Abstract
One of the most important prognostic factors in heart failure patients is physical capacity. Patients with very poor physical performance and otherwise eligible, may be listed as candidates for heart transplantation (HTx). After such surgery, life-long immunosuppression therapy is needed to prevent rejection of the new heart. The dark side of immunosuppression is the increased risk of infections, kidney failure, cancer and advanced atherosclerosis (cardiac allograft vasculopathy), with the two latter conditions as the main causes of later mortality. In a worldwide perspective, 50% of the HTx patients survive past 10 years. Poor aerobic capacity prior to graft deterioration is not only limited to the failing heart, but also caused by peripheral factors, such as limited function in the skeletal muscles and in the blood vessels walls. Exercise rehabilitation after HTx is of major importance in order to improve physical capacity and prognosis. Effects of high-intensity interval training (HIT) in HTx recipients is a growing field of research attracting worldwide focus and interest. Accumulating evidence has shown that HIT is safe and efficient in maintenance HTx recipients; with superior effects on physical capacity compared to conventional moderate exercise. This article generates further evidence to the field by summarizing results from a decade of research performed at our center supported by a broad, but not strict formal, literature review. In short, this article demonstrates a strong association between physical capacity measured after HTx and long-term survival. It describes the possible “HIT-effect” with increased levels of inflammatory mediators of angiogenesis. It also describes long-term effects of HIT; showing a positive effect in development of anxiety symptoms despite that the improved physical capacity was not sustained, due to downregulation of exercise and intensity. Finally, our results are linked to the ongoing HITTS study, which investigates safety and efficiency of HIT in de novo HTx recipients. Together with previous results, this study may have the potential to change existing guidelines and contribute to a better prognosis for the HTx population as a whole.
Keywords: High-intensity interval training, Peak oxygen uptake, Heart transplantation, Survival, Prognosis, Angiogenesis, Inflammation, Physical capacity, Exercise
Core tip: Despite the positive effects of regular exercise after heart transplantation (HTx), HTx recipients’ physical capacity remains subnormal, and a strong association between physical capacity and survival has been demonstrated. Thus, the positive effects of high-intensity interval training (HIT) are a growing field of research, attracting worldwide focus and interest. Although the “HIT-effect” is not fully understood, a possible contributing factor is the increased levels of inflammatory mediators of angiogenesis generated during exercise. More high-quality research is strongly warranted, but ongoing studies already have the potential to change existing guidelines and contribute to a better prognosis for the HTx population.
INTRODUCTION
Heart transplantation
For patients with heart failure (HF) the 5-year mortality rates are 62% for women and 75% for men[1], with even higher rates in patients with end-stage HF[2]. Although these are old references, recent findings conclude that survival in HF patients has hardly changed since the 90’s[3]. Heart transplantation (HTx) is an established treatment to improve survival in selected patients with end-stage HF. From 1983 and to date, more than 920 HTx have been performed at Oslo University Hospital in Norway.
After HTx, the patients require lifelong immunosuppression to prevent rejection of the graft. These drugs have a potential to give adverse complications such as diabetes, gout, hypertension and osteoporosis, and serious side effects, such as higher risk of infections, renal failure and cancer. These side effects are the leading causes of death in the long-term, together with an advanced HTx-specific process of atherosclerosis, called coronary allograft vasculopathy (CAV)[4].
According to the 2012 ISHLT registry, the median survival for all HTx patients is 10 years, but if surviving the first year, the survival rates are higher and show a 63% survival past 10 years[4]. Increased knowledge about CAV and immunosuppression has resulted in further improved survival. However, the HTx recipients still have a shorter estimated length of survival than the general population.
Physical capacity after HTx
The dynamics of physical capacity after HTx is illustrated in Figures 1 and 2. Physical capacity increases significantly after HTx as a result of therapy, as shown by peak oxygen uptake (VO2peak) levels above 12 mL/kg per minute in published studies (Figures 1 and 2). Osada et al[5] and these two figures show that the highest rate of increase is found within the first years. In nearly 70% of the studies, regardless of time after HTx, VO2peak is below 20 mL/kg per minute, also classified as Weber function class B-C[6]. Patients within function class B and C are shown to be similar to coronary artery disease (CAD) and HF patients referred to rehabilitation programs[7]. VO2peak is often used as the primary outcome measure in exercise intervention studies after HTx[8].
Figure 1.
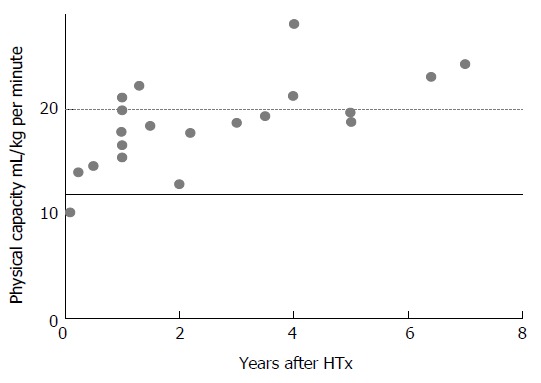
Physical capacity, as assessed by VO2peak after heart transplantation from published studies, illustrated by years after surgery. Black line at 12 mL/kg per minute, show the threshold to be candidates for HTx, dotted line at 20 mL/min per kilogram, show the start of Weber function class A, representing good physical condition. The measurements are carried out from exercise tests from; Bernandi et al[85], Carter et al[86], Dall et al[35], Ewert et al[87], Givertz et al[88], Gullestad et al[89], Habedank et al[90], Haykowski et al[91], Hermann et al[36], Hognestad et al[92], Karpolat et al[93], Kavanagh et al[94], Kemp et al[95], Kobashigawa et al[96], Nytrøen et al[37], Osada et al[5], Renlund et al[97], Schwaiblmair et al[98], Squires et al[99], Tegtbur et al[100], Wu et al[77]. HTx: Heart transplantation.
Figure 2.
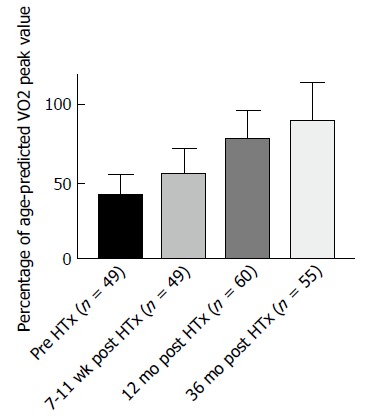
Illustration of the increase in peak VO2 from pre heart transplantation to 36 mo post heart transplantation. The data presented in the figure is from the unpublished Schedule trial.
Physical capacity as a prognostic variable
The gold standard measurement of physical capacity is VO2peak, and is defined as “the maximum ability of the cardiovascular system to deliver oxygen to exercising muscles and of the exercising muscle to extract oxygen from the blood”[9]. VO2peak is shown to be a strong predictor of survival in general populations[10,11], among patients with CAD[12], and in patients with severe HF[13]. Limited exercise capacity is the cardinal symptom in HF. The HF patients with VO2peak < 12 mL/kg per minute are considered to have the worst prognosis, despite optimal medical therapy, and can be appropriate candidates listed for HTx[14]. These patients are most likely men > 50 years of age[4]. When evaluating younger patients and women, it is found reasonable to include age and gender adjusted levels of exercise capacity, and values ≤ 50% percent of predicted VO2peak differentiate better in these populations[14].
However, studies addressing the relation between VO2peak and survival after HTx are currently lacking, although a number of other predictors have been identified through register-data analyses. These predictors are: Non-ischemic cardiomyopathy as the primary diagnosis, younger recipient age, younger donor-graft age and shorter allograft ischemic time; all associated with a better long-term prognosis[4,15,16]. The mortality beyond one-year after HTx has remained relatively constant, and Stehlik et al[4] predict that interventions resulting in a reduction of mortal events in the long-term are needed to achieve further improvements in survival after HTx.
