Abstract
OBJECTIVE
Improved risk assessment for patients with type 2 diabetes and elevated cardiovascular (CV) risk is needed. The Thrombolysis in Myocardial Infarction (TIMI) Risk Score for Secondary Prevention (TRS 2°P) predicts a gradient of risk in patients with prior myocardial infarction (MI) but has not been evaluated in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
CV event rates were compared by baseline TRS 2°P in 16,488 patients enrolled in SAVOR-TIMI 53 (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction 53) with type 2 diabetes and high CV risk or established CV disease. Calibration was tested in the diabetes cohort from the REACH (REduction of Atherothrombosis for Continued Health) Registry.
RESULTS
TRS 2°P revealed a robust risk gradient for the composite of CV death, MI, and ischemic stroke in the full trial population, with 2-year event rates of 0.9% in the lowest- and 19.8% in the highest-risk groups (Ptrend < 0.001). A clear risk gradient was present within the subgroups of all coronary artery disease (CAD), CAD without prior MI, CAD with prior MI, peripheral artery disease, and prior stroke (Ptrend < 0.001 for each), with consistent risk relationships across subgroups. The C-statistic (0.71 for CV death and 0.66 for the composite end point) was consistent in each subgroup. There was close calibration with the type 2 diabetes cohort from the REACH Registry (goodness-of-fit P = 0.78).
CONCLUSIONS
The expanded TRS 2°P provides a practical and well-calibrated risk prediction tool for patients with type 2 diabetes.
Introduction
Type 2 diabetes, which is expected to afflict 500 million people worldwide by 2030, is associated with at least a twofold increase in the risk of cardiovascular (CV) events (1–3). Concurrently, medical therapies for primary and secondary prevention of atherothrombotic events, including new antihyperglycemic (4–7), lipid-lowering (8,9), and antiplatelet (10,11) agents with potential or proven CV benefit, are expanding rapidly. These broadening therapeutic options for prevention heighten the need for accurate CV risk quantification; yet, limited tools exist for risk assessment for patients with type 2 diabetes and established CV disease, particularly tools based on contemporary data (12–17).
The recently reported Thrombolysis in Myocardial Infarction (TIMI) Risk Score for Secondary Prevention (TRS 2°P) was developed among patients with prior myocardial infarction (MI), with demonstrated robust risk assessment in that group (10,18). TRS 2°P additionally predicted degree of benefit with the thrombin receptor antagonist vorapaxar (18). The risk score was further validated in a large trial of patients who had recently experienced an acute coronary event and identified a subgroup of patients in whom the coadministration of ezetimibe was beneficial (8,19). TRS 2°P has not been investigated specifically in patients with type 2 diabetes with or without established CV disease.
Because TRS 2°P was derived in a cohort exclusively comprising patients with prior MI or recent acute coronary syndrome, this variable was not a component in the original score. Given the clinical importance of prior MI for patients with type 2 diabetes (20), this variable is included here in the risk score. We examined the ability of this expanded score to discriminate risk in patients with type 2 diabetes and established CV disease or high CV risk enrolled in the SAVOR-TIMI 53 (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction 53) trial (21,22). To assess TRS 2°P in a second large cohort of patients with type 2 diabetes, we additionally applied the risk score to the cohort with diabetes of the REACH (REduction of Atherothrombosis for Continued Health) Registry (23).
Research Design and Methods
Study Design and Participants
The study design, baseline patient characteristics, and primary findings of the SAVOR-TIMI 53 trial (NCT01107886) have previously been reported (21,22,24). SAVOR-TIMI 53 was a randomized, controlled, double-blind, multinational, event-driven trial among patients with type 2 diabetes and moderate-to-high CV risk as determined by prior manifest CV disease or multiple CV risk factors. Patients with a history of either end-stage renal disease on chronic dialysis, serum creatinine >6.0 mg/dL, or previous kidney transplant were excluded. Patients were randomized to treatment with the dipeptidyl peptidase-4 inhibitor saxagliptin or matching placebo with concurrent glucose-lowering medications and CV therapies, including diet and lifestyle modification, managed by the treating clinician.
End Points
The primary end point of SAVOR-TIMI 53 was the composite of CV death, MI, or ischemic stroke. A blinded, independent clinical events committee adjudicated each component of the primary end point as well as hospitalization for heart failure according to prespecified criteria (21,22,24).
TRS 2°P
Bohula et al. (18) derived TRS 2°P in the placebo arm of the TRA 2°P-TIMI 50 (Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events–Thrombolysis in Myocardial Infarction 50) trial (NCT00526474) (10,18) among patients whose qualifying event was MI (n = 8,598). The design and primary results of the TRA 2°P-TIMI 50 trial have previously been described (10,25). Univariate predictors of CV death, MI, or ischemic stroke were identified with Cox proportional hazards and then refined to a final set of nine variables by forward and backward selection with a selection threshold of P < 0.01 (18). Each variable was given even weighting, such that total risk would be defined by the sum of risk indicators. The nine predictor variables identified in this manner were age ≥75 years, diabetes, hypertension, current smoking, peripheral artery disease (PAD), prior stroke, prior coronary artery bypass grafting, history of heart failure, and renal dysfunction (estimated glomerular filtration rate <60 mL/min/1.73 m2 by the MDRD formula).
Statistical Analysis
TRS 2°P was calculated at baseline for each patient with a point value of 1 assigned for each clinical variable listed above (because TRS 2°P was derived in a cohort exclusively comprising patients with prior MI, this variable was not a component in the original score; in the present analysis, a history of MI was added as a tenth risk factor given the clinical importance of this variable in patients with diabetes) (20). Because all patients enrolled in SAVOR-TIMI 53 were required to have type 2 diabetes as an entry criterion, the lowest possible score was 1. Baseline patient characteristics were reported by bin of TRS 2°P (≤2, 3, 4, ≥5) with continuous variables described by median and interquartile range and categorical variables described by percentage. Baseline variables were compared between groups with the Kruskal-Wallis test for continuous variables or the χ2 test for categorical variables.
