Abstract
Aims
Combining mesenchymal stem cells (MSCs) and chondrocytes has great potential for cell-based cartilage repair. However, there is much debate regarding the mechanisms behind this concept. We aimed to clarify the mechanisms that lead to chondrogenesis (chondrocyte driven MSC-differentiation versus MSC driven chondroinduction) and whether their effect was dependent on MSC-origin. Therefore, chondrogenesis of human adipose-tissue-derived MSCs (hAMSCs) and bone-marrow-derived MSCs (hBMSCs) combined with bovine articular chondrocytes (bACs) was compared.
Methods
hAMSCs or hBMSCs were combined with bACs in alginate and cultured in vitro or implanted subcutaneously in mice. Cartilage formation was evaluated with biochemical, histological and biomechanical analyses. To further investigate the interactions between bACs and hMSCs, (1) co-culture, (2) pellet, (3) Transwell® and (4) conditioned media studies were conducted.
Results
The presence of hMSCs–either hAMSCs or hBMSCs—increased chondrogenesis in culture; deposition of GAG was most evidently enhanced in hBMSC/bACs. This effect was similar when hMSCs and bAC were combined in pellet culture, in alginate culture or when conditioned media of hMSCs were used on bAC. Species-specific gene-expression analyses demonstrated that aggrecan was expressed by bACs only, indicating a predominantly trophic role for hMSCs. Collagen-10-gene expression of bACs was not affected by hBMSCs, but slightly enhanced by hAMSCs. After in-vivo implantation, hAMSC/bACs and hBMSC/bACs had similar cartilage matrix production, both appeared stable and did not calcify.
Conclusions
This study demonstrates that replacing 80% of bACs by either hAMSCs or hBMSCs does not influence cartilage matrix production or stability. The remaining chondrocytes produce more matrix due to trophic factors produced by hMSCs.
Introduction
Cartilage has a very limited capacity for self-regeneration. Untreated lesions—caused by trauma, tumors, congenital malformation or age related degeneration—persist indefinitely and ultimately require surgical intervention. However, current treatments are unsuccessful for long-term repair; resulting in a need for novel repair strategies. Cell-based cartilage repair holds promise for restoring missing or destroyed cartilage and has the potential to overcome limitations of current treatments, while re-establishing the unique biological and functional properties of the tissue.
One of the major challenges herein is defining an appropriate cell source. Current cell-based surgical treatments for cartilage lesions are predominantly based on the use of either (1) chondrocytes or (2) mesenchymal stem cells (MSCs). These cell-based procedures are however associated with specific disadvantages. Chondrocytes from several anatomical locations (e.g. joint, rib, nose, ear, meniscus) have been investigated for their application in cartilage regeneration. [1–21] However, to generate a construct of reasonable size, large numbers of chondrocytes are required, necessitating the use of culture-expansion. In monolayer culture-expansion, chondrocytes dedifferentiate; they change phenotypically to a fibroblast-like morphology and lose their chondrogenic gene-expression capacity. Chondrocyte-dedifferentiation usually results in fibrous and mechanically inferior cartilage, making them less suitable for cell-based cartilage repair. [22] In contrast, multipotent cells, like MSCs, achieved considerable attention as alternative cells, as they can undergo multiple population doublings without losing their chondrogenic potential and have the capacity to differentiate into cartilage tissue under appropriate culture conditions. [23–27] Furthermore, MSCs are easily available from several tissues, including bone marrow and adipose tissue, which makes culture-expansion unnecessary. However, the single use of MSCs for cell-based cartilage repair is currently debated, since the cartilage tissue formed is unstable and predisposed to mineralization and ossification in vivo. [28–32]
Currently, combining both cell sources holds great promise for cell-based cartilage repair as it reduces the required number of chondrocytes and diminishes many disadvantages of both individual cell types. Moreover, by decreasing the amount of chondrocytes required (≤ 20% of the total cell mixture), culture-expansion is no longer necessary, which would allow the use of freshly isolated primary chondrocytes leading to improved cartilage formation. [33] Unfortunately, in depth understanding of the cellular interaction pathways between MSCs and chondrocytes is under debate in literature: It is thought that the co-culture effect is either credited by (1) chondrocyte driven MSC-differentiation or ascribed to (2) chondrocytes, whose cartilage-forming capacity and proliferation activity are enhanced in the presence of MSCs. [34] In recent years, the trophic and paracrine functions of MSCs appeared most critical in this process, rather than the simple chondrogenic differentiation of MSCs alone. However, little is known as to whether their trophic function is a general characteristic of MSCs or dependent on the origin of the MSC source. MSCs from several anatomical locations have been applied in co-culture. Independent on their origin, mixed cell cultures of chondrocytes and MSCs have been demonstrated to generally improve chondrogenesis as well as to reduce hypertrophy and tissue mineralization. [34–36] In contrast, three co-culture studies using adipose-tissue-derived MSCs (AMSCs) showed limited or decreased effects of MSCs on chondrogenesis. [37–39] Such effect was hardly seen in co-culture studies using bone-marrow-derived MSCs (BMSCs), which may propose that, compared to BMSCs, AMSCs are less efficient in co-culture. Due to methodological heterogeneity however, a direct comparable analysis between AMSCs and BMSCs in co-culture could not be easily made. So far, only three research groups have directly compared the effect of AMSCs and BMSCs on chondrocytes in co-culture. [40–42] Unfortunately, these studies demonstrate conflicting outcomes and have never translated to animal research.
Therefore, we aim to investigate whether MSCs undergo chondrogenic differentiation upon contact with chondrocytes or by trophic effects of MSCs on chondrocytes. Whether the co-culture effect is dependent on MSC-origin or a general characteristic of MSCs, is further elucidated. Therefore, chondrogenesis of human AMSCs (hAMSCs) and BMSCs (hBMSCs) combined with bovine articular chondrocytes (bACs) is compared. The xenogeneic set-up using hMSCs and bACs will allow conclusions about the cell type responsible for chondrogenesis. As cellular interactions can be influenced or overruled by exogenous growth factors, no growth factors are added to the culture system to study cartilage formation of the co-cultures in vitro. Moreover, cartilage formation will be evaluated after immediate subcutaneous implantation of the constructs in mice. To further elucidate the interactions between MSCs and ACs, different in-vitro culture systems will be used: (1) co-culture system of hMSC/bACs in alginate, (2) pellet co-culture system of hMSC/bACs, (3) Transwell® system of singular isolated hMSCs and bACs in alginate, and (4) conditioned media culture systems of conditioned medium of hMSCs on bACs and vice versa.
Materials and methods
Chemicals were obtained from Sigma-Aldrich, USA unless stated otherwise.
Cell sources
All human samples were obtained after approval by the Erasmus MC Medical Ethical Committee. Human mesenchymal stem cells (hMSCs) were isolated from either adipose tissue (hAMSCs) or bone-marrow aspirates (hBMSCs). hAMSCs were obtained from subcutaneous abdominal adipose tissue as waste material without the need for informed consent (protocol # MEC-2011-371) (n = 3 independent donors: F 52Y; F 51Y; F 53Y). hBMSCs were isolated from bone-marrow heparinized aspirates, after written informed consent had been acquired (protocol # MEC-2004-142 and Albert Schweitzer Hospital 2011/7) (n = 3 independent donors: M 67Y; F 75Y; M 22Y). Both hAMSCs and hBMSCs were seeded and cultured overnight in medium consisting of Minimum Essential Medium Alpha (MEM-α; Gibco, USA), supplemented with 10% fetal calf serum (FCS; Lonza, the Netherlands), 10−4 M L-ascorbic acid 2-phosphate, and 1 ng/mL basic Fibroblast Growth Factor 2 (bFGF2; AbD Serotec, UK). [43–45]
Articular chondrocytes (ACs) were selected, to study the trophic effect of hAMSCs or hBMSCs on chondrocytes. To obtain primary bovine articular chondrocytes (bACs), macroscopically intact cartilage was harvested from the metatarsophalangeal joints of calves ≤ 6 months old (T. Boer & Zn., Nieuwerkerk aan den IJssel, the Netherlands), and washed with saline (n = 4 pools of 3 donors each). To isolate cells, cartilage pieces were incubated for 1 hour with 2 mg/mL protease (type XIV derived from Streptomyces griseus), followed by overnight incubation with 1.5 mg/mL collagenase B (Roch Diagnostics, Germany) in High Glucose—Dulbecco's Modified Eagle's Medium (HG-DMEM; Gibco) with 10% FCS, 50 μg/mL gentamycin (Gibco), and 0.5 μg/mL amphotericin B (Fungizone; Life Technologies, Breda, the Netherlands). To extract small parts of undigested cartilage, the cell suspension was filtered through a nylon 100-μm mesh. Prior to cell culture, cell viability was tested using the trypan blue exclusion test, and cell number was calculated with a hemocytometer.
Chondrogenesis
For in-vitro and in-vivo studies, all cells were encapsulated in alginate (Batch MG-004, CellMed, Germany), a hydrogel known of its high biocompatibility [46] and chondrogenic capacity [47]. Moreover, alginate hydrogels enable homogeneous cell distribution and allow paracrine factors to access all cells equally [47], making them suitable scaffolds for following research purposes.
Second-passaged hMSCs and non-expanded primary bACs were harvested and cultured in a 3D-alginate hydrogel. Cells were suspended at a density of 4x106 cells/mL in clinical grade 1.1% low viscosity alginate solution dissolved in 0.9% NaCl as single-cell-type populations or as a combination of 80% hMSCs (either hAMSCs or hBMSCs) and 20% bACs. (Table 1) A 4:1 ratio was selected based on our previous experience [48] and that of others [49, 50].
Table 1. Construct conditions.
| Human stem cells | Bovine chondrocytes | |||
|---|---|---|---|---|
| Source | Cell density (x106) | Source | Cell density (x106) | |
| hAMSC | hAMSCs | 4 nc/mL | x | x |
| hBMSC | hBMSCs | 4 nc/mL | x | x |
| bAC | x | x | bACs | 4 nc/mL |
| hAMSC/bAC | hAMSCs | 3.2 nc/mL | bACs | 0.8 nc/mL |
| hBMSC/bAC | hBMSCs | 3.2 nc/mL | bACs | 0.8 nc/mL |
| Control bAC | x | x | bACs | 0.8 nc/mL |
Cell density is displayed as the number of cells (nc) in 1 milliliter of alginate. hAMSC = human Adipose-tissue-derived Mesenchymal Stem Cell; hBMSC = human Bone-marrow-derived Mesenchymal Stem Cell; bAC = bovine Articular Chondrocyte.
