Abstract
Objectives
To examine whether a mixed lipid emulsion reduces the incidence of parenteral nutrition associated cholestasis (PNAC) in extremely low birth weight (ELBW, <1000 g) infants.
Study design
This double-blind randomized trial of 230 ELBW infants (June 2012-October 2015) was performed at a single level IV neonatal intensive care unit. Patients received either a mixed lipid emulsion composed of soybean oil, medium chain triglycerides, olive oil, and fish oil-(intervention) or a soybean oil-based lipid emulsion (control) for parenteral nutrition. The primary outcome measure was PNAC (conjugated bilirubin >1.5 mg/dL [25 μmol/L] at 2 consecutive measurements). The study was powered to detect a reduction of PNAC from 25% to 10%.
Results
Reasons for noneligibility of 274 infants screened were refusal to participate (n = 16), death (n = 10), withdrawal of treatment (n = 5), higher order multiples (n = 9), and parents not available for consent (n = 4). Intention to treat analysis was carried out in 223 infants (7 infants excluded after randomization). Parenteral nutrition associated cholestasis was 11 of 110 (10.1%) in the intervention and 18 of 113 (15.9%) in the control group (P = .20). Multivariable analyses showed no statistically significant difference in the intention to treat (aOR 0.428, 95% CI 0.155-1.187; P = .10) or per protocol population (aOR 0.457, 95% CI 0.155-1.347; P = .16). There was no statistically significant effect on any other neonatal morbidity.
Conclusions
The incidence of parenteral nutrition associated cholestasis was not significantly reduced using a mixed lipid emulsion in ELBW infants.
Trial Registration
Extremely low birth weight (ELBW, birth weight <1000 g) infants depend on parenteral nutrition (PN) for several weeks.1 With ongoing PN, parenteral nutrition associated cholestasis (PNAC) is a well-described pathology2 that may progress to liver failure.3 Soybean oil-based lipid emulsions are rich in proinflammatory ω-6 long chain polyunsaturated fatty acids (LC-PUFAs) and phytosterols4 that are both accused to trigger PNAC.5,6 To overcome these untoward effects alternative oil-based lipid emulsions were developed. Here, the latest generation contains fish oil, which provides ω-3 LC-PUFAs—that are less proinflammatory compared with ω-6 LC-PUFAs—and is devoid of phytosterols.4 Infants with PNAC because of intestinal failure who were treated with a lipid emulsion exclusively based on fish oil showed marked improvements of liver function.7,8 Most recently, a mixed lipid emulsion composed of soybean oil, medium chain triglycerides (MCTs), olive oil, and fish oil (SMOF-LE) has become available, and is licensed for pediatric use in the European Union, but not the US because of insufficient data to date on adequate supply with essential fatty acids (EFA). Because of its mixed nature, SMOF-LE contains less ω-6 LC-PUFA4,9 and phytosterols10 but more ω-3 LC-PUFA and also more alpha-tocopherol4,9 compared with soybean oil-based lipid emulsion. Parenteral nutrition using SMOF-LE may, therefore, prevent PNAC compared with the current standard soybean oil-based lipid emulsion. We therefore hypothesized that PN using SMOF-LE would reduce PNAC in ELBW infants. The current trial was set up to compare SMOF-LE and soybean oil-based lipid emulsion for PN of ELBW infants with PNAC as the primary outcome.
Methods
This double-blind randomized controlled trial (RCT) (ClinicalTrials.gov: NCT01585935) was performed at the level IV11 neonatal care unit of the University Children’s Hospital Vienna (Medical University of Vienna, Vienna, Austria). The primary aim was to assess whether a mixed lipid emulsion (SMOF-LE) would reduce PNAC in ELBW infants compared with a soybean oil-based lipid emulsion. Secondary aims were to explore the impact on other morbidities. Recruitment was initiated in June 2012. Eligible participants were ELBW infants admitted before 24 hours of life. Infants with cholestasis (conjugated bilirubin >1.5 mg/dL [25 μmol/L]) before intervention and higher order multiples were not eligible. Infants with conditions associated with cholestasis independent of PN (ie, infection with cytomegalovirus, HIV, hepatitis B or C, rhesus mediated hemolysis, cystic fibrosis, inborn errors of metabolism or primary liver diseases) were not eligible or excluded postrandomization.12 Participants were randomized using permuted blocks (ratio 1:1, block size of 4) and stratified according to sex and birth weight (<750 vs ≥750 g) using a software13 prepared by an independent statistician, who kept the randomization sequence concealed until the end of the study. To account for correlation of twins, the first twin was randomized and the second assigned to the opposite treatment.14 Intervention was started within the first 120 hours of life. Infants received either a mixed lipid emulsion composed of 30% soybean oil, 30% MCT, 25% olive oil, and 15% fish oil (SMOFlipid 20%; Fresenius Kabi, Bad Homburg, Germany; SMOF-LE; ω-6:ω-3 ratio 2.5:1) or a soybean oil-based lipid emulsion (Intralipid 20%; Fresenius Kabi, Bad Homburg, Germany; soybean oil-based lipid emulsion; ω-6:ω-3 ratio 8:1) for PN over 24 hours. Both products have similar side effects and are registered for infants in Europe, but in the US SMOFlipid 20% (Fresenius Kabi, Bad Homburg, Germany) is only registered for adults. Based on a content of 35 mg/mL (range: 28-50) linoleic acid and a requirement of 0.25 g/kg/day15 to prevent EFA deficiency, SMOF-LE theoretically will prevent EFA deficiency at 1.4 g/kg/day (range: 1-1.8).
