.v12.i11.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng AY, Kong AP, Wong VW, So WY, Chan HL, Ho CS, Lam CW, Tam JS, Chow CC, Cockram CS, et al. Chronic hepatitis B viral infection independently predicts renal outcome in type 2 diabetic patients. Diabetologia. 2006;49:1777–1784. doi: 10.1007/s00125-006-0294-4. [DOI] [PubMed] [Google Scholar]
- 8.Lai KN, Li PK, Lui SF, Au TC, Tam JS, Tong KL, Lai FM. Membranous nephropathy related to hepatitis B virus in adults. N Engl J Med. 1991;324:1457–1463. doi: 10.1056/NEJM199105233242103. [DOI] [PubMed] [Google Scholar]
- 9.Peters MG. Special populations with hepatitis B virus infection. Hepatology. 2009;49:S146–S155. doi: 10.1002/hep.22965. [DOI] [PubMed] [Google Scholar]
- 10.Pişkinpaşa S, Akoğlu H, Özkayar N, Turgut D, Akyel F, Demir M, Çimen K, Turhan T, KOÇ E, ODABAŞ AR, et al. Seroprevalance of the hepatitis B and C in patients with chronic kidney disease without history of renal replacement therapy. Turk Neph Dial Transpl. 2013;22:171–176. [Google Scholar]
- 11.Urbánek P. Viral hepatitis infections in chronic kidney disease patients and renal transplant recipients. Kidney Blood Press Res. 2012;35:454–467. doi: 10.1159/000338309. [DOI] [PubMed] [Google Scholar]
- 12.Taal MW, Brenner BM. Predicting initiation and progression of chronic kidney disease: Developing renal risk scores. Kidney Int. 2006;70:1694–1705. doi: 10.1038/sj.ki.5001794. [DOI] [PubMed] [Google Scholar]
- 13.Pipili CL, Papatheodoridis GV, Cholongitas EC. Treatment of hepatitis B in patients with chronic kidney disease. Kidney Int. 2013;84:880–885. doi: 10.1038/ki.2013.249. [DOI] [PubMed] [Google Scholar]
- 14.Inoue J, Ueno Y, Shimosegawa T. Management of chronic hepatitis B patients: efficacy & limitation of nucleos(t)ide analogues. Indian J Med Res. 2011;133:11–13. [PMC free article] [PubMed] [Google Scholar]
- 15.Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486–1500. doi: 10.1056/NEJMra0801644. [DOI] [PubMed] [Google Scholar]
- 16.Jiang W, Liu T, Dong H, Xu Y, Liu LQ, Guan GJ, Liu XC. Relationship Between Serum DNA Replication, Clinicopathological Characteristics and Prognosis of Hepatitis B Virus-associated Glomerulonephritis with Severe Proteinuria by Lamivudine Plus Adefovir Dipivoxil Combination Therapy. Biomed Environ Sci. 2015;28:206–213. doi: 10.3967/bes2015.027. [DOI] [PubMed] [Google Scholar]
- 17.Tang S, Lai FM, Lui YH, Tang CS, Kung NN, Ho YW, Chan KW, Leung JC, Lai KN. Lamivudine in hepatitis B-associated membranous nephropathy. Kidney Int. 2005;68:1750–1758. doi: 10.1111/j.1523-1755.2005.00591.x. [DOI] [PubMed] [Google Scholar]
- 18.National Health Insurance Administration, Ministry of Health and Welfare, Taiwan. Available from: http://www.nhi.gov.tw.
