Abstract
The evolutionary causes and consequences of allopolyploidization, an exceptional pathway to instant hybrid speciation, are poorly investigated in animals. In particular, when and why hybrid polyploids versus diploids are produced, and constraints on sources of paternal and maternal ancestors, remain underexplored. Using the Palearctic green toad radiation (including bisexually reproducing species of three ploidy levels) as model, we generate a range-wide multi-locus phylogeny of 15 taxa and present four new insights: (i) at least five (up to seven) distinct allotriploid and allotetraploid taxa have evolved in the Pleistocene; (ii) all maternal and paternal ancestors of hybrid polyploids stem from two deeply diverged nuclear clades (6 Mya, 3.1–9.6 Mya), with distinctly greater divergence than the parental species of diploid hybrids found at secondary contact zones; (iii) allotriploid taxa possess two conspecific genomes and a deeply diverged allospecific one, suggesting that genomic imbalance and divergence are causal for their partly clonal reproductive mode; (iv) maternal versus paternal genome contributions exhibit asymmetry, with the maternal nuclear (and mitochondrial) genome of polyploids always coming from the same clade, and the paternal genome from the other. We compare our findings with similar patterns in diploid/polyploid vertebrates, and suggest deep ancestral divergence as a precondition for successful allopolyploidization.
Keywords: polyploidy, hybridization, divergence, multi-locus phylogeny, directional asymmetry, Bufo viridis subgroup
1. Introduction
How much hybridization and introgression events contribute to speciation and genome evolution is developing as an active research topic [1,2]. At least in plants (e.g. [2–5]), polyploid hybrid speciation appears more common than homoploid hybrid speciation. This question has been less investigated in animals, due to both lower incidence of polyploid hybrid speciation and smaller economic importance (cf. [6,7]). Research efforts in amphibians have mainly involved cytogenetics (overview: [6,8]). Advanced recent molecular approaches, allowing dating and genome-wide evidence, have been applied to Pipidae (e.g. [9–12,13]) and Ambystomatidae [14–17]. Nevertheless, important questions regarding hybrid speciation remain to be addressed, such as: what circumstances favour allopolyploid over diploid hybrid formation? And: what specific constraints govern allopolyploid formation, in terms of origin and differentiation of paternal and maternal genomes?
The divergence of parental lineages is expected to affect opportunities for hybrid speciation and allopolyploid formation [18]. Reproductive isolation between diploid lineages, and thus introgression, tends to scale with divergence [19–22], with complex effects on hybrid meiosis. In particular, hybridization between genetically similar lineages presents higher opportunities for multi-valent formation, mis-segregation and chromosome rearrangements during meiosis, which poses major challenges to early polyploid evolution [23,24]. By contrast, multi-valent formation is less likely if hybridizing lineages exhibit greater divergence and structural genome differentiation [23]. Accordingly, several meta-studies of hybrid plants have suggested that genetic divergence is greater for parents of polyploids than of homoploids [25] (but see [26]). Chapman & Burke [25] furthermore hypothesized that triploids arise from diploid hybrids via meiotic non-reduction (resulting in diploid gametes), followed by fertilization with haploid pollen. Thus, the production of unreduced gametes, associated with increased divergence time, has been considered as a mechanism facilitating allopolyploidization [27].
This complex relationship between divergence, meiosis and ploidy in asexual hybrids (also documented from vertebrates) has inspired the ‘balance hypothesis’ [28,29], which proposes that parental genome divergence has to be large enough to affect meiosis in hybrids (so as to produce enough unreduced gametes), but not too large to maintain some hybrid viability or fertility. The ratios of parental genomes may further affect hybrid meiosis by generating additional difficulties in AAB or ABB triploids (when compared with AABB allotetraploids), potentially leading to asexuality [29]. Extrapolating to animals Chapman & Burke's [25] suggestion, we therefore predict that (i) the parental species of polyploid hybrids should exhibit greater divergence than those involved in the formation of contact zones with variably introgressed diploid hybrids, and (ii) ameiotic hybrids should result from both profound parental divergence and unequal parental genome contributions.
In addition, hybrid and allopolyploid formation may be governed by the direction of hybridization. Reciprocal hybrids often show asymmetric fitness differences that stem from dominance effects in Dobzhansky–Muller incompatibilities [30], originating from sex chromosomal versus autosomal (e.g. Haldane's rule [31–33]) or cyto-nuclear interactions. Therefore, we further hypothesize that the evolution of allopolyploid lineages may show similar asymmetric interactions.
To test these questions in amphibians, Palearctic green toads (Bufo viridis subgroup) present a highly suitable system to compare diploid and polyploid hybridization within one radiation. This group comprises different diploid lineages forming secondary contact zones, with levels of introgression that scale with divergence [22,34]. Furthermore, bisexually reproducing species of three ploidy levels (2n, 3n and 4n) have been described from Central Asia [35]. Maternal ancestry has been inferred from mtDNA sequences plus nuclear microsatellites for two allopolyploids (3n B. baturae, 4n B. pewzowi) [36–38], and mtDNA only (entirely missing nuDNA evidence) for three other presumably allopolyploid species (B. oblongus, B. pseudoraddei and B. zugmayeri) [39]. Another six Eurasian diploid species have unclear nuclear relationships to the polyploids, which calls for integration into a comprehensive phylogenetic analysis. Diploid and tetraploid green toads reproduce meiotically ([39] incl. refs.), while one triploid species (B. baturae) has a partly ameiotic gametogenesis [40], possibly also found in two other triploid forms (systematic details: electronic supplementary material, text S1).
In this paper, we use new multi-locus nuclear sequence data, supplemented by mitochondrial DNA, to (i) identify allopolyploidization events in the Palearctic green toad radiation, (ii) infer the paternal and maternal ancestries of polyploids, (iii) compare the genetic divergence of lineages involved in diploid versus allopolyploid hybrid formation, and (iv) test for a possible directionality in hybridization events (namely, asymmetries in the origin and contribution of maternal versus paternal genomes to allopolyploid formation).
2. Material and methods
(a). Animal sampling and DNA extraction
Our study includes a total of 51 green toads from scientific collections as specified (figure 1a; electronic supplementary material, text S2 and table S1) [35,37,39], obtained between 1997 and 2012 from 32 localities across their Palearctic range. This comprises 15 taxa from all currently known major mtDNA clades [37], as well as three taxonomically unassigned toads, namely two tetraploids (X1, X2) and one triploid (X4), further abbreviated ‘UIL’ (unidentified lineage). Samples of B. bufo and B. raddei were used as outgroups.
