Abstract
Cytochrome P450 2J2 isoform (CYP2J2) is a drug-metabolizing enzyme that is highly expressed in adult ventricular myocytes. It is responsible for the bioactivation of arachidonic acid (AA) into epoxyeicosatrienoic acids (EETs). EETs are biologically active signaling compounds that protect against disease progression, particularly in cardiovascular diseases. As a drug-metabolizing enzyme, CYP2J2 is susceptible to drug interactions that could lead to cardiotoxicity. CYP2J2 has been shown to be resistant to induction by canonical CYP inducers such as phenytoin and rifampin. It is, however, unknown how cellular stresses augment CYP2J2 expression. Here, we determine the effects of oxidative stress on gene expression in adult ventricular myocytes. Further, we assess the consequences of CYP2J2 inhibition and CYP2J2 silencing on cells when levels of reactive oxygen species (ROS) are elevated. Findings indicate that CYP2J2 expression increases in response to external ROS or when internal ROS levels are elevated. In addition, cell survival decreases with ROS exposure when CYP2J2 is chemically inhibited or when CYP2J2 expression is reduced using small interfering RNA. These effects are mitigated with external addition of EETs to the cells. Finally, we determined the results of external EETs on gene expression and show that only two of the four regioisomers cause an increase in HMOX1 expression. This work is the first to determine the consequence of cellular stress, specifically high ROS levels, on CYP2J2 expression in human ventricular myocytes and discusses how this enzyme may play an important role in response to cardiac oxidative stress.
Introduction
Reactive oxygen species (ROS) are highly reactive entities that are naturally produced in mitochondria as byproducts of metabolism. ROS are normally released as part of aerobic respiration and include hydrogen peroxide, superoxide, and hydroxyl radicals, all of which are highly reactive and capable of oxidizing cellular lipids, proteins, and nucleic acids (Schieber and Chandel, 2014). ROS are typically associated with oxidative stress within cells but in recent years research has shown that ROS play a role in cell signaling and cell survival (Finkel, 2011; Bouitbir et al., 2012; Brieger et al., 2012). In healthy cells, ROS levels are carefully maintained and managed. Cellular antioxidants such as glutathione and enzymes such as superoxide dismutase and catalase ensure that ROS levels remain below toxic thresholds. In pathologic states, however, this careful balance is disrupted, and as ROS levels rise beyond healthy levels, cells enter a state of oxidative stress (Schieber and Chandel, 2014). Oxidative stress is associated with many disease states, including cardiovascular disease and diabetes (Giacco and Brownlee, 2010; Sugamura and Keaney, 2011).
Cardiovascular disease (CVD) is a major leading cause of mortality in the United States and worldwide. CVD is a family of diseases involving the heart and blood vessels. These include hypertension, arrhythmias, coronary artery disease, myocardial infarctions, hypertrophy, and heart failure. ROS and oxidative stress have been shown to be important factors in the onset and progression of CVD, but the exact role and mechanism of ROS involvement are still a matter of debate (Sugamura and Keaney, 2011). In addition to a possible role in the etiology of CVD, ROS are also implicated in the cardiovascular toxicities of some drugs. Doxorubicin (DOX), for example, is an anticancer agent that causes severe cardiotoxicity (Damiani et al., 2016). One mechanism leading to an adverse reaction to this drug has been shown to involve mitochondrial dysfunction and a subsequent rise in cellular ROS levels (Ichikawa et al., 2014; Damiani et al., 2016).
Arachidonic acid (AA), an omega-6 polyunsaturated fatty acid, is a precursor to a myriad of signaling molecules including eicosanoids, leukotrienes, prostaglandins, and thromboxanes (Sacerdoti et al., 2016). In the 1980s, cytochrome P450 (CYP) enzymes were discovered to convert AA to mono hydroxyl metabolites in addition to four regioisomers of epoxyeicosatrienoic acids (EETs) (Oliw et al., 1982; Laniado-Schwartzman et al., 1988; Oliw, 1994). Several animal models have demonstrated EETs to be protective in many disease states as well as against DOX-induced cardiotoxicity (Zhang et al., 2009; Cai et al., 2013; Ma et al., 2013; Westphal et al., 2013; Chen et al., 2015). EETs have gained increasing attention in CVD in the last two decades due to their involvement in angiogenesis, regulation of vasodilation, up-regulation of endothelial nitric oxide synthase, and interactions with cardiac ion channels, highlighting their importance in overall cardiovascular health (Larsen et al., 2007; Behm et al., 2009; Campbell and Fleming, 2010; Pfister et al., 2010). Although they were initially thought to elicit a response through activation of an EET receptor, which remains elusive, EETs have been shown to affect cellular changes by activating key signaling pathways, such as the mitogen-activated protein kinase/extracellular signal-regulated kinase and protein kinase B cascades (Yang et al., 2007).
CYP2J2, the major CYP expressed in human heart tissue, plays a prominent role in EET synthesis (Roman, 2002). Like other drug-metabolizing CYP isoforms, CYP2J2 is expressed in intestinal and hepatic tissue, but unlike other isoforms it is expressed in extrahepatic tissues, including the kidney, lungs, skeletal muscle, and most prominently the heart (Wu et al., 1996; Zeldin et al., 1997; DeLozier et al., 2007; Michaud et al., 2010; Evangelista et al., 2013). The active site cavity of CYP2J2, determined from homology models, is comparable to that of CYP3A4, the most prominent drug-metabolizing CYP isoform, resulting in a similarly wide substrate range (Lee et al., 2010, 2012). Examples of drugs metabolized by CYP2J2 include the antihistamines, terfenadine, astemizole, and ebastine, the chemotherapeutics tamoxifen and DOX, the immunosuppressant cyclosporine, and the antipsychotic thioridazine, among others (Matsumoto and Yamazoe, 2001; Hashizume et al., 2002; Matsumoto et al., 2002; Lee et al., 2012).
Our group and others have previously shown CYP2J2 to be the dominant CYP isoform expressed in the heart, specifically in ventricular myocytes (Wu et al., 1996; DeLozier et al., 2007; Michaud et al., 2010; Evangelista et al., 2013). In the heart, CYP2J2 is believed to be the predominant source of EETs. Given the importance of EETs in the heart, their reported abilities to protect against disease states, and the role for CYP2J2 as a primary source of EETs in ventricular myocytes, we investigated the effects of ROS on CYP2J2 expression in human ventricular myocytes. Further, we experimentally determined the effects of reduced function or expression of CYP2J2 on surviving ROS toxicity and whether external EETs may be are able to mitigate these effects.
Materials and Methods
Chemicals and Cell Culture Materials.
Danazol and thiazolyl blue tetrazolium bromide (MTT) were obtained from Sigma-Aldrich (St. Louis, MO) and used without further purification. Solvents and hydrogen peroxide were purchased from Fisher Scientific (Waltham, MA) and were also used without further purification. Adult derived primary human ventricular myocytes were obtained from Celprogen (cat. no. 36044-15; San Pedro, CA). Cell culture materials including media (complete growth media, cat. no. M36044-15S, and phenol and serum-free media, cat. no. M36044-15PN) and cell culture flasks and plates precoated with extracellular matrix (cat. no. E36044-15) were obtained from Celprogen. The media were further sterile filtered using a vacuum filter through a 0.22-μm polyether sulfone filter. DOX, (±)-5,6-cis epoxyeicosatrienoic acid (5,6-EET), (±)-8,9-cis epoxyeicosatrienoic acid (8,9-EET), (±)-11,12-cis epoxyeicosatrienoic acid (11,12-EET), and (±)-14,15-cis epoxyeicosatrienoic acid (14,15-EET) were obtained from Cayman Chemicals (Ann Arbor, MI).
