Abstract
Trichoderma spp., are saprophytic fungi that can improve plant growth through increased nutrient acquisition and change in the root architecture. In the present study, we demonstrate that Trichoderma asperellum T42 mediate enhancement in host biomass, total nitrogen content, nitric oxide (NO) production and cytosolic Ca2+ accumulation in tobacco. T42 inoculation enhanced lateral root, root hair length, root hair density and root/shoot dry mass in tobacco under deprived nutrients condition. Interestingly, these growth attributes were further elevated in presence of T42 and supplementation of NO3- and NH4+ nutrients to tobacco at 40 and 70 days, particularly in NO3- supplementation, whereas no significant increment was observed in nia30 mutant. In addition, NO production was more in tobacco roots in T42 inoculated plants fed with NO3- nutrient confirming NO generation was dependent on NR pathway. NO3- dependent NO production contributed to increase in lateral root initiation, Ca2+ accumulation and activities of nitrate transporters (NRTs) in tobacco. Higher activities of several NRT genes in response to T42 and N nutrients and suppression of ammonium transporter (AMT1) suggested that induction of high affinity NRTs help NO3- acquisition through roots of tobacco. Among the NRTs NRT2.1 and NRT2.2 were more up-regulated compared to the other NRTs. Addition of sodium nitroprusside (SNP), relative to those supplied with NO3-/NH4+ nutrition and T42 treated plants singly, and with application of NO inhibitor, cPTIO, confirmed the altered NO fluorescence intensity in tobacco roots. Our findings suggest that T42 promoted plant growth significantly ant N content in the tobacco plants grown under N nutrients, notably higher in NO3-, providing insight of the strategy for not only tobacco but probably for other crops as well to adapt to fluctuating nitrate availability in soil.
Keywords: ammonium, nitrate nutrition, nitrate transporter genes, nitric oxide, Trichoderma asperellum T42, root architecture
Introduction
Nitrate is one of the most important sources of nitrogen, not only for plant development but also for plant–microbe interactions (Dordas, 2008). Several reports have demonstrated that nitrate not only takes part in plant growth and development but its presence affects several other physiological processes such as disease management (Gupta et al., 2013), root organogenesis (Sun et al., 2015; Ruffel et al., 2016), flowering induction (Marín et al., 2011), crop growth and yield increment (Krapp et al., 2014). In agricultural systems, farmers generally prefer NO3-, NH4+ or a combination of both as N fertilizers for plant growth. Subsequently, NR activity reduces NO3- to NO2- and finally converted into NH4+, which is involved in amino acid biosynthesis through glutamine synthetase. Parallely, NH4+ can also incorporate in the soil biosystem either by degrading organic matter by the microorganisms or fixing atmospheric N. First time, Müller (1983) noticed that nia1/nia2 (nia28) double mutant tobacco plants were unable to utilize nitrate as a N source from medium whereas nia30 seedlings utilized nitrate at a very small rate. Recently, several researches had highlighted that nitrate participate in the regulation of NRT genes which help in uptake, transport and assimilation of nitrate (Medici and Krouk, 2014; Pii et al., 2014, 2016a; Noguero and Lacombe, 2016). Since, concentrations of nitrate vary from μM to mM in soil, therefore, for optimum uptake of nitrate from soil, plants have modulating nitrogen acquisition capacity and altering their own system such as root architecture (Miller et al., 2007; Song et al., 2013; Manoli et al., 2014). Several nitrate and AMTs are known which maximized uptake efficiency of nitrate through root system (Negi et al., 2008; Krapp, 2015). Based on their N uptake efficiency, NRTs are categorized into two groups. The low-affinity transporter allows the high capacity of external nitrate uptake (>0.5 mM) while high-affinity transporters (HATS) are essential for the uptake when the external concentration of NO3- is low (<0.5 mM) (Miller et al., 2007). Several NRTs have been identified in plant roots with a high-affinity for nitrate (Krouk et al., 2010; Kiba et al., 2012; Manoli et al., 2014; Pii et al., 2014, 2016a; Sun et al., 2015). However, our understanding on plants sensing of fluctuating soil nitrate concentrations and the signal induction that affects the root architecture remains narrow.
Nitric oxide (NO) is a signaling molecule generated by NR activity which regulates root traits development (Pii et al., 2007; Zhao et al., 2007; Manoli et al., 2014; Trevisan et al., 2014). Several evidences suggested that induction of NO signal is not only limited to root development but also regulated many plant physiological processes during nutrient assimilation (Correa-Aragunde et al., 2004; Chen et al., 2010; Chen and Kao, 2012; Meng et al., 2012; Nacry et al., 2013). Stöhr and Ullrich (2002) reported that root apex is the first site where NO is produced by NR activity during N acquisition. Interactions of NO with plant hormone like auxin further facilitated LR formation in tomato (Correa-Aragunde et al., 2004). But feedback inhibition of NR by NO was also noted in leaves of wheat (Rosales et al., 2011). Indeed, NR activation or inhibition by NO depends on external nitrate concentration in root of tomato (Jin et al., 2009). Furthermore, several reports highlighted that NO not only crosstalks with plant hormones in root organogenesis but also induced [Ca2+]cyt second messenger in the cGMP-dependent signaling pathways in plants that helps in cell wall synthesis, cellular homeostasis (Lamattina et al., 2003). White (2001) suggested that NO elevated release of Ca2+ in cytosol during root cell and root hair elongation. Similar case was also reported during root formation mediated through NO that induced Ca2+ signaling (Pagnussat et al., 2003). But complete knowledge about NO regulation in N uptake and interaction with Ca2+ in response to varying external nitrate concentrations is limited.
The agricultural soil has lots of beneficial microorganisms (Pseudomonas, Bacillus, Arbuscular mycorrhiza, Trichoderma sp., etc.) which are sustainably used for plant growth and development (Shoresh et al., 2010; Jain et al., 2012; Singh et al., 2015). They affect plant by increasing nutrient efficacy, nutrient recycling, seed germination, establishing induced systemic resistance and releasing plant growth promoting agents (Jeffries et al., 2003; Shoresh et al., 2010; Azarmi et al., 2011; Singh et al., 2014; Pii et al., 2015, 2016b; Giles et al., 2016; Scagliola et al., 2016). Plant growth promoting fungal interaction with plant roots does not have a similar efficiency for nutrient uptake throughout the life cycle of plant. Probably, microbial efficiency for nutrient uptake after reproductive phase of plants gets reduced (Jensen, 1986). In addition, Trichoderma sp. is one of the important rhizosphere microorganisms that can colonize at the outer epidermal layers of the roots and solubilize various nutrients such as N, Fe, Cu, Zn, Mn in soils (Singh et al., 2014). Similarly, Trichoderma harzianum T22 is reported to increase nitrogen utilization efficiency in maize (Harman, 2000). Plant nitrogen utilization increases with increased growth and yield at a certain point, but the response is not associated with increased yields in rising nitrogen fertilization. Seed treatment of wheat with Trichoderma has also increased yields (Harman, 2006). Besides wide range of effects on plant growth and yields, Trichoderma formulations have been preferred over chemical fertilizers in agriculture sector (Viterbo and Horwitz, 2010). Several evidences have shown that microbes like Trichoderma spp. are directly involved in NO generation mediated through NR expression in plants (Sherameti et al., 2005). Gupta et al. (2014) reported that T. asperelloides exhibited NO emission during interaction with plant roots. The ability of Trichoderma to elevate NO production through NR is well known, although specific knowledge of regulation of NRTs/AMTs and the induction of signal transduction during N acquisition and assimilation in the presence of N fertilizers remains limited. Given the mechanisms of action of Trichoderma strains and their broad efficiencies to enhance nitrogen utilization capacity in plants, a new strain was selected for improvement of nitrogen utilization efficiency in tobacco. We investigated the possible roles of T. asperellum T42 during interaction with tobacco roots and analyzed nitrogen acquisition and assimilation capacity in tobacco roots and correlated the effects with plant growth through possible mediation of NO under supplementation of different forms of N nutrients.
Materials and Methods
Microorganism, Tobacco Seeds and Experimental Setup
Seeds of tobacco (Nicotiana tabacum cv. Xanthi) were procured from CTRI, Rajahmundry, A.P., India. Seeds of a nitrate reductase (NR)-deficient nia30 mutant (cv. ‘Gatersleben’) were obtained from Prof. W. M. Kaiser, University of Würzburg, Germany. T. asperellum T42 (Gene bank accession: JN128894), referred herewith as T42 strain, was used as wild-type strain throughout this study. The T42 strain was used as biofertilizer for seed bio-priming of host tobacco seeds and propagated on potato dextrose agar (PDA, Himedia) medium at 28 ± 2°C.
Tobacco seeds were disinfected with 0.15% sodium hypochlorite and 0.01% of Tween-20 for 2 min. The seeds were bio-primed with T42 according to Yadav et al. (2013). Briefly, an aqueous suspension of T42 strain containing 1 × 106 cfu mL-1 was prepared in distilled water containing 1% CMC (carboxymethyl cellulose, Himedia). The seeds were dipped in the spore suspension for an hour and then air-dried under a laminar flow hood for 7–8 h. T42 strain treated tobacco seeds were allowed to germinate on petriplates containing moist filter paper soaked with and without N (nitrate and ammonium) nutrient solution under continuous day/night regime of 16/8 h, 22°C/20°C, and a relative humidity of 70% with artificial light of PPDF of 350–400 μmol m-2s-1. Non-bio-primed tobacco plants grown under nutrient deprived conditions served as a control. After 2 weeks, plants were transferred in Perspex tubes containing 1.8 L hydroponic solution (Planchet et al., 2005) for next 70 days. Hydroponic culture solution with nitrate (pH 6.3) supplement consisted of 10 mM KNO3, 1 mM CaCl2, 1 mM MgSO4, 25 μM NaFe-EDTA, 0.5 mM K2HPO4, 1 mM KH2PO4, while for ammonium nutrition, 3 mM NH4Cl, 1 mM CaCl2, 1 mM MgSO4, 25 μM NaFe-EDTA, 0.5 mM K2HPO4, 1 mM KH2PO4. Trace elements were used as described earlier (Johnson et al., 1957). The NR-deficient nia30 mutant plants were grown in nutrient solution having 10 mM KNO3, 3 mM NH4Cl, 1 mM CaCl2, 2 mM MgSO4, 25 μM Fe-EDTA, 2 mM KH2PO4/K2HPO4 and trace elements. The nutrient solutions were changed every 2 days during the course of experiment. Further, an experiment was conducted to determine the efficiency of NRT2.1, NR gene and NO emission in roots. A separate set of control tobacco plants were exposed to SNP (sodium nitroprusside, Sigma–Aldrich). The chemical composition of 10–200 μM SNP as NO donor was added in addition to NH4+ fed plants for 48 h. Oxidation of NH4+ was prevented by adding 10 μM dicyanimide in each pot. However, addition of 200 μM cPTIO [2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide; Sigma–Aldrich] was used as a NO scavenger. Three independent biological replicates were kept in each experiment. The tobacco plants were grown in nutrient solution filled in Perspex tubes (0.5 m high, 15 cm inner diameter) covered with a black plastic sheet to prevent light in a plant growth chamber. The tubes were filled with 1.8 L nitrogen nutrient solution as described above for each treated tobacco plant.
