ABSTRACT
Cytochrome c oxidases are members of the heme-copper oxidase superfamily. These enzymes have different subunits, cofactors, and primary electron acceptors, yet they all contain identical heme-copper (CuB) binuclear centers within their catalytic subunits. The uptake and delivery pathways of the CuB atom incorporated into this active site, where oxygen is reduced to water, are not well understood. Our previous work with the facultative phototrophic bacterium Rhodobacter capsulatus indicated that the copper atom needed for the CuB site of cbb3-type cytochrome c oxidase (cbb3-Cox) is imported to the cytoplasm by a major facilitator superfamily-type transporter, CcoA. In this study, a comparative genomic analysis of CcoA orthologs in alphaproteobacterial genomes showed that CcoA is widespread among organisms and frequently co-occurs with cytochrome c oxidases. To define the specificity of CcoA activity, we investigated its function in Rhodobacter sphaeroides, a close relative of R. capsulatus that contains both cbb3- and aa3-Cox. Phenotypic, genetic, and biochemical characterization of mutants lacking CcoA showed that in its absence, or even in the presence of its bypass suppressors, only the production of cbb3-Cox and not that of aa3-Cox was affected. We therefore concluded that CcoA is dedicated solely to cbb3-Cox biogenesis, establishing that distinct copper uptake systems provide the CuB atoms to the catalytic sites of these two similar cytochrome c oxidases. These findings illustrate the large variety of strategies that organisms employ to ensure homeostasis and fine control of copper trafficking and delivery to the target cuproproteins under different physiological conditions.
KEYWORDS: CcoA, cytochrome, copper homeostasis, copper transport, copper uptake, cytochrome biogenesis, cytochrome c oxidase
IMPORTANCE
The cbb3- and aa3-type cytochrome c oxidases belong to the widespread heme-copper oxidase superfamily. They are membrane-integral cuproproteins that catalyze oxygen reduction to water under hypoxic and normoxic growth conditions. These enzymes diverge in terms of subunit and cofactor composition, yet they all share a conserved heme-copper binuclear site within their catalytic subunit. In this study, we show that the copper atoms of the catalytic center of two similar cytochrome c oxidases from this superfamily are provided by different copper uptake systems during their biogenesis. This finding illustrates different strategies by which organisms fine-tune the trafficking of copper, which is an essential but toxic micronutrient.
INTRODUCTION
Copper (Cu) is an important micronutrient required for the survival of virtually all living organisms, as numerous cellular processes depend on cuproproteins (1, 2). At high concentrations, Cu is extremely toxic for cells and can cause severe oxidative damage by competing with other divalent metal cations (e.g., iron) or by triggering the Fenton reaction (3, 4). Indeed, both Cu deficiency and excess cause serious human disorders, including Menkes, Wilson’s, and Alzheimer’s diseases (5–7). Therefore, Cu homeostasis is crucial for organisms, and cells tightly control their Cu content, from its uptake to its incorporation into cuproproteins (8, 9).
Heme-copper oxidases (HCOs) are major Cu-containing enzymes located in the cytoplasmic membranes of bacteria and archaea and in the mitochondrial inner membranes (10, 11). They are widespread among all domains of life as key components of the respiratory electron transport chain, catalyzing the reduction of oxygen to water while pumping protons across the membrane. HCOs are classified in three major families (A, B, and C) based on conserved residues forming the proton pathways within their catalytic subunit I (12). Although they differ in the number of subunits and cofactor composition, they all contain a conserved catalytic subunit carrying a low-spin heme and a heterobinuclear metal center composed of a Cu atom (referred to as CuB) and a high-spin heme. The type A aa3-type cytochrome (cyt) c oxidases (aa3-Cox) are present in mitochondria and widespread in bacteria and archaea (13, 14). Two of the subunits of aa3-Cox harbor all of the cofactors required for catalysis. Subunit I (Cox1) is the conserved membrane integral catalytic subunit, which contains a low-spin heme a and a heterobinuclear center composed of a high-spin heme a (heme a3) and a CuB atom. Subunit II (Cox2) contains a homobinuclear Cu center (CuA) that receives electrons from a cyt c donor. Depending on the species, the aa3-Cox enzymes may contain additional subunits with no cofactors. Biogenesis of the Cu centers of mitochondrial aa3-Cox requires plasma membrane-integral Cu transporters (known as Ctr) that import Cu into the cytoplasm and multiple chaperones (11, 15, 16). In yeast mitochondria, Cu is imported into the mitochondrial matrix before being inserted into aa3-Cox (17). The Cu chaperone Cox17 conveys the Cu in the mitochondrial intermembrane space to Sco1/Sco2 proteins for incorporation into the CuA center in Cox2 or to Cox11 for assembly of the CuB center in Cox1 subunits, respectively (18–21). In bacteria, periplasmic chaperones (e.g., PCuAC-like [22]) act as functional homologues of mitochondrial Cox17 and, together with the homologues of Sco1/Sco2 (SenC [23] or PrrC [24]), deliver Cu to the CuA center of aa3-Cox (22). Similarly, Cox11 homologues are required for the insertion of CuB into bacterial aa3-Cox, but its source of Cu remains unknown (24, 25).
Class C HCOs are cbb3-type cyt c oxidases (cbb3-Cox) that are found only in bacteria (26). They are the most divergent type of HCO and differ from class A aa3-Cox by containing three functional subunits (instead of two) and different cofactors (27). CcoN (subunit I) is the main catalytic subunit, which is the functional analogue of Cox1. It is an integral membrane protein and contains a low-spin heme b and a heterobinuclear center composed of a high-spin heme b (heme b3) coupled to the CuB atom. cbb3-Cox has no structural homologue of Cox2 or CuA center (27). Instead, it contains a dihemic cyt c subunit (CcoP), acting as the primary electron acceptor of cbb3-Cox. CcoP transfers the electrons via the monohemic cyt c subunit (CcoO) to the low-spin heme b and finally to the binuclear center heme b3-CuB of the CcoN subunit (13, 28). CuB atom incorporation into CcoN requires several transporters and chaperones (27). The P1B-type ATPase CcoI (29) (also known as CopA2 [30] or CtpA [31]), which is similar to the Cu-detoxifying transporter CopA (32) (or CopA1 [30]), is a Cu exporter located in the cytoplasmic membrane and is required for cbb3-Cox biogenesis. The fate of the Cu exported by CcoI is currently unclear. Possibly, it can be delivered either directly to the catalytic center of CcoN or to other periplasmic Cu chaperones. At low Cu availability, the Cu chaperones SenC (in R. capsulatus [23]) and PrrC (in R. sphaeroides [24]), which are homologues of mitochondrial Sco1/Sco2, and their interacting partners PccA (of R. capsulatus [33]) and PCuAC (of R. sphaeroides [24]) are needed for cbb3-Cox biogenesis. Finally, a member of the major facilitator superfamily (MFS) of transporters, CcoA, is a Cu importer that is required for assembly of the CuB center of cbb3-Cox (32, 34, 35). R. capsulatus mutants lacking CcoA are impaired for Cu uptake and contain a very small amount of cbb3-Cox (34). This importer is the first MFS member involved in metal transport and defines a new family of “copper uptake porters” (2.A.1.81) among the MFS-type transporters (http://www.tcdb.org/) (36). Recent studies showed that conserved Met and His residues of CcoA are important for its function, possibly acting as metal ligands (37). It is noteworthy that both the Cu importer CcoA and the Cu exporter CcoI are required to incorporate the CuB center into CcoN, implying trafficking of Cu across the cytoplasmic membrane during cbb3-Cox biogenesis (32).
In this study, we conducted comparative genomic analyses of CcoA orthologs in alphaproteobacterial genomes. This search revealed a higher degree of co-occurrence of CcoA with cbb3-Cox than with aa3-Cox, suggesting that CcoA activity is specific to class C HCOs. To test this hypothesis, we investigated the function of CcoA in R. sphaeroides, a close relative of R. capsulatus, which contains both functional cbb3- and aa3-Cox with identical heme-CuB binuclear centers, belonging to different HCO families. Upon the identification of R. sphaeroides ccoA (RSP_2726), appropriate mutants were constructed and their physiological and biochemical properties were characterized. We also identified bypass suppressors of ΔccoA mutants in R. sphaeroides copA (RSP_2890) that restored cbb3-Cox activity at the expense of increased Cu2+ sensitivity. This study showed that CcoA is specific to cbb3-Cox and is not involved in the biogenesis of aa3-Cox. Therefore, we concluded that the Cu atoms needed for the formation of the heme-CuB binuclear centers in these two similar enzymes must be provided by distinct Cu uptake pathways.
RESULTS
Distribution of CcoA homologues in alphaproteobacteria.