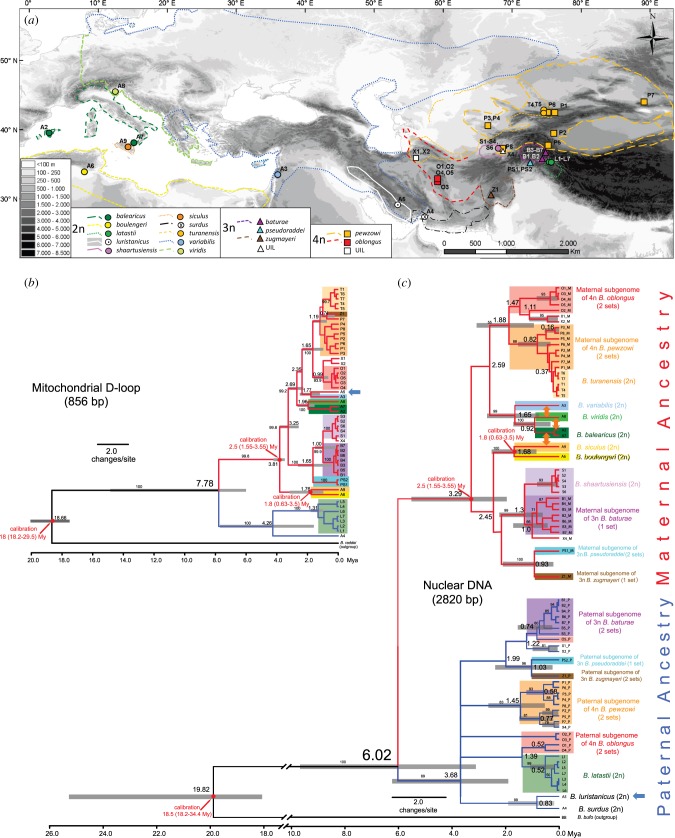
Figure 1.
Green toads in geographical and phylogenetic context. (a) Map with sampling localities, approximate range borders and sample abbreviations as used in (b,c) and electronic supplementary material, table S1. (b,c) Bayesian trees of approximately 856 bp of the mitochondrial D-loop (b) and of approximately 2820 bp of concatenated nuclear DNA (c) as obtained with the program BEAST v. 1.8.3. Subclades shown in red (maternal ancestry) are also referred to as ‘western clade’; subclades shown in blue (paternal ancestry) are referred to as ‘eastern clade’. Orange double arrows, in (c) between branches, within the western clade, indicate known natural hybridization between diploid lineages including the formation of diploid/diploid hybrid zones with introgression [22,34]. Blue arrows at sample A5 point to an incongruent mitochondrial versus nuclear phylogenetic position of B. luristanicus. Small numbers at branches show Bayesian posterior support values (greater than 50%); large numbers at nodes and time scales below trees in (b) and (c) show divergence time estimates (mean values) in millions of years ago (Mya); grey bars in trees indicate 95% confidence intervals for nodes with sufficient posterior support. An extended version of this figure is provided in the electronic supplementary material, figure S1.
(b). Amplification and sequencing of nuclear markers
Six nuclear sequence markers (CYP19, DMRT1, SF-1, SPAG6, SOX3, VLDLR), several of which are involved in vertebrate sexual development and differentiation, representing different linkage groups of the anuran genome were developed using orthologues on Xenopus tropicalis scaffolds 1, 3, 6, 8 and 19. Primers for cross-amplifying markers (electronic supplementary material, Text S2, and table S2) were designed using a B. viridis transcriptome (GenBank Biosample SAMN03993917 [41]). Markers were PCR amplified (electronic supplementary material, texts S1 and S3). Amplicons were extracted from agarose gels, purified using the Wizard SV Gel and PCR clean-up system (Promega) and a single final amplicon pool was obtained for each individual by mixing equimolar amounts of these products. Each individual pool was barcoded prior to further pooling of all 48 mixes, which were NGS-sequenced using the Roche/454 GS-FLX Titanium platform by LGC Genomics Corporation (UK) with a coverage of greater than 80× per PCR product. Alleles were then screened and edited manually to eliminate singletons, and contigs with greater than 15× coverage considered a true allele; the maximum number of alleles was inferred according to ploidy (2 in 2n, 3 in 3n, 4 in 4n). To complete the dataset for this radiation with three initially unconsidered polyploid species, for three samples exclusively (B. zugmayeri (Z1), B. siculus (A9) and B. pseudoraddei (PS2)), nuclear PCR products were cloned using the TOPO TA cloning kit (with pCR II-TOPO-vector system; Invitrogen) according to the manufacturer's protocol. To detect heterozygotes, at least 12 clones were Sanger-sequenced in diploids and 24 in triploids, edited to eliminate singletons, and added to the rest of the dataset.
(c). Sequencing and phylogenetic analyses of mtDNA
The mitochondrial control region (D-loop, approx. 880 bp) was amplified as described [37,42]. Products were Sanger-sequenced in both directions and contigs edited in Sequencher v. 4.9. Bayesian phylogenetic analyses were carried out with MrBayes v. 3.2.6 [43] using the best-fit model of sequence evolution (HKY + G, Bayesian information crit., BIC) as determined by jModeltest v. 2.1.7 [44] (electronic supplementary material, text S2). Stationarity and convergence of the runs were confirmed using the software Tracer v. 1.7.2 [45]. The first 25% of each run was discarded as burn-in.
(d). Subgenome inference and phylogenetic analyses of nuclear markers
Nuclear DNA sequences, in total 2820 bp, were aligned using ClustalW multiple alignment in BIOEDIT [46]. For each marker, a maximum-likelihood phylogenetic analysis was performed using PhyML (v. 3.0; [47]). The maternal ancestor of each allopolyploid was assigned according to mtDNA haplotype, from which the maternal (and by deduction, paternal) subgenomes could be inferred based on microsatellite allelic range similarity (in part identical samples as in [36]). To allow proper concatenation of ancestral nuclear markers, we retained only one consensus sequence from heterozygous fragments of diploid individuals, replacing SNPs with ‘Ns’ (i.e. any base). The same was done in allopolyploids, from which two consensus sequences were kept, corresponding to their inferred maternal and paternal subgenomes, respectively. Finally, all resulting nuclear sequences were concatenated in the 5′–3′ direction to obtain a ‘super-alignment’ for phylogenetic analyses (electronic supplementary material, text S2).