In a recent retrospective study from our center, investigating survival in two different HTx populations (n = 178, n = 133), we found that VO2peak and SF-36 physical function (PF) sum-score were strong predictors for survival in each population, respectively[17]. In the “VO2peak cohort” (n = 178), the mean age was 52 years, mean age after HTx was 2.5 years, mean VO2peak was 19.6 mL/kg per minute, 88% were men and mean observation time was 11 years. The most important predictors (analyzed by multiple Cox regression) for survival in this population were VO2peak (HR = 0.917, P < 0.001) age at time of test (HR = 1.045, P < 0.001) and CAV development (HR = 1.968, P = 0.001), and the group above the median VO2peak had an increased survival of four years. Similar results were found in the “SF-36 cohort” where the mean age was 54 years, mean time after HTx was 4.5 years, mean SF-36 physical function (PF) score was 90 and mean observation time was 10 years. The most important predictors (analyzed by multiple Cox regression) for survival in this population were the PF score (HR = 0.983, P < 0.001), age (HR = 1.077, P <0.001), smoking history (HR = 1.077, P = 0.016) and CAV development (HR = 1.674, P = 0.039), and the group above the median PF score value had an increased survival of four years.
Other well-known predictors of HTx survival such as diagnosis prior to HTx, ischemic time, donor age, measurements of cardiac output and kidney function by creatinine did not add any additional explanation to the regression models.
The relationship between physical health and long-term survival
Earlier studies addressing survival, have estimated how physical performance pre HTx is related to survival after HTx. Physical capacity (measured by VO2peak) in this population is well known to predict survival and supports the clinicians in the selection of HTx candidates[14]. Our study documented that also VO2peak measured after HTx is a strong predictor for long-term survival[17], and this result is in line with the only study we found that demonstrated a relationship between physical performance (measured by VE/VCO2 slope) and survival in a small sample of HTx patients (n = 49)[18]. Other related studies on this topic describe how VO2peak is related to soft end-points; how a beneficial VO2peak correlates with NYHA class 1-2 after HTx[19] and how the pre-transplant VO2peak, together with age, predict the gain in physical capacity post HTx[5]. Succeeding our study on survival, Rosenbaum et al[20] published new knowledge in this field, with a study investigating the effect of early rehabilitation on survival: They concluded that early cardiac rehabilitation participation after HTx could predict survival time.
The measurement of physical capacity requires CPET equipment and test personnel, and thus, is quite costly. Although VO2peak is the gold standard to examine exercise performance, there are other physical tests with limited costs that can be useful in the follow-up, found to correlate with CPET results. Such physical tests are the 6-min walk test and the shuttle walking test[21], but if these test are associated with prognosis remains to be determined. If resources are limited, we also found that the self-reported physical health (PF-score) showed a similar effect on long-term survival in the HTx population[17]. Research in general populations underscore the importance of physical activity and report a dose-response effect on survival rates[22,23], as well as a strong dose-response relation on self-reported health[24]. As shown in another of our studies[25], physical performance measured as VO2peak is highly correlated with SF-36 PF sum-scores, and both were found to be highly associated with prognosis in our survival analysis[17]. Accordingly, we suggest that such measures should be more frequently used after HTx to identify patients at higher risk for complications.
Exercise after HTx - the past and the future
To increase physical capacity and prevent long-term complications such as hypertension and diabetes, aerobic exercise after HTx has a positive effect, but HTx recipients’ physical capacity still remains subnormal in most studies[26]. High-intensity interval training (HIT) is proven to be a more efficient exercise modality than moderate-intensity continuous training (MICT) in order to increase VO2peak, shown in patients with HF[27], CAD[28], metabolic disease[29], as well as in healthy individuals[30]. The new knowledge has had a great impact on how general cardiac rehabilitation programs are organized today. These two different exercise modalities are illustrated in Figure 3. HIT corresponds to an intensity of 16-18 on Borg’s rated perceived exertion (RPE) 6-20 scale[31,32], and MICT to Borg 12-15.
Figure 3.
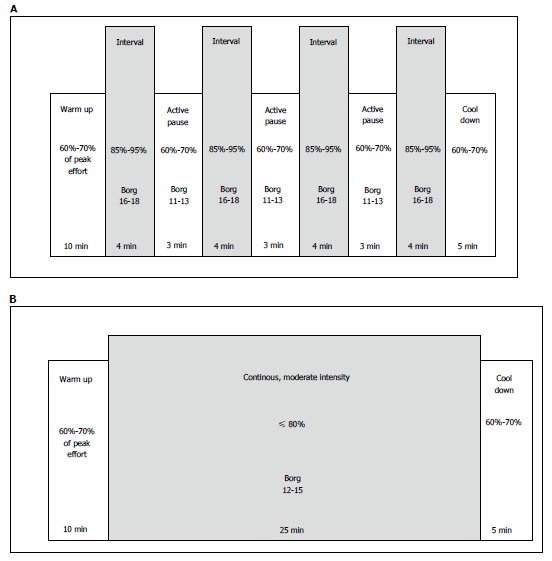
Illustration of two different exercise modalities. A: Illustration of a session with high-intensity interval training (HIT). HIT is an exercise strategy with alternating short periods of intense endurance exercise with less-intense recovery periods. A usual HIT session may include 4 × 4 min periods with high intensity (85%-95% of maximal capacity), with active recovery periods of 3min between each interval (with 60%-70% of maximal capacity); B: Illustration of a session with moderate intensity continuous training (MICT). MICT is an exercise strategy with moderate intensity (60%-70% of maximal capacity) of endurance exercise in periods for usually 25-30 min, with no recovery periods.
Rehabilitation after HTx has traditionally had, and still has, a more conservative approach, with MICT as traditionally recommended, mainly due to uncertainty and concerns regarding denervation with consequently chronotropic incompetence and parasympathetic impairment[33]. The heart rate (HR) will typically be higher at rest, with a slower increase during exercise, a lower maximum HR at peak exercise, and a slower HR decrease after exercise cessation (Figure 4).
Figure 4.
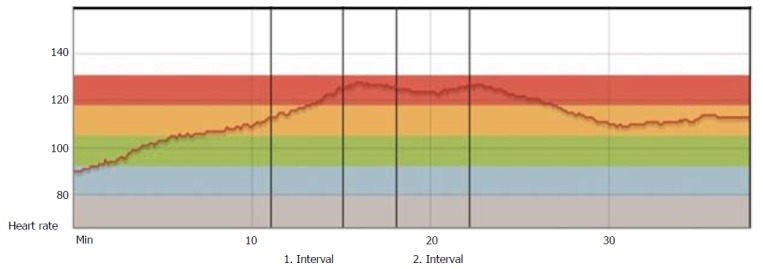
Heart rate during exercise 3 mo post heart transplantation. Patient from our hospital, 3 mo post-HTx: HR curve during warm-up, two high-intensity intervals divided by one recovery period and cool-down. The curve shows a typical pattern of impaired HR responses in the early stage after HTx. HTx: Heart transplantation.
The chronotropic incompetence is most prominent the first months after HTx and tends to be largely normalized in the majority of patients after 12 mo[34], as illustrated in Figure 5. Recent randomized controlled trials (RCTs), have investigated the effect of HIT in maintenance HTx recipients and have to a large extend overruled the traditional, conservative approach with MICT[35-37]. These studies showed that HIT increased VO2peak significantly compared to the control groups, and that a HIT intervention was safe and well tolerated. References to some of these results are mentioned in the most recent recommendations for cardiac rehabilitation from 2013[38]. The mechanisms of effect are probably multifactorial and might involve improved chronotropic response (CRI)[35,37], endothelial function[36] and less development of CAV after a long-term exercise intervention[39].
Figure 5.
