Abstract
For barnacle cypris larvae at the point of settlement, selection of an appropriate surface is critical. Since post-settlement relocation is usually impossible, barnacles have evolved finely tuned surface-sensing capabilities to identify suitable substrata, and a temporary adhesion system for extensive surface exploration. The pattern of exploratory behaviour appears complex and may last for several hours, imposing significant barriers to quantitative measurement. Here, we employ a novel tracking system that enables simultaneous analysis of the larval body movement of multiple individuals over their entire planktonic phase. For the first time, to our knowledge, we describe quantitatively the complete settlement process of cyprids as they explore and select surfaces for attachment. We confirm the ‘classic’ behaviours of wide searching, close searching and inspection that comprise a model originally proposed by Prof. Dennis Crisp FRS. Moreover, a short-term assay of cyprid body movement has identified inspection behaviour as the best indicator of propensity to settle, with more inspection-related movements occurring in conditions that also promote higher settlement. More than half a century after the model was first proposed by Crisp, there exists a precise method for quantifying cyprid settlement behaviour in wide-ranging investigations of barnacle ecology and applied studies of fouling management.
Keywords: barnacle larvae, cyprid, settlement behaviour, tracking, biofouling
1. Background
When humans refer to the ‘decision of a lifetime’, this is often hyperbole. For barnacle larvae at the point of settlement, however, the metaphor is more appropriate. The cypris larva, which is specialized for identification of suitable attachment sites and adhesion to them, has little margin for error. After the release of their permanent cement, most barnacles are unable to move any further. Surface selectivity has evolved to ensure their settlement under suitable environmental conditions, including surface physicochemical properties, local hydrodynamic conditions [1] and within proximity to a potential mate. The latter fulfils the requirement of most species for reproduction by pseudocopulation, akin to internal fertilization. Surface selection occurs both by innate species-specific preference for particular environmental conditions and/or direct identification of the presence of conspecifics [2,3]. The former may include perception of specific biofilms [4] substrate physicochemistry [5] or, for epibiotic or parasitic forms, the particular chemical cues released by the host [6,7]. For direct identification of potential mates, cyprids are able to detect and respond to a pheromone, the settlement-inducing protein complex [8]. The colonization of surfaces by barnacles is not a random process, therefore, but there remains surprisingly little insight into the means by which cyprids process information and select surfaces for attachment through, for example, releaser behaviour or aversive/appetitive memory [9].
When they encounter a surface, cyprids engage in an elaborate sequence of exploratory activity that has been subject to study for over half a century [9,10]. Theoretically, if we can understand the decision-making process of barnacles at settlement, we can design more effective, environmentally benign antifouling coatings to which they choose not to attach [11] or, indeed, stimulate settlement where this is desirable such as in the aquaculture of food species [12]. In practice, however, quantitative analysis of barnacle cyprid behaviour is a complex theoretical and practical challenge, and the software and hardware tools available to the field have so far proven inadequate (see Alsaab et al. [13] for detailed discussion).
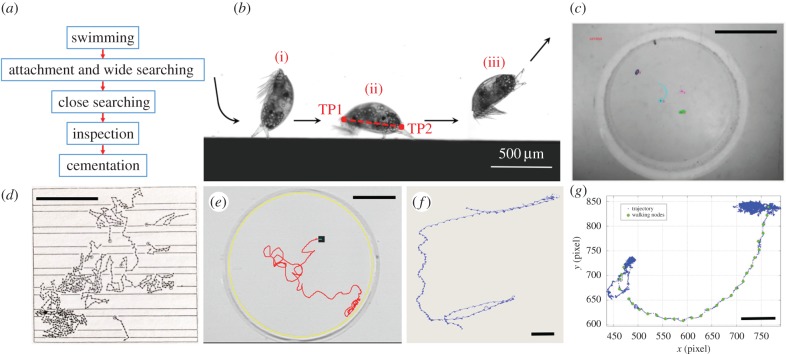
All recent studies of cyprid behaviour [14–22] have, to a greater or lesser extent, referred to the ‘classic’ model of cyprid exploration described by Crisp [9] (figure 1a). Crisp outlined a sequence whereby swimming cyprids encounter a surface, attach using their temporary adhesive mechanism and ‘walk’ in a bipedal fashion using a pair of highly specialized antennules, detecting local surface cues as they go (figure 1b). Their exploratory pattern was described by Crisp as broad, at first, covering a large area in straight lines or shallow arcs; ‘wide searching’. At any stage, the cyprid could detach and return to the water column, delaying settlement. However, if the surface remained acceptable, the cyprid could constrain its exploration to a smaller area, turning more frequently; ‘close searching’. Finally, before settlement, cyprids engaged in ‘inspection’. Inspection, as defined by Crisp, was attachment to the surface by one or both antennules while probing the local area [23]. In our analysis, we do not identify inspection based on the exact position of the antennules, but rather the relative locations of the terminal points of the cyprid body, as described by Alsaab et al. [13] (figure 1b). It was clear from early observations that ‘inspection’, by this definition, occurs throughout the exploratory period and is not restricted to the moments before permanent attachment. The reader is referred to the electronic supplementary material document for a fuller description of the behaviours.
Figure 1.
(a) The widely accepted stages of behaviour observed in the settlement process of barnacle cypris larvae. (b) An example of the initial stages of this process; (i) locating a surface, (ii) wide/close searching, and (iii) returning to the water column. TP1 and TP2 correspond to the posterior and anterior terminal points of the cyprid, the relative movements of which are used to define walking and inspection nodes (reproduced with permission from [13]). (c) An example image captured from the new tracking system described in Alsaab et al. [13]. (d) One of the first attempts by Crisp to track exploring cyprids [10]. (e) The evolution of more advanced methods began with the use of Noldus EthoVision 3.1 [14]. (f) This was followed by 3D tracking using SIMI Motion (our laboratory 2012, unpublished data). Periodic dots on the blue track indicate cyprid footsteps. (g) Finally, the system described by Alsaab et al. [13]. Scale bars: (c) 6 mm, (d) 10 mm, (e) 10 mm, (f) 2 mm, (g) 2 mm.
Briefly, our analysis pipeline is enabled by automatic identification of nodes, of which there are two types: ‘walking nodes’ and ‘inspection nodes’. A walking node is identified by the characteristic gate of a walking cyprid [13]. One node equates to one step and each step typically contains only one movement. Wide and close searching both contain walking nodes. Inspection nodes correspond to periods of time when a cyprid is attached by one or both antennules to a single point. Each inspection node can contain multiple body movements that correspond to the metronomic swinging of the body around the attachment point. Inspection and close searching both contain inspection nodes with multiple movements.
