Abstract
Shipping is the dominant marine anthropogenic noise source in the world's oceans, yet we know little about vessel encounter rates, exposure levels and behavioural reactions for cetaceans in the wild, many of which rely on sound for foraging, communication and social interactions. Here, we used animal-borne acoustic tags to measure vessel noise exposure and foraging efforts in seven harbour porpoises in highly trafficked coastal waters. Tagged porpoises encountered vessel noise 17–89% of the time and occasional high-noise levels coincided with vigorous fluking, bottom diving, interrupted foraging and even cessation of echolocation, leading to significantly fewer prey capture attempts at received levels greater than 96 dB re 1 µPa (16 kHz third-octave). If such exposures occur frequently, porpoises, which have high metabolic requirements, may be unable to compensate energetically with negative long-term fitness consequences. That shipping noise disrupts foraging in the high-frequency-hearing porpoise raises concerns that other toothed whale species may also be affected.
Keywords: anthropogenic disturbance, exposure rates, behavioural response, fitness consequences, foraging, DTAG
1. Introduction
Toothed whales rely on sound for communication, navigation and searching for food by echolocation [1], and may therefore be impacted negatively by increased levels of noise associated with human activities in the marine environment [2,3]. Effects may include physical damage and hearing loss for powerful transient noise sources, such as explosions or seismic airguns [2,4], whereas more frequent, lower-level noise exposures can cause masking and behavioural disruption that may be hard to detect, but can have cumulative long-term effects on populations [3]. Recent research efforts have focused on how odontocetes [5–9] respond to transient noise sources, including pile driving, airguns and military sonars, but little is known about the effects of shipping noise—the dominant anthropogenic noise source in the world's oceans [10]. The few studies on the effects of shipping noise have primarily focused on baleen whales owing to their communication, and thus probably sensitive hearing, at low frequencies that overlap with the maximum power outputs of large cargo vessels [11–13]. However, it has recently been shown that a diverse range of vessels produce substantial noise levels at even very high frequencies, where toothed whales hear well and use sound [14,15]. Moreover, boat traffic in many coastal areas is dominated by smaller vessels that generate noise at higher frequencies than large cargo vessels [16], raising the possibility that vessel noise may actually be a significant, but so far overlooked problem for odontocetes [17]. This concern may be particularly relevant for porpoises that live in areas with some of the highest shipping densities in the world [10].
Although data are sparse, harbour porpoises have been reported to react to ships at long ranges (800–1000 m) [18,19], where noise, rather than the physical presence of the vessel, is more likely to deliver the negative stimulus. Furthermore, recently, captive individuals have been shown to respond behaviourally to low levels of relatively high-frequency vessel noise [20]. This led us to hypothesize that broadband shipping noise may cause behavioural disruptions in porpoises despite them having poor low-frequency hearing compared with most other cetaceans [21]. As small marine mammals that live in cold water requiring high feeding rates year round [22,23], porpoises may be particularly vulnerable to disruption of, or increased energy expenditures associated with, foraging. Behavioural reactions that affect foraging time [24] and increase energy expenditure over short time periods may accumulate over repeated exposures and impact the long-term fitness of animals. In spite of these concerns, very little is known about vessel encounter rates, exposure levels and avoidance reactions of any small odontocetes in the wild, including porpoises. To address this, we here use sound recording tags to study the foraging rates of harbour porpoises as a function of the vessel noise they experience. We show that the tagged porpoises were exposed to vessel noise between 17 and 89% of the time, and that they interrupted foraging in the presence of high-noise levels, which may have adverse effects on populations in industrialized coastal waters.
2. Results
Wideband sound and movement recording tags (DTAGs [25]) were deployed on seven porpoises yielded high-quality recordings (i.e. with little sliding of the suction cup-attached tag, clear buzzes, low flow noise and long duration of between 11.9 and 23.7 h, table 1; electronic supplementary material, figure S1).
Table 1.
Tag deployment and data summary. (The age classes of the porpoises were determined using growth curves established for Danish porpoises [26].)
| animal ID | hp12_272a | hp12_293a | hp13_102a | hp13_170a | hp14_226b | hp15_117a | hp16_264a |
|---|---|---|---|---|---|---|---|
| deployment date | 28 Sep 2012 | 19 Oct 2012 | 12 Apr 2013 | 19 Jun 2013 | 14 Aug 2014 | 26 Apr 2015 | 20 Sep 2016 |
| age class and sex | juvenile ♀ | adult ♀ (with a calf) | juvenile ♂ | juvenile ♂ | juvenile ♂ | adult ♀ | adult ♀ (with a calf) |
| standard length (cm) | 122 | 163 | 114 | 122 | 126 | 170 | 163 |
| handling time (min) | 15 | 3 | 5.5 | 3.5 | 7.5 | 12 | 10 |
| recording duration (h) | 21.9 | 17.7 | 23.7 | 15.3 | 21.7 | 13 | 11.9 |
| time to first foraging buzz (h) | 4.1 | 1.4 | 1 | 0.1 | 0.2 | 0.6 | 0.2 |
(a). Foraging rates
The seven porpoises performed short (1–3 min long) foraging dives to depths of 5–50 m (e.g. figure 1), where they produced a total of 380–3400 buzzes (table 2), an indication of prey encounters [23], with an hourly rate of 0–550 buzzes. Excluding time intervals with rain (e.g. figure 1) or non-vessel sound transients, for example, owing to water splashing, the proportion of 1 min intervals with at least one buzz ranged from 18 to 76% and averaged approximately 50% (table 2; electronic supplementary material, table S1). While few data were collected during night-time for hp13_170a and hp16_264a, all but one porpoise (hp15_117a) seemed to forage primarily after dusk (table 2 and figure 1).
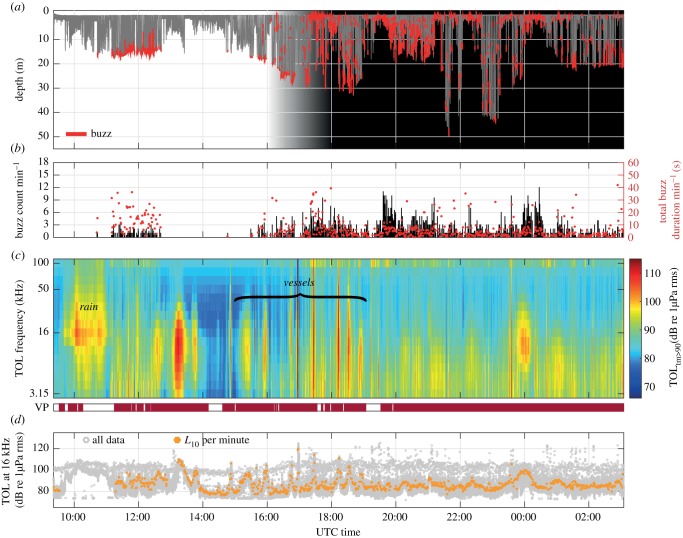
Figure 1.
