Abstract
The present study was aimed to isolate indigenous plant growth-promoting bacteria (PGPB) from Nanmangalam reserve forest, India and to analyze their positive impact on nursery plant species. In total, 160 isolates were obtained from different nitrogen-free Media (LGI, JMV, NFB). Amongst these, 12 isolates were shown positive for 5–8% of ammonia production nif H positive and then isolates were further tested for their plant growth-promoting (PGP) activity. Based on their PGP activity, nine isolates were selected, and applied in nurseries of twelve native plant species, along with organic manure and inorganic fertilizer. All the isolates were shown positive effects when compared to control. In that, five of these bacterial isolates, Paenibacillus sp. RRB2, Azospirillum brasilense RRAK5, Bacillus subtilis subsp. subtilis RRD8, Burholdria kururiensis RRAK1, and Pseudomonas stutzeri RRAN2, enhanced biomass production in several trees.
Keywords: Indigenous PGPB, Native plants, Biomass, Nutrient content
Introduction
Worldwide, forest degradation is a major environmental problem that leads to poor soil health (Wang et al. 2004). Soil degradation is a process that negatively affects the characteristics of a forest. For instance, soil degradation reduces the value of the goods and services provided by forests. The frequency, quality, extent, origin, and severity of soil degradation are highly variable. It can be caused by various natural and/or anthropogenic disturbances. It is important to enhance the carbon sequestration in the degraded forests of tropical countries (FAO 2009). The reversal of forest degradation through restoration increases carbon stocks. However, it is difficult to achieve natural regeneration and reforestation in tropical degraded forests because the soils have already lost their nutrient content (Lund 2009). This issue adversely affects plant growth and regeneration through the recruitment of seedlings (Ramachandran et al. 2007).
Compared to the agricultural sector, negligible quantities of chemical fertilizers are used in forests and forest nursery production. However, the use of this practice contributes to severe environmental damage in degraded forests (Putz and Redford 2009). The use of such additives to enhance soil nutrient levels and crop yield also pollutes the forest soil, placing the complex system of biogeochemical cycles under pressure (Radhapriya et al. 2015; Adesemoye and Kloepper 2009). Environmental degradation occurs when the leachate of added nutrients, such as nitrogen and phosphorus, mixes with runoff. Previous studies have shown that the microbial communities are also minimal in degraded forest soils (Radhapriya et al. 2014). From now, there is ever increasing attention in developing ecologically sustainable plant growth-promoting bacteria (PGPB), which increase the growth of forest seedlings and crops in an eco-friendly way. Different researchers have clearly explained the PGPB effect on the various crops positive yield and production.
Very limited studies were reported in enhanced growth and biomass of forest seedlings and plants using beneficial microorganisms and PGPB. Pajares and Bohannan (2016) explained about the nitrogen-fixing bacteria in tropical forest. Bashan et al. (2012) and Moreno et al. (2017) scientifically proved that the PGPB and native plant species enhanced plant growth and soil stability in degraded forest and desert soil. Bashan et al. (2012) and Liu et al. (2013) reported that these bacteria accelerated the development of plant shoots and roots, increased the number of branches, nutrients and improved the survival rate in different forest seedlings of Prosopis articulata, Parkinsonia microphylla, and Parkinsonia florida and Fraxinus americana, respectively. To the best of our knowledge, using indigenous plant growth-promoting bacteria to enhance biomass potential has not been attempted in Indian forests. In this manuscript, we describe the isolation of such bacteria from Nanmangalam Reserve Forest, and characterize their impact on growth, biomass, and nutrient content of native plant species.
Materials and methods
Study area and its present status
The study area NRF, located in the southern part of Chennai and spreads in an area of 321 ha (12°55′5″N to 12°56′13″N and 80°9′46″E to 80°10′57″E) of south west of Chennai adjoining the coastal track of Bay of Bengal, is a fragmented hill of Eastern Ghats of Tamil Nadu (Fig. 1).
Fig. 1.
Study Area Nanmangalam Reserve Forest (NRF), Tamil Nadu, India
In India, most forests in Eastern Ghats were placed under heavy, long-term need-based management and silvicultural practices, and are now scrub jungles with scattered tree growth, poor soil strata, and significantly altered species composition (Jayakumar et al. 2009). The present study area, Nanmangalam Reserve Forest (NRF), is in a similar state (Radhapriya et al. 2014). Different reforestation programmes in this forest, including planting of indigenous and exotic tree species, have been futile, and have transformed its original floristic composition to a succession of primary, secondary, and tertiary species (Palani 2004; Annamalai 1987; Tangam1959). Thus, proper afforestation techniques are urgently needed.
Isolation of soil bacteria
A systematic sampling method was followed, dividing the total study area location into 40 grid points with a grid space of 0.25 km2. A total of 40 triplicate soil samples were collected at 0–10 cm. However, to facilitate the process, samples were pooled into ten groups, each consisting of four samples thoroughly mixed and homogenized in field. Samples were then placed in sterile bags, immediately transported to the laboratory, and stored 4 °C until processed and analyzed.
To isolate and enrich plant growth-promoting bacteria, each pool was serially diluted and inoculated into three separate vials containing 5 mL LGI (Loitsyanskya, Gv and Ivchenko), JMV (Johanna Mannitol Vera), and NFB (New Fábio Pedrosa) nitrogen-free semisolid media at triplicates. Sucrose was used as carbon source in LGI media, and the pH was maintained at 6.0-6.2. Mannitol and malic acid were used as carbon source in JMV and NFB media, which had pH 6.0–6.2 and 6.0–6.5, respectively. Vials were incubated at 30 °C for 5 days. Formation of white subsurface pellicles and color change from yellowish green to blue indicated growth of diazotrophs (Jha et al. 2009). To confirm results, inocula growing in cultures of the highest dilutions of soil samples were transferred to fresh media. Confirmed isolates were then plated on nitrogen-free solid media to obtain single colonies, which were finally inoculated in 50% glycerol, and stored at − 80 °C for further analysis.
