Abstract
Ant colonies self-organize to solve complex problems despite the simplicity of an individual ant’s brain. Pavement ant Tetramorium caespitum colonies must solve the problem of defending the territory that they patrol in search of energetically rich forage. When members of 2 colonies randomly interact at the territory boundary a decision to fight occurs when: 1) there is a mismatch in nestmate recognition cues and 2) each ant has a recent history of high interaction rates with nestmate ants. Instead of fighting, some ants will decide to recruit more workers from the nest to the fighting location, and in this way a positive feedback mediates the development of colony wide wars. In ants, the monoamines serotonin (5-HT) and octopamine (OA) modulate many behaviors associated with colony organization and in particular behaviors associated with nestmate recognition and aggression. In this article, we develop and explore an agent-based model that conceptualizes how individual changes in brain concentrations of 5-HT and OA, paired with a simple threshold-based decision rule, can lead to the development of colony wide warfare. Model simulations do lead to the development of warfare with 91% of ants fighting at the end of 1 h. When conducting a sensitivity analysis, we determined that uncertainty in monoamine concentration signal decay influences the behavior of the model more than uncertainty in the decision-making rule or density. We conclude that pavement ant behavior is consistent with the detection of interaction rate through a single timed interval rather than integration of multiple interactions.
Keywords: agent-based model, aggressive behavior, ants, decision making, monoamines, octopamine, serotonin
Despite their miniaturized and simple brains, ants are able to solve complex problems when organized at the colony level. Ant colonies are regulated as non-hierarchical distributed systems (Camazine et al. 2001; Collignon and Detrain 2009; Gordon 2010; Robinson et al. 2014). Without an authority to direct the actions of workers, it is necessary for each individual to assess local information cues, integrate information in those cues, compare them to an inherent set of rules, and make decisions to change their behavior (Greene and Gordon 2003; Couzin et al. 2005; Arganda et al. 2012). Colony behavior changes collectively because of the cascade of individual decisions made by workers. Examples of collective decision making include choosing a new nest, allocating the proper number of workers to perform jobs to support colony homeostasis, forming foraging trails, and even tending gardens (Gordon 1986; Frederickson et al. 2005; Collignon and Detrain 2009; Sumpter and Pratt 2009). Thus, we witness the aggregation of simple deterministic components leading to a nuanced, variable system that natural selection can act on; the superorganism of the ant colony (Detrain and Deneubourg 2006; Gordon 2010).
A key component in the collective decision making of ants is the effect of interaction rate on behavior. Interaction rate is often used by insects as a proximate measurement of local density. It has been shown that in red harvester ants Pogonomyrmex barbatus interaction rate is associated with colonial task allocation in addition to the availability and dedication of reserve workers to foraging (Pinter-Wollman et al. 2013). In Temnothorax albipennis, interaction rate is shown to modulate the process of nest emigration (Pratt 2004). Interaction rate could be measured by a spectrum of methods with 2 extremes: 1) integration of all encounters in a given time or 2) response based only on the interval before first encounter (Pratt 2004). Indeed, there are examples of both occurring in insects. Integration over multiple interactions is observed during locust Schistocerca gregaria aggregation while nest construction in Polybia occidentalis is a result of single interval measurement (Pratt 2004).
Pavement ant Tetramorium caespitum workers perform random walks to search colony territory for foods high in sugar and fat content (Collignon and Detrain 2009; Countryman et al. 2015). In order to secure territory, pavement ant workers organize wars against neighboring colonies (Greene MJ, unpublished data; Plowes 2008). This organization occurs after individuals from neighboring colonies meet during the course of a random walk, touch antennae to the other’s cuticle, and assess nestmate recognition cues coded in cuticular hydrocarbon profiles (Greene MJ, unpublished data). A decision rule to fight a non-nestmate ant is satisfied when 2 conditions are met: 1) there is a mismatch in the information coded in the cues (Greene MJ, unpublished data; Martin and Drijfhout 2009) and 2) the ant had a recent history of interactions with nestmate ants (Greene MJ, unpublished data). The probability that a pavement ant worker will fight a non-nestmate increases with the density of nestmate ants as assessed by the interaction rate (Greene MJ, unpublished data). Fighting among conspecifics involves a ritualized pushing of mass between dyads that are locked together by mandibles and grasping appendages (Plowes 2008). The fights last for many hours during which few, if any, ants die (Plowes 2008). However, the dedication of the vast majority of available foragers to this behavior constitutes an opportunity cost as foraging efforts cease during the course of the war (Plowes 2008). Some workers do not fight, but instead recruit more nestmates to the war in a positive feedback loop (Plowes 2008). Collectively, the decisions to fight by many workers aggregated through feedback in recruitment lead to wars between neighboring colonies.