Cardiomyocyte Cell Culture.
Cell culture was performed following Celprogen’s protocols. The cells obtained and used for these studies are adult derived ventricular cardiomyocytes. All experiments were performed between passages 6 and 8 from receipt from Celprogen. Passage numbers were determined by number of times the cells were treated with trypsin since receipt from Celprogen, which was designated passage 1. Briefly, the cells were maintained and expanded using Celprogen precoated flasks and plates and complete growth medium with serum. All experimental procedures involving treatments were performed using serum- and phenol-free media unless otherwise stated. Cells used for RNA isolation and subsequent experiments were harvested by washing the cells still attached to the plate with 1× phosphate-buffered saline (PBS). After washing, all liquid was aspirated from the wells, and the entire plate was stored in −80°C until further processing.
Gene Expression after Treatment with Hydrogen Peroxide, Doxorubicin, or EETs.
Experiments to determine gene response to external factors were performed using 12-well precoated plates from Celprogen. Cells were plated onto the wells at a density of approximately 250,000 cells per well and allowed to attach overnight at 37°C and 5% CO2. The cells were then washed with PBS and treated with hydrogen peroxide (0.01% v/v final concentration) or DOX (20 or 5 μM final concentration) in serum-free media.
Two concentrations of DOX were used in this study to determine the effects of DOX toxicity on the cells with impaired CYP2J2 expression and activity. A high concentration (20 μM) was chosen to induce maximal ROS formation in the cells over 24 hours. The effects of a lower concentration are also reported (5 μM, Supplemental Material), which was chosen to more closely mimic in vivo concentrations. Previously, others have determined the maximal in vivo concentration to be within 2–5 μM (Greene et al., 1983; van Asperen et al., 1999; Barpe et al., 2010; Maillet et al., 2016). The highest observed in vivo concentration was selected to achieve the greatest rise in intracellular ROS levels.
The control vehicle treatments included serum-free media with PBS to serve as the hydrogen peroxide treatment controls and 0.1% DMSO for DOX treatment controls. The cells were treated for either 6 (H2O2) or 24 hours (DOX), after which they were washed and stored at −80°C until RNA extraction.
Inhibition of CYP2J2 Using Danazol.
Inhibition experiments were performed using 12-well precoated plates from Celprogen. Cells were plated at a density of 250,000 cells per well and allowed to attach overnight at 37°C and 5% CO2. Cells were then washed with PBS, and the growth media was replaced with serum- and phenol-free media containing 1 μM danazol (DAN) and either H2O2 (0.01%) or DOX (5 or 20 μM final concentration). The controls included cells treated with vehicle only, DAN only, or cells treated with hydrogen peroxide (0.01%) or DOX (5, or 20 μM) alone. Experiments treating with hydrogen peroxide were performed for 6 hours to avoid complete cell death, while DOX experiments were performed for 24 hours.
After each treatment period, cell viability was measured using an MTT assay as described below. A variation of the experiments outlined earlier were also performed in the presence or absence of pyruvate (10 mM final concentration) to determine the effects of an antioxidant present in the medium (Franco et al., 2007).
CYP2J2 Gene Silencing.
Silencing of CYP2J2 gene expression was achieved using the RNAiMAX lipofectamine (Thermo Fisher Scientific, Waltham, MA) and the CYP2J2 Trilencer small interfering RNA (siRNA) or scrambled siRNA (Origene, Rockville, MD), using the manufacturer’s suggested protocols, optimized for the cardiomyocyte system for time and siRNA concentrations. Silencing of the HPRT1 gene using the Origene siRNA served as positive control for all gene knockdown experiments. Lipofectamine was delivered using a reverse-transfection protocol. Briefly, the siRNA was reconstituted to a stock concentration of 20 μM per the manufacturer instructions and prepared with the lipofectamine using OptiMEM reduced serum media (Thermo Fisher Scientific) by diluting to a concentration of 50 nM. Cells were washed with warm (37°C) PBS and harvested using trypsin.
Following trypsinization, cells were pelleted, resuspended and diluted to a concentration of 200,000 cells/ml in complete medium. The lipofectamine/siRNA stocks were added to each well to a final concentration of 10 nM siRNA (250 μl volume of 50 nM siRNA/lipofectamine in OptiMEM), followed by the cells (1 ml of cell suspension) for a final volume of 1250 μl in each well. Cells were incubated with the lipofectamine/siRNA for 72 hours, after which follow-up experiments were performed.
Following siRNA silencing of cells, the wells were carefully washed with PBS before serum-free medium containing DOX (20 or 5 μM) or DMSO (0.1%) we added for 24 hours. Rescue experiments were also performed with the addition of pyruvate (10 mM) or 11,12-EET (5 or 50 nM) to determine whether DOX toxicity after gene silencing can be mitigated.
Measuring Gene Expression following EETs Treatment.
Experiments examining gene expression following EET addition were performed using 12-well precoated plates. The cells were plated at a density of approximately 250,000 cells per well and allowed to attach overnight at 37°C and 5% CO2. The following day, cells were washed with warm (37°C) PBS. After aspirating the PBS, cells were treated with EETs (50 nM final concentration), either in combination (mix) or separately (individual isomers) in serum-free media for 1 hour. Negative controls were treated with serum-free media with <0.1% ethanol. The cells were treated with external EETs for 1 hour, after which the media was aspirated, the cells were washed, and the plates were stored at −80°C until RNA isolation.
Total RNA was extracted using the MagMax 96 Total RNA Isolation kit (Thermo Fisher Scientific). The RNA quality (A260/A280) and quantity were determined using a Synergy HTX Multi-Mode Reader (BioTek, Winooski, VT). Total RNA was then used to synthesize cDNA using the High Capacity RNA-to-cDNA kit (Thermo Fisher Scientific). Reverse-transcription polymerase chain reaction was then performed using TaqMan (Thermo Fisher Scientific) FAM reporter primers for the various genes screened (CYP2J2, EPHX2, PLA2G4C, HMOX1, SOD1, SOD2, CAT, and GPX1) in addition to the housekeeping gene GusB.
Cycle threshold (CT) values and the ΔCT method followed by 2ΔCTcalculation were used to determine the relative quantity of CYP2J2 (and other genes) mRNA relative to the GusB mRNA levels. The mRNA levels were first normalized to the housekeeping gene using the ΔCT method, and then the levels of expression in treated cells were compared to the expression levels in untreated cells using the ΔΔCT calculation, and relative gene expression levels were reported using the 2−ΔΔCT calculation (Livak and Schmittgen, 2001).
MTT Assay for Cell Viability.
Cell viability was determined using MTT assays. Briefly, after treatment with siRNA and/or chemicals, cells were treated with 5 μl of 12 mM MTT per milliliter of medium (60 μM MTT final concentration). The cells were incubated with MTT for 20 minutes at 37°C. Afterward, the medium was aspirated carefully, and DMSO (600 μl) was added to each well, followed by 75 μl of Sorenson’s glycine (100 mM glycine, 100 mM sodium chloride). The plate was placed on an orbital shaker for 5 minutes at 400 rpm.
The absorbance from each well was measured using a Tecan Infinite M200 plate reader (Tecan, Männedorf, Switzerland) using the following protocol: 5 seconds of orbital shaking with an amplitude of 1 mm, followed by 10 seconds of wait time, and then absorbance measurement at 570 nm (9 nm bandwidth) using 670 nm (9 nm bandwidth) as the reference wavelength. A true zero signal was obtained by following the same protocol using plates that did not contain cells. Measurements were normalized to the absorbance in vehicle controlled wells (set as 100% viability).
