Abstract
Objective
To evaluate the implementation and effectiveness of an internet-based perioperative care programme for patients following gynaecological surgery for benign disease.
Design
Stepped-wedge cluster randomised controlled trial.
Setting
Secondary care, nine hospitals in the Netherlands, 2011–2014.
Participants
433 employed women aged 18–65 years scheduled for hysterectomy and/or laparoscopic adnexal surgery.
Interventions
An internet-based care programme was sequentially rolled out using a multifaceted implementation strategy. Depending on the implementation phase of their hospital, patients were allocated to usual care (n=206) or the care programme (n=227). The care programme included an e-health intervention equipping patients with tailored personalised convalescence advice.
Main outcome measures
The primary outcome was duration until full sustainable return to work (RTW). The degree of implementation of the care programme was evaluated at the level of the patient, healthcare provider and organisation by indicators measuring internet-based actions by patients and providers.
Results
Median time until RTW was 49 days (IQR 27–76) in the intervention group and 62 days (42–85) in the control group. A piecewise Cox model was fitted to take into account non-proportionality of hazards. In the first 85 days after surgery, patients receiving the intervention returned to work faster than patients in the control group (HR 2.66, 95% CI 1.88 to 3.77), but this effect was reversed in the small group of patients that did not reach RTW within this period (0.28, 0.17 to 0.46). Indicators showed that the implementation of the care programme was most successful at the level of the patient (82.8%) and professional (81.7%).
Conclusions
Implementation of an internet-based care programme has a large potential to lead to accelerated recovery and improved RTW rates following different types of gynaecological surgeries.
Trial registration number
NTR2933; Results.
Keywords: telemedicine, minimally invasive surgery, organisation of health services, quality in healthcare
Strengths and limitations of this study.
This study provides evidence that implementation of an internet-based care programme targeting the patient’s self-management throughout the entire surgical pathway can lead to accelerated postoperative recovery following benign gynaecological surgery.
The key strength of the study is its stepped-wedge cluster randomised design, minimising the risk of contamination between study groups and allowing assessment of both the implementation process and the effectiveness on patient level.
Due to a non-proportionality of hazards of the treatment effect, a piecewise Cox model was fitted with a time-dependent covariate.
The study only included employed women of which the majority was highly educated, thus caution is needed when generalising the findings.
Further research should focus on the identification of patients who might benefit the most from the care programme.
Introduction
At present, perioperative care is fragmented due to short hospitalisations and limited coordination of care among involved healthcare professionals following discharge.1–3 In addition, a lack of knowledge on appropriate postoperative recovery times and an absence of guidelines on convalescence advice hamper healthcare professionals to provide profound patient education and manage their patients’ expectancies adequately.4–6 As a consequence, patients are insufficiently prepared to engage in self-management and retreat to inappropriate recovery behaviour.7–9 Thus, several barriers at the levels of the patient, the healthcare professional and the organisation lead to suboptimal perioperative care.10 The current situation puts patients at risk for unnecessary prolonged postoperative recovery, which can lead to personal disease burden11 12 and high societal costs.13–15
We previously studied the feasibility of an internet-based care programme as an alternative to conventional management of postoperative gynaecological patients. Proof of concept was demonstrated in an efficacy randomised controlled trial (RCT), and the care programme resulted in improved return to work (RTW) rates in the intervention group compared with the control group.16 However, external validity was low due to strict guidance of patients and professionals by the research team in order to avoid protocol deviations.17 Following a process evaluation, several improvements were made to the care programme to facilitate implementation in real practice.17 18
The aim of the present study was to study the implementation of the care programme in daily practice in nine hospitals in the Netherlands. A multifaceted implementation strategy was employed, targeting the three identified levels of barriers. Due to a stepped-wedge design, effectiveness of the care programme could be assessed at patient level. The findings on the cost-effectiveness are reported in a separate paper.19
Methods
Study design and participants
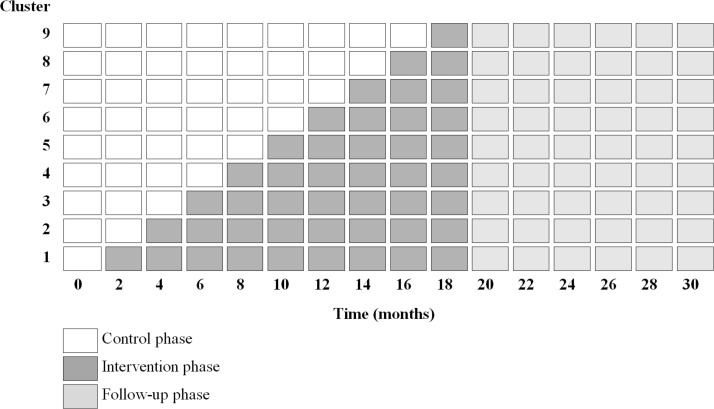
Between April 2011 and July 2014, we did a multicentre, stepped-wedge cluster randomised trial. In this unidirectional crossover design, the care programme was sequentially rolled out among the nine participating hospitals (figure 1). Hospitals served as the control group until the care programme was implemented. Outcomes were assessed at patient level. The trial protocol has been published previously in accordance to the Consolidated Standards of Reporting Trials extended guidelines.18
Figure 1.
Stepped-wedge design with nine clusters. At baseline, all clusters provide usual care. At 2-month intervals, the clusters cross over to the intervention. How long the care programme is implemented in a cluster at 20 months varies from 2 months (cluster 9) to 18 months (cluster 1).
Nine hospitals were selected before the start of the trial. Hospitals were eligible if they performed at least 100 hysterectomies or laparoscopic adnexal surgeries annually, and were located within 50 km of the VU University Medical Center in Amsterdam, the Netherlands.
Patients scheduled for hysterectomy (abdominal, vaginal or laparoscopic) and/or laparoscopic adnexal surgery in one of the participating hospitals were recruited from the waiting lists and were given verbal and written information about the study. Patients were eligible if they were between 18 and 65 years of age and were employed for at least 8 hours a week. We excluded patients who had severe benign comorbidity or a malignancy, were pregnant, were computer or internet illiterate, were involved in a lawsuit against their employer, were on disability sick leave before surgery or had insufficient command of Dutch.