- 19.Kuo HW, Tsai SS, Tiao MM, Yang CY. Epidemiological features of CKD in Taiwan. Am J Kidney Dis. 2007;49:46–55. doi: 10.1053/j.ajkd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS, Lin JT. Association between nucleoside analogues and risk of hepatitis B virus–related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308:1906–1914. doi: 10.1001/2012.jama.11975. [DOI] [PubMed] [Google Scholar]
- 21.Grams ME, Coresh J, Segev DL, Kucirka LM, Tighiouart H, Sarnak MJ. Vascular disease, ESRD, and death: interpreting competing risk analyses. Clin J Am Soc Nephrol. 2012;7:1606–1614. doi: 10.2215/CJN.03460412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 23.Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317:1309–1312. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 25.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S221–S266. [PubMed] [Google Scholar]
- 26.Sit D, Kadiroglu AK, Kayabasi H, Yilmaz ME, Goral V. Seroprevalence of hepatitis B and C viruses in patients with chronic kidney disease in the predialysis stage at a university hospital in Turkey. Intervirology. 2007;50:133–137. doi: 10.1159/000098239. [DOI] [PubMed] [Google Scholar]
- 27.Lee JJ, Lin MY, Yang YH, Lu SN, Chen HC, Hwang SJ. Association of hepatitis C and B virus infection with CKD in an endemic area in Taiwan: a cross-sectional study. Am J Kidney Dis. 2010;56:23–31. doi: 10.1053/j.ajkd.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Lai KN, Lai FM, Chan KW, Chow CB, Tong KL, Vallance-Owen J. The clinico-pathologic features of hepatitis B virus-associated glomerulonephritis. Q J Med. 1987;63:323–333. [PubMed] [Google Scholar]
- 29.Patton H, Tran TT. Management of hepatitis B during pregnancy. Nat Rev Gastroenterol Hepatol. 2014;11:402–409. doi: 10.1038/nrgastro.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Zhou JH, Yin XL, Wang FY. Treatment of hepatitis B virus-associated glomerulonephritis: a meta-analysis. World J Gastroenterol. 2010;16:770–777. doi: 10.3748/wjg.v16.i6.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yi Z, Jie YW, Nan Z. The efficacy of anti-viral therapy on hepatitis B virus-associated glomerulonephritis: A systematic review and meta-analysis. Ann Hepatol. 2011;10:165–173. [PubMed] [Google Scholar]
- 32.Wu CY, Lin JT, Ho HJ, Su CW, Lee TY, Wang SY, Wu C, Wu JC. Association of nucleos(t)ide analogue therapy with reduced risk of hepatocellular carcinoma in patients with chronic hepatitis B: a nationwide cohort study. Gastroenterology. 2014;147:143–151.e5. doi: 10.1053/j.gastro.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 33.Chan TM. Hepatitis B and Renal Disease. Curr Hepat Rep. 2010;9:99–105. doi: 10.1007/s11901-010-0042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He XY, Fang LJ, Zhang YE, Sheng FY, Zhang XR, Guo MY. In situ hybridization of hepatitis B DNA in hepatitis B-associated glomerulonephritis. Pediatr Nephrol. 1998;12:117–120. doi: 10.1007/s004670050417. [DOI] [PubMed] [Google Scholar]
- 35.Du W, Zhen J, Zheng Z, Ma S, Chen S. Expression of AIM2 is high and correlated with inflammation in hepatitis B virus associated glomerulonephritis. J Inflamm (Lond) 2013;10:37. doi: 10.1186/1476-9255-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen YC, Hwang SJ, Li CY, Wu CP, Lin LC. A Taiwanese Nationwide Cohort Study Shows Interferon-Based Therapy for Chronic Hepatitis C Reduces the Risk of Chronic Kidney Disease. Medicine (Baltimore) 2015;94:e1334. doi: 10.1097/MD.0000000000001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen YC, Lin HY, Li CY, Lee MS, Su YC. A nationwide cohort study suggests that hepatitis C virus infection is associated with increased risk of chronic kidney disease. Kidney Int. 2014;85:1200–1207. doi: 10.1038/ki.2013.455. [DOI] [PubMed] [Google Scholar]
- 38.Cacoub P, Saadoun D, Bourlière M, Khiri H, Martineau A, Benhamou Y, Varastet M, Pol S, Thibault V, Rotily M, et al. Hepatitis B virus genotypes and extrahepatic manifestations. J Hepatol. 2005;43:764–770. doi: 10.1016/j.jhep.2005.05.029. [DOI] [PubMed] [Google Scholar]