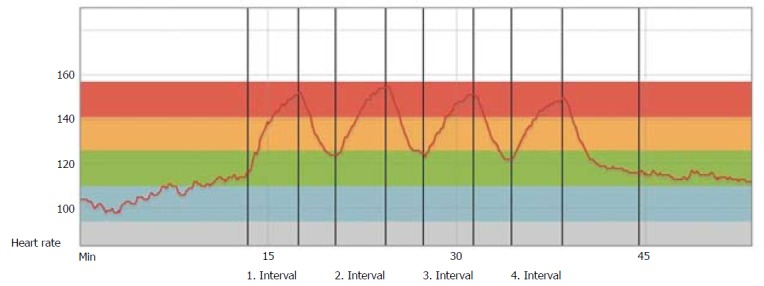
Heart rate during exercise 12 mo post heart transplantation. The same patient 12 mo post-HTx: Heart rate curve during warm-up four high-intensity intervals divided by 3 recovery periods and cool-down. The curve shows a largely normalized HR, with immediate HR adaptions to exercise intensity. HTx: Heart transplantation.
So far there are no studies on the effect of HIT in the novo patients, but a similar HIT intervention study is currently ongoing in Scandinavia[40]. One of the goals in this study is to update, optimize and implement new exercise prescriptions also in this group.
Meta-analyses in HF populations[41,42] find a possible long-term effect of exercise-based rehabilitation (MICT protocols) on survival and health related quality of life (HRQoL), and most importantly; a significantly decrease in re-hospitalization. Knowledge about exercise-based rehabilitation and the effect on mortality and hospital admissions in HTx recipients are currently missing, as recently stated in a 2017 Cochrane review on the effectiveness and safety of exercise-based rehabilitation in HTx recipients[43]. The lack of research regarding possible long-term benefits of exercise was also pointed out in the published meeting report from 2014: “Consensus recommendations for a research agenda in exercise in solid organ transplantation”[44].
HIT intervention and long-term effects
Regarding long-term effects of exercise in HTx recipients, we have conducted a 5-year follow-up study of a previous RCT investigating the effects of a HIT intervention[25]. Forty-eight maintenance HTx patients, mean four years after HTx were randomized to HIT intervention or control with 12 mo duration[37]. The study demonstrated a significant improved VO2peak (mean difference between groups: 3.6 mL/kg per minute), increased muscular capacity and less development of CAV compared to the control group[37,39]. However, at the 5-year follow-up (n = 41), the HIT group had not sustained the exercise intensity over time, and although the decline in VO2peak from baseline to 5-year follow-up was numerically lower in the HIT-group, there were no significant differences between the groups for the parameters described above (Figure 6). These findings were explained by the similar amount of daily (moderate) activity in both the HIT and the control group, measured at the 5-year follow-up. Our results differ from a study by Moholdt et al[45] who investigated long-term effects of a HIT intervention after myocardial infarction (MI). These MI-patients still had a significantly higher aerobic performance at the 30 mo follow-up compared to the control group, explained by more frequent exercise in the HIT group. Although the initial 1-year gain in physical capacity in the HIT group was not sustained and the mean difference between groups at the 5-year follow-up was non-significant, only the control group had a significant decrease within group from baseline to the 5-year follow-up. This significant decrease, corresponding to a 9% decline in mL/kg per minute, could mostly be explained by an expected age-related decrease in VO2peak. Healthy young adults show a decline of 3%-6% each decade, and this decline is shown to accelerate with age; a decline of 15% is found normal and corresponds to the age group of the TEX population[46]. This age related VO2-decline is related to decreasing maximal stroke volume, decreasing blood flow to skeletal muscles and mitochondrial dysfunction[47]. As for the HIT group, the decrease from baseline to the 5-year follow-up in VO2peak was less pronounced (-6%), and could possibly indicate a hidden long-term effect of the intervention. In contrast, the development of anxiety symptoms was significantly different between the groups; the exercise group showed decreased symptoms of anxiety, whereas the control group had an increased anxiety symptom score. This beneficial trend in anxiety development together with no negative trends in other secondary end points, support the statements of HIT as a safe exercise modality in HF patients[48], and in maintenance HTx patients[35-37].
Figure 6.
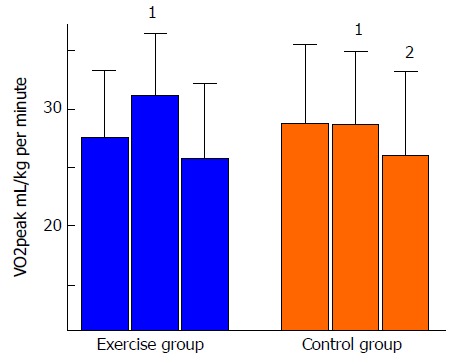
Measurements of VO2peak at baseline, 1-year and 5-year follow-up. 1Significant changes between groups; 2Significant changes from baseline to 5-year follow-up within group.
Nevertheless, more research is still needed regarding long-term effects of exercise, and to optimize the rehabilitation regimes and improve the HTx recipients’ future prognosis[43,44].
HIT intervention in de novo HTx recipients
While HIT already is an established exercise modality in patients with HF[27] and CAD[28], and more recently in maintenance HTx[35-37], the upcoming results from the HITTS study[40] will contribute to fill the gap of knowledge related to the effect of HIT among de novo HTx recipients. In addition to exercise capacity measurements, other important secondary outcomes are: development of CAV, improvements in chronotropic response and changes in cardiac and endothelial function. The results from the HITTS study will make a strong contribution to improve and increase the knowledge-base about how early HTx-rehabilitation should be organized in order to gain the most optimal results. The study is followed closely by our dedicated HTx-staffs in Scandinavia, and one of our main goals is to document knowledge about safety and effects of HIT, and thereby initiate an update of the current guidelines. If HIT is found to be safe (and with potentially beneficial effects) also among de novo HTx patients, the patients will have the possibility to participate in established cardiac rehabilitation programs, which usually combines both MICT and HIT exercise. These rehabilitation programs are usually group based, rather than only consistent individual physiotherapy, thus demanding less government resources.
Mechanisms behind the “HIT-effect”
As described previously, the effects of HIT interventions are so far mostly studied in healthy individuals, CAD and HF patients. The main mechanisms behind the increase in exercise capacity are shown to be through central factors, induced by a prominent improvement in cardiac output (CO)[27,49]. However, the “HIT-effect” in maintenance HTx recipients show different results, seemingly with peripheral factors as the main mechanisms; by improvement in skeletal muscle exercise capacity[37], endothelial function and vasodilatation[36], rather than an increased CO[50]. The underlying triggers behind these peripheral effects are poorly understood, and the potential of inflammatory signaling pathways are not explored in detail. Markers of inflammation have been studied as an additional effect of exercise through long-term steady state levels (before and after exercise intervention), showing mostly neutral results[36,39,51].
We hypothesized that investigation of immediate exercise effects in inflammatory signaling pathways during HIT could contribute to further explain the “HIT-effect” in the HTx recipients, and recently we performed such an exploratory study[52]. Fourteen patients were included in the randomized cross-over study, comparing HIT to MICT. Blood samples were drawn before, during and after exercise. The main results from the enzyme immunoassays analyses were that exercise, regardless of intensity, induced a significant immediate response in several vascular, angiogenetic and particularly in platelet derived inflammatory mediators in HTx recipients shown in Table 1. HIT showed trends to induce an increased response in von willebrand factor (vWF), vascular endothelial growth factor 1 (VEGF-1) and Angiopoetin-2 (Ang-2), and a decreased response in growth derived factor 15 (GDF-15), compared to MICT (Table 1).
Table 1.
A simplified illustration of the ANOVA results: The response in markers of inflammation and angiogenesis during high-intensity interval training and moderate intensity continuous training sessions
| MICT | HIT | |
| General inflammation | ||
| CRP | → | → |
| sTNFr-1 | ↑ | ↑ |
| Vascular inflammation | ||
| vWFd | ↓ | ↑ |
| VCAM | → | → |
| Blood platelets | ||
| PDGF | ↑ | ↑ |
| sCD40L | ↑ | ↑ |
| DKK-1 | ↑ | ↑ |
| Angiogenesis | ||
| VEGF-1 | ↑ | ↑↑ |
| Ang2 | ↑ | ↑↑ |
| Tie-2 | → | → |
| Endostatin | → | → |
| Cardiokine/myokine | ||
| GDF-15 | ↑ | ↓1 |
| ST2 | → | → |
| SPARC | ↑ | ↑ |
The decrease is found in the recovery period (0-2 h) after the exercise-session. Horizontal arrows illustrate non-significant response during exercise. Arrows pointing up illustrate a significant increase with exercise, regardless of intensity, and two arrows illustrate a significant increase by increasing intensity (HIT). An arrow pointing down, illustrates a significant decrease in response during exercise. HIT: High-intensity interval training; MICT: Moderate intensity continuous training.