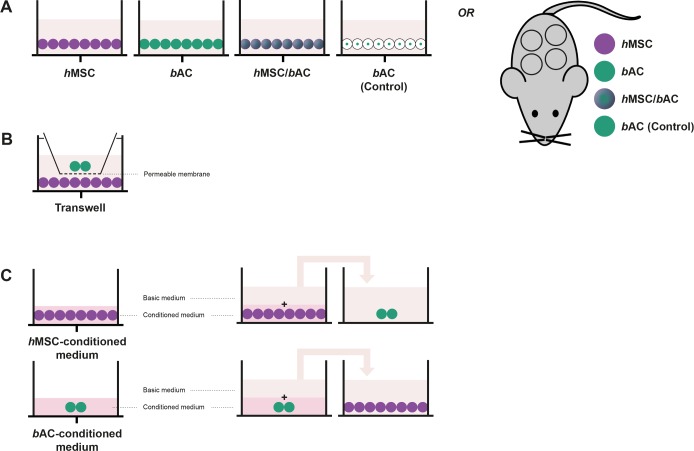
Flat constructs (8 mm diameter; 2 mm height) were processed as previously described. [2] In short, alginate suspensions were injected into a custom designed slab mold consisting of 2 calcium-permeable membranes (Durapore® 5.0 μm membrane filters, Millipore) rigidly supported by stainless-steel meshes and separated by a stainless-steel casting frame. Alginate was instantaneously gelated for 30 minutes in 102 mM CaCl2 and thereafter washed with 0.9% NaCl and HG-DMEM. Sterile biopsy punches (Spengler, Asnières sur Seine, France) were used to create alginate constructs suitable for mechanical testing. Constructs were either cultured in vitro or directly implanted subcutaneously in mice. (Fig 1A)
Fig 1. Cellular interaction.
Cells were encapsulated in alginate beads separately and alginate and pellet co-cultures (A, control conditions). Furthermore, hMSCs and bACs were co-cultured in (B) a Transwell® system as well as in (C) medium conditioned by the other cell type, to further understand the complex cellular communication pathways between hMSCs and bACs. In purple: hMSCs = human Mesenchymal Stem Cells; in green: bACs = bovine Articular Chondrocytes.
In vitro, constructs were cultured in ‘basic medium’ containing serum-free HG-DMEM supplemented with 50 μg/mL gentamycin; 0.5 μg/mL Fungizone; 1 mM sodium pyruvate (Gibco); 40 μg/mL L-proline; supplemented Insulin Transferrine Selenium (ITS+; B&D Bioscience, Bedford, MA, USA); 10−7 M dexamethason; and 25 μg/mL L-ascorbic acid 2-phosphate without the addition of growth factors. For each condition referred to in Table 1, 3 independent donors were used in triplicate (total n = 54). After 3 and 5 weeks, constructs were processed for biochemical and gene-expression analysis.
In-vivo studies were completed after 8 weeks of subcutaneous implantation. In total, 10 9-week-old, female NMRI nu/nu mice (Charles River Laboratories, the Netherlands) were used. Two separate incisions were made along the central line of the spine (1 at the shoulders and 1 at the hips), after which 4 separate subcutaneous dorsal pockets were prepared by blunt dissection. For each condition referred to in Table 1, 3 independent donors were used in duplicate (total n = 36). Moreover, cell-free constructs were used as controls (n = 4). For implantation, alginate constructs were randomly assigned to these 4 pockets. After 8 weeks, animals were sacrificed and samples were explanted for histological, biomechanical and biochemical analyses. Animal experiments were carried out to the guidelines prescribed by the Dutch National Institutes of Health, and were approved by the Dutch equivalent of the Institutional Animal Care and Use Committee, the Erasmus MC Dier Ethische Commissie (protocol # EMC 2429).
Cellular interaction
To further understand the complex cellular communication pathways between MSCs and ACs, cell types (hAMSCs (F 53Y); hBMSCs (M 22Y); bACs pool of 3 donors) were co-cultured as follows: (1) hMSCs and bACs were combined and cultured in alginate as previously described; (2) hMSCs and bACs were cultured in pellets, allowing direct cell-cell contact. Furthermore, hMSCs and bACs were encapsulated in alginate separately and co-cultured in (3) a Transwell® system; as well as (4) in medium conditioned by the other cell type. (Fig 1) The ratio of hMSCs to bACs in each culture system was kept 80:20 for all conditions. All constructs were cultured under standardized nutritional conditions. Medium was changed 3 times a week. After 3 weeks, alginate beads and pellets were processed for biochemical or gene-expression analysis.
(1) Co-culture
hMSCs and bACs were suspended at a density of 4x106 cells/mL in clinical grade alginate solution as a mixed-cell-type population at a 80:20 ratio as described above. (Fig 1A)
(2) Pellet culture
To study the effects of direct cell-cell contact in co-cultures, hMSCs and bACs were cultured in pellets. Therefore, a mixture of 80% hMSCs and 20% bACs was suspended in basic medium and a total number of 2,5x105 cells in 0.5 mL were transferred into polypropylene tubes and pellet were formed by centrifuging at 200 G for 8 minutes. To induce proper pellet formation, addition of Transforming Growth Factor β1 (TGFβ1; R&D Systems, USA) for 24 hours was required. This exposure was not sufficient to induce chondrogenesis in hMSCs (data not shown). After 24 hours, pellet were exposed to the ‘basis medium’ without addition of any growth factors. (Fig 1A)
(3) Transwell® system
hMSCs and bACs were suspended at a density of 4x106 cells/mL in clinical grade alginate solution as single-cell-type populations and transferred into a 10-mL sterile syringe. Thereafter, the cell-suspension was slowly passed through a 23-gauge needle to produce drops, which fell into a 102 mM CaCl2 creating alginate beads. Following instantaneous gelation, beads were allowed to further gelate for a period of 10 minutes in the CaCl2-solution. After being washed once with 0.9% NaCl and HG-DMEM, the beads were transferred to a Transwell® system (Corning Life Science, USA). The Transwell® inserts separated hMSCs and bACs by a porous membrane of 8 μm, allowing paracrine signaling between hMSCs and bACs. (Fig 1B)
(4) Conditioned medium
Alginate beads containing hMSCs or bACs were produced as described above and cultured in medium conditioned by the other cell types. To obtain bACs, hAMSCs and hBMSCs conditioned media, alginate beads were cultured in ‘basic medium’ for 3 days. After 3 days of culture, conditioned media were collected, enriched with 1:1 ‘basic medium’ and immediately added to alginate cultures of the other cell types. Again, a 80:20 ratio between hMSCs and bACs was maintained. (Fig 1C)
Biochemical evaluation of the extracellular matrix
Alginate constructs were digested overnight at 56°C in papain (250 μg/mL in 0.2 M NaH2PO4, 0.01 M EDTA, containing 5 mM L-cystein; pH 6.0); pellets were digested overnight at 56°C in proteinase K (1 mg/mL in Tris/EDTA buffer containing 185 μg/mL iodoacetamide and 1 μg/mL pepstatin A; pH 7.6). After digestion, samples were subjected to biochemical analyses to determine DNA, glycosaminoglycan (GAG), and hydroxyproline contents as described previously. [2] In short, the amount of DNA was determined by Ethidium bromide (GibcoBR1), using calf thymus DNA as a standard. Sulphated GAGs were quantified by the 1,9-Dimethylmethylene blue (DMMB) dye-binding assay, using shark chondroitin sulphate C as a standard. To be suitable for cell cultures containing alginate, the DMMB-pH-level was adjusted to pH 1.75, as described previously. [51] For the hydroxyproline content, digests were hydrolysed, dried and redissolved in 150 μL water. Hydroxyproline contents were measured using chloramine-T and dimethylaminobenzaldehyde as reagents and hydroxyproline (Merck, Germany) as a standard. Collagen content was subsequently estimated from the hydroxyproline content, assuming that one collagen triple helix molecule contains 300 hydroxyproline residues.
Histological evaluation
After 8 weeks of subcutaneous implantation, constructs were harvested, set in 2% agarose, fixed in 4% formalin in PBS and embedded in paraffin. Paraffin-embedded sections (6 μm) were deparaffinised and rehydrated.
To evaluate tissue calcification, Von Kossa staining was performed. Slides were immersed in 5% silver nitrate solution for 10 minutes, rinsed in MilliQ and exposed to light for another 10 minutes. Excess silver nitrate was removed with 5% sodium-thiosulphate and slides were rinsed in distilled water afterwards. Sections were counterstained with Nuclear fast red (Merck).
To examine proteoglycans present in the newly synthesized ECM, deparaffinised sections were stained with Safranin-O and fast green.
To allow the use of monoclonal mouse antibody collagen type II (II-II6B3 1:100; Developmental Studies Hybridoma Bank, USA) on constructs which had been implanted in mice, the primary antibody was pre-coupled overnight with goat anti-mouse biotin at 4°C (1:500; Jackson Laboratories, USA), followed by a 2-hour incubation in 0.1% normal mouse serum (CLB, the Netherlands), to prevent unwanted binding of the anti-mouse antibodies to mouse immunoglobulins. [52] Antigen retrieval was performed through incubation with 0.1% pronase for 30 minutes at 37°C, continued with a 30 minutes incubation with 1% hyaluronidase at 37°C. Non-specific binding sites were blocked with 10% goat serum and sections were stained with the pre-treated antibodies for 60 minutes. Sections were than incubated with enzyme-streptavidin conjugate (Label, 1:100, Biogenex, HK-321-UK, USA) in PBS/1% BSA, followed by incubation with Neu Fuchsin substrate (Chroma, Germany).
Biomechanical analysis
In order to distinguish the mechanical strength of alginate itself, cell containing constructs were prepared and directly taken for mechanical testing as described previously. [2] In short, for mechanical characterization of engineered cartilage constructs after in vivo cell culture, constructs 2.5 mm thick and 5 mm in diameter were used. The samples were placed in close-fitting Ø 5 mm stainless steel cylindrical wells. Mechanical testing was performed with a materials testing machine (Zwick Z005, Ulm, Germany) equipped with a 10 N load cell, a built-in displacement control, and a cylindrical, plane ended, stainless steel indenter (Ø 1.2 mm). During mechanical testing the samples were immersed in PBS. Stress-strain testing was performed: the samples were compressed to a final height of 0.5 mm at a loading rate of 5 mm per minute. An in-house Matlab® script was used to locate the sample surface and measure the sample thickness. Force-displacement curves were then converted to stress-strain curves. Measurements of compressive modulus at 40% strain, E40%, were determined for every sample.