Based on a prevalence of PNAC of 25% at our unit,16 a χ2 test indicated that 100 infants/group were required to detect a relative reduction by 60% (from 25% to 10%) with a 2-sided 5% significance level and a power of 80%. Assuming a dropout rate of 18%, we aimed at recruiting 122 infants/group in a 3-year period.
Participants, healthcare providers, data collectors, and outcome adjudicators were blinded. The investigational products were visually indistinguishable. Taste or smell was regarded irrelevant because of the closed mode of application; study lipids were stored at room temperature and applied using light protected syringes and infusion lines. A blinding team uninvolved in clinical decisions established the blinding code and masked the glass containers using opaque labels designated “Lipid A” or “Lipid B.” Labels were resistant to detachment, in particular by 70% alcohol used during aseptic preparation. Neonatal nurses who prepared the study lipids for PN were part of the blinding team. Discarded containers were controlled for blinding integrity. The attending physicians prescribed the study lipids together with PN using a computer program (catoPAN; Cato Software Solutions, Becton Dickinson, Vienna, Austria) customized to include the designations “Lipid A” and “Lipid B.” Participants received full PN from birth using soybean oil-based lipid emulsion (1 g/kg/day) and were switched to study lipids after enrollment. Lipids were dosed up to 3 g/kg/day at the discretion of the attending physicians and reduced in relation to enteral nutrition (increased up to 20 mL/kg/day). Serum triglycerides were measured at least weekly. Lipids were halted for 24 hours at triglyceride levels >400 mg/dL (4.5 mmol/L) or down titrated >250 mg/dL (2.8 mmol/L). Parenteral nutrition was stopped at 140-160 mL/kg/day of enteral feeds. Urodeoxycholic acid was administered to patients that developed cholestasis. Parenteral fish oil (Omegaven; Fresenius Kabi, Bad Homburg, Germany) was permitted as rescue therapy (1 g/kg/day) if conjugated bilirubin was >6 mg/dL (100 μmol/L). Infants were followed until their 44th week of postmenstrual age (PMA), discharge, or transfer to another hospital.
Patient data were collected from the electronic charts (ICCA; Phillips Medical Systems, Eindhoven, The Netherlands) and discharge letters. Data on PN were collected from the prescription software (catoPAN; Cato Software Solutions, Becton Dickinson, Vienna, Austria).
Demographic characteristics were recorded as shown in Table I.
Table I. Demographic characteristics.
| Parameter | SMOF-LE (n = 110) | S-LE (n = 113) |
|---|---|---|
| Obstetric parameters | ||
| Multiple pregnancy | 30 (27) | 36 (32) |
| Cesarean delivery | 100 (91) | 101 (89) |
| Prenatal steroids (full course) | 69 (63) | 65 (59) |
| PROM | 38 (35) | 42 (37) |
| Preeclampsia | 16 (15) | 16 (14) |
| Neonatal parameters | ||
| Umbilical artery pH | 7.31 [7.25 to 7.36] * | 7.30 [7.24 to 7.35] † |
| Apgar—5 min | 8 [8 to 9] | 8 [8 to 9] ‡ |
| Male sex | 64 (58) | 73 (65) |
| Surfactant | 97 (88) | 98 (87) |
| Gestational age (wk+d) | 25 + 6 [24 + 6 to 27 + 3] | 26 + 2 [25 + 0 to 28 + 0] |
| Birth weight (g) | 788 [648 to 891] | 760 [610 to 884] |
| Z score | −0.4 [−1.1 to −0.2] | −0.7 [−1.4 to − 0.2] |
| Birth length (cm) | 34 [31 to 35] ‡ | 33 [31 to 35] ‡ |
| Z score | −0.3 [−1.1 to 0.7] ‡ | −0.3 [−1.5 to 0.4] ‡ |
| Birth head circumference (cm) | 24 [23 to 25] § | 24 [23 to 25] |
| Z score | −0.1 [−0.7 to 0.6] § | −0.2 [−1.0 to −0.2] |
| Small for gestational age | 22 (20) | 38 (34) |
PROM, premature rupture of membranes; S-LE soybean oil-based lipid emulsion.
Analysis by ITT. Categorical data are presented as numbers with percentages in round parentheses and were tested using the χ2 test. Continuous data are presented as the median and IQR in squared parentheses and were tested using the Mann-Whitney U Test.
Data of 15 patients missing.
Data of 21 patients missing.
Data of 2 patients missing.
Data of 1 patient missing.