Unlike many other implementations of behavioural analysis in zooplankton ecology [24,25], in the study of cyprid behaviour, we are interested specifically in: (i) behaviour on a surface, excluding swimming in the water column; and (ii) what the animals are doing, rather than where they are going. Although subtle, these two distinctions have required a step-change in our approach from subjective interpretation of the path shape (figure 1c–f) of individuals that spend greater than 90% of their time swimming, to direct identification of the behaviours associated with settlement based on quantitative analysis of walking and inspection (figure 1g). To this end, our novel tracking and classification system [13] identifies walking and inspection behaviour of multiple cyprids directly from body movements, without reference to the track shape, enabling rapid, quantitative and objective analysis of only the behaviours of interest.
While the individual elements of the settlement process (figure 1a), and sometimes combinations thereof, can be observed easily in the laboratory, the time scales involved (h) and low frequency of the behaviours of interest have precluded validation of Crisp's model. It remains unclear whether the three phases of settlement behaviour exist as discrete activities [23,26], or whether they simply arise at different time points in a spectrum of gradually refined surface investigation. Further, it is not known how invariant the behaviours are between individuals and, thus, how useful a behavioural assay may be as an indicator of propensity to settle. Previous studies have been limited to interpretation of short snapshots of behaviour out of context [14,16–18,20,21]. A significant advantage of our system is that it enables us to track multiple cyprids, simultaneously, throughout the entire pelagic–benthic transition to settlement, providing detail about the sequence of behaviours as well as the ability to compare between experimental conditions. Importantly, and uniquely among studies separating these three exploratory behaviours, the opinion of the observer has been entirely removed.
This paper focuses on three objectives identified during development of the tracking system that have the potential to accelerate our understanding of habitat selection by barnacles. First, we describe the ability of the system to identify Crisp's behaviours. Second, we use automated assignment of behaviours to identify features that invariably indicate surface exploration prior to settlement. Third, we developed a short-term assay to allow use of cyprid pre-settlement behaviour as tool for comparison of experimental conditions. For illustration, we use cyprids of varying ages [27] and an artificial settlement inducer, 3-isobutyl-1-methylxanthine (IBMX) [28], to stimulate increased settlement by ‘natural’ and ‘unnatural’ means. We propose that analysis of cyprid behaviour has matured sufficiently to make a significant contribution to the development of novel coatings, materials and agents to combat the multibillion-dollar challenge of marine biofouling [29] and answer longstanding questions about the fundamentals of barnacle settlement behaviour.
2. Material and methods
The methods detailed here relate to the practical running of experiments. More detailed descriptions of the tracking system, software, node identification, definitions of behaviours and data analysis can be found in the electronic supplementary material.
(a). Barnacle cyprid culture
Stocks of adult Balanus amphitrite (=Amphibalanus amphitrite) were supplied from the Duke University Marine Laboratory, Beaufort, NC, USA, and used as per Alsaab et al. [13] to obtain cypris larvae, which were then stored at 6°C for experiments.
(b). Preparation of solutions
Artificial seawater (ASW; Tropic Marin) was produced in 100 l batches and stored for 2 days prior to use. For production of 10−5 M solutions of IBMX, the dry chemical was first dissolved in analytical grade dimethylsulfoxide (DMSO). Sufficient DMSO was used to result in a final working concentration of 1 : 10 000 with ASW. Once the IBMX was completely dissolved in the DMSO carrier, seawater was added slowly with frequent mixing to bring the solution to an intermediate concentration of 10−3 M for storage at −20°C. It was then further diluted to the working concentration of 10−5 M. The control solution for IBMX experiments was DMSO at the working concentration of 1 : 10 000.
(c). Settlement assay
Settlement assays were performed using larvae from the same batches as those monitored in tracking experiments. Untreated polystyrene 24-well plates (IWAKI™) were used. Two millilitres of ASW, IBMX or control solutions were added to 12 replicate wells for each treatment. Ten cyprids of the appropriate age were added to each well. For the IBMX experiment, cyprids were used at 3 days old [30]. The assay was incubated in the dark at 28°C for 24 h [30], at which time the numbers of swimming and settled cyprids were counted.
(d). Design of tracking experiments
Broadly, experiments were divided into long-term (figures 4–6) and short-term studies (figures 7 and 8); the short-term studies overcoming the intrinsic limitations on sample number experienced in long-term observations. For experiments using IBMX, the treatment and control recordings were made simultaneously. Those involving cyprids of different ages, by definition, occurred on different days using cyprids from the same culture. Long-term and short-term experiments were conducted at different times, using independent, batches of larvae.
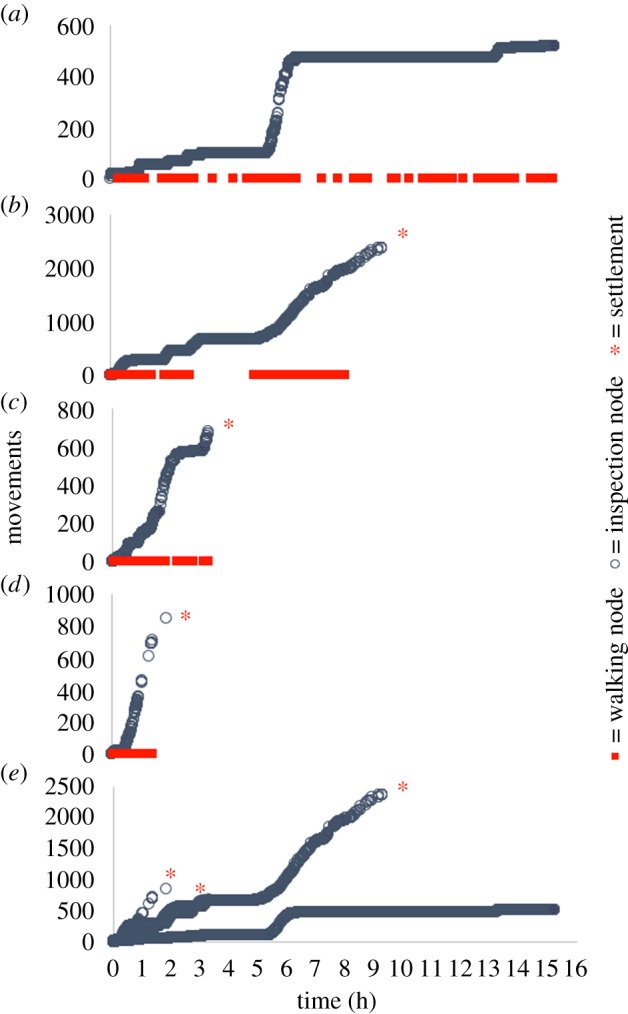
Figure 4.
