Abstract
The development of endoscopic ear surgery techniques promises to change the way we approach ear surgery. In this review paper, we explore the current evidence, seek to determine the advantages of endoscopic ear surgery, and see if these advantages are both measureable and meaningful. The wide field of view of the endoscope allows the surgeon to better visualize the various recesses of the middle ear cleft. Endoscopes make it possible to address the target pathology transcanal, while minimizing dissection or normal tissue done purely for exposure, leading to the evolution of minimally-invasive ear surgery and reducing morbidity. When used in chronic ear surgery, endoscopy appears to have the potential to significantly reduce cholesteatoma recidivism rates. Using endoscopes as an adjunct can increase the surgeon's confidence in total cholesteatoma removal. By doing so, endoscopes reduce the need to reopen the mastoid during second-look surgery, help preserve the canal wall, or even change post-cholesteatoma follow-up protocols by channeling more patients away from a planned second-look.
Keywords: Endoscopic ear surgery, Minimally-invasive ear surgery, Ear surgery outcomes, Ear surgery, Otologic surgery, Mastoid surgery
Introduction
Endoscopic ear surgery has recently become a hot topic in otology. There has been a recent explosion in the number of publications on this topic. The aim of this paper is to help the reader digest this voluminous material and boil it down to the issues that are important, even transformative in the field of ear surgery. The advent of endoscopic techniques is making possible the next steps in evolution of ear surgery. The otolaryngology professional organizations have noticed this growing interest in ear endoscopy. The first endoscopic ear surgery courses offered a few years ago were received with reservation and skepticism. Today, endoscopic ear surgery courses, seminars and hands-on workshops are available across the country and the world, to address this rising interest.
Discussion
-
a.
History of endoscopic ear surgery.
In the early 20th century, while medicine and surgery were already clearly separate, otolaryngology was just starting to establish itself as a distinct specialty. Ear surgeons in the early 20th century used loupes. In 1921, a Swedish otologist, Carl Olof Nylen, was the first to use a microscope to complete an ear surgery. Because the microscope was monocular and illumination was poor, the use of the microscope did not catch on immediately. In 1953, Carl Zeiss developed the first binocular otologic microscope with coaxial illumination, better depth of focus, and adjustable magnification. This microscope was a game changer in ear surgery. This technological advance spurred the rapid development of most of the modern ear surgery techniques we use today, an advance in technology so rapid and far-reaching, it greatly enhanced our ability to manage chronic ear disease, making it worthy of the epithet “revolutionary”. That was 1953, only 64 years ago. Technological advances can and often do allow the development of new therapies and new surgical techniques.
Shortly thereafter, another major technological advance came on the horizon. Developed in the 1960's, the Hopkins rod endoscope was a major improvement over the old endoscopes, with a wider field of view and much better optics. By the late 1960's it was possible to visualize unprecedented anatomic detail in vibrant true colors. Coupled with the cold light source, the Hopkins rod endoscope allowed the emergence of endoscopic minimally-invasive surgical techniques in many surgical specialties, including otolaryngology. In the beginning, these new techniques were ridiculed and shunned, running contrary to the well established dogma that promoted large incisions and wide exposure as immutable principles of surgical science.
It didn't take long before someone put an endoscope in an ear to look around, first in cadavers and animals ears, then in living human patients. In 1967, Mer et al1 reported on the use endoscopes to examine the middle ears of human cadavers and ears of living animals through iatrogenic myringotomies. In 1982, Nomura described endoscopic explorations of middle ears of living patients through a myringotomy.2 Then in the 1980's, a few pioneering ear surgeons started using endoscopes, not just to look, but also to perform parts of surgeries. Thomassin et al3 started investigating the advantages of this mode of endoscopic visualizations in ear surgery. The first publications on the application of endoscopy in ear surgery were seen in the 1990's.3 And so, endoscopic ear surgery was born.
-
b.
Impact of endoscopic ear surgery on outcomes.