Two-year Kaplan-Meier event rates were calculated by TRS 2°P in the full trial population for the primary composite end point and the individual components of CV death, MI, and ischemic stroke. Kaplan-Meier event rates were also computed by bin of TRS 2°P (≤2, 3, 4, ≥5). These analyses were repeated in the subgroups of patients with any coronary artery disease (CAD), CAD without prior MI, CAD with prior MI, PAD, prior stroke, and prior heart failure. CAD was defined as objective evidence of stenosis ≥50% in at least two coronary arteries, prior presumed spontaneous MI, or prior percutaneous intervention or bypass graft with revascularization of >1 artery (21). PAD was defined as intermittent claudication symptoms plus an ankle (or toe) brachial pressure index <0.90 within the preceding 12 months, prior peripheral revascularization, or prior amputation of the legs at any level due to arterial obstructive disease (21). Hazard ratios (HRs) were calculated for the primary end point, CV death, MI, and ischemic stroke using a Cox proportional hazards model for each TRS 2°P value in the full trial population relative to the minimum score. Harrell C-statistics were calculated for the primary end point and CV death in the full trial population and the key subgroups defined above.
The interaction between randomized treatment assignment and the relationship between TRS 2°P and outcomes was tested. Calibration was tested between SAVOR-TIMI 53 and the subgroup with type 2 diabetes (n = 15,852) from the REACH Registry, which enrolled >60,000 outpatients worldwide with or at high risk for atherothrombotic disease (12,26) using the Nam-D’Agostino test (27). The baseline patient characteristics and 1- and 4-year CV outcomes from the REACH Registry have previously been published (23,28,29). Forty-four percent of patients (n = 30,075) had type 2 diabetes at enrollment, 81.8% (n = 55,533) had hypertension, 59.3% (n = 40,258) had CAD, 27.8% (n = 18,843) had cerebrovascular disease, and 12.2% (n = 8,273) had PAD (26). Clinical events were not adjudicated (26).
All analyses were performed with a statistical software package (SAS, version 9.4; SAS Institute, Cary, NC). A two-sided P value of 0.05 was considered significant for all tests. All analyses were performed by the TIMI Study Group, and the authors take full responsibility for the integrity of the database and the analyses.
Results
Baseline Characteristics
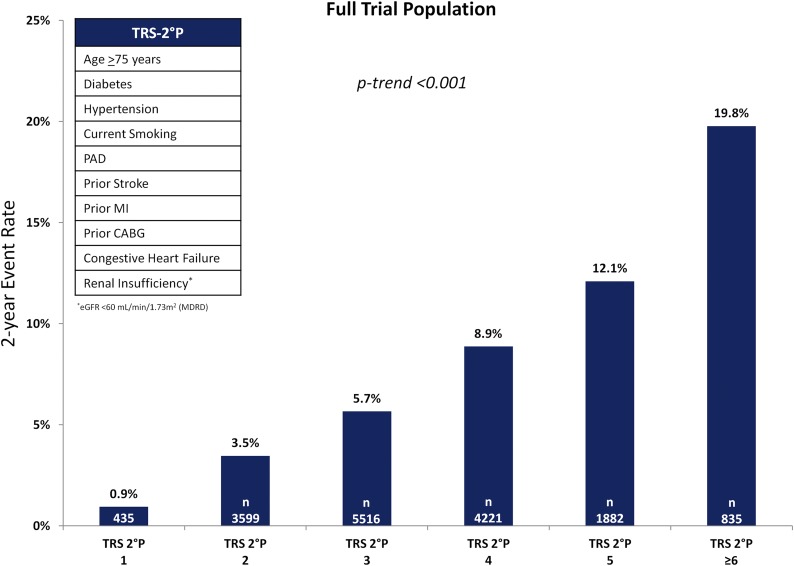
All 10 required baseline TRS 2°P variables were available for 16,488 of the 16,492 patients enrolled in SAVOR-TIMI 53, in whom 1,222 primary end point events, including 529 CV deaths, 543 MIs, and 298 ischemic strokes, occurred. The distribution of TRS 2°P values in the full trial population is shown in Fig. 1. A total of 10,276 patients were classified as having any CAD, 4,040 as CAD with no prior MI, 6,236 as prior MI, 1,958 as PAD, and 2,094 as prior stroke. Full baseline patient characteristics by TRS 2°P bin are presented in Table 1. All 10 of the TRS 2°P clinical variables except for hypertension were associated independently with the primary end point (Table 2). Higher TRS 2°P values were associated with a greater frequency of each of the component baseline variables in the score (Table 1). Outside of the TRS 2°P variables, patients with higher scores were more likely to be male (70.3% vs. 63.6%) and have a history of atrial fibrillation (17.8% vs. 4.5%) (Table 1).
Figure 1.
Two-year Kaplan-Meier rate of CV death, MI, or ischemic stroke in the full trial population. CABG, coronary artery bypass graft; eGFR, estimated glomerular filtration rate.
Table 1.