Ammonia production was estimated in all isolates according to Cappuccino and Sherman (1992). Isolates with the highest levels of ammonia were analyzed by the micro-Kjeldahl method for nitrogen accumulation (Bergersen and Turner 1980), and by Polymerized Chain Reaction (PCR) for copies of the nifH gene, using the primers 5ʹ-GCIWTYTAYGGIAARGGIGG-3ʹ and 5ʹ-AAICCRCCRCAIACIACRTC-3ʹ (Ueda et al. 1995). Organisms with nifH were tested for several traits of plant growth-promoting bacteria. Published methods were used to measure or demonstrate indole acetic acid production (Gordon and Weber 1951), hydrocyanic acid production (Kremer and Souissi 2001), phosphate solubilization (Gaur 1990), zinc and silicate solubilization (Bunt and Rovira 1955), siderophore production (Schwyn and Neilands 1987), ACC deaminase activity (Penrose and Glick 2003), total protein (Bradford 1976), salicylic acid (Meyer and Abdallah 1978), and acetylene reduction (Hardy 1968).
Molecular identification
DNA was extracted according to Sambrook et al. (1989) from bacterial isolates growing in nitrogen-free media. 16S rDNA was amplified by PCR using the forward primer 5′-AGAGTTTGATCCTGGCTCAG-3′and reverse primer 5′-GGTTACCTTGTTACGACTT-3′ and directly sequenced using the fluorescent dye terminator method (ABI Prism™ BigDye™ Terminator Cycle Sequencing-Ready Reaction Kit v.3.1). Sequencing products were purified with a Millipore Montage Dye Removal Kit and separated on an ABI3730XL capillary DNA sequencer. Sequences used to identify the isolate or its closest relative through the EzTaxon-e server (Kim et al. 2012). Isolates had 97–99% similarity to 16S rDNA sequences deposited in the NCBI database.
Bacterial inoculum and fertilizer
Isolates were grown in nitrogen-free media at 37 °C for 96 h, and harvested by centrifugation at 5000×g for 10 min at 4 °C. Cells were washed twice and resuspended in 0.1 M phosphate buffer to a density of 106 CFU mL−1.
Plants
Albizia lebbeck (L) Benth, Azadirachta indica Adr.Juss., Gmelina arborea Roxb, Madhuca longifolia (J.Koenig) Macbr, Pongamia pinnata (L.) Pierre, Pterocarpus santalinus L.f., Syzygium cummini (L.), Tamarindus indica L, Terminalia arjuna (DC.) Wight & Arn., Terminalia bellirica (Gaertner) Roxb., Thespesia populnea Sol. ex Correa, and Wrightia tinctoria (Roxb.)R.Br. were used in nursery studies. These are the predominant tree species in Nanmangalam Reserve Forest (NRF).
Nursery studies
Five mother beds 12.5 × 1.2 m each were prepared by digging and hoeing. For each treatment, 100 seeds (Nanmangalam Nursery, Tamil Nadu Forest Department, Chennai, India) were surface-sterilized with 0.1% HgCl2 for 2 min, rinsed six times with sterile distilled water, and sown in the mother bed. After 30 days, seedlings were transferred from the mother bed to 30 × 45 cm polyethylene bags containing 10 kg of NRF soil supplemented with farmyard manure, 4 mg N, 2 mg P, 2 mg K, and Bacillus sp. RRN12, Pseudomonas geniculata RRC11, Burholdria kururiensis RRAK1, Bacillus subtilis RRO7, Bacillus subtilis subsp. subtilis RRD8, Bacillus subtilis RRAR1, Azospirillum brasilense RRAK5, Paenibacillus sp. RRB2, and Pseudomonas stutzeri RRAN2. The forest soil had clayey texture, 1.02 bulk density, pH 6.8, 1.10% organic carbon, 0.19% nitrogen, 1.66 mg kg−1 available phosphorus, 1.51 cmol kg−1 available potassium; 7.6 mg kg−1 calcium, 3.92 mg kg−1 magnesium, 0.31 mg kg−1 iron, 0.28 mg kg−1 manganese, 0.28 mg kg−1 copper, and 0.16 mg kg−1 zinc. The experiment was conducted using a completely randomized block design with three replicates per treatment, each replicate consisting of 30 healthy seedlings. Forest department already using technique was used as control. For control treatments, 10 kg commercial biofertilizer (National Fertilizer Ltd., Noida, India) was mixed with 50 kg sand and applied over 1 acre of land. This biofertilizer contains 105 CFU each of non-native Azospirillum, Azotobacter, and Rhizobium per gram of dry lignite powder. Inorganic fertilizer (1.5 g) at 2:1:1 N:P:K ratio was added per cubic meter of soil.
Plant analysis
Plants were grown for 180 days. Plant height was measured once in each month. On the final day, polyethylene bags were flooded with water to loosen soil, after which seedlings were uprooted without injury. Roots, stems, and leaves were then separately harvested, and washed thoroughly with distilled water to remove soil and other debris. Samples were then placed in paper bags, kept at 70 °C until a constant weight was achieved, crushed, and passed through a 250 μm mesh sieve. N content was quantified using a CHNS-O elemental analyzer (Vario EL cube, Germany), following Wang and Anderson (1998). Total P was determined by the ascorbic acid method (Lu 1999), while K content was quantified spectrophotometrically using a Perkin Elmer Lambda 25 UV/Vis spectrophotometer, as described in Johnson and Ulrich (1959).
Statistical analysis
Significance was considered at the 95% of confidence was biomass and 95% confidence for growth parameter. Linear regression model in SPSS software version IBM.20.1 was used to analyze time courses of plant height.
