Abstract
Global variation in species richness is widely recognized, but the explanation for what drives it continues to be debated. Previous efforts have focused on a subset of potential drivers, including evolutionary rate, evolutionary time (maximum clade age of species restricted to a region), dispersal (migration from one region to another), ecological factors and climatic stability. However, no study has evaluated these competing hypotheses simultaneously at a broad spatial scale. Here, we examine their relative contribution in determining the richness of the most comprehensive dataset of tetrapods to our knowledge (84% of the described species), distinguishing between the direct influences of evolutionary rate, evolutionary time and dispersal, and the indirect influences of ecological factors and climatic stability through their effect on direct factors. We found that evolutionary time exerted a primary influence on species richness, with evolutionary rate being of secondary importance. By contrast, dispersal did not significantly affect richness patterns. Ecological and climatic stability factors influenced species richness indirectly by modifying evolutionary time (i.e. persistence time) and rate. Overall, our findings suggest that global heterogeneity in tetrapod richness is explained primarily by the length of time species have had to diversify.
Keywords: climatic stability, energy richness, evolutionary rate, evolutionary time, species richness, tetrapod
1. Introduction
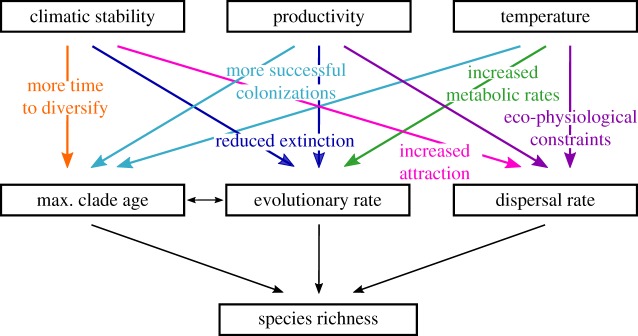
Species diversity is distributed unevenly across the planet. The clearest manifestation of this variation is an increase in the number of species towards the tropics—a pattern known as the latitudinal diversity gradient. With a few exceptions (e.g. mosses [1]), this pattern has been observed for diverse taxonomic groups (e.g. terrestrial vertebrates, fishes, insects and plants) on continents as well as in the ocean [2]. It has been recognized for more than 150 years [3], and can be said to have stimulated the development of community ecology [2]. However, the underlying cause for the latitudinal biodiversity gradient remains the subject of much debate. Accordingly, many hypotheses focusing on evolutionary, ecological and climatic factors have been proposed to explain it [4,5]. The hypothesized processes are not independent but organized hierarchically, and act concomitantly to shape biodiversity patterns. For example, evolutionary factors, which include evolutionary time, evolutionary rates (speciation, extinction and hence diversification rates) and dispersal, drive species richness patterns directly, whereas ecological and stability factors influence species richness indirectly through their effect on evolutionary factors (figure 1). However, the vast majority of studies to date have focused on either evolutionary, ecological or climatic stability hypotheses separately, or have not acknowledged the hierarchical structure of these effects on species richness [6–13]. For example, in two recent papers, the ‘rate hypothesis’ was evaluated through ecological variables and climatic stability [14,15], but the influence of clade age [13] was not tested. Here, we use structural equation models to evaluate the influence of direct (evolutionary time, rate and dispersal) and indirect (productivity, temperature and climatic stability) factors in shaping global tetrapod species richness patterns.
Figure 1.
Theoretical framework depicting the relationships, and the underlying hypotheses, between evolutionary, ecological, climatic stability and richness variables. max., maximum.
Direct factors are hypothesized to drive higher diversity in the tropics [16,17] via either a higher diversification rate (speciation minus extinction), a higher migration towards tropical areas (i.e. colonization from higher latitudes) or a longer evolutionary time (i.e. greater age of lineages). The ‘rate hypothesis’ suggests that evolutionary rates (speciation, extinction and hence diversification rates) are affected by various biological and ecological factors such as geographic area, mutation rate, generation time, ambient temperature, physiology and energy [5,18,19]. The ‘time hypothesis’ proposes that species richness is linked to the ages of clades. After a lineage or a species first colonizes an area, species accumulate at a nearly constant rate (constant diversification rate), producing a positive relationship between time and species richness [20]. Because many groups originated in the tropics, this hypothesis has been used to explain why tropical regions have higher species richness (e.g. [9,21,22]). In addition to ‘rate’ and ‘time’, species richness patterns can be directly influenced by dispersal [23], through range expansion, contraction and dispersal constraints [19,24–26]. The latitudinal diversity gradient could be either reinforced by the limited dispersal of tropical species into temperate regions because of dispersal constraints (i.e. niche conservatism) [24] or be mitigated by range expansion [26].
The main ecological hypothesis to explain global species richness is the ‘energy richness hypothesis’, which states that lower extinction rates occur in areas of higher productivity because these areas can support a higher number of individuals, and thus a higher number of species [27–29]. The effect of productivity on species richness is therefore indirect, through its effect on evolutionary rate (figure 1). This hypothesis is appealing because net primary productivity, which is largely dependent on solar radiation and water availability, increases markedly from the poles to the tropics [30]. Alternatively, species diversity may be indirectly influenced by temperature, with higher temperatures hypothesized to increase metabolic rates and, thus evolutionary rates [31] (figure 1). Moreover, temperature and productivity could also modulate species distributions through eco-physiological constraints on dispersal by limiting or promoting dispersal depending on the tolerance range of species [14,32,33] (figure 1). We can also assume older lineages to be predominant in more productive and warmer areas because, according to the energy richness hypothesis, areas of higher productivity are less prone to extinction [27–29].