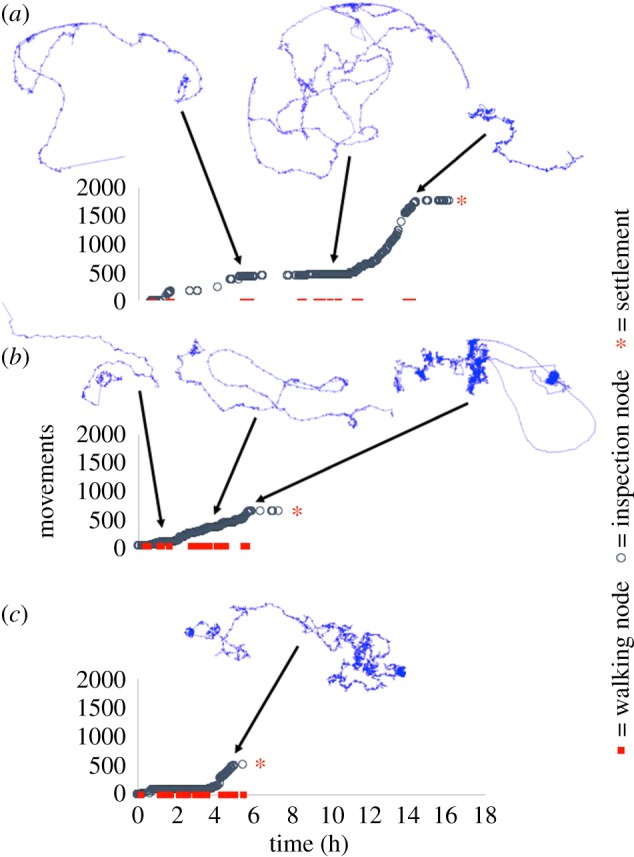
Cumulative plots of body movements recorded during the entire free-swimming/exploratory period of cyprids aged 1 (a), 3 (b) and 5 (c) days post-moult to the cyprid. The trajectories illustrated in blue are selected stretches of exploratory behaviour, their locations on the track data profile indicated by an arrow. (Online version in colour.)
Figure 6.
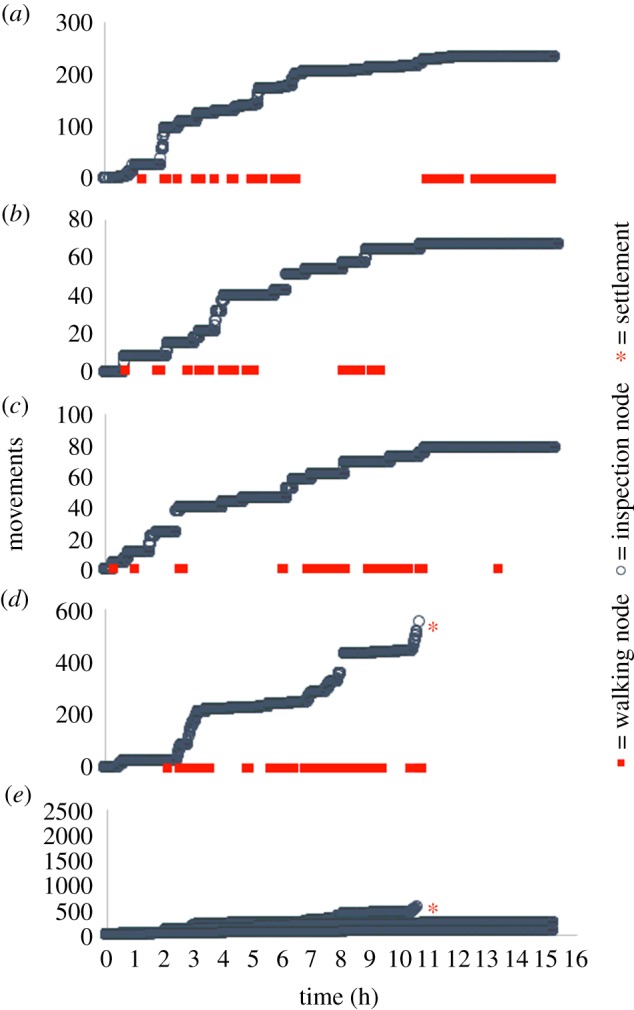
(a–d) As figure 5, but exposure to a 1 : 10000 control solution of DMSO in ASW. Plots (a–d) are presented on the same y-axis in (e). (Online version in colour.)
Figure 7.
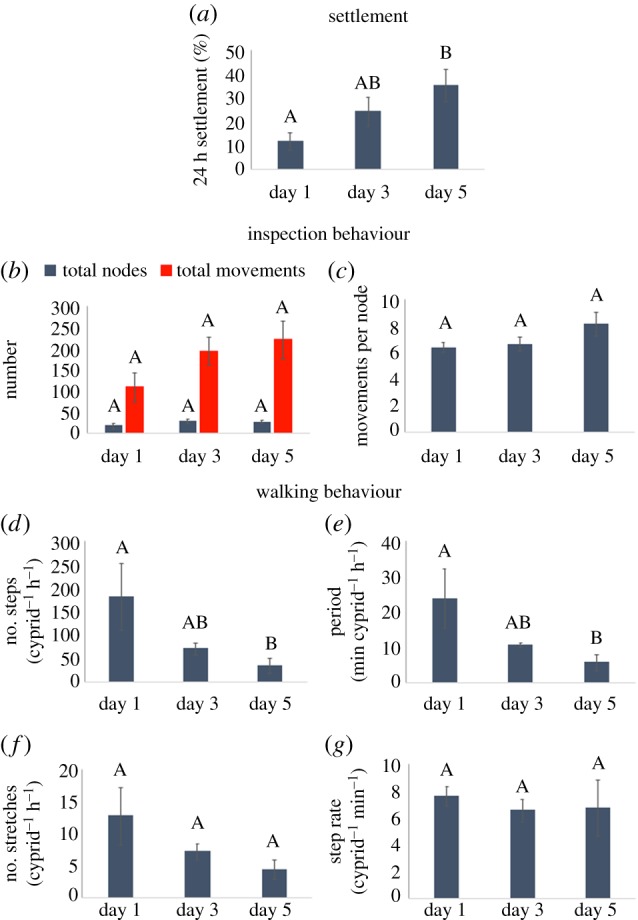
(a) Settlement of cyprids of three different ages after a standard 24 h settlement period (n = 12), (b) the total number of nodes and total number of movements in all nodes for all cyprids in 1 h long tracking experiments, (c) the data in (b) processed into an average number of movements per node for each cyprid age, (d) average number of walking steps taken per cyprid per hour, (e) average period of walking, (f) average number of stretches of walking behaviour and (g) the rate of walking for each cyprid age. All errors are standard errors of the mean. Bars that do not share a letter differ significantly (ANOVA) at an α-level of 0.05, n = 4 for (b–g). (Online version in colour.)
Figure 8.