Gene-expression analyses
For total RNA isolation, alginate was dissolved in ice-cold 55 mM sodium citrate and 20 mM Ethylene Diamintetraacetate (EDTA) in 150 mM NaCl and centrifuged. Each cell-pellet was subsequently suspended in 1 mL RNA-BeeTM (TEL-TEST, USA). For total RNA isolation from pellets, pellets were manually homogenized and suspended in 300 μL/pellet RNA-BeeTM. RNA was extracted with chloroform and purified from the supernatant using the RNAeasy Micro Kit (Qiagen, Germany) according to the manufacturer’s guidelines by on-column DNA-digestion. Extracted total RNA was quantified using NanoDrop® ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) at 260/280 nm. Total RNA of each sample was reverse transcribed into cDNA using RevertAidTM First Strand cDNA Synthesis Kit (MBI Fermentas, Germany).
For quantitative real-time Polymerase Chain Reaction (qRT-PCR) analysis, forward and reverse primers were designed using PrimerExpress 2.0 software (Applied Biosystems, USA) to meet TaqMan or SYBR Green requirements. Gene specificity of all primers was guaranteed by Basic Local Alignment Search Tool (BLASTN). Analysed genes are listed in Table 2. qRT-PCR was performed using qPCR Mastermix Plus for SYBR Green (Eurogentec, the Netherlands) according to the manufacturers’ guidelines and using ABIPRISM® 7000 with SDS software version 1.7 (Applied Biosystems, the Netherlands). Relative gene expressions were calculated by means of the 2-ΔCT formula.
Table 2. Sequences of primers for qRT-PCR.
| Primers and probes | |
|---|---|
| Human specific genes | |
| hsGAPDH | Fw: AGCTCACTGGCATGGCCTTC |
| Rev: CGCCTGCTTCACCACCTTCT | |
| hsACAN | Fw: CAGCCACCACCTACAAACGCAG |
| Rev: CTGGGTGGGATGCACGTCAGC | |
| hsCOL2A1 | Fw: ACGAGGCCTGACAGGTCCCA |
| Rev: GCCCAGCAAATCCCGCTGGT | |
| Bovine specific genes | |
| bsGAPDH | Fw: GTCAACGGATTTGGTCGTATTGGG |
| Rev: TGCCATGGGTGGAATCATATTGG | |
| bsACAN | Fw: GGACACTCCTTGCAATTTGAGAA |
| Rev: CAGGGCATTGATCTCGTATCG | |
| COL2A1 | Fw: GGCAATAGCAGGTTCACGTACA |
| Rev: CGATAACAGTCTTGCCCCACTT | |
GAPDH = GlycerAldehyde 3-Phosphate DeHydrogenase; ACAN = Aggrecan; COL2A1 = Collagen type 2; hs = human-specific; bs = bovine-specific.
Statistical analysis
All data were analyzed with PSAW statistics 20.0 (SPSS inc. Chicago, USA). For in vitro alginate co-cultures, the mean and standard deviation represents at least three independent donors per cell source performed in triplicate. For statistical evaluation, a mixed linear model was used followed by a Bonferroni's post-hoc comparisons test. Condition and time point were defined as fixed factors in the model. Donor and sample number were treated as random factors. For in vivo alginate co-cultures, the mean and standard deviation represents at least three independent donors per cell source performed in duplicate. For the evaluation of the cellular communication pathways between MSCs and ACs, the mean and standard deviation represents one donor per cell source performed in sextuple. For statistical evaluation, the Kruskal-Wallis followed by the Mann-Whitney-U tests was used followed by a Bonferroni's post-hoc comparisons test. For all tests, values of p<0.05 were considered statistically significant.
Results
Cartilage regeneration in co-cultures
In vitro outcomes
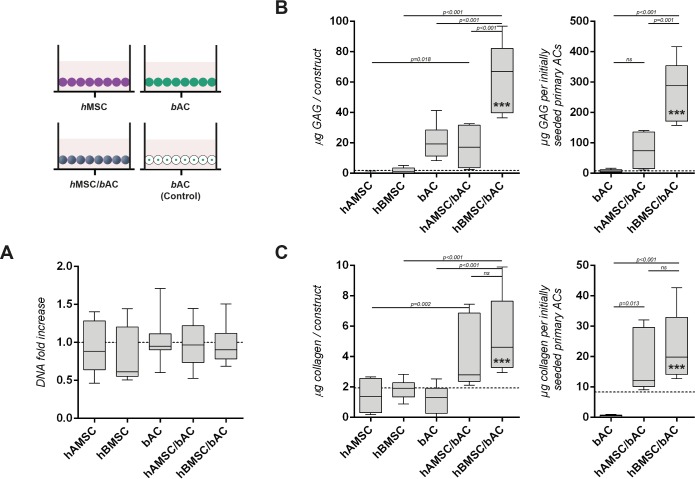
After 3 weeks, DNA content of alginate constructs containing either co-cultures of hMSCs and bACs or single-cell-type populations, did not change in relation to their initial DNA content. (Fig 2A) Because the amount of DNA had not changed significantly in any of the conditions, matrix deposition was expressed per construct and per initially seeded primary ACs. After 5 weeks, DNA content did significantly decrease in constructs containing hBMSCs only (p<0.001), but remained unchanged in the remaining culture conditions. (S1 Fig)
Fig 2. Cartilage matrix formation in constructs containing hMSCs and/or bACs, 3 weeks after in-vitro culture.
(A) The DNA content of none of the constructs had changed compared to their initial DNA content prior to cell-culture (dotted line). Biochemical evaluation of the GAG (B) and collagen (C) content, 3 weeks after culture in alginate. The left graphs demonstrate the amount of matrix components per construct, whereas for the right graphs matrix production is normalized to the initially seeded primary ACs. A control condition—containing similar amounts of bACs (0.8*106 nc/ml) without supplementation of hMSCs—was evaluated to determine the additional effect of hMSCs (3.2*106 nc/ml) on bACs in co-cultures (dotted line). *, ** or *** indicates p-values smaller than 0.05, 0.01 or 0.001 respectively compared to the control condition. Data are shown as mean ± SD. For statistical evaluation, a mixed model was used followed by a Bonferroni's post-hoc comparisons test. hAMSC = human Adipose-tissue-derived Mesenchymal Stem Cell (n = 3 experiments with 3 independent donors); hBMSC = human Bone-marrow-derived Mesenchymal Stem Cell (n = 3 experiments with 3 independent donors); bAC = bovine Articular Chondrocyte (n = 3 experiments with 3 pools of donors). Per experiment, 3 samples were used for analyses.
Since constructs were cultured in the absence of chondrogenic factors, constructs containing solely hAMSCs or hBMSCs produced very little GAG (Fig 2B) and collagen (Fig 2C). To demonstrate the additional effect of hMSCs in mixed-cell-type populations, a control condition—containing similar numbers of bACs (0.8*106 nc/mL) without the supplementation of hMSCs—was evaluated (Fig 2 dotted lines). The addition of either hAMSCs or hBMSCs to bACs demonstrated a significant increase in the production of GAG over their controls (hAMSC/bACs p = 0.018; hBMSC/bACs p<0.001). Compared to constructs containing single-cell-type populations, the deposition of GAG was most evidently enhanced in co-cultures combining hBMSCs and bACs (p<0.001). Constructs containing hAMSC/bACs deposited significantly less GAG compared to hBMSC/bACs (p<0.001) and equal amounts compared to constructs containing bACs only. (Fig 2B) The production of collagen was enhanced in co-cultures of both hAMSC/bACs and hBMSC/bACs compared to single-cell-type populations (hAMSC/bACs p = 0.002; hBMSC/bACs p<0.001). (Fig 2C) Normalization of the total GAG content to the initially seeded primary ACs revealed even more distinct differences between co-cultures and single-cell-type populations: hBMSC/bACs produced significantly more GAG compared to bACs only and co-cultures of hAMSC/bACs (both p<0.001); collagen production was significantly enhanced in both co-cultures (hAMSC/bACs p = 0.013; hBMSC/bACs p<0.001). (Fig 2B and 2C) Similar results were obtained after 5 weeks of culture. (Data not shown) These results demonstrate that co-cultures of hMSCs and bACs improve cartilage formation in vitro, depending on the hMSC-source used (hBMSC ≥ hAMSC).
In vivo outcomes
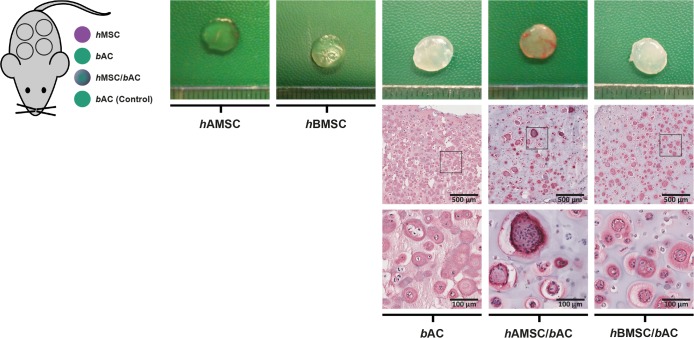
Cell-free alginate constructs (controls; n = 4) and alginate constructs containing hBMSC/bACs, hAMSC/bACs or hBMSC, hAMSC or bAC only, were generated and immediately implanted subcutaneously in athymic mice. After 8 weeks, all but 5 (n = 3 hAMSC, n = 2 hBMSC) of the 40 constructs could be identified and harvested. Unfortunately however, the remaining hMSC-constructs (either hAMSCs or hBMSCs) and cell-free alginate constructs were lost during the embedding process. Constructs containing bACs or hBMSC/bACs resembled cartilage tissue in both color and texture, while the appearance of constructs containing hAMSC/bACs was particularly donor-dependent. (Fig 3) None of the constructs had mineralized or ossified. Also, vascularization within the construct, was never observed. Cells were more heterogeneously distributed in constructs containing either hAMSC/bACs or hBMSC/bACs compared to bACs only. Collagen type II was abundantly present in constructs containing bAC or hBMSC/bACs. Again, hAMSC/bACs contained collagen type II in a donor-dependent manner. (Fig 3) A Safranin-O staining displayed similar results. (S2 Fig)
Fig 3. Macroscopic appearance and immunohistochemical analyses of constructs containing hMSCs and/or bACs, 8 weeks after subcutaneous implantation in mice.