Climate stability has also been hypothesized to drive biodiversity patterns through its effect on either or both evolutionary rate and evolutionary time (figure 1). Under the ‘climatic stability hypothesis', species persist longer and/or diversify more in regions that are climatically stable over geological time [9]. The constancy in resources resulting from climatic stability may enable finer specialization and adaptation possibilities (i.e. smaller niches) [34]. As a consequence, climatic stability could affect species richness via its effect on evolutionary rates if species in the more climatically stable tropics are able to persist when rare, while rare species in more climatically unstable temperate areas are driven to extinction (rate hypothesis). Alternatively, climatic stability could affect species richness via its effect on evolutionary time by providing lineages more time to diversify (time hypothesis) within a stable environment [9] (figure 1).
In this study, we examine all primary hypothesized drivers of global species richness simultaneously, using the most complete dataset of tetrapod vertebrates to date (approx. 27 000 species). Specifically, we tested for the direct influence of evolutionary factors (rate, time and dispersal) and the indirect influence of ecological (productivity and temperature) and climatic stability factors, on tetrapod species richness (figure 1 and table 1; see also electronic supplementary material, figure S2). Furthermore, we evaluate the relative importance of direct evolutionary effects in explaining tetrapod richness patterns. We provide a comprehensive picture of the main direct and indirect drivers of tetrapod species richness, and find conclusive evidence for the overarching importance of evolutionary time.
Table 1.
Recapitulation table of the variables used in these study to test the direct and indirect species richness predictors.
| species richness predictors | variables used in the analyses and description |
|---|---|
| direct factors | |
| dispersal | dispersal rate: dispersal rate from any bioregion to the focal bioregion |
| evolutionary rate | speciation (or diversification) rate: average rate of the cladesa of the focal bioregion |
| evolutionary time | maximum clade age: age of the oldest cladea of the focal bioregion |
| indirect factors | |
| climatic stability | area through time: integration of the focal bioregion area over the past 55 Myr |
| productivity | productivity: total productivity of the focal bioregion |
| temperature | temperature: mean temperature of the focal bioregion |
aWe only considered the clades in which all component species are found in the focal bioregion. See also the electronic supplementary material, figure S2.
2. Material and methods
We conducted global-scale analyses among 32 bioregion units, defined as the biomes nested within the world's main biogeographic realms, distributed as described by Olson et al. [35] (electronic supplementary material, figure S1). Species distribution data were used to obtain species lists for each of these bioregions (see the electronic supplementary material, methods for details). We performed structural equation modelling and variance partitioning analyses using richness, phylogenetic (dispersal rate, maximum clade age and speciation rate), ecological (productivity and temperature) and palaeoclimatic (area through time) data to evaluate evolutionary, ecological and climatic stability hypotheses for tetrapods overall, and individually for each of the four major groups of tetrapods (amphibians, birds, mammals and squamate reptiles) (table 1; electronic supplementary material, figure S2). For any given bioregion, the variable dispersal rate was the dispersal rate from any bioregion to the focal bioregion (calculated with the program GeoSSE [36]); maximum clade age was the age of the oldest clade in the focal bioregion (with all component species found in the focal bioregion), and represented the time during which species have successfully evolved and diversified; speciation rate was the average speciation rate of the clades belonging to the focal bioregion (calculated with the program BAMM [37]); productivity was the total productivity of the focal bioregion; temperature was the mean temperature of the focal bioregion; and area through time was the integration of the focal bioregion area over the past 55 Myr (see the electronic supplementary methods for details).
(a). Analyses
We evaluated the relationship between species richness and direct (maximum clade age, speciation rate and dispersal rate) and indirect (productivity, temperature and area through time) predictors for all tetrapods combined and for amphibians, birds, mammals and squamate reptiles. We did not use spatial models for bioregion analyses because of the strong independence of sampling in terms of response (low species overlap, 5, 12, 7 and 4% on average for amphibians, birds, mammals and squamates, respectively) and predictor variables (each bioregion is distinct) [15]. We first checked whether any variables were collinear by calculating variance inflation factors (VIFs) [38]. We found a low collinearity between our variables, with VIF scores between 1.56 and 3.34, and did not discard any variable. We also evaluated the relationship between each pair of variables by correlation analyses (Pearson coefficient) (electronic supplementary material, table S1).
(b). Structural equation modelling
We modelled the direct effects of evolutionary predictors (dispersal rate, maximum clade age and speciation rate) and the indirect effects of ecological (productivity and temperature) and climatic stability (area through time) predictors, through evolutionary predictors, on species richness using structural equation modelling with the package ‘lavaan’ [39] and 10 000 bootstrap iterations. Structural equations characterize the relationship between a set of variables through ordered networks of statistical dependence. The degree of relationship (i.e. the numerical effect of one variable upon another) is estimated by regression methods.
Because dispersal rate was not available for all bioregions, we evaluated two models, the full model (model 1) with all variables and model 2 without the variable dispersal rate. For model 1, we conducted the analysis among 25, 22, 28, 27 and 30 bioregions for amphibians, birds, mammals, squamates and tetrapods, respectively. For model 2, we conducted the analysis among 29, 32, 32, 29 and 32 bioregions for amphibians, birds, mammals, squamates and tetrapods, respectively. To test the effect of different combinations of variables, we used a model selection approach. We evaluated the four models and compared their AIC scores. The full model (model 1) and three alternative models, with all of the indirect but only two of the direct variables, were used to test if one variable could hide the influence of others on richness: maximum clade age and speciation rate (model 1a), maximum clade age and dispersal rate (model 1b), and speciation rate and dispersal rate (model 1c).