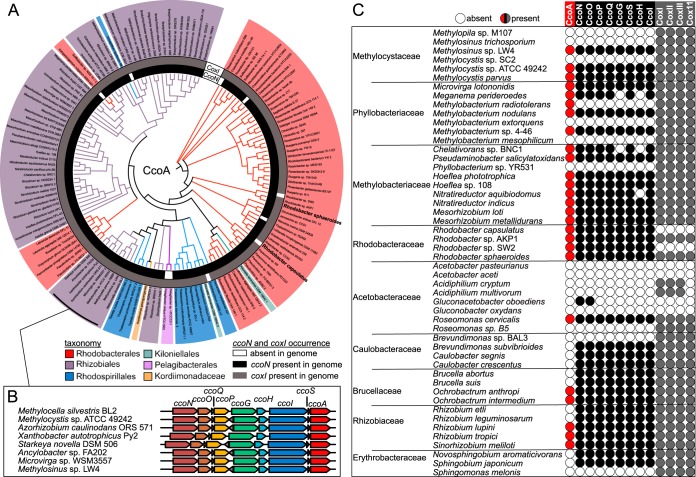
Using CcoA from R. capsulatus as a query, we identified 144 CcoA-like MFS proteins in 125 of the 327 alphaproteobacterial species interrogated, with several genomes containing up to three distinct copies of ccoA. We also compiled a phylogenetic profile of ccoA together with the presence of the cbb3-Cox and aa3-Cox structural genes (Fig. 1A). Most of the CcoA homologues were predicted to contain 12 transmembrane helices with conservation of the motifs MXXXM in helix 7 and HXXXM in helix 8, both of which are required for Cu uptake and for cbb3-Cox activity in R. capsulatus (37), with the exception of a group of CcoA-like proteins in Rhizobiales (Fig. 1B). These putative transporters also contained the two conserved motifs in helices 7 and 8 but were truncated at the C terminus, lacking predicted helix 12. Intriguingly, the genes encoding these truncated CcoA homologues were found right downstream of the ccoNOQP and ccoGHIS clusters, encoding the structural and assembly genes of cbb3-Cox, respectively (Fig. 1B), suggesting that the Rhizobiales CcoA-like proteins might play a role similar to that of R. capsulatus CcoA. For the complete set of data pertinent to Fig. 1, see Table S1 in the supplemental material.
FIG 1 .
Presence of CcoA homologues encoded in alphaproteobacterial genomes. (A) Evolutionary relationships between the CcoA homologues identified in sequenced alphaproteobacterial genomes. Branch lengths were ignored, and branch points with Shimodaira-Hasegawa scores of <0.5 were deleted. Branches and nodes are colored by order as shown at the bottom of panel A. The presence of ccoN (inner circle) or cox1 (outer circle) in each genome is represented by black or gray shading, respectively. Corresponding protein IDs are listed in Table S1. (B) Schematic representation of the cbb3-Cox structural (ccoNOQP) and assembly (ccoGHIS) gene clusters together with the ccoA homologue in the Rhizobiales genomes indicated. (C) Co-occurrence plot with circles indicating the presence or absence of ccoA (red or white, respectively), the cbb3-Cox structural and assembly gene clusters (black or white, respectively), and the aa3-Cox-related genes (gray or white, respectively). Not all species are shown because of space limitations, but for a complete profile and a summary, see Tables S1 and S2, respectively.
Co-occurrence of CcoA, aa3-Cox, and cbb3-Cox in alphaproteobacterial species. Download TABLE S1, XLSX file, 0.2 MB (245.2KB, xlsx) .
Copyright © 2018 Khalfaoui-Hassani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
We found that of the 327 alphaproteobacterial genomes analyzed, only 44 had no CcoA or Cox enzyme (Fig. 2; Table S2). Among the remaining genomes, 118 (62%) of 192 coding for cbb3-Cox (CcoNOQP) and 122 (46%) of 274 coding for aa3-Cox (CoxI, CoxII, and CoxIII) also contained CcoA. In contrast, 74 (23%) of these genomes had cbb3-Cox but not CcoA (mainly from Caulobacterales, Brucella, Rhizobiaceae, Hyphomonadaceae, Rhodospirillaceae, and Sphingomonadales), while 152 (46%) had aa3-Cox without CcoA (Fig. 2; Table S2). Thus, the data suggested a higher degree of co-occurrence of ccoA and cbb3-Cox than of ccoA and aa3-Cox. This co-occurrence was particularly evident in Methylocystaceae and Methylobacteriaceae, where species of the same genus would have both CcoA and cbb3-Cox or neither (Fig. 1C). In addition, we also observed strain differences; e.g., both Paracoccus denitrificans strains SD1 and PD1222 had cbb3-Cox and aa3-Cox but only strain PD1222 contained CcoA. Similarly, all of the Rhizobium leguminosarum strains analyzed had cbb3-Cox and aa3-Cox but individual biovars differed in the presence of CcoA. Finally, we found six species containing cbb3-Cox without CcoA and seven species containing CcoA but not cbb3-Cox, suggesting that CcoA-independent provision of Cu to cbb3-Cox and an additional unknown function(s) of CcoA, that is unrelated to Cu provision to the CuB center of this enzyme might exist in some species (Table S2).
FIG 2 .
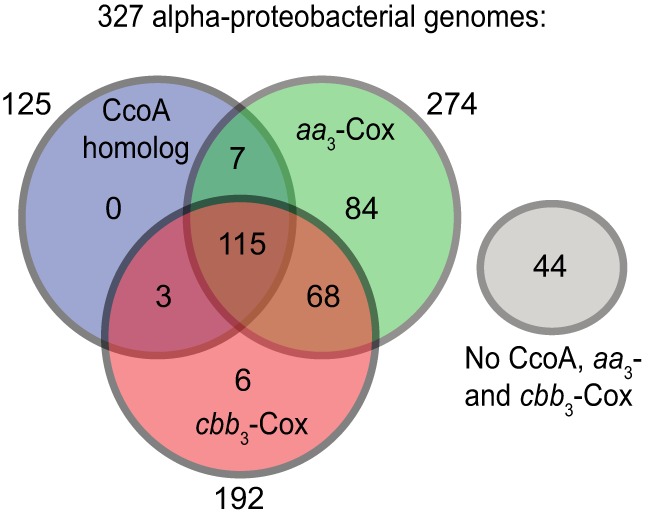
Distribution of CcoA, aa3-Cox, and cbb3-Cox homologues among the alphaproteobacterial genomes analyzed. Note that 44 genomes have no CcoA, aa3-Cox, or cbb3-Cox homologues, whereas 115 have all of them. Of the genomes that have no CcoA, 68 have both aa3-Cox or cbb3-Cox, while 84 have only aa3-Cox and 6 have only cbb3-Cox. Note that of the CcoA-containing genomes, only three have only cbb3-Cox and seven have only aa3-Cox.
Distribution of CcoA, cbb3-Cox, and aa3-Cox among the alphaproteobacterial genomes analyzed in this study. Download TABLE S2, DOCX file, 0.01 MB (14.8KB, docx) .
Copyright © 2018 Khalfaoui-Hassani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Phenotypes of ccoA mutants of R. sphaeroides.
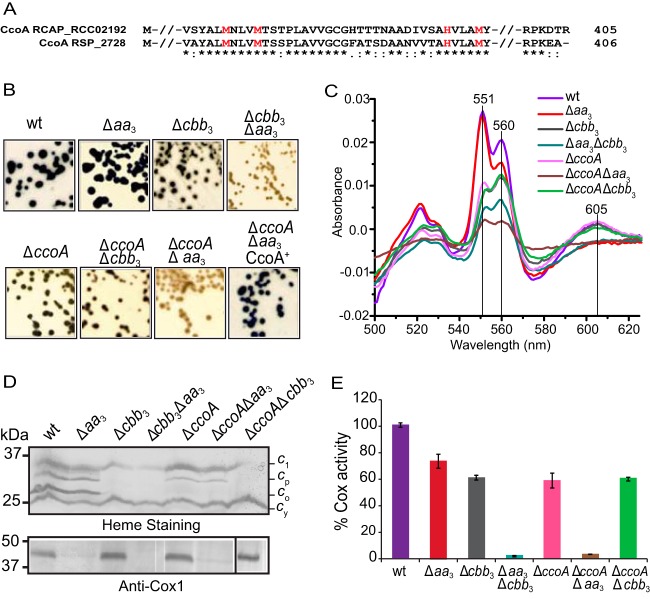
To understand the role of CcoA in the biogenesis of the CuB site of HCO and to test whether CcoA is involved in the provision of Cu to aa3-Cox, as it is in the provision of Cu to cbb3-Cox, we investigated the function of a CcoA ortholog in an organism that contains multiple HCOs. R. sphaeroides has a CcoA homologue (RSP_2726, previously annotated as a multidrug/metabolite efflux pump) containing the conserved Met motifs in transmembrane helices 7 and 8 (Fig. 3A). Unlike R. capsulatus, which is rare among the alphaproteobacterial species in having only one HCO (cbb3-Cox), R. sphaeroides also contains the canonical type A aa3-Cox. To assess the effect of lacking CcoA on both cbb3- and aa3-Cox activities, a ccoA deletion allele was introduced into appropriate R. sphaeroides strains. The wild-type (Ga) strain and the Δaa3 (JS100 [38]) and Δcbb3 (MT001 [39, 40]) mutant strains yielded the ΔccoA (HW3) single mutant and the ΔccoA Δaa3 (HW2) and ΔccoA Δcbb3 (HW4) double mutants, respectively (Table S3). The Δcbb3 Δaa3 double mutant (ME127 [40]), lacking both cbb3- and aa3-Cox activities, served as a negative control (Table S3). The Cox activities of these strains were visualized qualitatively by α-naphthol and N′N′-dimethyl-p-phenylenediamine (NADI; blue) staining of colonies grown aerobically on enriched medium (Fig. 3B). The wild-type and Δaa3 mutant strains were NADI+ (i.e., stained dark blue in seconds), whereas the Δcbb3 mutant was NADIslow (i.e., stained blue in a few minutes), indicating that cbb3-Cox provides most of the Cox activity under these growth conditions. The Δaa3 Δcbb3 double mutant was NADI− (i.e., no blue staining after 15 min), consistent with the absence of both Cox enzymes (39). Both the ΔccoA single mutant and the ΔccoA Δcbb3 double mutant had a NADIslow phenotype, similar to that observed when only aa3-Cox activity (Δcbb3) was present (Fig. 3B). Importantly, the double mutant lacking both CcoA and aa3-Cox (ΔccoA Δaa3) but containing the intact structural genes of cbb3-Cox was NADI− like the double mutant (Δcbb3 Δaa3) lacking both Cox activities (Fig. 3B). Upon complementation with a plasmid carrying a wild-type allele of R. sphaeroides ccoA, both the single (ΔccoA) and double (ΔccoA Δaa3) mutants lacking CcoA became NADI+ (Fig. 3B). Thus, the data indicated that in R. sphaeroides, the absence of ccoA affected cbb3-Cox, but not aa3-Cox, activity.
