Abstract
Objective
This investigation reports the cause and the quality of death certification in a community cohort of patients with Parkinson’s disease (PD) and controls at 18 years.
Setting
Denbighshire North Wales, UK.
Participants
The community-based cohorts consisted of 166 patients with PD and 102 matched controls.
Primary outcomes
All-cause mortality was ascertained at 18 years by review of hospitals’ primary care records and examination of death certificates obtained from the UK General Register Office. Mortality HRs were estimated using Cox proportional regression, controlling for covariates including age at study entry, age at death, gender, motor function, mood, health-related quality of life (HRQoL) and cognitive function.
Results
After 18 years, 158 (95%) of patients in the PD cohort and 34 (33%) in the control cohort had died. Compared with the general UK population, the PD cohort had a higher risk of mortality (standard mortality rate, 1.82, 95% CI 1.55 to 2.13). As the primary or underlying cause of death, PD was not reported in 75/158 (47%) of the death certificates. In addition, although 144/158 (91%) of the PD cohort had a diagnosis of dementia, this was reported in less than 10% of death certificates. The main cause of death reported in the PD cohort was pneumonia (53%), followed by cardiac-related deaths (21%). Compared with controls, patients with PD had a greater risk of pneumonia (2.03, 95% CI 1.34 to 3.6), poorer HRQoL and more likely to reside in institutional care at death (P<0.01).
Conclusion
This investigation found that PD was associated with an excess risk of mortality compared with the general population. However, PD as a primary or underlying cause of death recorded on certificates was found to be suboptimal. This suggests that the quality of mortality statistics drawn from death certificates alone is not a valid or reliable source of data.
Keywords: parkinson’s disease, mortality, death certification, dementia
Strengths and limitations of this study.
This study employs a community-based, longitudinal, follow-up cohort design.
All patients fulfilled the diagnostic criteria for Parkinson’s disease and/or dementia.
Baseline and subsequent repeated measure data capture allowed the analysis for predictive outcomes.
The cohort included prevalent and new cases of Parkinson’s disease. Although the varying disease duration in the Parkinson’s disease cohort is a possible source of bias, we found no differences in survival between the two groups.
The control cohort included subjects without known neurological conditions, and thus may have introduced bias in the outcome comparisons with patients with Parkinson’s disease.
Introduction
Data drawn from death certificates are often employed by epidemiological, public health and research scientists to capture the incidence, prevalence and mortality in populations. In addition, these statistics are often used in the evaluation of public health interventions, setting priorities for medical research and health services, planning of health services, and clinical assessment of the effectiveness of those services.1–3 The introduction of the revised International Classification of Diseases (ICD) system in 2001 aimed to improve the accuracy in the reported cause of death, where underlying conditions, mentioned in part 1 or 2 of death certificates, take priority over others.4 5 The underlying principle for this is that reporting of multiple causes of death should provide a better description of a particular disease or condition, allowing for more effective and meaningful data capture. The reliability of statistical information extracted from death certificates remains uncertain, where for example rather than the underlying chronic condition being reported, a secondary cause of death is often reported as the main cause of death.6–14
The projected elderly population demographic changes worldwide, along with exponential rises in chronic conditions, will most likely place greater social and fiscal demands on existing clinical, health and social services.15 To ensure that mortality and survival rates are more precisely captured for these chronic conditions, the relative contributions that different diseases have on survival and mortality need to be more accurately measured. The challenge is to ensure that vital health statistics collected are valid and reliable enough to allow for more efficient planning for healthcare services and clinical interventions. Parkinson’s disease (PD) is a progressive neurodegenerative disease strongly associated with increased mortality and lower life expectancy compared with the general population.16 In addition to the motor symptoms of PD, many patients often live with a significant number of other non-motor conditions that contribute to the symptomatology of the disease.17–22 In particular, dementia occurs frequently in elderly patients with PD and has been shown to be a strong predictor of increased mortality.23–33 This most probably has implications for the quality of death certification, which in previous investigations has been found to be inconsistent, under-recorded or an inaccurate record of the cause of death in patients.16 34–38 The methodological design of previous investigations, where cohorts have been drawn from clinical populations or pharmaceutical trials alone, may partially explain the variability between studies.39 40 Only a small number of investigations have employed prospective community study methods to ascertain the utility of death certification in PD, and furthermore few have included a comparison control group.
This investigation is a report of the outcomes from a community cohort of patients with PD and controls (without neurological disease) who have been regularly followed over the past 18 years in the county of Denbighshire in UK. It aims first to examine the reported cause and quality of death certification in these cohorts. Second, it will explore if PD and/or dementia are reported as a cause or underlying cause of death on certificates. Third, the demographic and motor and non-motor symptoms of PD will be explored to establish if they are associated with, or predictive of, an increased risk of mortality.
