ABSTRACT
Epithelia are highly polarised tissues and several highly conserved polarity protein complexes serve to establish and maintain polarity. The transmembrane protein Crumbs (Crb), the central component of the Crb protein complex, is required, among others, for the maintenance of polarity in most epithelia in the Drosophila embryo. However, different epithelia exhibit different phenotypic severity upon loss of crb. Using a transgenomic approach allowed us to more accurately define the role of crb in different epithelia. In particular, we provide evidence that the loss of epithelial tissue integrity in the ventral epidermis of crb mutant embryos is due to impaired actomyosin activity and an excess number of neuroblasts. We demonstrate that the intracellular domain of Crb could only partially rescue this phenotype, while it is able to completely restore tissue integrity in other epithelia. Based on these results we suggest a dual role of the extracellular domain of Crb in the ventral neuroectoderm. First, it is required for apical enrichment of the Crb protein, which in turn regulates actomyosin activity and thereby ensures tissue integrity; and second, the extracellular domain of Crb stabilises the Notch receptor and thereby ensures proper Notch signalling and specification of the correct number of neuroblasts.
KEY WORDS: Notch, Actomyosin, Polarity, Adhesion, Neurogenesis
Summary: Using a transgenomic approach we determine specific roles of the intra- and extracellular domain of the Crumbs protein for the maintenance of apico-basal epithelial polarity and epithelial morphogenesis in Drosophila embryos.
INTRODUCTION
Epithelia are highly polarised tissues that can be specialised for protection, absorption, secretion, transport or sensory perceptions. Hence, mechanisms controlling polarity and integrity of epithelial tissues are important for shaping tissues during development. In addition, maintaining polarity is essential for tissue homeostasis in adult organisms, which is reflected by the fact that 80-90% of all cancer types derive from epithelia (Cao et al., 2015; Grifoni et al., 2013; Laprise, 2011; McCaffrey and Macara, 2011; Tellkamp et al., 2014). Therefore, unravelling the basis of epithelial polarity and the mechanisms required to maintain tissue integrity is crucial to understand the origin of various diseases. Drosophila embryonic epithelia are excellent model tissues to study the genetic, molecular and cellular basis of development and maintenance of polarity. In particular, studies focussing on the embryonic epidermis have provided deep insight into the regulation of tissue polarity and integrity. The epidermis is subject to mechanical stress during various morphogenetic events, such as germ band extension or retraction, yet it is maintained as a properly polarised, coherent mono-layered sheet during these processes. Work from many groups have shown that elaborated adherens junctions (AJs), in particular the zonula adherens (ZA), a belt-like structure encircling the apex of the cell, is instrumental to provide adhesive strength in order to counteract mechanical forces, but at the same time is flexible to allow tissue movements and changes during morphogenesis (Harris and Tepass, 2010; Macara et al., 2014; Oda and Takeichi, 2011). Formation and maintenance of the ZA depends, among others, on proper apico-basal cell polarity. Polarity is established and maintained by a crosstalk between the polarised trafficking machinery and a polarised cytoskeleton, orchestrated by a sophisticated interplay of proteins forming the ‘epithelial polarity program’ (Rodriguez-Boulan and Macara, 2014). Three major evolutionarily conserved protein modules, the apical Par- and Crumbs (Crb)-complexes and the baso-lateral Lgl/Scrib/Dlg-module, act as key regulators of epithelial polarity and tissue integrity in various epithelia (reviewed in Campanale et al., 2017; Flores-Benitez and Knust, 2016; Macara et al., 2014; Tepass, 2012).
Initially discovered in a screen for genes with sequence homology to the neurogenic genes Notch and Delta (Knust et al., 1987), Drosophila Crb, the founding member of the Crb-complex, subsequently emerged as an evolutionarily conserved polarity regulator conserved from worms to human. crb genes encode type 1 transmembrane proteins, which are enriched at the sub-apical region right apical to the ZA. The cytoplasmic domains of Crb proteins are highly conserved and characterised by a C-terminal PDZ- (PSD-95, Dlg, ZO-1)-binding motif (PBM) and an N-terminal FERM- (4.1, ezrin, radixin, moesin)-domain binding motif (FBM). Similarly, the binding partners are highly conserved, including the PDZ-proteins Stardust (Sdt) and DmPar6 of Drosophila and their mammalian orthologues MPP5/PALS1 and Par6, respectively, and the FERM-proteins Moesin and Yurt/Mosaic eyes-like 1 (YMO1/EPB41L5) (Gosens et al., 2007; Le Bivic, 2013; Tepass, 2009). Loss or increased levels of Crb lead to disruption of apico-basal polarity and a breakdown of the mono-layered embryonic epithelial structure, followed by embryonic lethality (Tepass et al., 1990; Wodarz et al., 1993, 1995). This suggests that Crb levels at the apical membrane are crucial for the maintenance of polarity and tissue integrity. Multiple mechanisms contribute to maintain appropriate levels of Crb at the subapical region, including trafficking to and from the apical plasma membrane (Blankenship et al., 2007; Lin et al., 2015; Lu and Bilder, 2005; Pocha and Wassmer, 2011; Shivas et al., 2010; Zhou et al., 2011), as well as stabilisation at the membrane via the intra- or extracellular domain (Bachmann et al., 2001; Hong et al., 2001; Kempkens et al., 2006; Letizia et al., 2013).
Whereas Drosophila contains only one crb gene, Caenorhabditis elegans, zebrafish, mouse and human genomes encode more than one crb orthologues. In all crb genes described so far, the short intracellular domain (ICD) is highly conserved. In contrast, based on the extracellular domain (ECD), C. elegans and vertebrate crb genes can be subdivided into two groups: one group (Crb1 and Crb2) encodes proteins with a large ECD similar as the one found in Drosophila Crb, which is characterised by an array of variable numbers of epidermal growth factor (EGF)-like repeats interspersed by repeats with similarity to the globular domain of Laminin A. The second group (Crb3) encodes transmembrane proteins containing a very short ECD with no similarity to that of the first group. While many Crb-dependent functions, such as regulation of polarity and cytoskeleton activity, could be allocated to the short ICD, the role of the long ECD is still elusive. Earlier studies have suggested that homophilic interactions between the ECDs are responsible for stabilising the protein at the membrane, thus ensuring proper protein levels and maintenance of cell polarity in the embryonic epidermis (Letizia et al., 2013; Thompson et al., 2013). In addition, anisotropic distribution of Crb protein in neighbouring cells, mediated by the ECDs, has been proposed to be required for the recruitment of a circumferential actomyosin cable in cells with low Crb, which drives tissue invagination during tube formation in the embryo (Röper, 2012). In the zebrafish eye, homo- and heterophilic interactions between the ECDs of Crb2a/Crb2b in cone photoreceptor cells are required for proper patterning of the retina (Raymond et al., 2014; Zou et al., 2012; reviewed in Pocha and Knust, 2013; Thompson et al., 2013). In Drosophila eye and wing imaginal discs the ECD has been implicated in growth control (Richardson and Pichaud, 2010). Recently, we could show that the ECD of Crb stabilises the Notch-receptor in the apical membrane, thus preventing ligand-independent Notch signalling during vein formation in the pupal wing (Nemetschke and Knust, 2016).
Interestingly, although crb is expressed in all embryonic epithelia derived from the ectoderm, defects in epithelia of crb mutant embryos range from complete disintegration and widespread apoptosis (e.g. in some parts of the epidermis) to no obvious polarity defect at all (e.g. in the hindgut) (Tepass and Knust, 1990). The defects can even differ in the same tissue. For example, the ventral epidermis was shown to be more affected than the dorsal epidermis upon loss of crb (Kolahgar et al., 2011). The analysis to explain the different phenotypic severity has been hampered by two facts. (i) Most results obtained so far were based on overexpression studies of full-length Crb proteins or just part of it, using the Gal4/UAS system. Thereby it was shown that the membrane-bound ICD is able to restore polarity and tissue integrity in many epithelia to the same degree as the full-length protein. However, in this experimental set-up, neither the ICD nor the full-length protein can rescue embryonic lethality of crb mutant embryos (Klebes and Knust, 2000; Wodarz et al., 1995). In addition, expression of rescue constructs using the Gal4/UAS system results in excessive protein, thus hindering the analysis of tissue sensitivity towards differential levels of Crb. In contrast, a fosmid encoding the complete genomic locus of crb (called foscrb), the expression of which is under endogenous control, rescues all aspects associated with loss of crb and gives rise to viable and fertile adults (Klose et al., 2013). (ii) The second impediment to achieve an in-depth understanding of epithelia-specific roles of Crb is the lack of appropriate hypomophic alleles. Embryos homozygous mutant for amorphic crb alleles display severe defects in early embryogenesis leading to massive apoptosis, which makes it difficult to unveil tissue-specific functions of Crb.
In order to understand the full potential of the ICD and ECD of Crb and their requirements in different epithelia, we engineered foscrb to create flies containing either foscrbICD or foscrbECD, which encode the membrane-bound ICD and ECD, respectively. We show that fosmid-based expression of the ICD not only rescues polarity defects in most epithelia of crb mutant embryos, but also, and in contrast to results obtained from overexpression studies, is sufficient for proper invagination and morphogenesis of epithelial tubes and, strikingly, for viability in about 50% of cases. In addition, we provide data to show that the strong phenotype of the ventral epidermis of crb mutant embryos can be traced back to a neurogenic phenotype due to the development of an excess of neuroblasts. This phenotype could only partially be rescued by expressing the ICD only. This suggests an essential role of the ECD for maintaining the integrity of the neuroectoderm. Here, we propose two mechanisms by which the ECD mediates this function. First, it is required for apical enrichment of Crb, which, in turn, controls actomyosin activity; and second, the ECD ensures apical Notch localization and proper signalling in the neuroectoderm, and thus prevents the formation of supernumerary neuroblasts.