A full course of prenatal steroids was defined as 2 doses of betamethasone administered 24 hours apart. Surfactant (Curosurf; Chiesi, Parma, Italy) was administered to all infants <28 + 0 weeks of gestational age17 or else if deficiency was suspected (>35% oxygen with saturation ranges of 88%-96%). Anthropometry was performed by the attending nurses and z scores calculated using growth curves by Fenton et al.18,19 Small for gestational age was defined as birth weight <10th percentile (z score <–1.28).
The primary outcome PNAC, measures of liver function, and neonatal morbidities were recorded as shown in Table II.
Table II. Neonatal outcome.
| Outcome | SMOF-LE (n = 110) | S-LE (n = 113) | P |
|---|---|---|---|
| Primary outcome and liver function (peak levels throughout the study) | |||
| PNAC | 11 (10) | 18 (16) | .20 |
| Occurrence (day of life) | 23 [11-36] | 20 [9-42] | .95 |
| Death* | 2 (18) | 3 (16) | .91 |
| Rescue therapy using fish oil* | 3 (27) | 4 (22) | .76 |
| Normalized before discharge* | 6 (55) | 5 (28) | .15 |
| Conjugated bilirubin (mg/dL) | 0 [0-0.22] | 0 [0-0.38] | .67 |
| γ-GT (U/mL) | 148 [95-243] † | 157 [101-217] ‡ | .94 |
| AST (U/mL) | 41 [32-67] † | 48 [34-80] ‡ | .13 |
| ALT (U/mL) | 32 [30-41] † | 34 [29-45] ‡ | .40 |
| AP (U/mL) | 518 [396-665] † | 494 [376-671] ‡ | .70 |
| Neonatal morbidity | |||
| Death | 8 (7) | 8 (7) | .96 |
| Hospitalization (d) | 81 [59-105] | 79 [63-97] | .69 |
| Retinopathy of prematurity (any) | 60 (58) | 56 (55) | .69 |
| Highest grade (grade 1-5) | 1 [0-2] | 1 [0-2] | .63 |
| Requiring treatment (severe ROP) | 9 (8) | 10 (9) | .79 |
| Sepsis, culture proven | 24 (22) | 26 (23) | .83 |
| Intraventricular hemorrhage III/IV | 12 (11) | 9 (8) | .45 |
| Cystic periventricular leukomalacia | 3 (3) | 4 (4) | .73 |
| NEC ≥IIa | 8 (7) | 8 (7) | .96 |
| Focal intestinal perforation | 4 (4) | 5 (4) | .77 |
| Abdominal surgery | 13 (12) | 14 (12) | .90 |
| Days on mechanical ventilation | 6 [0-10] | 6 [0-10] | .51 |
| Chronic lung disease | 19 (17) | 21 (19) | .80 |
| Steroid treatment | 11 (10) | 17 (15) | .26 |
| PDA requiring treatment | 56 (51) | 68 (60) | .16 |
| Number of ibuprofen cycles | 2 [1-3] | 2 [1-3] | .82 |
| Surgical ligation | 5 (5) | 6 (5) | .80 |
| Pulmonary hypertension | 23 (21) | 31 (28) | .27 |
| iNO/sildenafil treatment | 19 (17) | 28 (25) | .18 |
ALT, Alanine transaminase; AST, Aspartate transaminase; AP, Alkaline phosphatase; GT, glutamyltransferase.
Analysis by ITT. Categorical data are presented as numbers with percentages in round parentheses and were tested using the chi-square test. Continuous data are presented as median and interquartile range in squared parentheses and were tested using the Mann-Whitney U test. P values <.05 were considered statistically significant.
SI conversion factors: To convert bilirubin to µmol/L multiply values by 17.1, to convert triglycerides to mmol/L multiply values by .011.
Percentage within the subgroup of infants with PNAC.
Data of 2 patient missing.
Data of 1 patients missing.
PNAC was defined as conjugated bilirubin levels >1.5 mg/dL (25 μmol/L) at 2 consecutive measurements16 by spectrophotometric quantitation (Vitros Chemistry System; Ortho Clinical Diagnostics, Raritan, New Jersey). Peak levels of liver enzymes (aspartate aminotransferase, alanine aminotransferase, γ-glutamyltransferase [GT], and alkaline phosphatase [AP]) during hospitalization were identified. Blood sampling was performed weekly as long as PN was required and then every 7-14 days. Retinopathy of prematurity (ROP) was screened for by indirect ophthalmoscopy starting at 5 weeks of age. Treatment (laser or intravitreal ranibizumab) was performed at ROP stage ≥3. Culture proven sepsis was detected by blood culture (BacT/Alert Pediatric FAN; BioMerieux, Marcy l’Etoile, France) drawn after birth and before any antibiotic treatment. Intraventricular hemorrhage and cystic periventricular leukomalacia were diagnosed by cerebral ultrasound performed every 7-14 days. Necrotizing enterocolitis (NEC) was diagnosed clinically (Bell’s stage ≥IIa20) or after surgical exploration. Focal intestinal perforation was defined as perforation in an otherwise healthy bowel. All infants received probiotics21 and lactoferrin.22 Bronchopulmonary dysplasia (BPD) was defined as supplementary oxygen after 36 + 0 weeks PMA. Persistent ductus arteriosus was treated if hemodynamically significant (enddiastolic blood flow in the left pulmonary artery >20 cm/s or backward flow in the abdominal aorta)23 using ibuprofen. Pulmonary hypertension was diagnosed by measurement of tricuspid regurgitation or right-to-left/bidirectional shunt across the ductus arteriosus24 and treated using inhaled nitric oxide, sildenafil or both.