Methods
Subjects
The patient and control recruitment methodology has been described in greater depth in previous reports.25 41 In brief, between December 1994 and January 1997, employing multiple sources of ascertainment, we recruited patients with newly diagnosed PD and patients with an existing diagnosis of probable PD based on the United Kingdom Parkinson’s Disease Society Brain Bank criteria (UKPDSBB).42 General practitioner (GP) records (n=74) in a defined area of North Wales (Denbighshire) were used to identify individuals in receipt of a defined group of antiparkinsonian drugs, which included levodopa, monoamine oxidase B inhibitors, dopamine agonists and antimuscarinic drugs. Additionally, hospital records were examined and patients who were not on active but known to medical services were also ascertained. In total, 402 patients were identified, of whom 213 fulfilled the criteria for clinically probable PD (n=213). Of the original PD cohort, 25 died before they could be consented into the investigation, 13 withdrew consent and the remaining patients (n=9) were lost to follow-up. This left at study entry 166 patients with probable PD for follow-up from December 1997 to January 2015.
The control cohort was randomly drawn from two GP practices within the same geographical area of the PD cohort and within the same time frame. The controls were matched for sex and age to patients with PD (±3 years), were not known to have a diagnosis of clinically probable PD, parkinsonism, Alzheimer’s or other dementia, stroke, and neurological disorder, not in receipt of psychoactive drugs, and with no known psychiatric, alcohol or substance abuse history. One hundred and sixty-four controls were invited to participate in the study, of whom 42 subsequently declined to participate and a further 6 withdrew consent at a later date. On initial baseline screening, eight were found to have previously suffered from a stroke, two had signs of parkinsonism, and four fulfilled the criteria for dementia and were excluded from further analysis, leaving a cohort of 102 control subjects.
Clinical assessment
The demographic details of PD and control cohorts were recorded, which included, age, gender, educational attainment, social class and smoking history. In addition to the demographic details, PD-specific variables were also recorded, which included age of diagnosis, duration of symptoms, Hoehn and Yahr staging, the Unified Parkinson’s Disease Rating Scale (UPDRS) motor subsection, the 15-item Geriatric Depression Scale (GDS-15), the Cambridge Examination for Mental Disorders of the Elderly, section B, Cambridge Cognition Examination (CAMCOG), the Parkinson’s Disease Activities of Daily Living Scale and the health-related quality of life (HRQoL) measure the EuroQol-5D (EQ-5D).43–47 These measures were reassessed at approximately 2 yearly intervals from the midpoint of the recruitment phase of the cohorts. Diagnosis of PD based on the UKPDS Brain Bank criteria was reassessed (RJM) at review to ensure diagnostic accuracy was maintained. The control cohort screening was also carried out approximately every 2 years from study entry, which included review and updating of demographic variables, and reassessment using the GDS-15, CAMCOG and EQ5-D. Analysis of the clinical assessments was the most recent prior to a subject’s reported death. The diagnosis of dementia for PD and controls was based on neuropsychological assessment, and patient and carer/informant interviews, along with the application of the Diagnostic and Statistical Manual of Mental Disorders 4th Edition criteria.48
Death certification collection and evaluation
Hospital and primary care records were reviewed to ascertain the number of individuals who were deceased. All of the death certificates in this investigation were obtained from the local births, deaths and marriages central record office for the PD and control cohorts. Primary and underlying causes of death, along with age of the subject and age of death, are recorded on all certificates in the UK. In addition, all certificates are completed by a doctor within the UK and are coded using the ICD-10 system as follows:
I(a): disease or condition leading directly to death
I(b): other disease or condition, if any, leading to I(a)
I(c): other disease or condition, if any, leading to I(b)
II: other significant conditions contributing to death but not related to the disease or condition causing it.
From the information recorded on the death certificates, we grouped primary and underlying causes of death into nine further categories, which were PD, sepsis, dementia, cerebrovascular, cardiac, cancer, pneumonia, chronic lung disease and other disorders.
Statistics
The age-specific and gender-specific standardised mortality ratios (SMR) were calculated by dividing the observed deaths in each cohort by the expected number of deaths. This is calculated by multiplying the number of person-years for each 5-year age group, gender and year by the corresponding general population age group, gender and year drawn from the UK Office of National Statistics 2016 interim life tables.
Descriptive statistics (mean, SD, median) were used for continuous variables, whereas categorical variables were described as percentages of subjects in each group. Student’s t-tests, χ2 test and univariate log-rank statistics were employed to examine between-group differences and between observed and expected survival curves. The relative risk (RR) was calculated by dividing the probability of an event occurring for PD cohort by the probability of an event occurring for the control cohort. Survival time was calculated from the date of baseline examination for each subject. The Kaplan-Meier estimates were used to calculate the observed survival curves. Cox proportional hazards (PH) analysis was used to investigate the effect of several variables on the time it takes for a specified event to happen. To satisfy the assumptions for PH modelling, visual inspections of the Kaplan-Meier curves were made. The Cox PH modelling was also employed to calculate the HR and the 95% CIs of the differences between groups defined by demographic and clinical features at baseline. The PH model covariates act as factors multiplying the HR, which is the probability of experiencing the event, which in this study is death. The PH model covariates included age at study entry, age at death, gender, motor function, mood, HRQoL and cognitive function. The HR reported in this study provides an estimate of the RR.
All data were analysed using the SPSS V.19 statistical package, and the RR and 95% CIs were calculated using the Altman formula and MedCalc software.49–51 The level of significance was set at P<0.05.