How much did the endoscope really change ear surgery since it was first used in the 1980's? Is the endoscope as transformative as the microscope was in the 1950's? Does endoscopic ear surgery give us any measurable and meaningful advantages over classic ear surgery techniques? Of course, the advantage should be both measurable and meaningful before any new technique is adopted. In current literature, surgeons are demonstrating that endoscopic ear surgery techniques can be equivalent to classic microscopic techniques. For example, endoscopic ear surgeons demonstrate that they can raise a tympanomeatal flap endoscopically, perform a tympanoplasty or stapedectomy endoscopically in a safe and effective manner. Surgeons comfortable with the microscope see no advantage in these endoscopic techniques, viewing them as equivalent at best, not superior. Are there any situations where the use of endoscopy leads to superior outcomes in ear surgery? If so, the case for endoscopic ear surgery would become much more compelling.
Let us explore the three statements below, each demonstrating that the use of endoscopy in ear surgery, either exclusively, or as an adjunct to the microscope, may be superior to classic ear surgery techniques. We will also summarize the literature and the evidence supporting some of these statements.
-
1.
Endoscopes allow the surgeon to see better.
-
2.
Endoscopes allow the surgeon to complete more tasks transcanal, reducing the need for postauricular incisions and avoiding the associated morbidity.
-
3.
Use of endoscopes in chronic ear surgery can reduce cholesteatoma recidivism rates.
-
c.
Statement #1: Endoscopes allow the surgeon to see better.
The wide field of view of the endoscope allows the surgeon to better visualize the various recesses of the middle ear cleft, a distinct advantage over the narrow field of view of the binocular microscope which is a line-of-sight instrument.
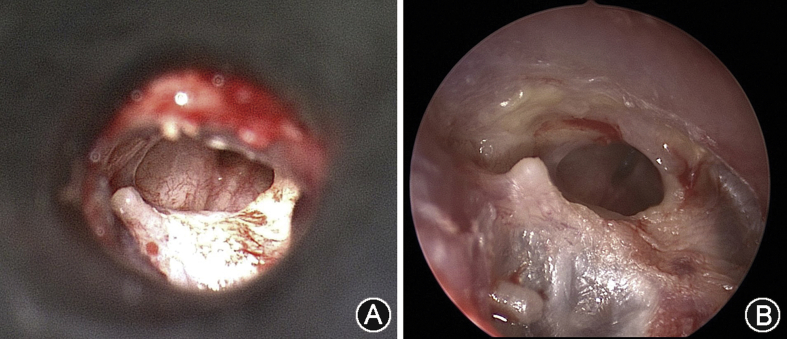
Fig. 1 illustrates the view of the same drum with a microscope and an endoscope. The endoscopic view is much more panoramic. The three dimensional view and depth perception of the microscope is not entirely lost with the very much monocular endoscope. The perception of depth can be recreated by simply moving the endoscope slightly around. Experienced ear endoscopists have not reported that the monocular nature of the endoscope presents a significant handicap.
Fig. 1.
A: View of a drum perforation as seen with a microscope through a speculum. B: View of the same drum with an endoscope. The endoscopic view is more panoramic.
Fig. 2 shows a panoramic view of the middle ear cavity as only seen with an endoscope. Many of the important landmarks can be seen concurrently, superiorly from the facial nerve and epitympanic isthmus, to the ossicles, oval and round windows, all the way inferiorly to the hypotympanic bony trabeculae, as well as the posterior recesses. The anterior mesotympanum and the Eustachian tube opening cannot be seen because the drum was not reflected off the malleus.
Fig. 2.
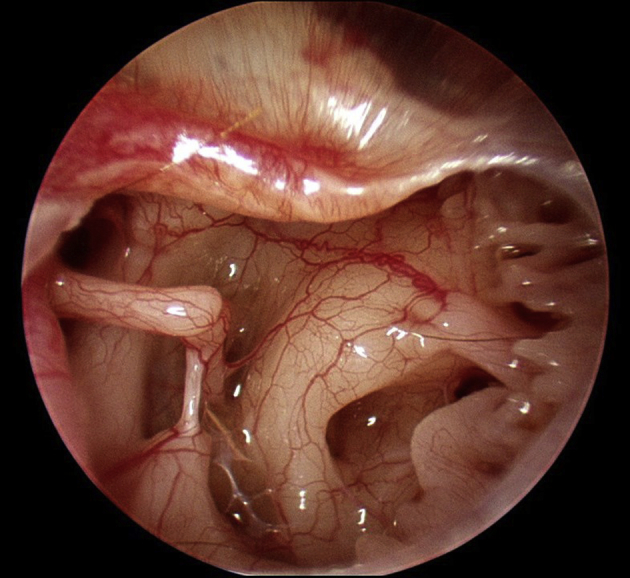
Panoramic view of the middle ear cavity as seen through a rigid Hopkins rod endoscope, 3 mm diameter, zero degree, with a high-definition, 3-chip camera.