Data from DTAG deployment on porpoise hp12_293a. (a) Dive profile. Individual buzzes are marked in red. Shading represents twilight and night. Given the bathymetry of the area, dives deeper than 25 m must have been performed in deeper-water channels. (b) Buzz counts per minute (black bars) and buzz durations, in seconds, summed in each minute (red circles). (c) TOLs. Shown are the TOL10, i.e. the noise levels in each third octave that are exceeded 10% of the time within each minute, excluding time spent by the animal at the surface during respirations and logging, which emphasizes the highest exposure levels, that is the levels most likely to explain any behavioural reaction. Periods with audible noise from vessels are marked in scarlet in the lower panel (vessels present, VP). (d) Noise levels in the third octave band centred at 16 kHz. Light-grey circles show 0.5 s trimmed mean averages prior to exclusion of segments dominated by loud transients (e.g. surface splashes, see Material and methods). Orange circles show 1 min TOL10 noise levels.
Table 2.
Overview of foraging buzz data, excluding time intervals dominated by rain, splashing and loud transients (see also electronic supplementary material, table S1), and estimates of vessel exposure rates for the entire recording period. (Night was assumed to start after civil dusk.)
| animal ID | hp12_272a | hp12_293a | hp13_102a | hp13_170a | hp14_226b | hp15_117a | hp16_264a |
|---|---|---|---|---|---|---|---|
| total buzz count | 1856 | 1381 | 3408 | 1222 | 3232 | 906 | 383 |
| number of minutes analysed | 897 | 907 | 1160 | 306 | 690 | 700 | 493 |
| buzz-positive minutes | 352 (39.2%) | 532 (58.7%) | 565 (48.7%) | 217 (70.9%) | 523 (75.8%) | 402 (57.4%) | 88 (17.8%) |
| daytime buzz-positive minutes | 65 (17.7%) | 83 (27.1%) | 124 (17.8%) | 114 (60.0%) | 383 (73.0%) | 304 (64.1%) | 22 (5.9%) |
| night-time buzz-positive minutes | 287 (54.2%) | 449 (74.7%) | 441 (95.0%) | 103 (88.8%) | 140 (84.9%) | 98 (43.4%) | 66 (55.0%) |
| vessel noise exposure rate (%) | 37 | 70 | 89 | 17 | 18 | 89 | 66 |
| vessel noise exposure rate-day/night (%) | 51/17 | 55/81 | 88/92 | 3/87 | 22/10 | 88/93 | 77/45 |
Prey pursuits involved significant increases in flow noise in the tag recordings, in some cases even at high frequencies (greater than 50 kHz) (electronic supplementary material, figure S2). However, 0.5 s averages of one third octave levels (TOLs, i.e. the root mean square (rms) sound pressure level in one third octave bands) in the 16 kHz band during foraging (i.e. 5 s before the start of each buzz and until the end of the buzz) were largely independent of the animals' swimming activity and rarely exceeded 90 dB re 1 µPa (figure 2; electronic supplementary material, figure S2).
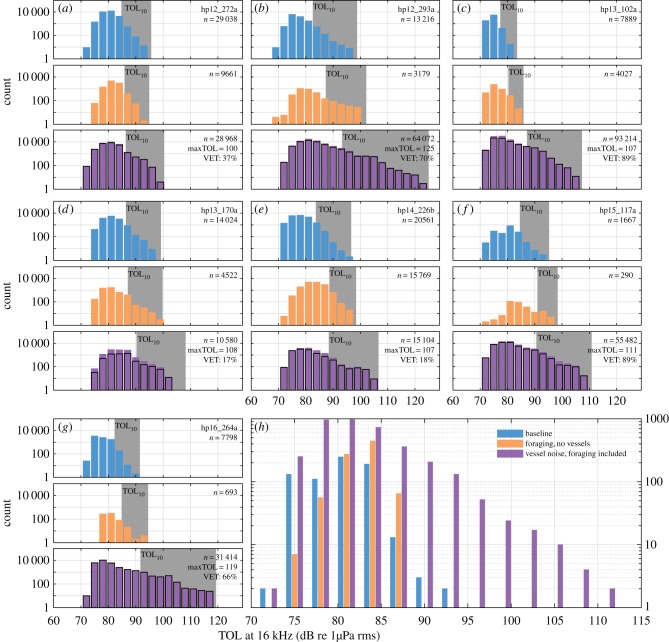
Figure 2.
Noise levels recorded on the seven porpoises (a–g) during three time categories: (i) baseline (i.e. outside of foraging or vessel noise exposure as judged by expert listeners; blue), (ii) during prey pursuit but outside of periods of vessel noise exposure (orange), and (iii) during vessel noise exposure, whether or not the porpoise was foraging (purple). The distribution of noise levels in the last category is overlaid with an outline of the distribution of levels during vessel noise exposure with time of prey pursuits excluded (black solid line) to illustrate the relative contribution of noise from vessels only. Noise levels are the 0.5 s trimmed mean average rms received levels in a 16 kHz third octave band for periods free of loud transients. The shaded areas correspond to the 16 kHz TOL exceeded 10% of the time, i.e. TOL10. VET gives per cent of audible vessel exposure time. (h) Distributions of 1 min TOL10 noise levels within the three categories with all individuals pooled.
(b). Vessel noise exposure
The proportion of time in which vessel noise was audible to expert listeners varied widely across the tagged animals, from approximately 17% for two animals to more than 65% for four animals (table 2 and figure 1). The high exposure rates of the latter individuals may be a consequence of the areas in which these animals stayed. Three of these porpoises were tagged in the narrow and heavily trafficked Great Belt (electronic supplementary material, figure S1) while the dive and movement profiles of the fourth animal (figure 1) suggest that it swam south to a narrow, relatively deep-water shipping route to Aarhus Harbour, the largest container port in Denmark (electronic supplementary material, figure S1; table 1). Vessel noise occurred primarily during daytime (table 2).
Most of the received vessel noise was of relatively low level at the frequencies that could be measured reliably, with L10 values (i.e. the noise level exceeded 10% of the time) in the 16 kHz third octave band 1–10 dB (median of 6 dB) above baseline (i.e. periods without foraging or vessel noise; figure 2a–g). Although for one animal (hp12_272a), only low-level vessel noise was recorded, the remaining animals experienced occasional high TOLs associated with vessel passes (maximum 1 min 16 kHz TOLs of 102–118 dB re 1 µPa rms, figure 2; electronic supplementary material, table S2). These high-noise events seemed to coincide with the absence of buzzes (figure 2, purple overlaid with black outline), raising the question of whether high-level exposures led to reduced foraging.