ROS Formation Assay.
ROS formation was measured using 2ʹ,7ʹ-dichlorodihydrofluorescein diacetate (H2DCFDA; Thermo Fisher Scientific). Cells were incubated in serum-free phenol-free media containing 20 μM H2DCFDA for 30 minutes following siRNA and/or drug treatments to determine the relative ROS levels in the cells. After incubation, 100-μl aliquots of the medium were transferred to a 96-well, black-walled, clear-bottom plate (Thermo Fisher Scientific). The fluorescence was captured and measured using a Synergy HTX Multi-Mode Reader (excitation wavelength of 485 nm/emission wavelength of 525 nm). Data were analyzed by normalizing signals to a control well subjected to similar conditions as described here but with no cells present as the 0 ROS formation control.
Data Analysis.
Experiments were performed as biologic triplicates, and the data reported as the mean ± S.D. All experiments were repeated at least 2 times on 2 separate days. Where appropriate, the reported values are the mean values of all experiments (representative of both interday and intraday variability). Despite the variation in interday knockdown efficiency using siRNA to reduce the CYP2J2 experiment, only experiments with >80% knockdown efficiency are presented in this report. Statistical significance was determined using t test with unequal variances, and using a threshold P value of 0.05. Statistical analyses were performed using GraphPad Prism version 6.07 for Windows (GraphPad Software, La Jolla, CA).
Results
Gene Expression in the Presence of ROS.
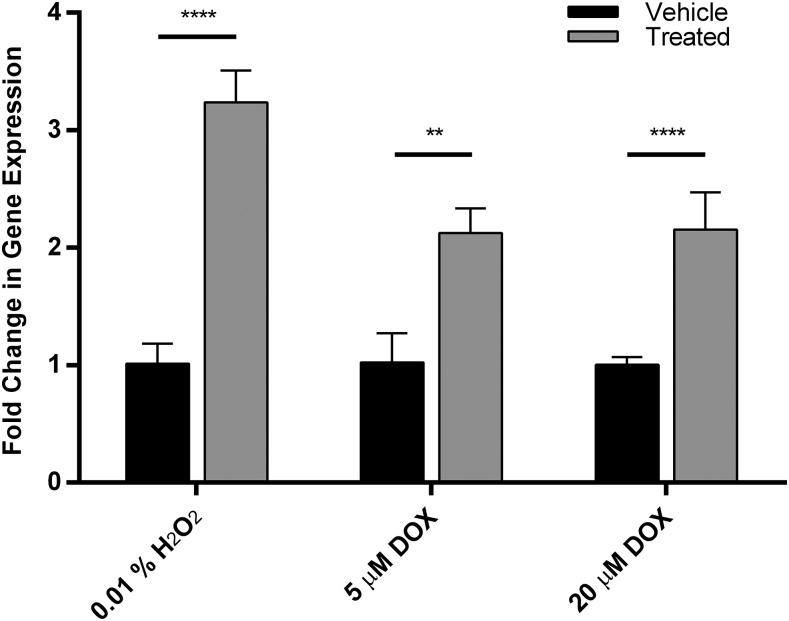
In the presence of increased ROS in adult ventricular myocytes, CYP2J2 expression is significantly increased by over 3-fold (Fig. 1). Treating the cells with DOX caused a dose-dependent increase in intracellular ROS levels (Supplemental Fig. 1). Treatment with DOX at 5 or 20 μM over a 24-hour period caused over 2-fold increases in CYP2J2 expression (Fig. 1). In addition, when cells are treated with 5 μM DOX, the expression of several other genes, specifically those that encode antioxidant proteins is also affected (Supplemental Fig. 2).
Fig. 1.
Ventricular myocytes treated with 0.01% H2O2 for 6 hours (left) or doxorubicin for 24 hours (right) result in a 3-fold and 2-fold up-regulation of CYP2J2 expression, respectively. Each experiment was performed in triplicate (nine data points per condition). Data were normalized to vehicle-treated cells (PBS for hydrogen peroxide and 0.1% DMSO for doxorubicin). Significance was determined using unpaired t test. ****P < 0.0001.
The most prominent increases were observed in HMOX1 (7-fold) and GPX1 (4-fold), which encode heme oxygenase 1 and glutathione peroxidase 1, respectively. Significant up-regulation were also observed in the gene expression of SOD1 and CAT, which encode superoxide dismutase 1 and catalase, respectively. Finally, modest, but not statistically significant, increases in superoxide dismutase 2 (SOD2) were also observed. The up-regulation in these genes was reversed, however, when cells were exposed to DOX in the presence of excess pyruvate, an antioxidant (Supplemental Fig. 2).
CYP2J2 Inhibition and Ventricular Myocytes Survival under Hydrogen Peroxide Stress.
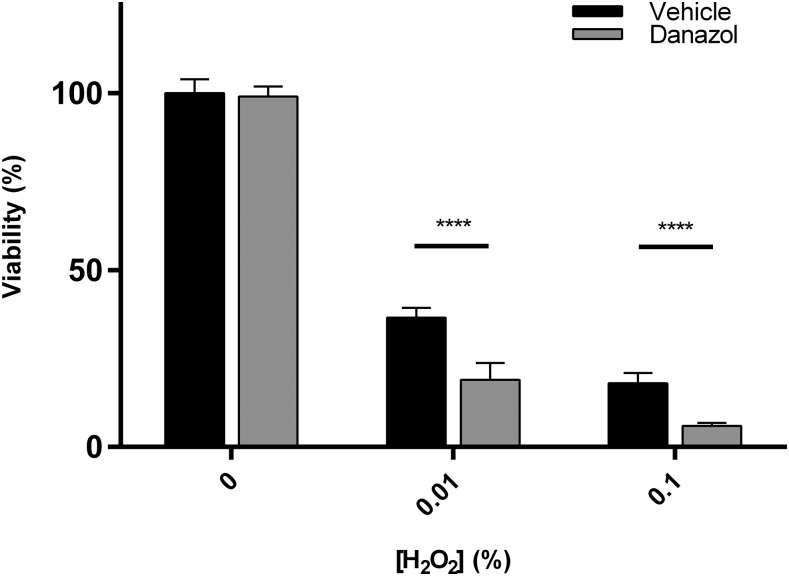
Cell survival in response to hydrogen peroxide in ventricular myocytes is dose dependent. There is significantly more cell death as the hydrogen peroxide concentration is increased. In the presence of DAN, a known CYP2J2 inhibitor (Lee et al., 2012; Evangelista et al., 2013), cells exposed to 0.01% hydrogen peroxide were less viable than cells not exposed to inhibitor (Fig. 2). In a series of three experiments performed all in triplicates, cells with chemically inhibited CYP2J2 activity were on average 2-fold less viable compared to cells exposed only to hydrogen peroxide. Of note, DAN treatment alone had no significant effect on cell viability.
Fig. 2.
Adult ventricular myocytes treated with varying concentrations of hydrogen peroxide for 6 hours. Cells were co-treated with vehicle (0.1% DMSO) or 1 μM danazol, a known CYP2J2 inhibitor. The data presented are the mean and S.D. of three separate experiments performed on three separate days, with each experiment done in triplicate. All data were normalized to untreated cells. Significance determined using unpaired t test. ****P < 0.0001.
Effects of CYP2J2 Silencing on Ventricular Myocytes.