Baseline patient characteristics*
| Characteristics | Full population (n = 16,488) | TRS 2°P |
|||
|---|---|---|---|---|---|
| ≤2 (n = 4,034 [24.5%]) | 3 (n = 5,516 [33.5%]) | 4 (n = 4,221 [25.6%]) | ≥5 (n = 2,717 [16.5%]) | ||
| Age (years) | 65.0 (60.0–71.0) | 63.0 (59.0–68.0) | 64.0 (58.0–69.0) | 66.0 (60.0–73.0) | 71.0 (63.0–77.0) |
| Female, n (%) | 5,454 (33.1) | 1,468 (36.4) | 1,852 (33.6) | 1,328 (31.5) | 806 (29.7) |
| Age >65 years, n (%) | 7,821 (47.4) | 1,503 (37.3) | 2,292 (41.6) | 2,210 (52.4) | 1,816 (66.8) |
| BMI (kg/m2) | 30.5 (27.2–34.4) | 30.5 (27.3–34.5) | 30.4 (27.1–34.4) | 30.4 (27.2–34.4) | 30.6 (27.4–34.5) |
| Hypertension, n (%) | 13,492 (81.8) | 2,397 (59.4) | 4,677 (84.8) | 3,852 (91.3) | 2,566 (94.4) |
| Dyslipidemia, n (%) | 11,738 (71.2) | 2,674 (66.3) | 3,794 (68.8) | 3,135 (74.3) | 2,135 (78.6) |
| Established atherosclerotic disease, n (%) | 13,139 (79.7) | 2,002 (49.6) | 4,489 (81.4) | 3,957 (93.7) | 2,691 (99.0) |
| CAD, n (%) | 10,276 (62.3) | 1,577 (39.1) | 3,245 (58.8) | 3,075 (72.9) | 2,379 (87.6) |
| Prior MI, n (%) | 6,236 (37.8) | 459 (11.4) | 1,816 (32.9) | 2,083 (49.3) | 1,878 (69.1) |
| Prior PCI >1 artery, n (%) | 4,039 (24.5) | 911 (22.6) | 1,309 (23.7) | 1,060 (25.1) | 759 (27.9) |
| Prior coronary revascularization, n (%) | 7,122 (43.2) | 1,041 (25.8) | 2,088 (37.9) | 2,147 (50.9) | 1,846 (67.9) |
| PAD, n (%) | 1,958 (11.9) | 156 (3.9) | 562 (10.2) | 604 (14.3) | 636 (23.4) |
| Prior heart failure, n (%) | 2,105 (12.8) | 18 (0.4) | 245 (4.4) | 696 (16.5) | 1,146 (42.2) |
| Prior atrial fibrillation, n (%) | 1,202 (7.3) | 139 (3.4) | 289 (5.2) | 372 (8.8) | 402 (14.8) |
| Prior ischemic stroke, n (%) | 2,094 (12.7) | 130 (3.2) | 697 (12.6) | 663 (15.7) | 604 (22.2) |
| Current smoker, n (%) | 2,219 (13.5) | 146 (3.6) | 676 (12.3) | 821 (19.5) | 576 (21.2) |
| eGFR (mL/min/1.73 m2) | 71.7 (57.1–86.4) | 80.6 (70.4–94.0) | 75.2 (63.7–89.2) | 64.4 (51.9–81.8) | 53.3 (43.0–64.7) |
| Glycated hemoglobin, %; mmol/mol | 7.6 (6.9–8.7); 60 (52–72) | 7.6 (6.9–8.7); 60 (52–72) | 7.7 (6.9–8.8); 61 (52–73) | 7.7 (7.0–8.8); 61 (53–73) | 7.6 (7.0–8.6); 60 (53–70) |
| Saxagliptin, n (%) | 8,278 (50.2) | 2,074 (51.4) | 2,762 (50.1) | 2,051 (48.6) | 1,391 (51.2) |
Continuous variables are presented as median (quartile 1–quartile 3). eGFR, estimated glomerular filtration rate; PCI, percutaneous coronary intervention.
*All P values <0.05 except for BMI and saxagliptin assignment.
Table 2.
Univariable clinical variables included in TRS 2°P and adjusted risk for the composite end point
| Predictor variable | N (%) | HR (95% CI) | P |
|---|---|---|---|
| Age >75 years | 2,330 (14.1) | 1.67 (1.46–1.92) | <0.001 |
| Diabetes | 16,488 (100.0) | — | — |
| Hypertension | 13,492 (81.8) | 1.11 (0.96–1.29) | 0.167 |
| Current smoking | 2,219 (13.5) | 1.21 (1.03–1.41) | 0.017 |
| PAD | 1,958 (11.9) | 1.58 (1.36–1.84) | <0.001 |
| Prior stroke | 2,094 (12.7) | 1.53 (1.32–1.77) | <0.001 |
| Prior MI | 6,236 (37.8) | 1.73 (1.55–1.94) | <0.001 |
| Prior CABG | 3,934 (23.9) | 1.46 (1.29–1.65) | <0.001 |
| Congestive heart failure | 2,105 (12.8) | 2.15 (1.89–2.46) | <0.001 |
| Renal insufficiency | 4,811 (29.2) | 1.99 (1.78–2.23) | <0.001 |
CABG, coronary artery bypass graft.
Full Trial Population
A risk gradient by increasing TRS 2°P was present in the full trial population, with 2-year event rates for the composite end point ranging from 0.9% in the lowest-risk group to 19.8% in the highest-risk group (Ptrend < 0.001) (Fig. 1). The risk of the primary composite end point for TRS 2°P ≥5 was over fourfold that for a score of ≤2 (HR 4.85 [95% CI 4.00–5.88]; P < 0.001). Similar risk gradients were observed for the individual outcomes of CV death, MI, and ischemic stroke (Table 3). The C-statistic was 0.71 (95% CI 0.69–0.73) for CV death and 0.66 (95% CI 0.64–0.67) for the primary end point.
Table 3.