RESULTS
The extracellular domain of Crb is not essential for embryogenesis
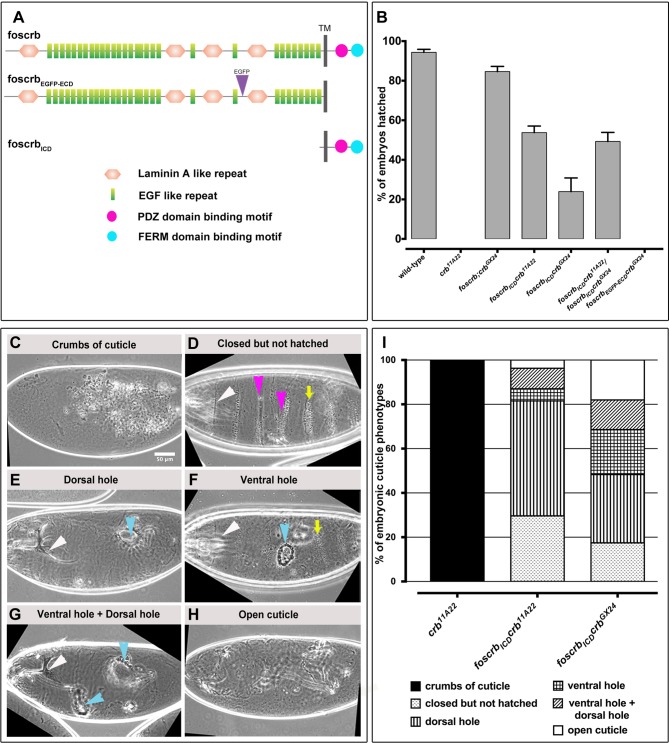
Gal4-mediated overexpression of the membrane-bound intracellular domain of Crb can suppress the phenotype of crb mutant embryos to the same degree as overexpression of a full-length Crb protein (Klebes and Knust, 2000; Wodarz et al., 1995). In contrast, fosmids containing the entire crb locus (foscrb and foscrb-EGFP) can completely rescue the lethality of amorphic crb11A22 and crbGX24 alleles (Klose et al., 2013). In order to dissect the functions of the intracellular and extracellular domain of Crb (here called ICD and ECD, respectively) in embryonic development under physiological conditions, we modified foscrb and foscrbEGFP to generate two transgenes. foscrbICD encodes a membrane bound ICD of Crb, in which the ECD was deleted with the exception of the C-terminal 8 amino acids (KEAYFNGS). foscrbECD-EGFP encodes an Enhanced Green Fluorescent Protein (EGFP)-tagged membrane-bound ECD, in which most of the ICD has been deleted with the exception of the N-terminal 7 amino acids (MARNKRAT) (Fig. 1A). foscrbICD, foscrbECD-EGFP as well as a fosmid encoding EGFP-tagged full-length Crb proteins (foscrbEGFP) were integrated into the VK00033 landing site (chromosomal location 65B2 on 3L). The three transgenic lines were recombined with crb11A22 and crbGX24 alleles. foscrbEGFP crb11A22 and foscrbEGFP crbGX24 were viable and fertile and could be maintained as homozygous stocks, similar as foscrb;crbGX24 (Klose et al., 2013).
Fig. 1.
The ICD of Crb restores epithelial integrity of crb mutant embryos. (A) Schematic representation of the fosmids. Depicted proteins are based on the Crb-PA isoform (2.146 amino acids). Adopted from Klose et al. (2013). (B) Analysis of embryonic lethality. The graph represents the percent of embryos that hatch. N>1000 embryos. The experiment was repeated 5 times. Error bars show standard error of the mean. (C-I) Classification of cuticle phenotypes of unhatched embryos. White arrowhead (D,E,F,G), intact head structure; yellow arrow (D,F), intact denticle belts; magenta arrowheads (D), fused or lost denticle belts; cyan arrowhead (E,F), dorsal/ventral hole, respectively; cyan arrowheads (G), ventral and dorsal hole. Scale bar: 50 μm. (J) Quantification of cuticle phenotypes. N>300 embryos. The experiment was repeated 3 times.
To test the rescuing activity of the ICD and ECD of Crb, we analysed the percentage of homozygous crb mutant larvae carrying two copies of the respective transgenes (Fig. 1B). While none of the homozygous crb11A22 embryos hatched, 95% of the wild-type embryos and 85% of crbGX24 mutant embryos expressing foscrb hatched. foscrbECD-EGFP completely failed to rescue homozygous crb null embryos (Fig. 1B). Strikingly, 50% and 25% of homozygous crb11A22 and crbGX24 embryos, respectively, that express foscrbICD, hatched, but these larvae died as first or second instar. Since 50% of crb11A22/crbGX24 transheterozygous embryos expressing foscrbICD hatched, the lower hatching rate in foscrbICD crbGX24 embryos is likely due to the genetic background. Therefore, further analyses were carried out with foscrbICD crb11A22 embryos (referred to as foscrbICD crb henceforth).
The crb locus was named according to its cuticle phenotype, which reveals only ‘crumbs’ of cuticle instead of a continuous cuticle, due to a complete breakdown and death of the epidermis (Jürgens et al., 1984; Tepass and Knust, 1990) (Fig. 1C). To further determine to what extent the different transgenes could suppress the crb mutant phenotype in those embryos that did not hatch, we quantified the cuticle phenotypes of embryos with different genotypes (Fig. 1C-H and I). Homozygous foscrbICD crb11A22 and foscrbICD crbGX24 embryos showed variable cuticle phenotypes. 20-30% of embryos formed a continuous, nearly wild-type cuticle (Fig. 1D) with intact head structures (white arrowhead) and denticle belts (yellow arrow), which were occasionally merged or absent (magenta arrowheads). Others showed a dorsal hole (cyan arrowhead in Fig. 1E), a ventral hole (cyan arrowhead in Fig. 1F) or ventral and dorsal holes (cyan arrowheads in Fig. 1G). foscrbECD-EGFP crb11A22 embryos did not show any rescuing activity and resemble crb mutant embryos without any transgene (data not shown). Together these results show that the ICD of Crb is indispensable for the function of Crb in maintaining embryonic epithelial integrity, but is insufficient to ensure robustness in completion of successful embryogenesis and proper larval development.
The ICD of Crb restores integrity of most embryonic epithelia
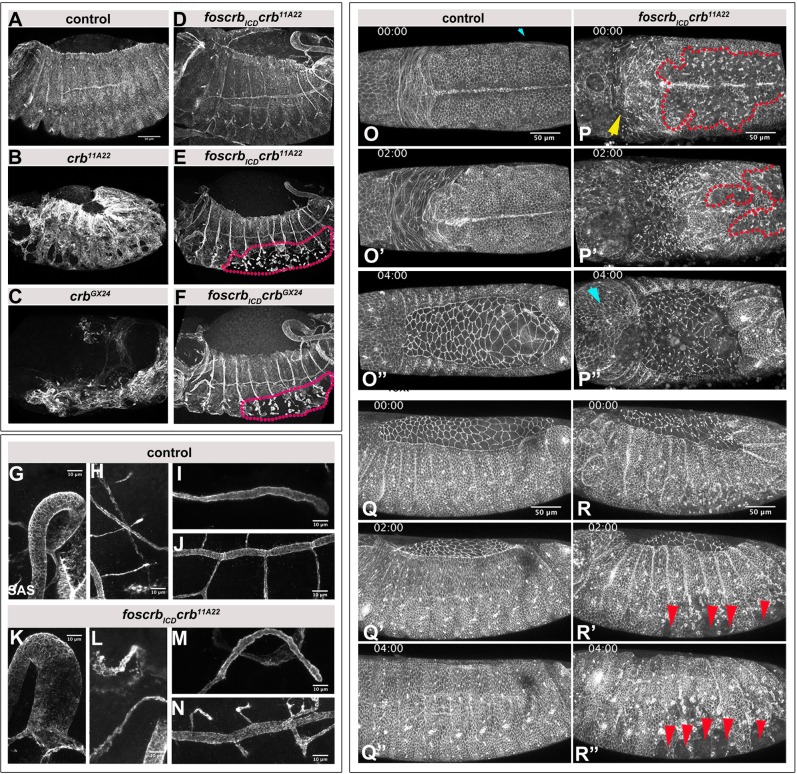
The dorsal and ventral holes in the cuticle of foscrbICD crb mutant embryos implied that the integrity of some epithelia was not completely restored in the absence of ECD. To corroborate this assumption, epithelial integrity was analysed in stage 12/13 embryos using the apical marker Stranded-at-second, SAS (Fig. 2). This revealed that the sheet-like structure and integrity of the epidermis as seen in control embryos (Fig. 2A) is completely lost in crb11A22 (Fig. 2B) and crbGX24 (Fig. 2C) embryos. Strikingly, some crb embryos expressing foscrbICD showed a completely restored epidermis (Fig. 1D). Other embryos had intact head and dorsal epidermis, but exhibited a disintegrated ventral epidermis (magenta region in Fig. 2E,F). In all cases, invagination and development of epithelial tubes, such as the hindgut, the Malpighian tubules, the salivary glands and the tracheae occurred properly in crb embryos expressing foscrbICD (Fig. 2G-N).
Fig. 2.
The Crb ICD is sufficient for tissue integrity of most embryonic epithelia. (A-F) Stage 12-13 embryos, stained for SAS. Dotted lines in E and F mark the disintegrated ventral epidermis. Dorsal is up, anterior left. Scale bar: 50 μm. The experiment was repeated 3 times. (G-N) Stage 12-13 foscrb; crbGX24 control (G-J) and foscrbICD crb11A22 (K-N) embryos, stained with anti-SAS. Polarity of epithelial tubes is restored in the hindgut (G,K), the Malpighian tubules (H,L), the salivary gland (I,M) and the trachea (J,N). Scale bar: 10 μm. (O-R″) Stills of time lapse movies of endogenously tagged DE-Cadherin-GFP lines. Dorsal (O-P″) and lateral (Q-R″) views of foscrb; crbGX24 control and foscrbICD crb11A22 embryos. Red dotted lines in P,P′ mark the disintegrated ventral epidermis. Yellow arrow in P, disintegrated head epidermis; cyan arrowhead in P″, recovered head epidermis; red arrowheads in R′,R″, ‘wounds’ in ventral epidermis. Anterior is to the left. Scale bar: 50 μm. The experiment was repeated 3 times.
To better understand the development of the mutant phenotype in the ventral epidermis of foscrbICD crb mutant embryos, we imaged endogenously tagged DE-Cadherin-GFP in embryos of the respective genotypes (Fig. 2O-O″,P-P″,Q-Q″,R-R″; Movies 1 and 2). The defects in the ventral epidermis (region demarcated by red dots in Fig. 2P,P′) were already evident in embryos prior to the onset of germ band retraction in foscrbICD crb mutant embryos. In addition, the head epidermis displayed loss of DE-Cadherin already at this stage (yellow arrow in Fig. 2P). The defects in the ventral epidermis (Fig. 2P′) persisted as germ band retraction proceeded, whereas the head epidermis seemed to recover by the end of germ band retraction (cyan arrow in Fig. 2P″). During the stage of dorsal closure, the ventral epidermis of foscrbICD crb mutant embryos displayed multiple, ‘wound’-like gaps (red arrows in Fig. 2R′ and R″), while the epidermis stayed intact during germ band retraction and dorsal closure of control embryos (Fig. 2O-O″ and Q-Q″). Closer examination of the ventral epidermis in the mutant embryos revealed that the cells gradually rearranged, resulting in the transformation of a mono-layered sheet into multiple, ‘cyst’-like structures (Fig. S1A-A″″, Movie 3). As development proceeds, the ventral epidermis of some of the crb mutant embryos expressing foscrbICD ripped apart (Fig. S1D-D″). In other embryos of the same genotype, however, the epidermis sealed these gaps later during embryogenesis (Fig. S1C-C″, Movie 4).