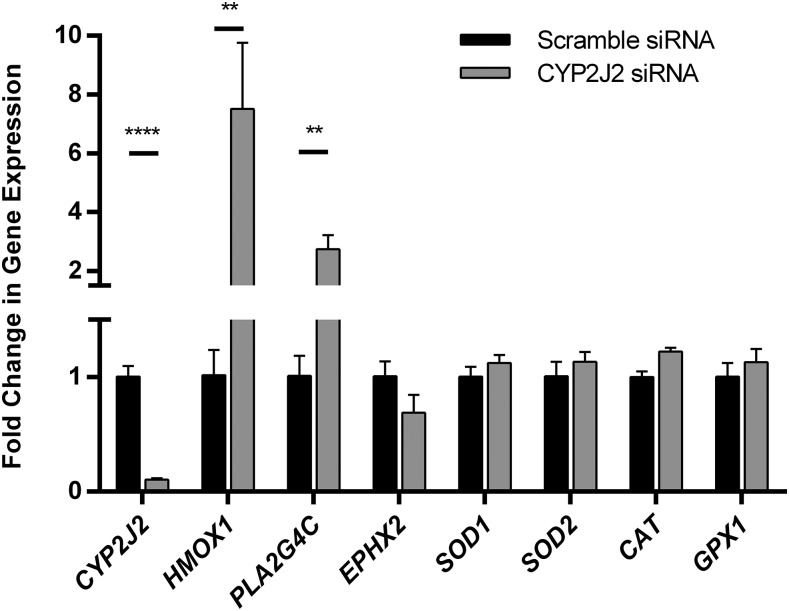
Using siRNA, CYP2J2 expression was consistently reduced in adult ventricular myocytes by >80% (Fig. 3). Experiments measuring CYP2J2 mRNA levels at various time points after 72 hours of silencing showed that CYP2J2 expression was restored to normal levels within 24 hours (data not shown). Silencing CYP2J2 had a remarkable effect on several other genes, specifically PLA2G4C and EPHX2, which encode human phospholipase A2 and soluble epoxide hydrolase proteins, respectively (Fig. 3). Alterations in the expression of either protein would be expected to affect EET levels by affecting free AA and EET degradation, respectively. Reduction in CYP2J2 resulted in 3-fold up-regulation of PLA2G4C and a 30% reduction of EPHX2 mRNA.
Fig. 3.
Representative figure of the effect of CYP2J2 knocked down using siRNA for 72 hours on several genes. The reported data are a mean of a single experiment done in triplicates and the S.D. The experiment was repeated multiple times on separate days. Data are normalized to control cells that were treated with scrambled siRNA, which were set to a value of 1, to determine the fold change in gene expression. Significance was determined using unpaired t test. **P < 0.01; ****P < 0.0001.
Gene silencing effects on the expression of HMOX1, SOD1, SOD2, CAT, and GPX1 were also probed. These genes encode heme oxygenase, superoxide dismutase (1 and 2), catalase, and glutathione peroxidase, respectively, all of which are ROS-responsive enzymes. The most remarkable change observed was the 7-fold up-regulation of HMOX1 (Fig. 3). The mRNA expression levels of the other four enzymes were also slightly elevated by CYP2J2 gene silencing, although these changes were not significant when compared to scramble-treated cells.
Decreasing CYP2J2 Increases Cell Death, an Effect That Is Reversed by External EETs.
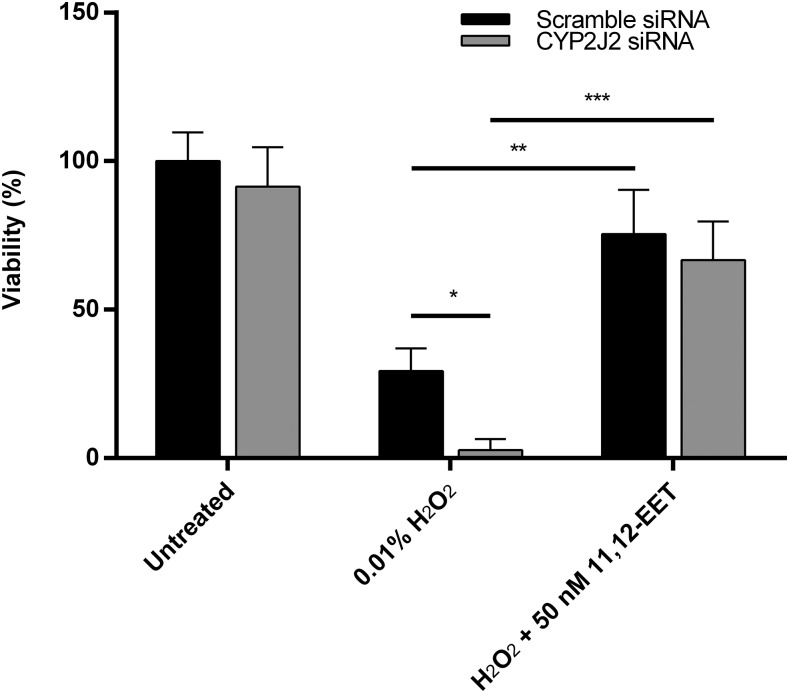
Reduction of CYP2J2 expression resulted in increased susceptibility to ROS toxicity (Fig. 4). While siRNA treatment by itself did not affect cell viability, exposing the cells to 0.01% H2O2 following CYP2J2 silencing resulted in significantly higher cell death. Cells treated with hydrogen peroxide after gene silencing had, on average, 2-fold greater cell death than cells treated with scramble siRNA.
Fig. 4.
The effects of hydrogen peroxide treatment and 11,12-EET rescue on cells with diminished CYP2J2 expression. Cells exposed to CYP2J2 siRNA were more susceptible to hydrogen peroxide toxicity compared with cells treated with scramble siRNA. 11,12-EET treatment 30 minutes before hydrogen peroxide reversed this effect. The data presented are the mean and S.D. from a single experiment performed in triplicate, and significance was determined using unpaired t test. *P < 0.05; **P < 0.01; ***P < 0.001.
Further, the effects of reduced viability in the presence of ROS were mitigated if the cells were exposed to external EETs prior to ROS exposure. Externally supplementing the cells with 50 nM 11,12-EET for 30 minutes before hydrogen peroxide exposure significantly increased the cells’ viability to levels similar to controls with normal CYP2J2 expression (Fig. 4).
Ventricular Myocytes with Compromised CYP2J2 Activity or Expression Are More Susceptible to Doxorubicin Toxicity.