Three week-old tobacco plantlets were grown hydroponically with different concentrations of 10–200 mM NO3- and 3–200 mM NH4+ nutrients as N source with other macronutrients and trace elements as discussed below for next 30 days. Nutrient media were replaced twice in a week. After 30 days, root proliferation in all concentrations was observed (Supplementary Figure S1).
Sampling, Measurements and Analyses
Length and diameter of tobacco roots were measured using traditional scale method at 40 and 70 days after bio-priming. Root intensity was calculated according to Thorup-Kristensen et al. (2009) and expressed as root intersection m-1 grid line. Root hairs from each replicate was randomly selected and carefully placed on petriplates. Root hair images were captured for all the tobacco roots on the main axis by ScopePhot (86×) camera fitted to an OLYMPUS OIC (105144) microscope at 10× magnification interfaced with a computer image board. Root hairs length was analyzed using ImageJ software. Root hair intensity and root hair length (RHL) were measured for more than 30 randomly selected root hairs. Root hair intensity was determined as the number of root hairs per mm root length. The root and shoot dry mass (RDM and SDM, respectively) were determined after oven drying at 65°C for 48 h.
Total Nitrogen Content Determination
Total N content was determined in roots of tobacco plants from different treatments. Roots were oven dried at 60°C for 48 h and their dry weight was determined. N concentration from 100 mg dried/powdered root samples was estimated by semi-automatic nitrogen analyzer (Kjeldahl, Pelican, Kelplus-classic DX VATS, E), adapting Kjeldahl methods (Mishra et al., 2016).
FT-IR Analysis of Root
FT-IR spectrometer (Bruker EQUINOX 55, Bruker, Ettlingen, Germany) with a range of infrared light source (4000–400 cm-1 wave numbers) was used to analyze the chemical finger-print of tobacco roots. The root samples were ground to a fine powder under high pressure and placed on a KBr microscope window and then examined. A background spectrum of the clear KBr window was recorded before acquisition of sample spectra and subtracted from the sample spectrum. To find more information about the sample’s homogeneity of the same treatment, spectra obtained from the same root samples (n = 3) were averaged and the average spectra further analyzed by the Origin8 software.
NO Content in Tobacco Roots
Nitric oxide content in the root was detected using 10 μM DAF-FM DA (fluorophore 4, 5-diaminofluorescein-FM diacetate, Alexis Biochemicals, Gruenberg, Germany) as described elsewhere (Fernández-Marcos et al., 2011). Tobacco root were dipped in 10 μM DAF-FM dye (whose stock solution was prepared in 10 mM HEPES-KOH, pH 7.5), for 30 min at 25°C in a dark room. After incubation, roots were rinsed three times for 5 min with HEPES-KOH buffer. In order to verify participation of a NO donor (100 μM SNP) and/or a scavenger (200 μM cPTIO) on NO accumulation, SNP and cPTIO were applied to 70 days old tobacco plants grown in nitrate and ammonium nutrient as well as T42 treated plant including control. Root fluorescence image and intensity was analyzed by Nikon Eclipse 90i fluorescence microscope (Nikon Instruments Inc., United States) with 500 nm excitation and 515 nm emission. Fluorescence intensity was expressed as arbitrary fluorescence units (A.U.). Three roots were used for each condition and independent analysis for each treatment.
Ca Accumulation in Tobacco Root
Roots from different treatments were collected and briefly washed with distilled water. The samples were then incubated in 10 μM Fluo-4 AM [4-(6-Acetoxymethoxy-2,7-difluoro-3-oxo-9-xanthenyl)-4′-methyl-2,2′-(ethylenedioxy)dianiline-N,N,N′, N′-tetraacetic acid tetra -kis (acetoxymethyl) ester; Sigma–Aldrich] in loading buffer [diluted from 5 mM stock solution in 10 mM MES-KOH; 2-(N-Morpholino) ethanesulfonic acid sodium salt, 4-Morpholineethanesulfonic acid sodium salt; pH 6.15] for 2 h at 4°C in darkness. Root samples were rinsed thrice with loading buffer to remove excess dye and kept at 24°C in the growth chamber for 1–2 h. Fluorescence intensity was increased when intracellular esterase removed AM from Fluo-4AM and bind to cytosolic Ca2+, measured at excitation (488 nm) and emission (510–545 nm) wavelength. All images were taken using Nikon Eclipse 90i fluorescence microscope (Nikon UK Ltd., Telford, United Kingdom). Higher concentration of Fluo-4AM may be due to binding of dye with other divalent cations like Mn2+, Pb2+, and Zn2+. For Ca2+ inhibitor studies, control plants were incubated in 10 mM Ca2+ channel blocker LaCl3 for 1 h prior to fluorescence imaging (Pei et al., 2000).
RNA Extraction, Reverse Transcription, and Quantitative PCR of Roots
Root samples (500 mg) were collected at 70 days and pooled from three plants in each treatment. Total RNA was isolated from root as described by Patel et al. (2016). Two micrograms of RNA was digested with DNase I (Fermentas) and total RNA was used as template for first-strand cDNA synthesis with High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Monza, Italy). Experiments were conducted using SYBR Green chemistry (PowerUpTM SYBR Green Master Mix, Applied Biosystems) with Applied Biosystems 7500 version 2.0.4. For each reaction, 50 ng cDNA was used as template. Reactions were performed with three biological replicates under the following conditions: an initial denaturation step (10 min at 95°C) followed by 40 cycles of denaturation at 95°C for 2 min, 40 repeats at 95°C for 20 s, 60°C for 30 s, and 72°C for 30 s. The data normalization with mean Ct (Cycle threshold) values of target and reference gene was calculated 2-ΔΔCT using established method (Schmittgen and Livak, 2008). For each transcript, the ratio between the expressions measured for a given treatment with own a control gene was considered.
Relative expression analysis was performed using six nitrogen acquisition and one NO response genes in tobacco. Primer sequences were designed using online software Primer3 (web tool version 0.4.01). The whole sequences for corresponding genes were retrieved from NCBI. The primers and gene locus numbers for the NtNRT1.2s (AB102807), NtNRT1.2t (AB102808), NtNRT1.1s (AB102805), NtNRT2.1 (AJ557583), NtNRT2.2 (AJ557584), NtAMT1 (KJ874416), N. tabaccum nia-1 for NR, NtNIA1 (X14058.1), for internal control N. tabacum β-tubulin (KP316400.1) are listed in Supplementary Table S2. Transcript levels were normalized to expression of the tobacco β-tubulin, a housekeeping gene and results are expressed as fold increase or down-regulated as compared to control.
Statistical Analysis
Values from different experiments shown in figures are mean ± standard error (SE) (n = 3). Statistical analyses were subjected to one way analysis of variance (ANOVA). For comparison between treatments, Duncan’s multiple range test at P < 0.05; P < 0.01 significance levels were applied and analyzed by SPSS ver. 16 (SPSS Inc., Chicago, IL, United States).
Results and Discussion
T42 and Nitrate Nutrition Promoted Root Architectures, Root/Shoot Dry Matter, and Thickness Higher in Wild-Type but to Lesser Extent in Tobacco nia30 Mutant
Nitrogen is one of the major elements among macronutrients for plant life. Crops are strongly dependent on chemical fertilizer throughout the world, thus causing adverse effects on environmental quality. NH4NO3 and CO(NH2)2 are common fertilizers that have been extensively used in agriculture fields by farmers. Nitrate form is easily available to the plant, but due to the negative charge on clay particle, it is easily leached out of the soil to become a major source of eutrophication (Peng and Zhu, 2006). Nitrogen acquisition is a major factor for development and productivity of crop plants because it is present either in a complex form or very limited amount that cannot be easily available to plants. Therefore, scientists continue to develop such type of crops with improved nutrient uptake efficiency under low nitrogen input agriculture system (Robertson and Vitousek, 2009; Xu et al., 2012) as well as identifying beneficial microbes which show mutual symbiotic relationships with a broad range of commercial crops, thus ensuring better mineral nutrient acquisition efficiency by the plant. Root architecture generally influences nutrient acquisition through increased root distribution over the soil layers with increased surface area for nutrient uptake (Lynch, 2013; White et al., 2013). Especially, root hairs were more important component of root traits that facilitate mineral nutrient uptake in several plant species, particularly in plants experiencing low mineral nutrient status (Yan et al., 2004; Brown et al., 2012; Wang et al., 2016; Aceves-García et al., 2016). However, relative significance of root hairs to N acquisition in NO3- and NH4+ fertilization in presence of beneficial rhizospheric fungi such as Trichoderma is still unclear. In the current experiment, inoculation of tobacco roots with T. asperellum T42 increased root differentiation comparatively more than control. Approximately, 109% increase in RDM was found in T42 bio-primed tobacco plants at 70 days after treatment compared to control, and no significant difference was observed in nia30 mutant (Table 1). Similar to RDM, approximately 115% increase in SDM was noticed in T42 treatment. Quantification of the RDM and SDM indicated that T42 had more impact on tobacco plants and increased primary root length (PRL), RD and number of LR, particularly in NO3- nutrient instead of NH4+ (Figure 1). Approximately 115% PRL, 168% RDM, and 149% SDM were increased in T42 inoculated tobacco roots fed with NO3- nutrient at 70 days of growth (Table 1). Subsequently, PRL, RDM, and SDM recorded 52, 84, and 94% increment, respectively, in non-bio-primed T42 tobacco plants fed with NO3- nutrient compared to control. Interestingly, PRL, RDM, and SDM were increased by 66, 58, and 60%, respectively; in NH4+ fed tobacco plants which were monitored lower compared to NO3- supplementation. But, tobacco plants inoculated with T42 fed with NH4+ nutrient further significantly elevated PRL (84%), RDM (125%), and SDM (72%) compared to control at 70 days, suggesting that T42 participated in nitrogen utilization in NH4+ fed plant. However, nia30 mutant showed very limited extent of root growth, root hair density and shoot growth probably due to the fact that double mutant nia30 line utilized very low nitrate from growth solution (Müller, 1983). Previous studies have shown that nitrate is easily taken up by plant roots improving lateral root growth and architecture (Sun et al., 2015; Ruffel et al., 2016). Here, we demonstrated that LR density was highest in T42 inoculated tobacco plants fed with NO3- nutrient. This result is in conformity with the fact that nitrate acts as a signal to trigger number of molecular changes and prevent acidification of root media that help in maintaining membrane potential associated with plant growth (Babourina et al., 2007; Gojon et al., 2011; Giehl et al., 2014). Subsequently, T. asperellum T42 inoculation induced modifications in the root architecture in response to N fertilizers that increased total absorptive surface area of roots, and T42 ultimately enhanced R/SDM, especially in NO3- supplementation condition.