Results
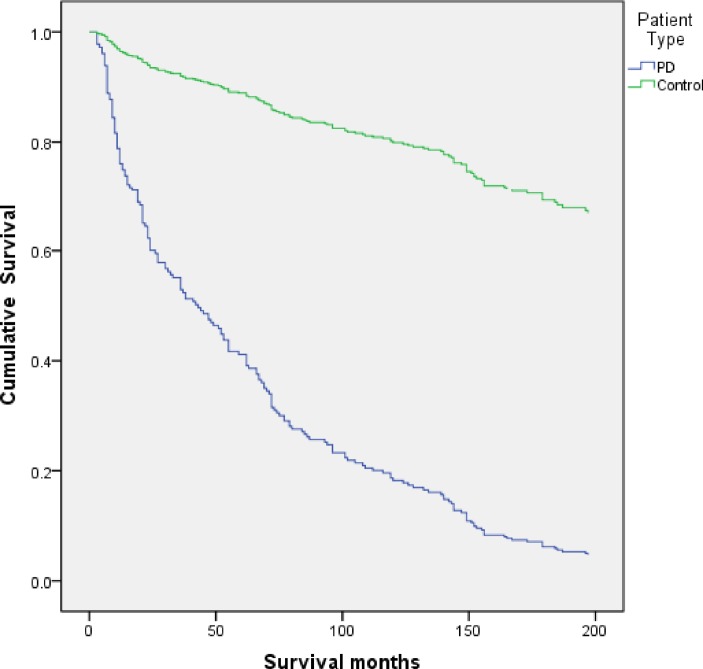
From baseline to the study end date (30 January 2015), 158/166 (96%) of the PD and 34/102 (33%) of the control cohorts were decedents. Table 1 shows the demographic and clinical outcomes of the PD and control cohorts. Figure 1 illustrates the Kaplan-Meier survival curves for all-cause mortality in the PD and control cohorts. The SMR for the whole PD cohort was 1.82 (95% CI 1.55 to 2.13). A subgroup analysis between new cases of PD (n=80) identified during the ascertainment phase of the investigation and existing cases of PD (n=78) revealed no excess mortality between the groups (P=0.186) despite the varying disease duration. By 18 years the cumulative survival in the PD cohort (figure 1) was approximately 5% and in the control cohort 67%. The mortality risk controlled for age and gender showed significantly higher risk in the PD cohort (HR 7.89, P=0.0001). Older age at entry into the current study was predictive of an increased risk of mortality in both cohorts (PD: HR 1.06, P=0.0001; control: HR 1.09, P=0.009). There were no statistical differences found in age at death between the PD and control cohorts (PD cohort: 80.7 (7.1); control cohort: 81.9 (6.3); P=0.552). The strongest predictor associated with mortality in the PD cohort after controlling for age and gender was worsening motor symptoms (HR 1.06, P<0.01).
Table 1.
Demographic and clinical outcomes (mean, SD) of the PD and control cohorts
| PD | Control | P<0.05 | |
| Gender (female, %) | 44 | 41 | NS |
| Age (entry into study) | 74.2 (8.6) | 74.8 (6.6) | NS |
| Age at death | 80.7 (7.1) | 81.9 (6.3) | NS |
| Institutional care (%) | 52 | 30 | 0.003 |
| Place of death (hospital) (%) | 37 | 74 | 0.002 |
| GDS-15 | 5.6 (2.2) | 3.7 (1.1) | 0.001 |
| EQ-5D (weighted health) | 0.58 (0.36) | 0.79 (0.28) | 0.001 |
| EQ-5D (VAS, %) | 55 (16.5) | 77 (17.6) | 0.001 |
| Onset of PD | 67.3 (10.7) | – | – |
| Duration of PD | 13.2 (8.8) | – | – |
| UPDRS (motor section) | 27.9 (11.7) | – | – |
| H&Y | 2.9 (0.74) | – | – |
| PADL | 3.1 (1.1) | – | – |
EQ-5D, EuroQol-5D; GDS-15, 15-Item Geriatric Depression Scale; H&Y, Hoehn and Yahr staging; PD, Parkinson’s disease; UPDRS, Unified Parkinson’s Disease Rating Scale; VAS, Visual Analogue Scale.
Figure 1.
Kaplan-Meier survival curves for all-cause mortality in the Parkinson’s disease (PD) and control cohorts.
As a primary cause of death (part 1(a) on UK death certificates), PD was recorded in just over 4% of the cohort. In sections 1(b) and 1(c) (conditions substantially contributing to death), PD was reported in 24% and 6% of cases, respectively. In section II of the death certificates (comorbid conditions substantially contributing to death), PD was reported in 19% of cases. Overall, PD as a contributing factor in the cause of death was not reported anywhere on 75/158 (47%) of the PD cohort’s certificates. The primary cause of death for the PD and control cohorts is shown in table 2. The most common causes of death reported within the PD cohort were pneumonia (53%), followed by cardiac-related deaths (21%). The most frequently recorded causes of death within the control cohort were cardiac disease (26%), cancer (24%) and pneumonia (18%). A comparison between the PD and control cohorts revealed that the PD cohort was nearly three times more likely to have pneumonia recorded as a primary cause of death (RR, 2.94, 95% CI 1.40 to 6.19). Controlling for patients and controls with dementia still revealed a higher risk of mortality in the PD cohort for pneumonia (RR, 2.03, 95% CI 1.34 to 3.6). The controls compared with the PD cohort had over a threefold increased risk of having a cancer-related disorder recorded as a primary or underlying cause of death (RR, 3.72, 95% CI 1.58 to 8.72). Examining the smoking history between the two cohorts found no significant differences in terms of frequency of current or former smokers (P=0.39), nor were there differences in cancer risk observed between the PD and control cohorts who never smoked (P=0.75). However, significantly more of the controls who were smokers prior to their death had cancer recorded as a primary or secondary cause of death (P<0.025).