Results
Isolation of soil bacteria
In total, 160 diazotrophs were obtained, of which 56, 57, and 47 were isolated on LGI, JMV, and NFB semisolid nitrogen-free media, respectively. Of these isolates, 12 produced 5–8% ammonia, the highest levels observed. Among these, 12 harbored copies of the nifH gene, and these isolates were assayed for traits of plant growth-promoting bacteria (Table 1). The isolates accumulated nitrogen, and produced indole acetic acid. Isolates solubilized P, Si, and Zn, viz., 12, 4 and 4, isolates, respectively. Eight isolates were positive for ACC deaminase activity. Isolates RRAK1, RRAK5, and RRAN2 showed the highest plant growth-promoting traits, followed by RRALC3, RRN12, and RRB2 (Table 1). Subsequently, nine isolates were selected for nursery tests based on these traits.
Table 1.
Traits of plant growth-promoting bacteria in soil isolates from Nanmangalam Reserve Forest
| No. | Strain name | Ammonia (ppm) | Total N (ppm) | NifH | IAA (µg mL−1) | ACC | Salicylic acid (µg mL−1) | ARA (nmol C2H4 mg protein−1 min−1) |
Siderophore | HCN | Solubilization | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | Si | Zn | |||||||||||
| 1 | RRN12 | 536.48 | 156.2 | + | 9.4 | + | 5.1 | 0.34 | + | − | + | − | − |
| 2 | RRB2 | 453.12 | 189.42 | + | 4.5 | + | 4.23 | 0.41 | + | − | + | − | − |
| 3 | RRD8 | 412.23 | 78.23 | + | 8.6 | − | 3.1 | 0.56 | − | − | + | − | + |
| 4 | RRC11 | 401.12 | 78.4 | + | 5.4 | + | 3.1 | 0.22 | − | − | + | − | − |
| 5 | RRR10 | 561.12 | 60.2 | + | 9.4 | + | 7.2 | 0.67 | + | − | + | − | − |
| 6 | RRAK1 | 593.1 | 136.4 | + | 9.8 | + | 6.8 | 0.79 | + | − | + | + | + |
| 7 | RR07 | 446.1 | 67.56 | + | 3.2 | − | 3.41 | 0.97 | − | − | + | − | − |
| 8 | RRALC3 | 601.34 | 158.2 | + | 8.4 | + | 7.23 | 0.78 | + | − | + | + | − |
| 9 | RRAJ2 | 484.22 | 68.6 | + | 5.6 | − | 0.7 | 0.59 | + | − | + | − | − |
| 10 | RRAK5 | 439.7 | 143.23 | + | 8.9 | + | 10.1 | 0.83 | + | − | + | + | + |
| 11 | RRAR1 | 456.16 | 156.2 | + | 8.5 | + | 1.2 | 0.61 | + | − | + | − | − |
| 12 | RRAN2 | 425.19 | 253.2 | + | 8.2 | − | 6 | 0.23 | + | − | + | + | + |
IAA indole acetic acid, HCN hydrocyanic acid, ACC 1-aminocyclopropane-1-carboxylic acid, ARA acetylene reduction assay
Sequencing of the 16S rDNA revealed that the 12 isolates are α-, β-, γ-Proteobacteria, Flavobacteria, Bacilli, and Firmicutes (Table 2). The strains RRO7, RRAR1, RRB2, RRACL3, RRAN2 and RRAJ2 had 99% sequence similarities to Bacillus subtilis, Paenibacillus sp., Pseudomonas aeruginosa, Pseudomonas stutzeri, and Lysinibacillus xylanilyticus. The remaining strains showed 97–99.67% similarities to Bacillus sp., Bacillus subtilis subsp. subtilis, Pseudomonas geniculata, Enterobacter asburiae, Burholdria kururiensis, and Azospirillum brasilense.
Table 2.
Identification of isolates by 16S rRNA sequence
| Strain no. | Gene accession number | Length (bp) | Microorganism | % Similarity |
|---|---|---|---|---|
| 1 | KF952291 | 1388 | Bacillus sp. RRN12 | 97 |
| 2 | KF952292 | 1629 | Paenibacillus sp. RRB2 | 99 |
| 3 | KF952294 | 797 | Bacillus subtilis subsp. subtilis RRD8 | 99.37 |
| 4 | KJ137013 | 1408 | Pseudomonas geniculata RRC11 | 99.67 |
| 5 | KJ137014 | 786 | Enterobacter asburiae RRR10 | 99.49 |
| 6 | KJ137015 | 1459 | Burholdria kururiensis RRAK1 | 99.49 |
| 7 | KF952293 | 850 | Bacillus subtilis RR07 | 99 |
| 8 | KF481965 | 997 | Pseudomonas aeruginosa RRACL3 | 99 |
| 9 | KJ911229 | 1222 | Azospirillum brasilense RRAK5 | 99 |
| 10 | KJ491071 | 997 | Pseudomonas stutzeri RRAN2 | 98.93 |
| 11 | KJ491072 | 720 | Lysinibacillus xylanilyticus RRAJ2 | 99 |
| 12 | KJ911230 | 1302 | Bacillus subtilis RRAR1 | 99 |
Nursery studies
Seedlings of 12 trees, native to Nanmangalam Reserve Forest, were grown for 6 months in soil supplemented with bacterial isolates. These plant growth-promoting bacteria generally enhanced biomass production in all tree species (Table 3). In particular, Paenibacillus sp. RRB2, Azospirillum brasilense RRAK5, Bacillus subtilis subsp. subtilis RRD8, Burholdria kururiensis RRAK1, and Pseudomonas stutzeri RRAN2 significantly enhanced the biomass in several plant species. In contrast, Bacillus subtilis RRAR1, Bacillus subtilis RRO7, Pseudomonas geniculata, and Bacillus sp. RRN12 did not significantly stimulate plant growth.
Table 3.