The monoamines serotonin (5-HT) and octopamine (OA) modulate many behaviors important to ant colony function, including: colony formation, reproductive dominance, division of labor, behavioral development, trophallaxis, predatory aggression, and nestmate recognition (Boulay et al. 2000; Wada-Katsumata et al. 2011; Aonuma and Watanabe 2012; Kamhi and Traniello 2013; Szczuka et al. 2013; Kamhi et al. 2015; Koyama et al. 2015). In pavement ants, brain concentrations of 5-HT and OA change according to social context (Greene MJ, unpublished data). Brain concentration of both 5-HT and OA increase after interactions with nestmate ants and rapidly return to baseline levels at or before 3 min. This presents a potential physiological mechanism for the fighting decision rule in pavements ants; we hypothesis that high 5-HT and OA brain concentrations correlate to recent interactions with nestmates and may prime the ant to fight (Greene MJ, unpublished data). A mismatch in nestmate recognition cues preceded by elevated brain concentrations of 5-HT and OA thus satisfies the proposed decision rule to initiate a fight.
To test the feasibility of this mechanism, we leverage the bottom-up approach of agent-based modelling. This modelling system was developed as a way to explore how system level characteristics emerge from simple, rule-based and stochastically interacting agents (Bankes 2002; Hare and Deadman 2004; Grimm et al. 2006; Holcombe et al. 2012). Use of the bottom-up approach took off rapidly in the field of eusocial insect behavior (Sumpter 2006) and has been used to explore the self-organization of the complex behaviors of ants including: aggregation (Morale et al. 2005), nest choice (Pratt et al. 2005), foraging (Robinson et al. 2008), task allocation (Momen 2013), and intraspecific battles (Martelloni et al. 2015). This modelling method integrates 2 key sources of variation that are often ignored in analytic analyses: distributed spatial structures and individual behavioral heterogeneity (Parunak et al. 1998).
Here, we build an agent-based model to conceptualize how changes in individual monoamine brain concentrations and the application of simple decision rules lead to the development of the observed colony behavior of fighting in pavement ant wars. In order to understand the organization of social insects, we must undertake an exploration of both the physiological mechanisms and contextual stimuli behind individual decision making, and how these decisions lead to system level patterns of behavioral organization in colonies.
Materials and Methods
Collection of ants
Pavement ants were collected along foraging trails in urban and suburban areas of Denver and Aurora, Colorado, United States. Ants were collected by aspiration on baited foraging trails. Collected ants were brought to the laboratory for experimental manipulations that were conducted between 24 and 36 h after collection. Ants were temporarily housed at room temperature (25°C) in plastic containers and allowed to drink ad libitum from glass tubes filled with water and plugged with cotton.
Worker density trials
In order to study the effects of increasing interaction rate on brain levels of biogenic amines, ants were placed in petri dishes with worker densities of 5, 20, or 100 ants per petri dish (area = 56.5 cm2). This allowed us to infer if ants detect interaction rate through integration of multiple interactions, or if they measure only a single time interval. There were 11 replicates of each density created using ants from 6 separate colonies. Petri dishes were all treated with the non-stick coating Insect-A-Slip (BioQuip, Rancho Dominguez, CA, USA) to prevent ants from escaping. Ants were allowed to interact for 10 min after which individuals were removed and their brains were dissected for measurement of brain monoamine levels.
Sample preparation and dissection
Brain removal and preparation was done after previously published methods (Bubak et al. 2013). Briefly, ants were rapidly decapitated under a dissection microscope immediately after behavioral trials using micro-scissors. A small medial–lateral incision was made directly behind the mandibles avoiding the disruption of brain tissue. Exposed brains were then removed with tweezers and submerged in 60 µL of ice-cold acetate buffer containing the internal standard, alpha-methyl DA. Samples were frozen immediately on dry ice and stored at −80°C until monoamine quantification. Each sample contained 2 brains with each dissection time averaging less than 1 min.
Quantification of monoamines
We quantified brain concentrations of the monoamines, OA, and 5-HT, using a high-performance liquid chromatography with electrochemical detection methods as described in Bubak et al. (2013) with slight modifications. Each sample was thawed, briefly sonicated, rapidly refrozen on dry ice, and allowed to thaw again before being centrifuged at 17,200 rpms. The supernatant (60 µL) was extracted from the samples, 50 µL was injected into a Waters Alliance e2695 separations module, and a C18 4 µm NOVA-PAK radial compression column (Waters Associates, Inc. Milford, MA, USA) was used for monoamine separation. The initial mobile phase (pH 4.1) was prepared using 8.6 g sodium acetate, 250 mg EDTA, 14 g citric acid, 130 mg octylsulfonic acid, and 160 mL methanol in 1 L of distilled water (chemicals were purchased through Sigma-Aldridge, St. Louis, MO, USA). Electrochemical detection of amines was accomplished using an LC 4 potentiostat and glassy carbon electrode (Bioanalytical Systems, West Lafayette, IN, USA) set at a sensitivity of 0.5 nA/V with an applied potential of + 1.0 V vs. an Ag/AgCl reference electrode. After dissolving the tissue pellet in 100 µL of 0.4 N NaOH, protein content was analyzed using the Bradford method (1976). A CSW32 data program (DataApex Ltd., Prague, Czech Republic) was used to determine OA, and 5-HT concentrations using peak heights calculated from standards. Corrections were made for injection vs. preparation volumes and monoamine concentrations were normalized by sample protein content (pg amine/µg protein).