Randomisation and blinding
Randomisation took place at the level of the clusters and determined the order in which the intervention was implemented in the nine participating hospitals. The sequence was delivered by a statistician using a computer-generated list of nine random numbers. A stepped-wedge approach was employed as it enabled us to study the implementation process as well.
Patients, clinicians and researchers could not be masked to intervention implementation. However, group allocation was concealed to patients until they had agreed to participate and had provided written informed consent. Data analysts (EVAB, PMvdV) were masked to group allocation.
Intervention care programme and implementation strategy
The development and content of the intervention care programme have been described before.18 20 In summary, the care programme was developed systematically applying the principles of intervention mapping, involving all stakeholders, including patients, gynaecologists, general physicians (GPs) and occupational physicians (OPs).21 The theory of planned behaviour was used as a theoretical framework for determinants of behaviour regarding recovery and RTW.22
The care programme targeted both the patient level and the cluster level. At the patient level, an interactive web portal facilitated self-management through the entire surgical pathway, by providing individual tailored convalescence advice preoperatively. These convalescence recommendations were developed previously through a Delphi method using an expert panel consisting of gynaecologists, GPs and OPs and are (therefore) in line with current typical beliefs on the resumption of activities following surgery in the Netherlands.23 Patients were not able to change the length of the recommended recovery times themselves. To illustrate, regarding full RTW, patients were advised to resume their work activities gradually in order to reach full RTW by 2 weeks after laparoscopic adnexal surgery, 4 weeks after a vaginal or laparoscopic hysterectomy and 6 weeks after an abdominal hysterectomy. An example of a personalised convalescence plan generated by the patient is presented in online supplementary file S1. Postoperatively, the web portal contained an interactive self-assessment tool to monitor recovery. Behaviours of healthcare professionals and the general organisation of care were targeted by a multifaceted implementation strategy, developed to achieve maximal adoption of the care programme. An overview of the care programme and the employed implementation strategies is presented in online supplementary file S2.
bmjopen-2017-017781supp001.pdf (240.1KB, pdf)
bmjopen-2017-017781supp002.pdf (16.7KB, pdf)
Usual care
Before the care programme was implemented in the hospitals, participating patients received usual care. Although considerable variation in usual care exists in the Netherlands, in general, postoperative patients receive verbal instructions at discharge by a nurse and/or physician, sometimes accompanied by a letter or brochure. Usually, a postoperative consultation is planned 6 weeks following surgery. Due to Dutch legislation, employed patients who do not resume work within 6 weeks after the surgery are invited for a consultation with their OP.
Outcomes
The effectiveness of the intervention care programme was assessed at patient level. As our intervention focused on recovery after discharge, sick leave duration until full sustainable RTW was the primary outcome of this trial. Full sustainable RTW was defined as the resumption of own work or other work with equal earnings, for at least 4 weeks without (partial or full) recurrence of sick leave.24 Sick leave data were collected by monthly, self-reported, electronic calendars.
Secondary outcomes were functional health status, assessed by 36-Item Short-Form Health Survey25 26; recovery, assessed by the Recovery Index-1027; self-efficacy, assessed by the General Self-Efficacy Scale28; coping, assessed by the Pearlin Mastery Scale29 and pain, assessed by the Von Korff questionnaire.30 Data on these secondary outcomes were collected by means of self-reported electronic questionnaires 2, 6, 12, 26 and 52 weeks after surgery.
Sociodemographic data, personal factors and work-related factors were collected before surgery to compare baseline characteristics between both study arms. Data on the surgical procedures and operative/postoperative complications were collected by review of surgical reports.
The degree to which the intervention care programme was successfully implemented was measured by three different indicators. Patient compliance was analysed by measuring patient activity on the web portal and by determining the proportion of patients that used the web portal as intended.17 To evaluate professional compliance, the number of electronic authorisations that were performed by gynaecologists at the web portal were recorded. The number of consultations that took place with the clinical OPs provided information about the impact of the programme on the organisational level.
Statistical analysis
We calculated the sample size with the method described by Hussey and Hughes.31 Based on our efficacy study, we assumed a hazard ratio (HR) of 1.5 on the primary outcome full sustainable RTW.16 To achieve a power of 0.8 with a two-tailed alpha of 0.05 with nine clusters, assuming an intraclass correlation coefficient of 0.05 and a dropout rate of 10%, the sample size was set at 454 patients.
The analyses were done at patient level, according to the intention-to-treat principle. To compare the baseline measurements of both groups, we used descriptive statistics. The primary outcome variable was the duration of sick leave until full sustainable RTW. The independent variable of interest was group allocation. Duration of sick leave in each of the two groups was depicted graphically using the Kaplan-Meier method. Duration of sick leave was compared between the two groups in Cox regression analyses. Here we corrected for possible confounders as indicated in our predefined analysis plan and the characteristics of the stepped-wedge cluster randomised trial design. The adjusted Cox regression model included the fixed effect for group together with (1) a random effect for hospital, (2) a fixed effect for type of surgery performed, (3) a fixed effect for time since start of the trial, (4) a fixed effect for time since implementation of the new intervention in the hospital which we set to zero for all observations in the control condition and (5) if necessary, clinically relevant dissimilarities between both study groups at baseline. HRs for RTW were calculated together with their 95% CIs. The proportional hazard assumption was checked visually and corrected for by including a time-varying covariate for group in the models. Crude analyses were performed in addition to these adjusted analyses.
Linear mixed models were used to assess differences in the longitudinal course of the secondary outcomes over the 52 weeks of follow-up. All of the available outcome measurements (2, 6, 12, 26 and 52 weeks) were used. Models included fixed effects for group, type of surgery, time since surgery, an interaction between group and time since surgery and, if available, the baseline value for the outcome measure. Random effects were included for hospital and patients nested within hospitals. Post hoc tests with Bonferroni correction were used to compare the means between groups separately at each time of follow-up.