Biomass production in seedlings inoculated with different isolates
| Plant species | Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Bacillus sp. RRN12 |
P. geniculata
RRC11 |
B. kururiensis
RRAK1 |
B. subtilis
RR07 |
B. subtilis subsp. RRD8 |
B. subtilis
RRAR1 |
A. brasilense
RRAK5 |
Pseudomonas sp. RRB2 |
P. stutzeri
RRAN2 |
Control | |
| A. lebbeck | 0.67* | 0.35 | 0.75* | 0.75* | 0.32 | 0.54 | 0.60 | 0.35 | 0.35 | 0.40 |
| A. indica | 0.45 | 0.40 | 0.34 | 0.60 | 0.25 | 0.78* | 0.53 | 0.68* | 0.72* | 0.25 |
| G. arborea | 0.56 | 0.54 | 0.46 | 0.45 | 0.39 | 0.50 | 0.67* | 0.74* | 0.82* | 0.50 |
| M. latifolia | 0.60 | 0.47 | 0.38 | 0.50 | 0.47 | 0.45 | 0.90* | 0.80* | 0.30 | 0.45 |
| P. pinnata | 0.75* | 0.75* | 0.54 | 0.35 | 0.78* | 0.34 | 0.55 | 0.42 | 0.45 | 0.35 |
| P. santalinus | 0.27 | 0.80* | 0.75* | 0.56 | 0.50 | 0.45 | 0.75* | 0.45 | 0.47 | 0.60 |
| S. cummini | 0.36 | 0.45 | 0.85* | 0.89* | 0.45 | 0.39 | 0.48 | 0.38 | 0.70* | 0.45 |
| T. indica | 0.48 | 0.58 | 0.60 | 0.37 | 0.35 | 0.25 | 0.50 | 0.90* | 0.58* | 0.47 |
| T. arjuna | 0.37 | 0.28 | 0.80* | 0.12 | 0.50 | 0.60 | 0.68* | 0.45* | 0.50 | 0.61 |
| T. bellirica | 0.45 | 0.45 | 0.75* | 0.67* | 0.36 | 0.58 | 0.74* | 0.45 | 0.35 | 0.57 |
| T. populnea | 0.80* | 0.58 | 0.43 | 0.45 | 0.81* | 0.40 | 0.35 | 0.50 | 0.60 | 0.45 |
| W. tinctoria | 0.47 | 0.68 | 0.74* | 0.50 | 0.56* | 0.68* | 0.48 | 0.45 | 0.54 | 0.48 |
*Statistically significant difference among isolates inoculated into the same plant species (p < 0.01)
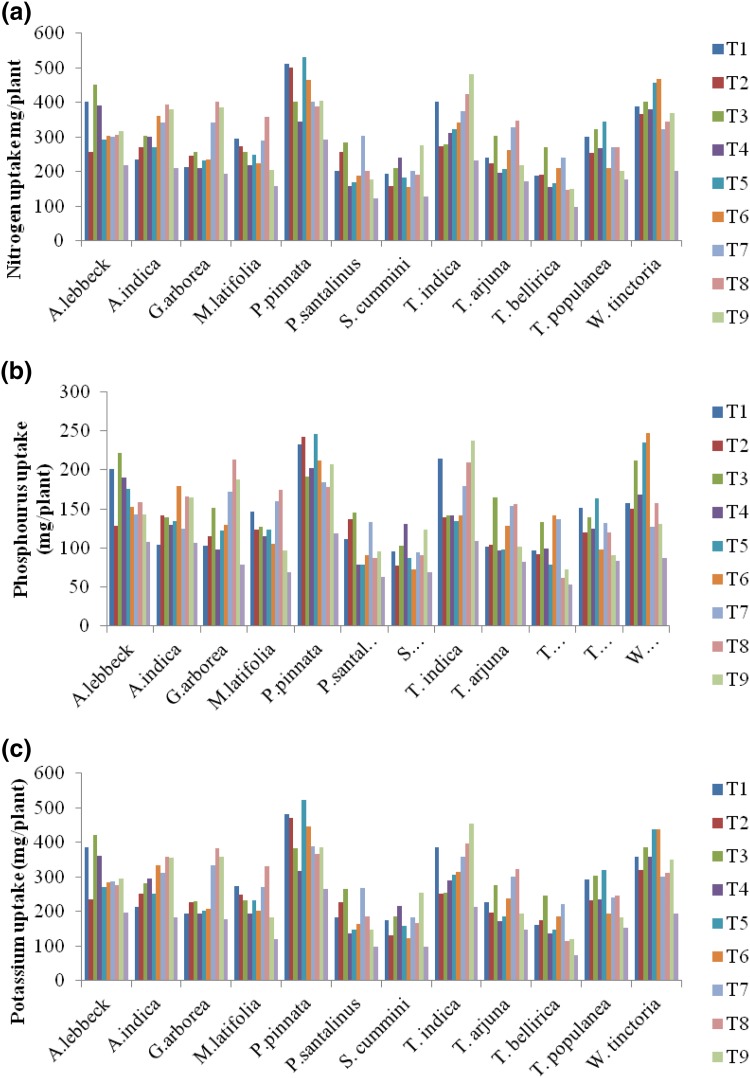
Seedlings treated with Paenibacillus sp. RRB2, A. brasilense RRAK5 and B. subtilis subsp. subtilis RRD8 showed significant nodal elongation, due to which seedlings were visibly taller than others. Regression analysis of plant height as a function of time indicated generally positive correlation, although the linearity and statistical significance varied among isolates, even within the same plant species (Table 4). Finally, analysis of plant N, P, and K content indicated that plant growth-promoting bacteria were key determinants of plant growth (Fig. 2).
Table 4.