Two-year Kaplan-Meier event rates by TRS 2°P and subgroup
| TRS 2°P |
Ptrend | ||||
|---|---|---|---|---|---|
| ≤2 | 3 | 4 | ≥5 | ||
| Full trial population | |||||
| n | 4,034 | 5,516 | 4,221 | 2,717 | |
| CV death | 1.0 | 2.1 | 4.1 | 6.8 | <0.001 |
| MI | 1.5 | 2.7 | 3.5 | 7.1 | <0.001 |
| Ischemic stroke | 1.0 | 1.4 | 2.4 | 3.0 | <0.001 |
| All CAD | |||||
| n | 1,577 | 3,245 | 3,075 | 2,379 | |
| CV death | 1.3 | 2.3 | 4.2 | 6.5 | <0.001 |
| MI | 2.9 | 3.4 | 3.9 | 7.5 | <0.001 |
| Ischemic stroke | 1.1 | 1.3 | 2.2 | 2.9 | <0.001 |
| CAD with no prior MI | |||||
| n | 1,118 | 1,429 | 992 | 501 | |
| CV death | 1.3 | 1.7 | 5.0 | 6.3 | <0.001 |
| MI | 2.5 | 3.7 | 2.8 | 6.5 | 0.019 |
| Ischemic stroke | 1.1 | 1.8 | 2.5 | 2.8 | 0.014 |
| Prior MI | |||||
| n | 459 | 1,816 | 2,083 | 1,878 | |
| CV death | 1.3 | 2.7 | 3.8 | 6.6 | <0.001 |
| MI | 3.7 | 3.3 | 4.4 | 7.8 | <0.001 |
| Ischemic stroke | 0.9 | 0.8 | 2.1 | 2.9 | <0.001 |
| PAD | |||||
| n | 156 | 562 | 604 | 6,363 | |
| CV death | 2.2 | 2.0 | 5.4 | 9.9 | <0.001 |
| MI | 0.9 | 2.0 | 2.2 | 10.2 | <0.001 |
| Ischemic stroke | 1.3 | 0.6 | 3.7 | 3.2 | 0.006 |
| Prior stroke | |||||
| n | 130 | 697 | 663 | 604 | |
| CV death | 0.0 | 1.6 | 2.4 | 7.5 | <0.001 |
| MI | 0.8 | 2.0 | 3.5 | 8.7 | <0.001 |
| Ischemic stroke | 4.3 | 2.5 | 4.0 | 5.1 | 0.039 |
Data are percentages unless otherwise indicated.
TRS 2°P was also associated with hospitalization for heart failure, with 2-year event rates of 0.7% in patients with a score of 1 compared with 12.9% in the highest-risk group (HR 12.51 [95% CI 8.73–17.92], P < 0.001, for TRS 2°P >5 vs. <2). For the composite of CV death and heart failure hospitalization, the 2-year event rates were 1.5% vs. 19.6% (HR 9.13 [95% CI 7.09–11.76], P < 0.001) (Supplementary Fig. 1).
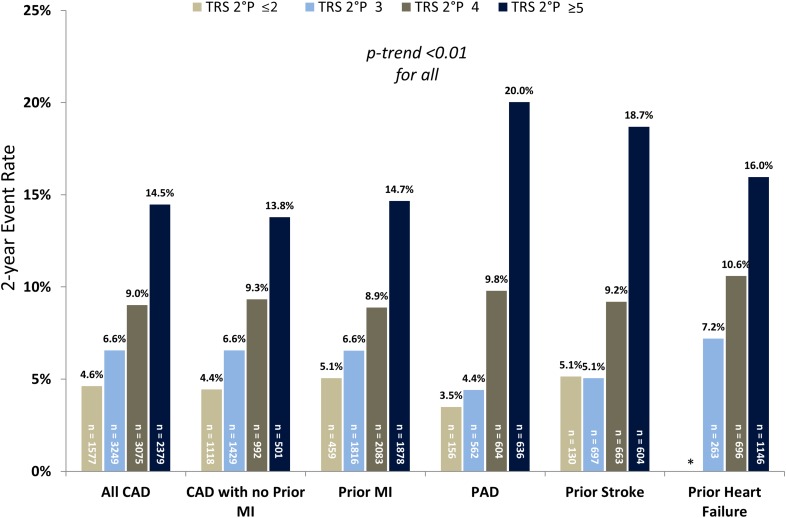
CAD
In the subgroups of patients with established coronary atherothrombotic disease (all CAD, CAD without prior MI, and CAD with prior MI), TRS 2°P again supported significant assessment of risk (Fig. 2). Patients with TRS 2°P ≥5 in the all CAD subgroup had threefold increased risk of the primary end point compared with patients with the TRS 2°P ≤2 (HR 3.33 [95% CI 2.58–4.28], P < 0.001). Similarly, in the subgroups of CAD patients without or with prior MI, the risk of the primary end point was significantly increased in patients with TRS 2°P ≥5 relative to ≤2 (HR 3.06 [95% CI 2.1–4.43], P < 0.001, for no prior MI and HR 3.19 [95% CI 2.09–4.88], P < 0.001, for prior MI). Risk stratification for additional end points was similar (Table 3). The C-statistics for TRS 2°P for CV death and the primary end point were 0.68 (95% CI 0.65–0.71) and 0.63 (0.61–0.65) in the all CAD cohort, 0.70 (0.66–0.75) and 0.63 (0.59–0.66) in the CAD with no prior MI cohort, and 0.68 (0.64–0.71) and 0.63 (0.61–0.66) in the prior MI cohort, respectively.
Figure 2.
Two-year Kaplan-Meier rate of CV death, MI, or ischemic stroke by established atherosclerotic disease subgroup. *Minimum TRS 2°P presented as ≤3 in the prior heart failure subgroup.
PAD and Prior Stroke
The 2-year rates of major adverse cardiovascular events by TRS 2°P for patients with PAD or prior stroke are shown in Fig. 2. The rates ranged from 3.5% and 5.1% for TRS 2°P ≤2 in the PAD and prior stroke subgroups, respectively, to 20.0% and 18.7% for TRS 2°P ≥5. Findings were similar for additional end points (Table 3). The risk of major adverse cardiovascular events for TRS 2°P ≥5 vs. ≤2 in these two groups was HR 5.60 (95% CI 2.46–12.71; P < 0.001) for patients with PAD and HR 3.70 (95% CI 1.72–7.95; P < 0.001) for patients with prior stroke. The C-statistics for CV death and the primary end point were 0.71 (95% CI 0.66–0.76) and 0.70 (0.66–0.73) in the PAD subgroup and 0.75 (0.69–0.80) and 0.67 (0.63–0.71) in the prior stroke subgroup, respectively.