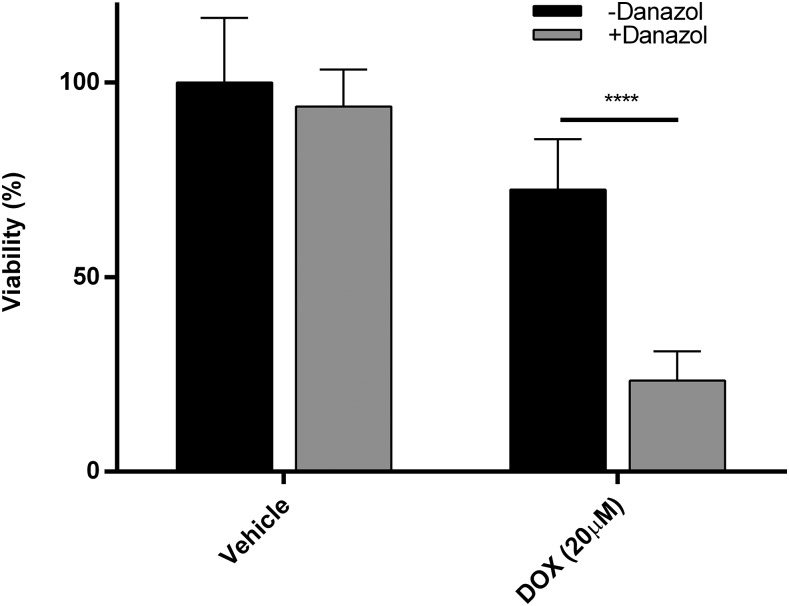
Cells exposed to DOX also experience dose-dependent toxicity. Further, treatment with DOX also results in dose-dependent increases in intracellular ROS levels. In the absence of additional stresses, exposure to 5 μM for approximately 24 hours resulted in a modest increase in ROS levels and about a 25% loss of cell viability (Supplemental Figs. 1 and 3). These numbers increase to approximately 50% higher intracellular ROS concentration and up to a 50% loss of cell viability when the dose is increased to 20 μM (Fig. 5; Supplemental Fig. 1). In addition, when CYP2J2 protein activity is inhibited by DAN, cell viability in the presence of DOX is much lower compared with cells not exposed to DAN at both concentrations tested. On average, cell viability was 1.5-fold and 3-fold higher in cells exposed only to DOX compared with cells exposed to DOX and DAN simultaneously at 5 and 20 μM, respectively (Fig. 5; Supplemental Fig. 3).
Fig. 5.
The effects of doxorubicin (20 μM for 24 hours) on adult ventricular myocyte viability in the presence and absence of the CYP2J2 inhibitor danazol. The data presented are the mean and S.D. of three separate interday experiments, each done in triplicate. The data were normalized to cells that were untreated. Significance was determined using unpaired t test. ****P < 0.0001.
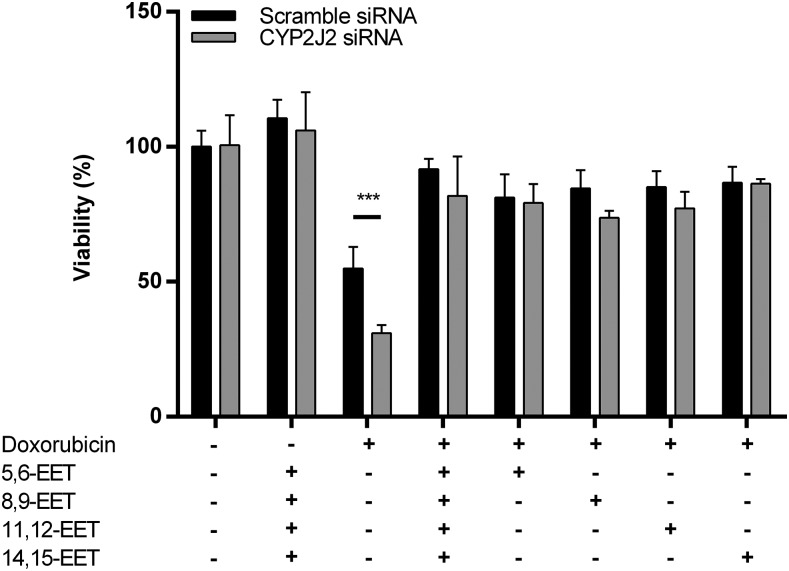
Finally, mirroring the results of cells exposed to hydrogen peroxide, adult ventricular myocytes are more susceptible to DOX toxicity when CYP2J2 expression is reduced. On average, lowering CYP2J2 expression reduced cell viability with DOX treatment by up to 40% compared with DOX-treated cells with normal CYP2J2 expression. In addition, this decrease in viability is mitigated when cells are simultaneously treated with EETs, either in a mixture or individually. There were no marked differences between the different regioisomers of EET in protecting against DOX toxicity as all four regioisomers appear to have similar effects (Fig. 6; Supplemental Fig. 4).
Fig. 6.
The effects of doxorubicin (20 μM, 24 hours) on adult cardiomyocyte viability with reduced CYP2J2 expression due to 72 hours of silencing with siRNA. Shown are the effects of cotreatment with EETs (50 nM total) on cell viability, using either combination or individual EETs. The data presented are the mean and S.D. of a single experiment in triplicate. Data were normalized to cells treated with scrambled siRNA for 72 hours, followed by vehicle treatment of 24 hours. The P values were determined using unpaired t test. ***P < 0.001.
EET Effects on Gene Expression.
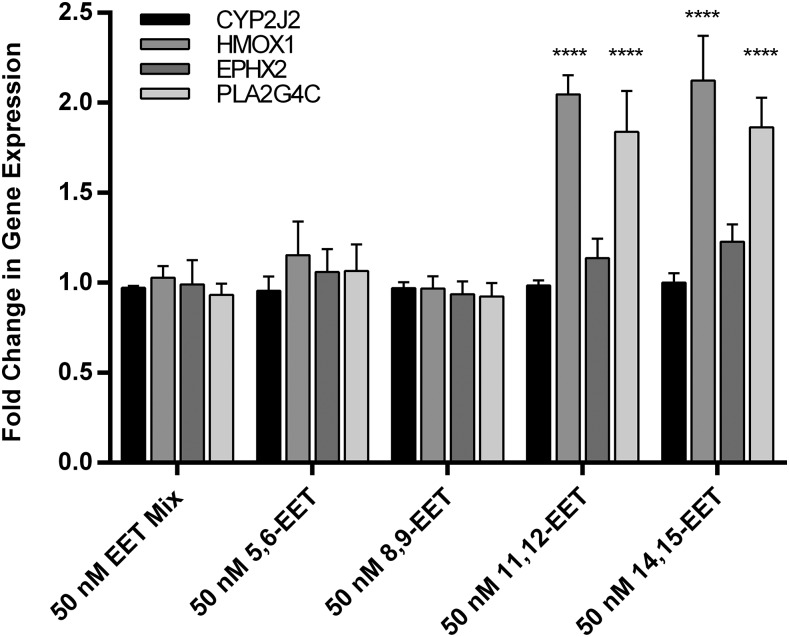
Cells were treated with EETs for one hour to capture the early effects on gene expression, before the possible esterification of external EETs into the cell membrane for storage or hydrolysis by soluble epoxide hydrolase. Cells exposed to external EETs for only one hour exhibited very little variability in CYP2J2 and EPHX2 gene expression. Similarly, the gene expression of HMOX1 and PLA2G4C changed very little over the 1-hour incubation (Fig. 7). The exceptions to this were when the cells were treated with external 11,12-EET and 14,15-EET separately. HMOX1 gene expression significantly increased by 2-fold over the 1-hour treatment. Similarly, PLA2G4C was significantly up-regulated by almost 2-fold (1.8-fold change over untreated, Fig. 7). Treatment with 11,12-EET and 14,15-EET exhibited similar effects on the genes that were surveyed. Neither 8,9-EET nor 5,6-EET had a significant effect on the surveyed gene expression.
Fig. 7.
Fold change in expression is compared to controls not treated with EETs.Relative gene expression when adult ventricular myocytes are treated with EETs, in combination or individually (50 nM total concentration). Significance and P values were determined using unpaired t test. ****P < 0.0001.
Discussion
In this study, we determined the effects of ROS on CYP2J2 expression in adult primary ventricular myocytes. One key finding was that CYP2J2 expression increases in response to oxidative stress in cardiomyocytes, either from exogenous ROS or DOX treatment. Other genes encoding antioxidant proteins were also up-regulated during DOX exposure. These effects were reduced in the presence of an antioxidant (pyruvate), indicating that gene up-regulation was, in part, due to increased ROS levels. Further, inhibition of CYP2J2 expression enhanced ROS-induced cell death, suggesting that CYP2J2 is part of the early defense mechanisms against oxidative stress in adult primary ventricular myocytes.