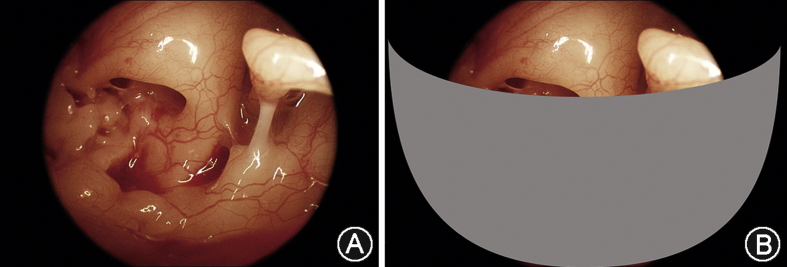
Endoscopes also allow us to see around corners, into the crevices of the middle ear cleft, formerly inaccessible without significant dissection. Fig. 3 A depicts a nice view of the posterior recesses with a 30° endoscope. The facial recess is easily seen with no curetting. The depth of the sinus tympani is more difficult to see. With the microscope this area cannot be seen at all. Fig. 3 B depicts a view expected with a microscope. A hint of the stapedius tendon can sometimes be seen without curetting. Of course, this anatomy varies among patients. Most tympanic sinuses can be examined, but the larger and deeper ones are still inaccessible, even with endoscopes. Overall, visualization of the posterior recesses is greatly enhanced using endoscopes.
Fig. 3.
A: Posterior recesses of the middle ear cleft as seen through an endoscope, 3 mm diameter, 30° angled. The facial recess is easily seen. The sinus tympani is seen as well, but the depth of this recess is not always fully appreciated even with the endoscope. B: View of the posterior recesses as expected using a microscope.
Fig. 4 shows a view with a 30° endoscope toward the epitympanum. Easily seen is the epitympanic isthmus between the long process of the incus and the cochleariform process, the main ventilation route from the middle ear to the mastoid. In this case, the isthmus is open and there are no obstructions. Also easily seen is the attachment of the tensor tympani tendon at the cochleariform process. The tensor tympani can be cut under endoscopic vision, without the need for any excessive dissection for exposure.4, 5 Understanding the anatomy of the middle ear recesses becomes much easier now that these recesses are visible. Endoscopes have been great for resident education in ear surgery.
Fig. 4.
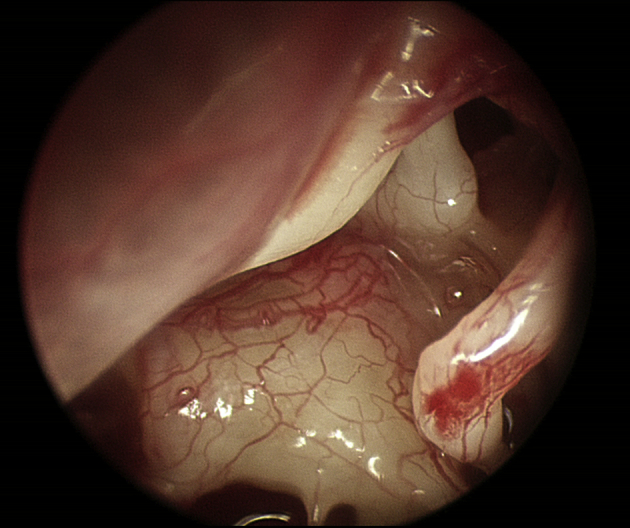
View of the epitympanum using an endoscope, 3 mm diameter, 30° angle. The epitympanic isthmus between the incus and cochleariform process is the main ventilation route between the middle ear and mastoid.