Interaction by Randomized Treatment
There was no interaction with saxagliptin for the primary outcome (Pinteraction = 0.66), CV death (Pinteraction = 0.60), or heart failure (Pinteraction = 0.72) in the full trial population after stratification by TRS 2°P.
Calibration
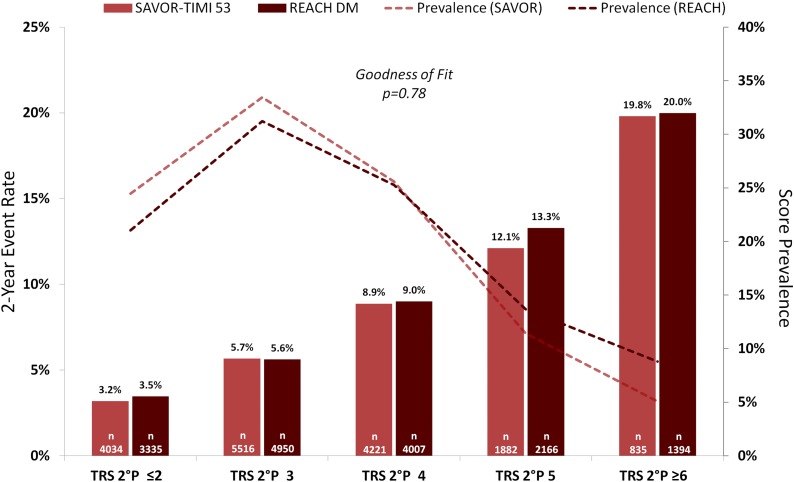
TRS 2°P calibration was tested between the patients in SAVOR-TIMI 53 and the patients with diabetes in the REACH Registry. The C-statistic for TRS 2°P in the REACH diabetes subgroup was 0.73 (95% CI 0.72–0.75) for CV death and 0.67 (0.66–0.69) for CV death/MI/ischemic stroke.
Two-year Kaplan-Meier event rates for the composite of CV death, MI, or ischemic stroke are shown for the two cohorts in Fig. 3. There was close calibration between these two populations (goodness-of-fit P = 0.78).
Figure 3.
Two-year Kaplan-Meier rate of CV death, MI, or ischemic stroke in SAVOR-TIMI 53 and patients in the REACH Registry with type 2 diabetes (REACH DM).
Conclusions
There is a need for simple, reliable, and clinically meaningful CV risk assessments in patients with type 2 diabetes. In this analysis, we demonstrate that TRS 2°P distinguishes a clear gradient of risk, with a fourfold higher risk of the primary composite end point in patients with TRS 2°P ≥5 compared with a score ≤2. The risk gradation was consistent across the different clinical end points and in key disease-specific subgroups, including patients with and patients without prior MI. Moreover, there was robust calibration of TRS 2°P in a second large population of patients with type 2 diabetes in the REACH Registry.
Risk Assessment
Options for secondary prevention of CV events are expanding rapidly, particularly for patients with type 2 diabetes (4–7,9–11,25). At the same time, medical costs and the complexity of individual patients’ medical regimens present an increasing challenge. Accurate quantification of CV risk allows for efficient and appropriate use of risk mitigation strategies.
The tools available for estimating the risk of recurrent CV events in patients with type 2 diabetes and established CV disease are limited (12,14–17,30). Further, there are no scoring systems for secondary prevention based on contemporary data with prospectively acquired and adjudicated outcomes. The prediction score generated from the REACH Registry provides risk stratification in patients with prior atherothrombosis and has the benefit of being derived from a large international cohort (12,29), although it is not specific to type 2 diabetes and requires many variables, limiting its use in a clinical setting. The UK Prospective Diabetes Study (UKPDS) risk engine estimates risk of a first coronary heart disease event (primary prevention) for patients with diabetes but is derived from a noncontemporary cohort of 4,540 patients enrolled between 1977 and 1991 (30). The American College of Cardiology/American Heart Association online risk calculator provides primary prevention risk stratification from contemporary data but is not specific to patients with type 2 diabetes and requires online computation (14). Finally, the Framingham Risk Score allows for primary prevention risk stratification (31), though it requires online computation and has not shown consistent discrimination in patients with type 2 diabetes (13).
Conversely, TRS 2°P was developed to predict atherothrombotic events in ∼35,000 patients with established ischemic heart disease. It was ultimately found to identify high-risk patients with a greater benefit from secondary preventative treatment such as vorapaxar and ezetimibe (18,19). Identifying these patients, who stand to derive the greatest benefit, is essential as clinicians and patients balance the competing considerations of treatment benefit, cost, and complexity in a field of expanding therapies.
In the present analysis, the expanded TRS 2°P showed a gradient of risk for the composite end point of CV death, MI, and ischemic stroke in the full trial population and in the subgroups of CAD with or without MI, PAD, and prior stroke. In the full trial population and each subgroup, the C-statistics were similar to those for other risk prediction tools (12). The C-statistic for prediction of a next CV event for the REACH score in the REACH Registry population was 0.67 (95% CI 0.66–0.68) and for CV death was 0.74 (95% CI 0.73–0.76) (12). The C-statistic for CV death was 0.71 for both the Framingham Risk Score and the American College of Cardiology/American Heart Association risk calculator in a large multiethnic cohort (32). Given similar risk discrimination, TRS 2°P provides several advantages over other risk estimators. First, it is calculated simply by the arithmetic sum of 10 readily available clinical variables. Second, there is consistent risk discrimination across and within disease-specific subgroups. Finally, the score was derived and validated in a contemporary data set with prospectively reported and adjudicated CV events (18,19).