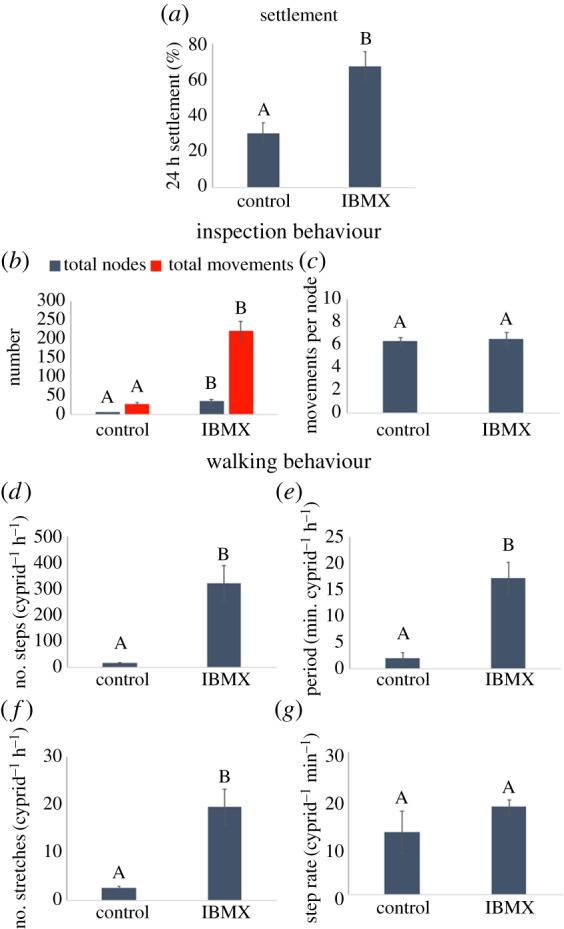
(a–g) As figure 7, but for settlement/behaviour of 3-day-old cyprids exposed to IBMX and control solutions. (Online version in colour.)
A typical long-term experiment involved addition of five cyprids, usually 3 days of age, to an experimental tube, which were then recorded from above for up to 18 h. Any cyprids that settled during this period were tracked either through their final exploratory period (figure 3) or for the entire pelagic and benthic periods leading up to settlement (figures 4–6).
Figure 3.
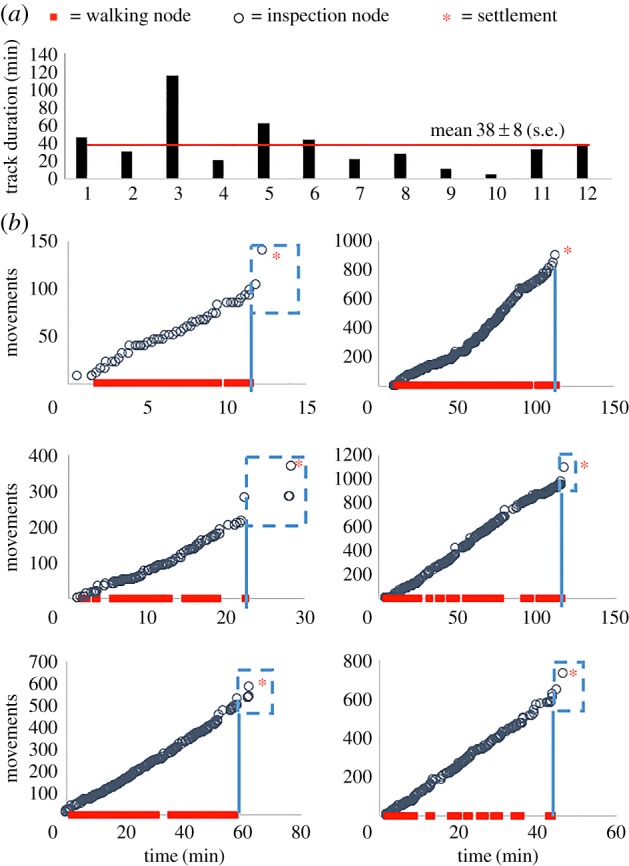
(a) The final period of surface exploration was tracked for 12 individual cyprids. (b) In most cases, this final period included a stretch of close searching behaviour (walking with linear increase in body movements; figure 2c) followed by several nodes of inspection behaviour, unassociated with walking—i.e. final inspection (blue boxes). (Online version in colour.)
The purpose of the short-term assay (figures 7 and 8) was to provide a higher throughput experimental design with greater replication and statistical power than the long-term assays. Repeated recordings of larger numbers of larvae were conducted, during which total behaviour was quantified per trial, rather than per cyprid. As well as generating replicate data, this approach had the additional advantage of eliminating the sometimes onerous process of identity re-assignment that was required during long-term tracking of individual cyprids. To this end, the short-term assay involved 1 h recordings of tubes containing 10 cyprids of the appropriate age (usually 3 days old) from four independent larval cultures for each experimental condition. Experiments were conducted at 23°C using cyprids gradually raised to room temperature and allowed 5 min to acclimatize prior to recording. Inspection or walking data recorded in the 1 h period were simply taken as a total for each recording and divided by the number of cyprids in the tube, providing a measure of average inspection events, movements and walking events per cyprid, per hour. These values were then averaged for the 4× replicate cultures to produce means ± standard error of the mean. Statistical analysis was performed between treatments using one-way analysis of variance (ANOVA) in Minitab version 17 at an α-level of 0.05. All data were normally distributed and showed similar variance, as identified from normal plots of residual values and plots of residuals versus fitted values.
3. Results
(a). Direct identification of cyprid settlement behaviour
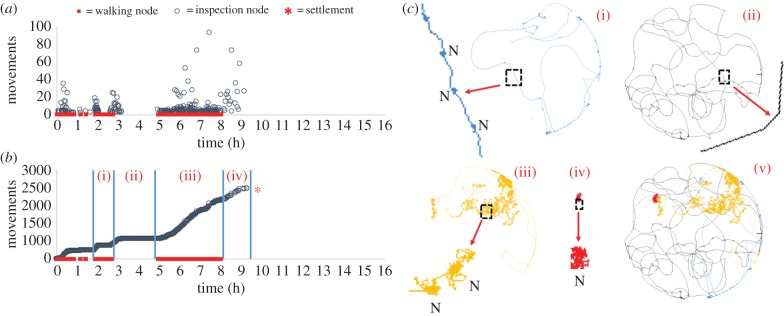
Through the automatic definition and subsequent analysis of walking and inspection nodes, all non-exploratory movements (e.g. swimming and resting) were ignored and simple distributions of the number of movements per inspection node, with time, could be produced (figure 2a), with periods of walking indicated on the x-axis. By presenting the same data as cumulative values (figure 2b), the three classic cyprid exploratory behaviours were clearly identifiable without reference to the track trajectory. The exploratory paths automatically produced during tracking analysis (figure 2c) were used only for quality control purposes. For each time point, the number of inspection movements was added to the sum of all previous time points. Wide searching behaviour produced no increase in the number of inspection nodes; however, the period of wide searching was identified as walking by the software and assigned walking nodes (figure 2b). Close searching behaviour was also identified as walking, but its definition required a concurrent increase in the number of inspection nodes and associated movements, since the close searching movement is essentially wide searching punctuated by inspection events (figure 2c, (i) and (iii)). Inspection resulted in a large increase in the number of body movements and a smaller increase in the number of inspection nodes, but did not coincide with walking (figure 2c, (iv)). With these three surface-specific behaviours assigned, all other periods of time with no identifiable nodes corresponded to non-exploratory behaviours. Most often, this would be swimming, but in the confines of an experimental arena also included any movements over and in contact with the surface other than via the antennules. In a major departure from past work, this classification process did not require the challenging interpretation of track morphology that has previously thwarted separation of these behaviours.