Data on study lipids, nutrition and growth were recorded as shown in Table III.
Table III. Nutrition and growth.
| Outcome | SMOF-LE (n = 110) | S-LE (n = 113) | P |
|---|---|---|---|
| Study drug and nutrition | |||
| Therapy adherence >80% | 109 (99) | 110 (97) | .33 |
| Time on PN (d) | 23 [17-37] | 24 [17-35] | .87 |
| Time on parenteral lipids (d) | 20 [15-35] | 21 [15-29] | .91 |
| Total parenteral lipids (g/kg) | 38.2 [26.4-68.5] | 39.0 [28.1-55.3] | .56 |
| Total study lipids (g/kg) | 32.6 [21.3-63.8] | 34.4 [23.0-50.8] | .57 |
| Highest triglycerides (mg/dL) | 208 [142-323] * | 207 [147-305] † | .85 |
| Parenteral lipids at measurement | 2.1 [1.5-2.5]* | 2.0 [1.2-2.5]† | .30 |
| Hypertriglyceridemia | 39 (39) | 38 (37) | .71 |
| Feeding first wk (mL per feed/kg) | 3.3 [2.3-4.1] | 3.1 [2.3-4.1] | .55 |
| Mothers milk at discharge (any) | 85 (75) | 80 (69) | .29 |
| Anthropometry at discharge ‡ | |||
| Weight at discharge (g) | 2594 [2124 to 3029] | 2479 [2175 to 2956] | .23 |
| Δ z score (birth to end of study) | −0.7 [−1.0 to −0.2] | −0.7 [−1.1 to −0.2] | .84 |
| Length (cm) | 45 [42.5 to 47] | 44 [41.5 to 47] | .14 |
| Δ z Score (birth to end of study) | −1.4 [−2.3 to 0.8] | −1.5 [−2.1 to 1.0] | .64 |
| Head circumference (cm) | 32 [30.6 to 33.5] | 32 [30.7 to 33.1] | .45 |
| Δ z Score (birth to end of study) | −1.0 [−1.7 to −0.3] | −1.0 [−1.6 to −0.3] | .92 |
Analysis by ITT. Categorical data are presented as numbers with percentages in round parentheses and were tested using the χ2 test. Continuous data are presented as median and IQR in squared parentheses and were tested using the Mann-Whitney U Test. P values <.05 were considered statistically significant.
Data of 11 patients missing.
Data of 10 patients missing.
For analysis of growth, infants who died were excluded (SMOF-LE, n = 104; S-LE, n = 101).
Therapy adherence was calculated as the percentage study lipids were correctly provided; >80% was considered highly adherent.25 Enteral feeds were provided every 3 hours; the median volume of a single feed per kg in the first week of life was calculated. For growth analysis (anthropometry with z score difference from birth to discharge), only survivors were analyzed to avoid distortion of measurements by perimortal edema.
Statistical Analyses
The χ2 test was used to examine the relationship between the intervention and PNAC. The primary outcome was additionally analyzed by binary logistic regression (Table IV). Possible confounders were specified according to literature (male sex,26 sepsis,27 compound outcome of NEC, focal intestinal perforation and gastrointestinal surgery,28 birth weight,29 total days on PN,3 z score of birth weight,16 and enteral nutrition in the first week of life per kg birth weight16) and tested in univariable logistic regression models for significant influence. Because of the low number of events the confounders included in the final model were restricted to those with univariate P < .01. Furthermore, the covariates of the final model were reduced according to Akaike information criterion to avoid overfitting. Secondary outcome measures were compared between the treatment groups with χ2 test for categorical data and Mann-Whitney U test for continuous data. Analyses were performed by intention to treat (ITT). As sensitivity analysis, calculations were additionally carried out on the per protocol set (treatment adherence ≥80%,25 admission ≥28 days according to the study protocol). Because of opposed allocation of twins, calculations including the mother as random factor were not necessary.14 There was no interim analysis.
Table IV. Multivariable analysis on the risk for PNAC.
| ITT (n = 223) |
Per protocol (n = 206) |
|||||
|---|---|---|---|---|---|---|
| Parameter | aOR | 95% CI | P | aOR | 95% CI | P |
| SMOF-LE | 0.428 | 0.155-1.187 | .10 | 0.457 | 0.155-1.347 | .16 |
| NEC/FIP/GI surgery | 5.481 | 1.894-15.868 | .002 | 5.016 | 1.528-16.464 | .008 |
| Time on PN (d) | 1.051 | 1.023-1.080 | <.001 | 1.059 | 1.027-1.092 | <.001 |
| Feeding first wk (mL per feed/kg) | 0.739 | 0.487-1.112 | .16 | 0.665 | 0.413-1.073 | .10 |
FIP, focal intestinal perforation; GI, gastrointestinal.