Linear regression models of seedling height as a function of time
| Treatment | Regression model | r 2 | p | Regression model | r 2 | P | Regression model | r 2 | P |
|---|---|---|---|---|---|---|---|---|---|
| A. lebbeck | P. pinnata | T. arjuna | |||||||
| Bacillus sp. RRN12 | y = 35.23 + 0.35x | 0.75 | 0.026 | y = 38.43 + 0.53x | 0.95 | 0.001 | y = 31.30 + 0.34x | 0.75 | 0.05 |
| P. geniculata RRC11 | y = 29.68 + 0.14x | 0.65 | 0.021 | y = 25.68 + 0.54x | 0.95 | 0.001 | y = 27.32 + 0.43x | 0.70 | 0.021 |
| B. kururiensis RRAK1 | y = 34.50 + 0.44x | 0.95 | 0.0013 | y = 16.20 + 0.11x | 0.90 | 0.02 | y = 22.20 + 0.09x | 0.92 | 0.001 |
| B. subtilis RR07 | y = 43.13 + 0.39x | 0.86 | 0.024 | y = 14.21 + 0.38x | 0.90 | 0.002 | y = 36.45 + 0.22x | 0.60 | Ns |
| B. subtilis subsp. RRD8 | y = 34.11 + 0.14x | 0.64 | 0.016 | y = 48.05 + 0.30x | 0.80 | 0.001 | y = 44.26 + 0.35x | 0.72 | 0.05 |
| B. subtilis RRAR1 | y = 28.69 + 0.16x | 0.48 | Ns | y = 25.45 + 0.26x | 0.75 | 0.05 | y = 24.14 + 0.13x | 0.75 | 0.05 |
| A. brasilense RRAK5 | y = 43.58 + 0.35x | 0.54 | Ns | y = 32.41 + 0.22x | 0.74 | 0.04 | y = 23.17 + 0.05x | 0.95 | 0.001 |
| Paenibacillus sp. RRB2 | y = 54.35 + 0.16x | 0.61 | Ns | y = 45.11 + 0.56x | 0.68 | 0.05 | y = 32.20 + 0.50x | 0.95 | 0.001 |
| P. stutzeri RRAN2 | y = 37.85 + 0.23x | 0.67 | 0.05 | y = 39.45 + 0.31x | 0.51 | Ns | y = 31.34 + 0.41x | 0.62 | Ns |
| Control | y = 42.43 − 0.09x | 0.75 | 0.02 | y = 21.15 + 0.12x | 0.68 | 0.02 | y = 27.55 + 0.51x | 0.65 | 0.02 |
| A. indica | P. santalinus | T. bellirica | |||||||
| Bacillus sp. RRN12 | y = 24.23 + 0.13x | 0.61 | Ns | y = 42.20 + 0.47x | 0.65 | Ns | y = 28.40 + 0.11x | 0.65 | 0.05 |
| P. geniculata RRC11 | y = 15.68 + 0.24x | 0.57 | Ns | y = 27.45 + 0.56x | 0.89 | 0.001 | y = 19.31 + 0.33x | 0.68 | 0.05 |
| B. kururiensis RRAK1 | y = 28.20 + 0.31x | 0.76 | 0.01 | y = 38.41 + 0.22x | 0.90 | 0.001 | y = 31.21 + 0.34x | 0.95 | 0.002 |
| B. subtilis RR07 | y = 33.21 + 0.18x | 0.81 | 0.03 | y = 51.30 + 0.17x | 0.60 | Ns | y = 43.02 + 0.26x | 0.75 | 0.05 |
| B. subtilis subsp. RRD8 | y = 52.15 + 0.21x | 0.70 | 0.021 | y = 45.46 + 0.67x | 0.58 | Ns | y = 48.43 + 0.02x | 0.60 | Ns |
| B. subtilis RRAR1 | y = 19.09 + 0.36x | 0.90 | 0.001 | y = 31.42 + 0.34x | 0.60 | Ns | y = 32.24 + 0.44x | 0.92 | 0.001 |
| A. brasilense RRAK5 | y = 43.58 + 0.35x | 0.70 | 0.05 | y = 29.10 + 0.13x | 0.80 | 0.02 | y = 38.58 + 0.25x | 0.95 | 0.001 |
| Paenibacillus sp. RRB2 | y = 45.35 + 0.56x | 0.95 | 0.002 | y = 37.58 + 0.14x | 0.67 | 0.05 | y = 55.15 + 0.26x | 0.75 | 0.05 |
| P. stutzeri RRAN2 | y = 39.56 + 0.61x | 0.90 | 0.001 | y = 27.34 + 0.17x | 0.55 | Ns | y = 41.26 + 0.31x | 0.70 | 0.05 |
| Control | y = 37.85 + 0.83x | 0.80 | 0.023 | y = 32.33 − 0.14x | 0.60 | Ns | y = 27.25 + 0.33x | 0.70 | 0.05 |
| G. arborea | S. cummini | T. populnea | |||||||
| Bacillus sp. RRN12 | y = 16.23 + 0.21x | 0.60 | Ns | y = 36.14 + 0.12x | 0.78 | 0.03 | y = 27.51 + 0.30x | 0.80 | 0.020 |
| P. geniculata RRC11 | y = 22.31 + 0.11x | 0.58 | Ns | y = 24.11 + 0.17x | 0.75 | 0.05 | y = 15.68 + 0.24x | 0.75 | 0.04 |
| B. kururiensis RRAK1 | y = 21.14 + 0.17x | 0.76 | 0.011 | y = 23.17 + 0.56x | 0.90 | 0.001 | y = 25.20 + 0.31x | 0.85 | 0.001 |
| B. subtilis RR07 | y = 23.61 + 0.38x | 0.58 | Ns | y = 43.19 + 0.45x | 0.96 | 0.001 | y = 30.11 + 0.18x | 0.70 | 0.04 |
| B. subtilis subsp. RRD8 | y = 47.35 + 0.21x | 0.55 | Ns | y = 28.22 + 0.34x | 0.70 | 0.05 | y = 62.45 + 0.21x | 0.90 | 0.001 |
| B. subtilis RRAR1 | y = 31.09 + 0.41x | 0.74 | 0.01 | y = 41.09 + 0.21x | 0.60 | Ns | y = 39.49 + 0.36x | 0.50 | Ns |
| A. brasilense RRAK5 | y = 61.11 + 0.77x | 0.95 | 0.001 | y = 21.34 + 0.26x | 0.59 | Ns | y = 43.51 + 0.25x | 0.45 | Ns |
| Paenibacillus sp. RRB2 | y = 32.45 + 0.61x | 0.93 | 0.001 | y = 25.23 + 0.34x | 0.58 | Ns | y = 36.32 + 0.36x | 0.54 | Ns |