HIT and the immediate responses in markers of inflammation and angiogenesis
Exercise training, regardless of intensity, led to an increase in multiple systemic, angiogenetic and platelet derived inflammatory mediators[52]. These results are in line with published research showing the pro-coagulation state during exercise, with blood platelet activation potentially reflecting the increase in catecholamines and shear stress[53], promotion of NO production from activated endothelial cells[54,55], and regulation of the growth and repair of blood vessels[56]. The activation of the endothelium and thereby induction of capillary growth in skeletal muscle through pro-angiogenetic mechanisms may play an important role in the beneficial effects of HIT. When we compared the response in inflammatory mediators during the HIT and MICT sessions, we observed a higher response in both Ang-2 and VEGF-1 with increased intensity. Kilian et al[57] have previously shown an increase in mRNA for VEGF in whole blood during HIT in healthy children. VEGF is dominantly secreted by working skeletal muscles, an essential factor to increase capillary density, oxygen delivery and thereby exercise performance[58-60]. Based on our previous results showing improved muscular exercise capacity after HIT[37], and now the finding of an increased VEGF response, we suggest that this mechanism is of high importance also in the HTx recipients. The fact that HIT markedly increased mediators of angiogenesis and neovascularization, may contribute to explain the different trigger mechanisms behind the two different exercise modalities.
CAV
CAV is characterized by intimal thickening and a more diffuse narrowing of the coronary arteries’ lumen than conventional atherosclerosis[61]. The mechanisms of development are described as both immunological and non-immunological, possibly modifiable factors[62]. It can be detected by coronary angiography, but intravascular ultrasound (IVUS) is now more frequently used, and is a superior diagnostic tool to detect early changes in intimal thickening (early CAV)[63]. The early CAV has been validated as a reliable surrogate marker for subsequent mortality, nonfatal major adverse cardiac events, and development of angiographic CAV following HTx[64,65]. CAV progression is a highly prioritized field of research among HTx clinicians and researchers, to further improve HTx prognosis. As a result, Kobashigawa et al[66] introduced statin therapy that showed to have beneficial effects on one-year survival and the incidence of CAV. Statins became routine therapy after HTx at our center from 1997. More recently, a Scandinavian multicenter RCT (The Schedule-study) has shown that early everolimus initiation with calcineurin inhibitor withdrawal reduces the progression of CAV in de-novo HTx recipients[67,68].
HIT and the effect on CAV
The effect of non-medical prevention strategies, such as HIT interventions, has also been studied by IVUS and have shown less progression of atherosclerosis both in mice[69] and in patients after MI[70]. We found the same trend in maintenance HTx recipients after a HIT intervention[39], but the positive effects were not sustained in the long-term as shown in the 5-year follow-up study[25]. Furthermore, exercise is shown to have a positive influence on the endothelium through increased nitric oxide production, and by reduction of inflammation[71,72]. This effect could possibly be enhanced through higher shear stress triggered with higher exercise intensity. A gain in endothelial function following a HIT intervention is found in CAD patients[73]. However, a relatively small sample size in the 1- and 5-year follow-up studies[25,39] limits our conclusion in the HTx population, and the effect of HIT on CAV should be examined in a larger sample and include a second intervention arm with MICT. It has been explored how early medical therapy can influence CAV progression in the long-term, and studies with everolimus are found to have positive impact on CAV severity in de novo HTx patients, whereas no effect is seen if everolimus is introduced later on[74]. The effect on CAV severity by an early initiation was also sustained in the long-term[67,68]. This illustrates an “opportunity window” during the first year after HTx. Knowing that the CAV development is most pronounced the first year after HTx, we anticipate that similar mechanisms may be seen with an early initiation of HIT. Results from the HITTS study[40] will contribute to a better understanding of the relationship between exercise and CAV development.
Health related quality of life
The HRQoL after HTx has been reported to increase significantly, with high levels of satisfaction in overall HRQoL; also stable over a 5-year period (measured from 5 to 10 years after HTx)[75]. Although, when HTx patients are compared with the general population, the HRQoL remains beneath normal values[76]. To improve HRQoL, and especially physical health, exercise interventions have shown to be successful and this is in contrast to the more neutral results reported in control groups[77,78]. Research on HRQoL after HTx regarding the effect of HIT (compared to MICT) is very limited, and the existing studies show mixed results; some studies show similar effects on HRQoL[51], while we and others have shown a beneficial effect with a significant increase after HIT[37,79].
Mental health, anxiety and depression
In the post-transplant stage the prevalence of significant depression and anxiety remains substantially above the general populations, and it tends to increase over time[80,81]. As it is found that depressed HTx recipients have a higher risk of mortality, screening for depressive symptoms during follow-up is recommended[81-83]. As an approach to increase mental health, the effect of exercise and HIT has been studied. The results showed that exercise decreases the burden of depression and anxiety, with HIT showing significant positive effects compared with usual care[79]. Additionally, the results align with the correlation between higher physical capacity and less depression and anxiety rates[25,83,84].
In our 5-year follow-up study after a HIT intervention[25], we measured physical and mental health as well as measures of physical capacity at each study visit, and at the 5-year follow-up there was significantly less development of anxiety symptoms in the HIT group compared to the control group. The long-term difference in anxiety between the HIT group and the control group is considered a valuable finding, as anxiety is a frequent health issue after HTx, especially in the long-term follow-up[80]. Overall, there was a positive correlation between the measured VO2 peak and the self-reported physical health (SF-36 PF sum-score). These findings might suggest that a 1-year “heavy” exercise intervention has a long-term value when it comes to self-confidence and trust regarding what your heart (and body) actually can tolerate of exertion, strain and physical work.
CONCLUDING REMARKS
Our findings, supported by a review of the existing literature, suggest that measures of physical health should be included frequently also after HTx, as they predict prognosis and survival in the long-term. A dose-response effect of physical capacity on survival was also found in the HTx population.
HIT is a feasible and efficient modality of exercise among maintenance HTx recipients, but the mechanisms behind this effect is poorly understood. Our results suggest that the beneficial effects seen in HTx recipients differ from CAD and HF patients, with more prominent peripheral effects from HIT exercise, rather than central adaptations with increased CO. We have showed that HIT significantly increased levels of inflammatory mediators of angiogenesis, suggesting that HIT can regulate and stimulate blood vessel formation in skeletal muscles and thus increase physical capacity.
Considering exercise prescription and future guidelines, our findings suggest that moderate levels of exercise and intensity are insufficient to maintain the improved VO2peak achieved after a HIT intervention. Thus, intermittent periods of HIT are likely to be necessary. Also, the number and length of HIT intervals needed in a HIT session should be further investigated. If a modified HIT protocol with shorter and fewer intervals has comparable effect to a 4 × 4 protocol, it could probably increase the patients’ motivation and adherence to exercise in the long-term. When considering other long-term effects, the benefit from a tough and intense HIT-intervention showed a positive effect on the development of anxiety symptoms. The exercise prescription in de novo HTx recipients is still conservative, consisting mainly of MICT exercise, but this traditional guideline might change when the ongoing HITTS study is completed. Existing gaps in knowledge are briefly mentioned in Table 2, and the results from the HITTS study will contribute to fill some of these gaps, and may also have the potential to update, optimize and possibly include HIT as a safe exercise modality in future guidelines.
Table 2.