Table 1.
Effect of T42 strain on nitrogen nutrient uptake and growth attributes in tobacoo roots.
| Plant treatments |
Root (total N content) (mg g-1 plant-1) |
RDM (g plant-1) |
SDM (g plant-1) |
Primary root length (cm) |
Primary root thickness (mm) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| 40 days | 70 days | 40 days | 70 days | 40 days | 70 days | 40 days | 70 days | 40 days | 70 days | |
| Control | 32.1 ± 0.8e | 33.6 ± 0.6e | 0.9 ± 0.2c | 1.02 ± 0.1d | 2.9 ± 0.2e | 3.8 ± 0.3e | 16.3 ± 0.9e | 21.3 ± 1.2d | 1.0 ± 0.0b | 1.3 ± 0.3d |
| T42 strain | 35.9 ± 0.4c | 38.1 ± 0.2c | 1.6 ± 0.1a | 2.1 ± 0.1bc | 5.4 ± 0.1bc | 8.2 ± 0.1b | 22.5 ± 0.5b | 34.9 ± 0.7b | 1.3 ± 0.3ab | 2.3 ± 0.3ab |
| NO3- fed | 38.2 ± 0.8b | 40.3 ± 0.4b | 1.4 ± 0.1b | 1.9 ± 0.1cd | 5.1 ± 0.1c | 7.4 ± 0.2c | 20.3 ± 1.2c | 32.3 ± 1.5b | 1.3 ± 0.3ab | 2.0 ± 0.0abc |
| NO3- plus T42 strain |
40.7 ± 0.4a | 44.0 ± 0.5a | 1.9 ± 0.1ab | 2.7 ± 0.2a | 6.4 ± 0.2a | 9.5 ± 0.2a | 28.7 ± 0.9a | 46.0 ± 1.7a | 1.7 ± 0.0a | 2.7 ± 0.3a |
| NH4+ fed | 34.1 ± 0.7d | 35.9 ± 0.2d | 1.2 ± 0.1b | 1.6 ± 0.0d | 4.1 ± 0.1d | 6.1 ± 0.3d | 17.0 ± 0.6de | 35.3 ± 0.9b | 1.0 ± 0.3b | 1.7 ± 0.3bc |
| NH4+ plus T42 strain |
35.8 ± 0.5cd | 37.4 ± 0.4cd | 1.7 ± 0.2ab | 2.3 ± 0.2b | 5.0 ± 0.1c | 6.6 ± 0.3d | 18.7 ± 0.3cd | 39.3 ± 0.9b | 1.7 ± 0.3ab | 2.0 ± 0.0abc |
| nia30 | 22.4 ± 0.4g | 23.7 ± 0.3g | 0.5 ± 0.0d | 0.7 ± 0.0f | 0.9 ± 0.1f | 1.3 ± 0.1f | 6.7 ± 0.3g | 10.7 ± 0.3e | 1.3 ± 0.3ab | 1.5 ± 0.3d |
|
nia30 plus T42 strain |
24.8 ± 0.3f | 30.8 ± 0.2f | 0.7 ± 0.0c | 0.9 ± 0.1e | 1.2 ± 0.1f | 1.5 ± 0.1f | 9.7 ± 0.3f | 14.3 ± 0.3d | 1.7 ± 0.3ab | 2.0 ± 0.0abc |
All values represent mean of eight replicates ± standard error. Total nitrogen content in roots was measured at P < 0.05 level. Different letters denote a statistically significant difference according to Duncan’s multiple range test (at P < 0.05). RDM stands for root dry mass whereas SDM for shoot dry matter.
FIGURE 1.
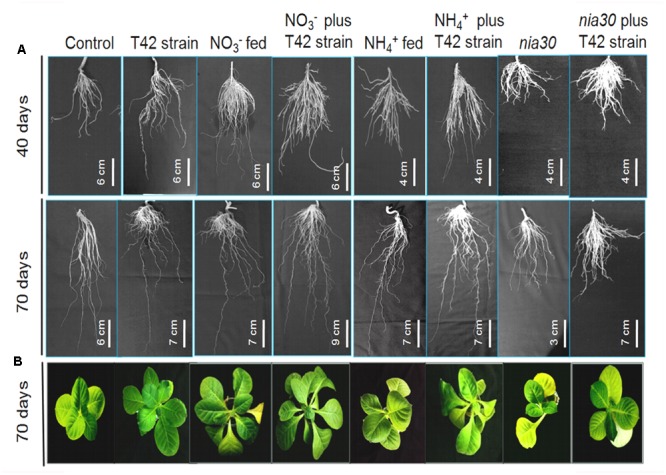
Root morphology variation in Nicotiana tabacum and nitrate reductase-(NR) nia30 mutant at 40 and 70 days of growth (A) and plant morphology (B) at 70 days. Bars in different figures show measurement of root length.
Similarly, root hair development as well as density and length were increased in T42 inoculated tobacco plants compared to control under deprived nutrient condition, after 70 days of plant’s growth (Figures 2A–C). But, in presence of the N nutrients, root density and root hairs were more effectively increased in NO3- compared to NH4+ fed. T42 inoculation with tobacco markedly induced both root density and root hairs under NO3- and NH4+ supplementation, especially more in NO3-. However, T42 inoculation influenced to a limited extent the root hair differentiation, density and length in nia30 mutant. The results suggested that T42 inoculation with tobacco participated in root proliferation more effectively in NO3- nutrient fed condition but did not in the nia30 mutant.
FIGURE 2.
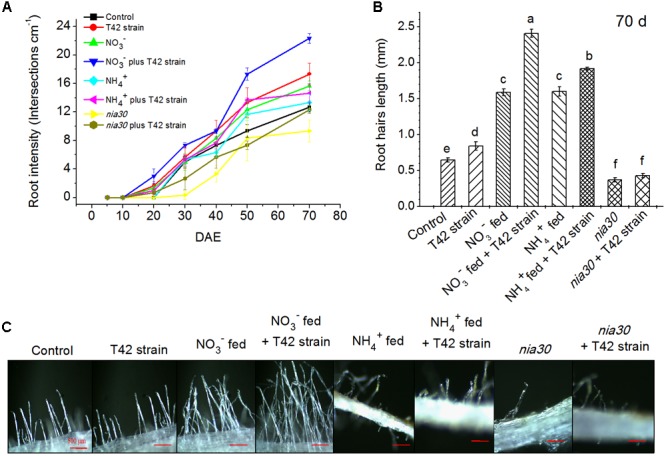
Root hair development in 70 days old tobacco roots. Root hair density (A, at P < 0.05), root hair length (B, at P < 0.01) and morphological view (C, Bar-500 μm) at 70 days under different conditions. Values are represented as means ± SE (n = 3). Different letters on bars indicate significant differences among treatments according to Duncan’s test. DAE stands for days after emergence.
T42 Influences Total N Content in Tobacco Roots under Nitrate and Ammonium Nutrition
It has been demonstrated that N is an essential mineral component for crop yields and plant quality (Hajjar and Hodgkin, 2007; Yun et al., 2008; Song et al., 2013; Sun et al., 2015). Although, N inequity in the plant correlates with host-cell sugar export to encourage pathogens susceptibility of French bean (Phaseolus vulgaris) to the anthracnose pathogen Colletotrichum lindemuthianum (Tavernier et al., 2007). Earlier, it was reported that Trichoderma colonizes on the root surface and help in increased nitrogen utilization efficiency and the stored pool of inorganic N plants (Shoresh et al., 2010; Singh et al., 2014; Courty et al., 2015). The putative effect of T42 strain on nitrogen content in tobacco roots under NO3- and NH4+ nutrition was assessed in both wild-type and nia30 mutant. T42 inoculation with tobacco roots significantly increased total N content by 13% compared to control at 70 days (Table 1). N content in tobacco plants fed with NO3-/NH4+ nutrient, increased by 20% in NO3- and 7% in NH4+ compared to control. Interestingly, highest total N content was increased by 31 and 11% in T42 inoculated tobacco roots fed in NO3- and NH4+ nutrient, respectively. However, 8% reduction was noticed in nia30 mutant, compared to control, but T42 inoculated nia30 plants significantly increased N content from those seen with non- T42 treated (Table 1). Thus, NR helped in N acquisition, and T42 treatment effectively promoted this mechanism.
Further, FT-IR spectrum was used to observe different functional groups corresponding to each active component present in tobacco root extracts based on the signal peak values (as wave number) appeared in the infrared radiation region. The assignment of bands for their corresponding functional groups are shown in Figure 3 and Supplementary Table S1. The peaks with highest chance to interfere with nitrate (1410–1340 cm-1), NO (1550–1475 cm-1) and ammonium (1450–1390 cm-1) were observed (Lambert et al., 1987). Comparison of the spectra presented in Figure 3 shows that there were slight differences in signals between them. The noticeable signal for nitrate was observed after comparing the spectra in 1410–1340 cm-1 wave number. The organic compound signal peaks varied due to differences in vibrational tweesting in the band between molecules. Signal peaks in N-O nitro compounds due to symmetric stretching normally lie in the range 1360–1290 cm-1 but shifts to 1550–1475 cm-1 if bond changes in asymmetric stretching. Similarly, the signal peak for NO3- compound lie between 1410 and 1340 cm-1 but if it is deformed due to covalent bond formation, then wave number lie in between 860 and 800 cm-1. Further, 1450–1475 cm-1 wave number is observed for NH4+ (bending). In the present study, we observed that group frequencies with a strong signal with different treatments appeared in these regions, but the weak signal was monitored in control roots in this range. The results obtained from signal peaks showed the presence of nitrate, NO and ammonium compounds in the root extract (Figure 3). Signal peaks in T42 strain pre-treated tobacco roots showed strong functional groups than untreated ones. These results suggested that T42 was able to increase total N content and its assimilation in the form of nitrate, nitro and amino group.
