Abstract
Introduction
People with rheumatoid arthritis (RA) frequently report reduced health-related quality of life (HRQoL), the impact one’s health has on physical, emotional and social well-being. There are likely numerous causes for poor HRQoL, but people with RA have identified sleep disturbances as a key contributor to their well-being. This study will identify sleep/wake rhythm-associated parameters that predict HRQoL in patients with RA.
Methods and analysis
This prospective cohort study will recruit 350 people with RA, aged 18 years or older. Following completion of a paper-based baseline questionnaire, participants will record data on 10 symptoms including pain, fatigue and mood two times a day for 30 days using a study-specific mobile application (app). A triaxial accelerometer will continuously record daytime activity and estimate evening sleep parameters over the 30 days. Every 10 days following study initiation, participants will complete a questionnaire that measures disease specific (Arthritis Impact Measurement Scale 2-Short Form (AIMS2-SF)) and generic (WHOQOL-BREF) quality of life. A final questionnaire will be completed at 60 days after entering the study. The primary outcomes are the AIMS2-SF and WHOQOL-BREF. Structural equation modelling and latent trajectory models will be used to examine the relationship between sleep/wake rhythm-associated parameters and HRQoL, over time.
Ethics and dissemination
Results from this study will be disseminated at regional and international conferences, in peer-reviewed journals and Patient and Public Engagement events, as appropriate.
Keywords: sleep medicine, rheumatology, epidemiology
Strengths and limitations of this study.
This study will take advantage of advances in mobile health to embed data collection into the daily lives of participants.
Using a patient codesigned smartphone/tablet app, it will capture objective and subjective sleep as well as self-reported symptoms.
Sophisticated structural equation modelling and latent trajectory models will enable the study to disentangle the complex relationship between sleep, health-related quality of life (HRQoL) and other symptoms.
While the use of actigraphy allows the collection of objective sleep data, it is not able to inform investigation of the relationship between HRQoL and sleep architectural parameters or sleep oscillations (eg, spindles, slow waves).
Potential sources of bias include (1) sampling bias, if those recruited to this study are only those who experience sleep disturbances and/or are comfortable/familiar with using smartphone apps and activity monitors and (2) loss to follow-up bias if those who do not complete the full data collection protocol are systematically different from those who do.
Introduction
Quality of life, sleep and rheumatoid arthritis (QUASAR) is a prospective cohort study which will use a patient-designed smartphone/tablet app and accelerometer to collect information on sleep and health-related quality of life (HRQoL) in rheumatoid arthritis (RA).
RA is a common chronic inflammatory disease characterised by joint damage, pain and disability.1 The occurrence of RA is between two and four times greater in women and as many as 70% of men and women of working age with RA stop work within 10–15 years of disease duration due to the condition.2 3 People with RA frequently report reduced HRQoL, which can be characterised as the impact one’s health has on physical, emotional and social well-being. People with RA have poorer HRQoL when compared with those with other rheumatic diseases4 or healthy peers.5 6 Poor HRQoL may even persist when the disease is well controlled.5
Although there are likely numerous causes for poor HRQoL, people with RA have identified sleep disturbances as a key contributor to their well-being.7–9 It is well known that people with RA report substantial sleep disturbances,10 11 such as less total sleeping time and unrefreshing sleep8 and greater night time awakenings.7 9 The cyclical nature of relationships between sleep disruptions and symptoms such as pain, fatigue and disability is relatively well characterised and likely cyclical.12–14However, few studies have determined the relationship between sleep and quality of life (QoL) among RA populations.
Studies which have investigated the relationship between sleep and HRQoL have tended to suggest that sleep problems are associated with poor HRQoL.15–18 However, these studies have been of low quality and have been hampered by a number of methodological challenges, which preclude accurate assessment and understanding of the relationship between sleep and HRQoL. First and foremost, sleep is a multifaceted behaviour which comprises objective and subjective components.19 Despite guidelines endorsing the measurement of both and the potential for discrepancy between subjective and objective reports,20 it is only subjective sleep which has been commonly measured within epidemiological studies because, historically, it has been difficult to objectively measure sleep outside of artificial laboratory settings. Studies have also tended to be cross-sectional in design or to have used low-resolution longitudinal data collection protocols which have not been able to collect data regarding the recurrent and fluctuating day-to-day changes in sleep and associated symptoms.
Now, advances in the availability of smartphone apps and wearables for health monitoring provide a hitherto unobtainable mechanism to collect regular self-reported symptoms and objective sleep data, while embedding data collection into participants’ everyday lives. This study will use a combination of patient codesigned smartphone/tablet app (provided by uMotif, London, UK) and a triaxial accelerometer MotionWatch8 (MW8; CamNtech, Cambridge, UK) to capture both subjective and objective assessments of sleep quality, continuity and duration as well as the timing and stability of the periods of time when someone is asleep or active.21 Furthermore, the uMotif app will be used to collect information about self-reported symptoms which were identified as priorities by patients in a series of focus groups and patient involvement activities. In doing so, this study aims to identify the relationship between sleep parameters, aspects of the sleep/wake rhythm-associated parameters and HRQoL in people with RA.
Study objectives
Describe baseline and period distribution of sleep/wake rhythm-associated parameters stratified by age, sex, socioeconomic status and disease characteristics.
Determine relative contributions of sleep/wake rhythm-associated parameters, pain, fatigue and mood to RA-HRQoL.
In an exploratory analysis to examine whether the relationship between disease severity, sleep/wake rhythm-associated parameters, pain, fatigue, mood and RA-HRQoL are moderated by age and sex.
Estimate the potential effect on RA-HRQoL of a successful intervention targeted at the key identified pathway(s).
Experimental design and methods: methods and analysis
Study design
We will conduct a prospective cohort study to investigate the relationships between sleep and HRQoL in people with RA.
Identifying and recruiting potential participants
Participants will be recruited via three channels. The primary source for participants will be the National Rheumatoid Arthritis Society (NRAS). NRAS is a national patient organisation with over 7000 members. Emails advertising the study will be sent to NRAS mailing list members and will include a copy of the study information pack comprising:
Study poster
Participant information sheet
Copy of the consent form (for information only)
A link to complete an online screening questionnaire
Following approval from the Health Research Authority and via arrangements with Clinical Commissioning Groups, participants will also be recruited via NHS mailing lists, where such mailing lists are available. NHS mailing lists provide the opportunity to search general practitioner (GP) records for potentially eligible participants. Local Clinical Research Network (CRN) teams will search GP records for patients aged 18 or older, with a diagnosis of RA and who are in receipt of a disease modifying antirheumatic drug (DMARD), as recommended by Muller et al.22 Letters will be sent to the identified patients, provided the GP or clinical team in the practice confirms that approaching the patient is acceptable. Reasons for not approaching patients may include previous refusal for records to be used for research, current hospitalisation or belief that the participant is not capable of participation. Within the letter, participants will be briefly introduced to the study and persons interested in participating will be asked to email the study team directly for the study information pack, the contents of which are detailed above.
Information about the study will be displayed on the NIHR CRN Portfolio to encourage NHS sites to support study recruitment by displaying posters advertising the study in any NHS Rheumatology clinics. NHS sites interested in supporting the study will contact the study team directly for further information and all relevant documentation needed to confirm they have the capacity and capability required to support the study. The displayed posters will provide brief information about the study and will ask persons interested in participating to email the study team directly for the study information pack, the contents of which are detailed above.