Figure 2.
(a) A plot of raw tracking data for a single cyprid. Each grey marker on the plot represents an inspection node. The number of movements per node is represented on the y-axis. The red markers on the x-axis signify walking behaviour. (b) The same data presented as a cumulative plot with: (i) zero or minimal increase in the number of body movements with time accompanied by walking = wide searching behaviour; (ii) zero or minimal increase in the number of body movements with time without walking = non-exploratory behaviour; (iii) linear increase in body movements with time, accompanied by walking = close searching behaviour; and (iv) linear increase in body movements with time without walking = inspection behaviour. Red asterisk, permanent attachment. (c) Tracks (i–v) illustrate the rationale behind the assignments in (b) (black box magnified for i–iv). The blue in (i) = walking behaviour. Walking behaviour can be identified visually by the periodic spacing of nodes (N) that correlate to pauses in cyprid body movement between steps. (ii) Non-exploratory behaviour refers to any movement that is not walking or inspection. Linear movement with no nodes means no walking or inspection. (iii) Close searching is a combination of walking (i) and inspection (iv), where inspection is movement of the body numerous times while attached to a single point. Each node in (iii) is therefore similar to (i), but the result of many movements per step. Inspection contains only body movements with no steps (iv), and (v) represents the entire trajectory of the track.
(b). Identifying and quantifying ‘typical’ settlement behaviour
The ability to track and classify multiple cyprids for prolonged periods enabled, for the first time to our knowledge, detailed analysis of cyprid behaviour in the final exploratory phase leading to settlement. Depending on the individual in question, this period of unbroken surface contact was anywhere from 5 min to 2 h (figure 3a). Any return to the water column, no matter how brief, reset the start of the tracking period. The consistency of behaviour in the final period before settlement was striking. The rationale described for interpreting figure 2b was applied to 12 tracks, including the six examples illustrated in figure 3b. The majority of cyprids, 10 out of 12 analysed, engaged in a period of close searching followed by inspection prior to settlement. All showed a largely unbroken stretch of walking behaviour accompanied by a linear increase in the number of inspection nodes and movements—i.e. close searching. As each cyprid approached settlement, the period between inspection nodes lengthened (less overlapping of circular markers) and the last few nodes of each plot (figure 3b) usually occurred after the last walking node on the x-axis, implying the transition to final inspection. What is more, the similarities between tracks extended beyond visual comparison. For the six final exploratory periods illustrated in figure 3b, the average frequency of inspection-related body movements during this period, identified from the slope of the frequency plots, was 9.7 movements per minute ± 0.72 (s.e.).
(c). Measuring effects of increasing larval age and the presence of a chemical inducer
The rate of cyprid settlement in laboratory assays can be manipulated by a variety of means. Storage of B. amphitrite cyprids at 6°C delays settlement but allows physiological ageing, resulting in an increase in the rate of settlement and a decrease in discrimination [31]. Alternatively, exposure to synthetic settlement inducers such as IBMX [28] can stimulate precocious settlement. A fundamental question is whether the patterns of behaviour that precede higher settlement of aged cyprids and those exposed to IBMX are similar or different.
We optimized the tracking method to enable analysis of the entire planktonic phase of cultured cyprids. Cyprids tracked through repeated surface encounters and returns to the water column increased their rate of settlement in response to increasing age and IBMX exposure; however, pre-settlement behaviour differed markedly between the two treatments. For each of these long-term experiments, the trends presented were observed in all (at least four) individuals tracked in each treatment.
Figure 4 illustrates representative tracks, leading to settlement, for cyprids 1, 3 and 5 days old. Older cyprids settled more quickly and the total number of movements recorded prior to settlement decreased with age, implying less inspection/close searching and reduced discrimination. These findings support previous studies of the effects of cyprid age on laboratory settlement [31]. Wide searching featured prominently in the tracks of 1- and 3-day-old cyprids, but was absent from tracks of 5-day-old individuals. All cyprids terminated their exploratory period with close searching behaviour. Figures 5 and 6, on the other hand, provide examples of 3-day-old cyprids in a 10−5 M solution of IBMX (figure 5) and a control solution of 1 : 10 000 DMSO (figure 6). While only one out of four cyprids settled in the control condition, three out of four settled in the presence of IBMX. There were also marked differences in the data profiles. While the maximum number of body movements prior to settlement was 2500 in the presence of IBMX (figure 5b), it was only 600 in the control condition (figure 6d), a figure closely matching the approximately 600 body movements observed for the 3-day-old cyprid in figure 4b. This highlighted a disparity between the effects of ageing and IBMX exposure on settlement behaviour. While both treatments increased the rate of settlement, the number of body movements reduced accordingly for cyprids of increasing age, from over 1500 on day 1 to 500 on day 5 (figure 4a–c), whereas the cyprid that settled in the control condition in figure 6 engaged in far fewer movements than those that settled more quickly in the IBMX solution (figure 5). For cyprids of increasing age, exploratory period and number of body movements reduced simultaneously, implying that the rate of activity was constant and independent of age. For those exposed to IBMX (figure 5), however, the total number of body movements was higher than in the control (figure 6), despite cyprids settling more quickly. This implied a stimulatory effect on searching activity by IBMX rather than simply a reduction in the stimulatory threshold required for settlement, as was the case for older cyprids.
Figure 5.
(a–d) Cumulative plots of body movements over the course of individual cyprid tracking experiments of up to 16 h in duration. The cyprids were exposed, throughout the experiment, to a 10−5 M solution of IBMX and 1 : 10 000 DMSO in ASW. Plots (a–d) are presented on the same y-axis in (e). (Online version in colour.)
(d). Development of a predictive assay based upon cyprid settlement behaviour
For most fundamental and applied applications of behavioural analysis, prolonged tracking of the entire pelagic period (e.g. figures 5 and 6) is both unnecessary and impractical. Rather, a rapid assay is required that can use specific aspects of behaviour to indicate propensity to settle and compare results reliably between conditions. Such an assay was developed using 1 h recordings of multiple cyprids in the previously applied experimental conditions.