FIGURE 3.
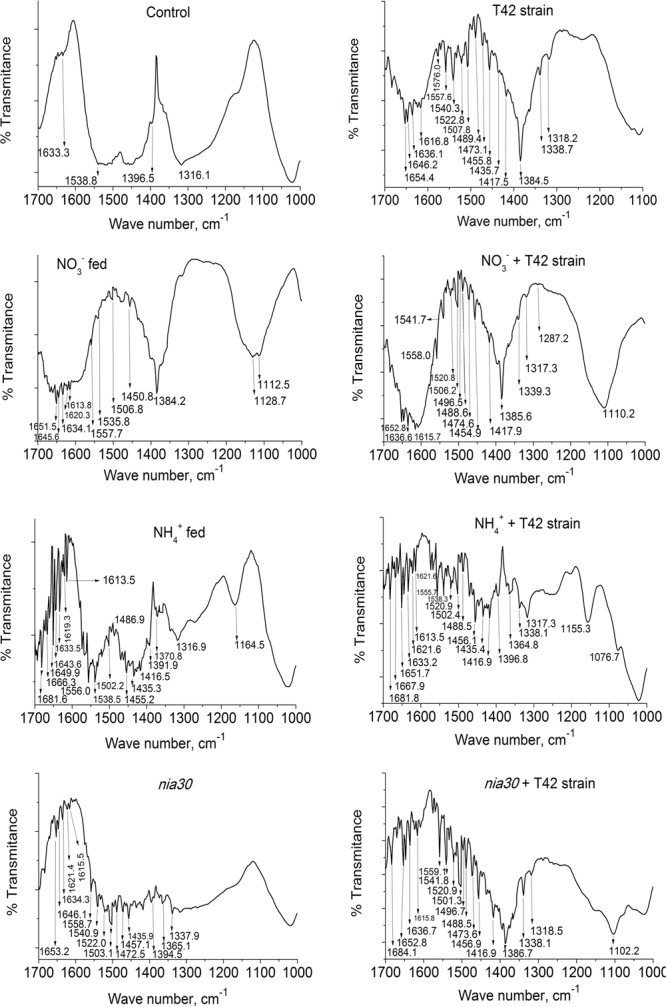
FT-IR spectrum in tobacco roots. Pictorial representation of different signal peaks lies in the range of wave number 1700–1000 cm-1 in tobacco root with different treatments: control (used distilled water), T42 strain treated, NO3- fed, NO3- fed plus T42 bio-primed, NH4+ fed, NH4+ fed plus T42 bio-primed, nitrate reductase mutant; nia30 and nia30 bio-primed with T42 strain at 70 days after treatment. Values are represented as % transmittance.
T42 and N Nutrition Affects NO Production and Release of Cytosolic Ca2+ in Tobacco Root
NO3- nutrient is considered as an essential mineral nutrient involved in development in root traits and crop yields. Nitrate also serves as a signal agent which activates several molecular and physiological changes in plant system that lead to increase N pool (Crawford et al., 1995; Stitt, 1999; Gojon et al., 2011). Recently, Trevisan et al. (2011) reported that NO plays a key role in nitrate sensing in maize roots when fed with NO3-. However, NO production in plants remains controversial whether it is produced through NR or NOS activities. However, application of NO inhibitor and scavenger that interferes with NO biosynthesis provided strong evidence that NO is produced by NR activity (Sun et al., 2015). NR is a key enzyme implicated in NO production under environmental stresses (Freschi et al., 2010; Kolbert et al., 2010). It is known that nitrate is essential for induction of NR expression, but NR dependent NO production in the presence of nitrate nutrient has remained controversial. Moreover, many reports demonstrated that NIA expression was essential for NO generation in plants (Planchet et al., 2005; Bright et al., 2006; Sun et al., 2015). However, nia1/nia2 double mutants for NR deficient showed lower NO level in the root of Arabidopsis compared to the wild-type (Schlicht and Kombrink, 2013). This means NIA dependent NR is a vital factor for NO production. In this study, the transcript level of NIA1 dependent NR in roots of tobacco increased by seven and threefold under NO3- and NH4+ treatment, respectively, compared to control, which is in agreement with the findings of Yun et al. (2008); however, down-regulation of the same was observed in nia30 mutant (Figure 7). It is possible that NR is essential for increasing NO level in the presence of nitrate nutrient. Application of NO3- and NH4+ supplement enhanced NO accumulation and DAF fluorescence intensity in the tobacco roots, comparatively higher in NO3- than NH4+ (Figures 4A,B). Similar results were also demonstrated where consistent accumulation of NO was found in response to nitrate in maize and rice during root development (Manoli et al., 2014; Trevisan et al., 2014; Sun et al., 2015). However, under the nutrient-deprived condition, T42 inoculation increased the expression level of NIA1 gene, analogous to tobacco roots where another microbe, i.e., Piriformospora indica induced NR expression (Sherameti et al., 2005). However, highest transcripts level of NIA1 was observed in T42 inoculated roots of tobacco fed with NO3- nutrient (12-fold) (Figure 7). These findings complement with NO generation, where maximum NO production and DAF fluorescence intensity showed in T42 inoculated roots of tobacco fed with NO3- nutrient (Figures 4A,B). Further, no significant difference in fluorescence intensity was observed in nia30 mutant, suggesting that NR is essential for NO production and nitrate might be involved in NR-mediated NO production. It was demonstrated that Trichoderma spp. inoculation with plants induces NR dependent NO production (Sherameti et al., 2005; Gupta et al., 2014). Thus, we can correlate our findings with those findings and conclude that T. asperellum T42 also induced NO production in tobacco roots and the intensity of NO DAF fluorescence was further enhanced in the presence of nitrate nutrient.
FIGURE 7.
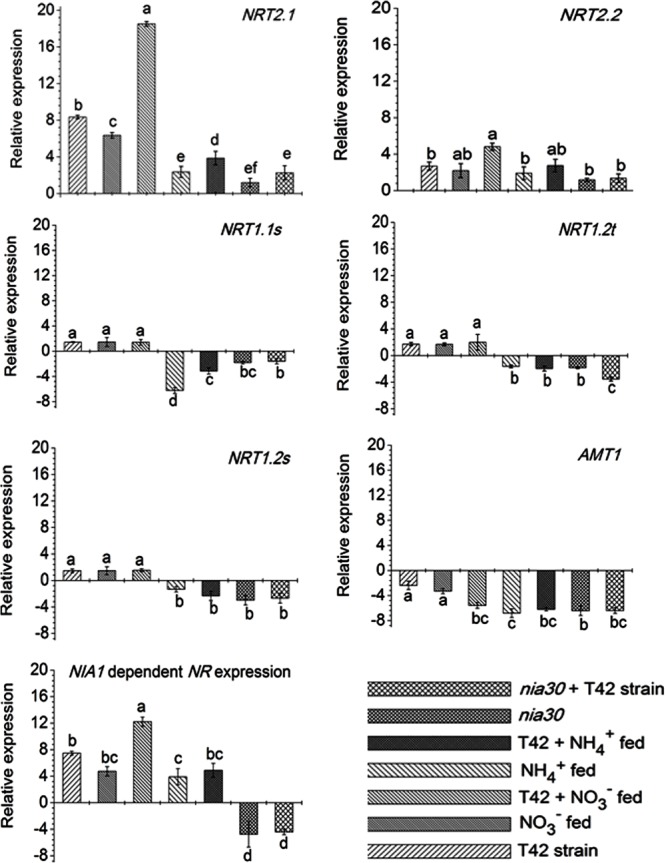
Transcript level of high affinity nitrate transporters (NRT2.1 and NRT2.2), low affinity transporters (NRT1.1s, NRT1.2s, and NRT1.2t), ammonium transporter (AMT1) and NR involved in N uptake in roots. Bars represent as SD (Standard Deviation) from means of three biological replicates. Different letters on bars show significant differences as analyzed by Duncan’s multiple rang test (at P < 0.01).
FIGURE 4.
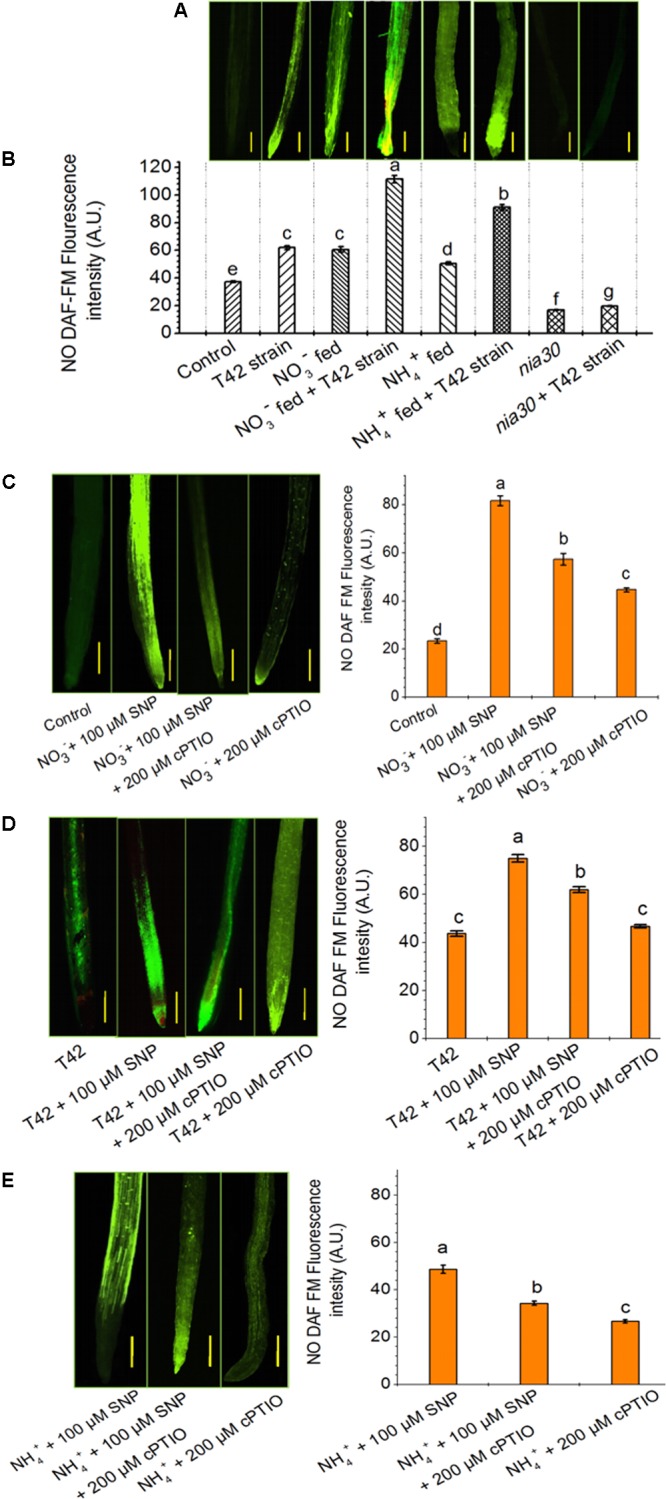
Nitric oxide (NO) production in the root of wild-type and nia30 mutant tobacco at 70 days. (A) NO production, as indicated by green fluorescence detected by DAF-FM staining in roots. The seeds of tobacco bio-primed with T42 were grown under NO3- and NH4+ nutrient supplement, and nia30 mutant plants (alone) as well as bio-primed with T42 strain were established in presence of nutrient supplements as described in section “Materials and Methods.” (B) NO production in root expressed as fluorescence intensity (A.U.) related to the same root regions of Figure 5B. NO production and its intensity under the exposure of 100 μM SNP, NO donor, in addition to NO3- (C), T42 treatment (D) and NH4+ (E) nutrient with control plants for 48 h in 70 days old plants. Application of NO donor in addition to NH4+ nutrient increased the NO emission in root. The addition of 200 μM cPTIO, NO scavenger reduced fluorescence emission. Values represent the average mean of eleven random selected sites for a definite area from roots (SE; n = 11). Letters on bars indicate significant differences in each treatment at P < 0.05, as determined by Duncan’s test (B–E).