We reasoned that the dorsal hole detected in cuticle preparations of foscrbICD crb mutant embryos may be due to defects in the development of the amnioserosa, an extraembryonic tissue that covers the dorsal side of the embryo. During dorsal closure, the lateral epidermis moves dorsal wards, while the amnioserosa is internalised. Finally, zippering of the two sides of the epidermis closes the embryo dorsally (Fig. S2A-A″″) (reviewed in Hayes and Solon, 2017). While in crb mutant embryos the amnioserosa disintegrates already during germband extension (Grawe et al., 1996; Tepass, 1996), expression of the ICD of Crb in these embryos prevents the collapse of the amnioserosa at early stages. However, at the end of germ band extension and later on, defects in DE-cadherin staining were obvious (Fig. S2B-B″). In addition, the zippering process is impaired in a subset of mutant embryos. Posterior zippering is not initiated (green arrows in Fig. S2B), and, as a consequence, dorsal closure fails (Fig. S2B-B″″).
These results demonstrate that the ICD of Crb is sufficient for maintaining integrity of the dorsal epidermis and for invagination of epithelial tubes, but is insufficient to maintain integrity of the ventral epidermis and the amnioserosa.
Apico-basal polarity is restored in the epidermis of crb embryos expressing the intracellular domain
Since Crb is required for the maintenance of apico-basal polarity in many embryonic epithelia, we reasoned that the defects observed in the ventral epidermis of foscrbICD crb mutant embryos may be due to incomplete restoration of polarity. Therefore, we scored mutant embryos for the distribution of apical SAS and lateral FasIII, both in the dorsal and in the ventral epidermis (Fig. 3). While the dorsal epidermis of crb mutant embryos showed defects in polarity and tissue integrity, the dorsal epidermis of foscrbICD crb embryos developed as a continuous, polarised epithelium, similar as in control embryos (Fig. 3A-I′). Numerous filopodial projections emerged from the dorsal-most epidermal cells of control embryos (magenta arrows in Fig. 3A) (Kaltschmidt et al., 2002). These filopodia were lost in crb mutant embryos (Fig. 3D-F), but only incompletely restored in foscrbICD crb mutant embryos (Fig. 3G). The sheet-like organisation of the ventral epidermis was severely disrupted in crb mutant embryos, which was associated with a complete loss of apico-basal polarity (compare Fig. 3M-O′ with J-L′). The ICD alone was unable to restore tissue integrity of the ventral epidermis of crb mutant embryos: multiple, cyst-like structures were visible, which developed, however, proper apico-basal polarity, with the apical side facing the centre of the cysts (Fig. 3P-R′). From these results, we conclude that cells in the ventral epidermis of foscrbICD crb mutant embryos are polarised, but are unable to maintain a coherent epithelial sheet.
Fig. 3.
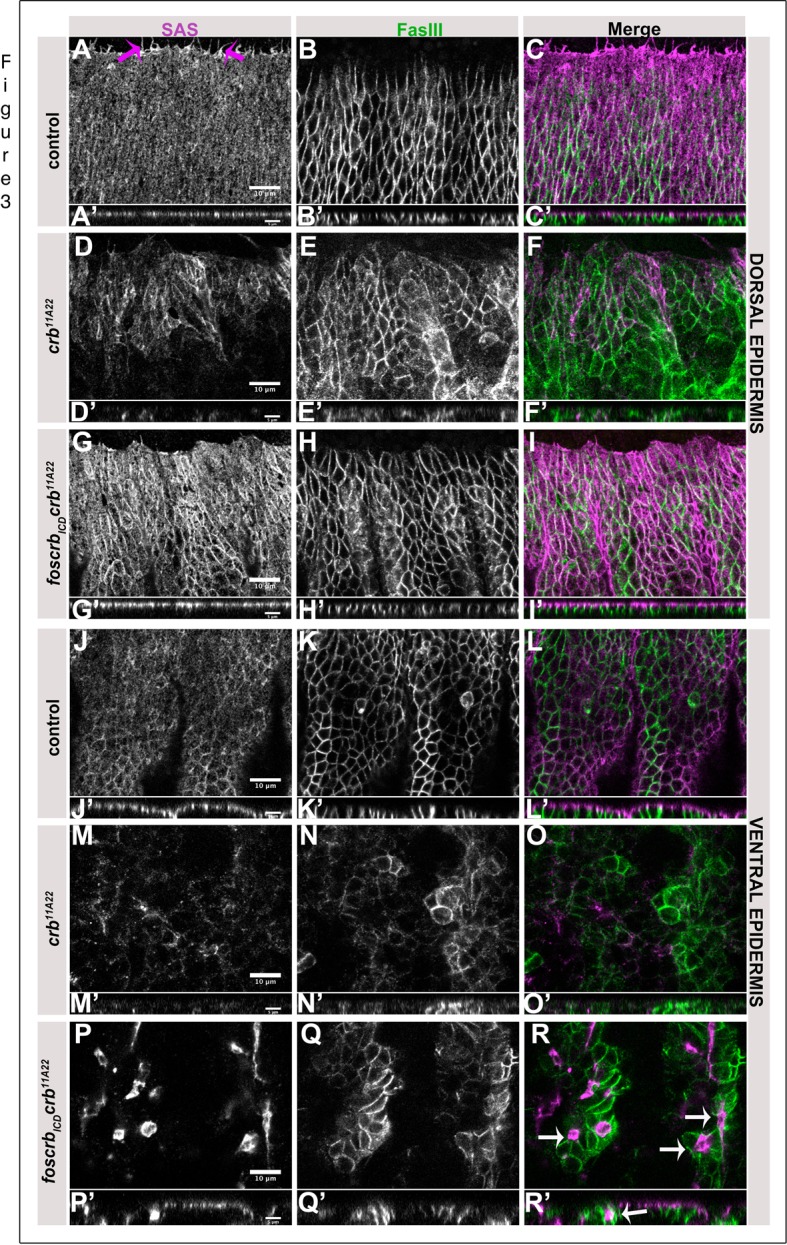
Apico-basal polarity is restored in foscrbICD crb embryos. Stage 13 foscrb; crbGX24 control (A-C′,J-L′), crb11A22 (D-F′,M-O′) and foscrbICD crb11A22 (G-I′,P-R′) embryos stained with anti-SAS (apical) and anti-FasIII (lateral). (A-I,J-R) Lateral view of the dorsal and ventral epidermis respectively. (A′-I′,J′-R′) XZ projections of images in A-I,J-R respectively. Magenta arrows in A point to filopodia. White arrows in R,R′, cyst-like structures with the apical membrane facing the lumen. Scale bar: 10 μm (A-R), 5 μm (A′-R′). The experiment was repeated twice.
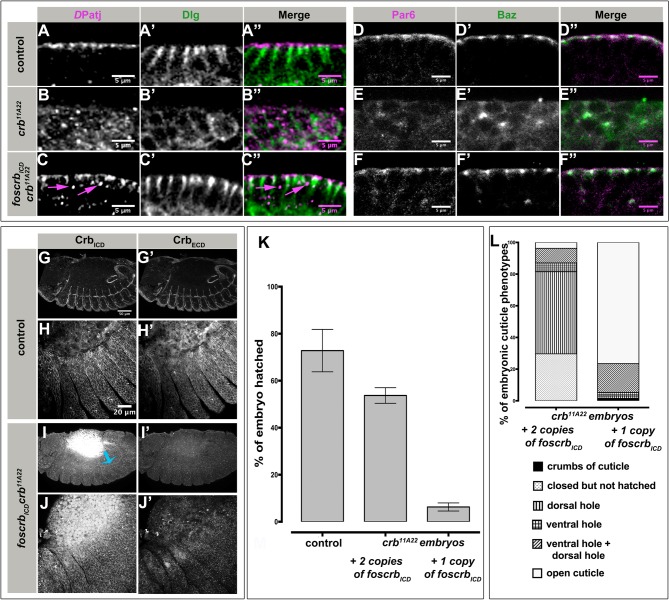
To further unravel more specifically the restoration of apico-basal polarity in the epidermis of foscrbICD crb mutant embryos, we analysed the localisation of the apical proteins DPatj, Par6 and Bazooka (Baz) and the lateral marker Dlg in stage 13 embryos (Fig. 4A-F″). In the epidermis of control embryos, DPatj and Dlg are localised in the subapical and the lateral region, respectively (Fig. 4A-A″). The clear segregation of these two proteins was completely lost in crb mutant embryos, in that Dlg outlines the whole cell and DPatj appeared in intracellular punctae (Fig. 4B-B″). In foscrbICD crb mutant embryos, the segregation of DPatj and Dlg into apical and lateral domains is recovered (Fig. 4C-C″), although some punctate staining of DPatj inside the cell was still observed (magenta arrows in Fig. 4C,C″). Similarly, the apical and junctional localisation of Par6 and Baz, respectively, was completely lost in crb mutant embryos (compare Fig. 4D-D″ and Fig. 4E-E″), but recovered in foscrbICD crb mutant embryos (Fig. 4F-F″). However, only minor amounts of apical Sdt were detected in foscrbICD crb mutant embryos (Fig. S3C-C″, magenta arrows) with punctate staining observed inside the cells (yellow arrow in Fig. S3C). Loss of apical DaPKC in epidermal cells of crb mutant embryos (compare Fig. S3D and E) was partially restored by foscrbICD (Fig. S3F, magenta arrows) but could still be detected intracellularly.
Fig. 4.
The Crb ICD partially rescues apico-basal polarity of crb mutant embryos. (A-F″) Stage 12-13 foscrb; crbGX24 control (A-A″,D-D″), crb11A22 (B-B″,E-E″) and foscrbICD crb11A22 (C-C″,F-F″) embryos, co-stained with anti-DPatj (magenta) and anti-Dlg (green) (A-C″) and anti-Par6 (magenta) and anti-Baz (green) (D-F″). Magenta arrows in C and C″, intracellular punctate accumulation of DPatj. Scale bar: 5 μm. The experiment was repeated 4 times. (G-J′) Stage 12-13 foscrb; crbGX24 control (G-H′) and foscrbICD crb11A22 (I-J′) embryos, co-stained with anti-CrbICD and anti-CrbECD. H-H′ and J-J′, magnifications of the epidermis shown in G-G′ and I-I′, respectively. Anterior is left, dorsal up. Cyan arrow in I′, hindgut. Scale bar: 50 μm (G,G′,I,I′) and 20 μm (H,H′,J and J′). The experiment was repeated 4 times. (K) Quantification of embryonic lethality. The graph shows the percentage of embryos that hatched. Control: foscrb; crbGX24. Note that embryos represented by the third column have only one copy of foscrbICD. The experiment was repeated 3 times. Error bars show standard error of the mean. (L) Quantification of the cuticle phenotypes of crb mutant embryos with one or two copies of foscrbICD. The experiment was repeated 3 times.