(c). Variance partitioning
To further evaluate the relative contribution to species richness of the two main direct hypotheses (age and rate), we used variance partitioning based on redundancy analysis ordination [40] as implemented in the R package ‘vegan’ [41]. The third direct variable, dispersal rate, was never associated with richness for any group, and removing this variable allowed us to increase the number of bioregions considered. We estimated the independent contribution to species richness variation for each predictor (individual fraction) as well as the shared contribution of each combination of covariate sets (shared fraction) using r² values adjusted for sample size, given the different number of predictors in each set. The sum of the individual and the shared fraction of each predictor set represented the predictor fraction. The total fraction was the variation explained by the complete model. Negative values can be generated in variance partitioning analyses; these should be interpreted as zeros as they correspond to cases where the explanatory variable explained less variation than would random variables [42].
The significance (i.e. whether the fraction explained a significant part of the variation) of each model and of the individual partitions was assessed by permutation (1000 permutations) [43]. For each of the predictors, we randomized the richness across regions and obtained the individual fractions (variance partitioning analysis) 1000 times to build a null distribution. We compared the observed individual fractions to the corresponding null distribution (one-tailed test, α = 0.05).
The individual fractions were compared by bootstrapping (1000 replications) [43]. We compared the evolutionary and ecological sets, the variables maximum clade age and speciation rate, and the variables productivity and temperature. The variables (response variable and the corresponding values of the explicative variables) were sampled with replacement to obtain a sample of the same size as the observed data and we calculated the individual fractions (variance partitioning analysis) 1000 times. We compared the distributions of each pair of individual fractions (one-tailed test, α = 0.05).
3. Results
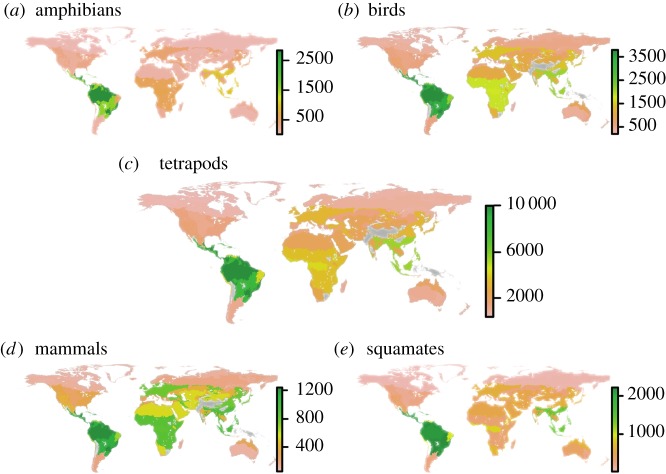
Newly available distributional data for reptiles along with existing amphibian, mammal and bird data highlight the uneven distribution of tetrapod species richness across the globe (figure 2). The different tetrapod groups all show a broad latitudinal gradient but different spatial patterns of species richness.
Figure 2.
Global species richness maps. Species richness was computed by bioregion for (a) amphibians, (b) birds, (c) tetrapods, (d) mammals and (e) squamate reptiles.
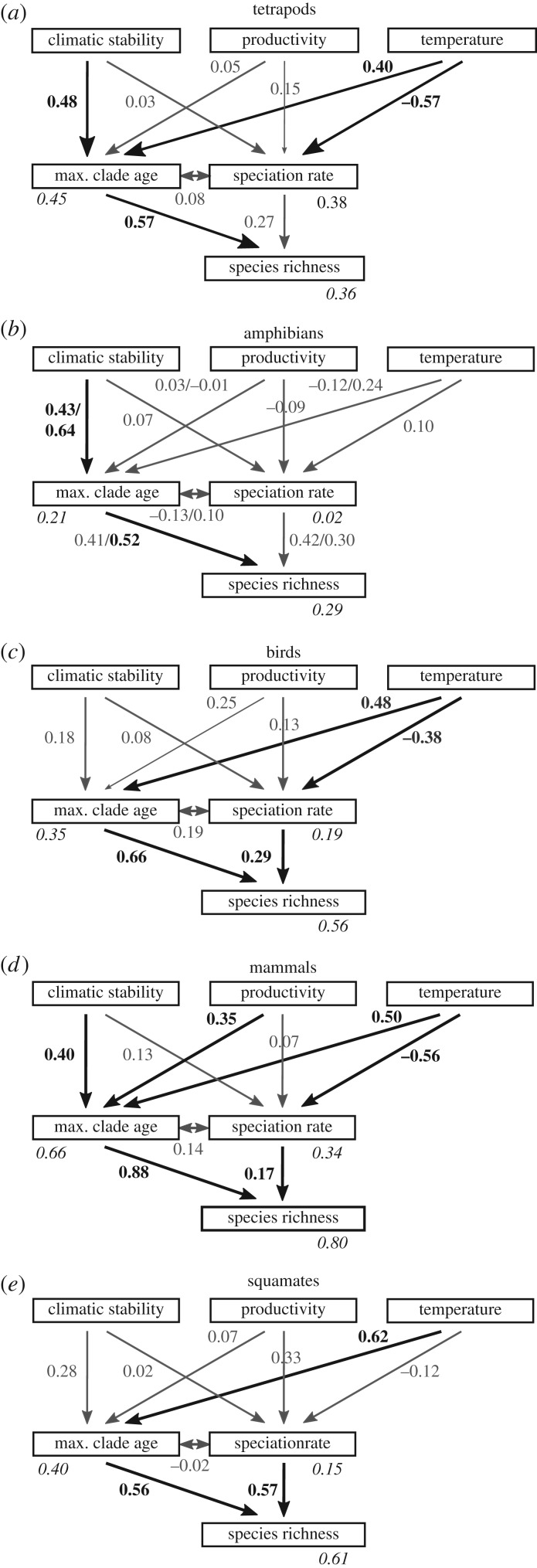
We used structural equation models to examine the direct effects of evolutionary factors (dispersal rate, maximum clade age and speciation rate) and indirect effects of ecological factors (productivity and temperature) and climatic stability (area through time) on species richness for tetrapods combined and for each of the four major tetrapod clades: amphibians, birds, mammals and squamates. We quantified the relationships between variables using standardized regression coefficients (β), which enable assessing the relative contribution of each effect. When considering the full model including all above effects (model 1; electronic supplementary material, figure S3), tetrapod species richness displayed a strong and significant association with maximum clade age (β = 0.55) and a non-significant association with speciation rate (electronic supplementary material, figure S3). Temperature had a significant indirect effect on species richness through both maximum clade age (β = 0.37) and speciation rate (β = −0.54), though the direction of these indirect effects was opposite. Tetrapod species richness was also indirectly influenced by area through time, our measure of climatic stability, through its effects on maximum clade age (β = 0.48). Among the subgroups of tetrapods, birds and squamates showed slightly different direct effects compared with tetrapods as a whole, with maximum clade age (β = 0.48 and 0.46, respectively) and speciation rate (β = 0.37 and β = 0.62, respectively) both significantly positively associated with species richness. However, only the variable temperature was significantly associated with speciation rate and dispersal rate for birds (β = −0.41 and β = −0.39 respectively) and with maximum clade age for squamates (β = 0.47). Maximum clade age was the only direct variable significantly associated with mammal richness (β = 0.88). Productivity was associated with maximum clade age (β = 0.35) and temperature was associated with maximum clade age (β = 0.50) and speciation rate (β = −0.51). No variable was significantly associated with amphibian