In this study, it was shown that NR was involved in NO production in response to nitrate nutrient and T42 in tobacco roots. To further assess the effective role of NO on NR expression, 10–200 μM SNP (NO donor) was applied in 70 days old tobacco plants for next 48 h. Highest transcript accumulation of NIA1 dependent NR expression (8.6-fold) and NO DAF fluorescence intensity were at 100 μM SNP (Figure 5). It can be concluded that 100 μM SNP was sufficient to induce maximum NR expression. A similar observation was also made in cabbage roots where SNP treatment increased the NR activity (Du et al., 2008). These findings also clearly indicated that NO positively stimulates NR expression at the post-translational level. However, NO production in NH4+ fed tobacco roots showed similarity with beech seedlings, where NO production was mediated at post-translational level during NH4+ uptake (Simon et al., 2009). Moreover, the role of NO in feedback regulation of NO3-/or NH4+ nutrient and T42 in root development was further confirmed through application of 100 μM SNP (NO donor) and a marked NO scavenger cPTIO (200 μM) in addition to NO3-/or NH4+ nutrient and T42 at 70 days (Figures 4C–E). We observed that SNP application elevated extraneous NO accumulation more in NO3- nutrient fed tobacco roots compared to T42 inoculation. However, lowest NO DAF fluorescence intensity was noticed in NH4+ plus SNP condition.
FIGURE 5.
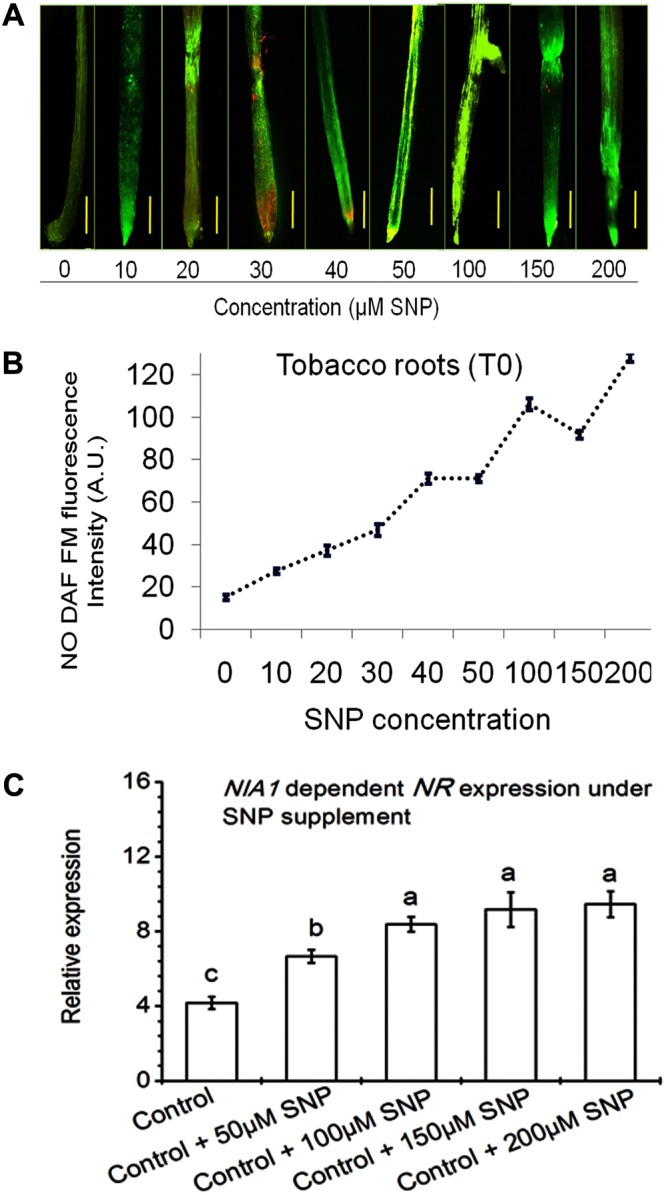
Effects of SNP on NIA1 dependent NO production in tobacco roots. Different concentrations of SNP (10–200 μM) were applied to 70 days old control tobacco plants under conditions as described in section “Materials and Methods.” DAF- FM Fluorescence was measured by fluorescence microscope (A) and intensity (B) was expressed as arbitrary units. Transcripts accumulation of NIA dependent NR gene was analyzed from tobacco after exposure of 50, 100, 150, and 200 μM SNP (C).
Several reports have been described in plants where NO-induced [Ca2+]cyt second messenger in the cGMP-dependent signaling pathways that help in cell wall synthesis and cellular homeostasis (Lamattina et al., 2003). Presence of Ca2+ in plants is associated with root cell and root hair elongation (White, 2001), while its deficiency causes hollow heart in potato (Palta, 2010). In the present study, we checked whether induced NO was linked to Ca2+ mediated root formation (Pagnussat et al., 2003). Roots were incubated with inhibitor solution prior to monitoring the stimulus-induced response of plasma membrane Ca2+ blocker lanthanum (III) chloride. Figure 6 shows that lanthanum remarkably reduced the relative fluorescence intensity for [Ca2+]cyt compared to control when incubated in the absence of inhibitor. Moreover, results obtained from different treatments in tobacco roots showed that T42 inoculation stimulated a release of relative fluorescence intensity for [Ca2+]cyt from 40 to 70 days either from mitochondria or ER to cytosol compared to control. However, the relative fluorescence intensity for [Ca2+]cyt was affected only slightly in T42 inoculated nia30 mutant. NO3- nutrient fed tobacco root (alone) was responsible for induced [Ca2+]cyt release, similar to tobacco cells where NO influenced Ca2+ influx across the plasma membrane (Lamotte et al., 2004). In contrast, NH4+ supplementation stimulated lesser extent of Ca2+ release as compared to NO3- nutrient (Figure 6). Our findings concluded that NO was generated in response to nitrate nutrient might possibly due to induced [Ca2+]cyt production in tobacco roots.
FIGURE 6.
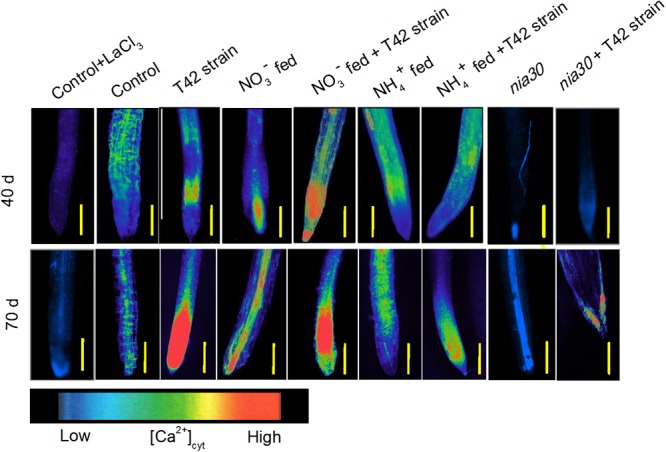
[Ca2+]cyt release in the root of wild-type tobacco and nia30 mutant was stained by 10 μM Fluo-4 AM dye at 40 and 70 days in different treatments. Gradient of color (scale) is used for comparing [Ca2+]cyt intensity. Release of cytosolic [Ca2+]cyt evaluated with addition of 10 mM Lacl3 was used as Ca2+ channel blocker with control tobacco roots. Bars are represented as 500 μm.