With endoscopes, we are also rediscovering the anatomy of the middle ear folds that has been known for many years, but perhaps forgotten because it has been hidden from view in most routine ear surgeries. Hammar in 1902, and later Proctor in 1964, described the anatomy of the middle ear, including the four embryonic pouches that expand and form the middle ear cleft.6, 7 The borders between these pouches form membranous folds that invest the ossicles and define middle ear ventilation routes. These membranes are routinely seen in endoscopic ear surgery. There are blood vessels and other mesoderm remnants coursing through these folds. Obstruction of these ventilation routes may cause localized retraction pockets, depending on which ventilation route is blocked, and also result in different patterns of cholesteatoma growth. Fig. 5 depicts these folds as seen during routine endoscopic ear surgery. The folds are completely encasing this ossicular chain. The interosseous fold is seen between the malleus and incus. This network of folds is seen easily with a wider field of view provided by the endoscope. There is even a membrane covering the round window niche, which also appears to be an embryonic remnant.
Fig. 5.
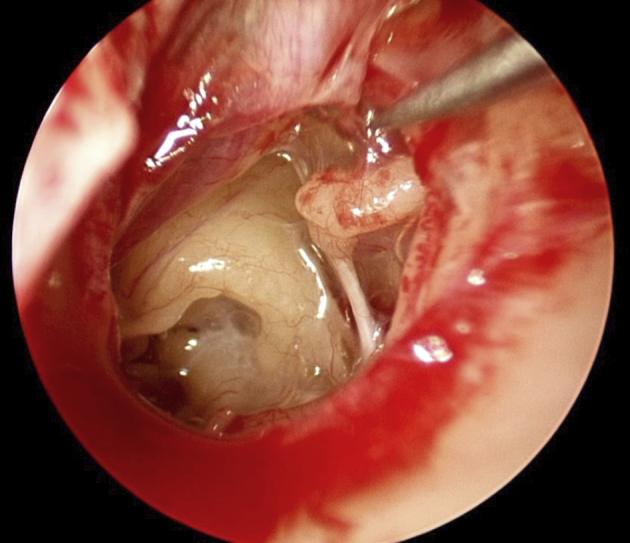
Membranous folds investing the ossicles are embryonic remnants. They are easily seen during this routine endoscopic ear surgery.
In summary, by using endoscopes exclusively or as an adjunct to the microscope, ear surgeons can greatly enhance their visualization of the target pathology and landmarks. Endoscopes do allow the surgeon to see better.
-
d.
Statement #2: Endoscopes allow the surgeon to complete more tasks transcanal, reducing the need for postauricular incisions and avoiding the associated morbidity.
This is a major reason why endoscopes deserve a place in mainstream ear surgery. There are, of course, multiple approaches to the middle ear cleft. The simplest route is the anterior tympanostomy, a transcanal route while holding a speculum. The speculum can be avoided if an endaural incision is made. Another approach is also an anterior tympanostomy, but via a postauricular approach. This approach allows better visualization of the anterior drum while using a microscope. The third approach is a posterior tympanotomy, through the mastoid and facial recess. Decisions on how to access middle ear pathology are subjective, often intraoperative choices. The decision between an open cavity and closed cavity mastoidectomy is also subjective to some degree (canal-wall-up vs canal-wall-down). Any type of extra dissection may come with extra morbidity. The surgeon's goal is to complete a task with the minimum amount of dissection of normal tissue that is done purely for exposure. Endoscopes, with their wide field of view, allow the surgeon to reduce dissection of normal tissue that is done simply for exposure.
Functional endoscopic ear surgery is a set of three principles that describe how the concept of minimally-invasive surgery applies to otology.8 The following are the three principles that define functional endoscopic ear surgery:
-
-
Use the ear canal as the natural conduit to the tympanic cavity.
-
-
Restore normal middle ear and mastoid ventilation routes.