Analysis by ITT and per protocol. Binary logistic regression analysis showing the odds for the effect of SMOF-LE on PNAC compared with S-LE, adjusted for “compound outcome of NEC, FIP, and GI surgery,” time on parenteral nutrition and the median volume per enteral feed per kg birth weight in the first week of life. P values < .05 were considered statistically significant.
The study was conducted in conformance with the Declaration of Helsinki, International Conference on Harmonisation / Good Clinical Practice guidelines, and the respective European Union directives embedded in the Austrian drug act. Written consent from 1 parent was sufficient due to low risk for participants.30 Patients were insured as legally required. The study was approved by the institution’s ethic committee (EK 2011/1030) and registered at European Clinical Trial Database (EudraCT 2011-005456-33) and clinicaltrials.gov (NCT01585935).
Results
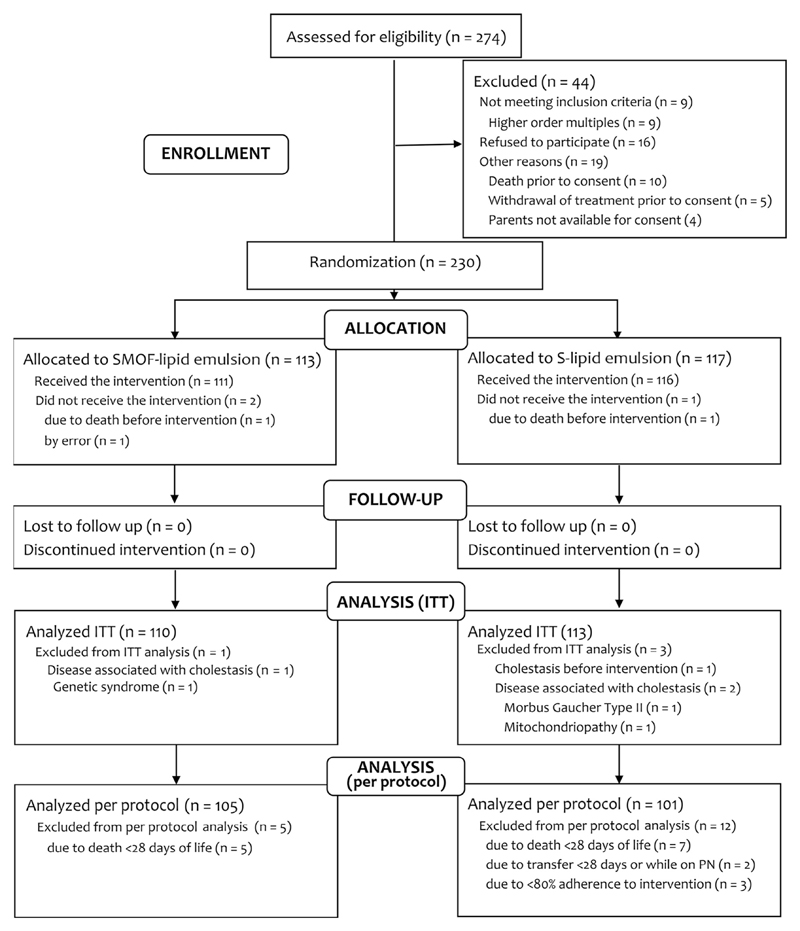
A total of 274 ELBW infants were screened: 223 infants were available for ITT analysis, 206 for per protocol analysis (Figure; available at www.jpeds.com). Three participants did not receive the intervention (death: n = 2; error: n = 1), 3 participants were withdrawn due to conditions associated with cholestasis (mitochondriopathy: n = 1; Gaucher disease type II: n = 1; undefined genetic syndrome: n = 1), and 1 participant was withdrawn due to cholestasis before intervention. Thus, 7 participants (SMOF-LE: n = 4; soybean oil-based lipid emulsion: n = 3) were excluded from ITT analysis after randomization.12 Recruitment ended after the intended number of participants was included (July 2015); follow-up lasted until October 2015. Premature unblinding (accidentally or as a result of a serious adverse event) did not occur. All analyses were prespecified according to the study protocol.
Figure.
Flow chart showing enrollment with reasons for study exclusion and analysis.
Demographic characteristics are shown in Table I. Study participants were born between 23 + 1 and 33 + 4 weeks PMA (median 26 + 0 weeks) and weighed between 390 and 998 g (median 775 g).
The primary outcome PNAC was 36% lower using SMOFLE compared with soybean oil-based lipid emulsion, a relative difference that was statistically not significant (Table II). We found no significant difference in PNAC characteristics, highest conjugated bilirubin, or liver enzymes. Mortality, hospitalization, culture proven sepsis and morbidity (visual, gastrointestinal, neurologic, cardiovascular, and pulmonary) did not differ significantly. Infants receiving fish oil as a rescue therapy did not differ between groups in mortality (SMOF-LE: 0/3 vs soybean oil-based lipid emulsion: 1/4; P = .35) or normalization of PNAC until discharge (SMOFLE: 1/3 vs soybean oil-based lipid emulsion: 0/4; P = .21). A multivariable model including relevant confounders showed no significant difference for the primary outcome (Table IV).