(c). Porpoise behaviour during high-level exposures
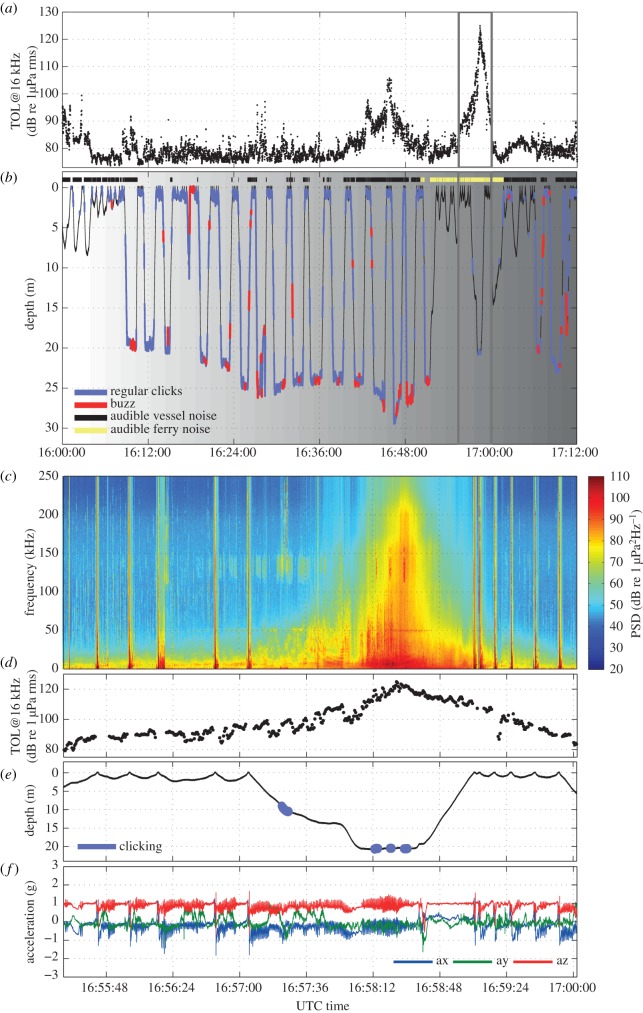
The behaviour of the porpoise that received the maximum noise exposure (hp12_293a) is shown in figure 3 and the electronic supplementary material, video S1. Vessel automatic identification system (AIS) data at the time of the noise exposure, together with the rapid increase and decrease in noise, suggest that the source was one of the fast ferries moving between the island of Zealand and the Jutland Peninsula (electronic supplementary material, figure S1). Doppler-shift analysis of the signal recorded by the tag indicates a speed of 33 knots and a closest approach to the porpoise of 140 m. Moreover, the spectral characteristics of the noise (figure 3c) strongly resemble those of the same fast ferries recorded at similar ranges [14]. This porpoise had been echolocating and foraging continuously prior to the exposure, but ceased regular echolocation at about the time when the ferry became audible in the recording (figure 3b), approximately 7 min before the point of the closest approach. Given the estimated speed of the vessel, this time corresponds to a reaction distance of approximately 7 km. As the 0.5 s 16 kHz TOL increased to 100 dB re 1 µPa, the porpoise dove away from the surface while fluking vigorously (figure 3c–f). When the noise levels decreased again, the animal resurfaced (figure 3c–f). Regular foraging behaviour resumed 8 min later, 15 min after it was first interrupted.
Figure 3.
Diving and foraging behaviour of porpoise hp12_293a around the time of passage of a presumed fast ferry. (c–f) A close-up of the period delineated by the grey frame in (a,b). (a,d) 16 kHz TOLs (0.5 s trimmed mean averages over 1 ms measurements). (b,e) Dive profile with the time during which the porpoise produced regular and buzz clicks marked in blue and red, respectively. The thick black lines above the profile in (b) show the times with audible vessel noise in the recordings. The yellow overlay marks the period when the ferry noise was clearly audible above other vessel noise. Shading marks the civil twilight and night-time. (c) Spectrogram composed of power spectral densities in 1 Hz bands. The broadband vertical bands indicate noise when the porpoise surfaces. (d,e) Detail of TOL and dive profile during the close-up interval. (f) Acceleration. The oscillations in the x- and z-axes (i.e. the animal's longitudinal and ventro-dorsal axes, respectively) indicate propulsive motions.
A similar reaction was recorded from another porpoise (hp14_226b), 2 years later (electronic supplementary material, figure S3c–f). The Doppler-shift method gave a speed estimate of 14.5 knots and a closest approach distance of 80 m, consistent with a maximum 0.5 s 16 kHz TOL of 107 dB re 1 µPa rms for this exposure. This porpoise also interrupted foraging and dove to deeper water when the vessel noise became audible; it resumed foraging soon after the vessel passed (electronic supplementary material, figure S3). Aurally and temporally, this vessel encounter and several others from the same recording (electronic supplementary material, figure S3a,b) were consistent with a fast ferry, implying that this porpoise was repeatedly passed by fast ferries during the 21.7 h tag attachment.
(d). Effects of vessel noise on foraging rates
To investigate whether repeated exposures to high-level vessel noise led to a pattern of reduced foraging, we performed a series of permutation tests, which compared the buzz count and total buzz duration in minutes with high- and low-level noise. This requires defining a threshold to separate high- and low-noise intervals. When averaged over 1 min, the vast majority of activity-related flow noise in the 16 kHz third octave band was below 90 dB re 1 µPa (figure 2; electronic supplementary material, table S2), making 96 dB the lowest usable threshold allowing a minimum 6 dB difference between low and high levels. Tougaard et al. [3] suggest that the threshold for behavioural reaction of porpoises to anthropogenic noise is approximately 100 dB re 1 µPa rms (averaged over 125 ms window) at 16 kHz making this a reasonable choice. Six of the seven porpoises were exposed to greater than 96 dB 16 kHz TOLs for a minimum of 5 min (electronic supplementary material, table S3). Of those, one individual produced significantly longer buzzes in the high-noise group, but showed no significant differences in buzz counts between the low- and high-noise groups. Another individual showed no significant differences in buzz count or duration. The four remaining porpoises produced fewer buzzes in the minutes with high-level vessel noise, with the differences being significant (p < 0.05, 10 000 permutations) at thresholds of 96 dB re 1 µPa for three animals and at 102 dB re 1 µPa for the fourth porpoise. For these four individuals, buzzes tended to be longer in the low-noise group, significantly so for three of them at a threshold of 96 dB re 1 µPa (electronic supplementary material, table S3). The exposure time to vessel noise levels that exceeded the threshold for reduced foraging was relatively short, ranging from 0.9 to 4.3% of the analysed minutes (electronic supplementary material, table S3).