Heart failure events are clinically and prognostically important in patients with diabetes. Though TRS 2°P was not originally derived as a tool to predict heart failure hospitalization, it appears to provide clear risk stratification for heart failure in this population with diabetes. This was true in the overall trial population as well as in the subgroup of patients with prior heart failure at baseline, suggesting prognostic utility in a broad population of patients with diabetes—not just those with a preexisting heart failure diagnosis.
Treatment Effect
In TRA 2°P-TIMI 50 and IMPROVE-IT, TRS 2°P predicted a greater absolute benefit with the protease-activated receptor-1 antagonist vorapaxar (18) and the lipid-lowering agent ezetimibe (19) in high-risk subgroups, respectively. In SAVOR-TIMI 53, the dipeptidyl peptidase-4 antagonist saxagliptin neither increased nor decreased CV risk (HR 1.00 [95% CI 0.89–1.12]) (21), and therefore it is not surprising that TRS 2°P did not identify differential treatment effect between risk categories for any of the CV outcomes. Even without a treatment-specific difference in relative effect, risk assessment can identify patients likely to derive the greatest absolute benefit from therapies with proven efficacy. A natural next step would be to apply TRS 2°P in a recent positive diabetes CV outcomes trial.
Calibration
Predicting absolute event rates across varied populations with a single tool is notoriously difficult because of different eligibility criteria (33). This challenge is particularly pronounced when comparing randomized controlled trial data with registry populations, given the typically less stringent entry criteria in the latter. Even so, the rates of CV death, MI, and ischemic stroke by TRS 2°P were quite similar in SAVOR-TIMI 53 and the REACH Registry cohort with type 2 diabetes, with excellent goodness of fit (Fig. 3). Further, the relative frequencies of each score were similar across the two cohorts. This close calibration suggests broad applicability across populations of patients with type 2 diabetes and CV disease.
These findings have implications for clinical practice as well as research. In the clinical setting, TRS 2°P is simple to calculate and is shown here to predict event rates in both clinical trial and real-world populations with high fidelity. Accurate risk assessment is additionally central to clinical trial design and patient selection, and TRS 2°P provides an important tool in this regard.
Limitations
The clinical trial cohort studied here provides important benefits and limitations. The large sample of patients with prospectively acquired and adjudicated outcomes is a key benefit, whereas strict inclusion and exclusion criteria reduce generalizability. Even so, the absolute event rates in the SAVOR-TIMI 53 cohort were quite similar to those in the REACH Registry. Also, because there was no overall benefit or harm with saxagliptin, there was limited opportunity to discern a differential treatment effect based on TRS 2°P. It would be informative to apply this score in a trial showing CV benefit in a large population of patients with type 2 diabetes. Finally, a central feature of the risk score is clinical utility through its relative parsimony. Indices of risk discrimination could be increased by the inclusion of additional variables such as biomarkers (34) or by altering the weighting of variables to better fit the score to this particular population with diabetes. Both of these changes would necessarily result in decreased generalizability and usability in a clinical setting.
Conclusion
TRS 2°P provides a practical and well-calibrated tool for risk assessment in patients with type 2 diabetes and CV disease based on 10 readily available clinical variables. Efficient risk discrimination is essential in the evolving landscape of cardiometabolic disease.
Supplementary Material
Article Information
Funding and Duality of Interest. SAVOR-TIMI 53 was sponsored by AstraZeneca and Bristol-Myers Squibb. B.A.B. was supported by National Institutes of Health grant T32HL007604, Training Grant in Cardiovascular Research, during the conduct of the study and has received consulting fees from Janssen. D.L.B. reports grants from AstraZeneca and Bristol-Myers Squibb during the conduct of the study. Outside of the conduct of this study, D.L.B. reports grants from Amarin, grants from AstraZeneca, grants from Bristol-Myers Squibb, grants from Eisai, grants from Ethicon, grants from Medtronic, grants from Sanofi, grants from The Medicines Company, other financial or business interests from FlowCo, other financial or business interests from PLx Pharma, other financial or business interests from Takeda, personal fees from Duke Clinical Research Institute, personal fees from Mayo Clinic, personal fees from Population Health Research Institute, personal fees from the American College of Cardiology, personal fees from Belvoir Publications, personal fees from Slack Publications, personal fees from WebMD, personal fees from Elsevier, other financial or business interests from Medscape Cardiology, other financial or business interests from Regado Biosciences, other financial or business interests from Boston VA Research Institute, personal fees and nonfinancial support from Society of Cardiovascular Patient Care, nonfinancial support from American Heart Association, personal fees from HMP Communications, grants from Roche, personal fees from Harvard Clinical Research Institute, other financial or business interests from Clinical Cardiology, personal fees from Journal of the American College of Cardiology, other financial or business interests from VA, grants from Pfizer, grants from Forest Laboratories, grants from Ischemix, other financial or business interests from St. Jude Medical, other financial or business interests from Biotronik, other