Agent-based model
This model description follows the Overview, Design concepts, Details (ODD) protocol for describing individual- and agent-based models (Grimm et al. 2006, 2010). The model was coded in Python 2.7.6 and is available in the SI.
Purpose
The purpose of this model is to determine whether the proposed individual decision rule for fighting, based on changes in brain concentrations of 5-HT and OA, can consistently lead to the development of wars between neighboring colonies, as observed in pavement ants. Additionally, we performed a sensitivity analysis to determine which parameters, if any, stand out as key forcing parameters for this behavior.
Entities, state variables, and scales
This model is built in 2 scales: individual ants and the arena. Individuals are described by the following state variables: colony, position (x,y), 5-HT concentration, OA concentration, 5-HT decay, and OA decay rates where decay rates represent time for the respective monoamines to return to baseline values, and decision rule. The arena is described by the following state variables: width, length, maximum number of ants per pixel, the identities and number of ants, the identities and number of fighting ants (Table 1). The arena is a discrete lattice with each pixel corresponding to the maximum speed at which an ant can traverse during the simulated time step (∼ 4.5 mm on a side).
Table 1.
Entities, processes, and parameters of the agent-based model, with default values
| Description | Default |
|---|---|
| Ant | |
| Colony identity | 1 or 2 |
| Position | Uniformly distributed on left or right column |
| 5-HT concentration | 0 |
| OA concentration | 0 |
| Decision rule (rate parameter) | 3 |
| Willing to fight | False |
| Is fighting | False |
| Ant processes | |
| Movement | Uniformly distributed movement across 8 adjacent pixels. |
| Nestmate interaction | Set 5-HT to 1; set OA to 1 |
| Non-nestmate interaction | Set OA to 1, if both ants are willing to fight set Is fighting to True |
| Check willingness | Compare (5-HT, OA) coordinate to decision rule. If (5-HT, OA) greater than decision slope, then set Willing to fight to True. |
| 5-HT decay rate | −1/540 per time step (equivalent to full decay in 3 min) |
| OA decay rate | −1/540 per time step (equivalent to full decay in 3 min) |
| Arena | |
| Time steps | 10,800 (equivalent to 1 h) |
| Width | 70 |
| Length | 105 |
| Pixel capacity | 2 |
| Number of ants | 100 (split evenly between colony 1 and 2) |
| Arena processes | |
| Move ants | Move all ants |
| Check pixel capacity | Return true is pixel is at or above the capacity set by pixel capacity |
| Interactions | Checks all pixels for those with occupancy >1. Each ant in those pixels will execute the appropriate interactions |
| Proportion fighting | Returns the proportion of total ants fighting at time t |
With the exception of the sensitivity analysis, these values are used in model initialization. During the sensitivity analysis, the targeted parameters are pulled from a uniform distribution between ± 50% of the default value before each simulation.
Process overview and scheduling
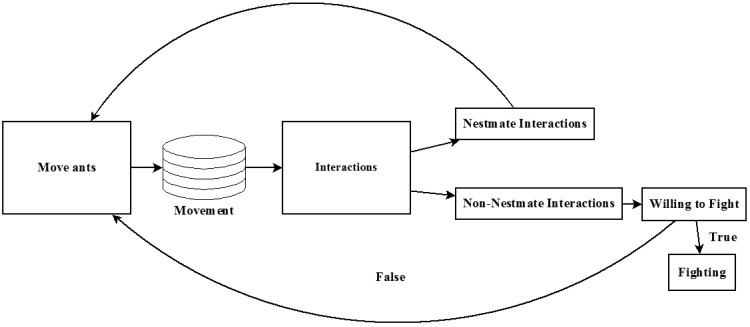
This model walks through discrete time at thirtieths of a second for a total of 1 h or 10,800 time steps. During each time step, 3 processes take place: movement, interactions, and fighting (Figure 1). In the movement process, each ant moves into 1 of the 8 adjacent pixels with equal probability, provided the pixel is not at capacity. Also during movement, each ant’s monoamines decay representing the lack of interaction during movement. In the interaction phase, the arena checks each pixel and selects those with 2 or more ants inside. The ants in each pixel update their monoamine levels simultaneously. If interacting with nestmates, OA, and 5-HT spike to their physiological maximal concentration. If interacting with non-nestmates, just OA spikes because of detection of non-nestmate cuticular hydrocarbons (Greene MJ, unpublished data). Because an ant will not fight unless primed over threshold levels of OA and 5-HT, non-nestmate cuticular hydrocarbon cues increase OA. Finally, during the fighting process, each pixel that had interacting non-nestmate ants in the previous process gets updated. Each ant in the pixel uses its associated decision rule to discern its willingness to fight. If both ants are willing to fight, they initiate a fight and will no longer update for the rest of the simulation. Otherwise, they move as normal in the next time step.