3. Discussion
Worldwide shipping, the primary source of underwater anthropogenic noise, is contributing to chronic acoustic pollution in many marine habitats [27,28]. But the overall impact of this large-scale environmental modification is difficult to assess because of the lack of comparable control areas without noise pollution. Effects are only measureable when there are step changes in the noise level above the gradually increasing baseline levels [28–30], e.g. owing to changes in vessel speed or routing. The few available reports on the effects of vessel activity on cetaceans mention short-term avoidance reactions [18,19], physiological stress responses [31] and habitat displacement [32]. Such reports have raised awareness of a potential problem (e.g. [33]) and have led to long-term noise monitoring programmes, e.g. as required to evaluate habitat quality under the European Marine Strategy Framework Directive [34–36]. However, data on how often individual toothed whales encounter vessels, the resulting noise exposure levels and the frequency and severity of reactions are scarce. Most importantly, almost nothing is known about whether vessel activity interferes with vital behaviours such as feeding (but see [37]) and if this occurs often enough to have biologically significant effects on the fitness of individuals and populations [38,39].
The present study addresses these knowledge gaps by measuring the vessel noise budget of free-ranging harbour porpoises under natural conditions in relation to their fine-scale foraging behaviour; to our knowledge the first for any toothed whale. Throughout data collection, we deliberately did not follow the tagged animals to avoid adding to their vessel noise exposure. This means that our results represent the actual authentic noise budget, but also that we are reliant on tag data both to measure exposure and to infer response. The multiple tag sensors and stereotyped acoustic behaviour of porpoises, verified in captive studies (e.g. [40,41]), make it possible to quantify their foraging behaviour with high accuracy. Quantifying noise exposure on free-ranging animals is more complicated owing to the presence of noise from water flowing around the tag, surface splashes and impact sounds, as well as sounds originating from the animal itself. We manually marked splash and impact events in all of the recordings and excluded these from spectral analysis. Clicks from the tagged animal were excluded by taking the trimmed mean of spectra computed over successive short intervals. Flow noise was minimized by using measurements at high frequencies as proxies for the total noise exposure. These frequencies, while falling on the low edge of the best hearing range of porpoises [21], and thus being highly relevant to these high-frequency specialists, make our results difficult to compare with long-term noise data, because most monitoring studies do not extend that high (e.g. [36]). However, given the typical spectra of vessel noise that decrease with increasing frequency, high levels at high frequencies very likely translate into higher levels at lower frequencies [14]. Our methodology does not allow for exploring the cues porpoises may use to assess the immediacy of threat from vessels. However, our aim was not to investigate such explanatory scenarios, but rather to assess whether wild porpoises respond to vessel passes and what impact responses could have. We argue that to achieve this objective, the proxy chosen here, i.e. the noise level actually experienced by the animal, is reasonable and can be measured robustly enabling comparison with other studies.
Evaluation of the tag recordings by experienced listeners revealed that the porpoises encountered vessels frequently (table 2), albeit primarily at long ranges, as indicated by the prevailing low received levels (figure 2; electronic supplementary material, table S2). The resultant lack of baseline data and the variable foraging strategies of porpoises (table 2; [23]) make statistical testing of effects of ship encounters on foraging rates challenging. Despite this, the data reveal a statistically significant decrease in prey capture attempts during exposures to vessel noise at values closely matching the reaction threshold predicted by Tougaard et al. [3], albeit with some interindividual variability (electronic supplementary material, table S3). While these results should be interpreted with caution owing to the small relative number of minutes with high-noise level (electronic supplementary material, table S3) and the lack of baseline noise-free periods, they strongly indicate that exposed porpoises produce fewer foraging buzzes in the presence of high-level vessel noise, whether the received noise level is an explanatory factor for the responses, or merely a corollary of vessel proximity [37]. Under the assumption that the foraging rates recorded under less acute exposure conditions reflect unperturbed foraging rates, the fact that relatively few disturbances were recorded by the tags would suggest a minimal fitness cost of exposure. Crucially, however, that assumption may be wrong and even just a few per cent of decrease in foraging may have significant effects on fitness of these small animals that must keep warm in cold waters [22,42,43], especially when accumulated with other disturbances [44]. The generally shorter total buzz duration during high-noise exposure (electronic supplementary material, table S3) suggests little if any increased effort per prey in the form of a longer pursuit, or perhaps premature termination of prey pursuits. Thus, a lower energy intake could result from lost foraging opportunities, a shift to an easier, lower quality prey, or failed prey captures, these effects probably being additive, context-dependent and accompanied by higher energy expenditure owing to increased swimming activity.
Two specific examples involving porpoises of different ages and sexes demonstrate energetic responses to close vessel passes despite their frequent exposure to more distant boat noise (table 2). In both cases, vessel noise had spectral and temporal characteristics consistent with a fast ferry (figure 3; electronic supplementary material, figure S3). Both animals dove deeper, increased swimming effort and interrupted their foraging activities during the vessel pass with one of them abandoning echolocation altogether. The responses therefore caused not only missed foraging opportunities, but also increased energy expenditure, as well as potentially a greater risk of swimming into fishing nets that would normally be detected by echolocation. The estimated reaction distance of 7 km for one of the porpoises, together with the poor underwater visibility in Danish waters (less than 10 m) and the very small fraction of time spent by the animals with their eyes out of the water, reinforces the notion that threat from vessels was primarily perceived acoustically [37], whether the response was triggered by noise level, rate of change of noise level, noise spectrum or all of the above. The observation of a 15 min cessation of foraging associated with a single close vessel pass suggests that the impact of vessels may extend longer than the interval in which noise levels exceed a high threshold, and the vessel is close. Those 15 min would correspond to 23 prey capture attempts, if the animal continued to buzz at the average rate recorded just prior to and just after the exposure, and up to 88 attempts, if maximum 15 min buzz count for this animal was assumed. Given the frequency of the fast ferry service in the area chosen by these animals for foraging, it is likely that they experience close passes often (electronic supplementary material, figure S3). Thus, the strong responses to high-level vessel passes reported here suggest that these animals have not habituated to the noise. This is in agreement with the findings of Dyndo et al. [20], who observed that porpoises showed a robust and stereotypical porpoising reaction to some boats, despite their long-term residence in a harbour enclosure.
AIS records for the study area indicate a wide spatial variation in traffic density consistent with the complex coastline and varying bathymetry (electronic supplementary material, figure S1). In particular, large ship traffic concentrates in deeper channels that allow access to ports or open water. Tagged porpoises did not appear to avoid such highly trafficked areas, perhaps because these overlapped with important foraging habitats. Locally deep waters may aggregate fish and offer distinctive and valuable resources (e.g. [45]). For porpoises, they may thus constitute ‘acoustic hotspots’ where noisy anthropogenic activities overlap with important habitats [46].