-
-
Preserve as much normal anatomy as possible, by minimizing dissection of bone and soft tissue that is done simply for exposure.
The corollary to the third statement is that ear pathology originates in the middle ear. The mastoid is often just an “innocent bystander”, a site where disease spreads rather than originates. Adding a mastoidectomy to tympanoplasty does not improve tympanoplasty outcomes.9 The cause of the problem is in the middle ear and this is where the surgeon's attention should be focused.
Many surgeons have noted that endoscopes help them complete more work transcanal and avoid unnecessary dissection, allow them to see the anterior part of the drum easily, even allow them to keep the canal wall up more often in cholesteatoma surgery. Endoscopes have greatly helped with visualization the anterior part of the drum, even if there is a sizable anterior canal bulge. Many tympanoplasties that once required a postauricular incision, are now being done endoscopically transcanal. Fig. 6 shows a large perforation, extending anteriorly, repaired transcanal, without using the speculum, and with no postauricular incision. The endoscope provided the view necessary to complete this operation, including placement of the fascia graft. Without the endoscope, this patient would have surely needed a postauricular incision. Tseng et al10 in 2016, published a series of 59 endoscopic transcanal tympanoplasties done for anterior perforations. Their graft success rate was 93%, a good success rate and comparable to historical controls, but they achieved these results without the need for any postauricular incisions, and without the need for canalplasty. They achieved these results by using minimally-invasive techniques, following the principles of functional endoscopic ear surgery.10
-
e.
Statement #3: The use of endoscopes in chronic ear surgery can reduce cholesteatoma recidivism rates.
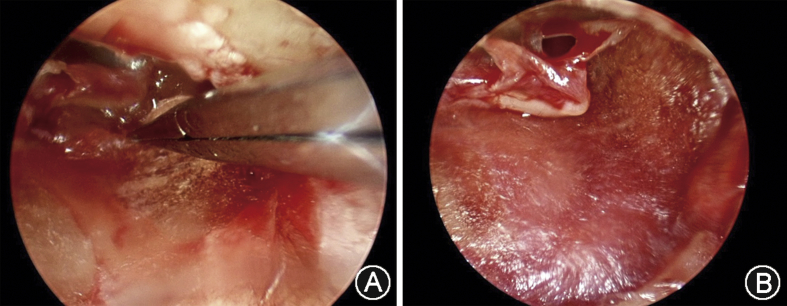
Fig. 6.
This large tympanic membrane perforation extends anteriorly to the malleus. It was repaired endoscopically, transcanal, without the need for a postauricular incision. A: Placing the fascia graft under endoscopic vision. B: View of the fascia graft in place, showing the antero-inferior extent of the perforation in relation to the malleus as a landmark.
This statement, if true, would represent a major advance in ear surgery. Cholesteatoma recidivism rates are notoriously high in chronic ear surgery with an intact canal wall. Hence the ongoing discussions and controversies surrounding management of the follow-up phase. Which patients need a second-look procedure, and what is the role of diffusion-weighted imaging (DWI) Propeller MRI in cholesteatoma follow-up?
Can the use of endoscopes really reduce cholesteatoma recidivism rates to a level low enough where our management protocols of the follow-up phase would change? There is a good volume of literature on this topic, with publications dating back to as early as 1990. The question of the utility of endoscopes in cholesteatoma surgery can be divided into several components:
-
1.
After removal of a cholesteatoma using a microscope, how often does the endoscope find residual disease?
-
2.
Does the use of endoscopes in primary cholesteatoma surgery reduce the rate of residual/recurrent cholesteatoma at the time of second-look?
-
3.
Does the use of endoscopes in primary cholesteatoma surgery reduce the need for a second-look? In other words, does it alter the postop follow-up protocol?
The following clinical vignette illustrates the issue in question 1: The surgeon performs a canal-wall-up (intact canal wall) tympanomastoidectomy for cholesteatoma. After her best effort, all visible cholesteatoma matrix is removed. Then the surgeon inserts an endoscope and looks around. How often will the endoscope reveal residual cholesteatoma matrix that was hidden from view when using a microscope? Several studies have addressed this question, as depicted in Table 1.
Table 1.