Figure 1.
Process scheduling in agent-based model of pavement ant fighting. During each time step all non-fighting ants move, check for interactions, and then either begin fighting or prepare to move in the next time step.
Design concepts
Basic principles: This model is based on the principle of stochastic interactions between pavement ant foragers during the course of a random walk. Using the results from our density trials on brain concentrations of the monoamines OA and 5-HT, we propose a new decision rule for individual ants consistent with single interval measurement. Here, we assume that levels of OA and 5-HT increase to the physiological maximum observed in samples taken from interacting ants, after 1 interaction with a nestmate or non-nestmate ant. Then, brain levels of OA and 5-HT decay with time. The higher the rate of interaction with other ants, the lower the time interval between interactions, and therefore less time for decay rate to affect the brain state.
Emergence: This model will determine whether the proposed individual decision rule can give rise to the emergence of the observed system level behavior of warfare between neighboring colonies of pavement ants.
Sensing: The ants in this model make decisions based on their position, colony, and brain concentrations of 5-HT and OA, and their willingness to fight. They also perfectly detect which colony an ant they interact with is from and whether or not an adjacent pixel is at maximum capacity.
Interaction: As specified in the process overview, ants interact with all other ants that share their pixel. If the pair is nestmates, then both ants experience a spike in brain concentrations of 5-HT and OA. If ants are from different colonies, they both experience a spike in brain concentrations of OA and then check their willingness to fight. If both ants are willing to fight, they do so and do not update for the rest of the model.
Stochasticity: Stochasticity is utilized for the process of ant movement. Ants can move to any of the 8 adjacent pixels with uniform probability provided the pixel is not at capacity (arena parameters Table 1).
Observation: The output from the model is the proportion of ants fighting over the course of the simulation. The proportion of ants fighting at the end of the time series is recorded and compared for the analyses in this article.
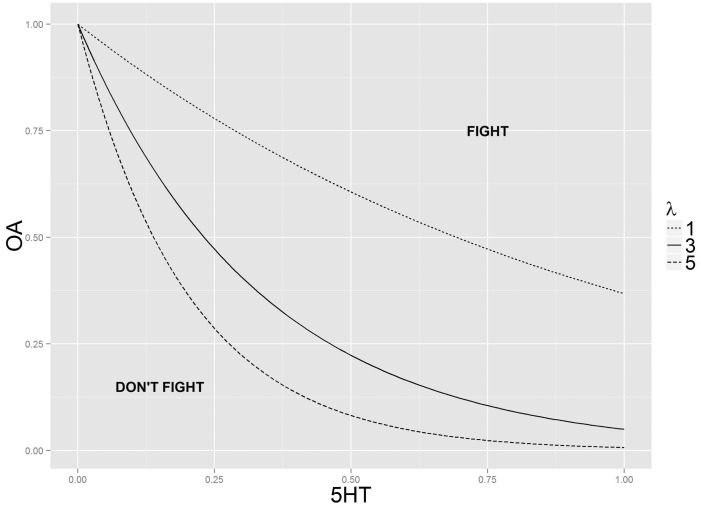
Initialization: The model is initialized as a 70 × 105 pixel lattice that corresponds to the 305 mm × 457 mm arena used in other laboratory experiments; ants from colony 1 are distributed randomly along the left edge of the arena, and ants from colony 2 on the right edge. Pixel capacity is initialized to 2 ants per pixel, which corresponds with the observed organization of aggressive dyads in the war (Plowes 2008) Ants are generated with OA and 5-HT brain concentrations set at zero (Table 1). Unless otherwise stated, 50 ants are generated from each colony with a decision rule with rate parameter λ = 3 (Figure 2) and a linear full decay of monoamine concentration in 540 time steps corresponding to the 3 min return to baseline concentrations observed in unpublished data from this laboratory.
Figure 2.
Physiological decision map. If an ant has a brain state that places it above some decision threshold, it will decide to fight. Lines represent the decision rule used in our agent-based model OA = exp (−λ*5-HT), where λ is the rate parameter of interest.
Submodels
Monoamine signal decay: 5-HT and OA brain concentrations are modeled to return linearly to baseline concentrations within 3 min. This is based off of the work done by Greene MJ (unpublished data), which presents 3 min as the finest time resolution available currently. For this reason, we model decay completion at 3 min as the most conservative estimate in keeping with observed data.
Fighting decision rule: The decision rule for fighting (Figure 2) is modeled on the unit square of 5-HT and OA. Concentration axes are unit-less and bounded between 0 and 1, where 0 represents basal levels associated with isolated ants and 1 represents the physiological maximum observed in samples taken from interacting ants. The functional form of the decision rule is an exponential decay curve: OA = exp (−λ*5-HT). This equational form was in keeping with the data obtained in our preliminary research. Specifically, pharmacologically induced increases in brain 5-HT and OA (resulting in coordinates above the unit square represented here) in isolated ants led to fighting behavior upon introduction of a non-nestmate, but increases in OA concentrations induced by exposure to non-nestmate cuticular hydrocarbons on glass beads (resulting in coordinates on the y axis of the unit square) where insufficient for development of fighting.