financial or business interests from Cardax, other financial or business interests from American College of Cardiology, other financial or business interests from Boston Scientific, grants from Amgen, grants from Eli Lilly, grants from Chiesi, grants from Ironwood, personal fees from Cleveland Clinic, personal fees from Mount Sinai School of Medicine, and other financial or business interests from Merck & Co. E.Br. reports grant support to his institution from AstraZeneca for this work. Outside of this work, E.Br. reports grant support to his institution from Novartis, Merck & Co., Daiichi-Sankyo, GlaxoSmithKline, and Duke University; uncompensated consultancies and lectures with Merck & Co. and Novartis; consultancies with The Medicines Company and Theravance; and personal fees for lectures from Medscape and Menarini International. D.A.M. reports grants from AstraZeneca during the conduct of the study. Outside of the conduct of the study, D.A.M. reports grants and personal fees from Abbott Laboratories, grants from Amgen, grants and personal fees from AstraZeneca, grants from Daiici-Sankyo, grants from Eisai, grants and personal fees from GlaxoSmithKline, grants and personal fees from Merck & Co., grants from Novartis, grants and personal fees from Roche Diagnostics, personal fees from diaDexus, personal fees from Peloton, personal fees from Verseon, and personal fees from Gilead. P.G.S. reports grants from AstraZeneca and Bristol-Myers Squibb during the conduct of the study. Outside the conduct of the study, P.G.S. reports grants and personal fees from Merck & Co., grants and personal fees from Sanofi, grants and personal feels from Servier, personal fees from Amarin, personal fees from Amgen, personal fees from Bayer, personal fees from Boehringer Ingelheim, personal fees from Bristol-Myers Squibb, personal fees from CSL Behring, personal fees from Daiichi-Sankyo, personal fees from GlaxoSmithKline, personal fees from Janssen, personal fees from Eli Lilly, personal fees from Novartis, personal fees from Pfizer, personal fees from Regeneron, and personal fees from The Medicines Company. A.C. discloses the following relationships: advisory board, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Glucome, Novo Nordisk, and Sanofi; speakers' bureau, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Merck Sharp & Dohme (MSD), Novo Nordisk, and Sanofi; and shareholder, Glucome. O.M. reports speaking fees from AstraZeneca, Bristol-Myers Squibb, Novo Nordisk, Eli Lilly, Sanofi, Novartis, MSD, and Kyowa Hakko Kirin; consulting fees from Novo Nordisk, Eli Lilly, Sanofi, Novartis, AstraZeneca, Boehringer Ingelheim, and Janssen; and research grants from AstraZeneca, Bristol-Myers Squibb, and Novo Nordisk. I.R. reports the following relationships: advisory board, AstraZeneca, Eli Lilly and Company, MSD, Novo Nordisk, Sanofi, Orgenesis, SmartZyme Innovation Ltd., Labstyle Innovations Ltd., and Boehringer Ingelheim; consultant, AstraZeneca/Bristol-Myers Squibb, Insuline Medical, Gili Medical, Kamada Ltd., FuturRx Ltd., and Diabetes Medical Center; speakers' bureau, AstraZeneca/Bristol-Myers Squibb, Eli Lilly and Company, Johnson & Johnson, MSD, Novartis Pharma AG, Novo Nordisk, Sanofi, Teva, and Boehringer Ingelheim; and stock/shareholder, Insuline Medical, Labstyle Innovations, Orgenesis, and Glucome Ltd. E.Bo. reports personal fees from Merck & Co. and Daiichi-Sankyo and grants from Eisai, Daiichi-Sankyo, and Merck & Co. outside this work. B.M.S. reports grants from AstraZeneca and Bristol-Myers Squibb during the conduct of the study. Outside the conduct of the study, B.M.S. reports personal fees from AstraZeneca; personal fees from Biogen Idec; personal fees from Boehringer Ingelheim; personal fees from Dr. Reddy's Laboratories; personal fees from Forest Laboratories; personal fees from GE Healthcare; personal fees from GlaxoSmithKline; personal fees from Health at Scale, Corp.; personal fees from Lexicon; personal fees from Merck & Co.; personal fees from St. Jude Medical; grants from AstraZeneca; grants from Eisai; grants from Merck & Co.; grants from Poxel; personal fees from Sanofi; and personal fees from Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
The SAVOR-TIMI 53 Trial Executive Committee comprises E.Br. (study chair), D.L.B. (co–principal investigator), I.R. (co–principal investigator), Jaime A. Davidson, Robert Frederich (nonvoting), Boaz Hirshberg (nonvoting), and P.G.S.
Author Contributions. B.A.B. contributed to study design, data interpretation, and drafting of the manuscript. D.L.B. contributed to study design, data collection, data interpretation, and critical review of the manuscript. E.Br. contributed to study design, data collection, data interpretation, and critical review of the manuscript. D.A.M. contributed to study design, data collection, data interpretation, and critical review of the manuscript. P.G.S. contributed to study design, data collection, data interpretation, and critical review of the manuscript. Y.G. contributed to study design, statistical analysis, and critical review of the manuscript. A.C. contributed to study design, data collection, data interpretation, and critical review of the manuscript. O.M. contributed to study design, data collection, data interpretation, and critical review of the manuscript. I.R. contributed to study design, data collection, data interpretation, and critical review of the manuscript. E.Bo. contributed to study design, data interpretation, and critical review of the manuscript. B.M.S. contributed to study design, data collection, data interpretation, and critical review of the manuscript. B.M.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the American College of Cardiology 66th Annual Scientific Session & Expo, Washington, DC, 17–19 March 2017.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-1736/-/DC1.
