Abstract
Tympanic membrane perforationsoccur frequently in children, and can result in hearing loss, otorrhea, pain, and cholesteatoma. Due to the narrower ear canal in children, a postauricular incision is often needed to access the tympanic membrane for surgical repair. Endoscopic approaches are increasingly being used for tympanic membrane repair, reducing the need for postauricular incisions. As the need for a postauricular incision decreases, the demand for non-autologous grafting material has increased. Acellular porcine small intestinal submucosa (SIS) has been described in the literature as an alternative to commonly used autologous grafts, and is well suited for use with transcanal endoscopic ear surgery as a minimally invasive approach. This paper describes techniques for use of SIS in endoscopic tympanic membrane repair in children.
Keywords: Pediatric, Endoscopic ear surgery, Tympanoplasty, Acellular matrix, Porcine small intestinal submucosa
Introduction
Tympanic membrane perforation is a common problem in children. A tympanic membrane perforation is readily diagnosed on otoscopic exam and can result in multiple sequelae including conductive hearing loss, chronic otorrhea, tinnitus, and cholesteatoma.1 Surgical repair of tympanic membrane perforation is performed via a wide range of techniques, through several approaches, and with numerous grafting materials. Recently, many surgeons have introduced rigid endoscopes into the surgical armamentarium for visualization during tympanic membrane repair. Advantages of visualization with an endoscope as compared to the microscope include a broader view, improved depth of field, and a minimally invasive approach via the external auditory canal.2 While improvements in visualization are enjoyed with any endoscopic ear surgery (EES), the benefit of a minimally invasive approach occurs only when the entire surgery is performed via the ear canal, with transcanal endoscopic ear surgery (TEES). The decision to perform TEES depends on many factors, however, a particular advantage is seen in children where most cases of tympanic membrane repair are performed via a postauricular approach simply due to the limited view afforded via the microscope with a speculum in the ear canal, and not for any special access to the mastoid or other anatomic structures. As a result, TEES in children can allow for a reduction in morbidity such as postop pain, hematoma or keloid scar (Fig. 1) by eliminating the need for a postauricular approach in many cases.3, 4, 5
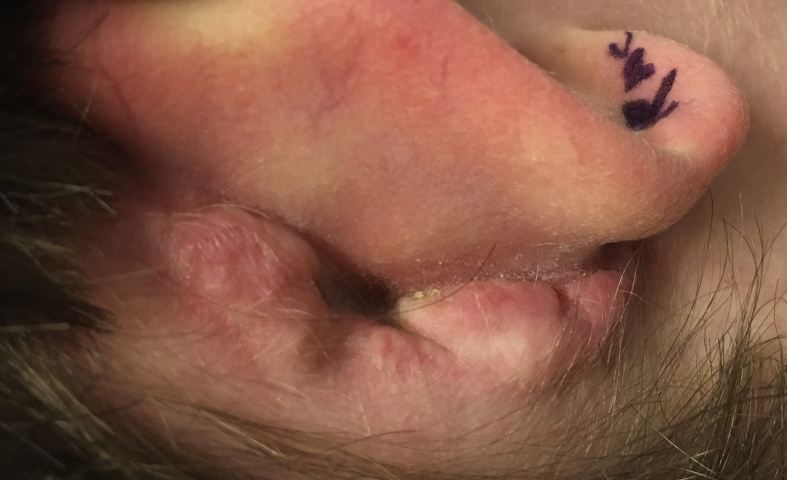
Fig. 1.
Keloid scar following post-auricular incision. Such complications can be prevented by eliminating this incision with transcanal endoscopic ear surgery.
There are many factors that affect surgical outcomes following tympanic membrane repair including age, location and size of the perforation, presence or absence of otorrhea, condition of the drum adjacent to the perforation edge, and technique used in performing the repair.6, 7 Although the use of those factors as a prognostic tool for predicting surgical success remains controversial, one cannot ignore the importance of considering them when choosing the right timing and techniques for tympanic membrane repair, particularly in the pediatric population. The degree to which those factors affect surgical outcomes has been widely reported.8
Another important consideration in tympanic membrane repair is the type of graft used to perform the repair. A common source of graft material is autologous temporalis fascia, which requires a small incision for harvest. As cases are increasingly performed via TEES, with a resultant decrease in the need for postauricular incisions, the availability of a robust, easily manipulated, cost-effective option for graft material is attractive. In this article, we discuss strategies for tympanic membrane repair in the pediatric population, with particular attention to the role of porcine small intestinal submucosa (SIS) as a graft material using an endoscopic approach. Techniques used by our group using SIS are described in-depth.