Since enrichment of Crb at the sub-apical region is crucial for localisation of other polarity proteins, and localisation of polarity and junctional proteins was restored in many epithelia upon expression of the ICD, we were interested to know whether the ICD expressed in foscrbICD crb embryos is correctly localised. Therefore, we co-stained embryos with an antibody directed against the ICD of Crb (CrbICD) and an antibody directed against the ECD of Crb (CrbECD). The CrbICD antibody detects apical Crb protein in all epithelial tissues of control embryos (Fig. 4G), including the epidermis (Fig. 4H) as does the CrbECD antibody (Fig. 4G′,H′). In contrast, in foscrbICD crb mutant embryos, only minimally apically enriched CrbICD protein was detected in the epidermis and the trachea (only upon enhancing the contrast) but no apically enriched staining was detected using anti-CrbECD, (Fig. 4I-J′).
Given that we observe only minimal apically enriched CrbICD but polarity is mostly rescued, we hypothesised that a small amount of apically enriched Crb is sufficient to restore major aspects of polarity. To test this hypothesis, we reduced the copy number of foscrbICD by half and analysed the rescue of embryonic lethality. Strikingly, while ∼55% of crb mutant embryos with two copies of foscrbICD hatched, only 8% of crb mutant embryos with only one copy of foscrbICD did so (Fig. 4K). Moreover, 80% of the unhatched crb mutant embryos with only one copy of foscrbICD display a severe cuticle phento (‘open cuticle’ class) (quantified in Fig. 4L), suggesting a widespread failure in maintaining epithelial integrity. In contrast, most of the crb mutant embryos with two copies of foscrbICD that did not hatch secrete a continuous cuticle with intact denticle belts and head structures (‘closed but not hatched’ category) or develop only a dorsal hole (Fig. 4L and Fig. 1). Together, these results suggest that the ICD of Crb is sufficient to restore apico-basal polarity in a dose-dependent manner, while the ECD is needed to ensure apical enrichment of Crb and hence complete rescue of all aspects of the embryonic crb mutant phenotype.
Loss of Crb or its ECD leads to a neurogenic phenotype due to impaired Notch signalling
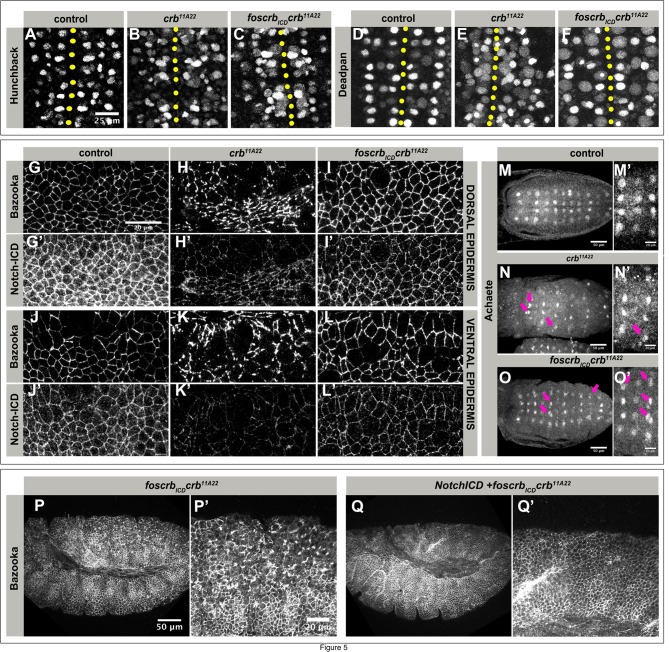
A more detailed look at the ventral epidermis of foscrbICD crb mutant embryos hinted to defects in neurogenesis. Therefore, we analysed the pattern of neuroblasts by staining for Deadpan (Dpn) and Hunchback (Hb), markers of early neuroblasts (Bier et al., 1992; Jiménez and Campos-Ortega, 1990). crb mutant embryos revealed an increase in the number of both Hb- and Dpn-positive cells (compare Fig. 5A and D with Fig. 5B and E, respectively). Expressing foscrbICD reduced the number of supernumerary Dpn-positive cells, while no obvious reduction was observed with respect to Hb-positive cells (Fig. 5F and C, respectively). The increased number of neuroblasts observed in crb mutant embryos is reminiscent of that observed in neurogenic mutants in which Notch-Delta signalling and hence lateral inhibition is compromised. This leads to specification of more than one neuroblast from a proneural cluster at the expense of epidermoblasts (Hartenstein and Wodarz, 2013). This led us to investigate Notch localisation and signalling in these embryos.
Fig. 5.
crb and foscrbICD crb mutant embryos develop a neurogenic phenotype. (A-F) Stage 9 foscrb; crbGX24 control (A,D), crb11A22 (B,E) and foscrbICD crb11A22 (C,F) embryos stained with anti-Hb (A-C) and anti-Dpn (D-F). Yellow dotted line marks the ventral midline. Scale bar: 25 μm. The experiment was repeated 4 times. (G-L′) Dorsal (G-I′) and ventral (J-L′) epidermis of stage 9 foscrb; crbGX24 control (G,G′,J,J′), crb11A22 (H,H′,K,K′) and foscrbICD crb11A22 (I,I′,L,L′) embryos, stained with anti-Baz and anti-Notch-ICD. Scale bar: 20 μm. The experiment was repeated 3 times. (M-O′) Stage 9 foscrb; crbGX24 control (M,M′), crb11A22 (N,N′) and foscrbICD crb11A22 (O,O′) embryos stained with anti-Achaete, anterior to the left. (M′,N′,O′) Close up of images shown in M-O, respectively. Magenta arrowheads in N,N′,O,O′ point to supernumerary neuroblasts. Scale bar: 50 μm (M,N,O) and 20 μm (M′,N′,O′). The experiment was repeated 3 times. (P-Q) Stage 9/10 embryos stained with anti-Bazooka. (P′,Q′) Close up of posterior ventral epidermis of embryos shown in P,Q. Anterior is left. Scale bar: 50 μm (P,Q) and 20 μm (P′-Q′). The experiment was repeated twice.
In fact, both crb and foscrbICD crb mutant embryos revealed a significant reduction of apical Notch in the dorsal and the ventral epidermis (Fig. 5G-L′, quantified in Fig. S4). To corroborate that Notch activity is weakened in these embryos, we analysed the expression of achaete (ac), a read-out of Notch activity. ac is a proneural gene, expression of which becomes restricted to a single cell within a proneural cluster due to lower Notch signalling in this cell in comparison to neighbouring cells, which experience high Notch activity and hence become specified as epidermoblasts (Skeath and Carroll, 1992) (Fig. 5M,M′). crb mutant embryos exhibited less proneural clusters, presumably due to enhanced apoptosis already at this stage. Some of the residual clusters showed more than one Ac-positive cell (magenta arrows in Fig. 5N,N′). foscrbICD crb mutant embryos revealed a pattern of clusters that is similar to that of control embryos. However, in many of these clusters, more than one cell retained expression of Ac (magenta arrows in Fig. 5O,O′).
From these results, we hypothesised that the loss of epithelial integrity observed in foscrbICD crb mutant embryos might be partly due to an increased number of delaminating neuroblasts. Specification of neuroblasts and hence neuroblast delamination can be blocked by expressing the constitutive active intracellular domain of Notch (Notchintra) (Lieber et al., 1993; Rebay et al., 1993; Struhl et al., 1993). Expression of Notchintra has been shown to prevent loss of ventral epidermal integrity of shotgun (shg) mutant or Cdc42 knock-down embryos (Harris and Tepass, 2008; Tepass et al., 1996). Therefore, we overexpressed Notchintra in foscrbICD crb mutant embryos. The fragmentation of the ventral epidermis was strongly suppressed in these embryos as revealed by the restoration of continuous staining of AJs using Baz (compare Fig. 5P,P′ to Q,Q′).
Taken together, these data suggest that the ECD of Crb is required for apical enrichment of Notch in the embryonic epidermis, and thus ensures proper neuroblast specification via Notch signalling.
Overexpression of DE-Cadherin or Flapwing restores tissue integrity of foscrbICD crb embryos
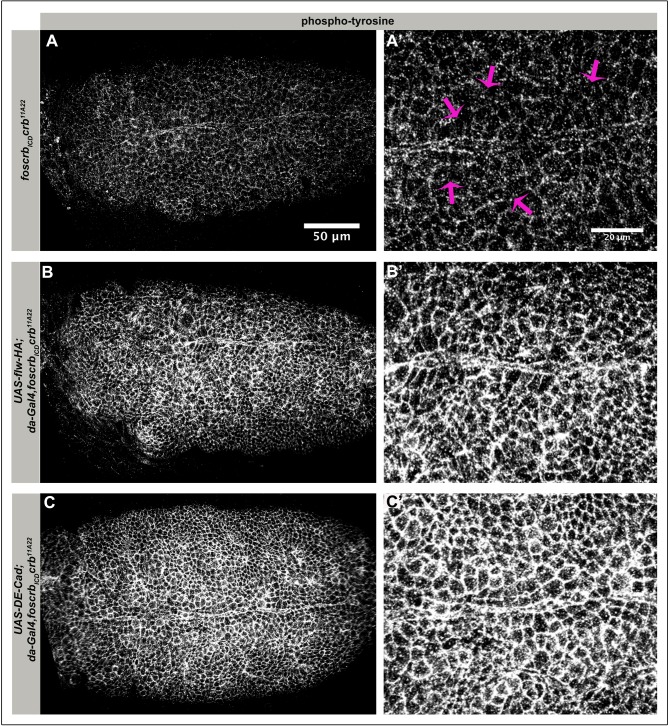
foscrbICD alone could not restore ventral epidermal integrity of crb mutant embryos (Fig. 2P), while overexpression of Notchintra in conjunction with foscrbICD could. From this we hypothesised that expression of Notchintra not only reduces the number of neuroblasts, but also relieves the ventral epidermis from morphogenetic stress due to reduced neuroblast delamination. It has recently been shown that neuroblast delamination requires increased actomyosin activity (An et al., 2017; Simões et al., 2017). In addition, studies from our lab have shown that Crb negatively regulates myosin contractility in the amnioserosa, thereby ensuring, among others, the maintenance of a proper adhesion belt (Flores-Benitez and Knust, 2015). Therefore, we wondered whether supressing actomyosin contractility or reinforcing AJs in foscrbICD crb mutant embryos could restore ventral epidermal integrity. To address this question, we overexpressed flapwing (flw) in foscrbICD crb embryos (Fig. 6). flw encodes the catalytic subunit of PP1β, a serine/threonine phosphatase, which negatively regulates the myosin regulatory light chain (MRLC), and hence myosin contractility (Vereshchagina et al., 2004). Strikingly, disintegration of the ventral epidermis was suppressed and AJ integrity was restored in these embryos (Fig. 6B,B′). Similarly, overexpressing DE-Cadherin in these mutants also rescued epithelial integrity and AJ in the ventral epidermis (Fig. 6C,C′).
Fig. 6.