Sensitivity analysis
To better understand the key parameters underlying this model, a sensitivity analysis was performed over the 3 explanatory parameters: worker density, decision-making rule, and monoamine concentration signal decay rate. To measure the effects of uncertainty in these parameters, we compared the proportion of ants fighting at the end of the model simulation runs under 5-parameter conditions: 1) no uncertainty, 2) uncertainty in all parameters, 3) uncertainty in monoamine concentration signal decay rate, 4) uncertainty in density parameter, and 5) uncertainty in decision-making rule. Uncertainty was introduced by allowing the uncertain parameters to be drawn from a uniform distribution before each run between 50 % and 150 % of the parameter estimates in the model free of uncertainty (Table 1).
Results
Worker density and monoamines
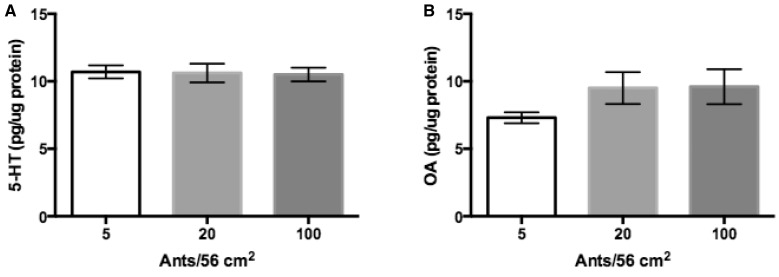
Concentrations of brain 5-HT recorded between density groups ranged from 4.2 and 14.9 pg/µg protein with a mean ± standard error of the mean (SEM) of 10.5 ± 2.1(n = 38). Concentrations of OA ranged between 3.3 and 17.1 pg/µg protein with a mean ± SEM of 8.2 ± 3.8 (n = 27). Worker density did not account for the observed variability in monoamines with ANOVA failing to support significant changes in variance: (5-HT ANOVA, P = 0.97 and OA ANOVA, P = 0.23; Figure 3).
Figure 3.
Increasing density of ants is not sufficient to explain variation in brain levels of monoamines in laboratory density test. Means with SEM. (A) 5-HT: no treatment is significantly different, ANOVA P = 0.97. N5 = 14, N20 = 13, N100 = 11. (B) Octopamine: no treatment is significantly different, ANOVA P = 0.23. N5 = 9, N20 = 9, and N100 = 9.
Agent-based model
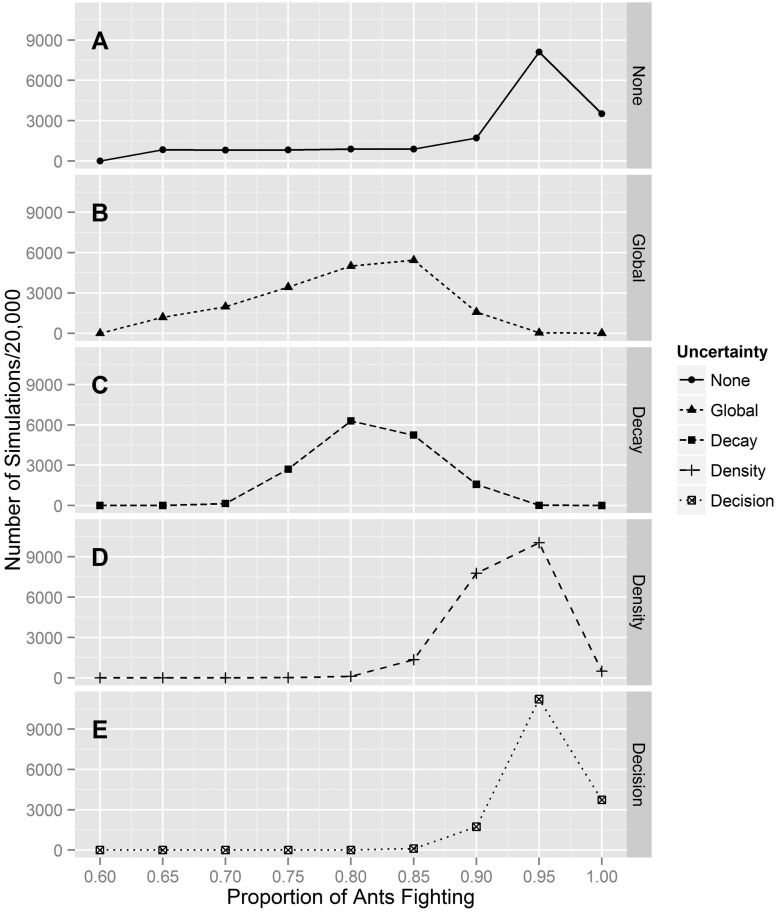
Simulated agents self-organized into wars, with a majority of the available workers engaged in fighting (mean = 0.907; 90% credible interval = 0.70, 1.0; Figure 4A). When uncertainty was added to the 3 key parameters of the model the distribution of proportion fighting at the end of each simulation shifted. (mean = 0.794; 90% credible interval = 0.65, 0.90; Figure 4B). The contributions to this shift were decomposed into the contributions of uncertainty in the parameter estimates of the rates of monoamine signal decay (mean = 0.817; 90% credible interval = 0.75, 0.90; Figure 4C), density (mean = 0.924; 90% credible interval = 0.85, 0.95; Figure 4D), and decision rule (mean = 0.955; 90% credible interval = 0.90, 1.0; Figure 4E). Although uncertainty introduced to the estimates for density and decision making only moderately shifted the distribution, uncertainty in the monoamine signal decay rate has a profound effect on the spread of the distribution.