To assess whether protocol deviations caused bias, a per-protocol analysis was performed. In addition, several subgroup analyses were performed. The predefined subgroups were: (1) hysterectomy (abdominal, vaginal, laparoscopic), (2) minimally invasive hysterectomy (vaginal, laparoscopic), (3) abdominal hysterectomy only and (4) laparoscopic adnexal surgery only.
All statistical analyses followed a predefined analysis plan and were done in SPSS V.16.0 and STATA V.12.0.
Results
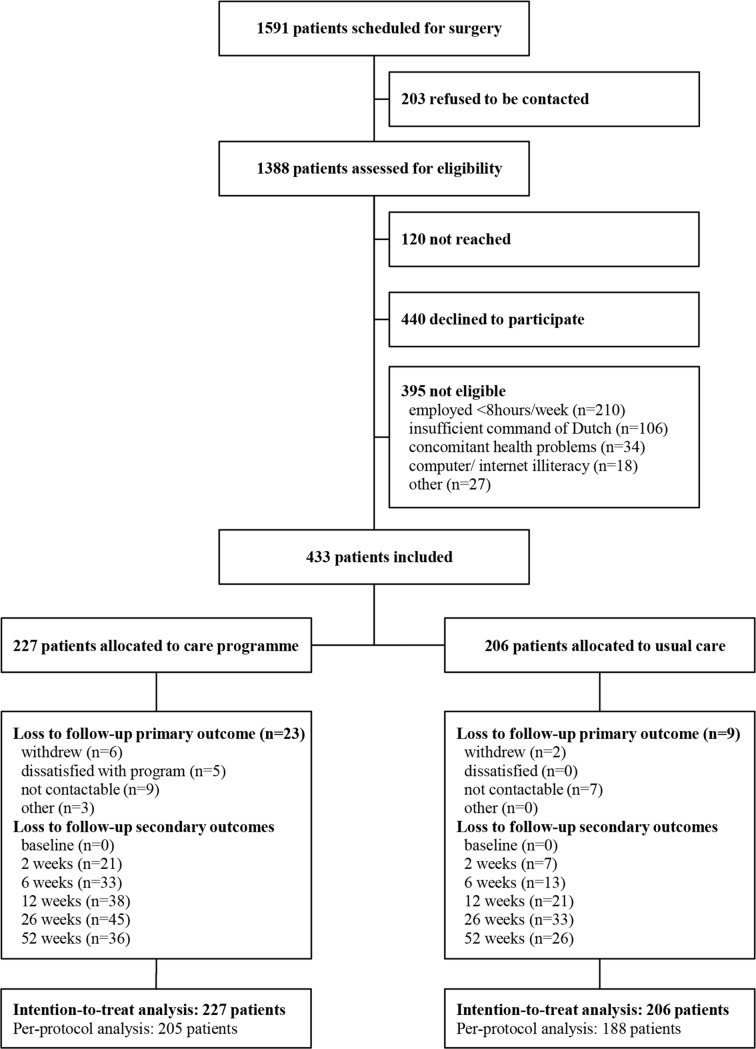
Nine hospitals participated in this trial. Between October 2011 and July 2013, 1591 patients were scheduled for a hysterectomy and/or laparoscopic adnexal surgery in these hospitals. In total, 433 patients were enrolled in the study, 206 patients during the control phase and 227 patients during the intervention phase (figure 2). The timing of crossover from usual care to the intervention of the eighth cluster was delayed by 2 months as the number of inclusions in the control group lagged behind, compared with the number of inclusions in the intervention group at that time. Although lengthening the total inclusion period would have led to reaching the number of patients calculated in the power analysis, this was decided against, as this would only have led to a greater misbalance between the number of patients in the control and intervention groups.
Figure 2.
Trial profile.
Patient characteristics
Most patient characteristics were well balanced between groups at baseline (table 1). However, baseline dissimilarities were present with type of surgery (P=0.038) and intention to RTW despite physical complaints (P=0.003). Because these variables are potentially associated with the outcome measures, they were added to the adjusted models.
Table 1.
Baseline characteristics of individual patients at baseline
| Care programme (n=227) | Usual care (n=206) |
|
| Patient characteristics | ||
| Age (years), mean±SD | 46.1±7.3 | 45.6±6.7 |
| Dutch nationality | 220 (96.9) | 202 (98.1) |
| Internet use (days per week) | ||
| <1 | 2 (0.9) | 3 (1.5) |
| 1–2 | 9 (4.0) | 10 (4.9) |
| 3–5 | 45 (19.8) | 42 (20.4) |
| >5 | 171 (75.3) | 151 (73.3) |
| Education level* | ||
| Low | 25 (11.0) | 17 (8.3) |
| Intermediate | 88 (38.8) | 100 (48.5) |
| High | 114 (50.2) | 89 (43.2) |
| Surgery-related characteristics | ||
| Type of surgery | ||
| Adnexal surgery | 74 (32.6) | 51 (24.8) |
| Laparoscopic hysterectomy | 65 (28.6) | 50 (24.3) |
| Vaginal hysterectomy | 36 (15.9) | 53 (25.7) |
| Abdominal hysterectomy | 52 (22.9) | 52 (25.2) |
| Health-related characteristics | ||
| Perceived health status, mean±SD | 75.8±16.5 | 76.9±16.7 |
| Work-related characteristics | ||
| Type of work | ||
| Salary employed | 194 (85.5) | 175 (85.0) |
| Self-employed | 28 (12.3) | 28 (13.6) |
| Voluntary work | 5 (2.2) | 3 (1.5) |
| Work hours per week, mean±SD | 29.7±9.3 | 28.7±8.2 |
| Sick leave (3 months before surgery) | ||
| Absence from work† | 88 (38.8) | 66 (32.0) |
| Number of sick leave days, median (IQR) | 4.0 (2–10) | 4.5 (2–11) |
| RTW expectation (long)‡ | 42 (18.5) | 38 (18.4) |
| RTW intention (low)§ | 45 (19.8) | 67 (32.5) |
Data are number of patients (%), unless otherwise indicated.