The spatial variability of vessel encounter rates (table 2; electronic supplementary material, figure S1) and the wide range of received noise levels (electronic supplementary material, table S2; figure 2) probably also reflect differences in the type of boat traffic. Vessel, engine and propeller design [14,16], as well as speed and load [14,15,47], all affect the spectral characteristics of the generated noise and the duration of the exposure. Such a wide range of noise sources may require animals to develop a number of strategies to cope with exposure. Many behavioural reactions may be subtle and so go unnoted, even though cumulatively they could represent a significant disturbance. As a result, convincingly demonstrating behavioural responses to noise under natural conditions is notoriously difficult (e.g. [6]), especially because the history of the animal's exposure to vessel noise is rarely known. In the consistently noisy inner Danish Waters, porpoises may have developed behavioural strategies and/or compensatory mechanisms, e.g. an increase in vocalization amplitude [48], to combat elevated noise levels, and the absence of a control population makes it impossible to assess the full cost of these. Here, we focus on the additional loss of foraging effort owing to close vessel passes as the most reliably quantifiable and biologically relevant response variable. In doing so, we probably underestimate the full effect of vessel noise on porpoises.
4. Conclusion
We quantified the vessel noise budget of seven harbour porpoises in their natural environment, to our knowledge the first time this has been achieved for any toothed whale. We show that porpoises in a busy coastal habitat are frequently exposed to vessel noise. Although most exposures are at low levels, occasional high-level exposures with rapid onset occur when vessels pass close to animals or at high speeds. Observed reactions to such vessel passes involved vigorous fluking, interrupted foraging and even cessation of echolocation. Such exposures led to a general pattern of reduced foraging effort in the presence of noise levels greater than 96 dB re 1 µPa rms in the 16 kHz third octave band, although we probably underestimate the total impact of noise because animals may have already adjusted to the elevated average noise levels or be affected by them offering no real baseline. Given the high metabolic requirements and near continuous foraging reported for porpoises in this area, missed foraging opportunities during frequent boat passes could have a significant cumulative effect on body condition and vital rates. As high-frequency echolocators, porpoises use signals well beyond the low frequencies predominantly produced by vessels, and thus, our results raise concerns about the effects of vessel noise on other lower-frequency toothed whale species.
5. Material and methods
(a). Study area
The study was conducted in the inner Danish waters of Kattegat and the Belt seas (electronic supplementary material, figure S1), which are relatively shallow with depths rarely exceeding 50 m and averaging 23 m. The Sound, Great Belt and eastern Kattegat serve as narrow, deeper-water connections between the Baltic Sea and the North Sea, making these straits heavily trafficked at all times of the day by large ships, such as tankers and bulk freighters, but also diverse smaller vessels, including fishing boats [49]. Ship traffic in southern Kattegat between the Jutland Peninsula and the island of Zealand includes a fast passenger ferry line operating up to 24 passes a day. From late spring to early autumn, the coastal waters are occupied by widespread leisure boating activities.
(b). Data collection
Between September 2012 and September 2016, 19 porpoises incidentally trapped in pound nets set by local fishermen were equipped with DTAG-3 tags [25]. Tagging was carried out within 24 h of discovering a porpoise in the net. For tagging, the porpoise was carefully lifted onboard a fishing boat and placed on a soft pad. Its sex was determined, body condition evaluated and morphometric measurements were taken. Only animals that seemed in good health from an external examination were equipped with a tag. The porpoise was handled on the boat for no more than 15 min (table 1) before being released several hundred metres from the net.
The suction cup-attached tag was placed dorsally approximately 5 cm behind the blowhole to ensure good quality recordings of the low-level clicks of foraging buzzes [40] and to minimize noise associated with the animal's propulsion. The tags measured 7 × 17 × 3.5 cm and weighed 221–321 g in air and were slightly positively buoyant in water to facilitate recovery. They sampled 16 bit stereo audio at 500 kHz (179 dB re 1 µPa clip-level; approximately flat frequency response at 0.5–150 kHz), as well as three-dimensional orientation and pressure sensors at 250–625 Hz (16 bit). To avoid biased estimates of noise pollution, the DTAG-equipped porpoises were not followed after release; the tags were detached actively or passively after 12 to more than 24 h and were recovered with the aid of aerial VHF radio tracking and in some cases ARGOS satellite telemetry.
(c). Data analysis
Data processing and analysis were performed using Matlab R2013b (MathWorks, Inc.). Tag acoustic recordings were evaluated by headphone-listening and visual inspection of spectrograms (Hamming window, fast Fourier transform (FFT) size = 512, 75% overlap) computed over consecutive 5 s segments of the data. A corresponding dive profile was displayed in the same plot (for Matlab code, see www.soundtags.org). All intervals with detectable vessel noise, rain or loud transients were marked, as were respirations, logging periods at the sea surface and high-repetition-rate click sequences. The high-rate click sequences were classified as pulsed communication calls [50] or foraging buzzes accompanying prey capture attempts by the tagged animal [40] using published criteria [23].
Intervals with audible vessel noise were checked on a dive-by-dive basis to remove short periods when the tag was out of the water from the total exposure time. Similarly, the durations of all respirations and logging events (with a 0.5 s guard window to account for masking when animals break the surface) were subtracted from the time with no detectable vessel noise. Periods when vessel noise was uncertain, for example, owing to masking during rain or high sea state, were considered vessel-free. Our vessel exposure rates are, therefore, conservative estimates.
Foraging and noise measures were quantified in consecutive 1 min segments of the data. This interval spans the approximate duration of a typical porpoise dive in the area and allows reliable estimates of rapidly fluctuating noise levels from vessels passing at high speeds. A dip in the distributions of inter-click-intervals at 15 ms was used to detect the start and end of buzzes [23]. Data prior to the first foraging buzz were excluded to allow for a post-tagging recovery period [6] and thereby minimize the potential for confound owing to a stress response to handling. This time interval varied from 0.2 to 4.1 h (table 1), but a minimum time of 1 h after tagging was excluded. As the animals switched between benthic, demersal, pelagic and surface foraging, they adapted their acoustic behaviour resulting in prolonged buzzes in some foraging modes. Such buzzes could represent a long pursuit of a repeatedly escaping prey, or a series of captures on several schooling prey. To allow for both possibilities, foraging effort was quantified by both the number of buzz sequences and their total duration in each 1 min segment. Noise level was quantified in a two-step procedure; to eliminate sound energy from the animal's powerful 100 µs clicks, the noise level was first measured in 1 ms intervals and averaged over a 0.5 s time window as a trimmed mean discarding the highest 10th percentile of the data in each one third octave band (see below). To estimate the highest noise level, i.e. the level most likely to explain any behavioural reaction, the 0.5 s averages were ordered within each minute and the 90th percentile identified. This corresponds to the L10 statistical noise level, a robust estimate of the highest noise level. Time spent by the animal at the surface with the tag out of the water during breathing and resting (typically 0.5–30 s) was excluded in each minute before ordering. Similarly, recording blocks dominated by rain, splash noise from the animal breaking the surface, breaking waves down to 2 m depth or loud transients that were not judged to come from vessels, but rather zero padding of rare undecodable data chunks or debris hitting the hydrophones, were excluded from further processing. Finally, time intervals dominated by the animal's calls or loud air recycling sounds were also excluded. If more than 40 s of a given 1 min segment were discarded, the whole minute was excluded.