What is known and unknown in this field?
| What is known in this field |
| A proper rehabilitation program including exercise training is recommended in all HTx patients |
| Good physical fitness is associated with improved outcome in HTx patients |
| The effect of HIT is superior to the effect of moderate training in general as well as for patients with coronary heart disease and heart failure |
| Accumulating evidence has shown that this is true also for HTx recipients 1-8 yr after HTx |
| Gaps in knowledge |
| There is no consensus on how, when and at which intensity exercise should be performed and organized after HTx |
| Because newly transplanted patients are totally denervated (without functional nerve supply resulting in impaired heart rate response), the effect of HIT has never been evaluated in this population, and the effect of HIT in de-novo HTx patients’ needs to be investigated |
| The effect of HIT on late complications after HTx as CAV, diabetes mellitus, gout, renal function and graft survival needs to be explored |
| Data on whether a HIT intervention should be carried out decentralized or in cooperation with the primary health care services as well as the safety and cost-effectiveness are scarce |
| How to optimize ways to maintain exercise training during long-term follow up needs to be investigated |
HIT: High-intensity interval training; HTx: Heart transplantation; CAV: Coronary allograft vasculopathy.
Footnotes
Conflict-of-interest statement: No potential conflicts of interest. No financial support.
Manuscript source: Invited manuscript
Peer-review started: November 17, 2017
First decision: November 30, 2017
Article in press: December 28, 2017
Specialty type: Transplantation
Country of origin: Norway
Peer-review report classification
Grade A (Excellent): A, A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cheungpasitporn W, Kita K, Therapondos G S- Editor: Ji FF L- Editor: A E- Editor: Li D
Contributor Information
Marianne Yardley, Department of Cardiology, Oslo University Hospital Rikshospitalet, Oslo 0424, Norway; Faculty of Medicine, University of Oslo, Oslo 0316, Norway.
Lars Gullestad, Department of Cardiology, Oslo University Hospital Rikshospitalet, Oslo 0424, Norway; Faculty of Medicine, University of Oslo, Oslo 0316, Norway.
Kari Nytrøen, Department of Cardiology, Oslo University Hospital Rikshospitalet, Oslo 0424, Norway; Faculty of Medicine, University of Oslo, Oslo 0316, Norway.
References
- 1.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 2.Zannad F, Mebazaa A, Juillière Y, Cohen-Solal A, Guize L, Alla F, Rougé P, Blin P, Barlet MH, Paolozzi L, et al. Clinical profile, contemporary management and one-year mortality in patients with severe acute heart failure syndromes: The EFICA study. Eur J Heart Fail. 2006;8:697–705. doi: 10.1016/j.ejheart.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Taylor CJ, Ryan R, Nichols L, Gale N, Hobbs FR, Marshall T. Survival following a diagnosis of heart failure in primary care. Fam Pract. 2017;34:161–168. doi: 10.1093/fampra/cmw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, Dobbels F, Kirk R, Rahmel AO, Hertz MI; International Society of Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report--2012. J Heart Lung Transplant. 2012;31:1052–1064. doi: 10.1016/j.healun.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Osada N, Chaitman BR, Donohue TJ, Wolford TL, Stelken AM, Miller LW. Long-term cardiopulmonary exercise performance after heart transplantation. Am J Cardiol. 1997;79:451–456. doi: 10.1016/s0002-9149(96)00785-0. [DOI] [PubMed] [Google Scholar]
- 6.Weber KT, Kinasewitz GT, Janicki JS, Fishman AP. Oxygen utilization and ventilation during exercise in patients with chronic cardiac failure. Circulation. 1982;65:1213–1223. doi: 10.1161/01.cir.65.6.1213. [DOI] [PubMed] [Google Scholar]
- 7.Soumagne D. Weber classification in cardiac rehabilitation. Acta Cardiol. 2012;67:285–290. doi: 10.1080/ac.67.3.2160716. [DOI] [PubMed] [Google Scholar]
- 8.Janaudis-Ferreira T, Mathur S, Konidis S, Tansey CM, Beaurepaire C. Outcomes in randomized controlled trials of exercise interventions in solid organ transplant. World J Transplant. 2016;6:774–789. doi: 10.5500/wjt.v6.i4.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dennis C. Braunwald’s Heart disease, a textbook of cardiovascular medicine, 9th ed. Philadelphia: Elsevier Saunders; 2012. Rehabilitation of patients with coronary artery disease; pp. 19611–19656. [Google Scholar]
- 10.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 11.Arena R, Myers J, Guazzi M. The future of aerobic exercise testing in clinical practice: is it the ultimate vital sign? Future Cardiol. 2010;6:325–342. doi: 10.2217/fca.10.21. [DOI] [PubMed] [Google Scholar]
- 12.Kavanagh T, Mertens DJ, Hamm LF, Beyene J, Kennedy J, Corey P, Shephard RJ. Prediction of long-term prognosis in 12 169 men referred for cardiac rehabilitation. Circulation. 2002;106:666–671. doi: 10.1161/01.cir.0000024413.15949.ed. [DOI] [PubMed] [Google Scholar]
- 13.Cahalin LP, Chase P, Arena R, Myers J, Bensimhon D, Peberdy MA, Ashley E, West E, Forman DE, Pinkstaff S, et al. A meta-analysis of the prognostic significance of cardiopulmonary exercise testing in patients with heart failure. Heart Fail Rev. 2013;18:79–94. doi: 10.1007/s10741-012-9332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehra MR, Kobashigawa J, Starling R, Russell S, Uber PA, Parameshwar J, Mohacsi P, Augustine S, Aaronson K, Barr M. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates--2006. J Heart Lung Transplant. 2006;25:1024–1042. doi: 10.1016/j.healun.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Jaramillo N, Segovia J, Gómez-Bueno M, García-Cosío D, Castedo E, Serrano S, Burgos R, García Montero C, Ugarte J, Martínez Cabeza P, et al. Characteristics of patients with survival longer than 20 years following heart transplantation. Rev Esp Cardiol (Engl Ed) 2013;66:797–802. doi: 10.1016/j.rec.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 16.Tallaj JA, Pamboukian SV, George JF, Kirklin JK, Brown RN, McGiffin DC, Acharya D, Loyaga-Rendon R, Melby SJ, Bourge RC, et al. Have risk factors for mortality after heart transplantation changed over time? Insights from 19 years of Cardiac Transplant Research Database study. J Heart Lung Transplant. 2014;33:1304–1311. doi: 10.1016/j.healun.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Yardley M, Havik OE, Grov I, Relbo A, Gullestad L, Nytrøen K. Peak oxygen uptake and self-reported physical health are strong predictors of long-term survival after heart transplantation. Clin Transplant. 2016;30:161–169. doi: 10.1111/ctr.12672. [DOI] [PubMed] [Google Scholar]
- 18.Grigioni F, Specchia S, Maietta P, Potena L, Bacchi-Reggiani ML, Ghetti G, Boriani G, Foschi E, Corazza I, Ionico T, et al. Changes in exercise capacity induced by heart transplantation: prognostic and therapeutic implications. Scand J Med Sci Sports. 2011;21:519–525. doi: 10.1111/j.1600-0838.2009.01065.x. [DOI] [PubMed] [Google Scholar]