Defects in the ventral epidermis of foscrbICD crb embryos are rescued by overexpression of DE-cadherin or flapwing. Ventral views of stage 9/10 foscrbICD crb11A22 embryos overexpressing HA-tagged flapwing (flw; B,B′) or DE-cadherin (C,C′), stained with an anti-phosphotyrosine antibody. Yellow lines mark the ventral midline. (A′,B′,C′) Close up of the ventral epidermis of embryos shown in A,B,C, respectively. Magenta arrows in A′ point to cells without proper junctional staining. Anterior to the left. Scale bar: 50 μm (A,B,C) and 20 μm (A′,B′,C′). The experiment was repeated twice.
Taken together, data presented here show that the ICD of Crb is sufficient to restore apico-basal polarity and integrity of most epithelia of crb mutant embryos, while morphogenesis of the neurogenic ectoderm additionally required the ECD. The ECD executes this function, first, by ensuring sufficient apical enrichment of Crb protein, thus stabilising junctions and prevent increased actomyosin activity and second, by stabilising the Notch receptor apically and thus controlling the proper number of delaminating neuroblasts.
DISCUSSION
By using foscrbICD (in the background of a crb null allele) we established a genotypic condition similar to a hypomorphic crb allele, which enabled us to unveil novel crb functions in the Drosophila embryo, which are normally hidden by massive polarity defects and apoptosis occurring in the null alleles. Thereby, we gained detailed mechanistic insight into stage- and tissue-specific functions of the ICD and ECD of Crb, which could not be achieved by overexpression studies. We show (i) that a threshold level of the CrbICD only is sufficient to rescue epithelial cell polarity and even lethality of crb mutant embryos. (ii) We provide compelling data for a novel role of the extracellular domain of Crb in embryonic neurogenesis by stabilizing the Notch receptor and thus ensuring proper Notch signaling. (iii) We further show that, in contrast to previous reports, the ECD of Crb is dispensable for the invagination of embryonic epithelial tubes, e.g. the salivary gland.
Earlier structure function analysis of Crb performed in Drosophila embryos based on overexpression already suggested an important role of the ICD, since it could rescue polarity defects in many epithelia to the same degree as the full-length protein (Letizia et al., 2013; Wodarz et al., 1995). Therefore, it was suggested that the ICD of Crb is sufficient to perform many Crb functions during embryonic development. Since a fosmid encompassing the whole crb locus can completely rescue lethality of crb mutant embryos (Klose et al., 2013), we used a similar approach to ask whether the membrane-bound intracellular domain, encoded by a fosmid, has the same rescuing capacity. We provide compelling data to show that the ICD of Drosophila Crb is sufficient to rescue lethality of about 50% of homozygous crb mutant embryos. It has to be pointed out, yet, that foscrbICD crb mutant animals die as first instar larvae due to various developmental defects, including defects in the maturation of trachea and Malpighian tubules (data not shown).
Furthermore, and in contrast to previous findings based on overexpression studies (Letizia et al., 2013; Röper, 2012), we show that even low amounts of CrbICD are sufficient to ensure normal invagination of the anlagen of the salivary glands and the tracheae. In addition, the ICD is sufficient to completely rescue AJs and the apical domain in the dorsal epidermis, while the rescue is incomplete in the ventral epidermis. In addition, localization of apical proteins, such as DPatj, Par6 and Bazooka/Par3, and the junctional protein DE-Cad, is completely restored in the dorsal epidermis. Interestingly, localization of the direct Crb binding partner Sdt was not completely restored under this experimental condition. This could be explained by the low levels of CrbICD itself, which was hardly detected by immunostainings (and was too low to be detected by western blots; S.D. and E.K., unpublished data). Two reasons can account for this low apical enrichment of CrbICD. (i) Homophilic interactions between the ECDs of Crb molecules have been suggested to stabilise the protein apically in Drosophila embryonic and follicle epithelia (Fletcher et al., 2012; Letizia et al., 2013; Röper, 2012), in the primitive streak of early mouse embryos (Ramkumar et al., 2016), and in the zebrafish retina (Zou et al., 2012). Even the short ECD present in mammalian Crb3 was shown to stabilize the protein at the apical membrane, when expressed in GP2-293 cells (Djuric et al., 2016). (ii) Alternatively, the low amount of Crb protein expressed in foscrbICD crb embryos could be explained by reduced trafficking of Crb to the apical membrane in the absence of the ECD. Studies in mouse embryos revealed that O-glycosylation of the EGF-like repeats in the ECD of Crb2 by Protein O-glucosyltransferase 1 (POGLUT1) is essential for proper trafficking to, and enrichment at, the apical membrane. As a consequence, mouse embryos mutant for POGLUT1 die during gastrulation due to defects in epithelial-mesenchymal transitions (Ramkumar et al., 2015), thus phenocopying defects of embryos lacking Crb2 (Ramkumar et al., 2016). Replacing all seven putative Rumi/POGLUT1 target sites in Drosophila Crb did not affect the viability of homozygous mutant flies (Haltom et al., 2014). This does not, however, exclude a role for other parts of the Crb ECD in apical targeting during embryogenesis. An apical targeting signal may also reside in the cytoplasmic tail of Crb. Targeting of Podocalyxin/Gp135, for example, to the apical membrane of Madine-Darbin-canine kidney (MDCK) cells depends on a bipartite signal, an O-glycosylation-rich region in the ECD and a C-terminal PDZ-domain binding motif in the ICD. During transport of newly synthesized Podocalyxin, EBP50 binds to its PDZ-domain binding motif at the Golgi, thereby inducing its oligomerization and sorting into a clustering complex, which facilitates apical sorting (Yu et al., 2007). It is tempting to speculate that the ICD of Crb may similarly interact with an unknown partner, which directs at least a small amount of CrbICD to the apical membrane, which is sufficient to rescue cell polarity defects in crb mutant embryos.
We are left with the question, how the apical domain of epithelial cells can be formed in the presence of such low levels of apical CrbICD and Sdt. Previous studies clearly showed that the amount of Crb protein is critical for proper apico-basal polarity. While loss of Drosophila crb/mouse Crb2 results in loss/reduction of the apical surface (Ramkumar et al., 2016; Wodarz et al., 1993, 1995), overexpression of the ICD of Drosophila Crb or mammalian Crb3 can lead to an expansion of the apical membrane of epithelial and photoreceptor cells (Klebes and Knust, 2000; Lemmers et al., 2004; Letizia et al., 2013; Muschalik and Knust, 2011; Pellikka et al., 2002; Wodarz et al., 1995). Results presented here suggest that a threshold level of apical Crb, and thus Sdt, is required and sufficient to maintain an apical domain. This assumption is supported by the observation that in the presence of just one copy of foscrbICD only 8% of crb mutant embryos hatch, compared to 50% in the presence of two copies of foscrbICD, while one copy of foscrb, which encodes full-length Crb proteins, is sufficient to fully rescue lethality of crb mutant embryos (Klose et al., 2013). Unlike Sdt, DaPKC was apically enriched in foscrbICD crb embryos, but was also detected within the cell. Removal of one copy of endogenous DaPKC in foscrbICD crb embryos enhanced embryonic lethality (S.D. and E.K.,unpublished data), making it unlikely that the phenotypes observed in foscrbICD crb embryos are due to increased phosphorylation of the CrbICD as a result of upregulation of DaPKC. Although phosphorylation of two threonine residues in CrbICD by aPKC was suggested to be functionally important (Sotillos et al., 2004), recent results showed that mutation of these residues to non-phosphorylatable alanine have no effect on viability and fertility of homozygous mutant flies (Cao et al., 2017). Interestingly, expression of a stable form of DE-cadherin can restore AJ formation and polarity in embryonic epithelia even in the absence of sdt or crb (Chen et al., 2017), suggesting other, Crb complex-independent mechanisms to ensure apico-basal polarity. Further investigations on the relationship between sdt, DaPKC and CrbICD are needed to completely understand the significance of the upregulation of DaPKC observed and its possible effect on embryonic epidermal integrity.
crb mutant embryos expressing two copies of foscrbICD, which fail to hatch, develop defects in the amnioserosa and the ventral epidermis, two tissues exhibiting a high degree of morphogenetic activity, which is in line with earlier proposals suggesting that Crb/the Crb-complex is particularly required in dynamic epithelia with high turnover of AJs (Campbell et al., 2009; Chen et al., 2017). The disruption of the mono-layered organisation of the ventral epidermis of foscrbICD crb mutant embryos goes along with the formation of ‘cyst’-like structures, probably due to weakened AJs. Similar defects in the ventral epidermis were observed in embryos in which Cdc42 was knocked down (Harris and Tepass, 2008) and in shotgun (shg) mutant embryos, which lack the gene encoding DE-cadherin (Tepass et al., 1996; Uemura et al., 1996). We would like to point out an important difference observed in the phenotypes of the ventral epidermis in foscrbICD crb embryos and in shg mutant embryos: while in both mutants AJs fail to be maintained, foscrbICD crb mutant embryos additionally show an increased number of neuroblasts, as revealed by an increased number of Hunchback (Hb)-positive cells, while shg mutant embryos do not show any defect in neuroblast numbers (Wang et al., 2004). Neuroblasts are the precursors of the ventral nerve cord, which delaminate from the ventral neurogenic ectoderm (Hartenstein and Campos-Ortega, 1984). Neuroblast delamination is characterised by an anisotropic loss of AJs, apical constriction due to periodic myosin pulsation, followed by the gradual disappearance of the apical membrane (An et al., 2017; Simões et al., 2017). Once neuroblasts have delaminated, the remaining cells within the epithelium have to close the gap by reforming AJs. Neuroblast number and spacing is controlled by the Notch signalling pathway, and loss of any of the neurogenic genes, which encode constituents of this pathway, results in a hyperplasia of the nervous system at the expense of the epidermis (Campos-Ortega and Knust, 1990; Hartenstein and Wodarz, 2013; Lehmann et al., 1981).
Defects in two, mutually not exclusive, mechanisms may account for the phenotype in the ventral epidermis associated with reduction of crb, namely reduced Notch signalling and enhanced uncontrolled actomyosin activity. First, reduced Notch levels observed in the absence of CrbICD lead to reduced Notch signalling, and consequently, to impaired lateral inhibition and an increased number of neuroblasts, which create additional stress during delamination. In fact, expression of the intracellular, constitutively active form of Notch, Notchintra, was able to suppress the disintegration of the ventral epidermis of foscrbICD crb mutant embryos. Two scenarios could account for impaired Notch signalling in the ventral neurogenic ectoderm in the absence of the ECD of Crb. (i) Reduced apical Notch protein in crb and in crb fosICD embryos due to impaired stabilisation by Crb or CrbECD results in reduced Notch signalling in the neuroectoderm. Similar observations were made in the developing dorsal telencephalon of Crb2 mutant mice, which is associated with premature expression of differentiation genes and an increase in basal neural progenitor cells at the cost of the apical progenitor pool (Dudok et al., 2016). This phenotype has striking similarity to that induced upon inactivation of Notch1, which is characterized by the loss of progenitor pools and premature neural differentiation (Mizutani et al., 2007). In other cases, loss/reduction of Crb can result in activation of the Notch pathway. In the developing pupal wing, depletion of Notch from the apical surface in the absence of Crb provokes the activation of the ligand-independent Notch pathway, followed by cell fate specification defects (Nemetschke and Knust, 2016). Similarly, zebrafish Crb was shown to interact with Notch when expressed in cell culture, and overexpression of Crb reduced Notch activity (Ohata et al., 2011). (ii) Alternatively, the absence or reduction of the Crb ECD may affect the Notch signalling pathway indirectly due to effects on apico-basal polarity, since in many cells receptors are enriched and activated at the apical pole. In fact, in zebrafish, an apico-basal gradient of Notch is instrumental for ensuring apical mitosis and proper cell fate decision, both in the neuroepithelium and the retinal epithelium. Expansion of the apical surface upon loss of Lgl1 (Clark et al., 2012) results in increased apical Notch activity, which prevents premature differentiation. To discriminate between these two possibilities, replacing the Crb ECD by a heterologous, extracellular dimerization domain may stabilise the CrbICD and thus lead to normal levels of apical Crb, but would not be able to stabilise Notch.