Discussion
Nomenclature
The nomenclature for tympanic membrane repair has many iterations and subtleties. For the purpose of this paper, myringoplasty refers to repair of the tympanic membrane limited to the drum head without elevation of a tympanomeatal flap. Tympanoplasty refers to all other repairs of the tympanic membrane, can be performed with or without ossicular chain reconstruction, and can be subdivided into lateral graft tympanoplasty and medial or underlay graft tympanoplasty.
Endoscopic vs microscopic ear surgery
The binocular operating microscope has long been the stalwart tool for visualization in otologic surgery. It offers high resolution, excellent depth perception, and fixed positioning allowing two free hands for surgical dissection. Limitations of the operating microscope include direct line-of-sight visualization, hindering the ability to see around anatomic obstacles such as the anterior canal wall or scutum. Furthermore, as magnification increases, illumination and depth of field decrease, making visualization poorer. Due to the limits of the external auditory canal diameter, particularly in children, many otologic procedures require a postauricular approach and division and retraction of the lateral hair-bearing skin of the ear canal, just to obtain a full view of the tympanic membrane. Even after these maneuvers have been performed, prominence of the anterior canal wall can limit a full view of the anterior tympanic membrane, and anterior canal skin elevation and canalplasty are necessary to obtain a full view of the drum. Over time, as rigid endoscopes became smaller and high resolution camera systems became available, endoscopes were used as a tool to diagnose and photograph diseases of the middle ear.9 Subsequently, the endoscope has moved beyond a tool for visualization alone, and techniques for performing otologic surgery under endoscopic visualization have emerged.4 Without question, endoscopes provide superior visualization of the tympanic membrane.10 In order to take advantage of this superior visualization, gradual incorporation of one-handed surgical techniques and strategies for managing ergonomics and hemostasis have evolved, allowing for minimally invasive approaches to common otologic pathology.4, 5, 11, 12, 13, 14, 15, 16, 17
Common themes among reports from authors who perform EES include an emphasis on optimal operating room layout, ergonomics, gradual incorporation of techniques, and progression from simpler to more complex cases. Safety considerations including management of heat from the endoscope tip and strategies for stabilization of the endoscope are critical. Patient selection, taking into consideration ear canal size, extent of disease, and presence of inflammation, can help to make earlier successes possible.
Grafting material
The purpose of tympanic membrane repair is to restore the integrity and function of the tympanic membrane in order to reestablish sound conduction and the protection of the middle ear structures. Historically, numerous materials have been used as graft material to achieve these goals.18, 19 Berthold first described the use of forearm skin graft for myringoplasty in 1878.20 Years later this technique was mastered and continued by others as a reliable modality for grafting the tympanic membrane.21, 22, 23 As technologies have progressed, other types of grafts were developed. Autologous grafts such as temporalis fascia, fascia lata, perichondrium, cartilage and fatty tissue have all been described as means to repair tympanic membrane perforations.24, 25, 26, 27 Temporalis fascia and tragal cartilage are among the most popular grafts used to reconstruct the tympanic membrane today, both requiring an incision for harvest.28, 29 In order to prevent donor-site morbidity, reduce operating time, and to avoid an extra incision in cases where that added step can be unnecessary, numerous allograft materials have been made available such as dura mater, pericardium, vein and aortic valve. In addition, xenograft and manufactured graft materials such as paper, absorbable gelatin sponge and acellular submucosal matrix have been described. The use of acellular human dermis (AlloDerm; Life Cell Corp., Bridgewater, NJ) in several reports as a tympanic membrane repair graft has been well described in both animals and humans.30, 31, 32, 33