(e). Molecular dating
Molecular dating for major lineages was performed based on the concatenated nuclear dataset, and separately for mtDNA, using the Bayesian relaxed-clock approach as implemented in BEAST v. 1.8.3 [48]. We determined the most suitable substitution models per partition (nuDNA), using PartitionFinder v. 1.1.1 [49] or for the entire mtDNA marker, using jModeltest v. 2.1.7 [44]; divergence time analyses were run with substitution models unlinked between partitions. We included an outgroup for both nuDNA and mtDNA, and imposed three available age constraints to the molecular clock (electronic supplementary material, text S2).
We generated a random starting tree and assumed an uncorrelated lognormal relaxed molecular clock and a Yule process as a model of speciation, as this prior is most appropriate for species-level divergences [48]. Two independent runs were performed with 200 million generations, sampling 10 000 trees and with a burn-in set to 25% of the samples. Convergence and stationary levels were verified with Tracer v. 1.7.2. We annotated the tree information with TreeAnnotator v. 2.3.1 and visualized it with FigTree v. 1.4.2 [48]. All runs were performed on the CIPRES Science Gateway [50].
3. Results
(a). Phylogenetic analyses of mitochondrial DNA
Bayesian analysis of maternally inherited mitochondrial DNA resulted in distinct haplotypes and clades that mostly coincide with the previously distinguished nominal taxa. This analysis unveiled a very deep divergence between B. surdus and B. latastii on one side (figure 1b, blue-marked clade, hereafter ‘eastern’; electronic supplementary material, figures S1 and S2) and the remaining taxa on the other side (red-marked clade, hereafter ‘western’), with an estimated divergence of 7.7 (4.4–12.7) Mya. Interestingly, several tetraploid (B. pewzowi, B. oblongus, plus X1 and X2) and one triploid species (B. zugmayeri) share a Pleistocene (1.65 (0.9–2.5) Mya) mitochondrial ancestor with the diploid B. turanensis, while other triploids (B. baturae, B. pseudoraddei and X4) share a similarly old Pleistocene (1.65 (0.72–2.76 Mya)) mtDNA-ancestry with a different diploid species (B. shaartusiensis). We further note that the diploid B. turanensis does not take a basal position in its subclade but appears to be derived from the polyploids (electronic supplementary material, text S4).
(b). Phylogenetic analyses of nuclear DNA
Phylogenies obtained from single genes are shown in electronic supplementary material, figures S3–S8. Allele numbers (haplotypes) therein varied between 1 and 6. The analyses of the concatenated sequences (2820 bp) yielded two highly supported clades (red and blue, figure 1c), which diverged about 6 Mya (95% HDP, 3.1–9.7 Mya). Surprisingly, all inferred maternal genomes of the polyploid species were assigned to the ‘western clade’ (red), and all paternal genomes to the ‘eastern clade’ (blue).
The western clade split about 3.29 (1.9–5.5) Mya into two major subclades. One contains a group comprising the diploid B. turanensis and the maternal ancestor of the allotetraploids (B. pewzowi and B. oblongus; plus UIL X1, X2), and is itself sister to several Eurasian diploid species. The other subclade constitutes a group formed by the Asian diploid B. shaartusiensis and the maternal ancestor of the Asian triploid B. baturae (plus UIL X4), itself sister to the maternal ancestor of the two other triploids (B. zugmayeri, B. pseudoraddei). Many Eurasian diploid lineages from this western clade are involved in diploid hybridization across secondary contact zones in Europe [22,34] (indicated by orange arrows in figure 1c; electronic supplementary material, figure S1c).
The eastern clade (figure 1c, blue) forms a large polytomy that split about 3.7 Mya (1.9–6.3), separating a clade of diploid species (B. surdus, B. luristanicus) from another diploid (B. latastii), and containing all the paternal subgenomes of allotriploid and allotetraploid species. The paternal subgenomes of the tetraploid B. oblongus are split among several subclades.
The topology and divergence-time estimates for the nuclear phylogeny largely agree with the mitochondrial tree, except for the diploid B. luristanicus, which appears as a weakly supported sister of B. variabilis in the mitochondrial phylogeny (figure 1b) but as a sister taxon of B. surdus in the nuclear phylogeny (figure 1c). This suggests a mitochondrial capture event by the lineage of B. luristanicus, possibly from the partly sympatric B. variabilis.
4. Discussion
The discovery of polyploid green toads in 1976 [51] was followed by initial studies of polyploidy origins using allozymes [52] and microsatellites [36]. Here, we extend these studies through the first phylogeny of this complex based on multi-locus mtDNA and nuclear sequences, providing insights into the relative ages and contributions of maternal and paternal ancestors to allopolyploidization.
(a). Allopolyploid origins and genome phylogenies
Our phylogenetic analysis highlighted at least five events of allopolyploidization that led to the evolution of two allotetraploids (B. pewzowi, B. oblongus) and three allotriploids (B. baturae, B. pseudoraddei, B. zugmayeri; figure 1c; electronic supplementary material, figure S2 (I–V) and text S5). Three additional allopolyploid forms (UIL X1, X2, X4) were also identified and characterized (figure 1c; electronic supplementary material, figure S2 and text S5), possibly corresponding to yet unrecognized taxa. The number of alleles (haplotypes), which varied between 1 and 6 in single-gene trees, did not allow further inferences regarding the number of hybridizations or routes to polyploidy (electronic supplementary material, figures S3–S8).
(b). Profoundly diverged lineages form hybrid polyploids, less diverged lineages form hybrid zones
Maternal and paternal ancestors of allopolyploid taxa (4n B. oblongus, 4n B. pewzowi, 3n B. baturae, 3n B. pseudoraddei, 3n B. zugmayeri) in each case belong to the relatively deeply diverged western and eastern clades (6 Mya, 3.1–9.6 Mya; figure 1c). Divergence times distinctly exceed the much younger ones (1.9–2.6 Mya) between diploid lineages that form hybrids at secondary contacts [22,34]. Thus, despite uncertainties inherent to the calibration procedure, our phylogenies are consistent with the hypothesis that ancestors of allopolyploids exhibit greater divergence than lineages that form diploid–diploid hybrid zones with various degrees of introgression [22,34]. Our results are in line with those from plants [25] and other vertebrate allopolyploids in which parental lineages have been shown to stem from deeply diverged ancestral lineages (e.g. Aspidocelis and Darevskia lizards [53–55]; Pelophylax [56–58]; Cobitis [59]; Squalius [60]). This suggests that allopolyploidization might occasionally overcome the decrease in hybrid fitness resulting from the accumulation of incompatibilities with increasing divergence time.