- 19.Chang AC, Shyr Y, Groves J, Chomsky DB, Davis SF, Wilson JR, Drinkwater DC, Pierson RN, Merrill WH. The utility of exercise testing after cardiac transplantation in older patients. J Surg Res. 1999;81:48–54. doi: 10.1006/jsre.1998.5484. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum AN, Kremers WK, Schirger JA, Thomas RJ, Squires RW, Allison TG, Daly RC, Kushwaha SS, Edwards BS. Association Between Early Cardiac Rehabilitation and Long-term Survival in Cardiac Transplant Recipients. Mayo Clin Proc. 2016;91:149–156. doi: 10.1016/j.mayocp.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 21.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 22.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT; Lancet Physical Activity Series Working Group. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380:219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holme I, Anderssen SA. [Physical activity, smoking and mortality among men who participated in the Oslo studies of 1972 and 2000] Tidsskr Nor Laegeforen. 2014;134:1743–1748. doi: 10.4045/tidsskr.14.0073. [DOI] [PubMed] [Google Scholar]
- 24.Eriksen L, Curtis T, Grønbæk M, Helge JW, Tolstrup JS. The association between physical activity, cardiorespiratory fitness and self-rated health. Prev Med. 2013;57:900–902. doi: 10.1016/j.ypmed.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 25.Yardley M, Gullestad L, Bendz B, Bjørkelund E, Rolid K, Arora S, Nytrøen K. Long-term effects of high-intensity interval training in heart transplant recipients: A 5-year follow-up study of a randomized controlled trial. Clin Transplant. 2017 doi: 10.1111/ctr.12868. 31: Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh PL, Wu YT, Chao WJ. Effects of exercise training in heart transplant recipients: a meta-analysis. Cardiology. 2011;120:27–35. doi: 10.1159/000332998. [DOI] [PubMed] [Google Scholar]
- 27.Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen Ø, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115:3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 28.Elliott AD, Rajopadhyaya K, Bentley DJ, Beltrame JF, Aromataris EC. Interval training versus continuous exercise in patients with coronary artery disease: a meta-analysis. Heart Lung Circ. 2015;24:149–157. doi: 10.1016/j.hlc.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Tjønna AE, Lee SJ, Rognmo Ø, Stølen TO, Bye A, Haram PM, Loennechen JP, Al-Share QY, Skogvoll E, Slørdahl SA, Kemi OJ, Najjar SM, Wisløff U. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118:346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wisløff U, Ellingsen Ø, Kemi OJ. High-intensity interval training to maximize cardiac benefits of exercise training? Exerc Sport Sci Rev. 2009;37:139–146. doi: 10.1097/JES.0b013e3181aa65fc. [DOI] [PubMed] [Google Scholar]
- 31.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–98. [PubMed] [Google Scholar]
- 32.Anderssen SA, Strømme SB. [Physical activity and health--recommendations] Tidsskr Nor Laegeforen. 2001;121:2037–2041. [PubMed] [Google Scholar]
- 33.Piña IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, Fletcher BJ, Fleg JL, Myers JN, Sullivan MJ; American Heart Association Committee on exercise, rehabilitation, and prevention. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107:1210–1225. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 34.Nytrøen K, Myers J, Chan KN, Geiran OR, Gullestad L. Chronotropic responses to exercise in heart transplant recipients: 1-yr follow-up. Am J Phys Med Rehabil. 2011;90:579–588. doi: 10.1097/PHM.0b013e31821f711d. [DOI] [PubMed] [Google Scholar]
- 35.Dall CH, Snoer M, Christensen S, Monk-Hansen T, Frederiksen M, Gustafsson F, Langberg H, Prescott E. Effect of high-intensity training versus moderate training on peak oxygen uptake and chronotropic response in heart transplant recipients: a randomized crossover trial. Am J Transplant. 2014;14:2391–2399. doi: 10.1111/ajt.12873. [DOI] [PubMed] [Google Scholar]
- 36.Hermann TS, Dall CH, Christensen SB, Goetze JP, Prescott E, Gustafsson F. Effect of high intensity exercise on peak oxygen uptake and endothelial function in long-term heart transplant recipients. Am J Transplant. 2011;11:536–541. doi: 10.1111/j.1600-6143.2010.03403.x. [DOI] [PubMed] [Google Scholar]
- 37.Nytrøen K, Rustad LA, Aukrust P, Ueland T, Hallén J, Holm I, Rolid K, Lekva T, Fiane AE, Amlie JP, et al. High-intensity interval training improves peak oxygen uptake and muscular exercise capacity in heart transplant recipients. Am J Transplant. 2012;12:3134–3142. doi: 10.1111/j.1600-6143.2012.04221.x. [DOI] [PubMed] [Google Scholar]
- 38.Mezzani A, Hamm LF, Jones AM, McBride PE, Moholdt T, Stone JA, Urhausen A, Williams MA; European Association for Cardiovascular Prevention and Rehabilitation; American Association of Cardiovascular and Pulmonary Rehabilitation; Canadian Association of Cardiac Rehabilitation. Aerobic exercise intensity assessment and prescription in cardiac rehabilitation: a joint position statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitation and the Canadian Association of Cardiac Rehabilitation. Eur J Prev Cardiol. 2013;20:442–467. doi: 10.1177/2047487312460484. [DOI] [PubMed] [Google Scholar]
- 39.Nytrøen K, Rustad LA, Erikstad I, Aukrust P, Ueland T, Lekva T, Gude E, Wilhelmsen N, Hervold A, Aakhus S, et al. Effect of high-intensity interval training on progression of cardiac allograft vasculopathy. J Heart Lung Transplant. 2013;32:1073–1080. doi: 10.1016/j.healun.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 40.Nytrøen K, Yardley M, Rolid K, Bjørkelund E, Karason K, Wigh JP, Dall CH, Arora S, Aakhus S, Lunde K, et al. Design and rationale of the HITTS randomized controlled trial: Effect of High-intensity Interval Training in de novo Heart Transplant Recipients in Scandinavia. Am Heart J. 2016;172:96–105. doi: 10.1016/j.ahj.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Piepoli MF, Davos C, Francis DP, Coats AJ; ExTraMATCH Collaborative. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH) BMJ. 2004;328:189. doi: 10.1136/bmj.37938.645220.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sagar VA, Davies EJ, Briscoe S, Coats AJ, Dalal HM, Lough F, Rees K, Singh S, Taylor RS. Exercise-based rehabilitation for heart failure: systematic review and meta-analysis. Open Heart. 2015;2:e000163. doi: 10.1136/openhrt-2014-000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson L, Nguyen TT, Dall CH, Burgess L, Bridges C, Taylor RS. Exercise-based cardiac rehabilitation in heart transplant recipients. Cochrane Database Syst Rev. 2017;4:CD012264. doi: 10.1002/14651858.CD012264.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mathur S, Janaudis-Ferreira T, Wickerson L, Singer LG, Patcai J, Rozenberg D, Blydt-Hansen T, Hartmann EL, Haykowsky M, Helm D, et al. Meeting report: consensus recommendations for a research agenda in exercise in solid organ transplantation. Am J Transplant. 2014;14:2235–2245. doi: 10.1111/ajt.12874. [DOI] [PubMed] [Google Scholar]
- 45.Moholdt T, Aamot IL, Granøien I, Gjerde L, Myklebust G, Walderhaug L, Hole T, Graven T, Stølen T, Mølmen-Hansen HE, et al. Long-term follow-up after cardiac rehabilitation: a randomized study of usual care exercise training versus aerobic interval training after myocardial infarction. Int J Cardiol. 2011;152:388–390. doi: 10.1016/j.ijcard.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 46.Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, Lakatta EG. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 47.Betik AC, Hepple RT. Determinants of VO2 max decline with aging: an integrated perspective. Appl Physiol Nutr Metab. 2008;33:130–140. doi: 10.1139/H07-174. [DOI] [PubMed] [Google Scholar]