*Low=preschool, primary school; intermediate=secondary school; high=tertiary school, university or postgraduate.
†Defined as at least 1 day of absence.
‡Defined as expectation longer than 3 weeks for adnexal surgery, longer than 6 weeks for laparoscopic or vaginal hysterectomy, or longer than 8 weeks for abdominal hysterectomy.
§Higher scores indicate a higher intention to return to work, despite symptoms (range 1–5). A low intention was defined as score 1 or 2.
RTW, return to work.
Lost to follow-up
Data for the primary outcome were obtained from self-reported sick leave calendars and were available for 401 participants (92.6%). Twenty-nine patients were lost to follow-up and three patients were censored for the primary endpoint because of the occurrence of an unforeseen independent incident before reaching full RTW (cerebral vascular accident, severe exacerbation of sarcoidosis and diagnosis of post-traumatic dystrophy shoulder). For the secondary outcomes, complete follow-up data were available for 334 patients (77.1%). Lost to follow-up rates did differ between both groups; patients in the intervention group were more likely to get lost to follow-up than patients in the usual care group (P=0.022).
Indicators of implementation
In the intervention group, the vast majority of patients logged in to the web portal at least once (215/227; 94.7%). A total of 188 patients (82.8%) used the website as intended and generated a personal convalescence plan online. Median time spent on the website was 97 min (IQR 55–167). Participants gave the web portal an overall score of 7.3 on a 10-point scale.
Gynaecologists electronically authorised 81.7% of all generated convalescence plans (170/208).
In total, 68 patients were eligible for a telephone consultation with a clinical OP before surgery due to a high risk for delayed recovery; however, only 23 patients (33.8%) received care by the OP as planned. Postoperatively, 126 patients were eligible for a telephone consultation with a clinical OP, of which 84 appointments took place (66.7%). In total, 65.7% of the patients (130/198) received clinical occupational care according to the protocol.
Primary outcome measure
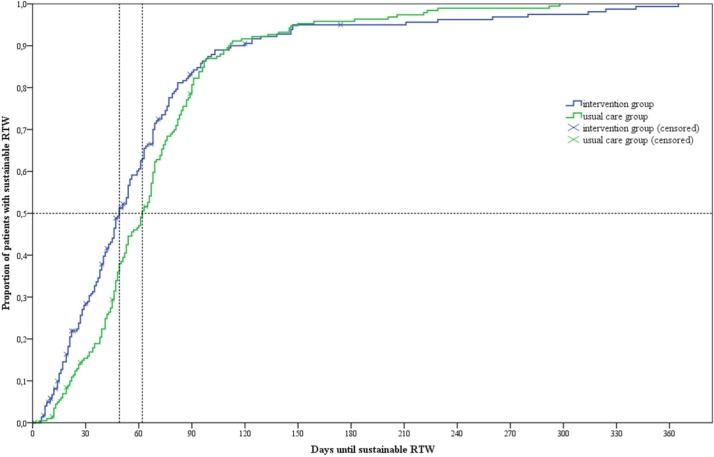
The median duration until full sustainable RTW was 49 days (IQR 27–76) in the intervention group and 62 days (IQR 42–85) in the usual care group (log-rank test P=0.153). Survival curves for duration until RTW diverged directly after surgery but converged again with time (figure 3). The proportional hazard hypothesis was tested and rejected as the time-dependent covariate for group was highly significant (P=0.001). Therefore, a piecewise Cox model was fitted taking into account the non-proportionality of hazards by creating two different time intervals. The cut-off for the time-dependent covariate was determined by plotting the HR over time and calculating the time period the HR was greater than one and smaller than one (online supplementary file S3). Duration to RTW was effectively reduced in the first 85 days after surgery: HR 2.66; 95% CI 1.88 to 3.77; P<0.001 (349 patients (191 in intervention group, 158 in control group); table 2). The effect was reversed if patients did not RTW within this period: HR 0.28, 95% CI 0.17 to 0.46; P<0.001 (84 patients (36 in intervention group, 48 in control group); table 2).
Figure 3.
Survival curves for duration until full sustainable return to work (RTW). Median time to full sustainable RTW in the control group was 62 days (95% CI 54.9 to 69.1) and in the intervention group 49 days (95% CI 44.2 to 53.8); log-rank test P=0.153.
Table 2.
Differences in duration until return to work between the intervention group and the usual care group
| Events/subjects | Cut-off | Subjects (n) | HR | 95% CI | |||
| UC | IC | Lower | Upper | ||||
| Unadjusted model | |||||||
| Intention to treat | 401/433 | T≤85 days | 158 | 191 | 2.55 | 2.02 | 3.21 |
| T>85 days | 48 | 36 | 0.26 | 0.18 | 0.39 | ||
| Per protocol | 368/393 | T≤85 days | 147 | 175 | 2.48 | 1.95 | 3.15 |
| T>85 days | 41 | 30 | 0.28 | 0.18 | 0.43 | ||
| Adjusted model 1* | |||||||
| Intention to treat | 401/433 | T≤85 days | 158 | 191 | 2.79 | 1.97 | 3.94 |
| T>85 days | 48 | 36 | 0.29 | 0.18 | 0.47 | ||
| Per protocol | 368/393 | T≤85 days | 147 | 175 | 2.79 | 1.95 | 3.97 |
| T>85 days | 41 | 30 | 0.31 | 0.19 | 0.52 | ||
| Adjusted model 2† | |||||||
| Intention to treat | 401/433 | T≤85 days | 158 | 191 | 2.66 | 1.88 | 3.77 |
| T>85 days | 48 | 36 | 0.28 | 0.17 | 0.46 | ||
| Per protocol | 368/393 | T≤85 days | 147 | 175 | 2.63 | 1.84 | 3.75 |
| T>85 days | 41 | 30 | 0.30 | 0.18 | 0.50 | ||
Results of the crude Cox regression models are not presented, due to violation of the proportional hazard assumption.