The relative ages of within-clade diversification for maternal (and mitochondrial) and paternal ancestors of allopolyploids vary between Lower (1.8 Mya) and Mid-Pleistocene (0.93 Mya; average of 1.4 Mya; figure 1; electronic supplementary material, figure S2 and text S5). If diversification dates coincide with polyploidization events, these ages suggest that such events were triggered by Pleistocene climatic oscillations, as supported by higher resistance of polyploids to climatic stresses [61]. Ficetola & Stöck [61] have also shown that allopolyploidization might be facilitated by occupation of transgressive ecological niches, unavailable to some of the parental species.
(c). Deeply diverged but unequal genome contributions in ameiotic forms
Whereas diploids and balanced allotetraploids reproduce by meiosis [39], ameiotic allotriploids show an unequal genomic configuration, comprising two conspecific heterozygous genomes (AA′) and a highly diverged clonal allospecific one (B) (figure 1c). In line with the balance hypothesis, this suggests that genomic imbalance and divergence are causal for their reproductive mode [28,29].
(d). Directional asymmetry in parental genome contributions to allopolyploidization
The five Pleistocene events (electronic supplementary material, figure S2 (I–V)) that led to allopolyploid species formation, as well as several possibly more recent events that produced allopolyploid hybrids with unclear taxonomic status, were all unidirectional in relation to maternal and paternal ancestors. Two allotetraploids (B. pewzowi, B. oblongus; as well as UIL X1 and X2) share nuclear maternal ancestors with the same diploid (B. turanensis), whereas the maternal ancestry of three allotriploids (B. baturae, B. zugmayeri, B. pseudoraddei; as well as UIL X4) can be traced to another diploid (B. shaartusiensis; figure 1c); all of these belong to the same major western clade. By contrast, the entire paternal ancestry goes back to one lineage (or several related and possibly extinct lineages), represented by a single extant diploid (B. latastii; figure 1c) from the eastern clade. Moreover, in a diploid–tetraploid contact zone (B. turanensis and B. pewzowi), adult triploid F1-hybrids mostly have diploid B. turanensis as their close maternal (mtDNA) ancestor and tetraploid B. pewzowi as paternal ancestor [35]. These shared patterns of directional asymmetry in hybridization point to strong evolutionary constraints during allopolyploidization in green toads.
Asymmetric contributions of paternal and maternal parents are known from homoploid hybrid plants (e.g. [62]), invertebrates (e.g. [63,64]) and vertebrates (e.g. [65,66]), including interspecies crosses in bufonid toads (e.g. [67,68]). However, asymmetries have rarely been documented in allopolypoid speciation. In plants, allopolyploid origins exhibit great diversity [69–75] with few examples of asymmetric ancestry in a whole complex [76]. In vertebrates, asymmetric genome contributions to allopolyploidization remain underexplored. Tetraploids forming the Hyla versicolor complex originated multiple times from extant diploid H. chrysoscelis and two apparently extinct lineages [77], but asymmetry has not been examined. This similarly applies to the Phyllomedusa burmeisteri complex [78,79]. In clawed frogs, Silurana comprises the diploid Silurana tropicalis and three derived tetraploid species; Xenopus includes 20 described species: 11 tetraploids, 7 octoploids and 2 dodecaploids [11,13]. Several ancestral diploid species (some extinct) are maternal genome donors for some allopolyploids and paternal donors for others (cf. [9]), thus contrasting with our results. A few other systems, however, call for further exploration of possible asymmetries in genome contributions. In the Pelophylax esculentus complex, allodiploid (P. esculentus, P. grafi, P. hispanica) and allotriploid (3n P. esculentus with either two P. lessonae, RLL, or two P. ridibundus genomes, RRL) gonochoristic hybrids perform multi-directional genetic interactions (among themselves and with diploid parental species), blurring potential signatures of asymmetric genome contributions [56,80]. In the largely unisexual Ambystoma jeffersonianum/A. laterale complex, all unisexual di- and polyploid hybrids derive their mtDNA from the diploid A. barbouri, from which all five nuclear unisexual species diverged 2.4–3.9 Mya [17], suggesting a ‘laterale-like’ asymmetric maternal contribution and various paternal contributions from other bisexual species. Allodiploid, triploid and tetraploids of the mostly all-female cyprinid Squalius alburnoides hybrid complex exhibit multiple polyploid origins and genetic interactions, while mtDNA asymmetrically stems from the common maternal ancestor (S. pyrenaicus), although with rare introgression [81,82]. Similarly, in Cobitis loaches, multiple all-female gynogenetic allodiploid and allopolyploid hybrid lineages (with few exceptions [83]) share Cobitis elongatoides mtDNA, and thus a maternal nuclear ancestor [84] with Miocene divergence from the paternal ancestors (greater than 7 Mya (3.83–10.28) [59]). Several other gynogenetic and polyploid teleost complexes (for a review: [85]) are often dominated by a common maternal (mitochondrial and thereby inferred nuclear) lineage; however, gynogenetic reproduction and possible stepwise ploidy elevation complicate evaluation of potential asymmetry. These examples from few allopolyploid vertebrate complexes show several similarities to our findings and suggest that asymmetric ancestry should be more carefully addressed by future research.
Asymmetric homoploid hybridization has been explained by imbalanced barriers to gene flow under pre- or post-zygotic isolation [62,65]. Pre-mating isolation in animals is attributed to mate choice behaviours, evolved in response to sexual selection [65]. Asymmetry in post-mating isolation often results from Dobzhansky–Muller incompatibilities that involve uniparentally inherited genetic factors, such as sex chromosomes, mitochondria, epigenetic programming or maternal effects (Darwin's corollary to Haldane's rule [68,86]). Alleles involved in hybrid incompatibilities are considered partly recessive, and those on sex chromosomes are more likely expressed in the heterogametic sex [31,86]. However, asymmetric dominance in allopolyploidization has not been investigated. As the dominance model of Haldane's rule assumes degenerated sex chromosomes, whereas those in green toads are homomorphic ([39] incl. refs) [87], nuclear–cytoplasmic incompatibilities may better explain directional asymmetry under allopolyploidization in this instance.
5. Conclusion
Our data provide four new major insights. First, we document at least five hybridization events (up to seven; electronic supplementary material, figure S2 and text S5) that resulted in the evolution of allopolyploid species. Second, molecular dating, based on mtDNA and nuDNA, shows that allopolyploid green toads presumably originated in the Pleistocene, from ancestors that had diverged in the Miocene to Pliocene period (6 Mya (3.1–9.6 Mya); i.e. much earlier than the parents of diploid hybrids forming at secondary contacts within the western clade [22,34]). This supports the hypothesis that allopolyploidization is facilitated by greater genomic divergence. Third, we note that allotriploid ameiotic taxa always possess two conspecific genomes and a deeply diverged allospecific clonal one, suggesting that genomic imbalance and divergence are causal. Fourth, we provide evidence for directional asymmetry in maternal versus paternal genome contributions, with the maternal nuclear (and mitochondrial) genome always coming from one phylogenetic clade, and the paternal nuclear genome from the other.