Noise level was quantified as one third octave levels, which approximate the filter-bank model of the mammalian auditory system [2,51]. Third octave bands with centre frequencies at 63 and 125 Hz have been suggested as proxies for general levels from shipping [34]. However, harbour porpoises have poor low-frequency hearing [21] with signal detection thresholds below 1 kHz probably higher than the ambient TOLs in southern Kattegat [14]. As porpoises have been shown to react to the high-frequency components of vessel noise [20], a third octave band centred at 2 or 10 kHz has been proposed as a more appropriate indicator of shipping noise relevant for these high-frequency specialists [14,52]. However, sound recordings made on a moving animal contain significant activity-dependent flow noise at low-to-mid frequencies, which complicates the measurement of ambient noise, especially during energetic pursuits of prey. To determine the lowest third octave band that is relatively free of flow noise in most activities, we examined the relationship between TOLs recorded in the absence of vessel noise, and log(J) a proxy for swimming activity (electronic supplementary material, figure S2), where J is the rms jerk [53] in a 0.5 s time window. For the 1 min averages, we computed the 90th percentile of the 0.5 s jerk measurements corresponding to the intervals included in the noise analysis. From this analysis, we chose the 16 kHz third octave band to characterize ambient noise.
Relative speed and closest point of approach (CPA) to the tagged animal were estimated for a subset of eligible vessels, by measuring the Doppler shift of tones generated by the vessels' engines, gearboxes and propellers [54] and recorded by the tag. The inflection point of the frequency shift of the tone was identified in the spectrogram of the vessel recording and a sigmoid curve was fitted to the data. Vessel velocity and CPA were estimated using the Doppler equation, assuming a stationary receiver and a sound speed of 1500 m s−1. The method requires high-quality recordings of the tones, which limited the dataset to less than 10 of the recorded vessels. In the remaining vessel passes, the tones were masked by cavitation noise and other broadband contributions from the vessel movement.
(d). Statistical analysis
Statistical analyses were carried out using R v. 3.3.2 (http://www.R-project.org) with the perm package.
Following an exploratory analysis of model fitting, we split the 1 min measurements for each animal into groups with low- and high-level noise and then tested for a difference in the distribution of buzz count and total buzz duration between groups using a two-sample permutation test corresponding to the central Fisher's exact test [55]. The noise level threshold for identifying the high-level group was increased stepwise in 3 dB intervals. An initial 6 dB buffer was used between the high- and low-level groups, i.e. minutes with average noise levels < threshold, but ≥(threshold-6 dB) were excluded from the analysis. The low-level group remained constant, i.e. number of minutes in the buffer increased as the high-level threshold increased. A one-sided permutation t-test evaluated whether minutes with high-level noise contained a lower number of buzzes than minutes with low-level noise. A two-sided test was used for total buzz duration, because more buzzing time could indicate an increased foraging activity, or an increased effort per prey. The permutation test was run if at least 5 min exceeded the threshold level for each animal. The p-values were estimated from 104 replications.
Supplementary Material
Acknowledgements
We thank S. Sveegaard, L. Mikkelsen, M.V. Jensen, L. Hermannsen, S. Elmegaard, M. de Freitas, L. Doñate-Rojano, M. Dyndo, B. McDonald, A. Hansen and B. Hansen as well as all the helpful fishermen and the skilled pilot (U. Gosewinkel) involved in tag deployments and recoveries. L. Doñate-Rojano, M. Dyndo, S. Videsen and A. Schrøder helped with data processing. T. Hurst at Woods Hole Oceanographic Institution and R. Swift at the University of St Andrews helped construct the tags. We thank L.A. Miller and J. Tougaard for comments on an earlier version of this manuscript.
Ethics
Tagging was carried out under permission from the Danish Forest and Nature Agency (NST-3446-00016) and the Animal Welfare Division (Ministry of Justice, 2010-561-1801).
Data accessibility
Scripts used to analyse the data and example datasets were deposited at doi:10.5281/zenodo.898733.
Authors' contributions
D.M.W., M.J., P.T.M., J.T. and U.S. designed the study. M.J. designed the measurement devices and processing software. D.M.W., J.T., P.T.M., A.G. and R.D. collected the data. D.M.W., M.J., P.T.M. and J.T. conducted data analysis and interpretation. D.M.W., P.T.M., M.J. and J.T. drafted the manuscript, and all authors contributed to finalize it.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by the German Federal Agency for Nature Conservation via a grant to U.S., J.T. and M.J. (contract Z1.2-5330/2010/14 and BfN-Cluster 7) and in part by grants from the Carlsberg Foundation and FNU to P.T.M. Tag development was supported, in part, by the Marine Alliance for Science and Technology Scotland and a Marie Skłodowska-Curie Career Integration Grant to M.J.