Figure 4.
Sensitivity analysis on agent-based model. (A) No uncertainty, (B) uncertainty in all parameters, (C) uncertainty in monoamine concentrations decay rate, (D) uncertainty in density parameter, and (E) Uncertainty in decision-making rule. It should be noted that uncertainty in concentration decay rate is the greatest contributor to the distributional shift associated with global uncertainty. This indicates that signal decay is the most sensitive parameter in the model.
Discussion
Pavement ant colonies self-organize to accomplish complex and nuanced tasks despite the relatively simple brains of the individual ants. The aggregation of simple deterministic rules in response to external stimuli can lead to subtle and wide-ranging societal behavioral changes at the colony level. Here, we use a model to demonstrate how changes in brain concentrations of the monoamines 5-HT and OA after interactions with nestmate ants could cause a brain state that leads to the engagement of fighting behavior of conspecific non-nestmate ants.
In particular, we examined the behaviors associated with nestmate recognition and the ritualized, aggressive exclusion of conspecific non-nestmates at the boundary between territories. Although it is known that pavement ants only engage in ritualized combat when they had sufficiently interacted with other nestmates (Greene MJ, unpublished data), it was assumed that the rate or abundance of interactions with nestmates over time was being integrated by the worker ants (Greene MJ, unpublished data). However, increased density and, therefore increased interaction rate, did not result in changes in brain monoamine concentrations (Figure 3; Greene MJ, unpublished data). Based on this result, we hypothesize that the ant’s decision making observes a hysteretic effect of their most recent interactions based on the return of brain monoamines to baseline concentrations and thus determine interaction rate through a single interval measurement instead of integration over multiple interactions.
This conceptualization of decision making via a “monoamine clock” timed by the rate of return of monoamines to baseline concentrations reconciles the apparent requirements of integrating complex information with the relatively simple organization of an individual ant brain. A key implication of this finding is that the density sensing apparatus of the pavement ant worker observes the mathematical forgetfulness or memoryless property (Leemis and McQueston 2008), that is, there is no difference between an ants 1st interaction and its 20th, the probability of having concentration levels above the decision threshold at some time (t) after each interaction is the same. This model, consistent with the proposed single interval measurement, demonstrates a way in which interaction rate would not lead to differences in monoamine concentrations but still affect decision making in ants. It further implies that the decision-making processes attributed to rate of interaction is more accurately attributed to the amount of time a worker spends primed for a decision after an increase in monoamine concentrations.
The proposed decision mechanism is further supported by our agent-based model as uncertainty in density had minimal effects on the system behavior compared with changes due to uncertainty in the rate of monoamine concentration decay. In the terms of a “monoamine clock,” this would indicate that the rate at which the clock ticks (decay rate) is more important than how often the clock is reset (interaction rate). To date, the dynamics for monoamine concentrations decay in the ant brain are unknown, and therefore estimates of this key parameter for decision making are by necessity highly uncertain.
In order to advance our understanding of how individual, simple, and physiologically driven decision making can lead to the development of complex, nuanced, and colonial responses, a better understanding of both the timescale and function of this “monoamine clock” must be established.
Future directions
Due to the small size of ant brains, HPLC analysis requires an aggregation of 2 brains per a sample. This course grain data is unable to resolve enough information to fully propose a mechanistic explanation of aggressive behavior in ants. To that end future research should look at specific aminergic pathways within the ant brain and their dynamics under the different social context that pavement ant workers experience in their life.
Having at length explored the necessary conditions for the engagement of aggressive conflicts between neighboring pavement ant colonies, we look forward to studying the processes that sustain and ultimately conclude this behavior. Although this article explores the interactions of ants along the territorial border, it is not well known why some ants decide to engage in active recruitment of sisters by returning to the nest instead of fighting. This critical positive feedback is a necessary component of the observed escalation in wars starting from dozens of ants to many thousand. Additionally, it has been suggested that in societal conflicts group size performs a similar function as body size does in conflicts between solitary organisms. In this way, recruitment of a colonies workforce is an important indicator of strength, and recruitment rate becomes a reliable indicator of victory in an engagement between colonies.