Due to violation of the proportional hazard assumption, a time-dependent covariate (T) was introduced, and therefore two HRs are presented. The cut-off was calculated by determining at what time the HR equalled value 1.
*Adjusted for hospital (random effect), type of surgery performed (fixed effect), time since start of trial (fixed effect), time since implementation (fixed effect).
†As adjusted model 1, including RTW intention (fixed effect).
IC, intervention care; RTW, return to work; T, time-dependent covariate; UC, usual care.
bmjopen-2017-017781supp003.pdf (130.3KB, pdf)
In the per-protocol analysis, a total of 40 patients were excluded because they, retrospectively, did not meet the inclusion criteria (n=3), had a significant larger surgery than planned (n=25) or needed a repeat surgery during follow-up (n=12). Findings from the per-protocol analysis were similar to those of the main analysis (table 2).
Subgroup analyses
Results of the prespecified subgroup analyses were also in concordance with the main analysis (online supplementary file S4). However, it is important to note that power was lost in some subgroups, due to the reduced sample sizes.
bmjopen-2017-017781supp004.pdf (32.5KB, pdf)
Secondary outcome measures
The results of the secondary outcome measures are presented in online supplementary file S5. For the outcome recovery-specific quality of life, a significant interaction between group allocation and time since surgery was found, indicating that there was a difference in the course of mean outcome over time in the two groups (P=0.003). Post hoc analyses showed a difference to be present at 2 weeks following surgery with patients in the intervention group having a higher score corresponding with a better recovery than patients in the control group (mean score of 30.07 in the intervention group vs 28.61 in the control group; P=0.046). However this difference disappeared with longer follow-up.
bmjopen-2017-017781supp005.pdf (173.5KB, pdf)
Similar findings were established for the outcome pain: 2 weeks following surgery, patients in the intervention group reported a lower pain intensity score than patients in the control group (mean score of 9.20 in the intervention group vs 10.55 in the control group; P=0.014), as well as a lower pain disability score (mean score of 11.83 in the intervention group vs 14.23 in the control group; P=0.000). Again, this difference disappeared with longer follow-up.
For the secondary outcomes functional health status, self-efficacy and coping, there were no differences in the course of mean outcomes over time in the two groups.
Discussion
In this study, an internet-based care programme was implemented in nine Dutch hospitals following a stepped-wedge design. Our results show that implementation was successful and that the internet-based care programme has a large potential to lead to accelerated recovery and improved RTW rates following different types of gynaecological surgeries.
Interpretation of the findings
The majority of patients benefited greatly from the care programme. Duration until full RTW was effectively reduced in the first 85 days after surgery in the intervention group compared with the control group. The reversed effect after 85 days of follow-up is an interesting finding of the study which accounted for a minority of the patients. We hypothesise that this shift may be caused by a statistical limitation, due to the application of a Cox regression model in a population with an overall good prognosis of RTW (99.8% of the population achieved full RTW within the year). In addition, we were confronted with non-proportional hazards of the treatment effect, for which we were forced to take into account the time-dependency of the HR. In case of non-proportional hazards, the power of the log-rank test may be low, and therefore, the outcome of a trial can be declared ‘negative’ when in fact a clinically relevant difference between groups was present.32 In our trial, the difference between median durations until full sustainable RTW between treatment groups was 13 days; however, this difference was not statistically significant using the log-rank test.
Patients in the intervention group scored slightly better on the outcomes recovery-specific quality of life and pain (both intensity score and disability score) at 2 weeks following surgery. The differences disappeared with longer follow-up. In addition, it is unknown if the small differences are of any clinical relevance.
Despite a restricted involvement of the research team following the initial instructions and training sessions, implementation at the patient level was quite successful. Due to user authentication, we were able to objectively measure usage of the e-health intervention by participants. The vast majority of the patients (82.8%) used the web portal as intended and generated a convalescence plan online. Compared with other internet-based interventions, this compliance rate is relatively high.33 34 However, these results are in concordance with our previous efficacy RCT.17
Participating gynaecologists electronically approved the convalescence plans of their patients in 81.7% of the cases. This implementation rate increased in comparison with the efficacy study, which might be attributable to the measures taken to increase the user-friendliness of the electronic procedures. In a survey among all involved gynaecologists, none agreed with the statement that the web portal was too time-consuming, and 94.7% of the responders thought the web portal was (very) easy to use.
At the level of the organisation of care processes, 65.7% of the patients received care according to the protocol. Taking into account the very poor implementation score at this level of 24.0% in the previous trial, adaptations made to the protocol and implementation strategies were highly rewarding. The most important change was to integrate occupational healthcare in clinical care, and therefore, postoperative appointments with a clinical OP were already planned at enrolment, which were to be cancelled in case full resumption of work was reached before the appointment.
Strengths and weaknesses of the study
A strength of our study is that the internet-based programme was developed with all involved stakeholders, including focus groups with patients. In addition, it was rigorously evaluated and adapted through different phases of research, including both an efficacy trial demonstrating proof of concept and a process evaluation. The current implementation study with a stepped-wedge approach provided not only important data on healthcare outcomes and adherence to the programme by its end users, but also valuable information about the organisational context. The latter has been identified as a striking absent outcome in studies reporting on electronic patient portals.35
In addition, we believe that our study is unique as the primary endpoint was sick leave duration until full sustainable RTW. WHO uses the International Classification of Functioning, Disability and Health which is a framework for the description of health and classifies functioning and disability associated with health conditions.36 By assessing participation restrictions on a social level, in our case sick leave following surgery, we integrated a biopsychosocial model and looked further than the illness and its treatment but also assessed the impact on the community.
Our study also has limitations. Regarding methodology, the cluster design of the study might have led to recruitment bias. This can be a threat to validity, when professionals recruit differently depending on the trial arm to which they are allocated. To minimise this, recruitment took place through the use of waiting lists and was performed independently from the professional invitation. Allocation was concealed to patients until informed consent was received. We believe that recruitment bias was minimised, as the proportion of patients included during the control phase, was broadly similar to the proportion of inclusions during the intervention phase, across all participating hospitals. In addition, the subgroup analyses show that our data are robust and confirmed in all subgroups.