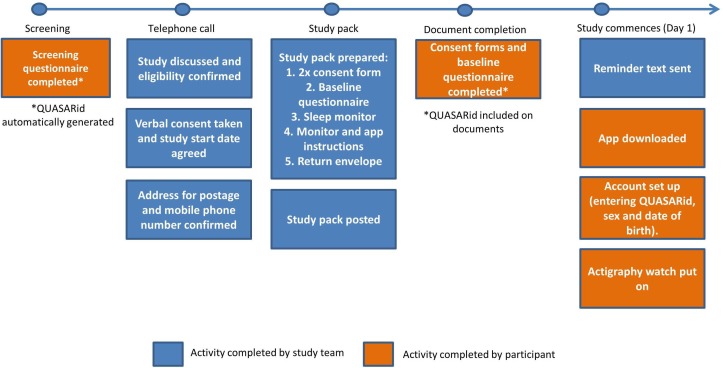
Following the receipt of the study information pack, potential participants follow an identical recruitment strategy (figure 1).
Figure 1.
Flow of participant entry into the study. QUASAR, quality of life, sleep and rheumatoid arthritis.
Screening questionnaire
Persons who receive the study information pack and are interested in participating in the study will be asked to complete the study screening questionnaire. Potential participants are asked to record their sex and date of birth and to confirm whether they (1) have a diagnosis of RA (including date of diagnosis), (2) are currently using DMARDs (biological or conventional synthetic), (3) have access to an Android or Apple smartphone or tablet. For the purposes of exclusion criteria, interested persons must also indicate whether they are currently employed in shift work. Finally, potential participants will be asked to give consent and contact details and preferred contact time (09:00 to 12:00 hours, 12:00 to 15:00 hours, 15:00 to 18:00 hours or 18:00 to 20:00 hours) to enable the study team to contact them to discuss participation in the study.
Eligibility criteria
Screening questionnaire respondents will be considered eligible for the study if they:
are aged ≥18 years,
have a diagnosis of RA,
are currently using DMARDs (biological or conventional synthetic),
have access to an Android or Apple smartphone or tablet.
Respondents would be excluded from the study if they:
are currently employed in a job that requires shift work.
QUASARid
Study participants will be asked to provide data via three different platforms: paper-based questionnaires, a study app (downloaded onto a smartphone or tablet) and a triaxial accelerometer that will measure daytime physical activity and sleep. To ensure that the data collected across these platforms can be consistently and accurately linked and matched to each participant, a unique participant identifier will be generated for each participant. The unique identifier, known as a QUASARid, will be automatically generated as the unique survey response which is allocated to the participant when they commence the screening questionnaire.
Telephone call
Potential participants meeting the study inclusion criteria will be telephoned by a member of the study team. To ensure they have had adequate time to review the participant information sheet provided in the study information email, telephone calls will take place no earlier than 24 hours after receipt of a completed screening questionnaire. A total of four attempts to contact the potential participant will be made on consecutive week days. During the phone call, a verbal summary of the information sheet will be discussed with the potential participant and they will be given the opportunity to ask as many questions about the study as they wish.
Verbal consent and agreement of study start date
Following the verbal summary of the study information sheet, participants will be given the option to enrol into the study, delay participation, request more time to think about participation or decline to take part in the study. Written consent will subsequently be obtained by the completion of two paper copies of the consent form sent to participants within the study pack. The provided consent forms will be signed and dated by the team member responsible for recruitment at the point of obtaining verbal consent, with participants asked to complete their own signature at the time of completing the baseline questionnaire. One copy of the consent form will be returned by the participant at the end of their first 30 days in QUASAR, along with the actigraph watch and baseline questionnaire. The second copy is for the patients’ personal records.
Once verbal consent has been obtained, participants will be asked to agree to a study start date, which should be within 14 days of the telephone call taking place. Participants who are unable to commence the study within 14 days will be asked to delay entry into the study. Those who agree a study start date will be asked to confirm their preferred postal address to which the study packs will be sent and a mobile phone number. The mobile phone number will be used to send participants text messages on the agreed study start date and throughout the study to encourage data completion and study compliance.
Study pack
The provision of verbal consent also enables the study pack to be prepared and posted to participants in advance of the agreed study start date. The study pack will include a letter of introduction, two copies of the consent form and a paper copy of the baseline questionnaire. Participants will also be provided with instructions to download and use the study app as well as an actigraph watch, called a MW8 (CamNtech) and associated instructions. The study pack will be sent from the University of Manchester using Special Delivery Guaranteed to arrive no less than 1 day prior to the study start date.
Document completion—Consent form and baseline questionnaire
Participants are requested to complete paper copies of the consent form and baseline questionnaire (sent within the study pack) on or before the first day of symptom monitoring. Initially, the baseline questionnaire was incorporated into the study app to be completed as part of the onboarding process. However, due to limits on the number of questions which could be displayed per screen and the associated time required for data completion, app testing with the study team and members of a focus group indicated that this method of delivery would be less acceptable to participants than a paper-based document.
The participant’s QUASARid will be written onto both the consent form and covering page of the baseline questionnaire to enable data linkage between these documents, the study app and actigraph watch. To encourage completion of the questionnaire in advance of the monitoring period, participants will be asked to indicate the date on which they have completed the questionnaire on the covering page. They will return the baseline questionnaire along with the monitor at the end of the study.
The baseline questionnaire will comprise three sections: demographic information, information about RA and health status information.
Demographics
Participants will record a number of demographic variables: sex, date of birth, weight and height, ethnicity, age left education, average weekly alcohol consumption, smoking status, occupational status, marital status and postcode. Participants will also indicate how likely they believe it is that sleep affects their QoL and the impact that RA has on their work productivity. A description of the reporting of these items is shown in table 1.
Table 1.
Baseline questionnaire domains and scales
| Domain | Scales/measurement |
| Demographics | |
| Sex | Tick box—male or female |
| Date of birth | Day, month, year |
| Height and weight | Free text (metric or imperial) |
| Ethnicity | Free text |
| Age left education | Free text |
| Smoking status | Tick box—current, ex-smoker or never smoker |
| Occupational status | Tick box—working full time, working part time, student, medically retired, voluntary worker, unemployed but seeking work or retired |
| Marital status | Tick box—single, in a relationship, cohabiting, married, civil partnership, separated, divorced or widowed |
| Alcohol consumption | Tick box—average units per week: 0, 1–5, 6–10, 11–15, 16–20, 21–40, >40 |
| Impact of RA on work | Work productivity question from WPAI-SHP |
| Postcode | Free text for first half of postcode |
| Belief that sleep affects HRQoL | 0–10 NRS: 0 ‘Not at all likely’, 10 ‘Very likely’ |
| Information about RA | |
| Date of RA diagnosis | Month and year |
| Disease activity | RAPID-3 |
| Comorbid rheumatic disease(s) and sleep-related problem(s) | Check list—osteoarthritis, spondyloarthropathy/ankylosing spondylitis, fibromyalgia/chronic widespread pain, gout or other crystal arthritis, Sjögren’s syndrome, restless leg syndrome, obstructive sleep apnoea/snoring, thyroid disorder, diabetes, multiple sclerosis, hypertension |
| Menopausal status | Tick box—yes/no |
| Current medication(s) and non-pharmacological intervention use | Check list—paracetamol, NSAIDs, other analgesics, weak opiates, strong opiates, drugs for neuropathic pain, glucocorticoids, synthetic DMARDs, biological DMARDs, sedatives (or hypnotics), mood stabilisers, antidepressants, other sleep medications Free-text box |
| Health status information | |
| Disease-specific quality of life | AIMS2-SF |
| Generic Quality of Life | WHOQOL-BREF |
| Prioritisation domains for good HRQoL | Participants are asked to use a free text box to indicate top three things which are most important to them to ensure they have good quality of life |
| Sleep quality | PSQI |
| Beliefs about sleep | DBAS-16 |
| Insomnia | SCI |
| Mood | HADS |
| Self-efficacy | ASES-8 |
| Cognitive flexibility | CAQ-8 |
AIMS2-SF, Arthritis Impact Measurement Scale 2-Short Form; ASES-8, Arthritis Self-Efficacy Scale-8 item; CAQ-8, Committed Action Questionnaire; DBAS-16, Dysfunctional Beliefs and Attitudes about Sleep questionnaire; DMARDs, disease modifying antirheumatic drugs; HADS, Hospital Anxiety and Depression Scale; HRQoL, health-related quality of life; NRS, numerical rating scale; NSAIDs, non-steroidal anti-inflammatory drugs; PSQI, Pittsburgh Sleep Quality Index; RA, rheumatoid arthritis; RAPID-3, Routine Assessment of Patient Index Data 3; SCI, Sleep Condition Indicator; WPAI-SHP, Work Productivity and Activity Impairment—specific health problem.