Settlement of cyprids on day 5 was significantly higher than for those on day 1 (figure 7a; ANOVA F2,3 = 4.17, p = 0.02). Although the total number of inspection-related movements doubled concurrently with the increase in settlement between day 1 and day 5 (figure 7b), this increase was not significant; a larger number of replicates would probably resolve this. As observed in the long-term tracks for cyprids of increasing age (figure 4), metrics of walking behaviour indicated a reduction with age (figure 7d–f). Cyprids on day 5 took significantly fewer steps per unit time (figure 7d; ANOVA F2,3 = 4.17, p = 0.05) and explored for less time than those on day 1 (figure 7e; ANOVA F2,3 = 4.64, p = 0.04). The number of inspection movements per node, the number of exploration periods per cyprid and the rate of walking (figure 7c,f,g) did not differ significantly between ages. This was of particular interest for the movements per node and step rate metrics, both of which relate to the mechanics of surface interaction rather than broader exploratory behaviour.
Short-term assays of cyprid behaviour in solutions of IBMX indicated significant differences for all metrics other than body movements per inspection node (figure 8c) and step rate during exploration (figure 8g). As expected, settlement was significantly higher in the IBMX condition (figure 8a; ANOVA F1,3 = 14.04, p ≤ 0.01), as were all measures of activity including the number of inspection events/nodes (figure 8b; ANOVA F1,3 = 24.01, p ≤ 0.01), the total number of inspection movements (figure 8b; ANOVA F1,3 = 20.96, p ≤ 0.01), the average rate of walking (figure 8d; ANOVA F1,3 = 13.26, p ≤ 0.01), average walking period (figure 8e; ANOVA F1,3 = 10.82, p = 0.02) and number of exploratory events (figure 8f; ANOVA F1,3 = 12.70, p = 0.01). Importantly, however, the direction of this difference was different relative to settlement when compared with the data for ageing cyprids in figure 7. Whereas cyprids of increasing age showed reduced walking activity as settlement increased, cyprids exposed to IBMX, where settlement was higher, showed increased walking behaviour. In both cases, the biomechanical aspects of motility in terms of step rate and body movements were unchanged. Since each inspection node for the two conditions contained a similar number of movements (figure 8c), the stark difference in data profiles between the IBMX and control conditions (figures 5 and 6) can be assigned primarily to a larger number of inspection events. Although not statistically significant in figure 7b, the number of recorded inspection nodes and/or movements in a given condition is the most reliable proxy with which to quantify the intensity of surface interaction and the best predictor of settlement drawn from the metrics calculated so far.
4. Discussion
We present here, to our knowledge, the first quantitative analysis of global barnacle settlement behaviour, using an automated, software-based tracking and classification system designed specifically for the task. Our novel approach enabled long-term monitoring and high-resolution, short-term quantification of exploratory behaviour that will enable both fundamental, hypothesis-driven studies of settlement cues, as well as applied investigations into the mechanism of action of novel antifouling coatings/agents.
The study had three objectives: first, to ascertain the validity of Crisp's behavioural model and to confirm the three classic settlement behaviours [9]. Second, to use data-driven assignment of behaviour to identify characteristic activities that cyprids engage in during the minutes to hours before settlement. Third, to develop a short-term assay that uses cyprid behaviour as a tool for comparison of experimental conditions. It can first be confirmed that Crisp's description of behaviour was broadly accurate and that these behaviours are well conserved between individual larvae; in general, wide searching leads to close searching which, in turn, leads to inspection before settlement [26]. However, behaviour meeting Crisp's definition of inspection also occurred at many other points. Exploratory behaviour (wide searching, close searching and inspection) occurred predominantly in the lead-up to settlement and less so during the indeterminate period of swimming prior to final engagement with a surface. Once settlement behaviour had been initiated, there were still brief returns to the water column, most often at the transition between wide and close searching. Wide and close searching indeed appear to be discrete behaviours with a clear transition from one to the next. Once close searching began, returns to the water column were increasingly rare and older cyprids tended to enter close searching directly upon encountering a surface, forgoing wide searching altogether. In the absence of external interruptions, close searching occurred only immediately before settlement.
When behaviour was analysed during the final period of surface contact before settlement (figure 3), clear consistencies emerged. While the period of time was highly variable, from less than 5 min to over 2 h, the behavioural parameters identifiable from cumulative plots of inspection and walking nodes remained highly consistent. Surface exploration always ended with close searching and/or inspection.
Prolonged experiments with cyprids of different ages and those in the presence of a settlement inducer, IBMX [27], indicated that propensity to settle and settlement behaviour were independent of one another. While older cyprids and those exposed to IBMX both settled more quickly, behaviour prior to settlement differed markedly. Older cyprids explored less than younger ones, engaged less in wide searching and settled more quickly (figure 4), and their rate of body movement throughout this process remained similar at all ages. Cyprids exposed to IBMX, on the other hand, settled quickly compared to those in the control but with greatly increased rates of body movement. While the inclination to settle more quickly was driven, in ageing cyprids, by the necessity to conserve a finite energy store [32,33], in cyprids exposed to IBMX, it was the result of increased exploratory activity initiated by exposure to the artificial stimulant.
Finally, a rapid assay was developed that allowed quantitative comparison of cyprid settlement behaviour between treatments. The total number of inspection-related nodes/movements (figures 7b and 8b) was the best comparative measure for experimental purposes, since it combined the effects of the number of inspection events and the number of movements in those events, providing a robust and consistent comparator between conditions. The direction of the increase/decrease for this metric was also consistent with increasing or decreasing settlement, unlike the measures of walking behaviour.
Measures of walking behaviour (figures 7d–f and 8d–f) yielded significant differences between treatments, but also highlighted the difference in behavioural response identified in long-term tracks (figures 5 and 6); i.e. reduced activity with age leading to higher settlement and increased activity with IBMX also leading to higher settlement. While the opposite response of these measures relative to settlement, depending on treatment, rendered them unreliable as simple predictors of settlement, they clearly have additional value. In an antifouling context, whether settlement reduction follows increased or decreased exploratory activity may reflect either selective rejection of the surface or a physical barrier to exploration.