Pavement ant wars last over 10 h but then disengage in under 30 min. The source of this synchronized withdrawal is not well understood. Preliminary data demonstrate that dyads of non-nestmate ants engaged in ritualized fighting experience a marked increase in dopamine both 3 min and 2 h after the start of the fight (Greene MJ, unpublished data). By extending the logic from this article, we propose that the length of fighting behavior is modulated by the rate at which this elevated concentration decreases to basal levels. We suspect that a superthreshold level of dopamine sustains fighting and the drop to subthreshold levels will signal a cessation of hostilities.
Finally, although this behavior has been attributed to territory defense there are at this time no studies that empirically study the cost and benefit of this behavior in the terms of colony fitness. Colonies will often return to sites of previous wars within 24 h, and a new war will begin in approximately the same location with no clearly demarked change in territory size. This pattern can last for 3–5 days, and it is not clear what benefit this prolonged dedication of colony resources achieves.
Supplementary Material
Acknowledgments
The authors thank Michael Wunder for statistical advice. We also thank Jennifer Larmore, William Schumann, Harper Jocque, and Allison Pierce for their insights in reviewing early drafts of this manuscript.
Funding
The research was supported, in part, by a Center for Brain and Behavioral Research (CBBRe) Pilot grant to K. J. R., University of South Dakota, and NSF grant # IOS 1256898 to J. G. S.
Supplementary Material
Supplementary material can be found at http://www.cz.oxfordjournals.org/.
References
- Aonuma H, Watanabe T, 2012. Octopaminergic system in the brain controls aggressive motivation in the ant Formica japonica. Acta Biologica Hungarica 63(Suppl 2):63–68. [DOI] [PubMed] [Google Scholar]
- Arganda S, Perez-Escudero A, de Polavieja GG, 2012. A common rule for decision making in animal collectives across species. Proc Natl Acad Sci U.S.A. 109:20508–20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankes SC, 2002. Agent-based modeling: a revolution? Proc Natl Acad Sci USA 99(Suppl 3):7199–7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulay R, Soroker V, Godzinska EJ, Hefetz A, Lenoir A, 2000. Octopamine reverses the isolation-induced increase in trophallaxis in the carpenter ant Camponotus fellah. J Exp Biol 203:513–520. [DOI] [PubMed] [Google Scholar]
- Bubak AN, Swallow JG, Renner KJ, 2013. Whole brain monoamine detection and manipulation in a stalk-eyed fly. J Neurosci Methods 219(1):124–130. [DOI] [PubMed] [Google Scholar]
- Camazine S, Deneubourg JL, Franks NR, Sneyd J, Theraulaz G et al. , 2001. Self-organization in Biological Systems. Princeton: Princeton University Press. [Google Scholar]
- Collignon B, Detrain C, 2009. Distributed leadership and adaptive decision-making in the ant Tetramorium caespitum. Proc Biol Sci 277:1267–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Countryman SM, Stumpe MS, Crow SP, Adler FR, Greene MJ et al. , 2015. Collective search by ants in microgravity. Frontiers in Ecology and Evolution. [cited 2015 March 26] Available from: http://dx.doi.org/10.3389/fevo.2015.00025. [Google Scholar]
- Couzin ID, Krause J, Franks NR, Levin SA, 2005. Effective leadership and decision–making in animal groups on the move. Nature 433:513–516. [DOI] [PubMed] [Google Scholar]
- Detrain C, Deneubourg JL, 2006. Self-organized structures in a superorganism: do ants “behave” like molecules? Phys Life Rev 3:162–187. [Google Scholar]
- Frederickson M, Greene MJ, Gordon D, 2005. Devil’s gardens; bedeviled by ants. Nature 437:495–496. [DOI] [PubMed] [Google Scholar]
- Gordon DM, 1986. The dynamics of the daily round of the harvester ant colony Pogonomyrmex barbatus. Anim Behav 5:1402–1419. [Google Scholar]
- Gordon DM, 2010. Ant Encounters: Interaction Networks and Colony Behavior. Princeton: Princeton University Press. [Google Scholar]
- Grimm V, Berger U, Bastiansen F, Eliassen S, Ginot V et al. , 2006. A standard protocol for describing individual-based and agent-based models. Ecol mod 198:115–126. [Google Scholar]
- Grimm V, Berger U, DeAngelis DL, Polhill JG, Giske J et al. , 2010. The ODD protocol: a review and first update. Ecol mod 221:2760–2768. [Google Scholar]
- Greene MJ, Gordon DM. 2003. Cuticular hydrocarbons inform task decisions. Nature 423:32. [DOI] [PubMed] [Google Scholar]
- Gronenberg W, Hölldobler B, 1999. Morphologic representation of visual and antennal information in the ant brain. J Comp Neurol 412:229–240. [DOI] [PubMed] [Google Scholar]