Information about RA
Disease activity
Participants will record the month and year of their diagnosis. Disease activity will be assessed using the Routine Assessment of Patient Index Data 3 (RAPID-3). The 15-item RAPID-3 is free to use and has been validated in RA populations. The measure has good validity and acceptable reliability and responsiveness.23
The RAPID-3 measures three domains: physical function (13 discrete response items), pain (1-item 0–10 numerical rating scale (NRS)) and global health (1-item 0–10 NRS)) in the past week. The first 10 items of the physical function domain are scored, transformed into a 0.3–10 scale and summed with the pain and global health domains to produce an overall score of 0–30. These overall scores are then converted into a weighed RAPID-3 score and categorised within the following disease activity score categories: near remission (0.3–1.0), low severity (1.3–2.0), moderate severity (2.3–4.0), high severity (4.3–10).
Comorbid rheumatic disease(s) and sleep-related problem(s) and menopausal status
Participants will be asked to record the presence of a variety of other rheumatic diseases, comorbidities which may affect sleep (eg, Sjögren’s syndrome, diabetes, multiple sclerosis, hypertension (table 1)) and menopausal status.
For pragmatic reasons, it was not possible to embed specific questionnaires about the presence, severity or impact of sleep disorders, as it was necessary to balance the availability of data with minimal participant burden of data completion. Nevertheless, because the presence of such disorders represent important covariates which should be adjusted for within data analysis, participants will be asked to record whether they experience restless leg syndrome and obstructive sleep apnoea/snoring. A full list of comorbidities collected is shown in table 1.
Insomnia is recorded using the Sleep Condition Indicator (SCI), an 8-item questionnaire which has good concurrent validity, high internal consistency and is sensitive to change.24 The SCI performs well to detect possible insomnia disorder and is the only validated and widely used insomnia measure that indexes insomnia disorder against contemporary criteria (eg, International Classification of Sleep Disorders (ICSD-3); The Diagnostic and Statistical Manual of Mental Disorders (DSM-5)).24
Medications
Participants will also be asked to record the use of medications including analgesics (eg, paracetamol, non-steroidal anti-inflammatories and opiates and DMARDs (eg, glucocorticoids, biological DMARDs). The use of sleep medications, including sedatives or hypnotics, mood stabilisers, antidepressants, other sleep medications (eg, chlorpromazine, haloperidol) will also be collected and participants will be given a free-text box to record other medications or coping strategies they use and the purpose for using the strategy (table 1).
Quality of life (QoL)
As is recommended,25 participants will complete a generic and disease specific measure of HRQoL. Generic HRQoL will be reported using the WHOQOL-BREF, a 26-item instrument which measures six domains in the past 14 days: overall perception of HRQoL (one discrete response item), health (one discrete response item), physical (seven discrete response items), psychological (six discrete response items), social relationships (three discrete response items) and environment (eight discrete response items). The items from each domain are summed and transformed into a 0–100 score, where higher scores indicate better HRQoL.26 The WHOQOL-BREF has good internal consistency, discriminant validity (ie, discriminating ill vs well groups) and sensitivity to change.26
Disease-specific HRQoL will be reported using the 26-item Arthritis Impact Measurement Scale 2-Short Form (AIMS2-SF). The AIMS2-SF uses discrete response items to capture five domains within a 4-week recall period: physical (13 items), symptom (3 items), affect (4 items), social interaction (4 items) and role (2 items). After some items are reverse coded, domain items are summed and converted into a 0–10 scale, where higher scores indicate poorer HRQoL.27 The AIMS2-SF shows good internal consistency and acceptable construct validity. The scale also generally demonstrates promising evidence of sensitivity to change, but there is less evidence supporting this for social interaction and role subscales.27
Participants will also be asked to use a free text box to indicate the top three things which are most important to them to ensure they have good QoL.
Sleep quality and beliefs about sleep
Sleep quality in the past month will be measured using the Pittsburgh Sleep Quality Index (PSQI), the most widely used measure within clinical and research settings.28 The 18-item instrument comprises seven domains: sleep quality (one discrete response item), sleep latency (one free-text item; one discrete response item), sleep duration (one free-text item), sleep efficiency (two free-text items), sleep disturbances (nine discrete response items), sleep medications (one discrete response item), daytime dysfunction (two discrete response items). The PSQI has good reliability and validity28 and demonstrates good sensitivity and specificity to distinguish good and poor sleepers.29
Participants will also record information regarding their beliefs about sleep using the 16-item Dysfunctional Beliefs and Attitudes about Sleep instrument (DBAS-16). Beliefs about causes, consequences and treatment of sleep problems are rated on 11-point Likert scales ranging from 0 (strongly disagree) to 10 (strongly agree). Scores are summed and averaged to produce a mean item score where higher scores indicate stronger endorsement of maladaptive beliefs about sleep.30 The DBAS-16 has good internal consistency and test-retest reliability.30
Mood, self-efficacy and cognitive flexibility
Finally, mood, self-efficacy and cognitive flexibility will be measured as important covariates which may affect the relationship between sleep and HRQoL. Mood, specifically anxiety and depression, will be measured using the 14-item Hospital Anxiety and Depression Scale (HADS).31 Anxiety (seven items) and depression (seven items) in the preceding week are reported using discreet response questions, scored 0–3. Subscale items are summed to produce a score of 0–21, where higher scores represent poorer mood.31 Scores rend to be categorised as 0–7 ‘normal’, 8–10 ‘borderline’, >11 ‘caseness’. The HADs is well accepted and has strong internal consistency and high test-retest reliability.31
Self-efficacy will be assessed using the Arthritis Self-Efficacy Scale-8 item (ASES-8),32 which will record information about participants’ self-efficacy for pain (2 0–10 NRS), preventing pain and fatigue interference (2 0–10 NRS) and for other symptoms (4 0–10 NRS). A self-efficacy score, ranging between 1 and 10, is produced by summing all items and calculating the mean value.32 Higher scores indicate better self-efficacy. The ASES-8 has been shown to have good reliability, validity and sensitivity to change.32
Cognitive flexibility will be recorded using the Committed Action Questionnaire (CAQ-8), to assess participants’ abilities to persist with or change behaviour in relation to their present situation.33 The CAQ-8 comprises four positive, and four negative, commitment to action subscales, which are recorded using 7-point Likert scales. An overall flexibility is scored by reverse coding the negative commitment subscales and summing all items, with higher scores indicating greater flexibility.33 The CAQ-8 has been shown to have good reliability and internal consistency.33
Study commences (day 1)
On the agreed study start date, participants will receive a text message on the mobile phone number provided during the recruitment process. The reminder text message will ask participants to put on their activity monitor and to download the study app and register for an account. It will also include a study password, which will unlock the app to allow the participant to commence full registration and a reminder of their QUASARid, which must be entered during the registration process to enable data linkage between the study app, actigraph watch and paper questionnaires.