This first dated nuclear phylogeny of Palearctic green toads offers new research avenues. Studies could be undertaken of sex determination in diploid ancestral versus allopolyploid derived species (cf. [87]) and whether asymmetry may not only be reflected in nuclear genome contributions but also in subgenome evolution after hybridization. This has been shown for African clawed frogs, Xenopus laevis, ‘with one chromosome set more often preserving ancestral states while the other experienced more gene loss, deletion, rearrangement, and reduced gene expression’ [12].
Supplementary Material
Acknowledgements
We thank Daniel Frynta, Theodore Papenfuss and Alessandra Sicilia for providing samples, Audrey Brown and Carsten Weißenborn for help in the laboratory, Maria João Collares-Pereira, Maria Manuela Coelho, Lukáš Choleva and Jörg Plötner for references, and the editor, Bryan Husband, and two anonymous reviewers for comments on a previous version of this paper. Photo credits (electronic supplementary material, figure S1): Mehregan Ebrahimi (B. surdus), Philip de Pous (B. variabilis), Sebastian Voitel (B. luristanicus).
Ethics
This research was performed in accordance with the laws, guidelines and ethical standards at the University of Lausanne (Switzerland), and the relevant Institutional Animal Care and Use Committee (IACUC); samples have been obtained from scientific collections under published collection permits [35,37,39], namely the Bundesamt für Veterinärwesen, BVET no. 1245/10, Bern, Switzerland, border veterinary control at Airport Frankfurt/Main, Germany (01/05/ 2010); and authorization no. 1798, Service de la consommation et des affaires vétérinaires, Canton de Vaud, Epalinges (Switzerland).
Data accessibility
Sequences have been deposited on GenBank and edited alignments have been archived on Dryad (doi:10.5061/dryad.7ns38).
Authors' contributions
M.S. and N.P. designed the study. M.S. and R.S. did fieldwork and provided samples. C.B.-C., R.S. and M.S. performed laboratory work. S.H., C.B.-C. and M.S. conducted analyses. M.S., C.B.-C., S.H. and N.P. wrote the manuscript, to which all co-authors contributed.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the German Research Foundation (DFG; grant Sto 493/3-1) and a Heisenberg-Fellowship (Sto 493/2-2) to M.S., and the Swiss National Science Foundation (grant 31003A_166323) to N.P.
References
- 1.Mallet J, Besansky N, Hahn MW. 2015. How reticulated are species? Bioessays 38, 140–149. ( 10.1002/bies.201500149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott RJ, Barton NH, Good JM. 2016. Genomics of hybridization and its evolutionary consequences. Mol. Ecol. 25, 2325–2332. ( 10.1111/j.1420-9101.2012.02599.x) [DOI] [PubMed] [Google Scholar]
- 3.Yakimowski SB, Rieseberg LH. 2014. The role of homoploid hybridization in evolution: a century of studies synthesizing genetics and ecology. Am. J. Bot. 101, 1247–1259. ( 10.3732/ajb.1400201) [DOI] [PubMed] [Google Scholar]
- 4.Lukhtanov VA, Shapoval NA, Anokhin BA, Saifitdinova AF, Kuznetsova VG. 2015. Homoploid hybrid speciation and genome evolution via chromosome sorting. Proc. R. Soc. B 282, 20150157 ( 10.1098/rspb.2015.0157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker MS, Arigo N, Baniaga AE, Li Z, Levin DA. 2016. On the relative abundance of autopolyploids and allopolyploids. New Phytol. 210, 391–398. ( 10.1111/nph.13698) [DOI] [PubMed] [Google Scholar]
- 6.Mable BK, Alexandrou MA, Taylor MI. 2011. Genome duplication in amphibians and fish: an extended synthesis . J. Zool. 284, 151–182. ( 10.1111/j.1469-7998.2011.00829.x) [DOI] [Google Scholar]
- 7.Allendorf FW, Bassham S, Cresko WA, Limborg MT, Seeb LW, Seeb JE. 2015. Effects of crossovers between homeologs on inheritance and population genomics in polyploid-derived salmonid fishes. J. Hered. 3, 217–227. ( 10.1093/jhered/esv015) [DOI] [PubMed] [Google Scholar]
- 8.Schmid M, Evans B, Bogart JP. 2015. Polyploidy in Amphibia. Cytogen. Genome Res. 145, 315–330. ( 10.1159/000431388) [DOI] [PubMed] [Google Scholar]
- 9.Evans BJ, Kelley DB, Melnick DJ, Cannatella DC. 2005. Evolution of RAG-1 in polyploid clawed frogs. Mol. Biol. Evol. 22, 1193–1207. ( 10.1093/molbev/msi104) [DOI] [PubMed] [Google Scholar]
- 10.Evans BJ. 2008. Genome evolution and speciation genetics in clawed frogs Xenopus and Silurana. Front. Biosci. 13, 4687–4706. ( 10.2741/3033) [DOI] [PubMed] [Google Scholar]
- 11.Evans BJ, Pyron AR, Wiens JJ. 2012. Polyploidization and sex chromosome evolution in amphibians. In Polyploidy and genome evolution (eds Soltis PS, Soltis DE), pp. 385–410. Berlin, Germany: Springer. [Google Scholar]
- 12.Session AM, et al. 2016. Genome evolution in the allotetraploid frog Xenopus laevis. Nature 538, 336–343. ( 10.1038/nature19840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans BJ, et al. 2015. Genetics, morphology, advertisement calls, and historical records distinguish six new polyploid species of African Clawed Frog Xenopus, Pipidae from West and Central Africa. PLoS ONE 10, e0142823 ( 10.1371/journal.pone.0142823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogart JP. 1980. Evolutionary significance of polyploidy in amphibians and reptiles. In Polyploidy: biological relevance (ed. Lewis WH.), pp. 341–378. New York, NY: Plenum Press. [Google Scholar]
- 15.Bogart JP. 2003. Genetics and systematics of hybrid species. In Reproductive biology and phylogeny of urodela (ed. Sever DM.), pp. 109–134. Enfield, NH: Science Publishers. [Google Scholar]
- 16.Bi K, Bogart JP. 2006. Identification of intergenomic recombinations in unisexual salamanders of the genus Ambystoma by genomic in situ hybridization GISH. Cytogen. Genome Res. 112, 307–312. ( 10.1159/000089885) [DOI] [PubMed] [Google Scholar]
- 17.Bi K, Bogart JP. 2013. Genetic and genomic interactions of animals with different ploidy levels. Cytogen. Genome Res. 140, 117–136. ( 10.1159/000351593) [DOI] [PubMed] [Google Scholar]
- 18.Seehausen O, et al. 2014. Genomics and the origin of species. Nat. Rev. Genet. 15, 176–192. ( 10.1038/nrg3644) [DOI] [PubMed] [Google Scholar]