Perhaps most interestingly, the two measures that were consistent between experimental conditions in both experiments were the step rate during walking and the number of movements per node during inspection. We suggest that the observable elements of cyprid exploratory behaviour could thus be divided into two categories: (i) those that control the biomechanics of movement involved in inspection/walking and that that appear to be consistent within an experiment, regardless of treatment; and (ii) those that incorporate these movements into more complex behaviours (i.e. walking/inspection) where there is flexibility regarding the timing/frequency of the component movements depending on environmental conditions. This is significant, not least, because most previous attempts to quantify cyprid exploration, including our own, have focused on metrics of wide searching behaviour—step length, step duration etc. [21]. In all likelihood, this emphasis on detailed analysis of walking behaviour has stemmed from practical limitations on the duration of previous tracking experiments. For example, in the field-based video observations of Prendergast et al. [22], wild cyprids of Semibalanus balanoides explored surfaces treated with a conspecific cue more frequently than an untreated control, and spent longer exploring treated surfaces. However, the work was limited by manual selection of cyprids followed by a largely manual and labour-intensive analysis pipeline. The correlation to settlement, which is known to be higher in the presence of a conspecific cue, was indirect since cyprids could only be tracked for up to 1 min. Similarly, the method of Chaw et al. [21] relied upon manual selection and largely manual tracking of cyprids exploring microstructured surfaces to present metrics of wide searching behaviour that correlated with settlement. All past results should now be considered in the context of our finding that it is possible for increased walking activity to precede either an increased or decreased settlement response. Based on our results, the involvement of wide searching in the decision to settle is indirect and the mechanics of the steps are probably ‘hard-wired’, remaining constant in varying non-stressful conditions.
The ability to correctly correlate the behaviour of barnacle larvae to settlement under a given condition would be a significant achievement, and this study represents an important step forward. By departing from traditional approaches towards interpretation of spatially calibrated tracks, we have generated these comparative data directly with minimal processing and interpretation. While the fundamentals presented here provide a framework for such interpretation, it should be noted that behavioural data are, and will remain, subject to large variability—particularly between experiments. However, our novel approach (figures 2–6) is underpinned by hard data and reduces the potential for observer bias to a minimum. We anticipate that the methods will enable new directions in barnacle ecology, albeit with the changes required to accommodate responses of different barnacle species to conspecifics or host organisms, as well as contributing significantly to improved selection of antifouling materials/agents based on deterrence of settling larvae.
Supplementary Material
Acknowledgements
We would also like to thank Prof. Dan Rittchof and Beatrice Orihueladiaz for continued provision of barnacle brood stock and Prof. Mike Detty/Dr Caitlyn Gatley for their longstanding collaboration during development of the tracking system.
Ethics
Handling of barnacle larvae adhered to Newcastle University's ethics policy with no external licenses/approval required for these organisms.
Data accessibility
Behavioural data produced by the classification system, as well as source code for the tracking and classification software, are available via Dryad: http://dx.doi.org/10.5061/dryad.64486 [34] and the authors encourage interested parties to contact them regarding its correct use.
Authors' contributions
N.A. designed and conducted the experiments, analysed the data and prepared the manuscript. A.A. designed and implemented the tracking and classification software and provided critical evaluation. A.S.C. secured funding for the research and provided input into the analysis and manuscript preparation.
Competing interests
We declare we have no competing interests.
Funding
We extend our gratitude to the Office of Naval Research (ONR) for funding support to develop our larval tracking system (N00014-08-1-1240 (A.S.C.), N00014-13-1-0633 (A.S.C.) and N00014-13-1-0634 (A.S.C. and N.A.)), and to the European Commission FP7 SEAFRONT project (A.S.C.).
References
- 1.Larsson AI, Granhag LM, Jonsson PR. 2016. Instantaneous flow structures and opportunities for larval settlement: barnacle larvae swim to settle. PLoS ONE 11, e0158957 ( 10.1371/journal.pone.0158957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crisp DJ, Meadows PS. 1962. The chemical basis of gregariousness in cirripedes. Proc. R. Soc. B 156, 500–520. ( 10.1098/rspb.1962.0052) [DOI] [Google Scholar]
- 3.Crisp DJ, Meadows PS. 1963. Adsorbed layers: the stimulus to settlement in barnacles. Proc. R. Soc. B 158, 362–387. ( 10.1098/rspb.1963.0053) [DOI] [Google Scholar]
- 4.De Gregoris TB, Khandeparker L, Anil AC, Mesbahi E, Burgess JG, Clare AS. 2012. Characterisation of the bacteria associated with barnacle, Balanus amphitrite, shell and their role in gregarious settlement of cypris larvae. J. Exp. Mar. Biol. Ecol. 413, 7–12. ( 10.1016/j.jembe.2011.11.014) [DOI] [Google Scholar]
- 5.Fino AD, Petrone L, Aldred N, Ederth T, Liedberg B, Clare AS. 2014. Correlation between surface chemistry and settlement behaviour in barnacle cyprids (Balanus improvisus). Biofouling 30, 143–152. ( 10.1080/08927014.2013.852541) [DOI] [PubMed] [Google Scholar]
- 6.Pasternak Z, Blasius B, Abelson A. 2004. Host location by larvae of a parasitic barnacle: larval chemotaxis and plume tracking in flow. J. Plankton Res. 26, 487–493. ( 10.1093/plankt/fbh040) [DOI] [Google Scholar]
- 7.Pasternak Z, Blasius B, Achituv Y, Abelson A. 2004. Host location in flow by larvae of the symbiotic barnacle Trevathana dentata using odour-gated rheotaxis. Proc. R. Soc. B 271, 1745–1750. ( 10.1098/rspb.2004.2765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dreanno C, Matsumura K, Dohmae N, Takio K, Hirota H, Kirby RR, Clare AS. 2006. An α2-macroglobulin-like protein is the cue to gregarious settlement of the barnacle Balanus amphitrite. Proc. Natl Acad. Sci. USA 103, 14396–14401. ( 10.1073/pnas.0602763103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crisp DJ. 1976. Two settlement responses in marine organisms. In Adaptation to environment. Essays on the physiology of marine animals (ed. Newell RC.), pp. 83–124. London, UK: Butterworths. [Google Scholar]