richness, even after the removal of Alytidae, a small outlier clade of 12 species. We identified outlier clades for amphibians, birds, mammals and squamate reptiles by comparing the mean and the maximum clade age for each bioregion. We found the largest difference (119 million years, Myr) in the Eurasian Mediterranean bioregion for amphibians (95 species), driven by that small clade of tree frogs (Alytidae). Smaller differences were found for birds (49 Myr), mammals (48 Myr) and squamate reptiles (78 Myr). Dispersal rate was never associated with richness for any group tested and removing this variable allowed us to include more bioregions; moreover, the model without dispersal rate had the lowest AIC (model 1a; electronic supplementary material, table S2). Therefore, we considered a model without dispersal rate and with more bioregions as an alternative model of richness (model 2).
After removing dispersal rate, maximum clade age had the strongest direct effect on species richness in all subgroups of tetrapods except squamates (figure 3). In squamates, maximum clade age and speciation rate were both strongly associated with richness (β = 0.56 and β = 0.57, respectively). When considering indirect effects, the significant associations were similar to model 1 except for area through time, which was significantly associated with maximum clade age in amphibians (β = 0.43; 0.64 after the removal of the small outlier clade) and temperature, which was significantly associated with maximum clade age in birds (β = 0.48).
Figure 3.
Structural equation models depicting the associations of direct and indirect variables with species richness (without dispersal rate, model 2). Maximum clade age and speciation rate are the direct variables and area through time, productivity and temperature the indirect variables, for (a) tetrapods, (b) amphibians, (c) birds, (d) mammals and (e) squamates. Bold path arrows represent significant (at p < 0.05) associations. Numbers adjacent to arrows represent β-standardized regression coefficients. r2-values associated with each exogenous variable are in italics. For amphibians, we also indicated β-standardized regression coefficients and r2-values after the removal of the outlier clade of Mediterranean Alytidae (12 species) if the result changed (value in second position). max., maximum.
In order to further discriminate the relative importance of the two evolutionary variables in explaining species richness patterns, we performed a variance partitioning analysis. This analysis revealed that maximum clade age explained a significant portion of the richness variation for all tetrapods combined and in the separate subgroups: amphibians (with or without the small outlier clade), birds, mammals and squamates (table 2). Speciation rate explained a significant portion of the richness at the tetrapod level and for amphibians, although not for the other groups or amphibians after the removal of the small outlier clade of Alytidae. Removing the outlier clades for the other groups (birds, mammals and squamate reptiles) did not change the results (electronic supplementary material, tables S3–S4). Maximum clade age explained a higher proportion of richness variation for tetrapods overall, and specifically for amphibians after the removal of the small outlier clade, birds and mammals specifically (table 3). Speciation rate never explained a higher proportion of the response variable than maximum clade age. In the case of squamate reptiles, the two factors explained an equal fraction of the richness (table 3), although only maximum clade age explained a significant fraction of the richness (table 2).
Table 2.
Results from partitioning the variance of maximum clade age and speciation rate variables in explaining patterns of species richness (model 3). The reported values are adjusted r² statistics representing the total (complete model without residuals), individual and shared (explained by both factors) proportion of richness variation explained by maximum clade age and speciation rate. In bold are the fractions explaining a significant portion of the richness variation (α < 0.05; electronic supplementary material, table S6). Negative values can be generated in variance partitioning analyses; these should be interpreted as zeros as they correspond to cases where the explanatory variable explained less variation than would random variables [42]. max., maximum.
| total fraction | max. clade age | speciation rate | shared fraction | |
|---|---|---|---|---|
| tetrapods | 0.606 | 0.595 | 0.113 | −0.101 |
| amphibians | 0.231 | 0.131 | 0.142 | −0.042 |
| amphibiansa | 0.334 | 0.234 | 0.063 | 0.038 |
| birds | 0.533 | 0.438 | 0.074 | 0.021 |
| mammals | 0.787 | 0.784 | 0.024 | −0.022 |
| squamates | 0.399 | 0.152 | 0.083 | 0.164 |
aAmphibians without an outlier clade of Mediterranean Alytidae (12 species).