FIG 3 .
Met motifs of CcoA and phenotypic and functional characterization of R. sphaeroides ΔccoA mutants. (A) Amino acid sequence alignment of the highly conserved region surrounding the Met and His motifs MXXXM and HXXXM (in red) from R. capsulatus CcoA (RCA_RCC02192) and its R. sphaeroides homologue (RSP_2726) (65% identity; 80% similarity). (B) Growth and NADI phenotypes of colonies of R. sphaeroides wild-type (wt) Ga and Δaa3 (JS100), Δcbb3 (MT001), and Δaa3 Δcbb3 (ME127) Cox mutants together with those of ΔccoA (HW3), ΔccoA Δcbb3 (HW24), and ΔccoA Δaa3 (HW2) CcoA mutants. Cells were grown aerobically at 35°C on LB medium, and the presence of Cox activity was visualized by NADI staining (see Materials and Methods). Colonies that contain wild-type levels of Cox activity turn dark blue within a few seconds (NADI+), while those that have low or no Cox activity show lighter blue (NADIslow) or no blue staining (NADI−) upon longer exposure, respectively. Note that the ΔccoA Δaa3 mutant is NADI– like the Δaa3 Δcbb3 mutant, unless it is complemented with a plasmid carrying a wild-type allele of ccoA (ΔccoA Δaa3 CcoA+). (C) Absorption difference spectra of membrane fractions of R. sphaeroides mutants recorded between 500 and 625 nm by using oxidized membrane preparations as the baseline and reducing the sample with an excess of sodium dithionite. The intensity of the peaks centered at 551, 560, and 605 nm indicates the contents of c-, b- and a-type hemes, respectively. (D) Steady-state levels of structural subunits of cbb3- and aa3-Cox enzymes in the membranes of R. sphaeroides mutants. (Top) Membrane preparations of R. sphaeroides mutants separated by SDS-PAGE and then stained with TMBZ. Four different cyts c (cyt c1 of the cyt bc1 complex, cyt cp [CcoP] and co [CcoO] subunits of cbb3-Cox, and the membrane-attached electron carrier cyt cy) can be seen in the wild-type strain (39). In the ΔccoA mutant, the steady-state levels of cyt cp and especially cyt co are very low. (Bottom) Membrane preparations of R. sphaeroides strains resolved by SDS-PAGE and subjected to immunoblot analysis. The presence of the Cox1 subunit of R. sphaeroides aa3-Cox was identified with P. denitrificans Cox1 polyclonal antibodies that cross-react with it. The white lines seen on the blot next to some lanes are scanning artifacts and do not reflect spliced gels. (E) cyt c activity of membrane fractions of R. sphaeroides ΔccoA mutants. Total Cox (cbb3-Cox plus aa3-Cox) activities were determined using membrane preparations of various R. sphaeroides strains by monitoring the rate of oxidation of reduced horse heart cyt c. R. sphaeroides wild-type strain Ga exhibited an activity of ~1.33 µmol of cyt c oxidized/min/mg of total membrane proteins, which was referred to as 100%. Three independent assays were carried out for each strain. The ΔccoA Δaa3 mutant has no activity, like the Δaa3 Δcbb3 mutant that lacks both Cox enzymes.
Strains and plasmids used in this study. Download TABLE S3, DOCX file, 0.03 MB (28.9KB, docx) .
Copyright © 2018 Khalfaoui-Hassani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The NADI– phenotypes of R. sphaeroides ΔccoA mutants were restored upon the addition of 5 μM Cu2+ to the growth medium, similar to that seen in the R. capsulatus ΔccoA mutant (34). In contrast, the ΔccoA Δcbb3 double mutant, which has only a functional aa3-Cox, remained NADIslow upon Cu2+ supplementation, suggesting that Cu2+ addition had no effect on aa3-Cox activity (data not shown).
Absence of CcoA affects heme and subunit compositions of cbb3-Cox but not aa3-Cox.
To assess how the absence of CcoA affects R. sphaeroides HCO biogenesis, the c-, b-, and a-type heme contents of membrane fractions derived from appropriate mutants were analyzed by using optical difference (dithionite-reduced minus ferricyanide-oxidized) spectra. In membranes of a wild-type R. sphaeroides strain, prominent peaks around 605, 560, and 551 nm, corresponding to the a-, b-, and c-type hemes, respectively, were readily detectable (39) (Fig. 3C). As expected, a significant decrease in the 605-nm peak and in the 560- and 551-nm peaks was observed in the Δaa3 and Δcbb3 mutants, respectively (41). Note that in R. sphaeroides membranes, only aa3-Cox has a-type heme but other proteins besides cbb3-Cox contain b- and c-type hemes (e.g., cyt bc1) under the growth conditions tested. Accordingly, in the double mutant (Δcbb3 Δaa3) lacking both Cox enzymes, all three peaks decreased substantially compared with the wild-type strain (Fig. 3C), as reported earlier (39). Remarkably, in the ΔccoA single mutant only the content of b- and c-type hemes decreased, as in the mutant lacking only cbb3-Cox (Δcbb3) or the ΔccoA Δcbb3 double mutant. Moreover, in the ΔccoA Δaa3 double mutant, all three peaks, corresponding to the a-, b-, and c-type hemes, decreased drastically, similar to what was seen in the double mutant (Δcbb3 Δaa3) (Fig. 3C). In summary, the data showed that in the absence of CcoA, the content of b- and c-type hemes in the membrane fraction (corresponding partly to cbb3-Cox) decreased significantly, whereas the a-type heme content (corresponding to aa3-Cox) remained unchanged, consistent with CcoA being involved in cbb3-Cox, but not aa3-Cox, production. We emphasize that these data are merely semiquantitative because of the presence of other b- and c-type cyts (in addition to cbb3-Cox) whose content may vary in the presence or absence of different HCOs.
Next, the steady-state amounts of cbb3-Cox subunits present in membranes from appropriate mutants were examined by SDS-PAGE and 3,3′,5,5′-tetramethylbenzidine (TMBZ) staining, which specifically reveals membrane-bound c-type cyts (42). In wild-type R. sphaeroides membranes, four distinct c-type cyts, including the CcoO (cyt co) and CcoP (cyt cp) subunits of cbb3-Cox, can be detected (Fig. 3D, top). As expected, in the absence of aa3-Cox, the c-type cyt profile remained unchanged, but in mutants lacking cbb3-Cox (Δcbb3 and Δcbb3 Δaa3), cyt co and cyt cp were not present, leaving only the cyt c1 subunit of cyt bc1 and the membrane-anchored electron carrier cyt cy. Remarkably, in strains lacking CcoA, such as the ΔccoA and ΔccoA Δaa3 mutants, the amounts of cyt co and cyt cp decreased at different levels, even though these strains contained an intact copy of the cbb3-Cox structural genes. These data, together with the spectral data showing that the amount of b-type heme, and hence that of CcoN, also decreased, indicated that production of cbb3-Cox was defective in the absence of CcoA. Finally, the presence of the Cox1 subunit of aa3-Cox was monitored by using polyclonal antibodies raised against Cox1 of Paraccocus denitrificans aa3-Cox (43) (Fig. 3D, bottom). As expected, Cox1 was absent from mutants lacking aa3-Cox, like the Δaa3, Δcbb3 Δaa3, and ΔccoA Δaa3 mutant strains. However, it was readily detected in strains lacking CcoA (ΔccoA mutant), cbb3-Cox (Δcbb3 mutant), or both proteins (ΔccoA Δcbb3 mutant) at levels comparable to those of the wild type, in agreement with the Cu-containing Cox1 subunit of aa3-Cox being unaffected by the absence of CcoA in R. sphaeroides.
Cox activities of mutants lacking CcoA.
The total cyt c oxidation activity (accounting for both aa3-Cox and cbb3-Cox activities) present in membranes of different R. sphaeroides strains was measured by using reduced horse heart cyt c. R. sphaeroides wild-type strain Ga exhibited an activity level of 1.33 μmol of cyt c oxidized/min/mg of total membrane proteins (referred to as 100%) (Fig. 3E). Addition of 200 μM KCN, a specific inhibitor of the HCO catalytic binuclear center, abolished this activity almost completely (96% inhibition). The mutants lacking aa3-Cox (Δaa3 mutant) and cbb3-Cox (Δcbb3 mutant) showed Cox activities corresponding to 73 and 62% of the wild-type level, respectively, whereas the Δcbb3 Δaa3 double mutant had no activity. A strain lacking only CcoA (ΔccoA mutant) or both CcoA and cbb3-Cox (ΔccoA Δcbb3 mutant) showed similar amounts of Cox activity, 59 and 60% of the wild-type level, respectively. In contrast, a strain lacking both CcoA and aa3-Cox (ΔccoA Δaa3 mutant), although it contained intact cbb3-Cox structural genes, had no Cox activity, similar to a Δcbb3 Δaa3 double mutant (Fig. 3E). Therefore, the absence of CcoA affected only cbb3-Cox, and not aa3-Cox, in R. sphaeroides.