Beside impaired Notch signalling and hence increased neuroblast delamination, our data support the conclusion that uncontrolled actomyosin activity contributes to the phenotype observed in foscrbICD crb mutant embryos. Coupling between actomyosin and AJ is essential for epithelial stability, and enhanced tensile forces due to increased actomyosin activity can be detrimental for AJ stability and epithelial integrity (Arnold et al., 2017; Citi et al., 2014; Heisenberg and Bellaïche, 2013). We previously showed that a mutation in the FERM-domain binding motif of Crb induces increased actomyosin activity in the amnioserosa, followed by severe disintegration of the epithelium and defects in dorsal closure. This defect could be rescued by overexpression of flapwing (flw) (Flores-Benitez and Knust, 2015). Based on the observation that overexpressing flw suppresses the disintegration of the ventral epidermis in foscrbICD crb mutant embryos as well, it is tempting to speculate that insufficient apical enrichment of CrbICD contributes to increased actomyosin activity also in the neuroectoderm. So, either reducing actomyosin activity by overexpression of flw or preventing neuroblast delamination by expressing Notchintra and thereby activating the Notch pathway can release excess morphogenetic stress in the neurogenic ectoderm of foscrbICD crb mutant embryos. Detailed measurements of actomyosin activity upon loss or reduction of Crb are needed to achieve further mechanistic insight into the role of Crb in actomyosin-mediated tension in the neuroectoderm.
In summary, the approach used here allowed us to systematically dissect the tissue-specific roles of different domains of the Crb protein during Drosophila embryogenesis. In the neurogenic ectoderm, the ECD of Crb is not only required to counteract increased tension due to neuroblast delamination, but also to ensure proper Notch signalling and thereby control the number of neuroblasts. Further studies will reveal how the activities of the different Crb protein domains are coordinated to ensure tissue homeostasis in different epithelia.
MATERIALS AND METHODS
Recombineering for generation of constructs
An improved counter-selection strategy that used the pABRG as the helper plasmid and phosphothioated oligonucleotides as ‘modification cassettes’ were used to generate the foscrb variants in this study (Bird et al., 2011). Single stranded oligonucleotides, with two 5′ phosphothioate bonds, that either target the endogenous lagging strand or the leading strand were used. A list of the ‘modification rpsl-neo cassettes’, the counter-selection oligonucleotides is mentioned in the supplementary Materials and Methods. All the exons in the newly created transgenic constructs were sequenced. A detailed, step-by-step description of the protocol used is available on request.
Generation of transgenic flies
Transgenic flies were generated with the help of phiC31 integrase mediated site-specific integration into VK00033 landing site y1, w*, P{nos-phiC31int.NLS}X; PBac{y+-attP-3B}VK00033 (BL-32542) (Sarov et al., 2016). Correct transformants were screened for red fluorescent eyes (from 3XP3-Dsred marker in fosmid backbone) (Ejsmont et al., 2009).
Fly stocks
Flies were maintained on standard food at 25°C. For most of the experiments, crb mutant alleles were balanced over fluorescent balancers to distinguish the homozygous mutant embryos from the rest of the embryos. In cases where the mutant flies could not be maintained over the fluorescent balancers, they were maintained over non-fluorescent balancers and the homozygous mutant embryos were distinguished by staining for Crb. All experiments were performed using the protein-null alleles crb11A22 or crbGX24, thus ensuring that the different versions of crb is expressed only from the fosmid. All fly stocks are listed in supplementary Materials and Methods. Drosophila manipulations were done in accordance with standard techniques.
Embryo collection and antibody staining
Embryos were collected in fly cages on apple juice agar plates at 25°C. For most experiments, 2 h or 1 h collections were done. Staging was based on control embryos (foscrb;crbGX24). Embryos of different genotypes used in the same experiment were collected and aged for the same time and fixed and stained in parallel. Embryos were dechorionated with 3% sodium hypchlorite (3 min) and fixed in 4% formaldehyde in phosphate-buffered saline (PBS)/heptane with a V/V of 1:1 on a rotating shaker (25 min). Embryos were devitellinized in a solution of heptane/methanol (1:1). For Achaete antibody staining, PEM buffer was used (4 ml PEM, 1 ml FA 37%, 5 ml heptane) and embryos were fixed for 20 min. For staining with Sdt-, Bazooka- and Notch-Intra antibodies, heat fixation of embryos was used (Müller, 2008). Fixed embryos were washed thrice and stored in 100% methanol at −20°C for future use. For staining, embryos were gradually rehydrated at room temperature in decreasing concentration of methanol (75%, 50%, 25%, 0%) in PBT (0.3 Tx-100). Embryos were incubated in blocking solution with 5% normal horse serum (NHS; Sigma-Aldrich H1270, St. Louis, Missouri, USA) in PBT (0.3 Tx-100) for two hours, followed by an overnight incubation with primary antibodies diluted in 5% NHS containing PBT (0.3Tx-100). The embryos were washed in PBT (0.3% Tx-100) four times for 15 min each and then incubated in the appropriate secondary antibodies (Alexa conjugated) diluted in 5% NHS/PBT (0.3Tx-100) for two hours. Embryos were washed in PMT (0.3% Tx-100) and mounted on glass slides using VectaShield. Homozygous crb mutant embryos were preselected prior to fixing by selecting against the GFP signal from the fluorescent balancers under a dissecting microscope. To identify crb mutant embryos at stage 9/10, embryos were stained with anti-Crb antibody and only those embryos without the Crb signal were imaged. Images were acquired using a Zeiss LSM 780 NLO confocal microscopy (ZEISS Microscopy) with a C-Apochromat 40×/1.2W Corr objective. Image analysis and pseudocolor assignment were done in Fiji and images were assembled in Adobe Photoshop CC. Image manipulation was fully compliant with the image guidelines for proper digital image handling outlined in Rossner and Yamada (2004).
Cuticle preparation
Cuticle preparations were performed as described recently (Flores-Benitez and Knust, 2015). Images of the cuticle were acquired by phase contrast with Zeiss Axio Imager.Z1 microscope with an EC Plan-NEOFLUAR 10× objective. Images were visualized and modified in Fiji and assembled in Adobe Photoshop.
Live imaging of embryos
Life imaging of embryos was essentially performed as described recently (Flores-Benitez and Knust, 2015). Embryos were imaged by multi-position and multi-time scanning using a Zeiss LSM 780 NLO confocal microscope with a W Plan-Apochormat 40×/1.0 objective. 4-D hyperstakcs were processed with Fiji. Image manipulation was fully compliant with the image guidelines for proper digital image handling outlined in Rossner and Yamada (2004).
Statistical analysis
Graphs were plotted and the statistical analyses were performed using GraphPad Prism6. Results are expressed as means±s.d. Statistical significance was calculated by an unpaired Kolmogorov–Smirnov test (Fig. S4).
Supplementary Material
Acknowledgements
We would like to thank D. Cavener, J. Urban, A. Wodarz, J. Skeath, T. Klein, the DSHB, the VDRC and the Bloomington Stock Centers for flies and antibodies. Special thanks go to our lab members D. Flores-Benitez and S. Hebbar for advice on experiments and critical reading of the manuscript. We thank the following MPI-CBG facilities: the Light Microscopy Facility, in particular J. Peychl and B. Schroth-Diez, for microscopy guidance. We are grateful to members of the Knust lab for fruitful and constant advice. We thank the fly keepers S. Ssykor, C. Mass and S. Wernicke for excellent care of our flies. S.D. was a member of the International Max Planck Research School for Cell, Developmental and Systems Biology and a doctoral student at Technische Universität Dresden.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: S.D., E.K.; Methodology: S.D., E.K.; Validation: S.D., E.K.; Formal analysis: S.D.; Investigation: S.D.; Writing - original draft: S.D.; Writing - review & editing: E.K.; Supervision: E.K.; Project administration: E.K.; Funding acquisition: E.K.
Funding
This work was supported by Max-Planck-Gesellschaft.