Table 3.
Bootstrapped p-values for comparisons of maximum clade age and speciation rate variables in explaining global tetrapod species richness. The significant results are in bold (α < 0.05). max., maximum; spe., speciation.
| max. clade age > spe. rate | spe. rate > max. clade age | |
|---|---|---|
| tetrapods | 0.003 | 0.998 |
| amphibians | 0.280 | 0.721 |
| amphibiansa | 0.027 | 0.974 |
| birds | 0.007 | 0.994 |
| mammals | 9.99 × 10−4 | 1 |
| squamates | 0.453 | 0.547 |
aAmphibians without an outlier clade of Mediterranean Alytidae (12 species).
Our findings were robust to a series of sources of uncertainty. We obtained similar results by replacing mean speciation rate with mean diversification rate, median speciation rate or median diversification rate for structural equation modelling (electronic supplementary material, figures S4 and S5, and table S2) and with diversification rate for variance partitioning analyses (electronic supplementary material, table S5). In addition, we also performed these analyses on New World squamates only, because most of the Old World squamate richness data were based on older IUCN Red List assessments (see Material and methods). We found largely comparable results except for a significantly higher influence of maximum clade age (β = 0.73) over speciation rate (β = 0.27) on species richness. Finally, we also obtained similar results by substituting maximum clade age with lower and upper bounds of the 95% confidence interval (age lower bound, age upper bound, random lower/upper age), or cumulative clade age (models 3a, 3b, 3c and 3d; electronic supplementary material, table S2).
4. Discussion
Relationships between the potential drivers of species richness patterns are complex and difficult to disentangle. In addition, a focus on some but not all major hypotheses in past analyses has prevented the evaluation of the relative and the structured contributions of evolution, ecology and climatic stability to species richness patterns. For example, one of the most inclusive studies conducted on birds and mammals [14], sharing similar results with our study (see below), omitted time and dispersal as potential predictors. This study concluded that historical and evolutionary processes explain species richness in birds and mammals, but could not discriminate among age, dispersal or climatic stability. Here, with a nearly complete global tetrapod dataset, we evaluated these hypotheses simultaneously in a structured framework (figure 1), using variables capturing the main components of each hypothesis. Despite mass extinctions and range reductions induced by anthropogenic factors from the Pleistocene to the present [44], we found that clade age is the most important factor explaining the distribution of species richness in tetrapods. Evolutionary rate emerged as a secondary factor and dispersal did not significantly affect richness patterns. We also detected indirect effects of ecological and climatic stability variables on species richness through their influence on clade age and evolutionary rates.
(a). Direct effects
Based on structural equation models, our analyses showed that clade age is the major predictor of species richness patterns in tetrapods. Clade age was strongly associated with richness and explained a significant portion of its variation in each group tested individually (figure 3 and table 2). Moreover, clade age explained a significantly higher fraction of richness variation than diversification rate at the tetrapod level and for amphibians (after the removal of the small outlier clade), birds and mammals (table 2). For squamates, despite a comparable association strength between squamate richness and clade age or evolutionary rate (figure 3), only clade age explained a significant fraction of their richness variation (table 2). These results are in line with several previous studies indicating that time, and not rate of diversification, best describes species richness patterns [9,13,20,45], with the richest areas being strongly associated with older lineages. Therefore, our results also largely corroborate studies supporting an expanding diversity [46], stating that species richness is correlated to time and not density-dependent.
By contrast, the ‘rate hypothesis’, often invoked to explain species richness patterns [6,7,11,12,47,48], was only weakly supported by our results, as was also the case in a recent global analysis [14]. The discrepancy between our results and previous studies supporting the ‘rate hypothesis’ over the ‘time hypothesis’ might be explained by methodological differences. For example, Scholl & Wiens [48] used stem age instead of crown age, even though the former has been shown to add noise to the age–richness relationship because the stem branch is statistically unrelated to crown age [46]. Scholl & Wiens [48] also used the same sampling fraction correction across their tree, despite the fact that the tree contained clades with different levels of uncertainty around their estimated richness. We are unable to compare our results with other studies that did not consider clade age in their analyses [11,12,47]. However, due to the difficulty in estimating extinction, we acknowledge that even in the absence of a strong association between diversification rate and richness, lower maximum crown ages in less diverse regions might be explained by higher extinction [49].
Finally, we did not find evidence of a significant role of dispersal in shaping tetrapod species distribution. However, our findings do not rule out the role of dispersal in shaping richness locally, and finer scale analyses would be helpful to better understand its role. Our results rather suggest that dispersal has a limited importance at global scale. The highest association between dispersal and richness was detected in birds (β=−0.24; electronic supplementary material, figure S3). Even if not significant, this trend probably reflects their higher dispersal ability, relative to other tetrapod groups, with species migrating preferentially towards the less inhabited areas (negative association). This result corroborates previous studies, showing a mitigating effect of dispersal on the latitudinal richness gradient by homogenizing richness across latitude [26].
(b). Indirect effects
Productivity has been postulated to increase regional richness by allowing more niches or larger population sizes, and thus by reducing extinction rates [31,50,51]. Temperature has also been predicted to shape distributional patterns by increasing individual metabolic rates and, in turn, reducing extinction rate [31,52,53]. Our results did not corroborate these hypotheses, as we did not detect a significant relationship between speciation (or diversification) rate and productivity, and found a negative association between speciation (or diversification) rate and mean annual temperature in tetrapods overall, and separately in birds and mammals. Indeed, for birds and mammals, most of the bioregions characterized by low speciation (and diversification) rate are hot regions, including deserts and tropical dry forests. A negative association between temperature and diversification rate has also been reported recently in mammals (e.g. [54]). The addition of more variables would help to understand why some (but not all) hot regions are characterized by low evolutionary rates for birds and mammals. Our results also showed a positive association between temperature and clade age for each group except amphibians (figure 3). This pattern might reflect better conditions for successful dispersal to warmer areas for birds, mammals and squamates. The biogeographic history of these groups, already invoked to explain time and rate patterns between biogeographic regions [13], could also explain this association, because most birds and mammals originated in Gondwana [55,56], whereas the oldest clades of amphibians are found in Laurasia, which was colder [57,58]. The origins of squamates and their major subclades are not yet established [59].