Suppressors of ΔccoA restore cbb3-Cox activity at the expense of Cu2+ hypersensitivity.
During the phenotypic characterization of ΔccoA mutants, we observed that the NADI– double mutant lacking both CcoA and aa3-Cox (ΔccoA Δaa3 mutant) readily yielded wild-type-like NADI+ revertants (Fig. 4A). Similar revertants had previously been obtained with R. capsulatus ΔccoA mutants, and their characterization showed that these suppressor mutations restored cbb3-Cox deficiency and conferred Cu2+ sensitivity (32). Using whole-genome sequencing, we determined that these mutations were single base-pair indels in a rare stretch of 10 conserved cytosine base pairs located in copA, which encoded the P1B-type ATP-dependent Cu exporter (CopA) (32). These indels caused translational frameshifts that inactivated copA and increased cellular Cu content and Cu2+ sensitivity (32).
FIG 4 .
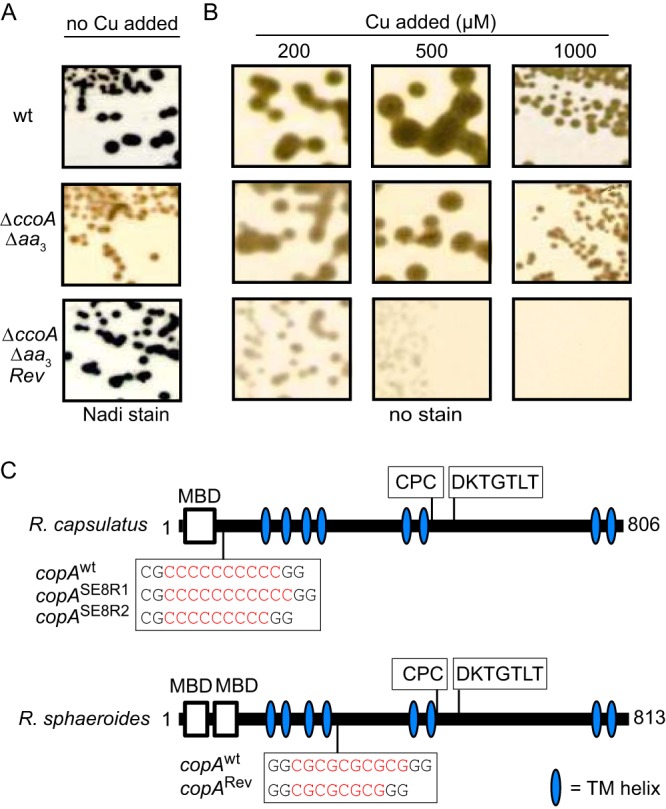
Bypass suppressors of R. sphaeroides mutants lacking CcoA are located in CopA and regain cbb3-Cox activity at the expense of Cu2+ hypersensitivity. (A) Spontaneous NADI+ bypass suppressors ΔccoA Δaa3/Revi that regained cbb3-Cox activity were isolated from the ΔccoA Δaa3 mutant. (B) The ΔccoA Δaa3/Revi suppressors exhibit hypersensitivity to Cu2+ compared with the wild type (wt) and the ΔccoA Δaa3 mutant. (C) The suppressor mutations corresponded to 2-bp (CG) deletions in copA (RSP_2829) of R. sphaeroides in a stretch of five CG repeats located immediately after the fourth transmembrane segment. They are compared with similar CcoA suppressors (CopASE8R1 and CopASE8R2) isolated previously from R. capsulatus (32). The latter mutations corresponded to a single-base-pair (C) indel located in a stretch of 10 C repeats of R. capsulatus CopA before its first transmembrane helix. In both species, the suppressor mutations led to translational frameshifts that abolished CopA activity, leaving intact the possibility of producing N-terminally truncated polypeptides that still carry the MBDs. The N-terminal MBD, the phosphorylation domain (DKTGT), and the transmembrane metal binding motif (CPC) are represented in CopA.
Intrigued by the occurrence of similar revertants of R. sphaeroides, we retained four independent NADI+ derivatives (HW2R1 to HW2R4) of the ΔccoA Δaa3 double mutant (Table S3) and tested their Cu2+ tolerance in enriched medium. Indeed, they were hypersensitive to Cu2+ (above ~200 μM) compared with their wild-type and ΔccoA Δaa3 mutant parents (tolerant to ~1 mM) (Fig. 4B). Thus, similar to R. capsulatus, these R. sphaeroides revertants regained cbb3-Cox activity at the expense of becoming hypersensitive to Cu2+. DNA sequencing of the genomic copies of R. sphaeroides copA (RSP_2890) (44) from these revertants showed that they all contained two base-pair (CG) deletions in copA (Fig. 4C). Remarkably, these deletions were located in a region of copA containing five consecutive CG repeats, presumably causing translational frameshifts that inactivated copA and increased the Cu2+ sensitivity of cells. The data indicated that in R. sphaeroides, as in R. capsulatus, suppression of the CcoA defect occurred via mutations (two base-pair CG deletions and single base-pair C indels, respectively), inducing translational frameshifts that inactivated CopA.
Distribution of the CcoA family among Bacteria and Eukarya.
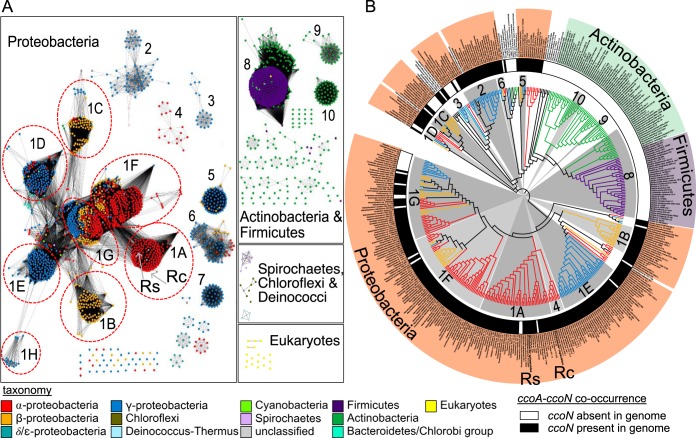
Given the functional specificity of CcoA for cbb3-Cox in Rhodobacter species, we widened our bioinformatic search for CcoA-like MFS transporters beyond the alphaproteobacteria and queried their co-occurrence with CcoN in the SEED database (45). We found CcoA homologues in all major classes of Proteobacteria, Bacteroidetes, and Spirochaetia and in all major divisions of the Terrabacteria group, including Chloroflexi and Deinococcus (Fig. 5A). Moreover, we found that most of the genomes that contained CcoA also encoded CcoN (i.e., cbb3-Cox), except Actinobacteria and Firmicutes (Fig. 5B). The CcoA-like proteins were also present in the nuclear genomes of eukaryotic algae, with both primary and secondary plastids, in two fungal genomes from Chytridiomycota (Fig. 5A), in addition to the group of Actinobacteria and Firmicutes (Fig. 5B), which are known to lack cbb3-Cox (26). Remarkably, these “orphan” CcoA-like transporters encountered in organisms lacking cbb3-Cox still contained the conserved MXXXM and HXXXM motifs in helices 7 and 8, suggesting that they might also transport Cu to other protein targets. Finally, we note that, similar to alphaproteobacterial genomes, about one-third of ccoN-containing organisms also contain ccoA and exhibited species level variation in its presence, which is particularly evident in Vibrio (Table S4). For the set of data pertinent to Fig. 5A and B, see Tables S4 and S5, respectively.
FIG 5 .
The CcoA-like family of putative transporters. (A) Protein similarity network of CcoA-like putative transporters identified in the Uniprot database. Each node (circle) represents a single protein sequence, and each edge (solid line) represents similarity between two proteins (threshold set at an alignment score of 80). Nodes are colored by taxonomy as shown at the bottom, and cluster designations (1 to 10) correspond to Table S4 and Fig. S1 PSN_CcoA.cys, which can be viewed in detail by using Cytoscape software. The locations of the nodes representing CcoA from R. sphaeroides (Rs) and R. capsulatus (Rc) are shown with gray arrows. (B) Evolutionary relationship between CcoA homologues identified in the SEED database. Branch lengths were ignored, and branch points with Shimodaira-Hasegawa scores of <0.5 were deleted. Branches are colored by taxonomy as shown at the bottom. The presence of ccoN (inner circle) in each genome is represented by black shading. Gray shading is used to color those clades that correspond to the numbered clusters (1A to 10) shown in panel A. Corresponding protein IDs are listed in Table S5. The positions of CcoA of R. sphaeroides (Rs) and R. capsulatus (Rc) in the tree are also indicated. Note that ccoA is present in Actinobacteria and Firmicutes (clusters 8 to 10) that are devoid of ccoN (i.e., cbb3-Cox).
Protein similarity network of CcoA-like putative transporters obtained by using the SEED database. Download TABLE S4, XLSX file, 0.8 MB (789KB, xlsx) .
Copyright © 2018 Khalfaoui-Hassani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Evolutionary relationship between CcoA homologues and the corresponding phylogenetic tree. Download TABLE S5, XLSX file, 0.2 MB (245.3KB, xlsx) .