- 19.Singhal S, Moritz C. 2013. Reproductive isolation between phylogeographic lineages scales with divergence. Proc. R. Soc. B 280, 20132246 ( 10.1098/rspb.2013.2246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montanari SR, Hobbs J-PA, Pratchett MS, Bay LK, Van Herwerden L. 2014. Does genetic distance between parental species influence outcomes of hybridization among coral reef butterflyfishes? Mol. Ecol. 23, 2757–2770. ( 10.1111/mec.12762) [DOI] [PubMed] [Google Scholar]
- 21.Taylor SA, Curry RL, White TA, Ferretti V, Lovette I. 2014. Spatiotemporally consistent genomic signatures of reproductive isolation in a moving hybrid zone. Evolution 68, 3066–3081. ( 10.1111/evo.12510) [DOI] [PubMed] [Google Scholar]
- 22.Dufresnes C, Bonato L, Novarini N, Betto-Colliard C, Perrin N, Stöck M. 2014. Inferring the degree of incipient speciation in secondary contact zones of closely related lineages of Palearctic green toads Bufo viridis subgroup. Heredity 113, 9–20. ( 10.1038/hdy.2014.26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lloyd A, Bomblies K. 2016. Meiosis in autopolyploid and allopolyploid Arabidopsis. Curr. Opin. Plant Biol. 30, 116–122. ( 10.1016/j.pbi.2016.02.004) [DOI] [PubMed] [Google Scholar]
- 24.Lenormand T, Engelstädter J, Johnston SE, Wijnker E, Haag CR. 2016. Evolutionary mysteries in meiosis. Phil. Trans. R. Soc. B 371, 20160001 ( 10.1098/rstb.2016.0001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapman MA, Burke JM. 2007. Genetic divergence and hybrid speciation. Evolution 61, 1773–1780. ( 10.1111/j.1558-5646.2007.00134.x) [DOI] [PubMed] [Google Scholar]
- 26.Buggs RJA, Solitis PS, Soltis DE. 2009. Does hybridization between divergent progenitors drive whole-genome duplication? Mol. Ecol. 18, 3334–3339. ( 10.1111/j.1365-294X.2009.04285.x) [DOI] [PubMed] [Google Scholar]
- 27.Mason AS, Pires JC. 2015. Unreduced gametes: meiotic mishap or evolutionary mechanism? Trends Gen. 31, 5–10. ( 10.1016/j.tig.2014.09.011) [DOI] [PubMed] [Google Scholar]
- 28.Wetherington J, Kotora K, Vrijenhoek R. 1987. A test of the spontaneous heterosis hypothesis for unisexual vertebrates. Evolution 41, 721–723. ( 10.1111/j.1558-5646.1987.tb05848.x) [DOI] [PubMed] [Google Scholar]
- 29.Moritz C, et al. 1989. Genetic diversity and the dynamics of hybrid parthenogenesis in Cnemidophorus Teeidae and Heteronotia Gekkonidae. In Evolution and ecology of unisexual vertebrates (eds Dawley R, Bogart JP), pp. 87–112. Albany, NY: New York State Museum. [Google Scholar]
- 30.Turelli M, Orr HA. 2000. Dominance, epistasis and the genetics of postzygotic isolation. Genetics 154, 1663–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haldane JBS. 1922. Sex-ratio and unidirectional sterility in hybrid animals. J. Genet. 12, 101–109. ( 10.1007/BF02983075) [DOI] [Google Scholar]
- 32.Schilthuizen M, Giesbers MCWG, Beukeboom LW. 2011. Haldane's rule in the 21st century. Heredity 107, 95–102. ( 10.1038/hdy.2010.170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delph LF, Demuth JP. 2016. Haldane's rule: genetic bases and their empirical support. J. Hered. 107, 383–391. ( 10.1093/jhered/esw026) [DOI] [PubMed] [Google Scholar]
- 34.Colliard C, Sicilia A, Turrisi GF, Moritz C, Perrin N, Stöck M. 2010. Strong reproductive barriers in a narrow hybrid zone of West-Mediterranean green toads Bufo viridis subgroup with Plio-Pleistocene divergence. BMC Evol. Biol. 10, 232 ( 10.1111/j.1755-0998.2009.02600.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stöck M, Ustinova J, Lamatsch DK, Schartl M, Perrin N, Moritz C. 2010. A vertebrate reproductive system involving three ploidy levels: hybrid origin of triploids in a contact zone of diploid and tetraploid Paleartic green toads Bufo viridis subgroup. Evolution 64, 944–959. ( 10.1111/j.1558-5646.2009.00876.x) [DOI] [PubMed] [Google Scholar]
- 36.Betto-Colliard C, Sermier R, Litvinchuk S, Perrin N, Stöck M. 2015. Origin and genome evolution of polyploid green toads in Central Asia: evidence from microsatellite markers. Heredity 114, 300–308. ( 10.1038/hdy.2014.100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stöck M, et al. 2006. Evolution of mitochondrial relationships and biogeography of Palearctic green toads Bufo viridis subgroup with insights in their genomic plasticity. Mol. Phylogenet. Evol. 41, 663–689. ( 10.1016/j.ympev.2006.05.026) [DOI] [PubMed] [Google Scholar]
- 38.Litvinchuk SN, et al. 2011. Influence of environmental conditions on the distribution of Central Asian green toads with three ploidy levels. J. Zool. Syst. Evol. Res. 9, 233–239. ( 10.1111/j.1439-0469.2010.00612.x) [DOI] [Google Scholar]
- 39.Stöck M, Steinlein C, Lamatsch DK, Schartl M, Schmid M. 2005. Multiple origins of tetraploid taxa in the Eurasian Bufo viridis subgroup. Genetica 124, 255–272. ( 10.1007/s10709-005-3085-9) [DOI] [PubMed] [Google Scholar]
- 40.Stöck M, Ustinova J, Betto-Colliard C, Schartl M, Moritz C, Perrin N. 2012. Simultaneous Mendelian and clonal genome transmission in a sexually reproducing all-triploid vertebrate. Proc. R. Soc. B 279, 1293–1299. ( 10.1098/rspb.2011.1738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerchen JF, Reichert SJ, Röhr JT, Dieterich C, Kloas W, Stöck M, Joger U. 2016. A single transcriptome of a green toad Bufo viridis yields candidate genes for sex determination and -differentiation and non-anonymous population genetic markers. PLoS ONE 11, e0156419 ( 10.1371/journal.pone.0156419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goebel AM, Donnelly JM, Atz ME. 1999. PCR primers and amplification methods for 12S ribosomal DNA, the control region, cytochrome oxidase I, and cytochrome b in bufonids and other frogs, and an overview of PCR primers which have amplified DNA in amphibians successfully. Mol. Phylogenet. Evol. 11, 163–199. ( 10.1006/mpev.1998.0538) [DOI] [PubMed] [Google Scholar]
- 43.Ronquist F, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. ( 10.1093/sysbio/sys029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat. Meth. 9, 772 ( 10.1038/nmeth.2109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rambaut A, Suchard MA, Xie D, Drummond AJ. 2014. Tracer v1.6. See http://beast.bio.ed.ac.uk/Tracer.