Given our finding that clade age largely predicts species richness patterns, a strong indirect association between species richness and climatic stability might be expected. This is because climatic stability reflects the amount of time a lineage could persist under unaltered climatic conditions. Our results corroborated this hypothesis for tetrapods, as well as for amphibians and mammals, as has previously been found [14]. Our finding that speciation or diversification rate is of lesser importance than clade age, and that climatic stability was never associated with evolutionary rates, suggests that climatic stability may be acting through exclusion, by altering species distributions, rather than causing species to go extinct. Under this model, lineages inhabiting unstable areas migrate towards more suitable areas or refuges when their climate becomes unsuitable, and later re-colonize the original area, when former climatic conditions are re-established. This process implies a re-set of clade age because some species (newly formed or older) might not return to the focal area, as a consequence, the clades for which all the species inhabit the area will be younger. However, conclusions based on this result are subject to uncertainty, as extinction rate estimates derived from phylogenies may not always be reliable [60,61].
New environments might also be colonized by already diversifying clades as long as the new conditions are suitable for them. This model is supported by our results for birds and squamates, with lineages (maximum clade age) being older than the occupied area. For example, tundra and boreal forests originated about 10 million years ago (Ma) and temperate grasslands 25 Ma [15], yet clades with ages of 20 and 40 Myr, respectively, inhabit these regions. This could explain why we did not detect an association between climatic stability and clade age in birds or squamates.
5. Conclusion
Our global analyses of more than 27 000 tetrapod species highlight the major importance of evolution in shaping geographic patterns of species richness. Of all potential drivers, clade age is of primary importance and is consistent with the notion of a speciation clock [46]. Nonetheless, temperature, productivity and climatic stability have also influenced tetrapod species richness indirectly via their influence on clade age and speciation rate.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the participants in the IUCN Red List workshops for sharing their knowledge about squamate distribution.
Data accessibility
This article has no additional data.
Authors' contributions
J.M. and S.B.H. planned the research with input from other authors. J.M. and G.R. prepared the datasets. J.M. conducted the analyses and wrote the first draft of the manuscript, and all authors contributed substantially to revisions.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by grants from the US National Science Foundation to S.B.H. (1136590 and 1455762), to T.M.B., G.C.C. and B.E.Y. (1136705), to C.H.G. (1136705) and to V.C.R. (1136592).
References
- 1.Geffert JL, Frahm JP, Barthlott W, Mutke J. 2013. Global moss diversity: spatial and taxonomic patterns of species richness. J. Bryol. 35, 1–11. ( 10.1179/1743282012Y.0000000038) [DOI] [Google Scholar]
- 2.Willig MR, Kaufman DM, Stevens RD. 2003. Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. System. 34, 273–309. ( 10.1146/annurev.ecolsys.34.012103.144032) [DOI] [Google Scholar]
- 3.Wallace AR. 1878. Tropical nature and other essays. London, UK: Macmillan and Company. [Google Scholar]
- 4.Pianka ER. 1966. Latitudinal gradients in species diversity: a review of concepts. Am. Nat. 100, 33–46. ( 10.1086/282398) [DOI] [Google Scholar]
- 5.Rohde K. 1992. Latitudinal gradients in species diversity: the search for the primary cause. Oikos 65, 514–527. ( 10.2307/3545569) [DOI] [Google Scholar]
- 6.Davies TJ, Savolainen V, Chase MW, Moat J, Barraclough TG. 2004. Environmental energy and evolutionary rates in flowering plants. Proc. R. Soc. Lond. B 271, 2195–2200. ( 10.1098/rspb.2004.2849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardillo M, Orme CDL, Owens IPF. 2005. Testing for latitudinal bias in diversification rates: an example using New World birds. Ecology 86, 2278–2287. ( 10.1890/05-0112) [DOI] [Google Scholar]
- 8.Graham CH, Moritz C, Williams SE. 2006. Habitat history improves prediction of biodiversity in rainforest fauna. Proc. Natl Acad. Sci. USA 103, 632–636. ( 10.1073/pnas.0505754103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jablonski D, Roy K, Valentine JW. 2006. Out of the tropics: evolutionary dynamics of the latitudinal diversity gradient. Science 314, 102–106. ( 10.1126/science.1130880) [DOI] [PubMed] [Google Scholar]
- 10.Werneck FP, Nogueira C, Colli GR, Sites JW, Costa GC. 2012. Climatic stability in the Brazilian Cerrado: implications for biogeographical connections of South American savannas, species richness and conservation in a biodiversity hotspot. J. Biogeogr. 39, 1695–1706. ( 10.1111/j.1365-2699.2012.02715.x) [DOI] [Google Scholar]