Second, external validity of the result might have been compromised. Only one of every three patients approached, ended up in the trial (31.2%). The other patients either declined to participate (31.7%), did not meet the inclusion criteria (28.5%) or were missed (8.6%). Therefore, as this study only included employed women who had access to internet and of which the majority was highly educated, caution is needed when generalising the findings. Possibly, clinical effectiveness is reduced when the intervention is accessible to the general audience. The most important reason for exclusion was not being employed for at least 8 hours a week. This criterion was put in place because of the primary outcome, sustainable RTW. It should be noted that the benefits of the care programme under study are probably not limited to work outcomes alone, but can also impact the resumption of other daily activities.
Finally, lost to follow-up rates differed significantly between both study groups with more participants withdrawing from the study in the intervention group than in the control group. Some participants judged the intervention programme in combination with the monthly trial questionnaires to be too time-consuming during their recovery. Also, there were a few participants in the intervention group who withdrew because they felt the focus of the care programme was too much on the resumption of work. Differences in lost to follow-up rates between study groups can lead to both overestimation and underestimation of the intervention effect. Since the results from the subgroup analysis with only complete cases were similar to those in the main analysis, we believe the effect in our trial to be minimal.
Comparison to other studies
In the last decade, e-health, defined by WHO as ‘the transfer of health-related resources and healthcare by electronic means, including information, support resources, assessments, interventions, and healthcare records’, has known an enormous growth.37 For patients with chronic disease such as diabetes or hypertension, and for patients with mental disorders such as depression, e-health programmes are numerous and already widespread.35 Currently, e-health solutions are also being developed for the care of surgical patients.38 39
Besides our own intervention, we are aware of two other internet-based interventions aimed at patients undergoing gynaecological surgery, both in an early stage of evaluation. Dukeshire et al developed the Studying Adverse Events From Elective Surgery Research self-care web application, designed to improve recovery after hysterectomy by providing patients timely, accurate information tailored to the patient’s stage of recovery.40 It also contained a screening tool to identify adverse symptoms. Feasibility was tested among 31 patients, of which 11 patients experienced an adverse event. Interviewed women (six) indicated that they used the provided information to guide themselves in seeking care for their complications.41 Andikyan et al evaluated the feasibility of an internet-based patient-reported outcome system in patients recovering from major gynaecological cancer surgery.42 They used a Symptom Tracking And Reporting for patients (STAR) system to identify adverse events postoperatively. The intervention was tested among 96 patients, of which the majority of patients found it helpful and would recommend it to other patients. Despite positive feedback from patients, clinical personnel found that STAR system increased their current workload without enhancing patient care.43 Although the results of those two feasibility studies are promising, we want to emphasise the importance of targeting the entire surgical pathway from the early preoperative phase, starting when the indication for surgery is set, until the late postoperative phase, ending with full recovery and resumption of all daily activities, in which our own internet-based care programme is unique.
Policy implications and recommendations
Affronted with increased pressure on current healthcare systems worldwide due to a combination of an ageing population, limited healthcare budgets and a shortage of the workforce, internet-based technology is widely accepted to play an essential role in revolutionising healthcare.
In the surgical field, there is an urgency to reorganise perioperative care as well, considering the escalation of the number of surgical procedures being performed and the transition of care from the hospital setting towards the home setting. In addition, there is considerable evidence that the length of recovery time after (gynaecological) surgery systematically exceeds the period considered as appropriate by specialists.3 8 44–46 Also in our study, the median time until RTW in the intervention group of 49 days can be considered as quite long. Policy-makers faced with the task to optimise perioperative care should consider the encouraging outcomes of this study demonstrating that our internet-based perioperative care programme provides an excellent platform to target all phases of the surgical pathway and is effective in facilitating self-management postoperatively, leading to accelerated recovery.
In addition, we showed that implementation was quite successful by employing a multifaceted implementation strategy, targeting both patients and healthcare professionals, as well as the organisation of healthcare. Key learnings from the current implementation study can be applied across other fields of surgical care; however, cost-effectiveness data will be essential to convince policy-makers that implementation of the care programme is worthwhile.
As there was a small group in our study population that did not benefit from the care programme, future research should focus on ways to discriminate between patients who might benefit most from the care programme, and patients who would need a more intensive form of postoperative guidance. In addition, in view of enhancing technologies, the web portal should evolve concurrently, with access to a mobile application being the first priority.
Conclusions
Our trial provides meaningful evidence that the internet-based intervention care programme can be highly beneficial for a majority of gynaecological patients, resulting in accelerated RTW rates following surgery. Key learnings from the current implementation study can be applied across all other fields of surgical care. Further research should focus on the identification of patients who might benefit most from the internet-based care programme.
Supplementary Material
Acknowledgments
We thank the participants of this trial. Derrick Stomp is thanked for his extensive role in developing the web portal. Dirk Knol is thanked for his statistical contributions in the earlier phases of this research. Arianne Scholten is thanked for her role in the recruitment of patients.
Footnotes
Contributors: All named authors made substantial contributions to this study and the article. EVAB, JAFH, AVN, FGS, HAMB and JRA participated in the design and/or execution of the study, the interpretation of data, and the drafting and/or revision of the article. PMvdV was involved in the statistical data analyses and interpretation of the data, and the revision of the article. SESK and PJMvK contributed to the execution of the study and to the revision of the article. All named authors approved the final version of the manuscript. EVAB and JRA are the study guarantors.
Funding: This study is funded by the Netherlands Organisation for Scientific Research and Development (ZonMw grants 171102015 and 92003590).
Disclaimer: ZonMw did not have any involvement in the study design, data collection, analysis or interpretation, or in writing the report and decision to submit for publication. The views expressed in this report are those of the authors and not necessarily of those of ZonMw.