Supplementary information
Supplementary information available online at http://bio.biologists.org/lookup/doi/10.1242/bio.031435.supplemental
References
- An Y., Xue G., Shaobo Y., Mingxi D., Zhou X., Yu W., Ishibashi T., Zhang L. and Yan Y. (2017). Apical constriction is driven by a pulsatile apical myosin network in delaminating Drosophila neuroblasts. Development 144, 2153-2164. 10.1242/dev.150763 [DOI] [PubMed] [Google Scholar]
- Arnold T. R., Stephenson R. E. and Miller A. L. (2017). Rho GTPases and actomyosin: partners in regulating epithelial cell-cell junction structure and function. Exp. Cell Res. 358, 20-30. 10.1016/j.yexcr.2017.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann A., Schneider M., Theilenberg E., Grawe F. and Knust E. (2001). Drosophila Stardust is a partner of Crumbs in the control of epithelial cell polarity. Nature 414, 638-643. 10.1038/414638a [DOI] [PubMed] [Google Scholar]
- Bier E., Vaessin H., Younger-Shepherd S., Jan L. Y. and Jan Y. N. (1992). deadpan, an essential pan-neural gene in Drosophila, encodes a helix-loop-helix protein similar to the hairy gene product. Genes Dev. 6, 2137-2151. 10.1101/gad.6.11.2137 [DOI] [PubMed] [Google Scholar]
- Bird A. W., Erler A., Fu J., Hériché J.-K., Maresca M., Zhang Y., Hyman A. A. and Stewart A. F. (2011). High-efficiency counterselection recombineering for site-directed mutagenesis in bacterial artificial chromosomes. Nat. Methods 9, 103-109. 10.1038/nmeth.1803 [DOI] [PubMed] [Google Scholar]
- Blankenship J. T., Fuller M. T. and Zallen J. A. (2007). The Drosophila homolog of the Exo84 exocyst subunit promotes apical epithelial identity. J. Cell Sci. 120, 3099-3110. 10.1242/jcs.004770 [DOI] [PubMed] [Google Scholar]
- Campanale J. P., Sun T. Y. and Montell D. J. (2017). Development and dynamics of cell polarity at a glance. J. Cell Sci. 130, 1201-1207. 10.1242/jcs.188599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K., Knust E. and Skaer H. (2009). Crumbs stabilises epithelial polarity during tissue remodelling. J. Cell Sci. 122, 2604-2612. 10.1242/jcs.047183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Ortega J. A. and Knust E. (1990). Genetics of early neurogenesis in Drosophila melanogaster. Annu. Rev. Genet. 24, 387-407. 10.1146/annurev.ge.24.120190.002131 [DOI] [PubMed] [Google Scholar]
- Cao F., Miao Y., Xu K. and Liu P. (2015). Lethal (2) giant larvae: an indispensable regulator of cell polarity and cancer development. Int. J. Biol. Sci. 11, 380-389. 10.7150/ijbs.11243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Xu R., Shi Q., Zhang D., Huang J. and Hong Y. (2017). FERM domain phosphorylation and endogenous 30UTR are not essential for regulating the function and subcellular localization of polarity protein Crumbs. J. Gen. Genomics 44, 409e412. [DOI] [PubMed] [Google Scholar]
- Chen Y.-J., Huang J., Huang L., Austin E. and Hong Y. (2017). Phosphorylation potential of Drosophila E-Cadherin intracellular domain is essential for development and adherens junction biosynthetic dynamics regulation. Development 144, 1242-1248. 10.1242/dev.141598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi S., Guerrera D., Spadaro D. and Shah J. (2014). Epithelial junctions and Rho family GTPases: the zonular signalosome. Small GTPases 5, e973760 10.4161/21541248.2014.973760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B. S., Cui S., Miesfeld J. B., Klezovitch O., Vasioukhin V. and Link B. A. (2012). Loss of Llgl1 in retinal neuroepithelia reveals links between apical domain size, Notch activity and neurogenesis. Development 139, 1599-1610. 10.1242/dev.078097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuric I., Siebrasse J. P., Schulze U., Granado D., Schlüter M. A., Kubitscheck U., Pavenstädt H. and Weide T. (2016). The C-terminal domain controls the mobility of Crumbs 3 isoforms. Biochim. Biophys. Acta 1863, 1208-1217. 10.1016/j.bbamcr.2016.03.008 [DOI] [PubMed] [Google Scholar]
- Dudok J. J., Murtaza M., Henrique Alves C., Rashbass P. and Wijnholds J. (2016). Crumbs 2 prevents cortical abnormalities in mouse dorsal telencephalon. Neurosci. Res. 108, 12-23. 10.1016/j.neures.2016.01.001 [DOI] [PubMed] [Google Scholar]
- Ejsmont R. K., Sarov M., Winkler S., Lipinski K. A. and Tomancak P. (2009). A toolkit for high-throughput, cross-species gene engineering in Drosophila. Nat. Methods 6, 435-437. 10.1038/nmeth.1334 [DOI] [PubMed] [Google Scholar]
- Fletcher G. C., Lucas E. P., Brain R., Tournier A. and Thompson B. J. (2012). Positive feedback and mutual antagonism combine to polarize Crumbs in the Drosophila follicle cell epithelium. Curr. Biol. 22, 1116-1122. 10.1016/j.cub.2012.04.020 [DOI] [PubMed] [Google Scholar]
- Flores-Benitez D. and Knust E. (2015). Crumbs is an essential regulator of cytoskeletal dynamics and cell-cell adhesion during dorsal closure in Drosophila. Elife 4, e07398 10.7554/eLife.07398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Benitez D. and Knust E. (2016). Dynamics of epithelial cell polarity in Drosophila: how to regulate the regulators? Curr. Opin. Cell Biol. 42, 13-21. 10.1016/j.ceb.2016.03.018 [DOI] [PubMed] [Google Scholar]
- Gosens I., Sessa A., den Hollander A. I., Letteboer S. J. F., Belloni V., Arends M. L., Le Bivic A., Cremers F. P. M., Broccoli V. and Roepman R. (2007). FERM protein EPB41L5 is a novel member of the mammalian CRB-MPP5 polarity complex. Exp. Cell Res. 313, 3959-3970. 10.1016/j.yexcr.2007.08.025 [DOI] [PubMed] [Google Scholar]
- Grawe F., Wodarz A., Lee B., Knust E. and Skaer H. (1996). The Drosophila genes crumbs and stardust are involved in the biogenesis of adherens junctions. Development 122, 951-959. [DOI] [PubMed] [Google Scholar]
- Grifoni D., Froldi F. and Pession A. (2013). Connecting epithelial polarity, proliferation and cancer in Drosophila: the many faces of lgl loss of function. Int. J. Dev. Biol. 57, 677-687. 10.1387/ijdb.130285dg [DOI] [PubMed] [Google Scholar]
- Haltom A. R., Lee T. V., Harvey B. M., Leonardi J., Chen Y.-J., Hong Y., Haltiwanger R. S. and Jafar-Nejad H. (2014). The protein O-glucosyltransferase Rumi modifies eyes shut to promote rhabdomere separation in Drosophila. PLoS Genet. 10, e1004795 10.1371/journal.pgen.1004795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K. P. and Tepass U. (2008). Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J. Cell Biol. 183, 1129-1143. 10.1083/jcb.200807020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T. J. C. and Tepass U. (2010). Adherens junctions: from molecules to morphogenesis. Nat. Rev. Mol. Cell Biol. 11, 502-514. 10.1038/nrm2927 [DOI] [PubMed] [Google Scholar]
- Hartenstein V. and Campos-Ortega J. A. (1984). Early neurogenesis in wild-type Drosophila melanogaster. Roux's Arch. Dev. Biol. 193, 308-325. 10.1007/BF00848159 [DOI] [PubMed] [Google Scholar]
- Hartenstein V. and Wodarz A. (2013). Initial neurogenesis in Drosophila. Wiley Interdiscip. Rev. Dev. Biol. 2, 701-721. 10.1002/wdev.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes P. and Solon J. (2017). Drosophila dorsal closure: an orchestra of forces to zip shut the embryo. Mech. Dev. 144, 2-10. 10.1016/j.mod.2016.12.005 [DOI] [PubMed] [Google Scholar]
- Heisenberg C.-P. and Bellaïche Y. (2013). Forces in tissue morphogenesis and patterning. Cell 153, 948-962. 10.1016/j.cell.2013.05.008 [DOI] [PubMed] [Google Scholar]
- Hong Y., Stronach B., Perrimon N., Jan L. Y. and Jan Y. N. (2001). Drosophila Stardust interacts with Crumbs to control polarity of epithelia but not neuroblasts. Nature 414, 634-638. 10.1038/414634a [DOI] [PubMed] [Google Scholar]
- Jiménez F. and Campos-Ortega J. A. (1990). Defective neuroblast commitment in mutants of the achaete-scute complex and adjacent genes of D. melanogaster. Neuron 5, 81-89. 10.1016/0896-6273(90)90036-F [DOI] [PubMed] [Google Scholar]
- Jürgens G., Wieschaus E., Nüsslein-Volhard C. and Kluding H. (1984). Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. II. Zygotic loci on the third chromosome. Roux's Arch. Dev. Biol. 193, 283-295. 10.1007/BF00848157 [DOI] [PubMed] [Google Scholar]
- Kaltschmidt J. A., Lawrence N., Morel V., Balayo T., Fernández B. G., Pelissier A., Jacinto A. and Martinez Arias A. (2002). Planar polarity and actin dynamics in the epidermis of Drosophila. Nat. Cell Biol. 4, 937-944. 10.1038/ncb882 [DOI] [PubMed] [Google Scholar]
- Kempkens Ö., Médina E., Fernandez-Ballester G., Özüyaman S., Le Bivic A., Serrano L. and Knust E. (2006). Computer modelling in combination with in vitro studies reveals similar binding affinities of Drosophila Crumbs for the PDZ domains of Stardust and DmPar-6. Eur. J. Cell Biol. 85, 753-767. 10.1016/j.ejcb.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Klebes A. and Knust E. (2000). A conserved motif in Crumbs is required for E-cadherin localisation and zonula adherens formation in Drosophila. Curr. Biol. 10, 76-85. 10.1016/S0960-9822(99)00277-8 [DOI] [PubMed] [Google Scholar]
- Klose S., Flores-Benitez D., Riedel F. and Knust E. (2013). Fosmid-based structure-function analysis reveals functionally distinct domains in the cytoplasmic domain of Drosophila Crumbs. G3 3, 153-165. 10.1534/g3.112.005074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knust E., Dietrich U., Tepass U., Bremer K. A., Weigel D., Vassin H. and Campos-Ortega J. A. (1987). EGF homologous sequences encoded in the genome of Drosophila melanogaster, and their relation to neurogenic genes. EMBO J. 6, 761-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolahgar G., Bardet P.-L., Langton P. F., Alexandre C. and Vincent J.-P. (2011). Apical deficiency triggers JNK-dependent apoptosis in the embryonic epidermis of Drosophila. Development 138, 3021-3031. 10.1242/dev.059980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprise P. (2011). Emerging role for epithelial polarity proteins of the Crumbs family as potential tumor suppressors. J. Biomed. Biotechnol. 2011, 868217 10.1155/2011/868217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bivic A. (2013). Evolution and cell physiology. 4. Why invent yet another protein complex to build junctions in epithelial cells? Am. J. Physiol. Cell Physiol. 305, C1193-C1201. 10.1152/ajpcell.00272.2013 [DOI] [PubMed] [Google Scholar]
- Lehmann R., Dietrich U., Jiménez F. and Campos-Ortega J. A. (1981). Mutations of early neurogenesis in Drosophila. Wilhelm Roux's Arch. Dev. Biol. 190, 226-229. 10.1007/BF00848307 [DOI] [PubMed] [Google Scholar]
- Lemmers C., Michel D., Lane-Guermonprez L., Delgrossi M.-H., Médina E., Arsanto J.-P. and Le Bivic A. (2004). CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol. Biol. Cell 15, 1324-1333. 10.1091/mbc.E03-04-0235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letizia A., Ricardo S., Moussian B., Martin N. and Llimargas M. (2013). A functional role of the extracellular domain of Crumbs in cell architecture and apicobasal polarity. J. Cell Sci. 126, 2157-2163. 10.1242/jcs.122382 [DOI] [PubMed] [Google Scholar]