- 11.Pyron RA, Wiens JJ. 2013. Large-scale phylogenetic analyses reveal the causes of high tropical amphibian diversity. Proc. R. Soc. B 280, 20131622 ( 10.1098/rspb.2013.1622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rolland J, Condamine FL, Jiguet F, Morlon H. 2014. Faster speciation and reduced extinction in the tropics contribute to the mammalian latitudinal diversity gradient. PLoS Biol. 12, e1001775 ( 10.1371/journal.pbio.1001775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marin J, Hedges SB. 2016. Time best explains global variation in species richness of amphibians, birds and mammals. J. Biogeogr. 43, 1069–1079. ( 10.1111/jbi.12709) [DOI] [Google Scholar]
- 14.Belmaker J, Jetz W. 2015. Relative roles of ecological and energetic constraints, diversification rates and region history on global species richness gradients. Ecol. Lett. 18, 563–571. ( 10.1111/ele.12438) [DOI] [PubMed] [Google Scholar]
- 15.Jetz W, Fine PVA. 2012. Global gradients in vertebrate diversity predicted by historical area-productivity dynamics and contemporary environment. PLoS Biol. 10, e1001292 ( 10.1371/journal.pbio.1001292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevens RD. 2011. Relative effects of time for speciation and tropical niche conservatism on the latitudinal diversity gradient of phyllostomid bats. Proc. R. Soc. B 278, 2528–2536. ( 10.1098/rspb.2010.2341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansson R, Rodríguez-Castañeda G, Harding LE. 2013. What can multiple phylogenies say about the latitudinal diversity gradient? A new look at the tropical conservatism, out of the tropics, and diversification rate hypotheses. Evolution 67, 1741–1755. ( 10.1111/evo.12089) [DOI] [PubMed] [Google Scholar]
- 18.Losos JB, Schluter D. 2000. Analysis of an evolutionary species–area relationship. Nature 408, 847–850. ( 10.1038/35048558) [DOI] [PubMed] [Google Scholar]
- 19.Allen AP, Gillooly JF. 2006. Assessing latitudinal gradients in speciation rates and biodiversity at the global scale. Ecol. Lett. 9, 947–954. ( 10.1111/j.1461-0248.2006.00946.x) [DOI] [PubMed] [Google Scholar]
- 20.McPeek MA, Brown JM. 2007. Clade age and not diversification rate explains species richness among animal taxa. Am. Nat. 169, E97–E106. ( 10.1086/512135) [DOI] [PubMed] [Google Scholar]
- 21.Willis JC. 1922. Age and area. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 22.Cronquist A. 1988. The evolution and classification of flowering plants. New York, NY: New York Botanical Garden. [Google Scholar]
- 23.Ricklefs RE. 2004. A comprehensive framework for global patterns in biodiversity. Ecol. Lett. 7, 1–15. ( 10.1046/j.1461-0248.2003.00554.x) [DOI] [Google Scholar]
- 24.Svenning JC, Skov F. 2007. Could the tree diversity pattern in Europe be generated by postglacial dispersal limitation? Ecol. Lett. 10, 453–460. ( 10.1111/j.1461-0248.2007.01038.x) [DOI] [PubMed] [Google Scholar]
- 25.Fløjgaard C, Normand S, Skov F, Svenning J-C. 2011. Deconstructing the mammal species richness pattern in Europe—towards an understanding of the relative importance of climate, biogeographic history, habitat heterogeneity and humans. Glob. Ecol. Biogeogr. 20, 218–230. ( 10.1111/j.1466-8238.2010.00604.x) [DOI] [Google Scholar]
- 26.Baselga A, Lobo JM, Svenning JC, Aragón P, Araújo MB. 2012. Dispersal ability modulates the strength of the latitudinal richness gradient in European beetles. Glob. Ecol. Biogeogr. 21, 1106–1113. ( 10.1111/j.1466-8238.2011.00753.x) [DOI] [Google Scholar]
- 27.Hutchinson GE. 1959. Homage to Santa Rosalia, or why are there so many kinds of animals? Am. Nat. 93, 145–159. ( 10.1086/282070) [DOI] [Google Scholar]
- 28.Brown JH. 1981. Two decades of homage to Santa Rosalia: toward a general theory of diversity. Am. Zool. 21, 877–888. ( 10.1093/icb/21.4.877) [DOI] [Google Scholar]
- 29.Wright DH. 1983. Species–energy theory: an extension of species–area theory. Oikos 41, 496–506. ( 10.2307/3544109) [DOI] [Google Scholar]
- 30.Brown JH. 2014. Why are there so many species in the tropics? J. Biogeogr. 41, 8–22. ( 10.1111/jbi.12228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen AP, Gillooly JF, Brown JH. 2007. Recasting the species-energy hypothesis: the different roles of kinetic and potential energy in regulating biodiversity. In Scaling biodiversity (eds Storch D, Marquet PA, Brown JH), pp. 283–299. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 32.Wiens JJ, et al. 2010. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 13, 1310–1324. ( 10.1111/j.1461-0248.2010.01515.x) [DOI] [PubMed] [Google Scholar]
- 33.Šímová I, Storch D, Keil P, Boyle B, Phillips OL, Enquist BJ. 2011. Global species–energy relationship in forest plots: role of abundance, temperature and species climatic tolerances. Glob. Ecol. Biogeogr. 20, 842–856. ( 10.1111/j.1466-8238.2011.00650.x) [DOI] [Google Scholar]
- 34.Klopfer PH. 1959. Environmental determinants of faunal diversity. Am. Nat. 93, 337–342. ( 10.1086/282092) [DOI] [Google Scholar]
- 35.Olson DM, et al. 2001. Terrestrial ecoregions of the world: a new map of life on earth. BioScience 51: 933–938. ( 10.1641/0006-3568(2001)051%5B0933:TEOTWA%5D2.0.CO;2) [DOI] [Google Scholar]