Table 2.
Primary cause of death (part 1a of death certificates) reported for the PD (n=158) and control (n=34) cohorts
| PD | Control | |
| Pneumonia | 84 (53.2%) | 6 (17.6%) |
| Cardiac | 33 (20.9%) | 9 (26.5%) |
| Cancer | 10 (6.3%) | 8 (23.5%) |
| Cerebrovascular | 12 (7.6%) | 4 (11.8%) |
| PD | 7 (4.4%) | 0 |
| Other* | 12 | 7 |
*Other includes sepsis, dementia, immobility, chronic obstructive pulmonary disease, old age, fracture neck of femur, multiorgan failure and motor neuron disease.
PD, Parkinson’s disease.
Disease progression within the PD cohort was significantly associated with a worsening HRQoL at death (P<0.0001). When compared with the controls, HRQoL was significantly poorer for the PD cohort (P<0.001). At the time of death, 83/158 (52%) of the PD and 9/34 (26%) of the control cohorts were living in institutional care (P<0.003). Overall, the PD cohort decedents had a threefold increased probability of living in institutional care at death (RR 3.23, 95% CI 1.4 to 7.41). Controlling for age and duration of illness, patients in the PD cohort living in institutional care were also more likely to be demented (RR 2.7, 95% CI 1.21 to 5.76). In contrast to the PD cohort, the control decedent’s place of death was more likely to be in the hospital (RR 1.97, 95% CI 1.48 to 2.62).
As a primary or underlying cause of death, dementia was under-reported on the certificates of both the PD and control cohorts. Although 144/158 (91%) of the PD cohort had a diagnosis of dementia before their deaths, it was reported in only 14/144 of certificates. Similarly, only two of the control cohort had dementia recorded anywhere as a primary or underlying cause of death. On review, however, a further four at the time of death had a confirmed diagnosis of dementia.
Discussion
This investigation reports the cause of death recorded on the death certificates of PD and age-matched control cohorts in Denbighshire in UK. We have previously reported that the life expectancy and average age at death in this PD cohort are much lower than the general population.16 In the current study, the overall SMR for our PD cohort was 1.82, indicating an excess mortality. This is similar to previous investigations where SMR has been reported to range from 0.9 to 3.8.16 However, recent community-based incident cohort and incident clinical cohort investigations have reported lower SMRs of 1.29 (95% CI 0.97 to 1.61) and 1.39 (95% CI 1.10 to 1.50), suggesting a moderately increased mortality compared with the general population.16 52 The shorter duration of PD diagnosis, lower number of recorded deaths and shorter follow-up period compared with the current investigation may partially explain the differences between the current and previously reported UK investigations. In addition, other European investigations were limited by the retrospective analysis of a data set from 1978 to 1998 and recruitment solely from a clinical population.
Overall death certification and clinical research data appear to provide disparate mortality data in PD. Although our PD cohort had confirmed UKPDSBB criteria for probable PD, as a primary cause of death (part 1(a)), it was recorded in just over 4% of the cohort. A further 30% had PD recorded in parts 1(b) and 1(c) of their death certificates, and on part II of certificates it was recorded in a further 19% of cases. Overall, PD was not cited anywhere on 47% of the death certificates, which falls approximately mid-way with previous certification studies of between 14% and 70%.16 34–38 The disparity reported between studies is most likely evidence of the differing methodologies employed, such as populations drawn from pharmaceutical trials alone, clinical samples, or retrospective case or chart record analysis.
Pneumonia was the most cited primary cause of death (52%) in the current study. This observation has also been frequently reported in other investigations.16 52 Patients with PD, particularly as they become frailer with the progression of their illness, are at greater risk for pulmonary complications, due to obstructive ventilation dysfunction, upper airway dysfunction and weakened respiratory muscles.53–55 The most frequently reported other causes of death were cardiovascular disease (21%), cerebrovascular disease (8%) and malignancy (6%).
This is the first study, to our knowledge, to describe under-reporting of dementia as a primary or underlying cause of death in a community-based PD cohort. We have previously reported in this cohort the high prevalence of dementia of around 90%.56 On review of the decedents’ death certificates, we found that less than 10% had any mention of dementia as an immediate or underlying cause of death. Previous general population investigations have also shown that rates of certification mentioning dementia as a main or underlying cause of death have been consistently under-reported.57–61 This perhaps is because death certification tends to focus on the immediate cause of death and does not really capture the multiple factors that contribute to death, particularly with elderly individuals with multiple comorbidities.