- 46.Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids 41, 95–98. [Google Scholar]
- 47.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321. ( 10.1093/sysbio/syq010) [DOI] [PubMed] [Google Scholar]
- 48.Drummond AJ, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214 ( 10.1186/1471-2148-7-214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lanfear R, Calcott B, Ho SY, Guindon S. 2012. Partitionfinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 29, 1695–1701. ( 10.1093/molbev/mss020) [DOI] [PubMed] [Google Scholar]
- 50.Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop GCE, 14 Nov. 2010, New Orleans, LA, pp. 45–52. Piscataway, NJ: IEEE. [Google Scholar]
- 51.Mazik EJ, Kadirova BK, Toktosunov AT. 1976. Karyotype patterns in the green toad Bufo viridis in Kyrgyzstan. Zoologichesky Zhurnal 55, 1740–1742. In Russian, with English summary. [Google Scholar]
- 52.Mezhzherin SV, Pisanets EM. 1995. Genetic structure and origin of the tetraploid toad Bufo danatensis Pisanetz, 1978 Amphibia, Bufonidae from Central Asia: differentiation of geographic forms and genetic relationships between diploid and tetraploid species. Genetika 31, 342–352. In Russian, with English summary. [PubMed] [Google Scholar]
- 53.Moritz C, Wright JW, Brown WM. 1992. Mitochondrial DNA analyses and the origin and relative age of parthenogenetic Cnemidophorus: phylogenetic constraints on hybrid origins. Evolution 46, 184–192. ( 10.1111/j.1558-5646.1992.tb01993.x) [DOI] [PubMed] [Google Scholar]
- 54.Moritz C, Bi K. 2011. Spontaneous speciation by ploidy elevation: laboratory synthesis of a new clonal vertebrate. Proc. Natl Acad. Sci. USA 108, 9733–9734. ( 10.1073/pnas.1106455108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murphy RW, Fu J, Maccolloch RD, Darevsky IS, Kuprianova LA. 2000. A fine line between sex and unisexuality: the phylogenetic constraints on parthenogenesis in lacertid lizards. Zool. J. Linn. Soc. 130, 527–549. ( 10.1006/zjls.1999.0241) [DOI] [Google Scholar]
- 56.Plötner J, et al. 2010. Genetic divergence and evolution of reproductive isolation in eastern Mediterranean water frogs. In Evolution in action (ed. Glaubrecht M.), pp. 373–403. Berlin, Germany: Springer. [Google Scholar]
- 57.Akin C, et al. 2010. Phylogeographic patterns of genetic diversity in eastern Mediterranean water frogs have been determined by geological processes and climate change in the Late Cenozoic. J. Biogeo. 37, 2111–2124. ( 10.1111/j.1365-2699.2010.02368.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffmann A, et al. 2015. Genetic diversity and distribution patterns of diploid and polyploid hybrid water frog populations Pelophylax esculentus complex across Europe. Mol. Phylogenet. Evol. 24, 4371–4391. ( 10.1111/mec.13325) [DOI] [PubMed] [Google Scholar]
- 59.Majtánová Z, Choleva L, Symonová R, Ráb P, Kotusz J, Pekárik L, Janko K, Laudet V. 2016. Asexual reproduction does not apparently increase the rate of chromosomal evolution: karyotype stability in diploid and triploid clonal hybrid fish Cobitis, Cypriniformes, Teleostei. PLoS ONE 11, e0146872 ( 10.1371/journal.pone.0146872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sousa-Santos C, Collares-Pereira MJ, Almada V. 2007. Reading the history of a hybrid fish complex from its molecular record. Mol. Phylogenet. Evol. 45, 981–996. ( 10.1016/j.ympev.2007.05.011) [DOI] [PubMed] [Google Scholar]
- 61.Ficetola GF, Stöck M. 2016. Do hybrid-origin polyploid amphibians occupy transgressive or intermediate ecological niches compared to their diploid ancestors? J. Biogeo. 43, 703–715. ( 10.1111/jbi.12667) [DOI] [Google Scholar]
- 62.Tiffin P, Olson MS, Moyle LC. 2001. Asymmetrical crossing barriers in angiosperms. Proc. R. Soc. B 268, 861–867. ( 10.1098/rspb.2000.1578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coyne JA, Orr HA. 1989. Patterns of speciation in Drosophila. Evolution 43, 362–381. ( 10.1111/j.1558-5646.1989.tb04233.x) [DOI] [PubMed] [Google Scholar]
- 64.Presgraves DC. 2002. Patterns of postzygotic isolation in Lepidoptera. Evolution 56, 1168–1183. ( 10.1554/0014-3820(2002)0561168:POPIIL.2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 65.Arnold SJ, Verrell PA, Tilley SG. 1996. The evolution of asymmetry in sexual isolation: a model and a test case. Evolution 50, 1024–1033. ( 10.1111/j.1558-5646.1996.tb02343.x) [DOI] [PubMed] [Google Scholar]
- 66.Wiwegweaw A, Seki K, Mori H, Asami T. 2009. Asymmetric reproductive isolation during simultaneous reciprocal mating in pulmonates. Biol. Lett. 5, 240–243. ( 10.1098/rsbl.2008.0714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malone JH, Fontenot BE. 2008. Patterns of reproductive isolation in toads. PLoS ONE 12, e3900 ( 10.1371/journal.pone.0003900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brandvain Y, Pauly GB, May M, Turelli M. 2015. Explaining Darwin's corollary to Haldane's Rule: the role of mitonuclear interactions in asymmetric postzygotic isolation among toads. Genetics 197, 743–747. ( 10.1534/genetics.113.161133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meimberg H, Rice KJ, Milan NF, Njoku CC, McKay JK. 2009. Multiple origins promote the ecological amplitude of allopolyploid Aegilops Poaceae. Am. J. Bot. 96, 1262–1273. ( 10.3732/ajb.0800345) [DOI] [PubMed] [Google Scholar]