- Lieber T., Kidd S., Alcamo E., Corbin V. and Young M. W. (1993). Antineurogenic phenotypes induced by truncated Notch proteins indicate a role in signal transduction and may point to a novel function for Notch in nuclei. Genes Dev. 7, 1949-1965. 10.1101/gad.7.10.1949 [DOI] [PubMed] [Google Scholar]
- Lin Y.-H., Currinn H., Pocha S. M., Rothnie A., Wassmer T. and Knust E. (2015). AP-2-complex-mediated endocytosis of Drosophila Crumbs regulates polarity by antagonizing Stardust. J. Cell Sci. 128, 4538-4549. 10.1242/jcs.174573 [DOI] [PubMed] [Google Scholar]
- Lu H. and Bilder D. (2005). Endocytic control of epithelial polarity and proliferation in Drosophila. Nat. Cell Biol. 7, 1232-1239. 10.1038/ncb1324 [DOI] [PubMed] [Google Scholar]
- Macara I. G., Guyer R., Richardson G., Huo Y. and Ahmed S. M. (2014). Epithelial homeostasis. Curr. Biol. 24, R815-R825. 10.1016/j.cub.2014.06.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey L. M. and Macara I. G. (2011). Epithelial organization, cell polarity and tumorigenesis. Trends Cell Biol. 21, 727-735. 10.1016/j.tcb.2011.06.005 [DOI] [PubMed] [Google Scholar]
- Mizutani K.-I., Yoon K., Dang L., Tokunaga A. and Gaiano N. (2007). Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature 449, 351-355. 10.1038/nature06090 [DOI] [PubMed] [Google Scholar]
- Müller H.-A. J. (2008). Immunolabeling of embryos. Methods Mol. Biol. 420, 207-218. 10.1007/978-1-59745-583-1_12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschalik N. and Knust E. (2011). Increased levels of the cytoplasmic domain of Crumbs repolarise developing Drosophila photoreceptors. J. Cell Sci. 124, 3715-3725. 10.1242/jcs.091223 [DOI] [PubMed] [Google Scholar]
- Nemetschke L. and Knust E. (2016). Drosophila Crumbs prevents ectopic Notch activation in developing wings by inhibiting ligand-independent endocytosis. Development 143, 4543-4553. 10.1242/dev.141762 [DOI] [PubMed] [Google Scholar]
- Oda H. and Takeichi M. (2011). Structural and functional diversity of cadherin at the adherens junction. J. Cell Biol. 193, 1137-1146. 10.1083/jcb.201008173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohata S., Aoki R., Kinoshita S., Yamaguchi M., Tsuruoka-Kinoshita S., Tanaka H., Wada H., Watabe S., Tsuboi T., Masai I. et al. (2011). Dual roles of Notch in regulation of apically restricted mitosis and apicobasal polarity of neuroepithelial cells. Neuron 69, 215-230. 10.1016/j.neuron.2010.12.026 [DOI] [PubMed] [Google Scholar]
- Pellikka M., Tanentzapf G., Pinto M., Smith C., McGlade C. J., Ready D. F. and Tepass U. (2002). Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature 416, 143-149. 10.1038/nature721 [DOI] [PubMed] [Google Scholar]
- Pocha S. M. and Knust E. (2013). Complexities of Crumbs function and regulation in tissue morphogenesis. Curr. Biol. 23, R289-R293. 10.1016/j.cub.2013.03.001 [DOI] [PubMed] [Google Scholar]
- Pocha S. M. and Wassmer T. (2011). A novel role for retromer in the control of epithelial cell polarity. Commun. Integr. Biol. 4, 749-751. 10.4161/cib.17658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramkumar N., Harvey B. M., Lee J. D., Alcorn H. L., Silva-Gagliardi N. F., McGlade C. J., Bestor T. H., Wijnholds J., Haltiwanger R. S. and Anderson K. V. (2015). Protein O-Glucosyltransferase 1 (POGLUT1) promotes mouse gastrulation through modification of the apical polarity protein CRUMBS2. PLoS Genet. 11, e1005551 10.1371/journal.pgen.1005551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramkumar N., Omelchenko T., Silva-Gagliardi N. F., McGlade C. J., Wijnholds J. and Anderson K. V. (2016). Crumbs2 promotes cell ingression during the epithelial-to-mesenchymal transition at gastrulation. Nat. Cell Biol. 18, 1281-1291. 10.1038/ncb3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond P. A., Colvin S. M., Jabeen Z., Nagashima M., Barthel L. K., Hadidjojo J., Popova L., Pejaver V. R. and Lubensky D. K. (2014). Patterning the cone mosaic array in zebrafish retina requires specification of ultraviolet-sensitive cones. PLoS ONE 9, e85325 10.1371/journal.pone.0085325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebay I., Fehon R. G. and Artavanis-Tsakonas S. (1993). Specific truncations of Drosophila Notch define dominant activated and dominant negative forms of the receptor. Cell 74, 319-329. 10.1016/0092-8674(93)90423-N [DOI] [PubMed] [Google Scholar]
- Richardson E. C. N. and Pichaud F. (2010). Crumbs is required to achieve proper organ size control during Drosophila head development. Development 137, 641-650. 10.1242/dev.041913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E. and Macara I. G. (2014). Organization and execution of the epithelial polarity programme. Nat. Rev. Mol. Cell Biol. 15, 225-242. 10.1038/nrm3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röper K. (2012). Anisotropy of Crumbs and aPKC drives myosin cable assembly during tube formation. Dev. Cell 23, 939-953. 10.1016/j.devcel.2012.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossner M. and Yamada K. M. (2004). What's in a picture? The temptation of image manipulation. J. Cell Biol. 166, 11-15. 10.1083/jcb.200406019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarov M., Barz C., Jambor H., Hein M. Y., Schmied C., Suchold D., Stender B., Janosch S., KJ V. V., Krishnan R. T. et al. (2016). A genome-wide resource for the analysis of protein localisation in Drosophila. Elife 5, e12068 10.7554/eLife.12068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivas J. M., Morrison H. A., Bilder D. and Skop A. R. (2010). Polarity and endocytosis: reciprocal regulation. Trends Cell Biol. 20, 445-452. 10.1016/j.tcb.2010.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões S., Oh Y., Wang M. F. Z., Fernandez-Gonzalez R. and Tepass U. (2017). Myosin II promotes the anisotropic loss of the apical domain during Drosophila neuroblast ingression. J. Cell Biol. 216, 1387-1404. 10.1083/jcb.201608038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeath J. B. and Carroll S. B. (1992). Regulation of proneural gene expression and cell fate during neuroblast segregation in the Drosophila embryo. Development 114, 939-946. [DOI] [PubMed] [Google Scholar]
- Sotillos S., Díaz-Meco M. T., Caminero E., Moscat J. and Campuzano S. (2004). DaPKC-dependent phosphorylation of Crumbs is required for epithelial cell polarity in Drosophila. J. Cell Biol. 166, 549-557. 10.1083/jcb.200311031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G., Fitzgerald K. and Greenwald I. (1993). Intrinsic activity of the Lin-12 and Notch intracellular domains in vivo. Cell 74, 331-345. 10.1016/0092-8674(93)90424-O [DOI] [PubMed] [Google Scholar]
- Tellkamp F., Vorhagen S. and Niessen C. M. (2014). Epidermal polarity genes in health and disease. Cold Spring Harb. Perspect. Med. 4, a015255 10.1101/cshperspect.a015255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U. (1996). Crumbs, a component of the apical membrane, is required for zonula adherens formation in primary epithelia of Drosophila. Dev. Biol. 177, 217-225. 10.1006/dbio.1996.0157 [DOI] [PubMed] [Google Scholar]
- Tepass U. (2009). FERM proteins in animal morphogenesis. Curr. Opin. Genet. Dev. 19, 357-367. 10.1016/j.gde.2009.05.006 [DOI] [PubMed] [Google Scholar]
- Tepass U. (2012). The apical polarity protein network in Drosophila epithelial cells: regulation of polarity, junctions, morphogenesis, cell growth, and survival. Annu. Rev. Cell Dev. Biol. 28, 655-685. 10.1146/annurev-cellbio-092910-154033 [DOI] [PubMed] [Google Scholar]
- Tepass U. and Knust E. (1990). Phenotypic and developmental analysis of mutations at the crumbs locus, a gene required for the development of epithelia in Drosophila melanogaster. Roux's Arch. Dev. Biol. 199, 189-206. 10.1007/BF01682078 [DOI] [PubMed] [Google Scholar]
- Tepass U., Theres C. and Knust E. (1990). crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell 61, 787-799. 10.1016/0092-8674(90)90189-L [DOI] [PubMed] [Google Scholar]
- Tepass U., Gruszynski-DeFeo E., Haag T. A., Omatyar L., Török T. and Hartenstein V. (1996). shotgun encodes Drosophila E-cadherin and is preferentially required during cell rearrangement in the neurectoderm and other morphogenetically active epithelia. Genes Dev. 10, 672-685. 10.1101/gad.10.6.672 [DOI] [PubMed] [Google Scholar]
- Thompson B. J., Pichaud F. and Röper K. (2013). Sticking together the Crumbs - an unexpected function for an old friend. Nat. Rev. Mol. Cell Biol. 14, 307-314. 10.1038/nrm3568 [DOI] [PubMed] [Google Scholar]
- Uemura T., Oda H., Kraut R., Hayashi S., Kotaoka Y. and Takeichi M. (1996). Zygotic Drosophila E-cadherin expression is required for processes of dynamic epithelial cell rearrangement in the Drosophila embryo. Genes Dev. 10, 659-671. 10.1101/gad.10.6.659 [DOI] [PubMed] [Google Scholar]
- Vereshchagina N., Bennett D., Szöőr B., Kirchner J., Gross S., Vissi E., White-Cooper H. and Alphey L. (2004). The essential role of PP1beta in Drosophila is to regulate nonmuscle myosin. Mol. Biol. Cell 15, 4395-4405. 10.1091/mbc.E04-02-0139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Dumstrei K., Haag T. and Hartenstein V. (2004). The role of DE-cadherin during cellularization, germ layer formation and early neurogenesis in the Drosophila embryo. Dev. Biol. 270, 350-363. 10.1016/j.ydbio.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Wodarz A., Grawe F. and Knust E. (1993). Crumbs is involved in the control of apical protein targeting during Drosophila epithelial development. Mech. Dev. 44, 175-187. 10.1016/0925-4773(93)90066-7 [DOI] [PubMed] [Google Scholar]
- Wodarz A., Hinz U., Engelbert M. and Knust E. (1995). Expression of Crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell 82, 67-76. 10.1016/0092-8674(95)90053-5 [DOI] [PubMed] [Google Scholar]
- Yu C.-Y., Chen J.-Y., Lin Y.-Y., Shen K.-F., Lin W.-L., Chien C.-L., ter Beest M. B. A. and Jou T.-S. (2007). A bipartite signal regulates the faithful delivery of apical domain marker podocalyxin/Gp135. Mol. Biol. Cell 18, 1710-1722. 10.1091/mbc.E06-07-0629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., Wu Y. and Lin X. (2011). Retromer regulates apical-basal polarity through recycling Crumbs. Dev. Biol. 360, 87-95. 10.1016/j.ydbio.2011.09.009 [DOI] [PubMed] [Google Scholar]
- Zou J., Wang X. and Wei X. (2012). Crb apical polarity proteins maintain zebrafish retinal cone mosaics via intercellular binding of their extracellular domains. Dev. Cell 22, 1261-1274. 10.1016/j.devcel.2012.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.