This investigation found that the PD cohort was more likely than the controls to be living in a long-term care setting before death. This may be a reflection of the duration, nature and the type of burden PD places on relatives, especially those who have physical frailty themselves. Caring within the home setting may therefore become impracticable and thus possibly precipitate entry into institutional care. The proportion of deaths in long-term care among patients in the PD cohort was also significantly higher than in the control cohort. A recent study reported wide variations in the place of death of people with PD throughout the world, concluding that individual preference, social and socioeconomic circumstances, cultural organisation, and provision of health and palliative care all contribute, to some extent, to the place of death.62
In common with previous mortality investigations, we found fewer recorded cancer deaths within the PD cohort.63 We did not observe differences in the current or smoking history frequency between our cohorts. Similarly, no differences were revealed between the cohorts and cancer risk in those who had never smoked. There were no differences seen on death certificates in terms of cancer type between smokers and non-smokers. However, in the group of current smokers, significantly more cancer-associated deaths were recorded in the control compared with the PD cohort. A possible explanation for this disparity may be that mutations of the PARK2 (Parkin) gene found in 6%–8% of patients with PD may act in some cancers as a tumour suppressor proteins.64 The absence or mutation of the Parkin gene is found in several tumour types, suggesting that the mechanisms of cell death in PD may play a role in the inhibition or formation of some cancers.64 65 The decreased risk of mortality from cancer has also been reported in other neurodegenerative diseases including Alzheimer’s and Huntington’s diseases, and in populations where mild to moderate cognitive impairment has also been observed.66–69 Further studies are needed to explore the associations, risks, possible genetic markers and underlying mechanisms of PD and other neurodegenerative conditions to improve and identify and understand the role of cell death and its decreased cancer risk.
We would caution the message often given to patients with PD that they die with, rather than die of, the condition. The non-motor features of PD such as dementia and autonomic dysfunction are frequently observed in all stages of the disease and most likely make a significant contribution to mortality.70 One recent investigation reported that autonomic dysfunction and dementia in PD were predictive of increased mortality particularly in patients with orthostatic hypotension (OH).71 Another meta-analysis that explored the association between OH and mortality in general populations also concluded that OH may confer a greater risk of mortality (RR, 1.40).72 The association between the non-motor features of PD disease and mortality needs further research to understand and determine if these are causal or not.
The strengths of this study include its robust follow-up over an 18-year period of a community-based cohort, all of whom fulfilled the criteria for PD, and that diagnostic re-evaluation was reviewed regularly over this period to ensure diagnostic accuracy. The repeated measure design of the study also allowed us to control for demographics, and motor and non-motor symptomatology. The limitations of the study are that the PD cohort had a mix of prevalent and incident cases, thus possibly overestimating the possible causal associations with mortality. However, controlling for prevalent and incident cases in our analysis did not reveal any between-group significant differences. Our control cohort was selected carefully in terms of age, gender and disease exposure, and may not be representative of the general population. We endeavoured to reduce this potential bias by randomly selecting controls from GP lists within defined catchment area as the PD cohort. However, excluding controls with known neurological, psychiatric illness and possibly better general health may be a potential source of error. The ideal comparison group in a cohort study would be the same as the cohort of interest, except that they would not have the condition under investigation. In older populations, the selection of any control group is often a compromise in ensuring that the control group differs enough with respect to the condition of interest, yet are similar as possible to explore what other factors influence the outcome under investigation. We believe that our selection of controls without neurological or psychiatric disease allowed us to control for confounding factors in the analysis in both cohorts, which would not have been possible in the general population.
Conclusions
In light of our findings, we feel that the current methods of capturing the cause of death from certification alone significantly underestimate the true population burden of PD. The under-reporting of dementia as an underlying cause of death in this cohort in addition to PD also suggests that the interpretation of and quality of mortality data currently are not a valid or reliable source of data. Furthermore, we would admonish the use of mortality statistics alone to plan for future service provision in this patient population.
Supplementary Material
Footnotes
Contributors: PH and RJM conceived and designed the study, collected the data and managed the database. PH managed the database, contributed to data cleaning and performed the statistical analyses. PH and RJM contributed to interpretation of the data and wrote the manuscript. Both authors critically reviewed the manuscript and approved the final version to be published.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: This study was approved by the North Wales Research and Ethics Committee (Central).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data available.