Copyright © 2018 Khalfaoui-Hassani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DISCUSSION
The R. capsulatus MFS-type transporter CcoA is the prototypical bacterial Cu importer and the key Cu provider to cbb3-Cox under limited Cu availability (32, 34, 37). Earlier, we observed that R. capsulatus mutants lacking either cbb3-Cox (ΔccoNOQP) or CcoA (ΔccoA) contained similar smaller amounts of total cellular Cu (~80% of the wild-type amount) (46), suggesting that the Cu imported by CcoA is allocated primarily to cbb3-Cox biogenesis. To assess the functional specificity of CcoA toward other cuproenzymes, we initiated a broad-based comparative genomic study to examine the presence of CcoA homologues and their co-occurrence with cyt c oxidases in organisms of known genome sequences. We found that the CcoA-like transporters are widespread in bacteria and some microbial eukaryotes. They are present in all major classes of Proteobacteria, Bacteroidetes, Spirochaetia, and Terrabacteria, as well as in the nuclear genomes of eukaryotic algae and fungi (Table S1). Interestingly, our all-inclusive bioinformatic analyses showed that not all CcoA family members are involved in cbb3-Cox biogenesis. Numerous species that have no cbb3-Cox, such as Actinobacteria and Firmicutes species, still contained CcoA-like transporters that possibly perform other functions that have yet to be uncovered. A closer look to the group of alphaproteobacteria showed that about one-third of these species contained at least one CcoA homologue together with the genes encoding cbb3-Cox or aa3-Cox, showing a high degree of co-occurrence of HCO with CcoA. Finally, similar to some genera of alphaproteobacteria, we observed species level variations in the presence of ccoA, which were particularly evident in Vibrio (Table S4), which may reflect that CcoA provides a selective advantage in some environmental niches.
Using R. sphaeroides, which contains both cbb3- and aa3-Cox (from C and A HCO families, respectively) and an ortholog of R. capsulatus CcoA (RSP_2726), we tested experimentally whether CcoA could also provide Cu to the canonical aa3-Cox, whose biogenesis has been studied (43, 47). Physiological, genetic, and biochemical data gathered by using appropriate ΔccoA mutants lacking either cbb3- or aa3-Cox established unequivocally that the absence of CcoA affected cbb3-Cox, but not aa3-Cox, production in this organism. Earlier work had shown that the absence of the Cu chaperone Cox11, which is required for CuB insertion into aa3-Cox, had no effect on cbb3-Cox production in R. sphaeroides (25) or Pseudomonas pseudoalcaligenes KF707 (48). Therefore, we concluded that the Cu atoms inserted into the binuclear centers of the cbb3-Cox and aa3-Cox enzymes are not only delivered by distinct pathways but also provided by different uptake systems (Fig. 6).
FIG 6 .
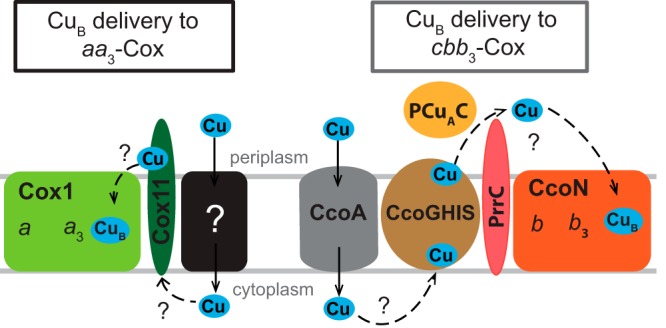
Schematic comparison of CuB incorporation into the active sites of bacterial aa3- and cbb3-Cox. A complete understanding of the pathways of Cu uptake and delivery to the heme-CuB binuclear site of HCOs is still missing. All known components involved in the biogenesis of the CuB center of bacterial aa3- and cbb3-Cox are depicted. In the case of aa3-Cox, the Cox1 subunit is thought to receive CuB from the Cu chaperone Cox11 either directly or via an unknown partner(s). How Cu is initially conveyed to Cox11 remains unknown. A putative Cu importer of unknown identity (black box) that is possibly functionally similar to mitochondrial Pic2 (71) is included. In the case of cbb3-Cox, Cu is imported by CcoA and is conveyed by the CcoGHIS, PCuAC, and PrrC (PccA and SenC homologs) assembly components to the CuB site of the CcoN subunit by a mechanism that remains elusive. Question marks indicate unknown Cu transfer steps.
CcoA being an exclusive Cu importer for cbb3-Cox was rather unexpected, especially because the catalytic subunits and the heme-Cu binuclear centers of all HCOs are very similar (11, 49). The existence of specialized Cu trafficking pathways for different cuproproteins has been documented in different organisms (50), and their specificity is generally conferred by target-specific chaperones rather than transporters (51). Thus, the independent Cu uptake systems operating during the biogenesis of different HCOs and the specificity of CcoA for cbb3-Cox are intriguing. Since the Sco-like and PCuAC-like Cu chaperones are involved in the biogenesis of both cbb3-Cox (23, 27, 33) and aa3-Cox (19, 22, 24, 52), they are less likely to confer specificity. Thus, a possibility is that CcoA may do so by conveying Cu either directly or via an unknown partner, to CcoI, which is the P1B-type ATPase required for cbb3-Cox production (29) (Fig. 6). Interestingly, the physical clustering of ccoA with the cbb3-Cox assembly genes ccoGHIS in members of the order Rhizobiales (Fig. 1B) suggests that these proteins function together and possibly interact during cbb3-Cox production. Undoubtedly, under low Cu availability, the occurrence of a membrane-integral complex containing both CcoI and CcoA would be advantageous for efficient biosynthesis of cbb3-Cox.
In both R. sphaeroides and R. capsulatus mutants lacking CcoA, the defect in cbb3-Cox production can be restored by providing a high concentration of exogenous Cu2+, which leads to an increase in cellular Cu content (34). The components of this putative CcoA-independent low-affinity Cu uptake pathway remain unknown. However, this pathway still relies on CcoI, whose absence cannot be palliated by Cu2+ supplementation, to provide Cu to cbb3-Cox. Alternatively, the defect in cbb3-Cox biogenesis can be bypassed via frameshift mutations in copA, which encodes the P1B-type ATPase involved in Cu export and detoxification, resulting in inactivation of CopA and consequent greater cellular Cu content and hypersensitivity to Cu2+ (32). Elucidation of how the Cu imported by CcoA is conveyed to CcoI is needed to understand how the increase in cellular Cu content bypasses the role of CcoA in cbb3-Cox biogenesis.
The molecular natures and locations of the suppressor mutations that inactivate CopA are different between the two Rhodobacter species. In R. capsulatus, these mutations are single base-pair C indels in a region of copA where 10 consecutive C base-pairs are located (bp 230 to 239) (32), whereas in R. sphaeroides, they are two base-pair CG deletions in a region of copA where five consecutive CG repeats are present (bp 863 to 872). Hypermutable nucleotide tandem repeats (NTRs), which are prone to DNA slippage during replication and increased recombination, are widespread in genomes of different organisms (53, 54), and they can reversibly inactivate or regulate the expression of specific coding sequences (55). Computational analyses suggested that in prokaryotes, the monomeric NTRs of G/C (e.g., C repeats of R. capsulatus copA) are more mutagenic than dimeric (e.g., CG repeats of R. sphaeroides copA) or trimeric NTRs (56). The different types of mutagenic NTRs located in copA may reflect different strategies used for Cu homeostasis governing Cu availability to cbb3-Cox via CcoA-independent pathways.
P1B-type ATPases such as CopA and CcoI contain conserved domains for ATP binding and for phosphorylation, in addition to their N-terminal metal-binding domains (MBDs), harboring a Cu-binding CXXC motif and a membrane-embedded Cu binding site (CPX) (57) (Fig. 4C). The frameshift mutations that inactivate CopA still conserve the genetic ability to produce truncated N-terminal CopA derivatives with intact N-terminal MBDs that, if produced and stable, could hypothetically facilitate Cu delivery to cbb3-Cox. An R. capsulatus CopA derivative would become soluble with a single MBD, whereas an R. sphaeroides CopA derivative would remain membrane attached and conserve its two MBDs, reminiscent of the Cu chaperone CupA in Streptococcus pneumoniae. The membrane-anchored CupA protein enhances Cu sequestration and mediates its binding to the MBD of CopA as an adaptation to Cu toxicity (58). Arabidopsis thaliana chaperone PCH1 is produced by alternative splicing of the P1B-type Cu+ ATPase PAA1 pre-mRNA and acts as its specific Cu chaperone (59). In Escherichia coli, a fragment of CopA containing the N-terminal MBD, resulting from programed ribosomal frameshifting during the translation of copA mRNA, is able to bind Cu and increase tolerance of Cu toxicity (60, 61). The molecular mechanisms underlying these cases are distinct from the NTR mutations in copA, yet they reflect similar responses that organisms have evolved to maintain Cu homeostasis and avoid its toxicity.
The isolation of mutations in copA of both R. capsulatus and R. sphaeroides may suggest that CcoA is not required for cbb3-Cox metalation, depending on the mechanisms of Cu homeostasis used by the organisms. Indeed, our comparative genomic analyses indicated that CcoA-like MFS proteins are absent from about one-third of cbb3-Cox-containing alphaproteobacterial species. That cbb3-Cox metallation in these species does not require CcoA while it does so in R. capsulatus and R. sphaeroides under low Cu availability is intriguing. These species may have other Cu acquisition pathways, similar to R. capsulatus ccoA mutants at high Cu2+ concentrations. As an example, P. denitrificans PD1222 has an MFS-type CcoA Cu importer and a typical P1B-type ATPase CopA ortholog with an N-terminal heavy-metal-associated (HMA) domain acting as its MBD. In contrast, P. denitrificans SD1 does not have CcoA but has a CopA homologue with a different MBD, an N-terminal TRASH domain (62). These differences are in agreement with the proposal that the copA NTR mutations occurring after the N-terminal MBD of CopA in both Rhodobacter species may result in HMA-containing derivatives acting as chaperones. Further investigation of these species and characterization of different strains with respect to their CcoA-independent Cu trafficking pathways will be informative.