Therapy adherence was high and equal between groups (Table III). Both groups received PN for a comparable time with similar amounts of lipids. Triglyceride levels were measured at similar intravenous lipid supply and did not differ significantly, as well as the incidence of hypertriglyceridemia. Feeding volumes in the first week of life and the use of mother’s milk were similar. There was no significant difference in anthropometry from birth to the end of the study.
Discussion
In this prospective, double-blind randomized trial in ELBW infants a mixed lipid emulsion composed of soybean oil, MCT, olive oil, and fish oil did not significantly reduce the incidence of PNAC compared with a soybean oil-based lipid emulsion. We found no effect on measures such as ROP, BPD, and growth.
Lipid emulsions based on soybean oil are the currently recommended product for provision of parenteral lipids in preterm infants,4,31 and the only licensed lipid emulsion for infants in the US.9 Because side effects of PN on liver function were ascribed to the specific properties of soybean oil, research focused on reducing the excess of ω-6 LC-PUFAs and phytosterols in lipid emulsions by admixing alternative oils (MCT and olive) and most recently fish oil providing ω-3 LC-PUFAs to counterbalance proinflammatory ω-6 effects.4 In this regard, SMOFLE is the most recent development and was shown to improve the supply with the crucial ω-3 LC-PUFA docosahexaenoic acid (DHA) while lowering soybean oil exposure by 70% and phytosterol exposure by roughly 50%.10 Also, the higher supply with vitamin E compared with soybean oil-based lipid emulsion may prevent PNAC as recently shown in preterm piglets.32 However, until now clinical trials have not provided enough evidence for improvement of clinical outcomes such as PNAC to justify a change of the current guidelines that recommend soybean oil-based lipid emulsion.
Four out of 6 trials that have tested SMOF-LE vs soybean oil-based lipid emulsion in 310 preterm infants reported on PNAC as secondary outcome,33–36 without a significant effect in a recent meta-analysis.31 However, the overall incidence of PNAC was 5% across studies, therefore, the relatively low number of infants analyzed may not suffice to exclude a clinically meaningful effect. Our trial was the first designed to investigate SMOF-LE for prevention of PNAC as primary outcome. To attain a high baseline incidence, we exclusively recruited the vulnerable group of ELBW infants with an anticipated PNAC incidence of 25% at our unit.16 However, the power to prove our hypothesis was lowered by an observed PNAC incidence of only 15.9% in the current trial, attributable to an accelerated weaning from PN compared with the planning phase (−10 days).16 This shorter time on PN is an important limitation of our study and possibly related to the implementation of probiotics at our unit before the start of this trial in 201021 and their preventive effect against NEC. Reduced fear of NEC possibly encouraged clinicians to establish full enteral feeds faster.
Although our study showed that SMOF-LE did not significantly prevent PNAC in ELBW, our results cannot be generalized to infants with a substantially longer time on PN such as those with intestinal failure. The same applies to infants with established PNAC. Here, a significant decline of conjugated bilirubin was demonstrated in a pilot RCT after switching from soybean oil-based lipid emulsion to SMOF-LE, however, only after adjusting the original analysis and eliminating a statistical outlier.37 In our study, PNAC also resolved more quickly, however, the numbers were not statistically significant (Table II).
Besides protection of liver function, other morbidities associated with prematurity such as BPD38 might have been affected by the intervention with SMOF-LE. So far meta-analyses did not show a significant influence on BPD.39 On the contrary, Collins et al recently reported their trial of fish oil supplementation in preterm infants and surprisingly found a significantly increased risk for BPD.40 In that study, fish oil was used as enteral add-on from birth to 36 weeks PMA providing a total DHA supply even beyond fetal accretion rates.40 This is in contrast to our study using SMOF-LE, where fish oil is provided parenterally and only as long as PN was needed. However, Collins et al also questioned the safety of PN using SMOF-LE. We and other RCTs found no difference in BPD using SMOF-LE compared with soybean oil-based lipid emulsion.33–36 Furthermore, we found no affection of other outcomes related to inflammatory processes such as pulmonary hypertension41 and persistent ductus arteriosus42 or of any other neonatal morbidity and death.