Macroscopic appearance (top row) of cartilage constructs, as well as a collagen type II immunohistochemical staining (bottom rows), 8 weeks after subcutaneous implantation. hAMSC = human Adipose-tissue-derived Mesenchymal Stem Cell (n = 3 experiments with 3 independent donors); hBMSC = human Bone-marrow-derived Mesenchymal Stem Cell (n = 3 experiments with 3 independent donors); bAC = bovine Articular Chondrocyte (n = 3 experiments with 3 pools of donors). Per experiment, 2 samples were used for analyses.
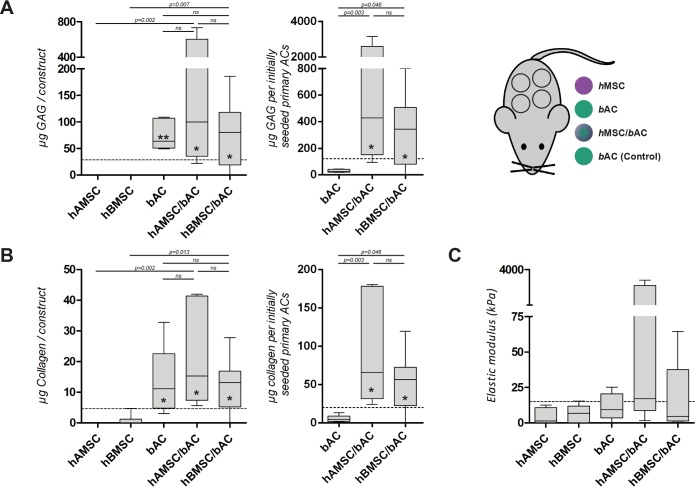
In vivo, DNA- and GAG-content were not detected in cell-free alginate constructs. (Data not shown) hAMSC/bACs and hBMSC/bACs contained similar quantities of cartilage matrix as constructs containing bACs only. Moreover GAG formation in co-cultures was independent of the origin of the hMSC-source used (p = 0.916). (Fig 4A) Collagen production demonstrated a similar trend, again without statistical significant differences between hAMSC/bACs and hBMSC/bACs (p = 1.000). (Fig 4B) Normalization of the data to their initially seeded primary ACs revealed more distinct differences between mixed-cell-type and single-cell-type populations: hAMSC/bACs and hBMSC/bACs produced significantly more GAG and collagen per initially seeded primary ACs compared to bACs (hAMSC/bACs p<0.01; hBMSC/bACs p<0.05). (Fig 4) After subcutaneous implantation, the elastic modulus was highest in constructs containing hAMSC/bACs and hBMSC/bACs, albeit this did not reach statistical significance due to the large variation between samples. (Fig 4C) These results confirm our in-vitro results by showing that co-cultures of hMSCs and bACs improve cartilage formation. However, in vivo this phenomenon seems independent of the hMSC-source used, although large donor variation is observed.
Fig 4. Cartilage matrix formation in constructs containing hMSCs and/or bACs, 8 weeks after subcutaneous implantation in mice.
Biochemical (GAG (A) and collagen (B) content) and biomechanical evaluation (C), 8 weeks after subcutaneous implantation. The left graphs in A and B, demonstrate the amount of matrix components per construct, whereas for the right graphs matrix production is normalized to the initially seeded primary ACs. A control condition—containing similar amounts of bACs (0.8*106 nc /ml) without supplementation of hMSCs—was evaluated to determine the additional effect of hMSCs (3.2*106 nc /ml) on bACs in co-cultures (dotted line). *, ** or *** indicates p-values smaller than 0.05, 0.01 or 0.001 respectively compared to the control condition. Data are shown as box-whisker plots. For statistical evaluation, a Kruskal-Wallis followed by the Mann-Whitney-U test was used followed by a Bonferroni's post-hoc comparisons test. hAMSC = human Adipose-tissue-derived Mesenchymal Stem Cell (n = 3 experiments with 3 independent donors); hBMSC = human Bone-marrow-derived Mesenchymal Stem Cell (n = 3 experiments with 3 independent donors); bAC = bovine Articular Chondrocyte (n = 3 experiments with 3 pools of donors). Per experiment, 2 samples were used for analyses.
Differentiation versus chondroinduction
Using a xenogeneic in-vitro culture system enabled us to determine the contribution of each individual cell type (i.e. hBMSCs, hBMSCs or bACs) to cartilage matrix production using species-specific gene-expression analyses.
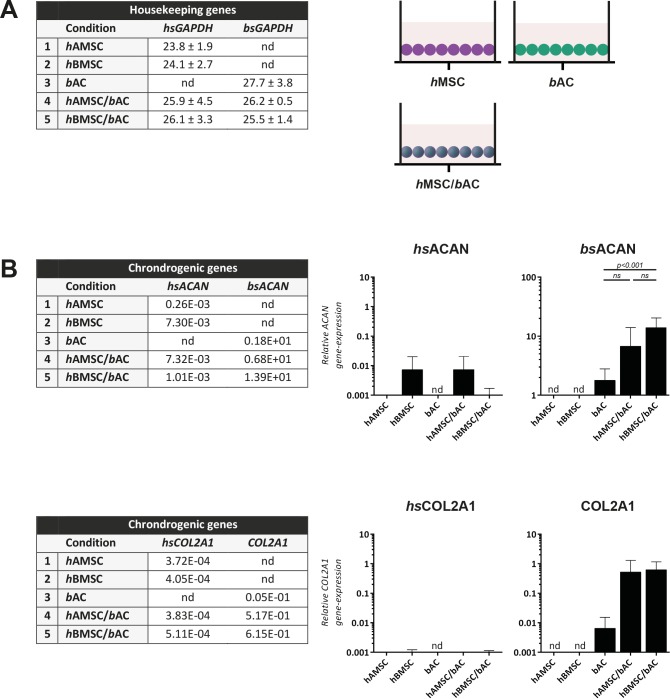
First, GAPDH-gene expression was analyzed after 5 weeks of in-vitro culture. hAMSC/bACs and hBMSC/bACs contained cells from both bovine (AC) and human (AMSC or BMSC) origin. (Fig 5A) Then, chondrogenic gene expression was evaluated by the ACAN and COL2A1 genes. In a growth-factor-free environment, hAMSCs and hBMSCs hardly expressed hsACAN and hsCOL2A1. Besides, chondrogenic genes were hardly expressed in hAMSC/bACs or hBMSC/bACs either. Conversely, hAMSC/bACs or hBMSC/bACs—containing solely 20% bovine articular chondrocytes—expressed as much or even higher levels of bsACAN compared to 100% bACs (hAMSC/bACs vs bACs p>0.05; hBMSC/bACs vs bACs p<0.001). hAMSC/bACs and hBMSC/bACs expressed COL2A1, although gene-expression of hsCOL2A1 was negligible. This means that the COL2A1 expressed was from bovine origin. (Fig 5B) These data indicate that the formed cartilage matrix was from bAC-origin, which suggests a more trophic role for hMSCs herein.
Fig 5. Gene-expression analysis, 5 weeks after in vitro culture.
Data are shown as mean CT-values ± SD of housekeeping genes (A) and average relative gene-expression of chondrogenic genes (B). nd = not detected (ct-value > 35.00); hsGAPDH = human-specific GAPDH; bsGAPDH = bovine-specific GAPDH; hsACAN = human-specific ACAN; bsACAN = bovine-specific ACAN; hsCOL2A1 = human-specific COL2A1; hAMSC = human Adipose-tissue-derived Mesenchymal Stem Cell (n = 3 experiments with 3 independent donors); hBMSC = human Bone-marrow-derived Mesenchymal Stem Cell (n = 3 experiments with 3 independent donors); bAC = bovine Articular Chondrocyte (n = 3 experiments with 3 pools of donors). Per experiment, 3 samples were used for analyses.
Cellular interactions
To further understand the complex cellular interaction between hMSCs and bACs, cells were encapsulated in separate alginate constructs and co-cultured in a Transwell® system as well as in medium conditioned by the other cell type. (Figs 6A and 7A) In addition cell combination were also cultured in pellets, allowing direct cell-cell contact.
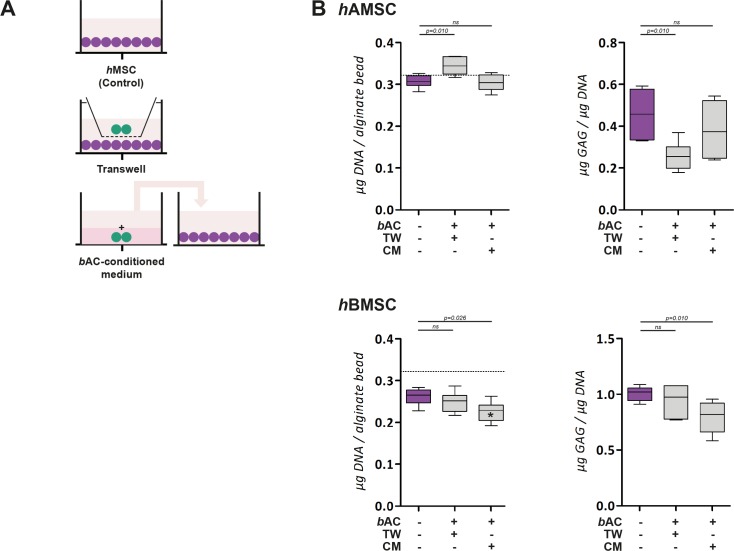
Fig 6. Paracrine effect of bACs on hAMSCs and hBMSCs.