In summary, this work established that Cu incorporation into the catalytic site of different HCOs, in particular cbb3-Cox and aa3-Cox, occurs not only via distinct delivery pathways but also via distinct uptake pathways (Fig. 6). While the MFS-type transporter CcoA is required for Cu incorporation into cbb3-Cox, it is not involved in the metallation of aa3-Cox. The occurrence of dedicated Cu uptake pathways, critical for the maintenance of intracellular Cu homeostasis, might be an evolutionary example of different strategies to improve fitness encountered in many organisms.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The E. coli and R. sphaeroides strains and plasmids used in this study are listed in Table S3. The standard molecular biology techniques used are described in reference 63, and all plasmid and strain constructions are described below Table S3. E. coli strains were grown at 37°C in Luria-Bertani (LB) broth supplemented with 100, 50, 50, 12.5, and 12 μg/ml (final concentrations) ampicillin, kanamycin (Kan), spectinomycin (Spe), tetracycline (Tet), and gentamicin (Gen), respectively (46). R. sphaeroides strains were grown in either minimal (64) or LB medium supplemented with 10, 10, 2.5, and 1 μg/ml (final concentrations) Kan, Spe, Tet, and Gen, respectively (39).
Biochemical and spectroscopic techniques.
R. sphaeroides cells grown under semiaerobic conditions on LB medium were harvested and resuspended in 50 mM Tris-HCl (pH 7.2), 1 mM KCl assay buffer. Intracytoplasmic membrane vesicles (chromatophores) were prepared as previously described (65). The protein concentration of membrane fractions was determined with the bicinchoninic acid assay (Sigma, Inc.). Visualization of c-type cyts was done by TMBZ staining following the separation of ~200 μg of total membrane proteins by 15% SDS-PAGE as done earlier (42). Immunoblot analysis to identify R. sphaeroides Cox1 was done with ~40 μg of total membrane proteins separated by 12% SDS-PAGE. Proteins were transferred onto polyvinylidene difluoride membranes and incubated with P. denitrificans anti-Cox1 specific polyclonal antibodies cross-reacting with R. sphaeroides protein (47). Alkaline phosphatase-conjugated secondary antibodies and 5-bromo-4-chloro-3-indolyl phosphate (BCIP)–nitroblue tetrazolium were used for visualization of Cox1 polypeptide.
Visible spectra were taken with 50 μg of total membrane proteins in 1 ml of assay buffer containing 0.2% n-dodecyl-β-d-maltoside (DDM). Samples were oxidized by the addition of a few grains of potassium ferricyanide, and the absorption spectra taken between 480 and 660 nm were saved as a baseline. After reduction of the samples by the addition of a small amount of sodium dithionite, the spectra were rerecorded in the same wavelength range (39).
Determination of Cox activities.
The cbb3-Cox activity of colonies was visualized by using the NADI reaction (α-naphthol + N′N′-dimethyl-p-phenylenediamine → indophenol blue + H2O) by staining the plates with a 1:1 (vol/vol) mixture of 35 mM α-naphthol and 30 mM N′,N′-dimethyl-p-phenylenediamine (66). Colonies with cbb3-Cox activity exhibited dark blue staining (NADI+) within 30 s to 1 min, while those with low activity or lacking it showed light blue (NADIslow) or no staining (NADI−) up to 15 min, respectively. Total aa3-Cox and cbb3-Cox activity levels were determined with reduced cyt c as a substrate as done previously (39). Chromatophore membranes were solubilized at room temperature by the addition of 1 mg of DDM/mg of total proteins. Activity assays were initiated by the addition of ~10 μg of solubilized membranes to 1 ml of assay buffer containing 25 μM reduced cyt c. Rates of cyt c oxidation were determined by monitoring the time-dependent decrease in absorbance at 550 nm and expressed in micromoles of cyt c oxidized/min/mg of total membrane proteins by using the extinction coefficient at 550 nm for cyt c (ε550 = 20.0 mM−1 cm−1). The specificity of Cox activity was confirmed by inhibition with 200 μM KCN, a specific inhibitor of HCO enzymes, which stopped cyt c oxidation almost completely. Any residual cyanide-insensitive cyt c oxidase activity (air oxidation was negligible) was subtracted from the final rates.
Bioinformatic analysis.
Genes encoding CcoA-like, CcoN, and Cox1 proteins were identified in the SEED database (45). In addition to amino acid sequence similarity, annotation of a protein as being CcoA-like required conservation of the MXXXM and HXXXM motifs of transmembrane helices 7 and 8. Patterns of co-occurrence and genomic colocalization were detected with the set of tools for comparative genome analysis available in SEED. For the phylogenetic trees of CcoA-like proteins, full-length amino acid sequences (Tables S1, S4, and S5) were aligned through the CIPRES web portal (67) with MAFFT on XSEDE (v. 7.305) (68) and an approximate maximum-likelihood estimation was performed with FastTreeMP on XSEDE (v. 2.1.9) (69). The resulting phylogenetic trees were visualized and annotated with the Interactive Tree of Life (iTOL) tool (70). A comprehensive identification of CcoA homologues in sequenced genomes was performed with a protein similarity network as implemented with the EFI-EST tool (http://efi.igb.illinois.edu/efi-est/) with R. capsulatus CcoA as the seed sequence, an E value of 1E-4 for the blast search, and an alignment score of 80. EFI retrieved 2,490 proteins (see Table S4), which were incorporated into the network and visualized with the yFiles organic layout provided with the Cytoscape software (http://www.cytoscape.org).
Primers used in this study. Download TABLE S6, DOCX file, 0.01 MB (14KB, docx) .
Copyright © 2018 Khalfaoui-Hassani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ACKNOWLEDGMENTS
This work was supported mainly by the Division of Chemical Sciences, Geosciences and Biosciences, Office of Basic Energy Sciences of the Department of Energy (DOE DE-FG02-91ER20052 to F.D.), and partly by the National Institutes of Health (NIH GM38237 to F.D.). We acknowledge the partial support provided by the Office of Biological and Environmental Research of the Department of Energy to C.E.B.-H. Support of H.-G.K. by the Deutsche Forschungsgemeinschaft (IRTG 1478 and RTG 2202) is greatly appreciated. The funding agencies had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We thank Bernd Ludwig for providing the polyclonal antibodies specific for the Cox1 subunit of P. denitrificans aa3-Cox, Shalini Paliwal for help constructing plasmid pSP1, and Stefan Steimle for critical reading of the manuscript.
Footnotes
Citation Khalfaoui-Hassani B, Wu H, Blaby-Haas CE, Zhang Y, Sandri F, Verissimo AF, Koch H-G, Daldal F. 2018. Widespread distribution and functional specificity of the copper importer CcoA: distinct Cu uptake routes for bacterial cytochrome c oxidases. mBio 9:e00065-18. https://doi.org/10.1128/mBio.00065-18.