Use of endoscopy frequently reveals residual disease during primary cholesteatoma surgery.
| Study | Year | Fraction of cases with residual cholesteatoma | Comments |
|---|---|---|---|
| Badr-El-Dine11 | 2002 | 23% | |
| El-Meselaty et al12 | 2003 | Intraoperative cholesteatoma remnants were detected with endoscope in both CWU and CWD procedures | |
| Ayache et al13 | 2008 | 44% overall 76% of cases of cholesteatoma that involved the retrotympanum |
|
| Sajjadi14 | 2013 | 22% | |
| Sarcu and Isaacson15 | 2016 | 17% |
Badr-El-Dine11 in 2002 published a series of 82 canal-wall-up (CWU) surgeries and 10 canal-wall-down (CWD) surgeries for cholesteatoma. After microscopic excision, endoscope detected residual disease in 23% of cases. Common sites of residual disease were the sinus tympani, facial recess and undersurface of the scutum. El-Meselaty et al12 in 2003 published a series of 82 cholesteatoma ears. The investigators found that intraoperative cholesteatoma remnants were detected by the endoscope in both CWU and CWD surgeries, mostly in the sinus tympani. Ayache et al13 in 2008 published a large retrospective review of 350 patients who had surgery for cholesteatoma. After completing the surgery using a microscope, the investigators introduced the endoscope and found residual cholesteatoma in 44% of cases overall, and in a staggering 76% of cases where cholesteatoma involved the retrotympanum. Sajjadi14 in 2013 published a large retrospective review of 249 primary cholesteatoma cases with a minimum follow-up of 2 years. The use of endoscopy at the time of primary cholesteatoma surgery revealed “cholesteatoma remnants” in 22% of open cavity cases. Sarcu and Isaacson15 in 2016 investigated the role of endoscopes in pediatric cholesteatoma surgery. He published a series of 42 pediatric cholesteatoma surgeries, all canal-wall-up. Seventeen percent had additional disease found on endoscopy that was missed by the microscope. Evidence seems to be mounting that endoscopes can find residual disease after cholesteatoma excision using a microscope, anywhere from 17% in pediatric cases to 76% of cases where cholesteatoma involves the posterior recesses. Endoscopes find hidden cholesteatoma remnants frequently. Endoscopes have the power to find cholesteatoma remnants hidden from view of the microscope.
The follow-up, question 2, practically asks itself: Does the use of endoscopes in primary cholesteatoma surgery reduce the rate of residual cholesteatoma at the time of second-look? Several authors have addressed this question in many of the same studies we already mentioned (Table 2). Some have noted a significant decrease in residual cholesteatoma at the time of second-look, others have not.
Table 2.
Cholesteatoma recidivism rates in chronic ear surgery when endoscopes were used as an adjunct.
Badr-El-Dine et al11 in 2002 published a series of 82 canal-wall-up cholesteatoma surgeries and 10 canal-wall-down surgeries. A second-look was done in 43% of cases. Three cholesteatoma recurrences were found, two of which were just pearls, making the recurrence rate 8.6%. Even though there was no control arm, this recurrence rate is low by historical standards for a canal-wall-up operation. The authors conclude that the use of endoscopes in cholesteatoma surgery reduces cholesteatoma recurrence rates. El-Meselaty et al12 in 2003 published a series of 82 cholesteatoma cases. This study had a control arm. Residual cholesteatoma rates were 25% for canal-wall-up and 5% for canal-wall-down, all in cases where intraoperative endoscopy was not used during primary surgery. Presumably no cholesteatoma recurrences were seen in cases where endoscopy was used as an adjunct, though the number of cases was small. Authors argue that the use of endoscopes in cholesteatoma surgery raised the surgeon's confidence about total removal of the cholesteatoma, thus encouraging the surgeon to leave the canal wall up, while removing cholesteatoma from hidden areas. Ayache et al13 in 2008 published a large retrospective case series of 350 surgeries for cholesteatoma. Authors state that adding ear endoscopy to cholesteatoma surgery allowed them to resort less to open cavity surgery (CWD), and address more lesions transcanal, consistent with principles of functional endoscopic ear surgery. It remained unclear whether adding the endoscope to look for residual disease did or did not reduce cholesteatoma recurrence rates at the time of second-look. Sajjadi14 in 2013 published a retrospective review of 249 primary cholesteatoma cases, another large series. The use of endoscopy during primary cholesteatoma surgery reduced the cholesteatoma residual rate to 9.7% at the time of second-look. Isaacson et al15 in 2016 published a series of 42 pediatric cholesteatoma surgeries, all canal-wall-up. Residual cholesteatoma rate at follow-up was 16.7% when using endoscopes as an adjunct during initial surgery.