| P. stutzeri RRAN2 | y = 39.56 + 0.61x | 0.90 | 0.001 | y = 20.15 + 0.57x | 0.90 | 0.001 | y = 33.43 + 0.43x | 0.60 | Ns |
| Control | y = 39.56 + 0.61x | 0.54 | Ns | y = 29.16 − 0.14x | 0.60 | 0.02 | y = 27.45 + 0.53x | 0.55 | Ns |
| M. latifolia | T. indica | W. tinctoria | |||||||
| Bacillus sp. RRN12 | y = 64.56 + 3.39x | 0.82 | 0.013 | y = 19.11 + 0.55x | 0.95 | 0.001 | y = 36.14 + 0.05x | 0.75 | 0.05 |
| P. geniculata RRC11 | y = 51.19 + 1.54x | 0.86 | 0.008 | y = 44.11 + 0.14x | 0.58 | Ns | y = 25.18 + 0.31x | 0.80 | 0.002 |
| B. kururiensis RRAK1 | y = 42.13 + 1.2x | 0.55 | Ns | y = 47.31 + 0.29x | 0.60 | Ns | y = 33.15 + 0.11x | 0.97 | 0.001 |
| B. subtilis RR07 | y = 45.90 + 2.37x | 0.63 | Ns | y = 25.46 + 0.19x | 0.55 | Ns | y = 23.11 + 0.13x | 0.70 | 0.05 |
| B. subtilis subsp. RRD8 | y = 68.38 + 3.07x | 0.77 | 0.05 | y = 26.01 + 0.10x | 0.58 | Ns | y = 45.35 + 0.12x | 0.95 | 0.001 |
| B. subtilis RRAR1 | y = 41.45 + 1.56x | 0.47 | Ns | y = 28.15 + 0.31x | 0.80 | 0.001 | y = 17.25 + 0.37x | 0.92 | 0.001 |
| A. brasilense RRAK5 | y = 53.13 + 5.2x | 0.95 | 0.001 | y = 49.21 + 0.29x | 0.75 | 0.02 | y = 36.44 + 0.26x | 0.60 | Ns |
| Paenibacillus sp. RRB2 | y = 45.09 + 2.37x | 0.83 | 0.001 | y = 25.41 + 0.65x | 0.90 | 0.001 | y = 41.22 + 0.15x | 0.68 | 0.05 |
| P. stutzeri RRAN2 | y = 68.38 + 3.07x | 0.77 | 0.021 | y = 51.32 + 0.46x | 0.95 | 0.001 | y = 35.15 + 0.14x | 0.75 | 0.05 |
| Control | y = 45.23 + 2.52x | 0.80 | 0.001 | y = 28.33 − 0.15x | 0.67 | 0.05 | y = 29.33 + 0.51x | 0.60 | Ns |
Ns not significant (p > 0.05)
Fig. 2.
Nitrogen, phosphorous, and potassium content in seedlings after 180 days of growth
Discussion
Forest ecosystems are very sensitive to external pressures such as clear felling, grazing, and fire. Nanmangalam Reserve Forest has been exposed to such pressures, and has degraded over 150 years (Jayakumar et al. 2009). Currently, trees in this forest exhibit stunted growth, with a maximum height of 2 m for several years running, crown cover < 30%, very low biomass, sporadic tree populations, and negligible regeneration (Radhapriya et al. 2014). The soil appears completely eroded and devoid of significant topsoil, with the parent rock mostly exposed. Indeed, the soil is deficient in total nitrogen, organic carbon, available potassium, calcium, magnesium, iron, and zinc (Radhapriya et al. 2014). The absence or minimal of natural regeneration and repeated failure of replanting or reseeding suggest that the top soil has lost beneficial plant-associated microorganisms, and consequently, its ability to support tree growth (Drezner et al. 2006; Bashan et al. 2012).
Reforestation work based on conventional techniques without application of beneficial microorganisms has been marginally successful. To the best of our knowledge, the application of native plant growth-promoting bacteria to enhance the biomass of forest seedlings has not yet been attempted in Indian forests. Therefore, we isolated such bacteria from Nanmangalam Reserve Forest soil, and characterized their impact on the growth, nutrient, and biomass content of 12 tree species indigenous to the forest.
All diazotrophs isolates produced ammonia when grown on a complex nitrogen source. In the process, these isolates could accumulate and supply nitrogen to host plants, and thereby enhance root proliferation, shoot elongation, and biomass, as observed by Marques et al. (2010) in Zea mays. In addition, the isolates produced enormous quantities of indole acetic acid, which directly stimulates biomass production above and below ground. In particular, indole acetic acid accelerates the development of lateral roots, and increases nutrient absorption through root hairs (Hussain and Hasnain 2011).