Activation of Nitrate/Ammonium Transporters Enhanced N Accumulation
Root architecture development depends on signals derived from nitrogen acquisition and assimilation (Corpas and Barroso, 2015). Stitt (1999) reported that nitrate signaling elevated NR expression and NRTs. But high nitrate fertilization preferentially inhibits root: shoot ratio and reduced lateral root formation (Grime et al., 1991). However, limited application of nitrogen fertilization leads to a proliferation of lateral root distribution. Nonetheless, the regulatory mechanism and controlling system of N acquisition as well as assimilation are not fully known in any single plant species. However, several factors that influence at the transcriptional level of the gene involved during nitrate acquisition have been identified in many plant species (Pii et al., 2014, 2016a; Sun et al., 2015; Vidal et al., 2015). In this context, several nitrate and AMTs were classified on the basis of nitrate/ammonium uptake affinity in plant roots (Pii et al., 2014). Few previous reports suggested that NRT2.1 and NRT2.2 participated in primary root development in maize and rice under nitrate nutrition (Sorgona et al., 2011; Sun et al., 2015; Pii et al., 2016a). In the current investigation, we focused on the expression pattern of different nitrate (viz. NRT2.1, NRT2.2, NRT1.1s, NRT1.2s, NRT1.2t) and ammonium (AMT1) transporters in roots of tobacco grown under different conditions (Figure 7). The transcripts level of two high-affinity NRTs viz., NRT2.1 and NRT2.2 were up-regulated during N availability, and particularly more with NO3- fed condition compared to NH4+ fed condition in Arabidopsis (Noguero and Lacombe, 2016). T42 treatment under nutrient deprived condition induced transcripts level of most of the genes except AMT1 in roots at 70 days, but significantly more in case of the NRT2.1 gene (Figure 7). Similarly, inoculation of T42 into tobacco roots and fed with N nutrition, up-regulated all those transporters except AMT1 in NO3- nutrition. NRT2.1 was highly up-regulated, i.e., 18-fold whereas, the same was up-regulated by only 4-fold in NH4+ fed condition. Besides, induction of NRT1 and NRT2 transporters, acts as an inducible high affinity NRTs; another worthy NRT, i.e., NRT3, analogous to NRT2 in plant roots, independently expressed during “induction” phenomenon in response to NO3- treatment, which might be involved NO3- acquisition in our experiment as well (data not shown) (Okamoto et al., 2006; Pii et al., 2014, 2016a). Transcriptional regulation patterns of these genes gave an overall picture but not a complete picture regarding regulation of the nitrate/AMTs in response to NO3-/NH4+ nutrient fed condition in tobacco roots inoculated with T. asperellum T42. Likewise, the role of T. asperellum T42 in higher expression of NRTs, another filamentous fungi, i.e., T. harzianum has been identified in plant roots which enhanced di/tri-peptide transporters (PTR2) under nitrogen starvation (Vizcaíno et al., 2006). Further investigating on how each component can show a discrepancy during N availability in the presence of Trichoderma spp. may reveal more interesting facts. Moreover, in this work, we observed that the transcript accumulation of NRT2.1 and NRT2.2 transporters were greater among all transporters and mutational study with nia30 tobacco plants further showed that only these two NRTs were responsible for partial nitrate uptake in the nia30 mutant (Müller, 1983). In addition to NO3- and T42 effects, we also examined how NO could elevate expression level of NRT2.1 and N accumulation in addition to NH4+ nutrient supplementation (Figure 8) through application of 10–150 μM SNP (NO donor) with NH4+ fed tobacco plants. Interestingly it was observed that expression of high-affinity transporter (NRT2.1) and total N content in NH4+ fed tobacco roots were increased significantly by SNP application. The results thus indicated that increase in N uptake is through NO regulated N acquisition and assimilation.
FIGURE 8.
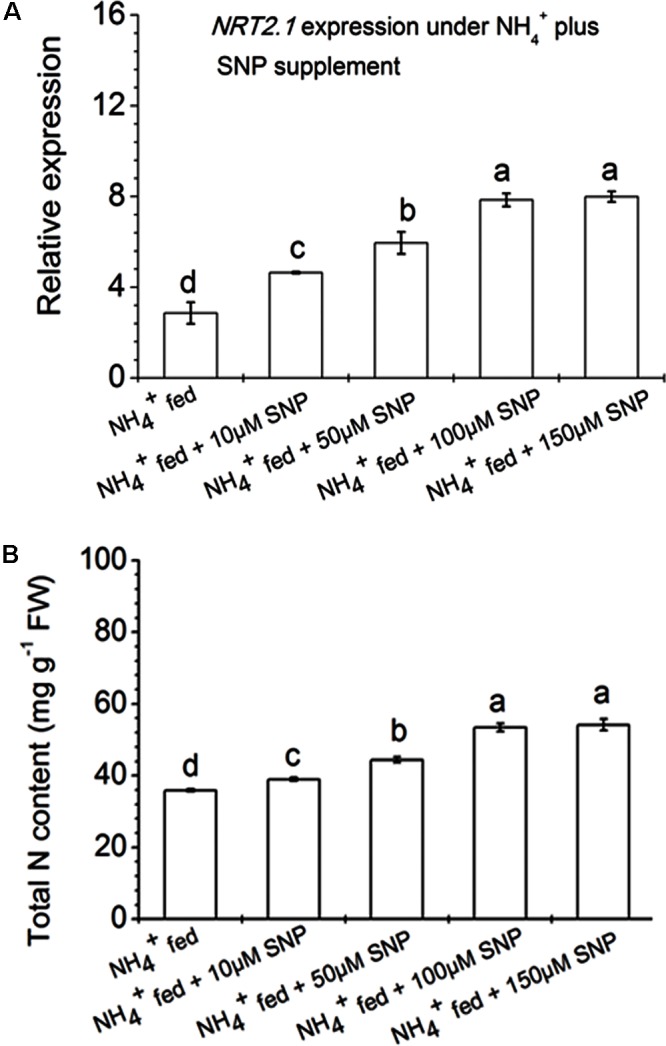
Relative expression of high affinity nitrate transporter NRT2.1 gene and total nitrogen content in the roots of tobacco. Plants were grown hydroponically under NH4+ nutrient solution as described in section “Materials and Methods,” and for assessment of effect of high-affinity nitrate transporter (NRT2.1) in N uptake, added 10, 50, 100, and 150 μM SNP in NH4+ nutrient solution for 48 h in 70 days old tobacco plants (A). Total nitrogen content was estimated with same treatments through Kjeldahl method after exposure of 48 h (B). Data represented as the mean ± standard deviation (n = 4), bars with different letters represent significant differences as analyzed by Duncan’s multiple range test at P < 0.05.
Conclusion
The quantitative analysis of different root traits provides novel insights into plant–microbe interactions and its long-term effects on N acquisition and assimilation. Exogenous supplement of N source(s) triggers the plant nutritional status exerting a major impact on individual root traits that have impacts on plant development. On top of that, nitrate supplement can stimulate individual root development and T. asperellum T42 helped in nitrogen utilization efficiency. These interesting data raise the question of the genetic control of nitrogen uptake and utilization efficiency on plant growth. The identification of corresponding Trichoderma based induction of transcriptional promoters for NRTs will uncover new molecular actors of N-controlled plant growth.
Author Contributions
Conceived and designed the experiments, performed the experiments, and wrote the paper: PD and BNS. Contributed reagents/materials/analysis tools/equipment facilities: PD, BKS, and HS. Analysis of data: BNS, PD, and GS.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer YP and handling Editor declared their shared affiliation.
Acknowledgments
We thank Professor W. M. Kaiser (University of Wurzburg, Germany) for providing nia30 mutant tobacco seeds. We also thank SERB-DST, Government of India, New Delhi for providing financial grant [SR/SO/PS-23/10(G)] in the form of research project to the corresponding author.
Abbreviations
- AMT
ammonium transporter
- CMC
carboxymethyl cellulose
- cPTIO
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- DAF-FM DA
fluorophore 4, 5-diaminofluorescein-FM diacetate
- Fluo-4 AM
4-(6-Acetoxymethoxy-2,7-difluoro-3-oxo-9-xanthenyl)-4′-methyl-2,2′-(ethylenedioxy)dianiline-N,N,N′, N′-tetraacetic acid tetra -kis (acetoxymethyl) ester
- MES-KOH
2-(N-Morpholino) ethanesulfonic acid sodium salt, 4-Morpholineethanesulfonic acid sodium salt
- NRT
nitrate transporter
- PPDF
photosynthetic photon flux density
Footnotes
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.00163/full#supplementary-material
References
- Aceves-García P., Álvarez-Buylla E. R., Garay-Arroyo A., García-Ponce B., Muñoz R., Sánchez M. P. (2016). Root architecture diversity and meristem dynamics in different populations of Arabidopsis thaliana. Front. Plant Sci. 7:858. 10.3389/fpls.2016.00858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarmi R., Hajieghrari B., Giglou A. (2011). Effect of Trichoderma isolates on tomato seedling growth response and nutrient uptake. Afr. J. Biotechnol. 10 5850–5855. [Google Scholar]
- Babourina O., Voltchanskii K., McGann B., Newman I., Renge Z. (2007). Nitrate supply affects ammonium transport in canola roots. J. Exp. Bot. 58 651–658. 10.1093/jxb/erl238 [DOI] [PubMed] [Google Scholar]
- Bright J., Desikan R., Hancock J. T., Weir I. S., Neill S. J. (2006). ABA induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 45 113–122. 10.1111/j.1365-313X.2005.02615.x [DOI] [PubMed] [Google Scholar]
- Brown L. K., George T. S., Thompson J. A., Wright G., Lyon J., Dupuy L., et al. (2012). What are the implications of variation in root hair length on tolerance to phosphorus deficiency in combination with water stress in barley (Hordeum vulgare)? Ann. Bot. 110 319–328. 10.1093/aob/mcs085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. W., Yang J. L., Qin C., Jin C. W., Mo J. H., Ye T., et al. (2010). Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiol. 154 810–819. 10.1104/pp.110.161109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. H., Kao C. H. (2012). Calcium is involved in nitric oxide-and auxin-induced lateral root formation in rice. Protoplasma 249 187–195. 10.1007/s00709-011-0277-2 [DOI] [PubMed] [Google Scholar]
- Corpas F. J., Barroso J. B. (2015). Functions of nitric oxide (NO) in roots during development and under adverse stress conditions. Plants 4 240–252. 10.3390/plants4020240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Aragunde N., Graziano M., Lamattina L. (2004). Nitric oxide plays a central role in determining lateral root development in tomato. Planta 218 900–905. 10.1094/MPMI-06-13-0160-R [DOI] [PubMed] [Google Scholar]
- Courty P. E., Smith P., Koegel S., Redecker D., Wipf D. (2015). Inorganic nitrogen uptake and transport in beneficial plant root-microbe interactions. Crit. Rev. Plant Sci. 34 4–16. 10.1080/07352689.2014.897897 [DOI] [Google Scholar]
- Crawford T. J., Haeger B., Kennard C., Reveley M. A., Henderson L. (1995). Saccadic abnormalities in psychotic patients. II. The role of neuroleptic treatment. Psychol. Med. 25 473–483. 10.1105/tpc.7.7.859 [DOI] [PubMed] [Google Scholar]
- Dordas C. (2008). Role of nutrients in controlling plant diseases in sustainable agriculture, a review. Agron. Sustain. Dev. 28 33–46. 10.1051/agro:2007051 [DOI] [Google Scholar]