(A) Schematic overview. In purple: hMSCs; in green: bACs. (B) The DNA and GAG content of hAMSCs and hBMSCs in the presence of paracrine factors of bACs via Transwell® system or bAC-conditioned medium. The DNA content after 3 weeks of culture was compared to the initial DNA content prior to cell-culture (dotted line). *, ** or *** indicates p-values smaller than 0.05, 0.01 or 0.001 respectively compared to the amount of DNA prior to cell culture. Data are shown as box-whisker plots of 6 samples of one experiment. For statistical evaluation, a Kruskal-Wallis followed by the Mann-Whitney-U test was use followed by a Bonferroni's post-hoc comparisons test. TW = Transwell; CM = Conditioned Medium; hAMSC = human Adipose-tissue-derived Mesenchymal Stem Cell; hBMSC = human Bone-marrow-derived Mesenchymal Stem Cell; bAC = bovine Articular Chondrocyte.
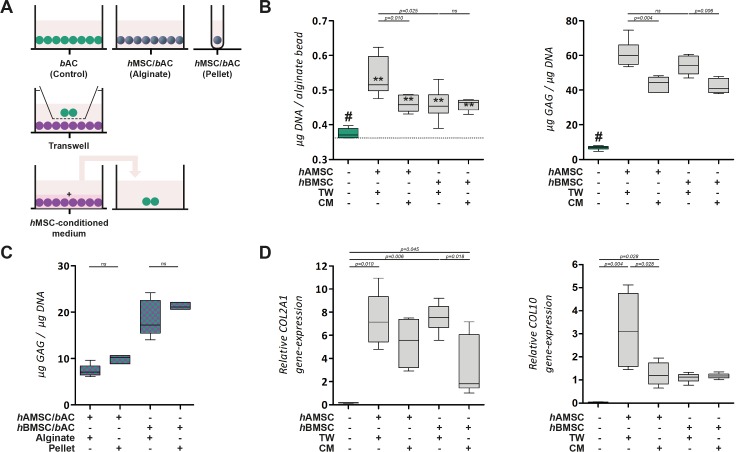
Fig 7. Paracrine effect of hAMSCs and hBMSCs on bACs.
(A) Schematic overview. In purple: hMSCs; in green: bACs. (B) The DNA and GAG content of bACs in the presence of paracrine factors of hMSCs via Transwell® system or hMSC-conditioned medium. The DNA content after 3 weeks of culture was compared to the initial DNA content prior to cell-culture (dotted line). (C) Co-culture in alginate constructs and pellet culture, allowing direct cell-cell contact. (D) Relative gene-expression analysis, 3 weeks after culture in alginate. *, ** or *** indicates p-values smaller than 0.05, 0.01 or 0.001 respectively compared to the amount of DNA prior to cell culture. # indicates significant differences from all conditions (p<0.01). Data are shown as box-whisker plots of 6 samples of one experiment. For statistical evaluation, a Kruskal-Wallis followed by the Mann-Whitney-U test was use followed by a Bonferroni's post-hoc comparisons test. TW = Transwell; CM = Conditioned Medium; hAMSC = human Adipose-tissue-derived Mesenchymal Stem Cell; hBMSC = human Bone-marrow-derived Mesenchymal Stem Cell; bAC = bovine Articular Chondrocyte.
Alginate constructs containing solely bACs, hAMSCs or hBMSCs cultured in ‘basic medium’ maintained their DNA content over the 3 weeks of culture. Exposure to paracrine factors of bAC via Transwell® system or bAC-conditioned medium, did not alter the amount of DNA in alginate constructs seeded with either hAMSCs or hBMSCs. (Fig 6B) The presence of factors secreted by hMSC significantly increased the total amount of DNA in constructs containing bACs (p<0.01). This effect was independent on the origin of the hMSCs (i.e. hAMSCs, hBMSCs) and co-culture system used (i.e. Transwell® system, hMSC-conditioned medium). (Fig 7B) This suggests MSC have paracrine effects on chondrocytes.
Alginate constructs containing hAMSCs or hBMSCs, formed very little GAG after 3 weeks of culture. GAG-production remained similarly low when hMSC-constructs were cultured in the presence of paracrine factors of bAC via Transwell® system or bAC-conditioned medium. (Fig 6B) The production of GAG was higher in constructs containing bACs. Exposure to paracrine factors of hMSC significantly increased GAG-production, irrespective to the hMSC-source used (i.e. hAMSCs, hBMSCs, p<0.01). Since the amount of DNA was also enhanced in these constructs, GAG content was adjusted to the amount of DNA, still showing pronounced differences. GAG formation was significantly increased in Transwell® system compared to constructs cultured with bAC-conditioned medium (p<0.01). (Fig 7B) Similar trends were observed at COL2A1 gene-expression level. (Fig 7D) This provides further indications that the effect of the combination of hMSCs and bACs on chondrogenesis is due to paracrine effect of hMSCs on chondrocytes.
Based on previous results, we further wanted to evaluate signs of hypertrophy in these constructs, since hypertrophic differentiation is an unwanted phenomenon in cartilage regeneration. bACs cultured in ‘basic medium’ expressed hardly any COL10 after 3 weeks of culture. In addition, when bACs were exposed to paracrine factors of hMSC either via Transwell® system or hMSC-conditioned medium, COL10-gene-expression was upregulated and significantly increased in constructs exposed to paracrine factors of hAMSCs (Transwell® system p = 0.004; hAMSC-conditioned medium p = 0.028). Although COL10-gene-expression was slightly upregulated in constructs exposed to paracrine factors of hBMSC, no significant differences could be observed in comparison with constructs cultured in ‘basic medium’ (both Transwell® system and hBMSC-conditioned medium p>0.05). (Fig 7D)
This indicates that hMSCs have the ability to improve cartilage matrix formation in co-culture, by improving bAC-proliferation capacity as well as increasing bAC-GAG-production. Moreover, when exposed to paracrine factors of hBMSC, hypertrophic differentiation was not significantly enhanced compared to untreated bACs. In pellet co-culture, matrix production was similarly produced as in 3D-alginate constructs, meaning that direct cell-cell contact is not required for co-cultures of hMSCs and bACs. (Fig 7C)
Discussion
Combining chondrocytes and MSCs holds great promise for cell-based cartilage repair as it reduces the required number of chondrocytes and diminishes many disadvantages of individually used cell types leading to enhanced cartilage matrix formation with low hypertrophic differentiation. In line with former research, hAMSC/bACs and hBMSC/bACs produced similar or even improved quantities of cartilage matrix components as constructs containing bACs only, both in vitro and in vivo. Moreover, hypertrophic gene expression (COL10) was not affected by hBMSCs, but slightly enhanced by hAMSCs. However, constructs containing either hAMSC/bACs or hBMSC/bACs appeared stable and did not calcify in vivo. This suggests that 80% of bACs can be replaced by either hAMSCs or hBMSCs without influencing cartilage matrix production nor stability. Therefore, mixed-cell-cultures of MSCs and chondrocytes could be very valuable for cell-based cartilage repair, as appropriate numbers of cells are more easily acquired from bone-marrow aspirates or adipose tissue than from cartilage biopsies.
The cellular mechanism responsible for enhanced cartilage production in co-culture is however still debated. Numerous cellular communication pathways have been hypothesized in order to explain the beneficial effect in co-cultures [53]. We found no evidence that cartilage formation was the consequence of chondrogenic lineage differentiation of hMSCs, as stated by others [41, 49, 54–62]. In contrast, cartilage matrix clearly originated from bACs, which suggests a predominantly trophic role for hMSCs in these constructs: both hAMSCs and hBMSCs improved bAC-proliferation as well as bAC-GAG-formation. This confirms previous studies were the co-culture effect has been ascribed to ACs, whose cartilage-forming capacity and proliferation activity appears to enhance in the presence of MSCs. [40, 50, 63–68] The trophic and paracrine function of MSCs herein appeared essential rather than MSCs actively undergoing chondrogenic differentiation. We show that this is a general feature that applies to both AMSCs and BMSCs.
To date, only three studies have compared the trophic effect of several MSC-sources—such as AMSCs and BMSCs—on ACs in co-culture. [40–42] Unfortunately, these studies demonstrate conflicting outcomes and have never translated to animal research. Therefore, to our knowledge, we are the first to systematically compare the cartilage forming capacity of either hAMSC/bACs and hBMSC/bACs in vitro and in vivo. In vitro, hBMSC/bACs contained significantly more cartilage matrix components than hAMSC/bACs. Cartilage formation after 8 weeks of subcutaneous implantation was, however, not different in constructs containing hAMSC/bACs and hBMSC/bACs, although large donor variations were observed, in particular in hAMSC/bACs. Our results support a general trophic or immunomodulatory role for hAMSCs and hBMSCs on bACs in co-culture, as stated by Wu [40] and Maumus et al [42]. Although both cell sources share comparable immunomodulatory modalities, they do not necessarily behave the same. In monocultures there are clear differences observed between hAMSCs and hBMSCs. For instance, they possess distinctive proliferation capacities and a dissimilar potential to chondrogenically differentiate. [2] Moreover, both cell sources secrete different subsets of paracrine factors: compared to hBMSCs, hAMSCs secrete significantly more VEGF-D [69], IGF-1 [69, 70], IL-8 [69] and IL-6 [69, 71], and significantly less SDF-1 [72] and TFGβ1 [72]. In co-cultures, differences between hMSC-cell sources appear less clear. Acharya et al. demonstrated enhanced chondrocyte proliferation capacity and improved GAG formation in pellets containing hBMSC/bACs compared to hAMSC/bACs. [41] Besides, 3 independent co-culture studies using AMSCs only showed limited or decreased effects of MSCs on chondrogenesis. [37–39] Such effect was hardly seen in co-culture studies using BMSCs only, which may propose that, compared to BMSCs, AMSCs seem less efficient in co-culture, Although we could not find a general beneficial effect of hBMSCs in co-cultures compared to hAMSCs in vitro and in vivo, we did show that in vitro, hBMSC/bACs outperformed hAMSC/bACs and hypertrophic gene expression was lower in hBMSC/bACs. True dissimilarities between hAMSCs and hBMSCs in co-culture are unfortunately hard to expose, as hMSC-cultures are highly heterogeneous and distinct population subsets will probably interfere with the reciprocal communication pathways in co-culture. Therefore, the purification of distinct subsets of hMSCs might enhance the particular capability of hAMSCs and hBMSCs in co-culture by eliminating interfering cells with limited potential, or even cells with inhibitory activity. Future research still needs to clarify whether the trophic role of MSCs in co-culture is truly a general MSC-characteristic produced by a distinct subset of the MSC-population or dependent on the original origin of the MSCs.