App installation and account set-up
Instructions about how to download the study app, which can be installed onto the participants’ Apple or Android smartphone, tablet or both, will be included within the study pack that will be posted to participants. Once the app is installed participants will be asked to:
complete a standard registration form (including creation of a username and password),
enter their unique QUASARid, sex and date of birth, to enable linkage between the study’s different data collection platforms.
Registrations will be monitored via a live database, held by uMotif, of the data collected by the study app, to which the study team is provided secure access. All data provided by participants using the study app will be immediately transferred to uMotif server and visible in the database in real-time via 3G/4G/Wifi. Any participants who have not registered with the app on their agreed start date, or within a 5-day window of that date, will be contacted by the study team to understand what issues may have arisen. If appropriate, participation in the study may be rescheduled for a later date.
Data collection
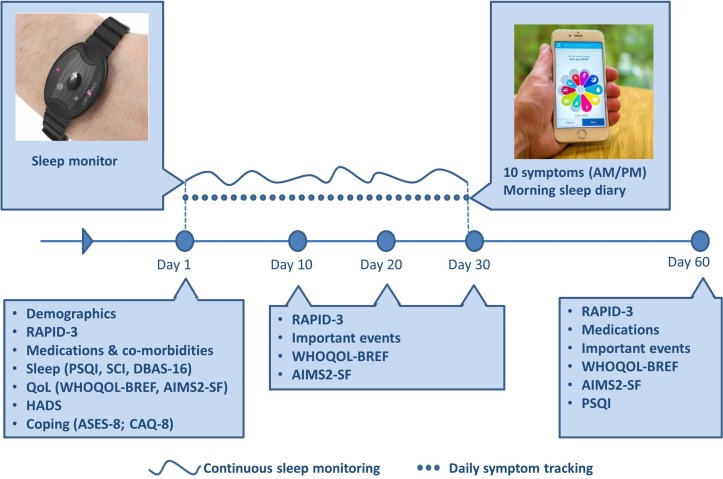
Following app-installation and the completion of the baseline questionnaire, participants will commence data collection lasting a total of 60 days. The first 30 days of the study comprise the continuous data collection phase of the study during which time participants wear the actigraph watch 24 hours a day and complete a sleep diary one time a day and symptom reports two times a day. During this 30-day monitoring period, participants will also complete follow-up questionnaires on days 10, 20 and 30 of the study. No data are recorded between days 31 and 59, but participants are asked to complete a final follow-up questionnaire on day 60 of the study.
During focus groups conducted to inform the design of this study, participants discussed a desire to have options for ongoing support from the study team via telephone contact. For that reason, we will send personalised messages on days 5, 15 and 25 to encourage participation and give the participant the option to email or telephone the study team if they would like to discuss any issues or concerns. Any emails or requests for telephone calls will be responded to by the study’s postdoctoral research associate, or principal investigator, at the earliest possible opportunity and no later than one working day after the response is received.
A full outline of the data collected is provided below.
Days 1–30—Actigraph watch and uMotif app
Continuous monitoring of sleep and physical activity
Participants will wear the MW8 (CamNtech) for 24 hours a day over the 30 days of continuous data collection. The MW8 is a Class I Medical device which conforms to the essential safety and health requirements and provisions of EC Council Directives 93/42.EEC, Annex VII. The MW8 requires a standard watch battery (CR2032) which is replaced each time the watch is used. The configuration of the MW8 for the present study will enable the watch to collect data for up to 45 days, providing an additional 2-week window for data to be collected in instances where study entry may have been delayed.
The MW8 monitors limb or bodily movements during daily living and sleep at 30 s time points (epochs) using a triaxial accelerometer. It is waterproof for up to 1 hour at 1 m and is therefore suitable for use while swimming or showering. Participants are instructed to wear the MW8 on their non-dominant wrist.
Actigraphy has been validated against polysomnography data.34 The MW8 has been shown to provides reliable estimates for both sleep (including sleep latency, duration, efficiency and fragmentation) and physical activity when worn for at least 14 days35–37 and has a number of practical features which make it suitable for the purposes of this study, including an event marker and prolonged battery life. Furthermore, the MW8 was specifically selected by participants invited to a research design focus group, who identified the MW8 as their preferred device due to the comfort of wearing it and the lightweight and unobtrusive design.
There is no real-time transmission of actigraphy data because the data are stored on the watches internal memory and can only be downloaded via a USB connection. It will therefore not be possible to assess participant compliance until after data collection has been completed and the actigraph watch is returned.
Following the receipt and download of actigraphy data the following variables will be extracted: total sleep time (the total time spent in sleep according to the epoch-by-epoch wake/sleep categorisation), sleep onset latency (the time which elapsed between the participant getting into bed and the participant falling asleep) and sleep efficiency (the total sleep time, expressed as a proportion of the total times pent in bed). The sleep fragmentation index, a measure of the degree of sleep discontinuity, will be calculated as a percentage of the total time categorised as mobile in the epoch-by-epoch mobile/immobile categorisation and the number of immobile bouts which were less than or equal to 1 min in length.
Daily symptom reporting
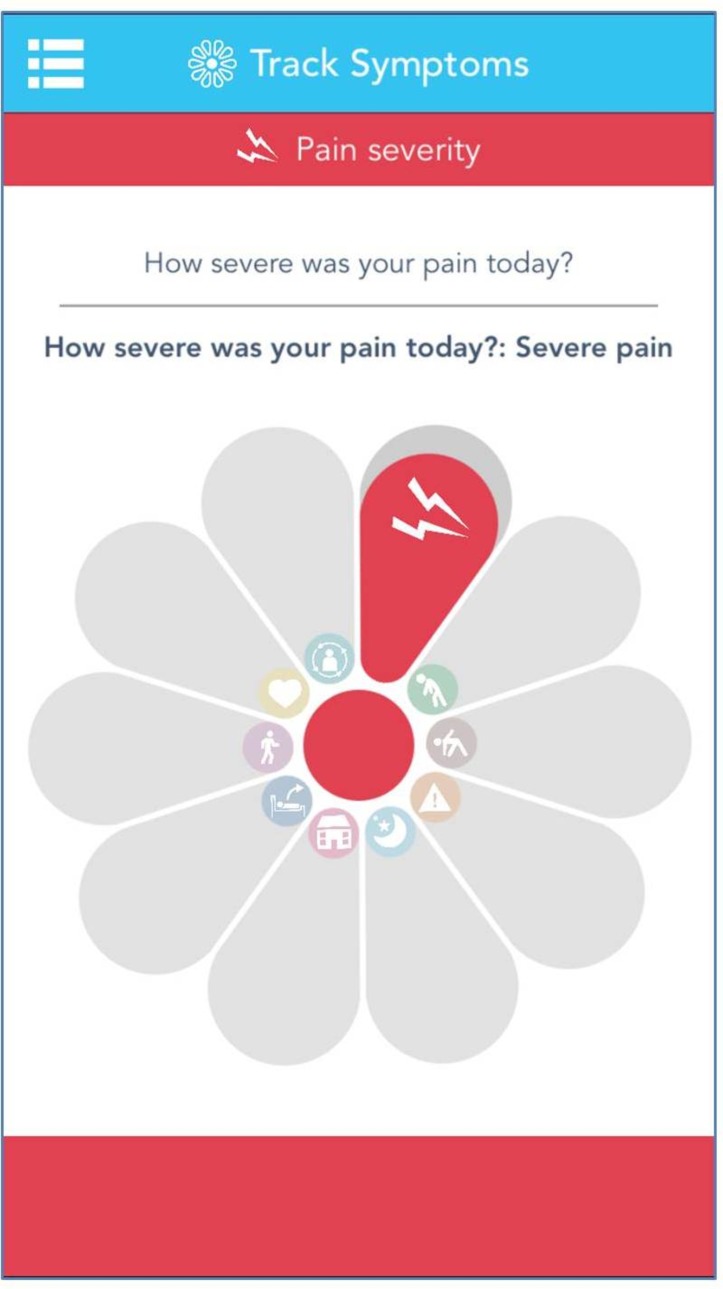
During the 30-day continuous data collection, participants will be asked to use the uMotif study app to report their experience of daily symptoms. Within the app, the unique ’motif' interface is used by patients to simply track their daily symptoms. The motif which comprises 10 symptom segments, such as pain severity as is highlighted in figure 2. The uMotif study app has been used in multiple ethics-approved studies, capturing over 64 million data points from patients using their own devices. The uMotif app has been specifically configured to capture the data required for the QUASAR study.