In recent years, the ω-3 LC-PUFA DHA was included into models of ROP as another nonoxygen-regulated factor.43 Supplementation with fish oil to improve DHA enrichment of the retina was suggested to prevent ROP.43 Here, SMOF-LE would not only provide DHA but also higher amounts of vitamin E4 known to prevent ROP.44 Two RCTs studied the effect of SMOF-LE33 or a pure fish oil LE45 on ROP as primary outcome. In the study by Beken et al, SMOF-LE significantly reduced any ROP.33 Pawlik et al showed that co-application of a pure fish oil LE and soybean oil-based lipid emulsion significantly prevented severe ROP.45 A recent systematic review suggested a preventive effect based on RCTs and observational studies.46 In the present study, we did not find any effect against any stage of ROP. Compared with the study by Pawlik et al, we provided less fish oil using SMOF-LE, which may indicate some dose dependency. However, the lack of effect compared with Beken et al (ROP reduced by 80%) who also used SMOF-LE is striking.33 In this context, it seems important that infants in our study were more immature by 4 weeks compared with Beken et al. As ROP typically occurs after 30 weeks PMA, the timing of fish oil supplementation from birth and retinal DHA enrichment in relation to the actual PMA probably matters. In this respect, well-designed pharmacologic studies on dosing and timing of SMOF-LE or pure fish oil LE in relation to the actual PMA are necessary. However, it is discouraging that the trial by Collins et al, who applied enteral fish oil in high amounts, also showed no effect on ROP.40
Safety and tolerance of SMOF-LE is another important issue inconsistently referred to in literature. Although lipid clearance was even improved in adults,47 serum triglycerides were found significantly higher in preterm infants using SMOF-LE compared with soybean oil-based lipid emulsion, though at doses exceeding current recommendations (>3 g/kg/day).34 In our study, serum triglyceride levels and the incidence of hypertriglyceridemia did not differ between SMOF-LE and soybean oil-based lipid emulsion.
Postnatal growth failure is a frequently observed problem in preterm infants.48 Although SMOF-LE and soybean oil-based lipid emulsion provide similar amounts of energy, improved supply with ω-3 LC-PUFAs might impact on head growth as DHA rapidly accumulates in the fetal brain in late pregnancy.49 Furthermore, a recent meta-analysis of biochemical aspects showed lower levels of arachidonic acid using SMOF-LE50 and raised concerns because of an association of low arachidonic acid levels and growth failure.51 In our study, we found no significant impact of SMOF-LE on anthropometry. Moreover, there is a lack of studies demonstrating the efficacy of SMOF-LE in the prevention of EFA deficiency in preterm infants. As we did not measure EFA in our study, we can only speculate. A supply of less than 0.25 g/kg/day of linoleic acid may cause EFA deficiency in preterm infants.15 It therefore seems prudent to aim at a supply of 2 g/kg/day SMOF-LE if enteral nutrition is low in the first week of life, or likely even at 2.5 g/kg/day in exclusively parenterally nourished ELBW infants, to safely prevent EFA deficiency.
Acknowledgments
We thank Eva Wissmann for excellent support with drug accountability.
Funded by the Austrian Science Fund (FWF, KLI99-B00). Study lipids were supplied free of charge by Herba Chemosan (Graz, Austria).
Glossary
- BPD
Bronchopulmonary dysplasia
- DHA
Docosahexaenoic acid
- EFA
Essential fatty acid
- ELBW
Extremely low birth weight
- ITT
Intention to treat
- LC-PUFA
Long chain polyunsaturated fatty acid
- MCT
Medium chain triglyceride
- NEC
Necrotizing enterocolitis
- PMA
Postmenstrual age
- PN
Parenteral nutrition
- PNAC
Parenteral nutrition associated cholestasis
- RCT
Randomized controlled trial
- ROP
Retinopathy of prematurity
- SMOF-LE
Lipid emulsion composed of soybean oil, medium chain triglycerides, olive oil, and fish oil
Footnotes
A.R. received funding from Fresenius Kabi (Graz, Austria) to employ a clinical research nurse The other authors declare no conflicts of interest.
References
- 1.Vohr BR, Wright LL, Dusick AM, Perritt R, Poole WK, Tyson JE, et al. Center differences and outcomes of extremely low birth weight infants. Pediatrics. 2004;113:781–9. doi: 10.1542/peds.113.4.781. [DOI] [PubMed] [Google Scholar]
- 2.Kelly DA. Liver complications of pediatric parenteral nutrition—epidemiology. Nutrition. 1998;14:153–7. doi: 10.1016/s0899-9007(97)00232-3. [DOI] [PubMed] [Google Scholar]
- 3.Zambrano E, El-Hennawy M, Ehrenkranz RA, Zelterman D, Reyes-Mugica M. Total parenteral nutrition induced liver pathology: an autopsy series of 24 newborn cases. Pediatr Dev Pathol. 2004;7:425–32. doi: 10.1007/s10024-001-0154-7. [DOI] [PubMed] [Google Scholar]
- 4.Vanek VW, Seidner DL, Allen P, Bistrian B, Collier S, Gura K, et al. A.S.P.E.N. position paper: clinical role for alternative intravenous fat emulsions. Nutr Clin Pract. 2012;27:150–92. doi: 10.1177/0884533612439896. [DOI] [PubMed] [Google Scholar]