- 36.Goldberg EE, Lancaster LT, Ree RH. 2011. Phylogenetic inference of reciprocal effects between geographic range evolution and diversification. Syst. Biol. 60, 451–465. ( 10.1093/sysbio/syr046) [DOI] [PubMed] [Google Scholar]
- 37.Rabosky DL, Grundler M, Anderson C, Shi JJ, Brown JW, Huang H, Larson JG. 2014. BAMMtools: an R package for the analysis of evolutionary dynamics on phylogenetic trees. Methods Ecol. Evol. 5, 701–707. ( 10.1111/2041-210X.12199) [DOI] [Google Scholar]
- 38.Fox J. 2002. An R and S-plus companion to applied regression. Thousand Oaks, CA: Sage. [Google Scholar]
- 39.Rosseel Y. 2016. Lavaan: latent variable analysis. R package version 0.5-23. Vienna, Austria: Comprehensive R Archive Network. [Google Scholar]
- 40.Borcard D, Legendre P, Drapeau P. 1992. Partialling out the spatial component of ecological variation. Ecology 73, 1045–1055. ( 10.2307/1940179) [DOI] [Google Scholar]
- 41.Oksanen J, et al. 2013. Vegan: community ecology package. R package version 2.0-10. Vienna, Austria: Comprehensive R Archive Network. [Google Scholar]
- 42.Legendre P. 2008. Studying beta diversity: ecological variation partitioning by multiple regression and canonical analysis. J. Plant Ecol. 1, 3–8. ( 10.1093/jpe/rtm001) [DOI] [Google Scholar]
- 43.Peres-Neto PR, Legendre P, Dray S, Borcard D. 2006. Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87, 2614–2625. ( 10.1890/0012-9658(2006)87%5B2614:VPOSDM%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 44.Faurby S, Svenning JC. 2015. Historic and prehistoric human-driven extinctions have reshaped global mammal diversity patterns. Divers. Distrib. 21, 1155–1166. ( 10.1111/ddi.12369) [DOI] [Google Scholar]
- 45.Soria-Carrasco V, Castresana J. 2012. Diversification rates and the latitudinal gradient of diversity in mammals. Proc. R. Soc. B 279, 4148–4155. ( 10.1098/rspb.2012.1393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hedges SB, Marin J, Suleski M, Paymer M, Kumar S. 2015. Tree of life reveals clock-like speciation and diversification. Mol. Biol. Evol. 32, 835–845. ( 10.1093/molbev/msv037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pyron RA. 2014. Temperate extinction in squamate reptiles and the roots of latitudinal diversity gradients. Glob. Ecol. Biogeogr. 23, 1126–1134. ( 10.1111/geb.12196) [DOI] [Google Scholar]
- 48.Scholl JP, Wiens JJ. 2016. Diversification rates and species richness across the Tree of Life. Proc. R. Soc. B 283, 20161334 ( 10.1098/rspb.2016.1334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schluter D, Pennell MW. 2017. Speciation gradients and the distribution of biodiversity. Nature 546, 48–55. ( 10.1038/nature22897) [DOI] [PubMed] [Google Scholar]
- 50.Evans KL, Warren PH, Gaston KJ. 2005. Species–energy relationships at the macroecological scale: a review of the mechanisms. Biol. Rev. 80, 1–25. ( 10.1017/S1464793104006517) [DOI] [PubMed] [Google Scholar]
- 51.Hurlbert AH, Jetz W. 2010. More than ‘more individuals’: the nonequivalence of area and energy in the scaling of species richness. Am. Nat. 176, E50–E65. ( 10.1086/650723) [DOI] [PubMed] [Google Scholar]
- 52.Allen AP, Brown JH, Gillooly JF. 2002. Global biodiversity, biochemical kinetics, and the energetic-equivalence rule. Science 297, 1545–1548. ( 10.1126/science.1072380) [DOI] [PubMed] [Google Scholar]
- 53.Currie DJ, et al. 2004. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett. 7, 1121–1134. ( 10.1111/j.1461-0248.2004.00671.x) [DOI] [Google Scholar]
- 54.Oliveira BF, Machac A, Costa GC, Brooks TM, Davidson AD, Rondinini C, Graham CH. 2016. Species and functional diversity accumulate differently in mammals. Glob. Ecol. Biogeogr. 25, 1119–1130. ( 10.1111/geb.12471) [DOI] [Google Scholar]
- 55.Cracraft J. 2001. Avian evolution, Gondwana biogeography and the Cretaceous–Tertiary mass extinction event. Proc. R. Soc. Lond. B 268, 459–469. ( 10.1098/rspb.2000.1368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kemp TS. 2005. The origin and evolution of mammals. Oxford, UK: Oxford University Press on Demand. [Google Scholar]
- 57.Feller AE, Hedges SB. 1998. Molecular evidence for the early history of living amphibians. Mol. Phylogenet. Evol. 9, 509–516. ( 10.1006/mpev.1998.0500) [DOI] [PubMed] [Google Scholar]
- 58.Bossuyt F, Roelants K.. 2009. Frogs and toads (Anura). In The timetree of life (eds Hedges SB, Kumar S), pp. 357–364. New York, NY: Oxford University Press. [Google Scholar]
- 59.McDowell SB. 1987. Systematics. In Snakes: ecology and evolutionary biology (eds Siegel RA, Collins JT, Novak SS), pp. 3–50. New York, NY: Macmillan Publishing Company. [Google Scholar]
- 60.Rabosky DL. 2010. Extinction rates should not be estimated from molecular phylogenies. Evolution 64, 1816–1824. ( 10.1111/j.1558-5646.2009.00926.x) [DOI] [PubMed] [Google Scholar]
- 61.Rabosky DL. 2016. Challenges in the estimation of extinction from molecular phylogenies: a response to Beaulieu and O'Meara. Evolution 70, 218–228. ( 10.1111/evo.12820) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.