- 10.Crisp DJ. 1984. Overview of research on marine invertebrate larvae, 1940–1980. In Marine biodeterioration: an interdisciplinary study (eds Costlow JD, Tipper RC), pp. 103–126. Annapolis, MD: Naval Institute Press. [Google Scholar]
- 11.Lejars M, Margaillan A, Bressy C. 2012. Fouling release coatings: a nontoxic alternative to biocidal antifouling coatings. Chem. Rev. 112, 4347–4390. ( 10.1021/cr200350v) [DOI] [PubMed] [Google Scholar]
- 12.Franco SC, Aldred N, Cruz T, Clare AS. 2016. Modulation of gregarious settlement of the stalked barnacle, Pollicipes pollicipes: a laboratory study. Sci. Mar. 80, 217–228. ( 10.3989/scimar.04342.01A) [DOI] [Google Scholar]
- 13.Alsaab A, Aldred N, Clare AS. 2017. Automated tracking and classification of the settlement behaviour of barnacle cyprids. J. R. Soc. Interface 14, 20160957 ( 10.1098/rsif.2016.0957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marechal JP, Hellio C, Sebire M, Clare AS. 2004. Settlement behaviour of marine invertebrate larvae measured by EthoVision 3.0. Biofouling 20, 211–217. ( 10.1080/08927010400011674) [DOI] [PubMed] [Google Scholar]
- 15.Aldred N, Phang IY, Conlan SL, Clare AS, Vancso GJ. 2008. The effects of a serine protease, Alcalase, on the adhesives of barnacle cyprids (Balanus amphitrite). Biofouling 24, 97–107. ( 10.1080/08927010801885908) [DOI] [PubMed] [Google Scholar]
- 16.Aldred N, Li GZ, Gao Y, Clare AS, Jiang SY. 2010. Modulation of barnacle (Balanus amphitrite Darwin) cyprid settlement behavior by sulfobetaine and carboxybetaine methacrylate polymer coatings. Biofouling 26, 673–683. ( 10.1080/08927014.2010.506677) [DOI] [PubMed] [Google Scholar]
- 17.Maleschlijski S, et al. 2012. Three dimensional tracking of exploratory behavior of barnacle cyprids using stereoscopy. Biointerphases 7, 50 ( 10.1007/s13758-012-0050-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maleschlijski S, Bauer S, Aldred N, Clare AS, Rosenhahn A. 2015. Classification of the pre-settlement behaviour of barnacle cyprids. J. R. Soc. Interface 12, 20141104 ( 10.1098/rsif.2014.1104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pradhan NN, Gohad NV, Orihuela B, Burg TC, Birchfield ST, Rittschof D, Mount AS. 2011. Development of an automated algorithm for tracking and quantifying barnacle cyprid settlement behavior. J. Exp. Mar. Biol. Ecol. 410, 21–28. ( 10.1016/j.jembe.2011.10.001) [DOI] [Google Scholar]
- 20.Maleshlijski S, Sendra GH, Aldred N, Clare AS, Liedberg B, Grunze M, Ederth T, Rosenhahn A. 2016. Imaging SPR combined with stereoscopic 3D tracking to study barnacle cyprid–surface interactions. Surf. Sci. 643, 172–177. ( 10.1016/j.susc.2015.08.027) [DOI] [Google Scholar]
- 21.Chaw KC, Dickinson GH, Ang K, Deng J, Birch WR. 2011. Surface exploration of Amphibalanus amphitrite cyprids on microtextured surfaces. Biofouling 27, 413–422. ( 10.1080/08927014.2011.577210) [DOI] [PubMed] [Google Scholar]
- 22.Prendergast G, Head R, Hansson LJ, Thomason J. 2008. Field-based video observations of wild barnacle cyprid behaviour in response to textural and chemical settlement cues. Biofouling 24, 449–459. ( 10.1080/08927010802340135) [DOI] [PubMed] [Google Scholar]
- 23.Maruzzo D, Conlan SL, Aldred N, Clare AS, Høeg JT. 2011. Video observation of surface exploration in cyprids of Balanus amphitrite: the movements of antennular sensory setae. Biofouling 27, 225–239. ( 10.1080/08927014.2011.555534) [DOI] [PubMed] [Google Scholar]
- 24.Ekvall MT, Bianco G, Linse S, Linke H, Backman J, Hansson L-A. 2013. Three-dimensional tracking of small aquatic organisms using fluorescent nanoparticles. PLoS ONE 8, e78498 ( 10.1371/journal.pone.0078498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uttieri M, Paffenhöfer GA, Mazzocchi MG. 2007. Prey capture in Clausocalanus furcatus (Copepoda: Calanoida). The role of swimming behaviour. Mar. Biol. 153, 925–935. ( 10.1007/s00227-007-0864-0) [DOI] [Google Scholar]
- 26.Lagersson NC, Høeg JT. 2002. Settlement and antennulary biomechanics in cypris larvae of Balanus amphitrite (Crustacea: Thecostraca: Cirripedia). Mar. Biol. 141, 513–526. ( 10.1007/s00227-002-0854-1) [DOI] [Google Scholar]
- 27.Rittschof D, Branscomb ES, Costlow JD. 1984. Settlement and behaviour in relation to flow and surface in larval barnacles, Balanus amphitrite Darwin. J. Exp. Mar. Biol. Ecol. 82, 131–146. ( 10.1016/0022-0981(84)90099-6) [DOI] [Google Scholar]
- 28.Clare AS, Thomas RF, Rittschof D. 1995. Evidence for the involvement of cyclic AMP in the pheromonal modulation of barnacle settlement. J. Exp. Biol. 198, 655–664. [DOI] [PubMed] [Google Scholar]
- 29.Davidson I, Scianni C, Hewitt CL, Holm E, Tanburri M, Ruiz G. 2016. Mini-review: assessing the drivers of ship biofouling management—aligning industry and biosecurity goals. Biofouling 32, 411–428. ( 10.1080/08927014.2016.1149572) [DOI] [PubMed] [Google Scholar]
- 30.Rittschof D, Clare AS, Gerhart DJ, Bonaventura J. 1992. Barnacle in vitro assays for biologically active substances: toxicity and settlement inhibition assays using mass cultured Balanus amphitrite amphitrite Darwin. Biofouling 6, 115–122. ( 10.1080/08927019209386217) [DOI] [Google Scholar]
- 31.Maréchal J-P, Matsumura K, Conlan SL, Hellio C. 2012. Competence and discrimination during cyprid settlement in Amphibalanus amphitrite. Inter. Biodeter. Biodeg. 72, 59–66. ( 10.1016/j.ibiod.2012.05.007) [DOI] [Google Scholar]
- 32.Thiyagarajan V, Harder T, Qian P-Y. 2002. Effect of the physiological condition of cyprids and laboratory-mimicked seasonal conditions on the metamorphic success of Balanus amphitrite Darwin (Cirripedia; Thoracica). J. Exp. Mar. Biol. Ecol. 280, 79–93. ( 10.1016/S0022-0981(02)00415-X) [DOI] [Google Scholar]
- 33.Tremblay R, Olivier F, Bourget E, Rittschof D. 2007. Physiological condition of Balanus amphitrite cyprid larvae determines habitat selection success. Mar. Ecol. Prog. Ser. 340, 1–8. ( 10.3354/meps340001) [DOI] [Google Scholar]
- 34.Aldred N, Alsaab A, Clare AS. 2018. Data from: Quantitative analysis of the complete larval settlement process confirms Crisp's model of surface selectivity by barnacles Dryad Digital Repository. ( 10.5061/dryad.64486) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Aldred N, Alsaab A, Clare AS. 2018. Data from: Quantitative analysis of the complete larval settlement process confirms Crisp's model of surface selectivity by barnacles Dryad Digital Repository. ( 10.5061/dryad.64486) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Behavioural data produced by the classification system, as well as source code for the tracking and classification software, are available via Dryad: http://dx.doi.org/10.5061/dryad.64486 [34] and the authors encourage interested parties to contact them regarding its correct use.