More recently, acellular porcine small intestinal submucosa (SIS) (Biodesign; Cook Medical Inc., Bloomington, IN) has been introduced into the United States market. The product consists of a 4 -layer sheet of decellularized, freeze-dried SIS and is provided in both pre-sized disks and square sheets. Badylak described SIS as conducive to effective tissue remodeling, and asserted that it could be successfully used for repair of the abdominal wall, bladder, and dura.34 Spiegel and Kessler studied the use of this graft in a chinchilla model, and found no rejection of the graft, no apparent antigenicity, and no evidence of transmission of diseases. They described it as easy to work with and inexpensive when compared to the other materials that are used in tympanoplasty.35 In 2015, D'Eredita conducted a randomized controlled study that compared the use of SIS to autologous temporalis fascia for type 1 tympanoplasty in children. The study found a similar rate of perforation closure for both materials. Among the advantages identified using SIS is that it was easy to use, suitable for revision cases, and reduced operative time by an average of 7.7 min. Use of SIS further reduced potential concerns with human allograft materials including expense and risk of transmission of infection due to latent viruses or prion disease.36, 37
Endoscopic tympanic membrane repair using SIS
Myringoplasty
SIS myringoplasty can be performed in an onlay or underlay technique. In our group, when a perforation is present in a drum without an ear tube at the start of the procedure, SIS is optimally used with an underlay technique. First, the margin of the perforation is freshened using a Rosen needle and cup forceps. The middle ear is then filled with absorbable gelatin sponge (Gelfoam; Pfizer Inc., New York, NY) soaked in an antibiotic solution. The middle ear should be slightly overfilled to closely oppose the graft material to the undersurface of the drum. The perforation is then measured with a gently-curved pick and a disk of SIS is cut to size, taking care to make the diameter 2–3 mm larger than the diameter of the perforation (Fig. 2). The disk is then tucked through the perforation and unfurled into position using a gently-curved pick, 90° house pick, or Thomassin dissector (Fig. 3). Additional Gelfoam is then placed onto the drum surface and Bacitracin ointment is instilled laterally into the ear canal.
Fig. 2.
Biodesign small intestinal submucosal graft in a square and cut into a disk.
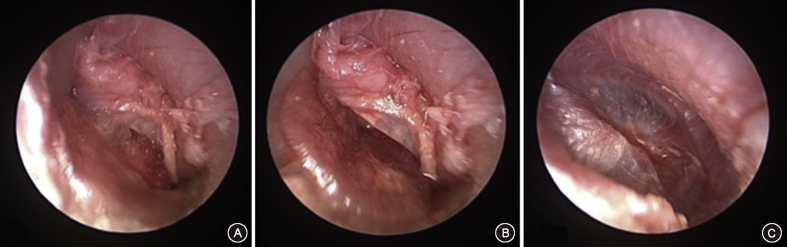
Fig. 3.
Endoscopic Myringoplasty. A: Endoscopic view of tympanic membrane perforation. B: After filling the middle ear with gelfoam through the perforation, the SIS graft is inserted with an underlay myringoplasty technique. C: Final graft position with underlay myringoplasty.
Underlay tympanoplasty
This technique is typically used in our group for perforations which are larger or abutting the tympanic annulus, but still small enough and located favorably on the drum to allow for an underlay of the tympanic membrane remnant. After injecting the canal with 1% lidocaine with 1:100,000 epinephrine, the perforation is freshened circumferentially with a gently curved pick and cup forceps. A tympanomeatal flap is then elevated with a Rosen knife or 20 gauge suction protected with 1 mm × 1 mm square of polyvinyl acetyl sponge (Fig. 4). The tympanic annulus is elevated out of its bony groove and the middle ear is inspected. If the margin of the perforation extends to the malleus, the long process of the malleus is skeletonized from the short process to the umbo. If there is adequate overhang of the drum around the malleus, then the remnant is left attached to the malleus. At this point, the perforation is measured and a sheet of SIS is cut to size, and designed to extend beyond the perforation by at least 1 mm on all sides, and to extend up onto the bony canal wall if the perforation is marginal. A slit or notch is cut into the graft to accommodate the malleus long process. It is critical when measuring the graft and cutting the notch or slit to consider how far the side opposite the notch will extend when the notch is snugged against the manubrium. As this can be maneuvered up to the malleus short process before meeting resistance, care must be taken that the graft will not be too short in its inferior and posterior extent after positioning against the malleus. An advantage of SIS is that if the graft is found to be inadequate in size, there is usually ample material from the 2.5 cm × 2.5 cm sheet to create a second graft of adequate size. An additional benefit is that the uniform thickness and consistency of the graft allows for precise trimming and placement of notches or slits to accommodate the 3-dimensional shape of the drum remnant, ossicles, and canal. After graft placement, the tympanomeatal flap is returned to its anatomic position. The drum can then be covered laterally with a very thin split-thickness skin graft, a layer of gelatin sheeting (Gelfilm, Upjohn, Kalamazoo, MI), a hyaluronic acid sheet (EpiFilm; Xomed-Medtronic, Jacksonville, FL, USA) or Gelfoam. Bacitracin ointment is then placed laterally in the ear canal.