References
- 1.Tyack PL, Clark CW. 2000. Communication and acoustic behavior of dolphins and whales. In Hearing by whales and dolphins (eds Au WWL, Fay R), pp. 156–224. New York, NY: Springer Handbook of Auditory Research Series. [Google Scholar]
- 2.Richardson WJ, Greene CR Jr, Malme CI, Thomson DH. 1995. Marine mammals and noise. London, UK: Academic Press. [Google Scholar]
- 3.Tougaard J, Wright AJ, Madsen PT. 2015. Cetacean noise criteria revisited in the light of proposed exposure limits for harbour porpoises. Mar. Pollut. Bull. 90, 196–208. ( 10.1016/j.marpolbul.2014.10.051) [DOI] [PubMed] [Google Scholar]
- 4.Gray H, van Waerebeek K. 2011. Postural instability and akinesia in a pantropical spotted dolphin, Stenella attenuata, in proximity to operating airguns of a geophysical seismic vessel. J. Nat. Conserv. 19, 363–367. ( 10.1016/j.jnc.2011.06.005) [DOI] [Google Scholar]
- 5.Tougaard J, Carstensen J, Teilmann J, Skov H, Rasmussen P. 2009. Pile driving zone of responsiveness extends beyond 20 km for harbor porpoises (Phocoena phocoena (L.)). J. Acoust. Soc. Am. 126, 11–14. ( 10.1121/1.3132523) [DOI] [PubMed] [Google Scholar]
- 6.Miller PJO, Johnson MP, Madsen PT, Biassoni N, Quero M, Tyack PL. 2009. Using at-sea experiments to study the effects of airguns on the foraging behavior of sperm whales in the Gulf of Mexico. Deep Sea Res. Part I Oceanogr. Res. Pap. 56, 1168–1181. ( 10.1016/j.dsr.2009.02.008) [DOI] [Google Scholar]
- 7.Miller P, Kvadsheim P, Lam F. 2012. The severity of behavioral changes observed during experimental exposures of killer (Orcinus orca), long-finned pilot (Globicephala melas), and sperm (Physeter macrocephalus) whales to naval sonar. Aquat. Mamm. 38, 362–401. ( 10.1578/AM.38.4.2012.362) [DOI] [Google Scholar]
- 8.DeRuiter SL, et al. 2013. First direct measurements of behavioural responses by Cuvier's beaked whales to mid-frequency active sonar. Biol. Lett. 9, 20130223 ( 10.1098/rsbl.2013.0223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller P, et al. 2015. First indications that northern bottlenose whales are sensitive to behavioural disturbance from anthropogenic noise. R. Soc. open sci. 2, 140484 ( 10.1098/rsos.140484) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hildebrand J. 2009. Anthropogenic and natural sources of ambient noise in the ocean. Mar. Ecol. Prog. Ser. 395, 5–20. ( 10.3354/meps08353) [DOI] [Google Scholar]
- 11.Payne R, Webb D. 1971. Orientation by means of long range acoustic signaling in baleen whales. Ann. NY Acad. Sci. 188, 110–141. ( 10.1111/j.1749-6632.1971.tb13093.x) [DOI] [PubMed] [Google Scholar]
- 12.Croll DA, Clark CW, Calambokidis J, Ellison WT, Tershy BR. 2001. Effect of anthropogenic low-frequency noise on the foraging ecology of Balaenoptera whales. Anim. Conserv. 4, 13–27. ( 10.1017/S1367943001001020) [DOI] [Google Scholar]
- 13.Castellote M, Clark CW, Lammers MO. 2012. Acoustic and behavioural changes by fin whales (Balaenoptera physalus) in response to shipping and airgun noise. Biol. Conserv. 147, 115–122. ( 10.1016/j.biocon.2011.12.021) [DOI] [Google Scholar]
- 14.Hermannsen L, Beedholm K, Tougaard J, Madsen PT. 2014. High frequency components of ship noise in shallow water with a discussion of implications for harbor porpoises (Phocoena phocoena). J. Acoust. Soc. Am. 136, 1640–1653. ( 10.1121/1.4893908) [DOI] [PubMed] [Google Scholar]
- 15.McKenna MF, Ross D, Wiggins SM, Hildebrand JA. 2012. Underwater radiated noise from modern commercial ships. J. Acoust. Soc. Am. 131, 92–103. ( 10.1121/1.3664100) [DOI] [PubMed] [Google Scholar]
- 16.Jensen FH, Bejder L, Wahlberg M, Soto NA, Johnson M, Madsen PT. 2009. Vessel noise effects on delphinid communication. Mar. Ecol. Prog. Ser. 395, 161–175. ( 10.3354/meps08204) [DOI] [Google Scholar]
- 17.Pirotta E, Milor R, Quick N, Moretti D, Di Marzio N, Tyack P, Boyd I, Hastie G. 2012. Vessel noise affects beaked whale behavior: results of a dedicated acoustic response study. PLoS ONE 7, e42535 ( 10.1371/journal.pone.0042535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barlow J. 1988. Harbor porpoise, Phocoena phocoena, abundance estimation for California, Oregon, and Washington: I. Ship surveys. Fish. Bull. 86, 417–432. [Google Scholar]
- 19.Palka DL, Hammond PS. 2001. Accounting for responsive movement in line transect estimates of abundance. Can. J. Fish. Aquat. Sci. 58, 777–787. ( 10.1139/cjfas-58-4-777) [DOI] [Google Scholar]
- 20.Dyndo M, Wisniewska DM, Rojano-Doñate L, Madsen PT. 2015. Harbour porpoises react to low levels of high frequency vessel noise. Sci. Rep. 5, 1–9. ( 10.1038/srep11083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kastelein RA, Hoek L, de Jong CAF, Wensveen PJ. 2010. The effect of signal duration on the underwater detection thresholds of a harbor porpoise (Phocoena phocoena) for single frequency-modulated tonal signals between 0.25 and 160 kHz. J. Acoust. Soc. Am. 128, 3211–3222. ( 10.1121/1.3493435) [DOI] [PubMed] [Google Scholar]
- 22.Lockyer CH, Desportes G, Hansen K, Labberté S, Siebert U. 2003. Monitoring growth and energy utilisation of the harbour porpoise (Phocoena phocoena) in human care. NAMMCO Sci. Publ. 5, 107–120. ( 10.7557/3.2743) [DOI] [Google Scholar]
- 23.Wisniewska DM, et al. 2016. Ultra-high foraging rates of harbor porpoises make them vulnerable to anthropogenic disturbance. Curr. Biol. 26, 1441–1446. ( 10.1016/j.cub.2016.03.069) [DOI] [PubMed] [Google Scholar]
- 24.Pirotta E, Brookes KL, Graham IM, Thompson PM. 2014. Variation in harbour porpoise activity in response to seismic survey noise. Biol. Lett. 10, 20131090 ( 10.1098/rsbl.2013.1090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson M, Aguilar Soto N, Madsen PT. 2009. Studying the behaviour and sensory ecology of marine mammals using acoustic recording tags: a review. Mar. Ecol. Prog. Ser. 395, 55–73. ( 10.3354/meps08255) [DOI] [Google Scholar]
- 26.Lockyer C, Kinze C. 2003. Status, ecology and life history of harbour porpoise (Phocoena phocoena), in Danish waters. NAMMCO Sci. Publ. 5, 143–176. ( 10.7557/3.2745) [DOI] [Google Scholar]
- 27.Malakoff D. 2010. A push for quieter ships. Science 328, 1502–1503. ( 10.1126/science.328.5985.1502) [DOI] [PubMed] [Google Scholar]
- 28.Slabbekoorn H, Bouton N, van Opzeeland I, Coers A, ten Cate C, Popper AN. 2010. A noisy spring: the impact of globally rising underwater sound levels on fish. Trends Ecol. Evol. 25, 419–427. ( 10.1016/j.tree.2010.04.005) [DOI] [PubMed] [Google Scholar]
- 29.Andrew RK, Howe BM, Mercer JA, Dzieciuch MA. 2002. Ocean ambient sound: comparing the 1960s with the 1990s for a receiver off the California coast. Acoust. Res. Lett. Online 3, 65–70. ( 10.1121/1.1461915) [DOI] [Google Scholar]
- 30.Tournadre J. 2014. Anthropogenic pressure on the open ocean: the growth of ship traffic revealed by altimeter data analysis. Geophys. Res. Lett. 41, 7924–7932. ( 10.1002/2014GL061786) [DOI] [Google Scholar]
- 31.Rolland RM, Parks SE, Hunt KE, Castellote M, Corkeron PJ, Nowacek DP, Wasser SK, Kraus SD. 2012. Evidence that ship noise increases stress in right whales. Proc. R. Soc. B 279, 2363–2368. ( 10.1098/rspb.2011.2429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bejder L, et al. 2006. Decline in relative abundance of bottlenose dolphins exposed to long-term disturbance. Conserv. Biol. 20, 1791–1798. ( 10.1111/j.1523-1739.2006.00540.x) [DOI] [PubMed] [Google Scholar]
- 33.Sutherland WJ, et al. 2013. Identification of 100 fundamental ecological questions. J. Ecol. 101, 58–67. ( 10.1111/1365-2745.12025) [DOI] [Google Scholar]
- 34.European Commission. 2008. Directive 2008/56/EC of the European Parliament and of the Council of 17th June 2008 establishing a framework for community action in the field of marine environmental policy (Marine Strategy Framework Directive), OJ L 164, 25.6.2008, pp. 19–40.