Overall, most of these studies are either retrospective reviews or prospective case series without a control arm. They report cholesteatoma recurrence rates at the time of follow-up and compare those numbers to historical controls. When endoscopes are used during primary cholesteatoma surgery as an adjunct (canal-wall-up), residual cholesteatoma rates found on follow-up range anywhere from zero to 17%. While only one of these studies had a control arm, these recurrence rates are quite low, almost as low as expected for canal-wall-down, or open cavity mastoidectomies. Endoscopes appear to have the potential to significantly reduce cholesteatoma recidivism rates. Further studies are needed, and better instruments also need to be developed in order to allow us to operate better in the areas that we can now see.
Finally, does the use of endoscopes in primary cholesteatoma surgery reduce the need for a second-look? This question is difficult to answer because of the subjective nature of the decision on whether or not to do a second-look, and because of the variety of post-cholesteatoma follow-up protocols that surgeons follow. Some authors have addressed this question in their publications, few have addressed it directly. In his retrospective review of 249 primary cholesteatoma cases, Sajjadi14 in 2013 addressed this question. This is a large cohort of surgeries where endoscopy was used every time. The author asserts that the use of endoscopy during primary cholesteatoma surgery significantly reduced the need to reopen the mastoid at the time of second-look, avoiding the associated morbidity. In a series of 42 pediatric cholesteatoma surgeries, Sarcu and Isaacson15 in 2016 found that by using the endoscope, he increased his confidence regarding total cholesteatoma removal. He was able to channel more children into a follow-up group without a planned second-look. A number of second-look procedures were safely avoided. Isaacson states that the use of the endoscope allowed him to change his post-cholesteatoma follow-up protocol in a manner that is favorable to the patient.
Conclusions
A new era of ear surgery is on the horizon. The wide field of view afforded by the endoscope allows the surgeon to better visualize the various recesses of the middle ear cleft. Understanding the anatomy of the middle ear recesses becomes easier now that these recesses are readily visible. Endoscopes have greatly helped with visualization of the anterior part of the drum, even if there is a sizable anterior canal bulge. Tympanoplasties that once required a postauricular incision, can now be done endoscopically transcanal.
The minimally-invasive nature of endoscopic ear surgery promises to reduce morbidity and improve outcomes. Endoscopes, with their wide field of view, allow the surgeon to reduce dissection of normal tissue that is done simply for exposure and complete more tasks transcanal, rather than via a postauricular incision.
In chronic ear and cholesteatoma surgery, evidence seems to be mounting that endoscopes find hidden cholesteatoma remnants frequently. Endoscopes have the power to find cholesteatoma remnants hidden from view of the microscope. Several authors state that adding endoscopy to cholesteatoma surgery allows them to be more confident about total cholesteatoma removal, allows them to preserve the canal wall, allowing the ear to remain in a more physiologic state. Endoscopes also may reduce the need to reopen the mastoid at the time of second-look, and even allow doctors to channel more patients into follow-up protocols without a planned second-look procedure. Significant morbidity related to chronic ear management can be avoided. Endoscopes appear to have the potential to significantly reduce cholesteatoma recidivism rates. Further studies are needed, and better instruments also need to be developed in order to allow us to operate in the areas that we can now see.
Every new technology has its advantages, disadvantages and a (needed) cohort of skeptics. So far, endoscopic ear surgery appears to be promising on our path to less morbidity and better outcomes.
Financial support and funding
None.
Conflicts of interest
None.
Financial disclosures
None.