References
- 1.Devis T, Rooney C. Death certification and the epidemiologist. Health Statistics Quarterly 1999;1:21–33. [Google Scholar]
- 2.Hanzlick R. Cause of death and the death certificate: Important information for physicians, coroners, medical examiners, and the public. Northfield, IL: College of American Pathologists, 2006. [Google Scholar]
- 3.World Health Organisation. International classification of diseases and related health problems. Tenth Revision Geneva: WHO, 1992. [Google Scholar]
- 4.Rooney C, Smith S. Implementation of ICD-10 for mortality data in England and Wales from January 2001. Health Statistics Quarterly;2000:41–50. [Google Scholar]
- 5.Rooney C, Griffiths C, Cook L. Implementation of ICD-10 for cause of death coding – some preliminary results from the bridge coding stud. Health Statistics Quarterly 2002;13:31–41. [Google Scholar]
- 6.Nielsen GP, Björnsson J, Jonasson JG. The accuracy of death certificates. Implications for health statistics. Virchows Arch A Pathol Anat Histopathol 1991;419:143–6. [DOI] [PubMed] [Google Scholar]
- 7.Sington JD, Cottrell BJ. Analysis of the sensitivity of death certificates in 440 hospital deaths: a comparison with necropsy findings. J Clin Pathol 2002;55:499–502. 10.1136/jcp.55.7.499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nashelsky MB, Lawrence CH. Accuracy of cause of death determination without forensic autopsy examination. Am J Forensic Med Pathol 2003;24:313–9. 10.1097/01.paf.0000097857.50734.c3 [DOI] [PubMed] [Google Scholar]
- 9.Smith Sehdev AE, Hutchins GM. Problems with proper completion and accuracy of the cause-of-death statement. Arch Intern Med 2001;161:277–84. 10.1001/archinte.161.2.277 [DOI] [PubMed] [Google Scholar]
- 10.Shibuya K, Scheele S, Boerma T. Health statistics: time to get serious. Bull World Health Organ 2005;83:722 doi:/S0042-96862005001000002 [PMC free article] [PubMed] [Google Scholar]
- 11.Cambridge B, Cina SJ. The accuracy of death certificate completion in a suburban community. Am J Forensic Med Pathol 2010;31:232–5. 10.1097/PAF.0b013e3181e5e0e2 [DOI] [PubMed] [Google Scholar]
- 12.Lu TH, Anderson RN, Kawachi I. Trends in frequency of reporting improper diabetes-related cause-of-death statements on death certificates, 1985-2005: An algorithm to identify incorrect causal sequences. Am J Epidemiol 2010;171:1069–78. 10.1093/aje/kwq057 [DOI] [PubMed] [Google Scholar]
- 13.Drummond MB, Wise RA, John M, et al. . Accuracy of death certificates in COPD: analysis from the TORCH trial. COPD 2010;7:179–85. 10.3109/15412555.2010.481695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rampatige R, Mikkelsen L, Hernandez B, et al. . Systematic review of statistics on causes of deaths in hospitals: strengthening the evidence for policy-makers. Bull World Health Organ 2014;92:807–16. 10.2471/BLT.14.137935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. World report on ageing and health. Geneva: WHO, 2015. [Google Scholar]
- 16.Macleod AD, Taylor KS, Counsell CE. Mortality in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 2014;29:1615–22. 10.1002/mds.25898 [DOI] [PubMed] [Google Scholar]
- 17.Hobson P, Islam W, Roberts S, et al. . The risk of bladder and autonomic dysfunction in a community cohort of Parkinson’s disease patients and normal controls. Parkinsonism Relat Disord 2003;10:67–71. 10.1016/j.parkreldis.2003.07.001 [DOI] [PubMed] [Google Scholar]
- 18.Hely MA, Morris JG, Reid WG, et al. . Sydney Multicenter Study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord 2005;20:190–9. 10.1002/mds.20324 [DOI] [PubMed] [Google Scholar]
- 19.Weerkamp NJ, Tissingh G, Poels PJ, et al. . Nonmotor symptoms in nursing home residents with Parkinson’s disease: prevalence and effect on quality of life. J Am Geriatr Soc 2013;61:1714–21. 10.1111/jgs.12458 [DOI] [PubMed] [Google Scholar]
- 20.Storch A, Schneider CB, Wolz M, et al. . Nonmotor fluctuations in Parkinson disease: severity and correlation with motor complications. Neurology 2013;80:800–9. 10.1212/WNL.0b013e318285c0ed [DOI] [PubMed] [Google Scholar]
- 21.Marras C, Chaudhuri KR. Nonmotor features of Parkinson’s disease subtypes. Mov Disord 2016;31:1095–102. 10.1002/mds.26510 [DOI] [PubMed] [Google Scholar]
- 22.Schapira AHV, Chaudhuri KR, Jenner P, et al. . Non-motor features of Parkinson disease. Nat Rev Neurosci 2017;18:435–50. 10.1038/nrn.2017.62 [DOI] [PubMed] [Google Scholar]
- 23.Aarsland D, Andersen K, Larsen JP, et al. . Risk of dementia in Parkinson’s disease: a community-based, prospective study. Neurology 2001;56:730–6. 10.1212/WNL.56.6.730 [DOI] [PubMed] [Google Scholar]
- 24.Foltynie T, Brayne CE, Robbins TW, et al. . The cognitive ability of an incident cohort of Parkinson’s patients in the UK. The CamPaIGN study. Brain 2004;127:550–60. 10.1093/brain/awh067 [DOI] [PubMed] [Google Scholar]