Figure 2.
Screenshot of uMotif study app.
Participants will receive prompts two times a day to complete the symptom ratings, once in the morning and once in the afternoon/evening. Symptom data are scored on an ordinal scale of 1–5 and are recorded by touching and sliding the relevant segment within the motif. The symptoms to be recorded (table 2) were defined and agreed with consultation of participants in focus groups.38
Table 2.
Daily symptoms to be captured by the uMotif study app
| Symptom | Question | Anchor 1 (centre of motif) | Anchor 5 (outside of motif) |
| Pain | How severe is your pain? | No pain | Very severe pain |
| Fatigue | How severe is your fatigue? | No fatigue | Very severe fatigue |
| Mood | How is your mood? | Depressed | Very happy |
| Well-being | How well do you feel? | Very well | Very unwell |
| Anxiety | How anxious do you feel? | Very well | Very anxious |
| Illness impact | How much is your illness impacting on your activities? | No impact | Very severe impact |
| Disease control | How much control do you feel you have over your symptoms? | No control | Very good control |
| Challenge | How challenging are you finding today? | Not challenging | Severely challenging |
| Sleepiness | How sleepy do you feel? | Not sleepy | Very sleepy |
| Concentration | How would you rate your concentration? | Poor | Excellent |
Throughout the study, participants will receive reminders via the study app to complete the symptom assessments at 08:00 and 18:00 hours. Participants will be able to provide additional symptom reports throughout the day at their discretion.
The completion of these symptom data can be continually monitored via a live database, held by uMotif, of the data collected by the study app, to which the study team is provided secure access. As all data provided by participants using the study app are immediately transferred to uMotif server in real-time via 3G/4G/Wifi, it will be possible for the study team to produce daily reports to monitor whether participants are completing their data in line with the study protocol.
A window of two consecutive days during the 30-day continuous monitoring period will be considered an acceptable period of non-completion for the daily symptom reports, sleep diaries and follow-up questionnaires. After this point, participants will receive a single reminder text message to encourage them to recommence data entry or contact the study team to discuss any concerns or issues, as appropriate.
Days 2–31 —Consensus Sleep Diary
The reminder sent to participants at 8am will also ask them to complete the 9-item Consensus Sleep Diary (CSD). This diary, which is completed in a separate section of the study app (figure 3), pertains to the previous night’s sleep. To ensure coverage of all 30 nights of actigraphy data collection, it will be completed on the morning of days 2–31. Participants will receive an automatically generated reminder every morning at 08:00 hours to complete the CSD, but are able to complete the diary earlier if they wish.
Figure 3.
Screenshot of consensus sleep diary within study app.
The 9-item CSD asks participants to record the time they got into bed, when they tried to sleep, how long it took to fall asleep, number and duration of night time awakenings, the time of final awakening, when they got out of bed, sleep quality and how refreshed they felt on awakening. The CSD is widely considered to be a gold-standard sleep diary, having been developed through the collaboration of sleep experts and potential users.39 40 An additional four items will ask participants to report the duration of morning stiffness (in min), emotional strength, motivation and worry about sleeping the previous evening.
As with the symptom reports, it will be possible to continually monitor sleep diary completion using the live database, held by uMotif. The study team will therefore produce daily reports to monitor whether participants are completing their sleep diaries in line with the study protocol. Any participant who does not complete their sleep diary for more than 2 days will receive a single reminder text message to encourage them to recommence data entry or contact the study team to discuss any concerns or issues, as appropriate. It will not be possible to retrospectively complete the sleep diaries for missing days.
Days 10, 20 and 30—HRQoL, disease activity and life events
A number of follow-up questionnaires will be used throughout the study(table 3). Prompts to complete the questionnaires will be sent. Requests to complete the follow-up questionnaires will be delivered to participants, via the app to participants, on the day they are to be completed when they open the study app. These reminders will be automatically generated and sent from uMotif and cannot be personalised. The questionnaires completed at days 10, 20 and 30 will capture the below items:
Table 3.
Summary of data collection via study questionnaires
| Preregistration | Day ≤1 (paper) |
Day 10 (app) |
Day 20 (app) |
Day 30 (app) |
Day 60 (app/paper) |
|
| Screening questionnaire | X | |||||
| Demographics | X | |||||
| Information about RA | X | |||||
| RAPID-3 | X | X | X | X | X | |
| Current medication(s) and non-pharmacological intervention use | X | X | ||||
| AIMS2-SF | X | X | X | X | X | |
| WHOQOL-BREF | X | X | X | X | X | |
| PSQI | X | X | ||||
| DBAS-16 | X | |||||
| SCI | X | |||||
| HADS | X | |||||
| ASES-8 | X | |||||
| CAQ-8 | X |
AIMS2-SF, Arthritis Impact Measurement Scale 2-Short Form; ASES-8, Arthritis Self-Efficacy Scale-8 item; CAQ-8, Committed Action Questionnaire; DBAS-16, Dysfunctional Beliefs and Attitudes about Sleep questionnaire; HADS, Hospital Anxiety and Depression Scale; PSQI, Pittsburgh Sleep Quality Index; RA, rheumatoid arthritis; RAPID-3, Routine Assessment of Patient Index Data 3; SCI, Sleep Condition Indicator.
Disease-specific QoL—AIMS2-SF.
Generic QoL—WHOQOL-BREF.
Disease activity—RAPID-3.
Occurrence of important events.
As with all data collected via the study app, the data recorded in each follow-up questionnaire will be immediately transferred to uMotif server in real-time via 3G/4G/Wifi. Completion of follow-up questionnaires will be monitored within daily reports produced by the study team and any participant who has not completed their questionnaire within 2 days of the required date will receive a single reminder text message to request that they complete the questionnaire as soon as possible. Participants will be given instructions to manually access and complete the follow-up questionnaire forms or contact the study team to discuss any concerns or issues, as appropriate.
Following the 30 days of data collection, participants will receive a text message to remind them to stop wearing the activity monitor and to send it back to the study team at the earliest convenient time, using the provided return addressed envelope. The envelopes, to which the relevant postage will be attached to return the package using Royal Mail Signed For 1st Class must be returned via the local post office to obtain proof of postage; participants will not be required to pay any additional postage to obtain this. In instances where the watch does not appear to have been dropped off at the Post Office within 5 days of the expected end date, the study team will contact the participant.