- Gronenberg W, 2008. Structure and function of ant (Hymenoptera: Formicidae) brains: strength in numbers. Myrmecol News 11:25–36. [Google Scholar]
- Hare M, Deadman P, 2004. Further towards a taxonomy of agent-based simulation models in environmental management. Math Comp Simul 64:25–40. [Google Scholar]
- Holcombe M, Adra S, Bicak M, Chin S, Coakley S et al. , 2012. Modelling complex biological systems using an agent-based approach. Integ Biol 4:53–64. [DOI] [PubMed] [Google Scholar]
- Kamhi JF, Traniello JFA, 2013. Biogenic amines and collective organization in a superorganism: neuromodulation of social behavior in ants. Brain Behav Evol 82:220–236. [DOI] [PubMed] [Google Scholar]
- Kamhi JF, Nunn K, Robson SK, Traniello JF, 2015, Polymorphism and division of labour in a socially complex ant: neuromodulation of aggression in the Australian weaver ant Oecophylla smaragdina. Proc R Soc B 282:20150704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Matsui S, Satoh T, Sasaki K, 2015. Octopamine and cooperation: octopamine regulates the disappearance of cooperative behaviours between genetically unrelated founding queens in the ant. Biol lett 11:20150206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemis LM, McQueston JT, 2008. Univariate distribution relationships. Am Statis 62:45–53. [Google Scholar]
- Martelloni G, Santarlasci A, Bagnoli F, Santini G, 2015. Modeling ant battles by means of a diffusion-limited Gillespie algorithm. arXiv preprint arXiv:1503.06094. [PubMed] [Google Scholar]
- Martin S, Drijfhout F, 2009. A review of ant cuticular hydrocarbons. J Chem Ecol 35:1151 1161. [DOI] [PubMed] [Google Scholar]
- Momen S, 2013. Ant-inspired decentralized task allocation strategy in groups of mobile agents. Proc Comp Sci 20:169–176. [Google Scholar]
- Morale D, Capasso V, Oelschläger K, 2005. An interacting particle system modelling aggregation behavior: from individuals to populations. J Math Biol 50:49–66. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Wada-Katsumata A, Fujikwaw K, Iwasaki M, Ykohari F et al. , 2005. Ant nestmate and non-nestmate discrimination by a chemosensory sensillum. Science 5732:311–314. [DOI] [PubMed] [Google Scholar]
- Parunak HV, Savit R, Riolo RL, 1998. Agent-based modeling vs. equation-based modeling: a case study and users’ guide. In: Sichman JS, Conte R, Gilert N, editors. Multi-Agent Systems and Agent-Based Simulation. Berlin/Heidelberg: Springer, 10–25. [Google Scholar]
- Pinter-Wollman N, Bala A, Merrell A, Queirolo J, Stumpe MC et al. , 2013. Harvester ants use interactions to regulate forager activation and availability. Anim Behav 86:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowes NJ, 2008. Self Organized Conflicts in Territorial Ants. Ann Arbor (USA): ProQuest. [Google Scholar]
- Pratt SC, 2004. Quorum sensing by encounter rates in the ant Temnothorax albipennis. [cited 2016 April 15] Available from: http://doi.org/10.1093/beheco/ari020. Behav Ecol16:488–496. [Google Scholar]
- Pratt SC, Sumpter DJ, Mallon EB, Franks NR, 2005. An agent-based model of collective nest choice by the ant Temnothorax albipennis. Anim Behav 70:1023–1036. [Google Scholar]
- Robinson EJ, Ratnieks FL, Holcombe M, 2008. An agent-based model to investigate the roles of attractive and repellent pheromones in ant decision making during foraging. J Theo Biol 255:250–258. [DOI] [PubMed] [Google Scholar]
- Robinson EJH, Feinerman O, Franks NR, 2014. How collective comparisons emerge without individual comparisons of the options. Proc R Soc Lond B 281 doi: 20140737, 11.07.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczuka A, Korczyńska J, Wnuk A, Symonowicz B, Szwacka AG et al. , 2013. The effects of serotonin, dopamine, octopamine and tyramine on behavior of workers of the ant Formica polyctena during dyadic aggression tests. Acta Neurobiol Exp 73:495–520. [DOI] [PubMed] [Google Scholar]
- Sturgis SJ, Gordon DM, Nestmate recognition in ants (Hymenoptera: Formicidae): a review. Myrmecol News 16:101–110. [Google Scholar]
- Sumpter DJ, 2006. The principles of collective animal behaviour. Philos Trans R Soc Lond B Biol Sci 361:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpter DJT, Pratt SC, 2009. Quorum responses and consensus decision-making. Philos Trans: Biol Sci 364:743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada-Katsumata A, Yamaoka R, Aonuma H, 2011. Social interactions influence dopamine and octopamine homeostasis in the brain of the ant Formica japonica. J Exp Biol 214:1707–1713. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.