Our data and that of others emphasize the importance of paracrine signaling pathways in co-culture comparatively to juxtacrine or gap-junctional signaling. Although the importance of direct cell-cell contact is still unclear in literature [63], such signaling pathways remained less important in our study, since alginate hydrogel impedes direct cell-cell contact and in pellet culture no beneficial effect of direct cell-cell contact was observed. On the contrary, bACs produced less cartilage matrix in Transwell® system with hMSCs and the amount of cartilage matrix was further reduced in hMSC-conditioned medium. Although direct cell-cell contact seems less significant than paracrine signaling, it seems correspondingly important to secure a certain cell-cell distance for optimal cell communication.
Furthermore, for optimal cell communication and subsequent cartilage regeneration, an optimal cell density and ratio of MSCs to ACs is imperative. Additionally, for cell-based cartilage repair, it would be ideal to only use low numbers of primary chondrocytes. Although Puelacher et al already recommended cell densities greater than 20x106 cells per milliliter [73], we could not increase the cell seeding density over 4x106 cells per milliliter, as the size of our experimental set-up did not enable higher densities. Additionally, we have replaced 80% of the bACs by hMSCs (at a 4:1 ratio), as described previously. [40, 50] However, no consensus on optimal co-culture ratios is yet available. Future research needs to clarify if we could increase cell density while further reduce the number of primary chondrocytes (increase the MSC-chondrocyte-ratio) without inhibiting cartilage matrix production and stability.
The species mismatch limited the translation of presented basic research to clinical application. However, the species mismatch was chosen to be able to discriminate between the role of the different cell types. We do not expect huge differences in fully human co-culture models, as both xenogeneic and autologous co-culture models have resulted in comparable outcomes, indicating that in both models comparable mechanisms are likely operational. [50] Our results confirmed previously published results of hMSCs combined with xenogeneic chondrocytes. [50, 74–76] Therefore, it appears to be an excellent model to study cell-specific contributions to tissue formation.
In conclusion, this study demonstrates that 80% of chondrocytes can be replaced by either hAMSCs or hBMSCs without influencing cartilage matrix production nor stability. Besides, our results support a general trophic role for hAMSCs and hBMSCs on chondrocytes in co-culture that does not need direct cell-cell contact. These data provide information that can be used to further optimize cell-based cartilage repair.
Supporting information
(A) The DNA content of none of the constructs had changed compared to their initial DNA content prior to cell-culture (dotted line). Biochemical evaluation of the GAG (B) and collagen (C) content, 5 weeks after culture in alginate. The left graphs demonstrate the amount of matrix components per construct, whereas for the right graphs matrix production is normalized to the initially seeded primary ACs. A control condition—containing similar amounts of bACs (0.8*106 nc/ml) without supplementation of hMSCs—was evaluated to determine the additional effect of hMSCs (3.2*106 nc/ml) on bACs in co-cultures (dotted line). *** indicates a p-value smaller than 0.001 compared to the control condition. Data are shown as mean ± SD. For statistical evaluation, a mixed model was used followed by a Bonferroni's post-hoc comparisons test. hAMSC = human Adipose-tissue-derived Mesenchymal Stem Cell (n = 3 experiments with 3 independent donors); hBMSC = human Bone-marrow-derived Mesenchymal Stem Cell (n = 3 experiments with 3 independent donors); bAC = bovine Articular Chondrocyte (n = 3 experiments with 3 pools of donors). Per experiment, 3 samples were used for analyses.
(TIF)
Macroscopic appearance (top row) of cartilage constructs, as well as a Safranin-O histochemical staining (bottom rows), 8 weeks after subcutaneous implantation. hAMSC = human Adipose-tissue-derived Mesenchymal Stem Cell (n = 3 experiments with 3 independent donors); hBMSC = human Bone-marrow-derived Mesenchymal Stem Cell (n = 3 experiments with 3 independent donors); bAC = bovine Articular Chondrocyte (n = 3 experiments with 3 pools of donors). Per experiment, 2 samples were used for analyses.
(TIF)
Acknowledgments
The authors would like to thank Jeanine Hendriks (CellcoTec, Bilthoven, the Netherlands) for her valuable ideas during preparation of this manuscript. We also acknowledge the Department of Orthopaedic Surgery (Erasmus MC, University Medical Center, Rotterdam, the Netherlands) for their assistance in obtaining bone marrow aspirates. The study was performed within the framework of EuroNanoMed (EAREG-406340-131009/1) and funded by SenterNovem.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was performed within the framework of EuroNanoMed (EAREG-406340-131009/1) and funded by SenterNovem.
References
- 1.Naumann A, Dennis JE, Aigner J, Coticchia J, Arnold J, Berghaus A, et al. Tissue engineering of autologous cartilage grafts in three-dimensional in vitro macroaggregate culture system. Tissue engineering. 2004;10(11–12):1695–706. Epub 2005/02/03. doi: 10.1089/ten.2004.10.1695 . [DOI] [PubMed] [Google Scholar]
- 2.Pleumeekers MM, Nimeskern L, Koevoet WL, Kops N, Poublon RM, Stok KS, et al. The in vitro and in vivo capacity of culture-expanded human cells from several sources encapsulated in alginate to form cartilage. European cells & materials. 2014;27:264–80; discussion 78–80. Epub 2014/04/08. . [DOI] [PubMed] [Google Scholar]
- 3.El Sayed K, Haisch A, John T, Marzahn U, Lohan A, Muller RD, et al. Heterotopic autologous chondrocyte transplantation—a realistic approach to support articular cartilage repair? Tissue engineering Part B, Reviews. 2010;16(6):603–16. Epub 2010/09/10. doi: 10.1089/ten.TEB.2010.0167 . [DOI] [PubMed] [Google Scholar]
- 4.Lohan A, Marzahn U, El Sayed K, Haisch A, Kohl B, Muller RD, et al. In vitro and in vivo neo-cartilage formation by heterotopic chondrocytes seeded on PGA scaffolds. Histochemistry and cell biology. 2011;136(1):57–69. Epub 2011/06/10. doi: 10.1007/s00418-011-0822-2 . [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Spector M. Comparison of three types of chondrocytes in collagen scaffolds for cartilage tissue engineering. Biomed Mater. 2009;4(4):045012 Epub 2009/07/29. doi: 10.1088/1748-6041/4/4/045012 . [DOI] [PubMed] [Google Scholar]
- 6.Isogai N, Kusuhara H, Ikada Y, Ohtani H, Jacquet R, Hillyer J, et al. Comparison of different chondrocytes for use in tissue engineering of cartilage model structures. Tissue engineering. 2006;12(4):691–703. Epub 2006/05/06. doi: 10.1089/ten.2006.12.691 . [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Lee E, Kim HY, Son Y. Comparison of articular cartilage with costal cartilage in initial cell yield, degree of dedifferentiation during expansion and redifferentiation capacity. Biotechnology and applied biochemistry. 2007;48(Pt 3):149–58. Epub 2007/05/12. doi: 10.1042/BA20060233 . [DOI] [PubMed] [Google Scholar]
- 8.Asawa Y, Ogasawara T, Takahashi T, Yamaoka H, Nishizawa S, Matsudaira K, et al. Aptitude of auricular and nasoseptal chondrocytes cultured under a monolayer or three-dimensional condition for cartilage tissue engineering. Tissue engineering Part A. 2009;15(5):1109–18. Epub 2008/12/09. doi: 10.1089/ten.tea.2007.0218 . [DOI] [PubMed] [Google Scholar]
- 9.Malicev E, Kregar-Velikonja N, Barlic A, Alibegovic A, Drobnic M. Comparison of articular and auricular cartilage as a cell source for the autologous chondrocyte implantation. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2009;27(7):943–8. Epub 2008/12/24. doi: 10.1002/jor.20833 . [DOI] [PubMed] [Google Scholar]
- 10.Kafienah W, Jakob M, Demarteau O, Frazer A, Barker MD, Martin I, et al. Three-dimensional tissue engineering of hyaline cartilage: comparison of adult nasal and articular chondrocytes. Tissue engineering. 2002;8(5):817–26. Epub 2002/12/03. doi: 10.1089/10763270260424178 . [DOI] [PubMed] [Google Scholar]
- 11.Johnson TS, Xu JW, Zaporojan VV, Mesa JM, Weinand C, Randolph MA, et al. Integrative repair of cartilage with articular and nonarticular chondrocytes. Tissue engineering. 2004;10(9–10):1308–15. Epub 2004/12/14. doi: 10.1089/ten.2004.10.1308 . [DOI] [PubMed] [Google Scholar]
- 12.Chung C, Erickson IE, Mauck RL, Burdick JA. Differential behavior of auricular and articular chondrocytes in hyaluronic acid hydrogels. Tissue engineering Part A. 2008;14(7):1121–31. Epub 2008/04/15. doi: 10.1089/tea.2007.0291 ; PubMed Central PMCID: PMC2667224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panossian A, Ashiku S, Kirchhoff CH, Randolph MA, Yaremchuk MJ. Effects of cell concentration and growth period on articular and ear chondrocyte transplants for tissue engineering. Plast Reconstr Surg. 2001;108(2):392–402. Epub 2001/08/10. . [DOI] [PubMed] [Google Scholar]
- 14.Tay AG, Farhadi J, Suetterlin R, Pierer G, Heberer M, Martin I. Cell yield, proliferation, and postexpansion differentiation capacity of human ear, nasal, and rib chondrocytes. Tissue engineering. 2004;10(5–6):762–70. Epub 2004/07/22. doi: 10.1089/1076327041348572 . [DOI] [PubMed] [Google Scholar]