Participants are not expected to continue tracking their symptoms or using the study app for continuous monitoring after day 30 and at this point participants may wish to uninstall the study app. For that reason, as the completion of the study’s final questionnaire will occur 30 days after the continuous data collection phase has ended (day 60), participants may no longer be using the app and may have uninstalled it from their devices. Within the text message sent on day 30 of the study to congratulate participants on completing the continuous monitoring phase, participants will be asked to advise the study team if they would like to receive a paper copy of the final follow-up questionnaire.
Day 60—final follow-up questionnaire
The study’s final follow-up questionnaire will be completed at day 60. It is assumed that those who do not request a paper copy will be happy to complete the questionnaire using the app. Participants will be prompted to complete the questionnaire by reminders automatically generated and sent from uMotif. As before these reminders are generic and cannot be personalised.
The questionnaire will capture:
Disease-specific QoL—AIMS2-SF.
Generic QoL—WHOQOL-BREF.
Prioritisation of domains—participants asked to use a free text box to indicate the top three things which are most important to them to ensure they have good QoL.
Disease activity—RAPID-3.
Current medication(s) and non-pharmacological intervention use.
Sleep quality—PSQI.
Occurrence of important events.
A complete overview of data collection and participant contact is shown in figure 4.
Figure 4.
Data collection and participant contact across 60 days for participants enrolled in QUASAR. AIMS2-SF, Arthritis Impact Measurement Scale 2-Short Form; ASES-8, Arthritis Self-Efficacy Scale-8 item; CAQ-8, Committed Action Questionnaire; DBAS-16, Dysfunctional Beliefs and Attitudes about Sleep questionnaire; HADS, Hospital Anxiety and Depression Scale; PSQI, Pittsburgh Sleep Quality Index; QoL, Quality of Life; QUASAR, quality of life, sleep and rheumatoid arthritis; RA, rheumatoid arthritis; RAPID-3, Routine Assessment of Patient Index Data 3; SCI, Sleep Condition Indicator.
Sample size
Following published guidance,41 42 a sample size calculation has been conducted to determine a minimum sample size required to enable the study to conduct structural equation modelling (SEM). We have based estimated the sample size to detect a conservative minimum effect size (defined as correlations between pairs of latent variables) of 0.2 (rated as small). Based on our hypothesised model using baseline and 60-day measurements, containing 13 observed variables and four latent variables, with a significance level of 5% and power of 80%, a minimum sample size of 166 participants is required. Recommended sample sizes for latent growth curve analyses are defined in relation to the number of participants and the number of repeated observations and suggest a minimum number of 100 participants should complete at least 3 data points.43 Our minimum sample size required and data collection protocol are therefore in excess of the requirements for latent growth curve analyses. In this study, 50 actigraph watches will be obtained. This means that the maximum capacity for concurrent data collection is 50 and that data collection will be conducted in waves.
Using data from previous studies, we conservatively estimate that: a total of 3500 questionnaires mailed will provide 1750 (50%) returned and completed screening questionnaires, 350 (20%) will agree to take part and 175 (50%) will provide complete useable data, in excess of the minimum 166 persons required for the analysis. It is important that a representative sample of people with RA is enrolled into the study. However, data regarding the characteristics (eg, age and sex) of persons who receive information about the study will not be available through the proposed recruitment channels. The presence of sampling bias will be examined for by comparing the age and sex distribution of UK population data (table 4) to (1) persons who complete and return screening questionnaires and (2) recruited participants to available.
Table 4.
The prevalence of rheumatoid arthritis in UK
| Age | Males (%) | UK estimate | Females (%) | UK estimate |
| 16–44 | 0.02 | 2500 | 0.12 | 15 100 |
| 45–64 | 0.58 | 42 900 | 1.67 | 126 900 |
| 65–74 | 1.14 | 27 100 | 2.56 | 67 800 |
| 75+ | 2.18 | 39 100 | 2.99 | 85 700 |
| Total adult population | 0.44 | 106 500 | 1.16 | 297 600 |
Recruitment
In order to manage the flow of participants into the study and given the limited number of actigraph watches available, NRAS have agreed to contact mailing list members in seven regionally stratified recruitment waves in order that actigraph watches can be returned by earlier participants, prepared and sent out to future participants. The dates of each wave are listed below:
Wave 1—8 May 2017
Wave 2—17 July 2017
Wave 3—25 September 2017
Wave 4—4 December 2017
Wave 5—12 February 2018
Wave 6—23 April 2018
Wave 7—2 July 2018
The identification of GP practices and NHS Rheumatology clinics able to support recruitment to the QUASAR study will occur throughout the study. Once identified, the letters sent to potential participants identified via the screening of GP records will be sent to coincide with the above mailing waves used by NRAS. Participants who see information about the study in NHS Rheumatology clinics will be free to contact the study team at any point, however their enrolment into the study will be restricted to coincide with the above mailing waves.
Targeted recruitment
The study’s third objective is to examine whether the relationships between sleep and HRQoL are moderated by age and sex. It is therefore important to ensure that a representative sample of people with RA are enrolled into the study. Table 4 displays the estimated UK prevalence of RA and demonstrates that women are around three times more likely to be affected than men and the increased prevalence of RA in older populations.2
From previous studies, we know that young men44 and older people of both sexes are often under-represented populations.41 42 They may be less likely to express an interest in taking part in studies41 or they may be excluded by the inclusion/exclusion criteria used.42 In anticipation that some of these ‘hard to reach’ groups may be under-represented in our sample, we propose to monitor the characteristics of participants who are enrolled in the study and apply stratified recruitment processes in later recruitment waves if required.
Data analysis plan
Analyses techniques used in descriptive epidemiology will address aim 1. Data will be presented as absolute numbers and percentages, presented for the whole group and stratified by age (18-44; 45-64; 65-74; 75+), sex and level of socioeconomic deprivation (Index of Multiple Deprivation,45 derived using participant postcodes and categorised as quartiles of most deprived, deprived, less deprived and least deprived).
Using SEM and latent trajectory models (LTM), we will address the study’s second aim: to examine if disrupted sleep patterns are associated with poor HRQoL.
SEM will assess whether the sleep and sleep/wake rhythm-associated parameters measured at baseline mediate the association between RAPID-3 score and HRQoL at 60 days (the end of the follow-up period) allowing intercorrelation between the sleep and sleep/wake rhythm-associated parameters and adjusting for putative confounders. The SEM analysis will be repeated adding to the model pain, fatigue and mood to assess the effect of on HRQoL (eg, figure 5). Finally, the analysis will be repeated including the data on mediators collected at day 30. This will allow assessment of whether RAPID-3 predicts change in the mediating factors and the subsequent impact on HRQoL at day 60. An exploratory multigroup SEM analysis will assess the moderating effects of age and sex and will thus address the third aim of this study.
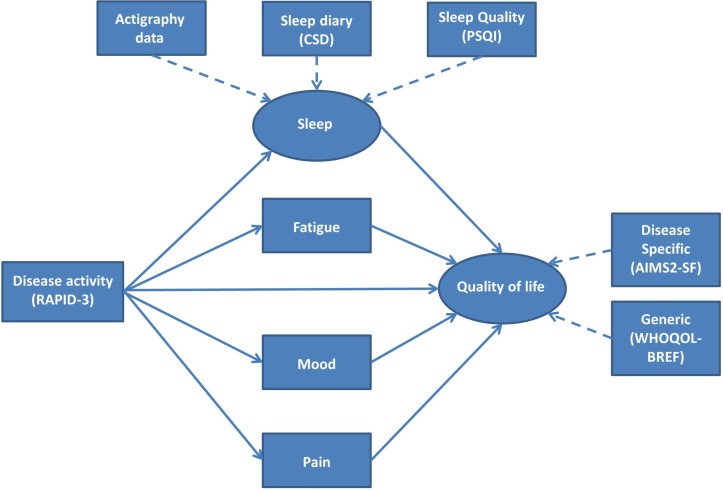
Figure 5.