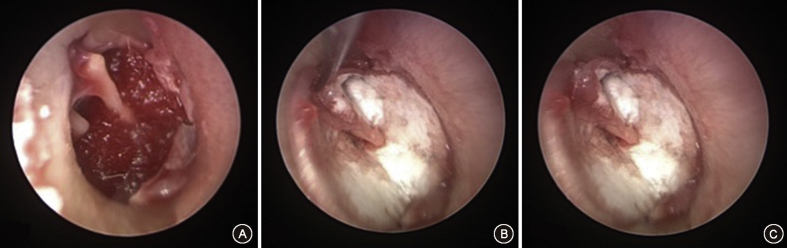
Fig. 4.
Endoscopic underlay tympanoplasty. A: Demonstrates an endoscopic view of the elevated tympanomeatal flap with Gelfoam place into the middle ear cavity. B: Positioning of the SIS graft under the tympanic membrane. The flap is then folded back over the graft. C: The final view of the repaired tympanic membrane.
Lateral graft tympanoplasty
This technique is used by our group for subtotal or total tympanic membrane perforations, or for large marginal perforations where underlay graft is not easily achieved. Using a TEES technique, the entire annulus can typically be seen without bone removal, and therefore the need for canalplasty is reduced or even eliminated in some cases. Again the procedure begins after canal injection with freshening the margin of the perforation if any drum remnant is present. A tympanomeatal flap is elevated and carried to the level of the annulus. The lateral layer of the drum is then separated from the fibrous annulus, leaving it within its bony groove. The incision is carried circumferentially, extending about 2 mm lateral to the annulus anteriorly.
After complete de-epithelialization of the tympanic membrane remnant, the bony canal sulcus is assessed, and if inadequate to support a graft, canalplasty is performed. Using TEES, this is increasingly unnecessary as the graft can be visualized and positioned circumferentially without bone removal. The middle ear is then filled with Gelfoam soaked in antibiotic solution and the defect is measured. Several notches may be cut into the anteroinferior aspect of the graft to support it folding upward against the exposed anterior canal wall and to prevent standing deformities of the graft in this area. These fine and precise notches can be readily created in SIS. Posteriorly, a notch is cut to accommodate the malleus manubrium. Once positioned, several squares of split thickness skin graft are placed laterally to the SIS graft (Fig. 5).
Fig. 5.
Endoscopic lateral graft tympanoplasty. A: Endoscopic view of the de-epithelialized tympanic annulus and medial canal wall, with Gelfoam in the middle ear cavity. B: SIS in lateral graft position, with notch cut to fold around malleus manubrium. C: Final endoscopic view of the SIS lateral graft in place.
A rosebud dressing comprised of strips of Owen's silk (a non-adhesive dressing) folded over a central core of cotton is used to hold the graft layers in place. Additional gelfoam pieces and bacitracin ointment are then placed laterally.
Conclusion
The use of acellular small intestinal submucosa (SIS) for tympanic membrane repair can offer many advantages to other graft materials, particularly in pediatric TEES.
Financial disclosures
None.
Conflicts of interest
None.