- 25.Hobson P, Meara J. Risk and incidence of dementia in a cohort of older subjects with Parkinson’s disease in the United Kingdom. Mov Disord 2004;19:1043–9. 10.1002/mds.20216 [DOI] [PubMed] [Google Scholar]
- 26.Hely MA, Reid WG, Adena MA, et al. . The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord 2008;23:837–44. 10.1002/mds.21956 [DOI] [PubMed] [Google Scholar]
- 27.Williams-Gray CH, Evans JR, Goris A, et al. . The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain 2009;132:2958–69. 10.1093/brain/awp245 [DOI] [PubMed] [Google Scholar]
- 28.Williams-Gray CH, Foltynie T, Brayne CE, et al. . Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain 2007;130:1787–98. 10.1093/brain/awm111 [DOI] [PubMed] [Google Scholar]
- 29.Nussbaum M, Treves TA, Inzelberg R, et al. . Survival in Parkinson’s disease: the effect of dementia. Parkinsonism Relat Disord 1998;4:179–81. 10.1016/S1353-8020(98)00039-X [DOI] [PubMed] [Google Scholar]
- 30.Levy G, Tang MX, Louis ED, et al. . The association of incident dementia with mortality in PD. Neurology 2002;59:1708–13. 10.1212/01.WNL.0000036610.36834.E0 [DOI] [PubMed] [Google Scholar]
- 31.Hughes TA, Ross HF, Mindham RH, et al. . Mortality in Parkinson’s disease and its association with dementia and depression. Acta Neurol Scand 2004;110:118–23. 10.1111/j.1600-0404.2004.00292.x [DOI] [PubMed] [Google Scholar]
- 32.de Lau LM, Schipper CM, Hofman A, et al. . Prognosis of Parkinson disease: risk of dementia and mortality: the Rotterdam Study. Arch Neurol 2005;62:1265–9. 10.1001/archneur.62.8.1265 [DOI] [PubMed] [Google Scholar]
- 33.Buter TC, van den Hout A, Matthews FE, et al. . Dementia and survival in Parkinson disease: a 12-year population study. Neurology 2008;70:1017–22. 10.1212/01.wnl.0000306632.43729.24 [DOI] [PubMed] [Google Scholar]
- 34.Paulson GW, Gill WM. Are death certificates reliable to estimate the incidence of Parkinson’s disease? Mov Disord 1995;10:587–8. 10.1002/mds.870100525 [DOI] [PubMed] [Google Scholar]
- 35.Phillips NJ, Reay J, Martyn CN. Validity of mortality data for Parkinson’s disease. J Epidemiol Community Health 1999;53:587–8. 10.1136/jech.53.9.587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pressley JC, Tang MX, Marder K, et al. . Disparities in the recording of Parkinson’s disease on death certificates. Mov Disord 2005;20:315–21. 10.1002/mds.20339 [DOI] [PubMed] [Google Scholar]
- 37.Benito-León J, Louis ED, Villarejo-Galende A, et al. . Under-reporting of Parkinson’s disease on death certificates: a population-based study (NEDICES). J Neurol Sci 2014;347:188–92. 10.1016/j.jns.2014.08.048 [DOI] [PubMed] [Google Scholar]
- 38.Donnan PT, Steinke DT, Stubbings C, et al. . Selegiline and mortality in subjects with Parkinson’s disease: a longitudinal community study. Neurology 2000;55:1785–9. 10.1212/WNL.55.12.1785 [DOI] [PubMed] [Google Scholar]
- 39.Marras C, McDermott MP, Rochon PA, et al. . Survival in Parkinson disease: thirteen-year follow-up of the DATATOP cohort. Neurology 2005;64:87–93. 10.1212/01.WNL.0000148603.44618.19 [DOI] [PubMed] [Google Scholar]
- 40.Chen H, Zhang SM, Schwarzschild MA, et al. . Survival of Parkinson’s Disease in a Large Prospective cohort of male Health Professionals. Mov Disord 2006;27:1002–7. [DOI] [PubMed] [Google Scholar]
- 41.Meara RJ, Bhowmick BK, Hobson JP. Accuracy of diagnosis in patients with presumed Parkinson’s disease in a community based disease register. Age Ageing 1999;28:99–02. [DOI] [PubMed] [Google Scholar]
- 42.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 1988;51:745–52. 10.1136/jnnp.51.6.745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fahn S, Elton RL. et al. , Members of the UPDRS Development Committee. Unified Parkinson’s Disease Rating Scale : Fahn S, Marsden CD, Calne DB, Goldstein M, Recent developments in Parkinson’s disease. Florham Park, NJ: Macmillan Health Care Information, 1987;Volume 2:153–64. [Google Scholar]
- 44.Sheikh JI, Yesavage JA, Geriatric Depression Scale (GDS). Recent evidence and development of a shorter version Brink TL, Clinical gerontology: A guide to assessment and intervention. New York: The Haworth Press, Inc., 1986:165–73. [Google Scholar]
- 45.Roth M, Tym E, Mountjoy CQ, et al. . CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry 1986;149:698–9. 10.1192/bjp.149.6.698 [DOI] [PubMed] [Google Scholar]
- 46.Hobson JP, Edwards NI, Meara RJ. The Parkinson’s Disease Activities of Daily Living Scale: a new simple and brief subjective measure of disability in Parkinson’s disease. Clin Rehabil 2001;15:241–6. 10.1191/026921501666767060 [DOI] [PubMed] [Google Scholar]
- 47.Williams A; EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 48.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed Washington, DC: Author, 2000. [Google Scholar]
- 49.IBM Corp. IBM SPSS Statistics for Windows. Version 19.0 Armonk, NY: IBM Corp; 2010. [Google Scholar]
- 50.Altman DG. Practical statistics for medical research. London: Chapman and Hall, 1991. [Google Scholar]