- Du S., Zhang Y., Lin X. Y., Tang C. (2008). Regulation of nitrate reductase by nitric oxide in Chinese cabbage pakchoi (Brassica chinensis L.). Plant Cell Environ. 31 195–204. 10.1111/j.1365-3040.2007.01750.x [DOI] [PubMed] [Google Scholar]
- Fernández-Marcos M., Sanz L., Lewis D. R., Muday G. K., Lorenzo O. (2011). Nitric oxide causes root apical meristem defects and growth inhibition while reducing PIN-FORMED 1 (PIN1)-dependent acropetal auxin transport. Proc. Natl. Acad. Sci. U.S.A. 108 18506–18511. 10.1073/pnas.1108644108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freschi L., Rodrigues M. A., Domingues D. S., Purgatto E., Van Sluys M. A., Magalhaes J. R., et al. (2010). Nitric oxide mediates the hormonal control of crassulacean acid metabolism expression in young pineapple plants. Plant Physiol. 152 1971–1985. 10.1104/pp.109.151613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giehl F. H. R., Gruber B. D., von-Wiren N. (2014). Its time to make changes: modulation of root system architecture by nutrient signals. J. Exp. Bot. 65 769–778. 10.1093/jxb/ert421 [DOI] [PubMed] [Google Scholar]
- Giles C., Paterson E., Robertson-Albertyn S., Cesco S., Mimmo T., Pii Y., et al. (2016). Plant-microbiota interactions as a driver of the mineral Turnover in the Rhizosphere. Adv. Appl. Microbiol. 95 1–67. 10.1016/bs.aambs.2016.03.001 [DOI] [PubMed] [Google Scholar]
- Gojon A., Krouk G., Perrine-Walker F., Laugier E. (2011). Nitrate transceptor(s) in plants. J. Exp. Bot. 62 2299–2308. 10.1093/jxb/erq419 [DOI] [PubMed] [Google Scholar]
- Grime J. P., Campbell B. D., Mackey J. M. L., Crick J. C. (1991). “Root plasticity, nitrogen capture and competitive ability,” in Plant Root Growth: An Ecological Perspective ed. Atkinson D. (Oxford: Blackwell Scientific Publications; ) 381–397. [Google Scholar]
- Gupta K. J., Brotman Y., Segu S., Zeier T., Zeier J., Persijn S. T., et al. (2013). The form of nitrogen nutrition affects resistance against Pseudomonas syringae pv. Phaseolicola in tobacco. J. Exp. Bot. 64 553–568. 10.1093/jxb/ers348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K. J., Mur L. A. J., Brotman Y. (2014). Trichoderma asperelloides suppresses nitric oxide generation elicited by Fusarium oxysporum in Arabidopsis roots. Mol. Plant Microbe Interact. 27 307–314. 10.1094/MPMI-06-13-0160-R [DOI] [PubMed] [Google Scholar]
- Hajjar R., Hodgkin T. (2007). The use of wild relatives in crop improvement: a survey of developments over the last 20 years. Euphytica 156 1–13. 10.1007/s10681-007-9363-0 [DOI] [Google Scholar]
- Harman G. E. (2000). Myths and dogmas of biocontrol. Changes in perceptions derived from research on Trichoderma harzianum T-22. Plant Dis. 84 377–393. 10.1094/PDIS.2000.84.4.377 [DOI] [PubMed] [Google Scholar]
- Harman G. E. (2006). Overview of mechanisms and uses of Trichoderma spp. Phytopathology 96 190–194. 10.1094/PHYTO-96-0190 [DOI] [PubMed] [Google Scholar]
- Jain A., Singh S., Kumar Sarma B., Bahadur Singh H. (2012). Microbial consortium–mediated reprogramming of defence network in pea to enhance tolerance against Sclerotinia sclerotiorum. J. Appl. Microbiol. 112 537–550. 10.1111/j.1365-2672.2011.05220.x [DOI] [PubMed] [Google Scholar]
- Jeffries P., Gianinazzi S., Perotto S., Turnau K., Barea J. M. (2003). The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils 37 1–16. 10.1007/s00374-002-0546-5 [DOI] [Google Scholar]
- Jensen E. S. (1986). The influence of rate and time of nitrate supply on nitrogen fixation and yield in pea (Pisum sativum L.). Fertil. Res. 10 193–202. 10.1007/BF01049349 [DOI] [Google Scholar]
- Jin C. W., Du S. T., Zhang Y. S., Lin X. Y., Tang C. X. (2009). Differential regulatory role of nitric oxide in mediating nitrate reductase activity in roots of tomato (Solanum lycocarpum). Ann. Bot. 104 9–17. 10.1093/aob/mcp087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C., Sout P., Broyer T., Carlton A. (1957). Comparative chlorine requirements of different plant species. Plant Soil 8 337–353. 10.1007/BF01666323 [DOI] [Google Scholar]
- Kiba T., Feria-Bourrellier A. B., Lafouge F., Lezhneva L., Boutet-Mercey S., Orsel M., et al. (2012). The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants. Plant Cell 24 245–258. 10.1105/tpc.111.092221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbert Z., Ortega L., Erdei L. (2010). Involvement of nitrate reductase (NR) in osmotic stress-induced NO generation of Arabidopsis thaliana L. roots. J. Plant Physiol. 167 77–80. 10.1016/j.jplph.2009.08.013 [DOI] [PubMed] [Google Scholar]
- Krapp A. (2015). Plant nitrogen assimilation and its regulation: a complex puzzle with missing pieces. Curr. Opin. Plant Biol. 25 115–122. 10.1016/j.pbi.2015.05.010 [DOI] [PubMed] [Google Scholar]
- Krapp A., David L. C., Chardin C., Girin T., Marmagne A., Leprince A. S., et al. (2014). Nitrate transport and signalling in Arabidopsis. J. Exp. Bot. 65 789–798. 10.1093/jxb/eru001 [DOI] [PubMed] [Google Scholar]
- Krouk G., Lacombe B., Bielach A., Perrine-Walker F., Malinska K., Mounier E., et al. (2010). Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev. Cell 18 927–937. 10.1016/j.devcel.2010.05.008 [DOI] [PubMed] [Google Scholar]
- Lamattina L., Garcia-Mata C., Graziano M., Pagnussat G. (2003). Nitric oxide: the versatility of an extensive signal molecule. Ann. Rev. Plant Biol. 54 109–136. 10.1146/annurev.arplant.54.031902.134752 [DOI] [PubMed] [Google Scholar]
- Lambert J. B., Shurvell H. F., Lightner D. A., Cooks G. (1987). Introduction to Organic Spectroscopy. New York, NY: Macmillan. [Google Scholar]
- Lamotte O., Gould K., Lecourieux D., Sequeira-Legrand A., Lebrun-Garcia A., Durner J., et al. (2004). Analysis of nitric oxide signaling functions in tobacco cells challenged by the elicitor cryptogein. Plant Physiol. 135 516–529. 10.1104/pp.104.038968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J. P. (2013). Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 112 347–357. 10.1093/aob/mcs293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoli A., Begheldo M., Genre A., Lanfranco L., Trevisan S., Quaggiotti S. (2014). NO homeostasis is a key regulator of early nitrate perception and root elongation in maize. J. Exp. Bot. 65 185–200. 10.1093/jxb/ert358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín I. C., Loef I., Bartetzko L., Searle I., Coupland G., Stitt M., et al. (2011). Nitrate regulates floral induction in Arabidopsis, acting independently of light, gibberellin and autonomous pathways. Planta 233 539–552. 10.1007/s00425-010-1316-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici A., Krouk G. (2014). The primary nitrate response: a multifaceted signalling pathway. J. Exp. Bot. 65 5567–5576. 10.1093/jxb/eru245 [DOI] [PubMed] [Google Scholar]
- Meng Z. B., Chen L. Q., Suo D., Li G. X., Tang C. X., Zheng S. J. (2012). Nitric oxide is the shared signalling molecule in phosphorus-and iron-deficiency-induced formation of cluster roots in white lupin (Lupinus albus). Ann. Bot. 109 1055–1064. 10.1093/aob/mcs024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. J., Fan X., Orsel M., Smith S. J., Wells D. M. (2007). Nitrate transport and signalling. J. Exp. Bot. 58 2297–2306. 10.1093/jxb/erm066 [DOI] [PubMed] [Google Scholar]
- Mishra B. K., Srivastava J. P., Lal J. P., Sheshshayee M. S. (2016). Physiological and biochemical adaptations in Lentil genotypes under drought stress. Russ. J. Plant Physiol. 63 695–708. 10.1134/S1021443716040117 [DOI] [Google Scholar]
- Müller A. J. (1983). Genetic analysis of nitrate reductase-deficient tobacco plants regenerated from mutant cells. Evidence for duplicate structural genes. Mol. Gen. Genet. 192 275–281. 10.1007/BF00327678 [DOI] [Google Scholar]
- Nacry P., Bouguyon E., Gojon A. (2013). Nitrogen acquisition by roots: physiological and developmental mechanisms ensuring plant adaptation to a fluctuating resource. Plant Soil 370 1–29. 10.1007/s11104-013-1645-9 [DOI] [Google Scholar]
- Negi J., Matsuda O., Nagasawa T., Oba Y., Takahashi H., Kawai-Yamada M., et al. (2008). CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452 483–486. 10.1038/nature06720 [DOI] [PubMed] [Google Scholar]
- Noguero M., Lacombe B. (2016). Transporters involved in root nitrate uptake and sensing by Arabidopsis. Front. Plant Sci. 7:1391. 10.3389/fpls.2016.01391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M., Kumar A., Li W., Wang Y., Siddiqi M. Y., Crawford N. M., et al. (2006). High-affinity nitrate transport in roots of Arabidopsis depends on expression of the NAR2-like gene AtNRT3.1. Plant Physiol. 140 1036–1046. 10.1104/pp.105.074385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat G. C., Lanteri M. L., Lamattina L. (2003). Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol. 132 1241–1248. 10.1104/pp.103.022228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palta J. P. (2010). Improving potato tuber quality and production by targeted calcium nutrition: the discovery of tuber roots leading to a new concept in potato nutrition. Potato Res. 53 267–275. 10.1007/s11540-010-9163-0 [DOI] [Google Scholar]
- Patel J. S., Sarma B. K., Singh H. B., Upadhyay R. S., Kharwar R. N., Ahmed M. (2016). Pseudomonas fluorescens and Trichoderma asperellum enhance expression of Gα subunits of the Pea heterotrimeric G-protein during Erysiphe pisi infection. Front. Plant Sci. 6:1206. 10.3389/fpls.2015.01206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z., Murata Y., Benning G., Thomine S., Èsener B. K., Allen J., et al. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406 731–734. 10.1038/35021067 [DOI] [PubMed] [Google Scholar]