- 70.Clarkson JJ, Knapp S, Garcia VF, Olmstead RG, Leitch AR, Chase MW. 2004. Phylogenetic relationships in Nicotiana Solanaceae inferred from multiple plastid DNA regions. Mol. Phylogenet. Evol. 33, 75–90. ( 10.1016/j.ympev.2004.05.002) [DOI] [PubMed] [Google Scholar]
- 71.Clarkson JJ, Kelly LJ, Leitch AR, Knapp S, Chase MW. 2010. Nuclear glutamine synthetase evolution in Nicotiana: phylogenetics and the origins of allotetraploid and homoploid diploid hybrids. Mol. Phylogenet. Evol. 55, 99–112. ( 10.1016/j.ympev.2009.10.003) [DOI] [PubMed] [Google Scholar]
- 72.Leitch IJ, Hanson L, Lim KY, Kovarik A, Chase MW, Clarkson JJ, Leitch AR. 2008. The ups and downs of genome size evolution in polyploid species of Nicotiana, Solanaceae. Ann. Bot. 101, 805–814. ( 10.1093/aob/mcm326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prakash S, Bhat SR, Quiros CF, Kirti PB, Chopra VL. 2009. Brassica and its close allies: cytogenetics and evolution. Plant Breed. Rev. 31, 21–188. ( 10.1002/9780470593783.ch2) [DOI] [Google Scholar]
- 74.Kadereit JW, Uribe-Convers S, Westberg E, Comes HP. 2006. Reciprocal hybridization at different times between Senecio flavus and Senecio glaucus gave rise to two polyploid species in north Africa and south-west Asia. New Phytol. 169, 431–441. ( 10.1111/j.1469-8137.2005.01604.x) [DOI] [PubMed] [Google Scholar]
- 75.Ge S, Sang T, Lu BR, Hong DY. 1999. Phylogeny of rice genomes with emphasis on origins of allotetraploid species. Proc. Natl Acad. Sci. USA 96, 14 400–14 405. ( 10.1073/pnas.96.25.14400) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adams KL, Wendel JF. 2004. Exploring the genomic mysteries of polyploidy in cotton. Biol. J. Linn. Soc. 82, 573–581. ( 10.1111/j.1095-8312.2004.00342.x) [DOI] [Google Scholar]
- 77.Holloway AK, Cannatella DC, Gerhardt HC, Hillis DH. 2006. Polyploids with different origins and ancestors form a single sexual polyploid species. Am. Nat. 167, E88–E101. ( 10.1086/501079) [DOI] [PubMed] [Google Scholar]
- 78.Brunes TO, Sequeira F, Haddad CFB, Alexandrino J. 2010. Gene and species trees of a neotropical group of treefrogs: genetic diversification in the Brazilian Atlantic forest and the origin of a polyploid species. Mol. Phylogenet. Evol. 57, 1120–1133. ( 10.1016/j.ympev.2010.08.026) [DOI] [PubMed] [Google Scholar]
- 79.Barth A, Vences M, Solé M, Costa MA. 2014. Molecular cytogenetics and phylogenetic analysis of Brazilian leaf frog species of the genera Phyllomedusa and Phasmahyla Hylidae: Phyllomedusinae. Canad. J. Zool. 92, 795–802. ( 10.1139/cjz-2013-0301) [DOI] [Google Scholar]
- 80.Plötner J. 2005. Die Westpaläarktischen Wasserfrösche. Zeitschrift Feldherpetologie Beiheft, vol. 9 Bielefeld, Germany: Laurenti Verlag; In German. [Google Scholar]
- 81.Collares-Pereira MJ, Matos I, Morgado-Santos M, Coelho MM. 2013. Natural pathways towards polyploidy in animals: the Squalius alburnoides fish complex as a model system to study genome size and genome reorganization in polyploids. Cytogen. Genome Res. 140, 97–116. ( 10.1159/000351729) [DOI] [PubMed] [Google Scholar]
- 82.Morgado-Santos M, Carona S, Magalhaes MF, Vicente L, Collares-Pereira MJ. 2016. Reproductive dynamics shapes genomotype composition in an allopolyploid complex. Proc. R Soc. B 283, 20153009 ( 10.1098/rspb.2015.3009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Janko K, Kotusz J, De Gelas K, Slechtovà V, Opoldusovà Z, Drozd P, Choleva L, Popiołek M, Baláž M. 2012. Dynamic formation of asexual diploid and polyploid lineages: multilocus analysis of Cobitis reveals the mechanisms maintaining the diversity of clones. PLoS ONE 79, e45384 ( 10.1371/journal.pone.0045384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choleva L, Apostolou A, Rab P, Janko K. 2008. Making it on their own: sperm-dependent hybrid fishes Cobitis switch the sexual hosts and expand beyond the ranges of their original sperm donors. Phil. Trans. R. Soc. B. 363, 2911–2919. ( 10.1098/rstb.2008.0059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lamatsch DK, Stöck M. 2009. Sperm-dependent parthenogenesis and hybridogenesis in teleost fishes. In Lost sex: the evolutionary biology of parthenogenesis (eds Schoen I, Martens K, van Dijk P), pp. 399–432. Berlin, Germany: Springer. [Google Scholar]
- 86.Turelli M, Moyle LC. 2007. Asymmetric postmating isolation: Darwin's corollary to Haldane's rule. Genetics 176, 1059–1088. ( 10.1534/genetics.106.065979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stöck M, Savary R, Betto-Colliard C, Biollay S, Jourdan-Pineau H, Perrin N. 2013. Low rates of XY recombination, not turnovers, account for homomorphic sex chromosomes in several diploid species of Palearctic green toads Bufo viridis subgroup. J. Evol. Biol. 3, 674–682. ( 10.1111/jeb.12086) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequences have been deposited on GenBank and edited alignments have been archived on Dryad (doi:10.5061/dryad.7ns38).