Edited by Xin Jin
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Gladstone H.B., Jackler R.K., Varav K. Tympanic membrane wound healing. An overview. Otolaryngol Clin North Am. 1995;28:913–932. [PubMed] [Google Scholar]
- 2.Yong M., Mijovic T., Lea J. Endoscopic ear surgery in Canada: a cross-sectional study. J Otolaryngol Head Neck Surg. 2016;45:4. doi: 10.1186/s40463-016-0117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James A.L. Endoscope or microscope-guided pediatric tympanoplasty? Comparison of grafting technique and outcome. Laryngoscope. 2017 Mar 17;127(11):2659–2664. doi: 10.1002/lary.26568. [DOI] [PubMed] [Google Scholar]
- 4.Cohen M.S., Landegger L.D., Kozin E.D., Lee D.J. Pediatric endoscopic ear surgery in clinical practice: lessons learned and early outcomes. Laryngoscope. 2016;126:732–738. doi: 10.1002/lary.25410. [DOI] [PubMed] [Google Scholar]
- 5.Hunter J.B., Zuniga M.G., Sweeney A.D. Pediatric endoscopic cholesteatoma surgery. Otolaryngol Head Neck Surg. 2016;154:1121–1127. doi: 10.1177/0194599816631941. [DOI] [PubMed] [Google Scholar]
- 6.Uyar Y., Keleş B., Koç S., Oztürk K., Arbağ H. Tympanoplasty in pediatric patients. Int J Pediatr Otorhinolaryngol. 2006;70:1805–1809. doi: 10.1016/j.ijporl.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Sarkar S., Roychoudhury A., Roychaudhuri B.K. Tympanoplasty in children. Eur Arch Otorhinolaryngol. 2009;266:627–633. doi: 10.1007/s00405-008-0908-1. [DOI] [PubMed] [Google Scholar]
- 8.Palva T., Ramsay H. Myringoplasty and tympanoplasty–results related to training and experience. Clin Otolaryngol Allied Sci. 1995;20:329–335. doi: 10.1111/j.1365-2273.1995.tb00053.x. [DOI] [PubMed] [Google Scholar]
- 9.Mer S.B., Derbyshire A.J., Brushenko A., Pontarelli D.A. Fiberoptic endotoscopes for examining the middle ear. Arch Otolaryngol. 1967;85:387–393. doi: 10.1001/archotol.1967.00760040389009. [DOI] [PubMed] [Google Scholar]
- 10.Bottrill I.D., Poe D.S. Endoscope-assisted ear surgery. Am J Otol. 1995;16:158–163. [PubMed] [Google Scholar]
- 11.Harugop A.S., Mudhol R.S., Godhi R.A. A comparative study of endoscope assisted myringoplasty and micrsoscope assisted myringoplasty. Indian J Otolaryngol Head Neck Surg. 2008;60:298–302. doi: 10.1007/s12070-008-0099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lade H., Choudhary S.R., Vashishth A. Endoscopic vs microscopic myringoplasty: a different perspective. Eur Arch Otorhinolaryngol. 2014;271:1897–1902. doi: 10.1007/s00405-013-2673-z. [DOI] [PubMed] [Google Scholar]
- 13.Isaacson G. Endoscopic anatomy of the pediatric middle ear. Otolaryngol Head Neck Surg. 2014;150:6–15. doi: 10.1177/0194599813509589. [DOI] [PubMed] [Google Scholar]
- 14.Usami S., Iijima N., Fujita S., Takumi Y. Endoscopic-assisted myringoplasty. ORL J Otorhinolaryngol Relat Spec. 2001;63:287–290. doi: 10.1159/000055759. [DOI] [PubMed] [Google Scholar]
- 15.Karhuketo T.S., Ilomäki J.H., Puhakka H.J. Tympanoscope-assisted myringoplasty. ORL J Otorhinolaryngol Relat Spec. 2001;63:353–357. doi: 10.1159/000055773. discussion 358. [DOI] [PubMed] [Google Scholar]
- 16.Yadav S.P., Aggarwal N., Julaha M., Goel A. Endoscope-assisted myringoplasty. Singapore Med J. 2009;50:510–512. [PubMed] [Google Scholar]
- 17.Mohindra S., Panda N.K. Ear surgery without microscope; is it possible. Indian J Otolaryngol Head Neck Surg. 2010;62:138–141. doi: 10.1007/s12070-010-0033-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheehy J.L., Anderson R.G. Myringoplasty. A review of 472 cases. Ann Otol Rhinol Laryngol. 1980;89:331–334. doi: 10.1177/000348948008900407. [DOI] [PubMed] [Google Scholar]