CYP2J2 has long been identified as a protective enzyme in the heart, through its metabolism of AA to EETs (Murray, 2016). In animal models, others have shown that overexpression of human CYP2J2 mitigates DOX cardiotoxicity (Zhang et al., 2009). Zordoky et al. (2010) demonstrated that treating rats with DOX for 24 hours showed a significant up-regulation of CYP2J3, the rat ortholog of CYP2J2, and Alsaad et al. (2012) demonstrated that chronic DOX (14-day) treatment of rats showed no statistically significant change in CYP2J3 expression, the rat ortholog of CYP2J2. These data suggest a rapid transient response to the drug.
Our study is, the first report where CYP2J2 expression is up-regulated in response to DOX due to an increase in ROS in human adult ventricular myocytes. Further, while typical chemical cytochrome P450 inducers such as phenobarbital and rifampicin are unable to affect CYP2J2 expression levels in cardiomyocytes, disease and stress markers have been shown to affect expression levels (Bystrom et al., 2013; Evangelista et al., 2013). Bystrom et al. (2013) demonstrated that in response to bacterial lipopolysaccharides, CYP2J2 is induced in human peripheral blood mononuclear cells and stimulates anti-inflammatory action in response to the presence of bacteria. These results indicate that regulation of CYP2J2 expression is a general protective response against stresses that may increase ROS in different cell types.
The protective outcomes that EETs exert, particularly where cardiac health is concerned, are numerous, though the exact mechanisms are not well defined. Of the cytochrome P450 isoforms, EET formation has been linked predominantly to the CYP2 family, with CYP2J2, CYP2C8, and CYP2C9 putatively the most active isoforms involved in the cardiovascular system (Chaudhary et al., 2009). We have previously shown CYP2J2 to be the most prominently expressed drug-metabolizing CYP isoform expressed in ventricular myocytes (Evangelista et al., 2013). CYP2C8 and CYP2C9, in contrast, have greater expression levels in the endothelium of the vasculature, where CYP2J2 is present at lower levels in comparison with other EET-forming enzymes (DeLozier et al., 2007; Michaud et al., 2010). Other groups have shown induction of CYP2J2 in various cell lines such as hepG2 cells using chemical inducers, but induction in cardiomyocytes was not observed (Lee and Murray, 2010; Evangelista et al., 2013). As the primary source of EETs in ventricular myocytes, it is likely that CYP2J2 expression is tightly regulated to preserve cardiac function, thus providing an explanation to its resistance to typical CYP inducers such as phenobarbital and rifampicin.
Data from our study demonstrate that adult ventricular myocytes respond to elevated ROS levels by up-regulating CYP2J2 expression. In addition, this response is triggered either through the direct exposure of the cells to ROS or indirectly through exposure to DOX and subsequent intracellular ROS elevation. The up-regulation of CYP2J2 is likely a response designed to increase EET levels to counteract the threat of increased ROS levels. In this work, cells that were either pretreated or cotreated with EETs, when CYP2J2 expression was knocked down, experienced greater cell survival in response to ROS toxicity. In cases where we treated with DOX, increases in ROS levels were mitigated by cotreatment with EETs. In addition, rescue by EETs against DOX toxicity has an identical effect to rescue with excess amounts of antioxidant, specifically pyruvate. The mechanism for this protection remains unknown although it is likely a result of EET signaling rather than direct reactions between ROS and EETs. The stoichiometry of EETs compared with hydrogen peroxide (i.e., 50 nM external EETs to rescue against the effects of millimolar hydrogen peroxide or micromolar DOX concentrations) provides evidence against the latter mechanism. It is unlikely that EETs would be able to directly protect against levels that are in vast excess, therefore signal amplification is the most likely cause of mitigation of any ROS toxicity observed.
This response to ROS by CYP2J2 is unique to this isozyme because other drug-metabolizing CYPs are typically down-regulated in times of disease and stress (Xu et al., 2006; Morgan et al., 2008; Morgan, 2009). This is of special interest considering evidence suggesting an interplay between heme oxygenase 1 (HO-1) and EET analogs as mitigators of adipogenesis during metabolic syndrome, as demonstrated by Sodhi et al. (2009) and others (Sacerdoti et al., 2016). Data from this study suggest a link between CYP2J2 and HO-1, specifically at the transcriptional level. We showed that transient silencing of CYP2J2 expression consistently led to HMOX1 up-regulation. Additionally, when 11,12-EETs or 14,15-EETs were added externally to the cells, HMOX1 and PLA2G4C expression increased (Fig. 7).
HMOX1, the gene that encodes HO-1, is a gene sensitive to the redox state of the cell. Phospholipase A2, encoded by the PLA2G4C gene, cleaves stored AA and EETs from the cell membrane, promoting increases in EET levels. The same effects, however, are not observed when a mixture of the four EETs is introduced (Fig. 7). This discrepancy could be due to reduced overall concentrations of the 11,12-EET and 14,15-EET isomers, which may not have reached a threshold necessary to affect HMOX1 expression.
These findings are intriguing for several reasons. First, all EET regioisomers, including 5,6-EET and 8,9-EET, protected the cell from ROS toxicity, but only 11,12-EET and 14,15-EET were capable of increasing HMOX1 mRNA transcripts, suggesting one or more alternate pathways by which EET regioisomers protect against ROS damage.
Others have also reported that the various regioisomers exhibit different effects. For example, Mitra et al. (2011) found that in some tumors, 14,15-EET affects human tumor growth while the other isomers had no affect While 11,12-EET and 14,15-EET separately appear to protect cardiomyocytes through the heme oxygenase pathway, introducing a mixture of EETs to the cardiomyocytes diminished any effect on the gene expression of HMOX1. In combination, a different pathway or pathways to rescue the cells could be activated by EETs. It is difficult to tease out the exact mechanism, especially given that very little is known about the 5,6-EET and 8,9-EET isomers, including how they might exert their effects on the cells and the elusive nature of an EET receptor.
Taken together, these data suggest a role, perhaps multiple roles, for EETs and CYP2J2 in the oxidative stress response of ventricular myocytes. Investigating the dose-response and time-response effects of the separate EETs on ventricular myocytes are necessary to tease out the role of each regioisomer.
Increased production of ROS and subsequent oxidative stress are characteristic of many diseases, including diabetes, neurodegenerative disease, and heart disease (Giacco and Brownlee, 2010; Sugamura and Keaney, 2011; Tsutsui et al., 2011; Brieger et al., 2012). Although these are not the sole causative factors in any of these diseases, they are prominent aspects. Our results suggest an interplay between CYP2J2 expression and the oxidative state of the cell. Based on the data obtained here and by others, one possible mechanism is that EETs, or at least two of the EET isomers as indicated earlier, trigger signaling cascades that result in the up-regulation of HMOX1 and other antioxidant enzymes. HO-1 has long been identified as a protective enzyme with regards to oxidative stress. It catalyzes the metabolism of heme into biliverdin and free iron and in the process releases carbon monoxide, a gasotransmitter that can act as an antioxidant (Rochette et al., 2013; Otterbein et al., 2016).
Small amounts of ROS are beneficial to the cell as they trigger cascades that promote cell survival and proliferation (Zhang et al., 2016). In this context, where EETs have previously been shown to promote cell growth and survival, up-regulation of CYP2J2 might be expected. Further, up-regulation of HMOX1 by EET isomers may represent a mechanism which signals triggered by ROS are terminated. In this manner, CYP2J2 would be part of a response signal to trigger growth and survival while simultaneously initiating the reduction of ROS levels through the up-regulation of heme oxygenase, ensuring that cells are not overwhelmed by prolonged damage stemming from elevated ROS levels. Our work suggests that CYP2J2 through EETs can protect the cell against increased ROS levels, but the pathway that leads to this protection has not been elucidated and will be further characterized in future work.