Competing interests: JRA reports a chair in insurance medicine paid by the Dutch Social Security Agency, and he is a stockholder of Evalua. JAFH reports grants from Samsung, Gideon Richter and Celonova, outside the submitted work. HAMB reports grants from Olympus and personal fees from Nordic Farma, during the conduct of the study. JRA and JAFH intend to set up a spin-off company concerning the implementation of a mobile application concerning the ikherstel intervention in the Netherlands. The remaining authors have nothing to disclose.
Patient consent: Obtained.
Ethics approval: The study protocol was approved by the Institutional Review Board of the VU University Medical Centre (16 May 2011, number 2011/142) and by the medical ethics committees of Onze Lieve Vrouwe Gasthuis Oost (Amsterdam), Meander Medical Center (Amersfoort), Amstelland Hospital (Amsterdam), Medical Center Alkmaar (Alkmaar), Diakonessenhuis (Utrecht), Spaarne Gasthuis (locations Haarlem and Hoofddorp) and Flevo Hospital (Almere).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available, though details on statistical analyses are available from the corresponding author on request.
References
- 1.Majeed AW, Brown S, Williams N, et al. Variations in medical attitudes to postoperative recovery period. BMJ 1995;311:296 10.1136/bmj.311.7000.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cox H. Recovery from gynaecological day surgery: are we underestimating the process. Ambul Surg 2003;10:114–21. 10.1016/S0966-6532(03)00007-6 [DOI] [Google Scholar]
- 3.Evenson M, Payne D, Nygaard I. Recovery at home after major gynecologic surgery: how do our patients fare? Obstet Gynecol 2012;119:780–4. 10.1097/AOG.0b013e31824bb15e [DOI] [PubMed] [Google Scholar]
- 4.Møller C, Ottesen M, Kehlet H, et al. (Convalescence recommendations after hysterectomy. a study of opinions among Danish physicians). Ugeskr Laeger 2001;163:7043–7. [PubMed] [Google Scholar]
- 5.Ottesen M, Møller C, Kehlet H, et al. Substantial variability in postoperative treatment, and convalescence recommendations following vaginal repair. a nationwide questionnaire study. Acta Obstet Gynecol Scand 2001;80:1062–8. [PubMed] [Google Scholar]
- 6.Naidu M, Sultan AH, Thakar R. Convalescence advice following gynaecological surgery. J Obstet Gynaecol 2012;32:556–9. 10.3109/01443615.2012.693983 [DOI] [PubMed] [Google Scholar]
- 7.Young J, O’Connell B, Mcgregor S. Day surgery patients convalescence at home: does it make a difference. Nurs Health Sci 2000;2:29–39. [Google Scholar]
- 8.Horvath KJ. Postoperative recovery at home after ambulatory gynecologic laparoscopic surgery. J Perianesth Nurs 2003;18:324–34. 10.1016/S1089-9472(03)00181-3 [DOI] [PubMed] [Google Scholar]
- 9.Bradshaw C, Beard C, Pritchett CJ, et al. How quickly do patient’s recover from operations: the postoperative recovery study (P.O.R.S). Journal of One-Day Surgery 2008;18:48–51. [Google Scholar]
- 10.Grol R, Wensing M. Implementatie - effectieve verbetering van de patientenzorg. 4th edn Reed Business, 2011. [Google Scholar]
- 11.Waddell G, Burton A. Is work good for your health and well-being? UK: Centre for Psychosocial and Disability Research, Cardiff University, 2006. [Google Scholar]
- 12.van Oostrom SH, Driessen MT, de Vet HC, et al. Workplace interventions for preventing work disability. Cochrane Database Syst Rev 2009;2:CD006955. [DOI] [PubMed] [Google Scholar]
- 13.Lenihan JP, Kovanda C, Cammarano C. Comparison of laparoscopic-assisted vaginal hysterectomy with traditional hysterectomy for cost-effectiveness to employers. Am J Obstet Gynecol 2004;190:1714–20. 10.1016/j.ajog.2004.02.059 [DOI] [PubMed] [Google Scholar]
- 14.Roumm AR, Pizzi L, Goldfarb NI, et al. Minimally invasive: minimally reimbursed? An examination of six laparoscopic surgical procedures. Surg Innov 2005;12:261–87. 10.1177/155335060501200313 [DOI] [PubMed] [Google Scholar]
- 15.Epstein AJ, Groeneveld PW, Harhay MO, et al. Impact of minimally invasive surgery on medical spending and employee absenteeism. JAMA Surg 2013;148:641–7. 10.1001/jamasurg.2013.131 [DOI] [PubMed] [Google Scholar]
- 16.Vonk Noordegraaf A, Anema JR, van Mechelen W, et al. A personalised e health programme reduces the duration until return to work after gynaecological surgery: results of a multicentre randomised trial. BJOG 2014;121:1127–36. 10.1111/1471-0528.12661 [DOI] [PubMed] [Google Scholar]
- 17.Bouwsma EV, Vonk Noordegraaf A, Szlávik Z, et al. Process evaluation of a multidisciplinary care program for patients undergoing gynaecological surgery. J Occup Rehabil 2014;24:425–38. 10.1007/s10926-013-9475-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouwsma EV, Anema JR, Vonk Noordegraaf A, et al. The cost effectiveness of a tailored, web-based care program to enhance postoperative recovery in gynecologic patients in comparison with usual care: protocol of a stepped wedge cluster randomized controlled trial. JMIR Res Protoc 2014;3:e30 10.2196/resprot.3236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouwsma EVA, Huirne JAF, van Dongen JM, et al. Cost-effectiveness of an internet-based perioperative care programme to enhance postoperative recovery in gynaecological patients: economic evaluation alongside a stepped-wedge cluster-randomised trial. BMJ Open 2017. 10.1136/bmjopen-2017-017782 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vonk Noordegraaf A, Huirne JA, Pittens CA, et al. eHealth program to empower patients in returning to normal activities and work after gynecological surgery: intervention mapping as a useful method for development. J Med Internet Res 2012;14:e124 10.2196/jmir.1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartholomew L, Parcel G, Kok G, et al. An intervention mapping approach. 2nd edn San Francisco, CA: Jossey-Bass, 2006. [Google Scholar]