REFERENCES
- 1.Öhrvik H, Aaseth J, Horn N. 2017. Orchestration of dynamic copper navigation—new and missing pieces. Metallomics 9:1204–1229. doi: 10.1039/c7mt00010c. [DOI] [PubMed] [Google Scholar]
- 2.Smith AD, Logeman BL, Thiele DJ. 2017. Copper acquisition and utilization in fungi. Annu Rev Microbiol 71:597–623. doi: 10.1146/annurev-micro-030117-020444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macomber L, Imlay JA. 2009. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci U S A 106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besold AN, Culbertson EM, Culotta VC. 2016. The yin and yang of copper during infection. J Biol Inorg Chem 21:137–144. doi: 10.1007/s00775-016-1335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J. 1993. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat Genet 3:7–13. doi: 10.1038/ng0193-7. [DOI] [PubMed] [Google Scholar]
- 6.Tanzi RE, Petrukhin K, Chernov I, Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L, Brzustowicz LM, Devoto M, Peppercorn J, Bush AI, Sternlieb I, Honig B, Edelman IS, Soares MB, Scheinberg IH, Gilliam TC. 1993. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet 5:344–350. doi: 10.1038/ng1293-344. [DOI] [PubMed] [Google Scholar]
- 7.Hung YH, Bush AI, Cherny RA. 2010. Copper in the brain and Alzheimer’s disease. J Biol Inorg Chem 15:61–76. doi: 10.1007/s00775-009-0600-y. [DOI] [PubMed] [Google Scholar]
- 8.Baker ZN, Cobine PA, Leary SC. 2017. The mitochondrion: a central architect of copper homeostasis. Metallomics 9:1501–1512. doi: 10.1039/c7mt00221a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barwinska-Sendra A, Waldron KJ. 2017. The role of intermetal competition and mis-metalation in metal toxicity. Adv Microb Physiol 70:315–379. doi: 10.1016/bs.ampbs.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 10.García-Horsman JA, Barquera B, Rumbley J, Ma J, Gennis RB. 1994. The superfamily of heme-copper respiratory oxidases. J Bacteriol 176:5587–5600. doi: 10.1128/jb.176.18.5587-5600.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Timón-Gómez A, Nývltová E, Abriata LA, Vila AJ, Hosler J, Barrientos A 7 September 2017. Mitochondrial cytochrome c oxidase biogenesis: recent developments. Semin Cell Dev Biol doi: 10.1016/j.semcdb.2017.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira MM, Santana M, Teixeira M. 2001. A novel scenario for the evolution of haem-copper oxygen reductases. Biochim Biophys Acta 1505:185–208. doi: 10.1016/S0005-2728(01)00169-4. [DOI] [PubMed] [Google Scholar]
- 13.Michel H, Behr J, Harrenga A, Kannt A. 1998. Cytochrome c oxidase: structure and spectroscopy. Annu Rev Biophys Biomol Struct 27:329–356. doi: 10.1146/annurev.biophys.27.1.329. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Hiser C, Ferguson-Miller S. 2017. Role of conformational change and K-path ligands in controlling cytochrome c oxidase activity. Biochem Soc Trans 45:1087–1095. doi: 10.1042/BST20160138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dancis A, Haile D, Yuan DS, Klausner RD. 1994. The Saccharomyces cerevisiae copper transport protein (Ctr1p). Biochemical characterization, regulation by copper, and physiologic role in copper uptake. J Biol Chem 269:25660–25667. [PubMed] [Google Scholar]
- 16.Dancis A, Yuan DS, Haile D, Askwith C, Eide D, Moehle C, Kaplan J, Klausner RD. 1994. Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell 76:393–402. doi: 10.1016/0092-8674(94)90345-X. [DOI] [PubMed] [Google Scholar]
- 17.Cobine PA, Ojeda LD, Rigby KM, Winge DR. 2004. Yeast contain a non-proteinaceous pool of copper in the mitochondrial matrix. J Biol Chem 279:14447–14455. doi: 10.1074/jbc.M312693200. [DOI] [PubMed] [Google Scholar]
- 18.Horng YC, Cobine PA, Maxfield AB, Carr HS, Winge DR. 2004. Specific copper transfer from the Cox17 metallochaperone to both Sco1 and Cox11 in the assembly of yeast cytochrome c oxidase. J Biol Chem 279:35334–35340. doi: 10.1074/jbc.M404747200. [DOI] [PubMed] [Google Scholar]
- 19.Leary SC, Kaufman BA, Pellecchia G, Guercin GH, Mattman A, Jaksch M, Shoubridge EA. 2004. Human SCO1 and SCO2 have independent, cooperative functions in copper delivery to cytochrome c oxidase. Hum Mol Genet 13:1839–1848. doi: 10.1093/hmg/ddh197. [DOI] [PubMed] [Google Scholar]
- 20.Carr HS, George GN, Winge DR. 2002. Yeast Cox11, a protein essential for cytochrome c oxidase assembly, is a Cu(I)-binding protein. J Biol Chem 277:31237–31242. doi: 10.1074/jbc.M204854200. [DOI] [PubMed] [Google Scholar]
- 21.Baker ZN, Jett K, Boulet A, Hossain A, Cobine PA, Kim BE, El Zawily AM, Lee L, Tibbits GF, Petris MJ, Leary SC. 2017. The mitochondrial metallochaperone SCO1 maintains CTR1 at the plasma membrane to preserve copper homeostasis in the murine heart. Hum Mol Genet 26:4617–4628. doi: 10.1093/hmg/ddx344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banci L, Bertini I, Ciofi-Baffoni S, Katsari E, Katsaros N, Kubicek K, Mangani S. 2005. A copper(I) protein possibly involved in the assembly of CuA center of bacterial cytochrome c oxidase. Proc Natl Acad Sci U S A 102:3994–3999. doi: 10.1073/pnas.0406150102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swem DL, Swem LR, Setterdahl A, Bauer CE. 2005. Involvement of SenC in assembly of cytochrome c oxidase in Rhodobacter capsulatus. J Bacteriol 187:8081–8087. doi: 10.1128/JB.187.23.8081-8087.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson AK, Gray J, Liu A, Hosler JP. 2012. The roles of Rhodobacter sphaeroides copper chaperones PCuAC and Sco (PrrC) in the assembly of the copper centers of the aa3-type and the cbb3-type cytochrome c oxidases. Biochim Biophys Acta 1817:955–964. doi: 10.1016/j.bbabio.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiser L, Di Valentin M, Hamer AG, Hosler JP. 2000. Cox11p is required for stable formation of the CuB and magnesium centers of cytochrome c oxidase. J Biol Chem 275:619–623. doi: 10.1074/jbc.275.1.619. [DOI] [PubMed] [Google Scholar]
- 26.Ducluzeau AL, Ouchane S, Nitschke W. 2008. The cbb3 oxidases are an ancient innovation of the domain bacteria. Mol Biol Evol 25:1158–1166. doi: 10.1093/molbev/msn062. [DOI] [PubMed] [Google Scholar]
- 27.Ekici S, Pawlik G, Lohmeyer E, Koch HG, Daldal F. 2012. Biogenesis of cbb3-type cytochrome c oxidase in Rhodobacter capsulatus. Biochim Biophys Acta 1817:898–910. doi: 10.1016/j.bbabio.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buschmann S, Warkentin E, Xie H, Langer JD, Ermler U, Michel H. 2010. The structure of cbb3 cytochrome oxidase provides insights into proton pumping. Science 329:327–330. doi: 10.1126/science.1187303. [DOI] [PubMed] [Google Scholar]
- 29.Koch HG, Winterstein C, Saribas AS, Alben JO, Daldal F. 2000. Roles of the ccoGHIS gene products in the biogenesis of the cbb3-type cytochrome c oxidase. J Mol Biol 297:49–65. doi: 10.1006/jmbi.2000.3555. [DOI] [PubMed] [Google Scholar]
- 30.González-Guerrero M, Raimunda D, Cheng X, Argüello JM. 2010. Distinct functional roles of homologous Cu+ efflux ATPases in Pseudomonas aeruginosa. Mol Microbiol 78:1246–1258. doi: 10.1111/j.1365-2958.2010.07402.x. [DOI] [PubMed] [Google Scholar]
- 31.Hassani BK, Astier C, Nitschke W, Ouchane S. 2010. CtpA, a copper-translocating P-type ATPase involved in the biogenesis of multiple copper-requiring enzymes. J Biol Chem 285:19330–19337. doi: 10.1074/jbc.M110.116020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ekici S, Turkarslan S, Pawlik G, Dancis A, Baliga NS, Koch HG, Daldal F. 2014. Intracytoplasmic copper homeostasis controls cytochrome c oxidase production. mBio 5:e01055-13. doi: 10.1128/mBio.01055-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trasnea PI, Utz M, Khalfaoui-Hassani B, Lagies S, Daldal F, Koch HG. 2016. Cooperation between two periplasmic copper chaperones is required for full activity of the cbb3 -type cytochrome c oxidase and copper homeostasis in Rhodobacter capsulatus. Mol Microbiol 100:345–361. doi: 10.1111/mmi.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ekici S, Yang H, Koch HG, Daldal F. 2012. Novel transporter required for biogenesis of cbb3-type cytochrome c oxidase in Rhodobacter capsulatus. mBio 3:e00293-11. doi: 10.1128/mBio.00293-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beaudoin J, Ekici S, Daldal F, Ait-Mohand S, Guérin B, Labbé S. 2013. Copper transport and regulation in Schizosaccharomyces pombe. Biochem Soc Trans 41:1679–1686. doi: 10.1042/BST2013089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saier MH Jr., Reddy VS, Tsu BV, Ahmed MS, Li C, Moreno-Hagelsieb G. 2016. The Transporter Classification Database (TCDB): recent advances. Nucleic Acids Res 44:D372–D379. doi: 10.1093/nar/gkv1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khalfaoui-Hassani B, Verissimo AF, Koch HG, Daldal F. 2016. Uncovering the transmembrane metal binding site of the novel bacterial major facilitator superfamily-type copper importer CcoA. mBio 7:e01981-15. doi: 10.1128/mBio.01981-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shapleigh JP, Gennis RB. 1992. Cloning, sequencing and deletion from the chromosome of the gene encoding subunit I of the aa3-type cytochrome c oxidase of Rhodobacter sphaeroides. Mol Microbiol 6:635–642. doi: 10.1111/j.1365-2958.1992.tb01511.x. [DOI] [PubMed] [Google Scholar]