- 48.Ismail H, McFarlane JR, Nojoumian AH, Dieberg G, Smart NA. Clinical outcomes and cardiovascular responses to different exercise training intensities in patients with heart failure: a systematic review and meta-analysis. JACC Heart Fail. 2013;1:514–522. doi: 10.1016/j.jchf.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 49.Kemi OJ, Wisloff U. High-intensity aerobic exercise training improves the heart in health and disease. J Cardiopulm Rehabil Prev. 2010;30:2–11. doi: 10.1097/HCR.0b013e3181c56b89. [DOI] [PubMed] [Google Scholar]
- 50.Rustad LA, Nytrøen K, Amundsen BH, Gullestad L, Aakhus S. One year of high-intensity interval training improves exercise capacity, but not left ventricular function in stable heart transplant recipients: a randomised controlled trial. Eur J Prev Cardiol. 2014;21:181–191. doi: 10.1177/2047487312469477. [DOI] [PubMed] [Google Scholar]
- 51.Dall CH, Gustafsson F, Christensen SB, Dela F, Langberg H, Prescott E. Effect of moderate- versus high-intensity exercise on vascular function, biomarkers and quality of life in heart transplant recipients: A randomized, crossover trial. J Heart Lung Transplant. 2015;34:1033–1041. doi: 10.1016/j.healun.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Yardley M, Ueland T, Aukrust P, Michelsen A, Bjørkelund E, Gullestad L, Nytrøen K. Immediate response in markers of inflammation and angiogenesis during exercise: a randomised cross-over study in heart transplant recipients. Open Heart. 2017;4:e000635. doi: 10.1136/openhrt-2017-000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallén NH, Goodall AH, Li N, Hjemdahl P. Activation of haemostasis by exercise, mental stress and adrenaline: effects on platelet sensitivity to thrombin and thrombin generation. Clin Sci (Lond) 1999;97:27–35. [PubMed] [Google Scholar]
- 54.Crimi E, Ignarro LJ, Cacciatore F, Napoli C. Mechanisms by which exercise training benefits patients with heart failure. Nat Rev Cardiol. 2009;6:292–300. doi: 10.1038/nrcardio.2009.8. [DOI] [PubMed] [Google Scholar]
- 55.Hambrecht R, Adams V, Erbs S, Linke A, Kränkel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, et al. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107:3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Li M, Dong F, Zhang J, Zhang F. Physical exercise-induced protection on ischemic cardiovascular and cerebrovascular diseases. Int J Clin Exp Med. 2015;8:19859–19866. [PMC free article] [PubMed] [Google Scholar]
- 57.Kilian Y, Wehmeier UF, Wahl P, Mester J, Hilberg T, Sperlich B. Acute Response of Circulating Vascular Regulating MicroRNAs during and after High-Intensity and High-Volume Cycling in Children. Front Physiol. 2016;7:92. doi: 10.3389/fphys.2016.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Delavar H, Nogueira L, Wagner PD, Hogan MC, Metzger D, Breen EC. Skeletal myofiber VEGF is essential for the exercise training response in adult mice. Am J Physiol Regul Integr Comp Physiol. 2014;306:R586–R595. doi: 10.1152/ajpregu.00522.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olfert IM, Baum O, Hellsten Y, Egginton S. Advances and challenges in skeletal muscle angiogenesis. Am J Physiol Heart Circ Physiol. 2016;310:H326–H336. doi: 10.1152/ajpheart.00635.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoier B, Hellsten Y. Exercise-induced capillary growth in human skeletal muscle and the dynamics of VEGF. Microcirculation. 2014;21:301–314. doi: 10.1111/micc.12117. [DOI] [PubMed] [Google Scholar]
- 61.Avery RK. Cardiac-allograft vasculopathy. N Engl J Med. 2003;349:829–830. doi: 10.1056/NEJMp038124. [DOI] [PubMed] [Google Scholar]
- 62.Jansen MA, Otten HG, de Weger RA, Huibers MM. Immunological and Fibrotic Mechanisms in Cardiac Allograft Vasculopathy. Transplantation. 2015;99:2467–2475. doi: 10.1097/TP.0000000000000848. [DOI] [PubMed] [Google Scholar]
- 63.Tuzcu EM, Kapadia SR, Sachar R, Ziada KM, Crowe TD, Feng J, Magyar WA, Hobbs RE, Starling RC, Young JB, et al. Intravascular ultrasound evidence of angiographically silent progression in coronary atherosclerosis predicts long-term morbidity and mortality after cardiac transplantation. J Am Coll Cardiol. 2005;45:1538–1542. doi: 10.1016/j.jacc.2004.12.076. [DOI] [PubMed] [Google Scholar]
- 64.Kobashigawa JA, Tobis JM, Starling RC, Tuzcu EM, Smith AL, Valantine HA, Yeung AC, Mehra MR, Anzai H, Oeser BT, et al. Multicenter intravascular ultrasound validation study among heart transplant recipients: outcomes after five years. J Am Coll Cardiol. 2005;45:1532–1537. doi: 10.1016/j.jacc.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 65.Potena L, Masetti M, Sabatino M, Bacchi-Reggiani ML, Pece V, Prestinenzi P, Dall’Ara G, Taglieri N, Saia F, Fallani F, et al. Interplay of coronary angiography and intravascular ultrasound in predicting long-term outcomes after heart transplantation. J Heart Lung Transplant. 2015;34:1146–1153. doi: 10.1016/j.healun.2015.01.990. [DOI] [PubMed] [Google Scholar]
- 66.Kobashigawa JA, Katznelson S, Laks H, Johnson JA, Yeatman L, Wang XM, Chia D, Terasaki PI, Sabad A, Cogert GA. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333:621–627. doi: 10.1056/NEJM199509073331003. [DOI] [PubMed] [Google Scholar]
- 67.Arora S, Andreassen AK, Andersson B, Gustafsson F, Eiskjaer H, Bøtker HE, Rådegran G, Gude E, Ioanes D, Solbu D, Sigurdardottir V, Dellgren G, Erikstad I, Solberg OG, Ueland T, Aukrust P, Gullestad L; SCHEDULE (SCandinavian HEart transplant everolimus De novo stUdy with earLy calcineurin inhibitors avoidancE) Investigators. The Effect of Everolimus Initiation and Calcineurin Inhibitor Elimination on Cardiac Allograft Vasculopathy in De Novo Recipients: One-Year Results of a Scandinavian Randomized Trial. Am J Transplant. 2015;15:1967–1975. doi: 10.1111/ajt.13214. [DOI] [PubMed] [Google Scholar]
- 68.Andreassen AK, Andersson B, Gustafsson F, Eiskjaer H, Rådegran G, Gude E, Jansson K, Solbu D, Karason K, Arora S, et al. Everolimus Initiation With Early Calcineurin Inhibitor Withdrawal in De Novo Heart Transplant Recipients: Three-Year Results From the Randomized SCHEDULE Study. Am J Transplant. 2016;16:1238–1247. doi: 10.1111/ajt.13588. [DOI] [PubMed] [Google Scholar]
- 69.DeCampli WM. Of mice and men ... does exercise decrease progression of transplant coronary vasculopathy? J Thorac Cardiovasc Surg. 2015;149:337–339. doi: 10.1016/j.jtcvs.2014.10.107. [DOI] [PubMed] [Google Scholar]
- 70.Madssen E, Moholdt T, Videm V, Wisløff U, Hegbom K, Wiseth R. Coronary atheroma regression and plaque characteristics assessed by grayscale and radiofrequency intravascular ultrasound after aerobic exercise. Am J Cardiol. 2014;114:1504–1511. doi: 10.1016/j.amjcard.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 71.Lavie CJ, Arena R, Swift DL, Johannsen NM, Sui X, Lee DC, Earnest CP, Church TS, O’Keefe JH, Milani RV, et al. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ Res. 2015;117:207–219. doi: 10.1161/CIRCRESAHA.117.305205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lavie CJ, Church TS, Milani RV, Earnest CP. Impact of physical activity, cardiorespiratory fitness, and exercise training on markers of inflammation. J Cardiopulm Rehabil Prev. 2011;31:137–145. doi: 10.1097/HCR.0b013e3182122827. [DOI] [PubMed] [Google Scholar]
- 73.Cornish AK, Broadbent S, Cheema BS. Interval training for patients with coronary artery disease: a systematic review. Eur J Appl Physiol. 2011;111:579–589. doi: 10.1007/s00421-010-1682-5. [DOI] [PubMed] [Google Scholar]