- 22.de Vries H, Dijkstra M, Kuhlman P. Self-efficacy: the third factor besides attitude and subjective norm as a predictor of behavioural intentions. Health Educ Res 1988;3:273–82. 10.1093/her/3.3.273 [DOI] [Google Scholar]
- 23.Vonk Noordegraaf A, Huirne JA, Brölmann HA, et al. Multidisciplinary convalescence recommendations after gynaecological surgery: a modified Delphi method among experts. BJOG 2011;118:1557–67. 10.1111/j.1471-0528.2011.03091.x [DOI] [PubMed] [Google Scholar]
- 24.de Vet HC, Heymans MW, Dunn KM, et al. Episodes of low back pain: a proposal for uniform definitions to be used in research. Spine 2002;27:2409–16. 10.1097/01.BRS.0000030307.34002.BE [DOI] [PubMed] [Google Scholar]
- 25.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 26.Aaronson NK, Muller M, Cohen PD, et al. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol 1998;51:1055–68. 10.1016/S0895-4356(98)00097-3 [DOI] [PubMed] [Google Scholar]
- 27.Kluivers KB, Hendriks JC, Mol BW, et al. Clinimetric properties of 3 instruments measuring postoperative recovery in a gynecologic surgical population. Surgery 2008;144:12–21. 10.1016/j.surg.2008.03.027 [DOI] [PubMed] [Google Scholar]
- 28.Teeuw B, Schwarzer R, Jerusalem M. Dutch adaptation of the general self-efficacy scale. Berlin, Germany: Bart Teeuw, Ralf Schwarzer & Matthias Jerusalem, 1994. http://www.webcitation.org/6NnSKKgm3 [Google Scholar]
- 29.Pearlin LI, Schooler C. The structure of coping. J Health Soc Behav 1978;19:2–21. 10.2307/2136319 [DOI] [PubMed] [Google Scholar]
- 30.Von Korff M, Ormel J, Keefe FJ, et al. Grading the severity of chronic pain. Pain 1992;50:133–49. 10.1016/0304-3959(92)90154-4 [DOI] [PubMed] [Google Scholar]
- 31.Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials 2007;28:182–91. 10.1016/j.cct.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 32.Royston P, Parmar MK. An approach to trial design and analysis in the era of non-proportional hazards of the treatment effect. Trials 2014;15:314 10.1186/1745-6215-15-314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eysenbach G. The law of attrition. J Med Internet Res 2005;7:e11 10.2196/jmir.7.1.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelders SM, Kok RN, Ossebaard HC, et al. Persuasive system design does matter: a systematic review of adherence to web-based interventions. J Med Internet Res 2012;14:e152 10.2196/jmir.2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldzweig CL, Orshansky G, Paige NM, et al. Electronic patient portals: evidence on health outcomes, satisfaction, efficiency, and attitudes: a systematic review. Ann Intern Med 2013;159:677–87. 10.7326/0003-4819-159-10-201311190-00006 [DOI] [PubMed] [Google Scholar]
- 36.WHO. International Classification of Functioning: Disability and Health, 2001. [Google Scholar]
- 37.Oh H, Rizo C, Enkin M, et al. What is eHealth (3): a systematic review of published definitions. J Med Internet Res 2005;7:e1 10.2196/jmir.7.1.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waller A, Forshaw K, Carey M, et al. Optimizing patient preparation and surgical experience using e-health technology. JMIR Med Inform 2015;3:e29 10.2196/medinform.4286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Meij E, Anema JR, Otten RH, et al. The effect of perioperative e-health interventions on the postoperative course: a systematic review of randomised and non-randomised controlled trials. PLoS One 2016;11:e0158612 10.1371/journal.pone.0158612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dukeshire S, Gilmour D, MacDonald N, et al. Development and evaluation of a web site to improve recovery from hysterectomy. Comput Inform Nurs 2012;30:164–75. 10.1097/NCN.0b013e31823eb8f9 [DOI] [PubMed] [Google Scholar]
- 41.Gilmour DT, MacDonald NJ, Dukeshire S, et al. Diagnosis of adverse events after hysterectomy with postoperative self-care web applications: a pilot study. Health Informatics J 2017;23:279–90. 10.1177/1460458216647759 [DOI] [PubMed] [Google Scholar]
- 42.Andikyan V, Rezk Y, Einstein MH, et al. A prospective study of the feasibility and acceptability of a Web-based, electronic patient-reported outcome system in assessing patient recovery after major gynecologic cancer surgery. Gynecol Oncol 2012;127:273–7. 10.1016/j.ygyno.2012.07.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cowan RA, Suidan RS, Andikyan V, et al. Electronic patient-reported outcomes from home in patients recovering from major gynecologic cancer surgery: a prospective study measuring symptoms and health-related quality of life. Gynecol Oncol 2016;143:362–6. 10.1016/j.ygyno.2016.08.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johansen P, Al-Khafagi SK, Thøstesen LM, et al. (Analysis of need for sick leave after hysterectomy). Ugeskr Laeger 2008;170:1465–8. [PubMed] [Google Scholar]
- 45.Rasmussen KL, Hansen V, Madzak F, et al. (Feeling of illness after hysterectomy. Women’s own assessment). Ugeskr Laeger 2001;163:7040–2. [PubMed] [Google Scholar]
- 46.Vonk Noordegraaf A, Anema JR, Louwerse MD, et al. Prediction of time to return to work after gynaecological surgery: a prospective cohort study in the Netherlands. BJOG 2014;121:487–97. 10.1111/1471-0528.12494 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-017781supp001.pdf (240.1KB, pdf)
bmjopen-2017-017781supp002.pdf (16.7KB, pdf)
bmjopen-2017-017781supp003.pdf (130.3KB, pdf)
bmjopen-2017-017781supp004.pdf (32.5KB, pdf)
bmjopen-2017-017781supp005.pdf (173.5KB, pdf)