- 35.Merchant ND, Pirotta E, Barton TR, Thompson PM. 2014. Monitoring ship noise to assess the impact of coastal developments on marine mammals. Mar. Pollut. Bull. 78, 85–95. ( 10.1016/j.marpolbul.2013.10.058) [DOI] [PubMed] [Google Scholar]
- 36.Merchant ND, Brookes KL, Faulkner RC, Bicknell AWJ, Godley BJ, Witt MJ. 2016. Underwater noise levels in UK waters. Sci. Rep. 6, 1–10. ( 10.1038/srep36942) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pirotta E, Merchant ND, Thompson PM, Barton TR, Lusseau D. 2015. Quantifying the effect of boat disturbance on bottlenose dolphin foraging activity. Biol. Conserv. 181, 82–89. ( 10.1016/j.biocon.2014.11.003) [DOI] [Google Scholar]
- 38.National Research Council. 2005. Marine mammal populations and ocean noise: determining when noise causes biologically significant effects. Washington, DC: National Academy Press. [Google Scholar]
- 39.New LF, et al. 2013. Modelling the biological significance of behavioural change in coastal bottlenose dolphins in response to disturbance. Funct. Ecol. 27, 314–322. ( 10.1111/1365-2435.12052) [DOI] [Google Scholar]
- 40.DeRuiter SL, Bahr A, Blanchet M-A, Hansen SF, Kristensen JH, Madsen PT, Tyack PL, Wahlberg M. 2009. Acoustic behaviour of echolocating porpoises during prey capture. J. Exp. Biol. 212, 3100–3107. ( 10.1242/jeb.030825) [DOI] [PubMed] [Google Scholar]
- 41.Verfuß UK, Miller LA, Pilz PKD, Schnitzler H-U. 2009. Echolocation by two foraging harbour porpoises (Phocoena phocoena). J. Exp. Biol. 212, 823–834. ( 10.1242/jeb.022137) [DOI] [PubMed] [Google Scholar]
- 42.Kastelein RA, Hardeman J, Boer H. 1997. Food consumption and body mass of harbour porpoises (Phocoena phocoena). In The biology of the harbour porpoise (eds Read AJ, Wiepkema PR, Nachtigall PE), pp. 217–233. Woerden, The Netherlands: DeSpil Publishers. [Google Scholar]
- 43.MacLeod CD, Santos MB, Reid RJ, Scott BE, Pierce GJ. 2007. Linking sandeel consumption and the likelihood of starvation in harbour porpoises in the Scottish North Sea: could climate change mean more starving porpoises? Biol. Lett. 3, 185–188. ( 10.1098/rsbl.2006.0588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nabe-Nielsen J, Sibly RM, Tougaard J, Teilmann J, Sveegaard S. 2014. Effects of noise and by-catch on a Danish harbour porpoise population. Ecol. Modell. 272, 242–251. ( 10.1016/j.ecolmodel.2013.09.025) [DOI] [Google Scholar]
- 45.Nilsson L, Thygesen UH, Lundgren B, Nielsen BF, Nielsen JR, Beyer JE. 2003. Vertical migration and dispersion of sprat (Sprattus sprattus) and herring (Clupea harengus) schools at dusk in the Baltic Sea. Aquat. Living Resour. 16, 317–324. ( 10.1016/S0990-7440(03)00039-1) [DOI] [Google Scholar]
- 46.McCarthy E. 2004. Hotspots: sensitive areas of intense acoustic activity. In International regulation of underwater sound: establishing rules and standards to address ocean noise pollution (ed. McCarthy E.), pp. 62–81. Boston, MA: Kluwer Academic Publishers. [Google Scholar]
- 47.McKenna MF, Wiggins SM, Hildebrand JA. 2013. Relationship between container ship underwater noise levels and ship design, operational and oceanographic conditions. Sci. Rep. 3, 1–10. ( 10.1038/srep01760) [DOI] [Google Scholar]
- 48.Hotchkin C, Parks S. 2013. The Lombard effect and other noise-induced vocal modifications: insight from mammalian communication systems. Biol. Rev. 88, 809–824. ( 10.1111/brv.12026) [DOI] [PubMed] [Google Scholar]
- 49.Stankiewicz M, Backer H, Vlasov Nikolay. 2010. Maritime activities in the Baltic Sea. An integrated thematic assessment on maritime activities and response to pollution at sea in the Baltic Sea region. Balt. Sea Environ. Proc. 123, 1–65. [Google Scholar]
- 50.Clausen KT, Wahlberg M, Beedholm K, Deruiter S, Madsen PT. 2010. Click communication in harbour porpoises Phocoena phocoena. Bioacoustics 20, 1–28. ( 10.1080/09524622.2011.9753630) [DOI] [Google Scholar]
- 51.Fletcher H. 1940. Auditory patterns. Rev. Mod. Phys. 12, 47–65. ( 10.1103/RevModPhys.12.47) [DOI] [Google Scholar]
- 52.Hermannsen L, Tougaard J, Beedholm K, Nabe-Nielsen J, Madsen PT. 2015. Characteristics and propagation of airgun pulses in shallow water with implications for effects on small marine mammals. PLoS ONE 10, e0133436 ( 10.1371/journal.pone.0133436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ydesen KS, Wisniewska DM, Hansen JD, Beedholm K, Johnson M, Madsen PT. 2014. What a jerk: prey engulfment revealed by high-rate, super-cranial accelerometry on a harbour seal (Phoca vitulina). J. Exp. Biol. 217, 2239–2243. ( 10.1242/jeb.100016) [DOI] [PubMed] [Google Scholar]
- 54.Holz F, Schuster M.2016. Tag data analysis regarding ship distance and speed. Auswirkungen des Unterwasserschalls der Offshore-Windenergieanlagen auf marine Säuger—Unterwasserschall Effekte (UWE).
- 55.Fay M. 2010. Two-sided exact tests and matching confidence intervals for discrete data. R J. 2, 53–58. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Scripts used to analyse the data and example datasets were deposited at doi:10.5281/zenodo.898733.