Notably, studies demonstrate that commercially available plant growth-promoting bacteria have limited capacity to rehabilitate degraded soil, in contrast to indigenous bacteria well adapted to the local environment (Bashan et al. 2009; García. 2004; Radhapriya et al. 2015). Furthermore, introduction of a non-indigenous microbe may impact the indigenous rhizosphere population (Whipps 2001). Indeed, Bashan et al. (2012) demonstrated revegetation in desert soil using native trees indigenous plant growth-promoting bacteria, arbuscular mycorrhizal fungi, and limited supplementation with compost and water. Their results indicate that native plant growth-promoting bacteria enhanced plant survival and growth in desert soil. Similarly, we found native plant growth-promoting bacteria to increase N, P, and K content in indigenous trees. Presumably, this is due to the production of organic acids by the plants and the bacteria in the rhizosphere, a process that acidifies the soil and mobilizes micronutrients, as demonstrated in several studies (Liu et al. 2013; Sundra et al. 2002; Shen et al. 2004). However, only five strains enhanced biomass production, even though almost all strains had characteristics that could stimulate plant growth. This result suggests specific, productive interactions between plants and microbes, as has been noted in agricultural experiments (Raaijmakers et al. 2009; Bergsma-Vlami et al. 2005; Wieland et al. 2001).
In conclusion, nine indigenous PGPB were isolated from NRF based on their plant growth-promoting activity. Then these isolates were given for treatment to 12 native tree species. In that, five bacterial isolates were given positive significant results in most of the tree species. Degraded soil treated with native plant growth-promoting bacteria and inorganic fertilizer enhanced the growth, biomass production, and nutrient content of tree species native to the soil. Hence, this study emphasizes the potential of niche-specific microorganisms and native plant species to accelerate the rehabilitation of degraded forests.
Acknowledgements
The first author (PR) gratefully acknowledges the University Grants Commission (UGC-BSR), Government of India, UGC Sanction./-7/2007 (BSR), dated 16.11.2009 for providing the financial support for this research work. Authors thank the Tamil Nadu Forest Department for allowing nursery treatments in Nanmangalam Reserve Forest. We are extremely grateful to Forest Department staff for valuable assistance and continuous support during the study.
Author contributions
Conception and design of experiments: AR, PR. Manuscript preparation: PR, AR, PP. Data analysis: PR.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Adesemoye AO, Kloepper JW. Plant microbes interactions in enhanced fertilizer-use efficiency. Appl Micobiol Biotechnol. 2009;85:1–12. doi: 10.1007/s00253-009-2196-0. [DOI] [PubMed] [Google Scholar]
- Annamalai R. Working plan for Chengalpattu Division, under the guidance of the Principal Chief Conservator of Forests. India: Government of Tamil Nadu; 1987. p. 1987. [Google Scholar]
- Bashan Y, Salazar B, Puente ME. Responses of native legume desert trees used for reforestation in the Sonoran Desert to plant growth-promoting microorganisms in screen house. Biol Fertil Soils. 2009;45:655–662. doi: 10.1007/s00374-009-0368-9. [DOI] [Google Scholar]
- Bashan Y, Salazar BG, Moreno M, Lopez BR, Linderman RG. Restoration of eroded soil in the Sonoran desert with native leguminous trees using plant growth-promoting microorganisms and limited amounts of compost and water. J Environ Manag. 2012;102:26–36. doi: 10.1016/j.jenvman.2011.12.032. [DOI] [PubMed] [Google Scholar]
- Bergersen FJ, Turner GL. Properties of terminal oxidase systems of bacteroids from root nodules of soybean and cowpea and of N2-fixing bacteria grown in continuous culture. J Gen Microbiol. 1980;118:235–252. [Google Scholar]
- Bergsma-Vlami M, Prins ME, Raaijmakers JM. Influence of plant species on population dynamics, genotypic diversity and antibiotic production in the rhizosphere by indigenous Pseudomonas spp. FEMS Microbiol Ecol. 2005;52:59–69. doi: 10.1016/j.femsec.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bunt JS, Rovira AD. Microbiological studies of some subantarctic soils. J Soil Sci. 1955;6:119–128. doi: 10.1111/j.1365-2389.1955.tb00836.x. [DOI] [Google Scholar]
- Cappuccino JG, Sherman N. Microbiology: a laboratory manual. New York: Addison-Wesley; 1992. p. 1992. [Google Scholar]
- Drezner TD. The regeneration of a protected Sonoran desert cactus since 1800 A.D. over 50,000 km2 of its range. Plant Ecol. 2006;183:171–176. doi: 10.1007/s11258-005-9015-1. [DOI] [Google Scholar]
- FAO (2009)Towards defining degradation, by Markku Simula. FRA Working Paper 154. Rome
- García JAL, Domenech J, Santamaría C, Camacho M, Daza A, Gutierrez Mañero FJ. Growth of forest plants (pine and holm-oak) inoculated with rhizobacteria: relationship with microbial community structure and biological activity of its rhizosphere. Environ Exp Bot. 2004;52:239–251. doi: 10.1016/j.envexpbot.2004.02.003. [DOI] [Google Scholar]
- Gaur AC. Phosphate solubilizing microorganisms as biofertilizer. New Delhi: Omega Scientific Publishers; 1990. [Google Scholar]
- Gordon SA, Weber RP. Colorimetric estimation of indole acetic acid. Plant Physiol. 1951;26:192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy RW, Holsten RD, Jackson EK, Burns RC. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968;43:1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A, Hasnain S. Interactions of bacterial cytokinins and IAA in the rhizosphere may alter phytostimulatory efficiency of rhizobacteria. World J Microbiol Biotechnol. 2011;27:2645–2654. doi: 10.1007/s11274-011-0738-y. [DOI] [Google Scholar]