- 74.Arora S, Erikstad I, Ueland T, Sigurdardottir V, Ekmehag B, Jansson K, Eiskjaer H, Bøtker HE, Mortensen SA, Saunamaki K, et al. Virtual histology assessment of cardiac allograft vasculopathy following introduction of everolimus--results of a multicenter trial. Am J Transplant. 2012;12:2700–2709. doi: 10.1111/j.1600-6143.2012.04234.x. [DOI] [PubMed] [Google Scholar]
- 75.Grady KL, Naftel DC, Kobashigawa J, Chait J, Young JB, Pelegrin D, Czerr J, Heroux A, Higgins R, Rybarczyk B, et al. Patterns and predictors of quality of life at 5 to 10 years after heart transplantation. J Heart Lung Transplant. 2007;26:535–543. doi: 10.1016/j.healun.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karam VH, Gasquet I, Delvart V, Hiesse C, Dorent R, Danet C, Samuel D, Charpentier B, Gandjbakhch I, Bismuth H, et al. Quality of life in adult survivors beyond 10 years after liver, kidney, and heart transplantation. Transplantation. 2003;76:1699–1704. doi: 10.1097/01.TP.0000092955.28529.1E. [DOI] [PubMed] [Google Scholar]
- 77.Wu YT, Chien CL, Chou NK, Wang SS, Lai JS, Wu YW. Efficacy of a home-based exercise program for orthotopic heart transplant recipients. Cardiology. 2008;111:87–93. doi: 10.1159/000119695. [DOI] [PubMed] [Google Scholar]
- 78.Karapolat H, Eyigor S, Durmaz B, Nalbantgil S, Yagdi T, Zoghi M. The effect of functional performance, respiratory function and osteopenia on the quality of life after heart transplantation. Int J Cardiol. 2008;124:381–383. doi: 10.1016/j.ijcard.2006.12.053. [DOI] [PubMed] [Google Scholar]
- 79.Christensen SB, Dall CH, Prescott E, Pedersen SS, Gustafsson F. A high-intensity exercise program improves exercise capacity, self-perceived health, anxiety and depression in heart transplant recipients: a randomized, controlled trial. J Heart Lung Transplant. 2012;31:106–107. doi: 10.1016/j.healun.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 80.Dew MA, DiMartini AF. Psychological disorders and distress after adult cardiothoracic transplantation. J Cardiovasc Nurs. 2005;20:S51–S66. doi: 10.1097/00005082-200509001-00007. [DOI] [PubMed] [Google Scholar]
- 81.Sivertsen B, Relbo A, Gullestad L, Hellesvik M, Grov I, Andreassen A, Simonsen S, Geiran O, Havik OE. [Self-assessed health and psychological symptoms after heart transplantation] Tidsskr Nor Laegeforen. 2007;127:3198–3201. [PubMed] [Google Scholar]
- 82.Dew MA, Rosenberger EM, Myaskovsky L, DiMartini AF, DeVito Dabbs AJ, Posluszny DM, Steel J, Switzer GE, Shellmer DA, Greenhouse JB. Depression and Anxiety as Risk Factors for Morbidity and Mortality After Organ Transplantation: A Systematic Review and Meta-Analysis. Transplantation. 2015;100:988–1003. doi: 10.1097/TP.0000000000000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karapolat H, Eyigor S, Durmaz B, Yagdi T, Nalbantgil S, Karakula S. The relationship between depressive symptoms and anxiety and quality of life and functional capacity in heart transplant patients. Clin Res Cardiol. 2007;96:593–599. doi: 10.1007/s00392-007-0536-6. [DOI] [PubMed] [Google Scholar]
- 84.Ulubay G, Ulasli SS, Sezgin A, Haberal M. Assessing exercise performance after heart transplantation. Clin Transplant. 2007;21:398–404. doi: 10.1111/j.1399-0012.2007.00658.x. [DOI] [PubMed] [Google Scholar]
- 85.Bernardi L, Radaelli A, Passino C, Falcone C, Auguadro C, Martinelli L, Rinaldi M, Viganò M, Finardi G. Effects of physical training on cardiovascular control after heart transplantation. Int J Cardiol. 2007;118:356–362. doi: 10.1016/j.ijcard.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 86.Carter R, Al-Rawas OA, Stevenson A, Mcdonagh T, Stevenson RD. Exercise responses following heart transplantation: 5 year follow-up. Scott Med J. 2006;51:6–14. doi: 10.1258/RSMSMJ.51.3.6. [DOI] [PubMed] [Google Scholar]
- 87.Ewert R, Wensel R, Bruch L, Mutze S, Bauer U, Plauth M, Kleber FX. Relationship between impaired pulmonary diffusion and cardiopulmonary exercise capacity after heart transplantation. Chest. 2000;117:968–975. doi: 10.1378/chest.117.4.968. [DOI] [PubMed] [Google Scholar]
- 88.Givertz MM, Hartley LH, Colucci WS. Long-term sequential changes in exercise capacity and chronotropic responsiveness after cardiac transplantation. Circulation. 1997;96:232–237. doi: 10.1161/01.cir.96.1.232. [DOI] [PubMed] [Google Scholar]
- 89.Gullestad L, Myers J, Edvardsen T, Kjekshus J, Geiran O, Simonsen S. Predictors of exercise capacity and the impact of angiographic coronary artery disease in heart transplant recipients. Am Heart J. 2004;147:49–54. doi: 10.1016/j.ahj.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 90.Habedank D, Ewert R, Hummel M, Wensel R, Hetzer R, Anker SD. Changes in exercise capacity, ventilation, and body weight following heart transplantation. Eur J Heart Fail. 2007;9:310–316. doi: 10.1016/j.ejheart.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 91.Haykowsky M, Taylor D, Kim D, Tymchak W. Exercise training improves aerobic capacity and skeletal muscle function in heart transplant recipients. Am J Transplant. 2009;9:734–739. doi: 10.1111/j.1600-6143.2008.02531.x. [DOI] [PubMed] [Google Scholar]
- 92.Hognestad A, Holm T, Simonsen S, Kjekshus J, Andreassen AK. Serial measurements of peripheral vascular reactivity and exercise capacity in congestive heart failure and after heart transplantation. J Card Fail. 2005;11:447–454. doi: 10.1016/j.cardfail.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 93.Karapolat H, Eyigor S, Zoghi M, Yagdi T, Nalbantgil S, Durmaz B, Ozbaran M. Effects of cardiac rehabilitation program on exercise capacity and chronotropic variables in patients with orthotopic heart transplant. Clin Res Cardiol. 2008;97:449–456. doi: 10.1007/s00392-008-0648-7. [DOI] [PubMed] [Google Scholar]
- 94.Kavanagh T, Mertens DJ, Shephard RJ, Beyene J, Kennedy J, Campbell R, Sawyer P, Yacoub M. Long-term cardiorespiratory results of exercise training following cardiac transplantation. Am J Cardiol. 2003;91:190–194. doi: 10.1016/s0002-9149(02)03108-9. [DOI] [PubMed] [Google Scholar]
- 95.Kemp DL, Jennison SH, Stelken AM, Younis LT, Miller LW. Association of resting heart rate and chronotropic response. Am J Cardiol. 1995;75:751–752. doi: 10.1016/S0002-9149(99)80674-2. [DOI] [PubMed] [Google Scholar]
- 96.Kobashigawa JA, Leaf DA, Lee N, Gleeson MP, Liu H, Hamilton MA, Moriguchi JD, Kawata N, Einhorn K, Herlihy E, et al. A controlled trial of exercise rehabilitation after heart transplantation. N Engl J Med. 1999;340:272–277. doi: 10.1056/NEJM199901283400404. [DOI] [PubMed] [Google Scholar]
- 97.Renlund DG, Taylor DO, Ensley RD, O’Connell JB, Gilbert EM, Bristow MR, Ma H, Yanowitz FG. Exercise capacity after heart transplantation: influence of donor and recipient characteristics. J Heart Lung Transplant. 1996;15:16–24. [PubMed] [Google Scholar]
- 98.Schwaiblmair M, von Scheidt W, Uberfuhr P, Reichart B, Vogelmeier C. Lung function and cardiopulmonary exercise performance after heart transplantation: influence of cardiac allograft vasculopathy. Chest. 1999;116:332–339. doi: 10.1378/chest.116.2.332. [DOI] [PubMed] [Google Scholar]
- 99.Squires RW. Exercise therapy for cardiac transplant recipients. Prog Cardiovasc Dis. 2011;53:429–436. doi: 10.1016/j.pcad.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 100.Tegtbur U, Busse MW, Jung K, Pethig K, Haverich A. Time course of physical reconditioning during exercise rehabilitation late after heart transplantation. J Heart Lung Transplant. 2005;24:270–274. doi: 10.1016/j.healun.2003.12.010. [DOI] [PubMed] [Google Scholar]