- 15.Xu JW, Zaporojan V, Peretti GM, Roses RE, Morse KB, Roy AK, et al. Injectable tissue-engineered cartilage with different chondrocyte sources. Plast Reconstr Surg. 2004;113(5):1361–71. Epub 2004/04/03. . [DOI] [PubMed] [Google Scholar]
- 16.Van Osch GJ, Mandl EW, Jahr H, Koevoet W, Nolst-Trenite G, Verhaar JA. Considerations on the use of ear chondrocytes as donor chondrocytes for cartilage tissue engineering. Biorheology. 2004;41(3–4):411–21. Epub 2004/08/10. . [PubMed] [Google Scholar]
- 17.Afizah H, Yang Z, Hui JH, Ouyang HW, Lee EH. A comparison between the chondrogenic potential of human bone marrow stem cells (BMSCs) and adipose-derived stem cells (ADSCs) taken from the same donors. Tissue engineering. 2007;13(4):659–66. Epub 2007/03/21. doi: 10.1089/ten.2006.0118 . [DOI] [PubMed] [Google Scholar]
- 18.Hellingman CA, Verwiel ET, Slagt I, Koevoet W, Poublon RM, Nolst-Trenite GJ, et al. Differences in cartilage-forming capacity of expanded human chondrocytes from ear and nose and their gene expression profiles. Cell Transplant. 2011;20(6):925–40. doi: 10.3727/096368910X539119 . [DOI] [PubMed] [Google Scholar]
- 19.Henderson JH, Welter JF, Mansour JM, Niyibizi C, Caplan AI, Dennis JE. Cartilage tissue engineering for laryngotracheal reconstruction: comparison of chondrocytes from three anatomic locations in the rabbit. Tissue engineering. 2007;13(4):843–53. Epub 2007/03/31. doi: 10.1089/ten.2006.0256 ; PubMed Central PMCID: PMC2562571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kusuhara H, Isogai N, Enjo M, Otani H, Ikada Y, Jacquet R, et al. Tissue engineering a model for the human ear: assessment of size, shape, morphology, and gene expression following seeding of different chondrocytes. Wound Repair Regen. 2009;17(1):136–46. Epub 2009/01/21. WRR451 [pii] doi: 10.1111/j.1524-475X.2008.00451.x . [DOI] [PubMed] [Google Scholar]
- 21.Karlsson C, Brantsing C, Svensson T, Brisby H, Asp J, Tallheden T, et al. Differentiation of human mesenchymal stem cells and articular chondrocytes: analysis of chondrogenic potential and expression pattern of differentiation-related transcription factors. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2007;25(2):152–63. Epub 2006/10/31. doi: 10.1002/jor.20287 . [DOI] [PubMed] [Google Scholar]
- 22.Von Der Mark K, Gauss V, Von Der Mark H, Mueller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977;267(5611):531–2. doi: 10.1038/267531a0 [DOI] [PubMed] [Google Scholar]
- 23.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Experimental cell research. 1998;238(1):265–72. doi: 10.1006/excr.1997.3858 . [DOI] [PubMed] [Google Scholar]
- 24.Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue engineering. 2005;11(7–8):1198–211. doi: 10.1089/ten.2005.11.1198 . [DOI] [PubMed] [Google Scholar]
- 25.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. . [DOI] [PubMed] [Google Scholar]
- 26.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Molecular biology of the cell. 2002;13(12):4279–95. doi: 10.1091/mbc.E02-02-0105 ; PubMed Central PMCID: PMC138633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue engineering. 2001;7(2):211–28. doi: 10.1089/107632701300062859 . [DOI] [PubMed] [Google Scholar]
- 28.Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis and rheumatism. 2006;54(10):3254–66. doi: 10.1002/art.22136 . [DOI] [PubMed] [Google Scholar]
- 29.Farrell E, Both SK, Odorfer KI, Koevoet W, Kops N, O'Brien FJ, et al. In-vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells. BMC musculoskeletal disorders. 2011;12:31 Epub 2011/02/02. doi: 10.1186/1471-2474-12-31 ; PubMed Central PMCID: PMC3045394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farrell E, van der Jagt OP, Koevoet W, Kops N, van Manen CJ, Hellingman CA, et al. Chondrogenic priming of human bone marrow stromal cells: a better route to bone repair? Tissue Eng Part C Methods. 2009;15(2):285–95. Epub 2009/06/10. doi: 10.1089/ten.tec.2008.0297 . [DOI] [PubMed] [Google Scholar]
- 31.Farrell MJ, Fisher MB, Huang AH, Shin JI, Farrell KM, Mauck RL. Functional properties of bone marrow-derived MSC-based engineered cartilage are unstable with very long-term in vitro culture. Journal of biomechanics. 2014;47(9):2173–82. Epub 2013/11/19. doi: 10.1016/j.jbiomech.2013.10.030 ; PubMed Central PMCID: PMC3995895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scotti C, Piccinini E, Takizawa H, Todorov A, Bourgine P, Papadimitropoulos A, et al. Engineering of a functional bone organ through endochondral ossification. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(10):3997–4002. Epub 2013/02/13. doi: 10.1073/pnas.1220108110 ; PubMed Central PMCID: PMC3593845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meretoja VV, Dahlin RL, Wright S, Kasper FK, Mikos AG. Articular chondrocyte redifferentiation in 3D co-cultures with mesenchymal stem cells. Tissue Eng Part C Methods. 2014;20(6):514–23. Epub 2014/01/07. doi: 10.1089/ten.tec.2013.0532 ; PubMed Central PMCID: PMC4025602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Windt TS, Hendriks JA, Zhao X, Vonk LA, Creemers LB, Dhert WJ, et al. Concise review: unraveling stem cell cocultures in regenerative medicine: which cell interactions steer cartilage regeneration and how? Stem cells translational medicine. 2014;3(6):723–33. doi: 10.5966/sctm.2013-0207 ; PubMed Central PMCID: PMC4039458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leijten JC, Georgi N, Wu L, van Blitterswijk CA, Karperien M. Cell sources for articular cartilage repair strategies: shifting from monocultures to cocultures. Tissue engineering Part B, Reviews. 2013;19(1):31–40. doi: 10.1089/ten.TEB.2012.0273 . [DOI] [PubMed] [Google Scholar]
- 36.Hubka KM, Dahlin RL, Meretoja VV, Kasper FK, Mikos AG. Enhancing chondrogenic phenotype for cartilage tissue engineering: monoculture and coculture of articular chondrocytes and mesenchymal stem cells. Tissue engineering Part B, Reviews. 2014;20(6):641–54. doi: 10.1089/ten.TEB.2014.0034 ; PubMed Central PMCID: PMC4241977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hildner F, Concaro S, Peterbauer A, Wolbank S, Danzer M, Lindahl A, et al. Human adipose-derived stem cells contribute to chondrogenesis in coculture with human articular chondrocytes. Tissue Eng Part A. 2009;15(12):3961–9. doi: 10.1089/ten.TEA.2009.0002 . [DOI] [PubMed] [Google Scholar]
- 38.Lee. Adipose stem cells can secrete angiogenic factors that inhibit hyaline cartilage regeneration. Stem Cell Research & Therapy. 2012;3(35). doi: 10.1186/scrt126 PubMed Central PMCID: PMCPMC3580473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopa S, Colombini A, Sansone V, Preis FW, Moretti M. Influence on chondrogenesis of human osteoarthritic chondrocytes in co-culture with donor-matched mesenchymal stem cells from infrapatellar fat pad and subcutaneous adipose tissue. International journal of immunopathology and pharmacology. 2013;26(1 Suppl):23–31. doi: 10.1177/03946320130260S104 . [DOI] [PubMed] [Google Scholar]
- 40.Wu L, Prins HJ, Helder MN, van Blitterswijk CA, Karperien M. Trophic effects of mesenchymal stem cells in chondrocyte co-cultures are independent of culture conditions and cell sources. Tissue Eng Part A. 2012;18(15–16):1542–51. Epub 2012/03/21. doi: 10.1089/ten.TEA.2011.0715 . [DOI] [PubMed] [Google Scholar]
- 41.Acharya C, Adesida A, Zajac P, Mumme M, Riesle J, Martin I, et al. Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. Journal of cellular physiology. 2012;227(1):88–97. Epub 2011/10/26. doi: 10.1002/jcp.22706 . [DOI] [PubMed] [Google Scholar]
- 42.Maumus M, Manferdini C, Toupet K, Peyrafitte JA, Ferreira R, Facchini A, et al. Adipose mesenchymal stem cells protect chondrocytes from degeneration associated with osteoarthritis. Stem cell research. 2013;11(2):834–44. doi: 10.1016/j.scr.2013.05.008 . [DOI] [PubMed] [Google Scholar]
- 43.Gharibi B, Hughes FJ. Effects of medium supplements on proliferation, differentiation potential, and in vitro expansion of mesenchymal stem cells. Stem cells translational medicine. 2012;1(11):771–82. doi: 10.5966/sctm.2010-0031 ; PubMed Central PMCID: PMC3659663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin I, Muraglia A, Campanile G, Cancedda R, Quarto R. Fibroblast growth factor-2 supports ex vivo expansion and maintenance of osteogenic precursors from human bone marrow. Endocrinology. 1997;138(10):4456–62. doi: 10.1210/endo.138.10.5425 . [DOI] [PubMed] [Google Scholar]
- 45.Choi KM, Seo YK, Yoon HH, Song KY, Kwon SY, Lee HS, et al. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. Journal of bioscience and bioengineering. 2008;105(6):586–94. doi: 10.1263/jbb.105.586 . [DOI] [PubMed] [Google Scholar]
- 46.Jork A, Thurmer F, Cramer H, Zimmermann G, Gessner P, Hamel K, et al. Biocompatible alginate from freshly collected Laminaria pallida for implantation. Applied microbiology and biotechnology. 2000;53(2):224–9. . [DOI] [PubMed] [Google Scholar]
- 47.Hauselmann HJ, Aydelotte MB, Schumacher BL, Kuettner KE, Gitelis SH, Thonar EJ. Synthesis and turnover of proteoglycans by human and bovine adult articular chondrocytes cultured in alginate beads. Matrix. 1992;12(2):116–29. Epub 1992/04/01. . [DOI] [PubMed] [Google Scholar]
- 48.Pleumeekers MM, Nimeskern L, Koevoet WL, Karperien M, Stok KS, van Osch GJ. Cartilage Regeneration in the Head and Neck Area: Combination of Ear or Nasal Chondrocytes and Mesenchymal Stem Cells Improves Cartilage Production. Plast Reconstr Surg. 2015;136(6):762e–74e. doi: 10.1097/PRS.0000000000001812 . [DOI] [PubMed] [Google Scholar]