- Jayakumar S, Ramachandran A, Bhaskaran G, Heo J. Forest Dynamics in the Eastern Ghats of Tamil Nadu, India. Environ Manag. 2009;21:326–345. doi: 10.1007/s00267-008-9219-y. [DOI] [PubMed] [Google Scholar]
- Jha B, Thakur MC, Gontia I, Albrecht V, Stoffels M, Schmid M, et al. Isolation, partial identification and application of diazotrophic rhizobacteria from traditional Indian rice cultivars. Eur J Soil Biol. 2009;45:62–72. doi: 10.1016/j.ejsobi.2008.06.007. [DOI] [Google Scholar]
- Johnson CM, Ulrich A. Analytical methods for use in plant analysis. Berkeley: University of California; 1959. [Google Scholar]
- Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol. 2012;62:716–721. doi: 10.1099/ijs.0.038075-0. [DOI] [PubMed] [Google Scholar]
- Kremer RJ, Souissi T. Cyanide production by rhizobacteria and potential for suppression of weed seedling growth. Curr Microbiol. 2001;43:182–186. doi: 10.1007/s002840010284. [DOI] [PubMed] [Google Scholar]
- Liu F, Xing S, Ma H, Du Z, Ma B. Plant growth-promoting rhizobacteria affect the growth and nutrient uptake of Fraxinus americana container seedlings. Appl Microbiol Biotechnol. 2013;97:4617–4625. doi: 10.1007/s00253-012-4255-1. [DOI] [PubMed] [Google Scholar]
- Lu RK. Analytical methods for soil and agrochemistry. Beijing: Chinese Agriculture Science and Technology Press; 1999. [Google Scholar]
- Lund HG (2009) What is a Degraded Forest? White Paper on Forest Degradation Definitions Prepared for FAO. Forest Information Services, Gainesville, VA. Available at: http://home.comcast.net/gyde/2009forest_degrade.doc (accessed 17.04.13)
- Marques APGC, Pires C, Moreira H, Rangel AOSS, Castro PML. Assessment of the plant growth promotion abilities of six bacterial isolates using Zea mays as indicator plant. Soil Biol Biochem. 2010;42:1229–1235. doi: 10.1016/j.soilbio.2010.04.014. [DOI] [Google Scholar]
- Meyer JM, Abdallah MA. The florescent pigment of Pseudomonas fluorescens: biosynthesis, purification and physicochemical properties. J Gen Microbiol. 1978;107:319–328. doi: 10.1099/00221287-107-2-319. [DOI] [Google Scholar]
- Moreno M, de-Bashan L, Lopez BR, Bashan Y. Success of long-term restoration of degraded arid land using native trees planted 11 years earlier. Plant Soil. 2017;421:83–92. doi: 10.1007/s11104-017-3438-z. [DOI] [Google Scholar]
- Pajares S, Bohannan BJM. Ecology of Nitrogen Fixing, Nitrifying, and Denitrifying Microorganisms in Tropical Forest Soils. Front Microbiol. 2016;7:1045–328. doi: 10.3389/fmicb.2016.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palani (2004) Working plan for Chengalpattu Division, under the guidance of the Principal Chief Conservator of Forests, p 69
- Penrose DM, Glick BR. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant. 2003;118:10–15. doi: 10.1034/j.1399-3054.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- Putz FE, Redford KH. Dangers of carbon-based conservation. Global Environ Change. 2009;19:400–401. doi: 10.1016/j.gloenvcha.2009.07.005. [DOI] [Google Scholar]
- Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moënne-Loccoz Y. The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil. 2009;321:341–361. doi: 10.1007/s11104-008-9568-6. [DOI] [Google Scholar]
- Radhapriya P, Ramachandran A, Dhanya P, Remya K, Malini P. An appraisal of physico-chemical and microbiological characteristics of Nanmangalam Reserve Forest soil. J Environ Biol. 2014;35:1137–1144. [PubMed] [Google Scholar]
- Radhapriya P, Ramachandran A, Anandham R, Mahalingam S. Pseudomonas aeruginosa RRALC3 enhances the biomass, nutrient and carbon contents of Pongamia pinnata seedlings in degraded forest soil. PLoS One. 2015;10(10):0139881. doi: 10.1371/journal.pone.0139881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Jayakumar S, Haroon ARM, Bashkaran A. Carbon management in forest floor—an agenda of 21st century in Indian forestry scenario. Indian Forest. 2007;133:25–40. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: a laboratory manual. 2. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Shen J, Li R, Zhang F, Fan J, Tang C, Rengel Z. Crop yields, soil fertility and phosphorus fractions in response to long-term fertilization under rice monoculture system on a calcareous soil. Field Crops Res. 2004;86:225–238. doi: 10.1016/j.fcr.2003.08.013. [DOI] [Google Scholar]
- Sundra B, Natarajan V, Hari K. Influence of phosphorous solubilizing bacteria on the changes in soil available phosphorus and sugarcane and sugar yields. Field Crops Res. 2002;77:43–49. doi: 10.1016/S0378-4290(02)00048-5. [DOI] [Google Scholar]
- Tangam . Working plan for Saidapet Division, under the guidance of the Principal Chief Conservator of Forests. India: Government of Tamil Nadu; 1959. p. 1959. [Google Scholar]
- Ueda T, Suga Y, Yahiro N, Matsuguchi T. Remarkable N2-fixing bacterial diversity detected in rice roots by molecular evolutionary analysis of nifH gene sequences. J Bacteriol. 1995;177:1414–1417. doi: 10.1128/jb.177.5.1414-1417.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DL, Anderson DW. Direct measurement of organic carbon content in soils by the Leco CR-12 carbon analyzer. Commun Soil Sci Plant Anal. 1998;29:15–21. doi: 10.1080/00103629809369925. [DOI] [Google Scholar]
- Wang X, Dong Z, Jhang J, Liu L. Modern dust storms in China: an overview. J Arid Environ. 2004;58:559–574. doi: 10.1016/j.jaridenv.2003.11.009. [DOI] [Google Scholar]
- Whipps JM. Microbial interactions and biocontrol in the rhizosphere. J Exp Bot. 2001;52:487–511. doi: 10.1093/jxb/52.suppl_1.487. [DOI] [PubMed] [Google Scholar]
- Wieland G, Neumann R, Backhaus H. Variation of microbial communities in soil, rhizosphere, and rhizoplane in response to crop species, soil type, and crop development. Appl Environ Microbiol. 2001;67:5849–5854. doi: 10.1128/AEM.67.12.5849-5854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]