To conclude, CYP2J2 gene expression is responsive to increasing ROS levels in human ventricular cardiomyocytes. Cells treated with either hydrogen peroxide or DOX, which causes an increase in ROS levels, exhibit an up-regulation of CYP2J2 expression. In addition to modulation of expression by ROS, other markers of cardiac disease should be investigated to determine other mechanisms that alter CYP2J2 levels. Given the wide range of effects exhibited by EETs, it is highly likely that CYP2J2 may respond to multiple stress factors that threaten proper cardiac function.
Abbreviations
- AA
arachidonic acid
- CT
cycle threshold
- CVD
cardiovascular disease
- CYP
cytochrome P450 isoforms
- DAN
danazol
- DMSO
dimethylsulfoxide
- DOX
doxorubicin
- EET
epoxyeicosatrienoic acid
- H2DCFDA
2ʹ,7ʹ-dichlorodihydrofluorescein diacetate
- HO-1
heme oxygenase 1
- MTT
thiazolyl blue tetrazolium bromide
- PBS
phosphate-buffered saline
- ROS
reactive oxygen species
- siRNA
small interfering RNA
Authorship Contributions
Participated in research design: Evangelista, Totah, Gharib, Sotoodehnia, Lemaitre.
Conducted experiments: Evangelista.
Contributed new reagents or analytic tools: Evangelista.
Performed data analysis: Evangelista, Totah.
Wrote or contributed to the writing of the manuscript: Evangelista, Totah, Gharib, Sotoodehnia, Lemaitre.
Footnotes
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [R01HL08845, R01HL130880, R01HL096706, and R01HL128809], the National Center for Advancing Translational Sciences [TL1TR000422], and the National Institute of General Medical Sciences [T32GM007750].
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Alsaad AMS, Zordoky BNM, El-Sherbeni AA, El-Kadi AOS. (2012) Chronic doxorubicin cardiotoxicity modulates cardiac cytochrome P450-mediated arachidonic acid metabolism in rats. Drug Metab Dispos 40:2126–2135. [DOI] [PubMed] [Google Scholar]
- Barpe DR, Rosa DD, Froehlich PE. (2010) Pharmacokinetic evaluation of doxorubicin plasma levels in normal and overweight patients with breast cancer and simulation of dose adjustment by different indexes of body mass. Eur J Pharm Sci 41:458–463. [DOI] [PubMed] [Google Scholar]
- Behm DJ, Ogbonna A, Wu C, Burns-Kurtis CL, Douglas SA. (2009) Epoxyeicosatrienoic acids function as selective, endogenous antagonists of native thromboxane receptors: identification of a novel mechanism of vasodilation. J Pharmacol Exp Ther 328:231–239. [DOI] [PubMed] [Google Scholar]
- Bouitbir J, Charles AL, Echaniz-Laguna A, Kindo M, Daussin F, Auwerx J, Piquard F, Geny B, Zoll J. (2012) Opposite effects of statins on mitochondria of cardiac and skeletal muscles: a ‘mitohormesis’ mechanism involving reactive oxygen species and PGC-1. Eur Heart J 33:1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieger K, Schiavone S, Miller FJ, Jr, Krause KH. (2012) Reactive oxygen species: from health to disease. Swiss Med Wkly 142:w13659. [DOI] [PubMed] [Google Scholar]
- Bystrom J, Thomson SJ, Johansson J, Edin ML, Zeldin DC, Gilroy DW, Smith AM, Bishop-Bailey D. (2013) Inducible CYP2J2 and its product 11,12-EET promotes bacterial phagocytosis: a role for CYP2J2 deficiency in the pathogenesis of Crohn’s disease? PLoS One 8:e75107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Zhao G, Yan J, Liu W, Feng W, Ma B, Yang L, Wang JA, Tu L, Wang DW. (2013) CYP2J2 overexpression increases EETs and protects against angiotensin II-induced abdominal aortic aneurysm in mice. J Lipid Res 54:1448–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WB, Fleming I. (2010) Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch 459:881–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary KR, Batchu SN, Seubert JM. (2009) Cytochrome P450 enzymes and the heart. IUBMB Life 61:954–960. [DOI] [PubMed] [Google Scholar]
- Chen G, Xu R, Zhang S, Wang Y, Wang P, Edin ML, Zeldin DC, Wang DW. (2015) CYP2J2 overexpression attenuates nonalcoholic fatty liver disease induced by high-fat diet in mice. Am J Physiol Endocrinol Metab 308:E97–E110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani RM, Moura DJ, Viau CM, Caceres RA, Henriques JAP, Saffi J. (2016) Pathways of cardiac toxicity: comparison between chemotherapeutic drugs doxorubicin and mitoxantrone. Arch Toxicol 90:2063–2076. [DOI] [PubMed] [Google Scholar]
- Delozier TC, Kissling GE, Coulter SJ, Dai D, Foley JF, Bradbury JA, Murphy E, Steenbergen C, Zeldin DC, Goldstein JA. (2007) Detection of human CYP2C8, CYP2C9, and CYP2J2 in cardiovascular tissues. Drug Metab Dispos 35:682–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista EA, Kaspera R, Mokadam NA, Jones JP, III, Totah RA. (2013) Activity, inhibition, and induction of cytochrome P450 2J2 in adult human primary cardiomyocytes. Drug Metab Dispos 41:2087–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. (2011) Signal transduction by reactive oxygen species. J Cell Biol 194:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R, Panayiotidis MI, Cidlowski JA. (2007) Glutathione depletion is necessary for apoptosis in lymphoid cells independent of reactive oxygen species formation. J Biol Chem 282:30452–30465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacco F, Brownlee M. (2010) Oxidative stress and diabetic complications. Circ Res 107:1058–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene RF, Collins JM, Jenkins JF, Speyer JL, Myers CE. (1983) Plasma pharmacokinetics of adriamycin and adriamycinol: implications for the design of in vitro experiments and treatment protocols. Cancer Res 43:3417–3421. [PubMed] [Google Scholar]
- Hashizume T, Imaoka S, Mise M, Terauchi Y, Fujii T, Miyazaki H, Kamataki T, Funae Y. (2002) Involvement of CYP2J2 and CYP4F12 in the metabolism of ebastine in human intestinal microsomes. J Pharmacol Exp Ther 300:298–304. [DOI] [PubMed] [Google Scholar]
- Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Naga Prasad SV, Mutharasan RK, Naik TJ, Ardehali H. (2014) Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest 124:617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laniado-Schwartzman M, Davis KL, McGiff JC, Levere RD, Abraham NG. (1988) Purification and characterization of cytochrome P-450-dependent arachidonic acid epoxygenase from human liver. J Biol Chem 263:2536–2542. [PubMed] [Google Scholar]
- Larsen BT, Campbell WB, Gutterman DD. (2007) Beyond vasodilatation: non-vasomotor roles of epoxyeicosatrienoic acids in the cardiovascular system. Trends Pharmacol Sci 28:32–38. [DOI] [PubMed] [Google Scholar]
- Lee AC, Murray M. (2010) Up-regulation of human CYP2J2 in HepG2 cells by butylated hydroxyanisole is mediated by c-Jun and Nrf2. Mol Pharmacol 77:987–994. [DOI] [PubMed] [Google Scholar]