- 49.Bian L, Zhai DY, Mauck RL, Burdick JA. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng Part A. 2011;17(7–8):1137–45. Epub 2010/12/15. doi: 10.1089/ten.TEA.2010.0531 ; PubMed Central PMCID: PMC3063700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu L, Leijten JC, Georgi N, Post JN, van Blitterswijk CA, Karperien M. Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng Part A. 2011;17(9–10):1425–36. doi: 10.1089/ten.TEA.2010.0517 . [DOI] [PubMed] [Google Scholar]
- 51.Enobakhare BO, Bader DL, Lee DA. Quantification of sulfated glycosaminoglycans in chondrocyte/alginate cultures, by use of 1,9-dimethylmethylene blue. Anal Biochem. 1996;243(1):189–91. Epub 1996/12/01. S0003-2697(96)90502-3 [pii] doi: 10.1006/abio.1996.0502 . [DOI] [PubMed] [Google Scholar]
- 52.Hierck BP, Iperen LV, Gittenberger-De Groot AC, Poelmann RE. Modified indirect immunodetection allows study of murine tissue with mouse monoclonal antibodies. J Histochem Cytochem. 1994;42(11):1499–502. Epub 1994/11/01. doi: 10.1177/42.11.7930532 . [DOI] [PubMed] [Google Scholar]
- 53.Hendriks J, Riesle J, van Blitterswijk CA. Co-culture in cartilage tissue engineering. J Tissue Eng Regen Med. 2007;1(3):170–8. doi: 10.1002/term.19 . [DOI] [PubMed] [Google Scholar]
- 54.Fischer J, Dickhut A, Rickert M, Richter W. Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis and rheumatism. 2010;62(9):2696–706. Epub 2010/05/25. doi: 10.1002/art.27565 . [DOI] [PubMed] [Google Scholar]
- 55.Aung A, Gupta G, Majid G, Varghese S. Osteoarthritic chondrocyte-secreted morphogens induce chondrogenic differentiation of human mesenchymal stem cells. Arthritis and rheumatism. 2011;63(1):148–58. doi: 10.1002/art.30086 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang HN, Park JS, Na K, Woo DG, Kwon YD, Park KH. The use of green fluorescence gene (GFP)-modified rabbit mesenchymal stem cells (rMSCs) co-cultured with chondrocytes in hydrogel constructs to reveal the chondrogenesis of MSCs. Biomaterials. 2009;30(31):6374–85. doi: 10.1016/j.biomaterials.2009.07.062 . [DOI] [PubMed] [Google Scholar]
- 57.Ahmed N, Dreier R, Gopferich A, Grifka J, Grassel S. Soluble signalling factors derived from differentiated cartilage tissue affect chondrogenic differentiation of rat adult marrow stromal cells. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2007;20(5):665–78. doi: 10.1159/000107728 . [DOI] [PubMed] [Google Scholar]
- 58.Hwang NS, Varghese S, Puleo C, Zhang Z, Elisseeff J. Morphogenetic signals from chondrocytes promote chondrogenic and osteogenic differentiation of mesenchymal stem cells. Journal of cellular physiology. 2007;212(2):281–4. doi: 10.1002/jcp.21052 . [DOI] [PubMed] [Google Scholar]
- 59.Cooke ME, Allon AA, Cheng T, Kuo AC, Kim HT, Vail TP, et al. Structured three-dimensional co-culture of mesenchymal stem cells with chondrocytes promotes chondrogenic differentiation without hypertrophy. Osteoarthritis Cartilage. 2011;19(10):1210–8. Epub 2011/08/06. doi: 10.1016/j.joca.2011.07.005 ; PubMed Central PMCID: PMC3188316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee JS, Im GI. Influence of chondrocytes on the chondrogenic differentiation of adipose stem cells. Tissue Eng Part A. 2010;16(12):3569–77. Epub 2010/07/06. doi: 10.1089/ten.TEA.2010.0218 . [DOI] [PubMed] [Google Scholar]
- 61.Carlesimo M, Rossi A, La Pietra M, Narcisi A, Verga E, Arcese A, et al. Diffuse plane xanthoma and monoclonal gammopathies. European journal of dermatology: EJD. 2009;19(6):640–1. doi: 10.1684/ejd.2009.0774 . [DOI] [PubMed] [Google Scholar]
- 62.Liu X, Sun H, Yan D, Zhang L, Lv X, Liu T, et al. In vivo ectopic chondrogenesis of BMSCs directed by mature chondrocytes. Biomaterials. 2010;31(36):9406–14. Epub 2010/11/09. doi: 10.1016/j.biomaterials.2010.08.052 . [DOI] [PubMed] [Google Scholar]
- 63.Zuo Q, Cui W, Liu F, Wang Q, Chen Z, Fan W. Co-cultivated mesenchymal stem cells support chondrocytic differentiation of articular chondrocytes. International orthopaedics. 2013;37(4):747–52. doi: 10.1007/s00264-013-1782-z ; PubMed Central PMCID: PMC3609966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meretoja VV, Dahlin RL, Kasper FK, Mikos AG. Enhanced chondrogenesis in co-cultures with articular chondrocytes and mesenchymal stem cells. Biomaterials. 2012;33(27):6362–9. doi: 10.1016/j.biomaterials.2012.05.042 ; PubMed Central PMCID: PMC3392514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsuchiya K. The effect of coculture of chondrocytes with mesenchymal stem cells on their cartilaginous phenotype in vitro. Materials Science and Engineering C. 2004;24:391–6. doi: 10.1016/j.msec.2003.12.014 [Google Scholar]
- 66.Levorson EJ, Santoro M, Kasper FK, Mikos AG. Direct and indirect co-culture of chondrocytes and mesenchymal stem cells for the generation of polymer/extracellular matrix hybrid constructs. Acta biomaterialia. 2014;10(5):1824–35. Epub 2013/12/25. doi: 10.1016/j.actbio.2013.12.026 ; PubMed Central PMCID: PMC3976699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Platas J, Guillen MI, del Caz MD, Gomar F, Mirabet V, Alcaraz MJ. Conditioned media from adipose-tissue-derived mesenchymal stem cells downregulate degradative mediators induced by interleukin-1beta in osteoarthritic chondrocytes. Mediators of inflammation. 2013;2013:357014 Epub 2013/12/24. doi: 10.1155/2013/357014 ; PubMed Central PMCID: PMC3864089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang M, Rahnama R, Cheng T, Grotkopp E, Jacobs L, Limburg S, et al. Trophic stimulation of articular chondrocytes by late-passage mesenchymal stem cells in coculture. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2013;31(12):1936–42. Epub 2013/09/17. doi: 10.1002/jor.22466 . [DOI] [PubMed] [Google Scholar]
- 69.Hsiao ST, Asgari A, Lokmic Z, Sinclair R, Dusting GJ, Lim SY, et al. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem cells and development. 2012;21(12):2189–203. doi: 10.1089/scd.2011.0674 ; PubMed Central PMCID: PMC3411362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. Journal of the American College of Cardiology. 2012;59(10):942–53. doi: 10.1016/j.jacc.2011.11.029 ; PubMed Central PMCID: PMC3292778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoo KH, Jang IK, Lee MW, Kim HE, Yang MS, Eom Y, et al. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cellular immunology. 2009;259(2):150–6. doi: 10.1016/j.cellimm.2009.06.010 . [DOI] [PubMed] [Google Scholar]
- 72.Dmitrieva RI, Minullina IR, Bilibina AA, Tarasova OV, Anisimov SV, Zaritskey AY. Bone marrow- and subcutaneous adipose tissue-derived mesenchymal stem cells: differences and similarities. Cell cycle. 2012;11(2):377–83. doi: 10.4161/cc.11.2.18858 . [DOI] [PubMed] [Google Scholar]
- 73.Puelacher WC, Kim SW, Vacanti JP, Schloo B, Mooney D, Vacanti CA. Tissue-engineered growth of cartilage: the effect of varying the concentration of chondrocytes seeded onto synthetic polymer matrices. International journal of oral and maxillofacial surgery. 1994;23(1):49–53. Epub 1994/02/01. . [DOI] [PubMed] [Google Scholar]
- 74.Mo XT, Guo SC, Xie HQ, Deng L, Zhi W, Xiang Z, et al. Variations in the ratios of co-cultured mesenchymal stem cells and chondrocytes regulate the expression of cartilaginous and osseous phenotype in alginate constructs. Bone. 2009;45(1):42–51. doi: 10.1016/j.bone.2008.07.240 . [DOI] [PubMed] [Google Scholar]
- 75.Hendriks J, Miclea R, Schotel R, De Bruijn E, Moroni L, Karperien M, et al. Primary chondrocytes enhance cartilage tissue formation upon co-culture with a range of cell types. Soft Matter. 2010;6(20):5080–8. doi: 10.1039/C0SM00266F [Google Scholar]
- 76.Tsuchiya K, Chen G, Uchida K, Matsuno T, Tateishi T. The effect of coculture of chondrocytes with mesenchymal stem cells on their cartilaginous phenotype in vitro. Materials Science and Engineering: C. 2004;24(3):391–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) The DNA content of none of the constructs had changed compared to their initial DNA content prior to cell-culture (dotted line). Biochemical evaluation of the GAG (B) and collagen (C) content, 5 weeks after culture in alginate. The left graphs demonstrate the amount of matrix components per construct, whereas for the right graphs matrix production is normalized to the initially seeded primary ACs. A control condition—containing similar amounts of bACs (0.8*106 nc/ml) without supplementation of hMSCs—was evaluated to determine the additional effect of hMSCs (3.2*106 nc/ml) on bACs in co-cultures (dotted line). *** indicates a p-value smaller than 0.001 compared to the control condition. Data are shown as mean ± SD. For statistical evaluation, a mixed model was used followed by a Bonferroni's post-hoc comparisons test. hAMSC = human Adipose-tissue-derived Mesenchymal Stem Cell (n = 3 experiments with 3 independent donors); hBMSC = human Bone-marrow-derived Mesenchymal Stem Cell (n = 3 experiments with 3 independent donors); bAC = bovine Articular Chondrocyte (n = 3 experiments with 3 pools of donors). Per experiment, 3 samples were used for analyses.
(TIF)
Macroscopic appearance (top row) of cartilage constructs, as well as a Safranin-O histochemical staining (bottom rows), 8 weeks after subcutaneous implantation. hAMSC = human Adipose-tissue-derived Mesenchymal Stem Cell (n = 3 experiments with 3 independent donors); hBMSC = human Bone-marrow-derived Mesenchymal Stem Cell (n = 3 experiments with 3 independent donors); bAC = bovine Articular Chondrocyte (n = 3 experiments with 3 pools of donors). Per experiment, 2 samples were used for analyses.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.