- Peng Y. Z., Zhu G. B. (2006). Biological nitrogen removal with nitrification and denitrification via nitrite pathway. Appl. Microbiol. Biotechnol. 73 15–26. 10.1007/s00253-006-0534-z [DOI] [PubMed] [Google Scholar]
- Pii Y., Alessandrini M., Dall’Osto L., Guardini K., Prinsi B., Espen L., et al. (2016a). Time-resolved investigation of molecular components involved in the induction of NO3– high-affinity transport system in maize roots. Front. Plant Sci. 7:1657. 10.3389/fpls.2016.01657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pii Y., Alessandrini M., Guardini K., Zamboni A., Varanini Z. (2014). Induction of high-affinity NO3– uptake in grapevine roots is an active process correlated to the expression of specific members of the NRT2 and plasma membrane H+-ATPase gene families. Funct. Plant Boil. 41 353–365. 10.1071/FP13227 [DOI] [PubMed] [Google Scholar]
- Pii Y., Crimi M., Cremonese G., Spena A., Pandolfini T. (2007). Auxin and nitric oxide control indeterminate nodule formation. BMC Plant Biol. 7:21. 10.1186/1471-2229-7-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pii Y., Marastoni L., Springeth C., Fontanella M. C., Beone G. M., Cesco S., et al. (2016b). Modulation of Fe acquisition process by Azospirillum brasilense in cucumber plants. Environ. Exp. Bot. 130 216–225. 10.1016/j.envexpbot.2016.06.011 [DOI] [Google Scholar]
- Pii Y., Mimmo T., Tomasi N., Terzano R., Cesco S., Crecchio C. (2015). Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 51 403–415. 10.1007/s00374-015-0996-1 [DOI] [Google Scholar]
- Planchet E., Gupta K. J., Sonoda M., Kaiser W. M. (2005). Nitric oxide emission from tobacco leaves and cell suspensions: rate limiting factors and evidence for the involvement of mitochondrial electron transport. Plant J. 41 732–743. 10.1111/j.1365-313X.2005.02335.x [DOI] [PubMed] [Google Scholar]
- Robertson G. P., Vitousek P. M. (2009). Nitrogen in agriculture: balancing the cost of an essential resource. Annu. Rev. Environ. Res. 34 97–125. 10.1146/annurev.environ.032108.105046 [DOI] [Google Scholar]
- Rosales E. P., Iannone M. F., Groppa M. D., Benavides M. P. (2011). Nitric oxide inhibits nitrate reductase activity in wheat leaves. Plant Physiol. Biochem. 49 124–130. 10.1016/j.plaphy.2010.10.009 [DOI] [PubMed] [Google Scholar]
- Ruffel S., Poitout A., Krouk G., Coruzzi G. M., Lacombe B. (2016). Long-distance nitrate signaling displays cytokinin dependent and independent branches. J. Int. Plant Boil. 58 226–229. 10.1111/jipb.12453 [DOI] [PubMed] [Google Scholar]
- Scagliola M., Pii Y., Mimmo T., Cesco S., Ricciuti P., Crecchio C. (2016). Characterization of plant growth promoting traits of bacterial isolates from the rhizosphere of barley (Hordeum vulgare L.) and tomato (Solanum lycopersicon L.) grown under Fe sufficiency and deficiency. Plant Physiol. Biochem. 107 187–196. 10.1016/j.plaphy.2016.06.002 [DOI] [PubMed] [Google Scholar]
- Schlicht M., Kombrink E. (2013). The role of nitric oxide in the interaction of Arabidopsis thaliana with the biotrophic fungi, Golovinomyces orontii and Erysiphe pisi. Front. Plant Sci. 4:351. 10.3389/fpls.2013.00351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen T. D., Livak K. J. (2008). Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3 1101–1108. 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- Sherameti I., Shahollari B., Venus Y., Altschmied L., Varma A., Oelmüller R. (2005). The endophytic fungus Piriformospora indica stimulates the expression of nitrate reductase and the starch-degrading enzyme glucan water dikinase in tobacco and Arabidopsis roots through a homeodomain transcription factor that binds to a conserved motif in their promoters. J. Biol. Chem. 280 26241–26247. 10.1074/jbc.M500447200 [DOI] [PubMed] [Google Scholar]
- Shoresh M., Harman G. E., Mastouri F. (2010). Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Pytopathol. 48 21–43. 10.1146/annurev-phyto-073009-114450 [DOI] [PubMed] [Google Scholar]
- Simon J., Stoelken G., Rienks M., Rennenberg H. (2009). Rhizospheric NO interacts with the acquisition of reduced N sources by the roots of European beech (Fagus sylvatica L.). FEBS Lett. 583 2907–2910. 10.1016/j.febslet.2009.07.052 [DOI] [PubMed] [Google Scholar]
- Singh B. N., Singh A., Singh G. S., Dwivedi P. (2015). Potential role of Trichoderma asperellum T42 strain in growth of pea plant for sustainable agriculture. J. Pure Appl. Microbiol. 9 1069–1074. [Google Scholar]
- Singh S. P., Singh H. B., Singh D. K., Rakshit A. (2014). Trichoderma-mediated enhancement of nutrient uptake and reduction in incidence of Rhizoctonia solani in tomato. Egypt. J. Biol. 16 29–38. 10.4314/ejb.v16i1.4 [DOI] [Google Scholar]
- Song W., Sun H., Li J., Gong X., Huang S., Zhu X., et al. (2013). Auxin distribution is differentially affected by nitrate in roots of two rice cultivars differing in responsiveness to nitrogen nutrients. Ann. Bot. 112 1383–1393. 10.1093/aob/mct212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgona A., Lupini A., Mercati F., Di Dio L., Sunseri F., Abenavoli M. R. (2011). Nitrate uptake along the maize primary root: an integrated physiological and molecular approach. Plant Cell Environ. 34 1127–1140. 10.1111/j.1365-3040.2011.02311.x [DOI] [PubMed] [Google Scholar]
- Stitt M. (1999). Nitrate regulation of metabolism and growth. Curr. Opin. Plant Biol. 2 178–186. 10.1016/S1369-5266(99)80033-8 [DOI] [PubMed] [Google Scholar]
- Stöhr C., Ullrich W. R. (2002). Generation and possible roles of NO in plant roots and their apoplastic space. J. Exp. Bot. 53 2293–2303. 10.1093/jxb/erf110 [DOI] [PubMed] [Google Scholar]
- Sun H., Li J., Song W., Tao J., Huang S., Chen S., et al. (2015). Nitric oxide generated by nitrate reductase increases nitrogen uptake capacity by inducing lateral root formation and inorganic nitrogen uptake under partial nitrate nutrition in rice. J. Exp. Bot. 66 2449–2459. 10.1093/jxb/erv030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernier V., Cadiou S., Pageau K., Laugé R., Reisdorf-Cren M., Langin T., et al. (2007). The plant nitrogen mobilization promoted by Colletotrichum lindemuthianum in Phaseolus leaves depends on fungus pathogenicity. J. Exp. Bot. 58 3351–3360. 10.1093/jxb/erm182 [DOI] [PubMed] [Google Scholar]
- Thorup-Kristensen K., Cortasa M. S., Loges R. (2009). Winter wheat roots grow twice as deep as spring wheat roots, is this important for N uptake and N leaching losses? Plant Soil 322 101–114. 10.1007/s11104-009-9898-z [DOI] [Google Scholar]
- Trevisan S., Manoli A., Begheldo M., Nonis A., Enna M., Vaccaro S., et al. (2011). Transcriptome analysis reveals coordinated spatiotemporal regulation of hemoglobin and nitrate reductase in response to nitrate in maize roots. New Phytol. 192 338–352. 10.1111/j.1469-8137.2011.03822.x [DOI] [PubMed] [Google Scholar]
- Trevisan S., Manoli A., Quaggiotti S. (2014). NO signaling is a key component of the root growth response to nitrate in Zea mays L. Plant Signal. Behav. 9:e28290. 10.4161/psb.28290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal E. A., Álvarez J. M., Moyano T. C., Gutiérrez R. A. (2015). Transcriptional networks in the nitrate response of Arabidopsis thaliana. Curr. Opin. Plant Biol. 27 125–132. 10.1016/j.pbi.2015.06.010 [DOI] [PubMed] [Google Scholar]
- Viterbo A., Horwitz B. A. (2010). “Mycoparasitism,” in Cellular and Molecular Biology of Filamentous Fungi eds Borkovich K., Ebbole D. J. (Washington DC: ASM Press; ) 676–693. 10.1128/9781555816636.ch42 [DOI] [Google Scholar]
- Vizcaíno J. A., Cardoza R. E., Hauser M., Hermosa R., Rey M., Llobell A., et al. (2006). ThPTR2, a di/tri-peptide transporter gene from Trichoderma harzianum. Fungal Genet. Biol. 43 234–246. 10.1016/j.fgb.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Wang Y., Thorup-Kristensen K., Jensen L. S., Magid J. (2016). Vigorous root growth is a better indicator of early nutrient uptake than root hair traits in spring Wheat Grown under low fertility. Front. Plant Sci. 7:865. 10.3389/fpls.2016.00865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. J. (2001). The pathways of calcium movement to the xylem. J. Exp. Bot. 52 891–899. 10.1093/jexbot/52.358.891 [DOI] [PubMed] [Google Scholar]
- White P. J., George T. S., Dupuy L. X., Karley A. J., Valentine T. A., Wiesel L., et al. (2013). Root traits for infertile soils. Front. Plant Sci. 4:193. 10.3389/fpls.2013.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G., Fan X., Miller A. J. (2012). Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 63 153–182. 10.1146/annurev-arplant-042811-105532 [DOI] [PubMed] [Google Scholar]
- Yadav S. K., Dave A., Sarkar A., Singh H. B., Sarma B. K. (2013). Co-inoculated biopriming with Trichoderma, Pseudomonas and Rhizobium improves crop growth in Cicer arietinum and Phaseolus vulgaris. Int. J. Agric. Environ. Biotechnol. 6 255–259. [Google Scholar]
- Yan X. L., Liao H., Beebe S. E., Blair M. W., Lynch J. P. (2004). QTL mapping of root hair and acid exudation traits and their relationship to phosphorus uptake in common bean. Plant Soil 265 17–29. 10.1007/s11104-005-0693-1 [DOI] [Google Scholar]
- Yun C., Fan X., Sun S., Xu G., Hu J., Shen Q. (2008). Effect of nitrate on activities and transcript levels of nitrate reductase and glutamine synthetase in rice. Pedosphere 18 664–673. 10.1016/S1002-0160(08)60061-2 [DOI] [Google Scholar]
- Zhao M. G., Tian Q. Y., Zhang W. H. (2007). Nitric oxide synthase-dependent nitric oxide production is associated with salt tolerance in Arabidopsis. Plant Physiol. 144 206–217. 10.1104/pp.107.096842 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