- Lee CA, Jones JP, III, Katayama J, Kaspera R, Jiang Y, Freiwald S, Smith E, Walker GS, Totah RA. (2012) Identifying a selective substrate and inhibitor pair for the evaluation of CYP2J2 activity. Drug Metab Dispos 40:943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CA, Neul D, Clouser-Roche A, Dalvie D, Wester MR, Jiang Y, Jones JP, III, Freiwald S, Zientek M, Totah RA. (2010) Identification of novel substrates for human cytochrome P450 2J2. Drug Metab Dispos 38:347–356. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) method. Methods 25:402–408. [DOI] [PubMed] [Google Scholar]
- Ma B, Xiong X, Chen C, Li H, Xu X, Li X, Li R, Chen G, Dackor RT, Zeldin DC, et al. (2013) Cardiac-specific overexpression of CYP2J2 attenuates diabetic cardiomyopathy in male streptozotocin-induced diabetic mice. Endocrinology 154:2843–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet A, Tan K, Chai X, Sadananda SN, Mehta A, Ooi J, Hayden MR, Pouladi MA, Ghosh S, Shim W, et al. (2016) Modeling doxorubicin-induced cardiotoxicity in human pluripotent stem cell derived-cardiomyocytes. Sci Rep 6:25333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Hirama T, Matsubara T, Nagata K, Yamazoe Y. (2002) Involvement of CYP2J2 on the intestinal first-pass metabolism of antihistamine drug, astemizole. Drug Metab Dispos 30:1240–1245. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Yamazoe Y. (2001) Involvement of multiple human cytochromes P450 in the liver microsomal metabolism of astemizole and a comparison with terfenadine. Br J Clin Pharmacol 51:133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud V, Frappier M, Dumas MC, Turgeon J. (2010) Metabolic activity and mRNA levels of human cardiac CYP450s involved in drug metabolism. PLoS One 5:e15666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Guo Z, Milani M, Mesaros C, Rodriguez M, Nguyen J, Luo X, Clarke D, Lamba J, Schuetz E, et al. (2011) CYP3A4 mediates growth of estrogen receptor-positive breast cancer cells in part by inducing nuclear translocation of phospho-Stat3 through biosynthesis of (±)-14,15-epoxyeicosatrienoic acid (EET). J Biol Chem 286:17543–17559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan ET. (2009) Impact of infectious and inflammatory disease on cytochrome P450-mediated drug metabolism and pharmacokinetics. Clin Pharmacol Ther 85:434–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan ET, Goralski KB, Piquette-Miller M, Renton KW, Robertson GR, Chaluvadi MR, Charles KA, Clarke SJ, Kacevska M, Liddle C, et al. (2008) Regulation of drug-metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab Dispos 36:205–216. [DOI] [PubMed] [Google Scholar]
- Murray M. (2016) CYP2J2—regulation, function and polymorphism. Drug Metab Rev 48:351–368. [DOI] [PubMed] [Google Scholar]
- Oliw EH. (1994) Oxygenation of polyunsaturated fatty acids by cytochrome P450 monooxygenases. Prog Lipid Res 33:329–354. [DOI] [PubMed] [Google Scholar]
- Oliw EH, Guengerich FP, Oates JA. (1982) Oxygenation of arachidonic acid by hepatic monooxygenases. Isolation and metabolism of four epoxide intermediates. J Biol Chem 257:3771–3781. [PubMed] [Google Scholar]
- Otterbein LE, Foresti R, Motterlini R. (2016) Heme oxygenase-1 and carbon monoxide in the heart: the balancing act between danger signaling and pro-survival. Circ Res 118:1940–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister SL, Gauthier KM, Campbell WB. (2010) Vascular pharmacology of epoxyeicosatrienoic acids. Adv Pharmacol 60:27–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochette L, Cottin Y, Zeller M, Vergely C. (2013) Carbon monoxide: mechanisms of action and potential clinical implications. Pharmacol Ther 137:133–152. [DOI] [PubMed] [Google Scholar]
- Roman RJ. (2002) P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82:131–185. [DOI] [PubMed] [Google Scholar]
- Sacerdoti D, Pesce P, Di Pascoli M, Bolognesi M. (2016) EETs and HO-1 cross-talk. Prostaglandins Other Lipid Mediat 125:65–79. [DOI] [PubMed] [Google Scholar]
- Schieber M, Chandel NS. (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24:R453–R462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi K, Inoue K, Gotlinger KH, Canestraro M, Vanella L, Kim DH, Manthati VL, Koduru SR, Falck JR, Schwartzman ML, et al. (2009) Epoxyeicosatrienoic acid agonist rescues the metabolic syndrome phenotype of HO-2-null mice. J Pharmacol Exp Ther 331:906–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugamura K, Keaney JF., Jr (2011) Reactive oxygen species in cardiovascular disease. Free Radic Biol Med 51:978–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui H, Kinugawa S, Matsushima S. (2011) Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol 301:H2181–H2190. [DOI] [PubMed] [Google Scholar]
- van Asperen J, van Tellingen O, Tijssen F, Schinkel AH, Beijnen JH. (1999) Increased accumulation of doxorubicin and doxorubicinol in cardiac tissue of mice lacking mdr1a P-glycoprotein. Br J Cancer 79:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal C, Spallek B, Konkel A, Marko L, Qadri F, DeGraff LM, Schubert C, Bradbury JA, Regitz-Zagrosek V, Falck JR, et al. (2013) CYP2J2 overexpression protects against arrhythmia susceptibility in cardiac hypertrophy. PLoS One 8:e73490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Moomaw CR, Tomer KB, Falck JR, Zeldin DC. (1996) Molecular cloning and expression of CYP2J2, a human cytochrome P450 arachidonic acid epoxygenase highly expressed in heart. J Biol Chem 271:3460–3468. [DOI] [PubMed] [Google Scholar]
- Xu D-X, Wang J-P, Sun M-F, Chen Y-H, Wei W. (2006) Lipopolysaccharide downregulates the expressions of intestinal pregnane X receptor and cytochrome P450 3a11. Eur J Pharmacol 536:162–170. [DOI] [PubMed] [Google Scholar]
- Yang S, Lin L, Chen JX, Lee CR, Seubert JM, Wang Y, Wang H, Chao ZR, Tao DD, Gong JP, et al. (2007) Cytochrome P-450 epoxygenases protect endothelial cells from apoptosis induced by tumor necrosis factor-alpha via MAPK and PI3K/Akt signaling pathways. Am J Physiol Heart Circ Physiol 293:H142–H151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeldin DC, Foley J, Goldsworthy SM, Cook ME, Boyle JE, Ma J, Moomaw CR, Tomer KB, Steenbergen C, Wu S. (1997) CYP2J subfamily cytochrome P450s in the gastrointestinal tract: expression, localization, and potential functional significance. Mol Pharmacol 51:931–943. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, Dong W. (2016) ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev 2016:4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, El-Sikhry H, Chaudhary KR, Batchu SN, Shayeganpour A, Jukar TO, Bradbury JA, Graves JP, DeGraff LM, Myers P, et al. (2009) Overexpression of CYP2J2 provides protection against doxorubicin-induced cardiotoxicity. Am J Physiol Heart Circ Physiol 297:H37–H46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zordoky BNM, Anwar-Mohamed A, Aboutabl ME, El-Kadi AOS. (2010) Acute doxorubicin cardiotoxicity alters cardiac cytochrome P450 expression and arachidonic acid metabolism in rats. Toxicol Appl Pharmacol 242:38–46. [DOI] [PubMed] [Google Scholar]