Edited by Yu-Xin Fang
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Mer S.B., Derbyshire A.J., Brushenko A., Pontarelli D.A. Fiberoptic endotoscopes for examining the middle ear. Arch Otolaryngol. 1967;85(4):387–393. doi: 10.1001/archotol.1967.00760040389009. [DOI] [PubMed] [Google Scholar]
- 2.Nomura Y. Effective photography in otolaryngology – head and neck surgery: endoscopic photography of the middle ear. Otolaryngol Head Neck Surg. 1982;90:395–398. doi: 10.1177/019459988209000406. [DOI] [PubMed] [Google Scholar]
- 3.Thomassin J.M., Duchon-Doris J.M., Emram B., Rud C., Conciatory J., Vilcoq P. Endoscopic ear surgery. Ann Otolaryngol Chir Cervicofac. 1990;107(8):564–570. [PubMed] [Google Scholar]
- 4.Pollak N., Azadarmaki R., Ahmad S. Feasibility of endoscopic treatment of middle ear myoclonus: a cadaveric study. ISRN Otolaryngol. 2014;2014:175268. doi: 10.1155/2014/175268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pollak N., Azadarmaki R., Ahmad S. Endoscopic treatment of middle ear myoclonus with stapedius and tensor tympani section: a new minimally-invasive approach. Br J Med Med Res. 2014;4(17):3398–3405. [Google Scholar]
- 6.Hammar J.A. Abtheilung: allgeraeine morphologie der schlundspalten beim menschen. entwicklnng des mittelohrraumes und des äusseren gehörganges. Studien über die entwicklung des vorderarms und einiger angrenzenden organe. Archiv für Mikroskopische Anatomie. 1902;59(1):471–628. [Google Scholar]
- 7.Proctor B. The development of the middle ear spaces and their surgical significance. J Laryngol Otol. 1964;78:631–648. doi: 10.1017/s002221510006254x. [DOI] [PubMed] [Google Scholar]
- 8.Pollak N. Principles of endoscopic ear surgery. In: Pollak N., editor. Endoscopic Ear Surgery. Plural Publishing, Inc.; San Diego: 2014. pp. 1–17. [Google Scholar]
- 9.Eliades S.J., Limb C.J. The role of mastoidectomy in outcomes following tympanic membrane repair: a review. Laryngoscope. 2013;123:1787–1802. doi: 10.1002/lary.23752. [DOI] [PubMed] [Google Scholar]
- 10.Tseng C.C., Lai M.T., Wu C.C., Yuan S.P., Ding Y.F. Endoscopic transcanal myringoplasty for anterior perforations of the tympanic membrane. JAMA Otolaryngol Head Neck Surg. 2016;142:1088–1093. doi: 10.1001/jamaoto.2016.2114. [DOI] [PubMed] [Google Scholar]
- 11.Badr-El-Dine M. Value of ear endoscopy in cholesteatoma surgery. Otol Neurotol. 2002;23:631–635. doi: 10.1097/00129492-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 12.El-Meselaty K., Badr-El-Dine M., Mandour M., Mourad M., Darweesh R. Endoscope affects decision making in cholesteatoma surgery. Otolaryngol Head Neck Surg. 2003;129:490–496. doi: 10.1016/S0194-59980301577-8. [DOI] [PubMed] [Google Scholar]
- 13.Ayache S., Tramier B., Strunski V. Otoendoscopy in cholesteatoma surgery of the middle ear: what benefits can be expected. Otol Neurotol. 2008;29:1085–1090. doi: 10.1097/MAO.0b013e318188e8d7. [DOI] [PubMed] [Google Scholar]
- 14.Sajjadi H. Endoscopic middle ear and mastoid surgery for cholesteatoma. Iran J Otorhinolaryngol. 2013;25:63–70. [PMC free article] [PubMed] [Google Scholar]
- 15.Sarcu D., Isaacson G. Long-term results of endoscopically assisted pediatric cholesteatoma surgery. Otolaryngol Head Neck Surg. 2016;154:535–539. doi: 10.1177/0194599815622441. [DOI] [PubMed] [Google Scholar]