- 51. MedCalc for Windows. version 15.0 Ostend, Belgium: MedCalc Software. [Google Scholar]
- 52.Duarte J, García Olmos LM, Mendoza A, et al. . The natural history of Parkinson’s disease in the province of Segovia: mortality in a longitudinal study (20-year follow-up). Acta Neurol Scand 2013;127:295–300. 10.1111/ane.12003 [DOI] [PubMed] [Google Scholar]
- 53.D’Amelio M, Ragonese P, Morgante L, et al. . Long-term survival of Parkinson’s disease: a population-based study. J Neurol 2006;253:33–7. 10.1007/s00415-005-0916-7 [DOI] [PubMed] [Google Scholar]
- 54.Estenne M, Hubert M, De Troyer A. Respiratory-muscle involvement in Parkinson’s disease. N Engl J Med 1984;311:1516–7. 10.1056/NEJM198412063112314 [DOI] [PubMed] [Google Scholar]
- 55.Sabaté M, González I, Ruperez F, et al. . Obstructive and restrictive pulmonary dysfunctions in Parkinson’s disease. J Neurol Sci 1996;138:114–9. 10.1016/0022-510X(96)00003-2 [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Shao WB, Gao L, et al. . Abnormal pulmonary function and respiratory muscle strength findings in Chinese patients with Parkinson’s disease and multiple system atrophy-comparison with normal elderly. PLoS One 2014;9:e116123 10.1371/journal.pone.0116123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hobson JP, Meara RJ. The Denbighshire cohort risk and incidence of Mild Cognitive Impairment and its progression to Parkinson’s disease dementia at 16-years. Int J Geriatr Psychiatr 2015;30:1048–55. [DOI] [PubMed] [Google Scholar]
- 58.Romero JP, Benito-León J, Mitchell AJ, et al. . Under reporting of dementia deaths on death certificates using data from a population-based study (NEDICES). J Alzheimers Dis 2014;39:741–8. 10.3233/JAD-131622 [DOI] [PubMed] [Google Scholar]
- 59.Sleeman KE, Ho YK, Verne J, et al. . Reversal of English trend towards hospital death in dementia: a population-based study of place of death and associated individual and regional factors, 2001-2010. BMC Neurol 2014;14:59 10.1186/1471-2377-14-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perera G, Stewart R, Higginson IJ, et al. . Reporting of clinically diagnosed dementia on death certificates: retrospective cohort study. Age Ageing 2016;45:667–72. 10.1093/ageing/afw077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia-Ptacek S, Kåreholt I, Cermakova P, et al. . causes of death according to death certificates in individuals with dementia: a cohort from the swedish dementia registry. J Am Geriatr Soc 2016;64:e137–42. 10.1111/jgs.14421 [DOI] [PubMed] [Google Scholar]
- 62.Moens K, Houttekier D, Van den Block L, et al. . Place of death of people living with Parkinson’s disease: a population-level study in 11 countries. BMC Palliat Care 2015;14:28 10.1186/s12904-015-0021-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bajaj A, Driver JA, Schernhammer ES. Parkinson’s disease and cancer risk: a systematic review and meta-analysis. Cancer Causes Control 2010;21:697–707. 10.1007/s10552-009-9497-6 [DOI] [PubMed] [Google Scholar]
- 64.Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat Rev Neurosci 2006;7:207–19. 10.1038/nrn1868 [DOI] [PubMed] [Google Scholar]
- 65.Dawson TM, Dawson VL. The role of parkin in familial and sporadic Parkinson’s disease. Mov Disord 2010;25 Suppl 1:S32–S39. 10.1002/mds.22798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sørensen SA, Fenger K. Causes of death in patients with Huntington’s disease and in unaffected first degree relatives. J Med Genet 1992;29:911–4. 10.1136/jmg.29.12.911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Romero JP, Benito-León J, Louis ED, et al. . Alzheimer’s disease is associated with decreased risk of cancer-specific mortality: a prospective study (NEDICES). J Alzheimers Dis 2014;40:465–73. 10.3233/JAD-132048 [DOI] [PubMed] [Google Scholar]
- 68.Driver JA, Beiser A, Au R, et al. . Inverse association between cancer and Alzheimer’s disease: results from the Framingham Heart Study. BMJ 2012;344:e1442 10.1136/bmj.e1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Benito-León J, Romero JP, Louis ED, et al. . Faster cognitive decline in elders without dementia and decreased risk of cancer mortality: NEDICES Study. Neurology 2014;82:1441–8. 10.1212/WNL.0000000000000350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goetz CG, Lutge W, Tanner CM. Autonomic dysfunction in Parkinson’s disease. Neurology 1986;36:73–5. 10.1212/WNL.36.1.73 [DOI] [PubMed] [Google Scholar]
- 71.Stubendorff K, Aarsland D, Minthon L, et al. . The impact of autonomic dysfunction on survival in patients with dementia with Lewy bodies and Parkinson’s disease with dementia. PLoS One 2012;7:e45451 10.1371/journal.pone.0045451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xin W, Lin Z, Mi S. Orthostatic hypotension and mortality risk: a meta-analysis of cohort studies. Heart 2014;100:406–13. 10.1136/heartjnl-2013-304121 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.