Hypothetical model of the pathways of relationship between rheumatoid arthritis and quality of life disease severity, sleep, fatigue, mood and pain—simple model. In this figure, rectangles represent observed variables and circles represent the constructs those variables represent. Solid arrows represent the pathways to be tested. AIMS2-SF, Arthritis Impact Measurement Scale 2-Short Form; CSD, Consensus Sleep Diary; PSQI, Pittsburgh Sleep Quality Index; RAPID-3, Routine Assessment of Patient Index Data 3.
The SEM analysis assumes that the identified associations are consistent across participants and use only selected time points. LTM will use all of the repeated measures data to fully explore the longitudinal relationships between RAPID-3, mediators and HRQoL and their variation between participants. First, multilevel growth models, accounting for the clustering of repeated measures (level 1) within participants (level 2), will assess the prospective associations of RAPID-3 with key observed pathways from the SEM analysis and the prospective associations with HRQoL. Second, we will assess if distinct clusters of participants can be identified with different longitudinal courses using dual trajectory latent class growth analysis. We will assess how these trajectories are associated with change in HRQoL over 60 days and the sociodemographic and clinical characteristics associated with these different trajectories.
Finally, causal mediation analysis methods, specifically the mediational g-formula, will be used to estimate how successful treatments that improve sleep might be for people with RA. G-formula will be applied to the SEM models outlined above. This analysis will estimate the potential effect of a hypothetical intervention targeted at the key causal pathways between the observed variables and HRQoL. The hypothetical interventions which most improve HRQoL will indicate the best treatment targets to be tested in future intervention studies. For example, if disrupted circadian pathways were found to be important drivers of poor QoL, a future study might investigate the impact of advancing or delaying them using phototherapy.46 However, it is likely that multiple pathways (eg, sleep-wake cycle; sleep-wake cycle and pain) will impact on HRQoL and an intervention ‘package’ (eg, phototherapy and behavioural therapy47) will need to be developed based on those pathways.
Ethics and dissemination
This study underwent a full NHS Research Ethics Committee (REC) review and was allocated to the National Research Ethics Service (NRES) Committee North West—Liverpool Central REC.
Results from this study will be disseminated at regional and international conferences and in peer-reviewed journals. Results will also be disseminated at Patient and Public Engagement events where opportunities arise and are appropriate.
Discussion
People with RA frequently report reduced HRQoL, which may be caused by sleep disturbances. Few studies have determined the relationship between sleep and QoL among RA populations and those which have are laden with methodological challenges, which preclude accurate assessment and understanding of the relationship between sleep and HRQoL. The QUASAR study is a comprehensive study which has been designed to overcome these challenges. The results of this study will inform future intervention studies by answering key questions regarding the link between sleep and poor HRQoL.
Supplementary Material
Footnotes
Contributors: JM led the conception and design of the study. All authors made substantial contributions to the conception and design of the study. JM and ML planned the statistical analysis. KLD wrote the first draft of the protocol manuscript. LC, VS, SM, BH, BJ, ML, SDK, WGD and JM critically reviewed the protocol manuscript. All authors approved the final version of the document.
Funding: This study is supported by Arthritis Research UK grant number 21188. The study is also supported by infrastructure support from the Arthritis Research UK Centre for Epidemiology (grant reference 20380).
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: National Research Ethics Service (NRES) Committee North West—Liverpool Central REC (reference 17/NW/0217).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: We intend to make data available for data sharing after the data collection has been completed and the primary aims of the study are met.
References
- 1.NICE. Rheumatoid arthritis: national clinical guideline for management and treatment in adults. Clinical guideline [CG79]: National Institute of Health and Clinical Excellence, 2015. [Google Scholar]
- 2.Symmons D, Turner G, Webb R, et al. . The prevalence of rheumatoid arthritis in the United Kingdom: new estimates for a new century. Rheumatology 2002;41:793–800. 10.1093/rheumatology/41.7.793 [DOI] [PubMed] [Google Scholar]
- 3.Verstappen SM, Bijlsma JW, Verkleij H, et al. . Overview of work disability in rheumatoid arthritis patients as observed in cross-sectional and longitudinal surveys. Arthritis Rheum 2004;51:488–97. 10.1002/art.20419 [DOI] [PubMed] [Google Scholar]
- 4.Dominick KL, Ahern FM, Gold CH, et al. . Health-related quality of life among older adults with arthritis. Health Qual Life Outcomes 2004;2:5 10.1186/1477-7525-2-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerhold K, Richter A, Schneider M, et al. . Health-related quality of life in patients with long-standing rheumatoid arthritis in the era of biologics: data from the German biologics register RABBIT. Rheumatology 2015;54:1858–66. 10.1093/rheumatology/kev194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geryk LL, Carpenter DM, Blalock SJ, et al. . The impact of co-morbidity on health-related quality of life in rheumatoid arthritis and osteoarthritis patients. Clin Exp Rheumatol 2015;33:366–74. [PMC free article] [PubMed] [Google Scholar]
- 7.Drewes AM, Svendsen L, Taagholt SJ, et al. . Sleep in rheumatoid arthritis: a comparison with healthy subjects and studies of sleep/wake interactions. Br J Rheumatol 1998;37:71–81. 10.1093/rheumatology/37.1.71 [DOI] [PubMed] [Google Scholar]
- 8.Roehrs T, Diederichs C, Gillis M, et al. . Nocturnal sleep, daytime sleepiness and fatigue in fibromyalgia patients compared to rheumatoid arthritis patients and healthy controls: a preliminary study. Sleep Med 2013;14:109–15. 10.1016/j.sleep.2012.09.020 [DOI] [PubMed] [Google Scholar]
- 9.Crosby LJ. Factors which contribute to fatigue associated with rheumatoid arthritis. J Adv Nurs 1991;16:974–81. 10.1111/j.1365-2648.1991.tb01803.x [DOI] [PubMed] [Google Scholar]
- 10.Wolfe F, Walitt BT, Katz RS, et al. . Symptoms, the nature of fibromyalgia, and diagnostic and statistical manual 5 (DSM-5) defined mental illness in patients with rheumatoid arthritis and fibromyalgia. PLoS One 2014;9:e88740 10.1371/journal.pone.0088740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YC, Chibnik LB, Lu B, et al. . The relationship between disease activity, sleep, psychiatric distress and pain sensitivity in rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther 2009;11:1–11. 10.1186/ar2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev 2006;10:357–69. 10.1016/j.smrv.2005.08.001 [DOI] [PubMed] [Google Scholar]
- 13.Goodchild CE, Treharne GJ, Booth DA, et al. . Daytime patterning of fatigue and its associations with the previous night’s discomfort and poor sleep among women with primary Sjögren’s syndrome or rheumatoid arthritis. Musculoskeletal Care 2010;8:107–17. 10.1002/msc.174 [DOI] [PubMed] [Google Scholar]