- 19.Mallo M. Formation of the middle ear: recent progress on the developmental and molecular mechanisms. Dev Biol. 2001;231:410–419. doi: 10.1006/dbio.2001.0154. [DOI] [PubMed] [Google Scholar]
- 20.Berthold E. Uebermyringoplastik. Wien Med Blätter. 1878;26:627–639. [Google Scholar]
- 21.Ely E.T. Skin-grafting in chronic suppuration of the middle ear. Arch Otolaryngol. 1880;9:343–345. [Google Scholar]
- 22.Wullstein H.L. Functional operations in the middle ear with split-thickness skin graft. Arch Othorhinolaryngol. 1953;161:422–435. [Google Scholar]
- 23.Zollner F. The principles of plastic surgery of the sound-conducting apparatus. J Laryngol Otol. 1955;69:637–652. [PubMed] [Google Scholar]
- 24.House H., House W., Tabb H., Wullstein H., Zollner F. Panel on myringoplasty methods. Arch Otolaryngol. 1963;78:296–304. doi: 10.1001/archotol.1963.00750020306011. [DOI] [PubMed] [Google Scholar]
- 25.Örtegren U. Myringoplasty. Acta Otolaryngol Suppl. 1964;139:1. [Google Scholar]
- 26.Jansen C. Cartilage tympanoplasty. Laryngoscope. 1963;73:1288–1301. doi: 10.1288/00005537-196310000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Ringenberg J.C. Fat graft tympanoplasty. Laryngoscope. 1962;72:188–192. doi: 10.1288/00005537-196202000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Awad O.G., Hamid K.A. Endoscopic type 1 tympanoplasty in pediatric patients using tragal cartilage. JAMA Otolaryngol Head Neck Surg. 2015;141:532–538. doi: 10.1001/jamaoto.2015.0601. [DOI] [PubMed] [Google Scholar]
- 29.Ayache S. Cartilaginous myringoplasty: the endoscopic transcanal procedure. Eur Arch Otorhinolaryngol. 2013;270:853–860. doi: 10.1007/s00405-012-2056-x. [DOI] [PubMed] [Google Scholar]
- 30.Youssef A.M. Use of acellular human dermal allograft in tympanoplasty. Laryngoscope. 1999;109:1832–1833. doi: 10.1097/00005537-199911000-00020. [DOI] [PubMed] [Google Scholar]
- 31.McFeely W.J., Bojrab D.I., Kartush J.M. Tympanic membrane perforation repair using AlloDerm. Otolaryngol Head Neck Surg. 2000;123:17–21. doi: 10.1067/mhn.2000.105920. [DOI] [PubMed] [Google Scholar]
- 32.Laidlaw D.W., Costantino P.D., Govindaraj S., Hiltzik D.H., Catalano P.J. Tympanic membrane repair with a dermal allograft. Laryngoscope. 2001;111:702–707. doi: 10.1097/00005537-200104000-00025. [DOI] [PubMed] [Google Scholar]
- 33.Saadat D., Ng M., Vadapalli S., Sinha U.K. Office myringoplasty with alloderm. Laryngoscope. 2001;111:181–184. doi: 10.1097/00005537-200101000-00033. [DOI] [PubMed] [Google Scholar]
- 34.Badylak S.F. Seminars in immunology special issue foreword. Semin Immunol. 2017;29:1. doi: 10.1016/j.smim.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Spiegel J.H., Kessler J.L. Tympanic membrane perforation repair with acellular porcine submucosa. Otol Neurotol. 2005;26:563–566. doi: 10.1097/01.mao.0000169636.63440.4e. [DOI] [PubMed] [Google Scholar]
- 36.D′Eredità R. Porcine small intestinal submucosa (SIS) myringoplasty in children: a randomized controlled study. Int J Pediatr Otorhinolaryngol. 2015;79:1085–1089. doi: 10.1016/j.ijporl.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 37.Shi L., Ronfard V. Biochemical and biomechanical characterization of porcine small intestinal submucosa (SIS): a mini review. Int J Burns Trauma. 2013;3:173–179. [PMC free article] [PubMed] [Google Scholar]