- 39.Daldal F, Mandaci S, Winterstein C, Myllykallio H, Duyck K, Zannoni D. 2001. Mobile cytochrome c2 and membrane-anchored cytochrome cy are both efficient electron donors to the cbb3- and aa3-type cytochrome c oxidases during respiratory growth of Rhodobacter sphaeroides. J Bacteriol 183:2013–2024. doi: 10.1128/JB.183.6.2013-2024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toledo-Cuevas M, Barquera B, Gennis RB, Wikström M, García-Horsman JA. 1998. The cbb3-type cytochrome c oxidase from Rhodobacter sphaeroides, a proton-pumping heme-copper oxidase. Biochim Biophys Acta 1365:421–434. doi: 10.1016/S0005-2728(98)00095-4. [DOI] [PubMed] [Google Scholar]
- 41.Hosler JP, Fetter J, Tecklenburg MM, Espe M, Lerma C, Ferguson-Miller S. 1992. Cytochrome aa3 of Rhodobacter sphaeroides as a model for mitochondrial cytochrome c oxidase. Purification, kinetics, proton pumping, and spectral analysis. J Biol Chem 267:24264–24272. [PubMed] [Google Scholar]
- 42.Thomas PE, Ryan D, Levin W. 1976. Improved staining procedure for detection of peroxidase activity of cytochrome P-450 on sodium dodecyl-sulfate polyacrylamide gels. Anal Biochem 75:168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- 43.Gurumoorthy P, Ludwig B. 2015. Deciphering protein-protein interactions during the biogenesis of cytochrome c oxidase from Paracoccus denitrificans. FEBS J 282:537–549. doi: 10.1111/febs.13160. [DOI] [PubMed] [Google Scholar]
- 44.Peuser V, Glaeser J, Klug G. 2011. The RSP_2889 gene product of Rhodobacter sphaeroides is a CueR homologue controlling copper-responsive genes. Microbiology 157:3306–3313. doi: 10.1099/mic.0.051607-0. [DOI] [PubMed] [Google Scholar]
- 45.Overbeek R, Begley T, Butler RM, Choudhuri JV, Chuang HY, Cohoon M, de Crécy-Lagard V, Diaz N, Disz T, Edwards R, Fonstein M, Frank ED, Gerdes S, Glass EM, Goesmann A, Hanson A, Iwata-Reuyl D, Jensen R, Jamshidi N, Krause L, Kubal M, Larsen N, Linke B, McHardy AC, Meyer F, Neuweger H, Olsen G, Olson R, Osterman A, Portnoy V, Pusch GD, Rodionov DA, Rückert C, Steiner J, Stevens R, Thiele I, Vassieva O, Ye Y, Zagnitko O, Vonstein V. 2005. The subsystems approach to genome annotation and its use in the project to annotate 1000 genomes. Nucleic Acids Res 33:5691–5702. doi: 10.1093/nar/gki866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ekici S, Jiang X, Koch HG, Daldal F. 2013. Missense mutations in cytochrome c maturation genes provide new insights into Rhodobacter capsulatus cbb3-type cytochrome c oxidase biogenesis. J Bacteriol 195:261–269. doi: 10.1128/JB.01415-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schimo S, Wittig I, Pos KM, Ludwig B. 2017. Cytochrome c oxidase biogenesis and metallochaperone interactions: steps in the assembly pathway of a bacterial complex. PLoS One 12:e0170037. doi: 10.1371/journal.pone.0170037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sandri F, Fedi S, Cappelletti M, Calabrese FM, Turner RJ, Zannoni D. 2017. Biphenyl modulates the expression and function of respiratory oxidases in the polychlorinated-biphenyls degrader Pseudomonas pseudoalcaligenes KF707. Front Microbiol 8:1223. doi: 10.3389/fmicb.2017.01223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pereira MM, Sousa FL, Veríssimo AF, Teixeira M. 2008. Looking for the minimum common denominator in haem-copper oxygen reductases: towards a unified catalytic mechanism. Biochim Biophys Acta 1777:929–934. doi: 10.1016/j.bbabio.2008.05.441. [DOI] [PubMed] [Google Scholar]
- 50.Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O’Halloran TV. 1999. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science 284:805–808. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 51.O’Halloran TV, Culotta VC. 2000. Metallochaperones, an intracellular shuttle service for metal ions. J Biol Chem 275:25057–25060. doi: 10.1074/jbc.R000006200. [DOI] [PubMed] [Google Scholar]
- 52.Glerum DM, Shtanko A, Tzagoloff A. 1996. SCO1 and SCO2 act as high copy suppressors of a mitochondrial copper recruitment defect in Saccharomyces cerevisiae. J Biol Chem 271:20531–20535. doi: 10.1074/jbc.271.34.20531. [DOI] [PubMed] [Google Scholar]
- 53.Lovett ST. 2004. Encoded errors: mutations and rearrangements mediated by misalignment at repetitive DNA sequences. Mol Microbiol 52:1243–1253. doi: 10.1111/j.1365-2958.2004.04076.x. [DOI] [PubMed] [Google Scholar]
- 54.Moxon R, Bayliss C, Hood D. 2006. Bacterial contingency loci: the role of simple sequence DNA repeats in bacterial adaptation. Annu Rev Genet 40:307–333. doi: 10.1146/annurev.genet.40.110405.090442. [DOI] [PubMed] [Google Scholar]
- 55.Zhou K, Aertsen A, Michiels CW. 2014. The role of variable DNA tandem repeats in bacterial adaptation. FEMS Microbiol Rev 38:119–141. doi: 10.1111/1574-6976.12036. [DOI] [PubMed] [Google Scholar]
- 56.Lin WH, Kussell E. 2012. Evolutionary pressures on simple sequence repeats in prokaryotic coding regions. Nucleic Acids Res 40:2399–2413. doi: 10.1093/nar/gkr1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gourdon P, Liu XY, Skjørringe T, Morth JP, Møller LB, Pedersen BP, Nissen P. 2011. Crystal structure of a copper-transporting PIB-type ATPase. Nature 475:59–64. doi: 10.1038/nature10191. [DOI] [PubMed] [Google Scholar]
- 58.Fu Y, Tsui HC, Bruce KE, Sham LT, Higgins KA, Lisher JP, Kazmierczak KM, Maroney MJ, Dann CE III, Winkler ME, Giedroc DP. 2013. A new structural paradigm in copper resistance in Streptococcus pneumoniae. Nat Chem Biol 9:177–183. doi: 10.1038/nchembio.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blaby-Haas CE, Padilla-Benavides T, Stübe R, Argüello JM, Merchant SS. 2014. Evolution of a plant-specific copper chaperone family for chloroplast copper homeostasis. Proc Natl Acad Sci U S A 111:E5480–E5487. doi: 10.1073/pnas.1421545111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drees SL, Beyer DF, Lenders-Lomscher C, Lübben M. 2015. Distinct functions of serial metal-binding domains in the Escherichia coli P1 B -ATPase CopA. Mol Microbiol 97:423–438. doi: 10.1111/mmi.13038. [DOI] [PubMed] [Google Scholar]
- 61.Meydan S, Klepacki D, Karthikeyan S, Margus T, Thomas P, Jones JE, Khan Y, Briggs J, Dinman JD, Vázquez-Laslop N, Mankin AS. 2017. Programmed ribosomal frameshifting generates a copper transporter and a copper chaperone from the same gene. Mol Cell 65:207–219. doi: 10.1016/j.molcel.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ettema TJ, Huynen MA, de Vos WM, van der Oost J. 2003. TRASH: a novel metal-binding domain predicted to be involved in heavy-metal sensing, trafficking and resistance. Trends Biochem Sci 28:170–173. doi: 10.1016/S0968-0004(03)00037-9. [DOI] [PubMed] [Google Scholar]
- 63.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 64.Sistrom WR. 1960. A requirement for sodium in the growth of Rhodopseudomonas spheroides. J Gen Microbiol 22:778–785. doi: 10.1099/00221287-22-3-778. [DOI] [PubMed] [Google Scholar]
- 65.Gray KA, Davidson E, Daldal F. 1992. Mutagenesis of methionine-183 drastically affects the physicochemical properties of cytochrome c1 of the bc1 complex of Rhodobacter capsulatus. Biochemistry 31:11864–11873. doi: 10.1021/bi00162a027. [DOI] [PubMed] [Google Scholar]
- 66.Marrs B, Stahl CL, Lien S, Gest H. 1972. Biochemical physiology of a respiration-deficient mutant of the photosynthetic bacterium Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A 69:916–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees, p 45–52. In Proceedings of the Gateway Computing Environments Workshop (GCE 2010), New Orleans, LA, 14 November 2010 Institute of Electrical and Electronics Engineers, Piscataway, NJ: http://www.phylo.org/sub_sections/portal/sc2010_paper.pdf. [Google Scholar]
- 68.Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Price MN, Dehal PS, Arkin AP. 2010. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS One 5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Letunic I, Bork P. 2016. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 44:W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boulet A, Vest KE, Maynard MK, Gammon MG, Russell AC, Mathews AT, Cole SE, Zhu X, Phillips CB, Kwong JQ, Dodani SC, Leary SC, Cobine PA. 13 December 2017. The mammalian phosphate carrier SLC25A3 is a mitochondrial copper transporter required for cytochrome c oxidase biogenesis. J Biol Chem doi: 10.1074/jbc.RA117.000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Co-occurrence of CcoA, aa3-Cox, and cbb3-Cox in alphaproteobacterial species. Download TABLE S1, XLSX file, 0.2 MB (245.2KB, xlsx) .
Copyright © 2018 Khalfaoui-Hassani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Distribution of CcoA, cbb3-Cox, and aa3-Cox among the alphaproteobacterial genomes analyzed in this study. Download TABLE S2, DOCX file, 0.01 MB (14.8KB, docx) .
Copyright © 2018 Khalfaoui-Hassani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Strains and plasmids used in this study. Download TABLE S3, DOCX file, 0.03 MB (28.9KB, docx) .
Copyright © 2018 Khalfaoui-Hassani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Protein similarity network of CcoA-like putative transporters obtained by using the SEED database. Download TABLE S4, XLSX file, 0.8 MB (789KB, xlsx) .
Copyright © 2018 Khalfaoui-Hassani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Evolutionary relationship between CcoA homologues and the corresponding phylogenetic tree. Download TABLE S5, XLSX file, 0.2 MB (245.3KB, xlsx) .
Copyright © 2018 Khalfaoui-Hassani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Primers used in this study. Download TABLE S6, DOCX file, 0.01 MB (14KB, docx) .
Copyright © 2018 Khalfaoui-Hassani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.