References
- 1.Cavender MA, Steg PG, Smith SC Jr, et al.; REACH Registry Investigators . Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death: outcomes at 4 years from the REduction of Atherothrombosis for Continued Health (REACH) Registry. Circulation 2015;132:923–931 [DOI] [PubMed] [Google Scholar]
- 2.Rao Kondapally Seshasai S, Kaptoge S, Thompson A, et al.; Emerging Risk Factors Collaboration . Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whiting DR, Guariguata L, Weil C, Shaw J. IDF Diabetes Atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011;94:311–321 [DOI] [PubMed] [Google Scholar]
- 4.Marso SP, Bain SC, Consoli A, et al.; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 5.Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 7.U.S. Food and Drug Administration. FDA approves Jardiance to reduce cardiovascular death in adults with type 2 diabetes [article online], 2016. Available from https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm531517.htm. Accessed 1 August 2017
- 8.Cannon CP, Blazing MA, Giugliano RP, et al.; IMPROVE-IT Investigators . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–2397 [DOI] [PubMed] [Google Scholar]
- 9.Sabatine MS, Giugliano RP, Keech AC, et al.; FOURIER Steering Committee and Investigators . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722 [DOI] [PubMed] [Google Scholar]
- 10.Morrow DA, Braunwald E, Bonaca MP, et al.; TRA 2P–TIMI 50 Steering Committee and Investigators . Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med 2012;366:1404–1413 [DOI] [PubMed] [Google Scholar]
- 11.Cavender MA, Scirica BM, Bonaca MP, et al. Vorapaxar in patients with diabetes mellitus and previous myocardial infarction: findings from the thrombin receptor antagonist in secondary prevention of atherothrombotic ischemic events-TIMI 50 trial. Circulation 2015;131:1047–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson PW, D'Agostino R, Bhatt DL, et al. An international model to predict recurrent cardiovascular disease. Am J Med 2012;125:695–703.e1 [DOI] [PubMed] [Google Scholar]
- 13.Coleman RL, Stevens RJ, Retnakaran R, Holman RR. Framingham, SCORE, and DECODE risk equations do not provide reliable cardiovascular risk estimates in type 2 diabetes. Diabetes Care 2007;30:1292–1293 [DOI] [PubMed] [Google Scholar]
- 14.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2935–2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allan GM, Nouri F, Korownyk C, Kolber MR, Vandermeer B, McCormack J. Agreement among cardiovascular disease risk calculators. Circulation 2013;127:1948–1956 [DOI] [PubMed] [Google Scholar]
- 16.Bertoluci MC, Rocha VZ. Cardiovascular risk assessment in patients with diabetes. Diabetol Metab Syndr 2017;9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Dieren S, Beulens JW, Kengne AP, et al. Prediction models for the risk of cardiovascular disease in patients with type 2 diabetes: a systematic review. Heart 2012;98:360–369 [DOI] [PubMed] [Google Scholar]
- 18.Bohula EA, Bonaca MP, Braunwald E, et al. Atherothrombotic risk stratification and the efficacy and safety of vorapaxar in patients with stable ischemic heart disease and previous myocardial infarction. Circulation 2016;134:304–313 [DOI] [PubMed] [Google Scholar]
- 19.Bohula EA, Morrow DA, Giugliano RP, et al. Atherothrombotic risk stratification and ezetimibe for secondary prevention. J Am Coll Cardiol 2017;69:911–921 [DOI] [PubMed] [Google Scholar]
- 20.Di Angelantonio E, Kaptoge S, Wormser D, et al.; Emerging Risk Factors Collaboration . Association of cardiometabolic multimorbidity with mortality. JAMA 2015;314:52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scirica BM, Bhatt DL, Braunwald E, et al.; SAVOR-TIMI 53 Steering Committee and Investigators . Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–1326 [DOI] [PubMed] [Google Scholar]
- 22.Scirica BM, Bhatt DL, Braunwald E, et al. The design and rationale of the saxagliptin assessment of vascular outcomes recorded in patients with diabetes mellitus-thrombolysis in myocardial infarction (SAVOR-TIMI) 53 study. Am Heart J 2011;162:818–825.e6 [DOI] [PubMed] [Google Scholar]
- 23.Bhatt DL, Steg PG, Ohman EM, et al.; REACH Registry Investigators . International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006;295:180–189 [DOI] [PubMed] [Google Scholar]
- 24.Mosenzon O, Raz I, Scirica BM, et al. Baseline characteristics of the patient population in the Saxagliptin Assessment of Vascular Outcomes Recorded in patients with diabetes mellitus (SAVOR)-TIMI 53 trial. Diabetes Metab Res Rev 2013;29:417–426 [DOI] [PubMed] [Google Scholar]
- 25.Morrow DA, Scirica BM, Fox KA, et al. ; TRA 2 P-TIMI 50 Investigators. Evaluation of a novel antiplatelet agent for secondary prevention in patients with a history of atherosclerotic disease: design and rationale for the Thrombin-Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2 P)-TIMI 50 trial. Am Heart J 2009;158:335–341.e3 [DOI] [PubMed] [Google Scholar]
- 26.Ohman EM, Bhatt DL, Steg PG, et al. ; REACH Registry Investigators. The REduction of Atherothrombosis for Continued Health (REACH) Registry: an international, prospective, observational investigation in subjects at risk for atherothrombotic events: study design. Am Heart J 2006;151:786.e1–10 [DOI] [PubMed] [Google Scholar]
- 27.Demler OV, Paynter NP, Cook NR. Tests of calibration and goodness-of-fit in the survival setting. Stat Med 2015;34:1659–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steg PG, Bhatt DL, Wilson PW, et al.; REACH Registry Investigators . One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007;297:1197–1206 [DOI] [PubMed] [Google Scholar]
- 29.Bhatt DL, Eagle KA, Ohman EM, et al.; REACH Registry Investigators . Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA 2010;304:1350–1357 [DOI] [PubMed] [Google Scholar]
- 30.Stevens RJ, Kothari V, Adler AI, Stratton IM, Holman RR. The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56). Clin Sci 2001;101:671–679 [PubMed] [Google Scholar]
- 31.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–1847 [DOI] [PubMed] [Google Scholar]
- 32.DeFilippis AP, Young R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med 2015;162:266–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen LA, Matlock DD, Shetterly SM, et al. Use of risk models to predict death in the next year among individual ambulatory patients with heart failure. JAMA Cardiol 2017;2:435–441 [DOI] [PubMed] [Google Scholar]
- 34.Scirica BM, Bhatt DL, Braunwald E, et al. Prognostic implications of biomarker assessments in patients with type 2 diabetes at high cardiovascular risk: a secondary analysis of a randomized clinical trial. JAMA Cardiol 2016;1:989–998 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.