- 14.Irwin MR, Olmstead R, Carrillo C, et al. . Sleep loss exacerbates fatigue, depression, and pain in rheumatoid arthritis. Sleep 2012;35:537–43. 10.5665/sleep.1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sariyildiz MA, Batmaz I, Bozkurt M, et al. . Sleep quality in rheumatoid arthritis: relationship between the disease severity, depression, functional status and the quality of life. J Clin Med Res 2014;6:44–52. 10.4021/jocmr1648w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purabdollah M, Lakdizaji S, Rahmani A, et al. . Relationship between sleep disorders, pain and quality of life in patients with rheumatoid arthritis. J Caring Sci 2015;4:233–41. 10.15171/jcs.2015.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goes ACJ, Reis LAB, Silva MBG, et al. . Rheumatoid arthritis and sleep quality. Revista Brasileira de Reumatologia 2017;57:294–8. 10.1016/j.rbre.2016.07.011 [DOI] [PubMed] [Google Scholar]
- 18.Guo G, Fu T, Yin R, et al. . Sleep quality in Chinese patients with rheumatoid arthritis: contributing factors and effects on health-related quality of life. Health Qual Life Outcomes 2016;14:151 10.1186/s12955-016-0550-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tafti M. Genetic aspects of normal and disturbed sleep. Sleep Med 2009;10:S17–21. 10.1016/j.sleep.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 20.Buysse DJ, Ancoli-Israel S, Edinger JD, et al. . Recommendations for a standard research assessment of insomnia. Sleep 2006;29:1155–73. 10.1093/sleep/29.9.1155 [DOI] [PubMed] [Google Scholar]
- 21.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep 2014;37:9–17. 10.5665/sleep.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller S, Hider SL, Raza K, et al. . An algorithm to identify rheumatoid arthritis in primary care: a Clinical Practice Research Datalink study. BMJ Open 2015;5:e009309 10.1136/bmjopen-2015-009309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson J, Caplan L, Yazdany J, et al. . Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res 2012;64:640–7. 10.1002/acr.21649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Espie CA, Kyle SD, Hames P, et al. . The sleep condition indicator: a clinical screening tool to evaluate insomnia disorder. BMJ Open 2014;4:e004183 10.1136/bmjopen-2013-004183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott DL, Garrood T. Quality of life measures: use and abuse. Baillieres Best Pract Res Clin Rheumatol 2000;14:663–87. 10.1053/berh.2000.0106 [DOI] [PubMed] [Google Scholar]
- 26.World Health Organisation. Programme on mental health: WHOQOL user manual, 2012. [Google Scholar]
- 27.Gignac MA, Cao X, Mcalpine J, et al. . Measures of disability: Arthritis Impact Measurement Scales 2 (AIMS2), Arthritis Impact Measurement Scales 2-Short Form (AIMS2-SF), The Organization for Economic Cooperation and Development (OECD) Long-Term Disability (LTD) Questionnaire, EQ-5D, World Health Organization Disability Assessment Schedule II (WHODASII), Late-Life Function and Disability Instrument (LLFDI), and Late-Life Function and Disability Instrument-Abbreviated Version (LLFDI-Abbreviated). Arthritis Care Res 2011;63(Suppl 11):S308–4. [DOI] [PubMed] [Google Scholar]
- 28.Mollayeva T, Thurairajah P, Burton K, et al. . The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med Rev 2016;25:52–73. 10.1016/j.smrv.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 29.Buysse DJ, Reynolds CF, Monk TH, et al. . The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 30.Morin CM, Vallières A, Ivers H. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16). Sleep 2007;30:1547–4. 10.1093/sleep/30.11.1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herrmann C. International experiences with the Hospital Anxiety and Depression Scale--a review of validation data and clinical results. J Psychosom Res 1997;42:17–41. 10.1016/S0022-3999(96)00216-4 [DOI] [PubMed] [Google Scholar]
- 32.Brady TJ. Measures of self-efficacy: Arthritis Self-Efficacy Scale (ASES), Arthritis Self-Efficacy Scale-8 Item (ASES-8), Children’s Arthritis Self-Efficacy Scale (CASE), Chronic Disease Self-Efficacy Scale (CDSES), Parent’s Arthritis Self-Efficacy Scale (PASE), and Rheumatoid Arthritis Self-Efficacy Scale (RASE). Arthritis Care Res 2011;63(Suppl 11):S473–S485. 10.1002/acr.20567 [DOI] [PubMed] [Google Scholar]
- 33.McCracken LM, Chilcot J, Norton S. Further development in the assessment of psychological flexibility: a shortened Committed Action Questionnaire (CAQ-8). Eur J Pain 2015;19:677–85. 10.1002/ejp.589 [DOI] [PubMed] [Google Scholar]
- 34.Sadaka Y, Sadeh A, Bradbury L, et al. . Validation of actigraphy with continuous video-electroencephalography in children with epilepsy. Sleep Med 2014;15:1075–81. 10.1016/j.sleep.2014.04.021 [DOI] [PubMed] [Google Scholar]
- 35.Falck RS, Landry GJ, Brazendale K, et al. . Measuring physical activity in older adults using motionwatch 8 actigraphy: how many days are needed? J Aging Phys Act 2017;25:51–7. 10.1123/japa.2015-0256 [DOI] [PubMed] [Google Scholar]
- 36.Landry GJ, Best JR, Liu-Ambrose T. Measuring sleep quality in older adults: a comparison using subjective and objective methods. Front Aging Neurosci 2015;7:166 10.3389/fnagi.2015.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elbaz M, Yauy K, Metlaine A, et al. . Validation of a new actigraph motion watch versus polysomnography on 70 healthy and suspected sleep-disordered subjects. J Sleep Res 2012;21(Suppl 1):218. [Google Scholar]
- 38.Short V, McBeth J, Druce KL, et al. . Fluctuating, unpredictable and challenging: how pain, fatigue and sleep disturbance impact on quality of life in people with rheumatoid arthritis. Ann Rheumatic Dis 2017;76 10.1136/annrheumdis-2017-eular.1849 [DOI] [Google Scholar]
- 39.Maich KHG, Lachowski AM, Carney CE. Psychometric properties of the consensus sleep diary in those with Insomnia disorder. Behav Sleep Med 2018;16:1–18. 10.1080/15402002.2016.1173556 [DOI] [PubMed] [Google Scholar]
- 40.Carney CE, Buysse DJ, Ancoli-Israel S, et al. . The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep 2012;35:287–302. 10.5665/sleep.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christopher Westland J, Westland JC. Lower bounds on sample size in structural equation modeling. Electron Commer Res Appl 2010;9:476–87. 10.1016/j.elerap.2010.07.003 [DOI] [Google Scholar]
- 42.Soper DS. A-priori sample size calculator for structural equation models (Online Software). version 4.0 2012.
- 43.Curran PJ, Obeidat K, Losardo D. Twelve frequently asked questions about growth curve modeling. J Cogn Dev 2010;11:121–36. 10.1080/15248371003699969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Druce KL, McBeth J, van der Veer SN, et al. . Recruitment and ongoing engagement in a UK smartphone study examining the association between weather and pain: cohort study. JMIR Mhealth Uhealth 2017;5:e168 10.2196/mhealth.8162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Department of the Envrionment, Transport and the regions. Indices of deprivation 2000, 2000. [Google Scholar]
- 46.Dodson ER, Zee PC. Therapeutics for circadian rhythm sleep disorders. Sleep Med Clin 2010;5:701–15. 10.1016/j.jsmc.2010.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ashburn MA, Staats PS. Management of chronic pain. The Lancet 1999;353:1865–9. 10.1016/S0140-6736(99)04088-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.