- 5.Carter BA, Taylor OA, Prendergast DR, Zimmerman TL, Von Furstenberg R, Moore DD, et al. Stigmasterol, a soy lipid-derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR. Pediatr Res. 2007;62:301–6. doi: 10.1203/PDR.0b013e3181256492. [DOI] [PubMed] [Google Scholar]
- 6.Van Aerde JE, Duerksen DR, Gramlich L, Meddings JB, Chan G, Thomson AB, et al. Intravenous fish oil emulsion attenuates total parenteral nutrition-induced cholestasis in newborn piglets. Pediatr Res. 1999;45:202–8. doi: 10.1203/00006450-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 7.de Meijer VE, Gura KM, Le HD, Meisel JA, Puder M. Fish oil-based lipid emulsions prevent and reverse parenteral nutrition-associated liver disease: the Boston experience. JPEN J Parenter Enteral Nutr. 2009;33:541–7. doi: 10.1177/0148607109332773. [DOI] [PubMed] [Google Scholar]
- 8.Diamond IR, Sterescu A, Pencharz PB, Kim JH, Wales PW. Changing the paradigm: omegaven for the treatment of liver failure in pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr. 2009;48:209–15. doi: 10.1097/MPG.0b013e318182c8f6. [DOI] [PubMed] [Google Scholar]
- 9.Biesboer AN, Stoehr NA. A product review of alternative oil-based intravenous fat emulsions. Nutr Clin Pract. 2016;31:610–8. doi: 10.1177/0884533616661174. [DOI] [PubMed] [Google Scholar]
- 10.Vanek VW, Seidner DL, Allen P, Bistrian B, Collier S, Gura K, et al. Update to A.S.P.E.N. position paper: clinical role for alternative intravenous fat emulsions. Nutr Clin Pract. 2014;29:841. doi: 10.1177/0884533614555235. [DOI] [PubMed] [Google Scholar]
- 11.American Academy of Pediatrics. Levels of neonatal care. Pediatrics. 2012;130:587–97. doi: 10.1542/peds.2012-1999. [DOI] [PubMed] [Google Scholar]
- 12.Fergusson D, Aaron SD, Guyatt G, Hebert P. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ. 2002;325:652–4. doi: 10.1136/bmj.325.7365.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Errath M, Berghold A, Ofner P, Quehenberger F. Randomizer for clinical trials 1.8.1. [Accessed July 31, 2017]; https://www.meduniwien.ac.at/randomizer/web/about.php.
- 14.Shaffer ML, Kunselman AR, Watterberg KL. Analysis of neonatal clinical trials with twin births. BMC Med Res Methodol. 2009;9:12. doi: 10.1186/1471-2288-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koletzko B, Goulet O, Hunt J, Krohn K, Shamir R, Parenteral Nutrition Guidelines Working G et al. 1. Guidelines on Paediatric Parenteral Nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), Supported by the European Society of Paediatric Research (ESPR) J Pediatr Gastroenterol Nutr. 2005;41(Suppl 2):S1–87. doi: 10.1097/01.mpg.0000181841.07090.f4. [DOI] [PubMed] [Google Scholar]
- 16.Repa A, Lochmann R, Unterasinger L, Weber M, Berger A, Haiden N. Aggressive nutrition in extremely low birth weight infants: impact on parenteral nutrition associated cholestasis and growth. PeerJ. 2016;4:e2483. doi: 10.7717/peerj.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klebermass-Schrehof K, Wald M, Schwindt J, Grill A, Prusa AR, Haiden N, et al. Less invasive surfactant administration in extremely preterm infants: impact on mortality and morbidity. Neonatology. 2013;103:252–8. doi: 10.1159/000346521. [DOI] [PubMed] [Google Scholar]
- 18.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens T, Fenton TR. Research bulk calculator 2013. [Accessed July 31, 2017]; http://ucalgary.ca/fenton/2013chart.
- 20.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33:179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Repa A, Thanhaeuser M, Endress D, Weber M, Kreissl A, Binder C, et al. Probiotics (Lactobacillus acidophilus and Bifidobacterium infantis) prevent NEC in VLBW infants fed breast milk but not formula [corrected] Pediatr Res. 2015;77:381–8. doi: 10.1038/pr.2014.192. [DOI] [PubMed] [Google Scholar]
- 22.Pammi M, Abrams SA. Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2015;(5) doi: 10.1002/14651858.CD007137.pub4. CD007137. [DOI] [PubMed] [Google Scholar]
- 23.Jain A, Shah PS. Diagnosis, evaluation, and management of patent ductus arteriosus in preterm neonates. JAMA Pediatr. 2015;169:863–72. doi: 10.1001/jamapediatrics.2015.0987. [DOI] [PubMed] [Google Scholar]
- 24.Steiner M, Salzer U, Baumgartner S, Waldhoer T, Klebermass-Schrehof K, Wald M, et al. Intravenous sildenafil i.v. as rescue treatment for refractory pulmonary hypertension in extremely preterm infants. Klin Padiatr. 2014;226:211–5. doi: 10.1055/s-0034-1375697. [DOI] [PubMed] [Google Scholar]
- 25.Morrison A, Stauffer ME, Kaufman AS. Defining medication adherence in individual patients. Patient Prefer Adherence. 2015;9:893–7. doi: 10.2147/PPA.S86249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albers MJ, de Gast-Bakker DA, van Dam NA, Madern GC, Tibboel D. Male sex predisposes the newborn surgical patient to parenteral nutrition-associated cholestasis and to sepsis. Arch Surg. 2002;137:789–93. doi: 10.1001/archsurg.137.7.789. [DOI] [PubMed] [Google Scholar]
- 27.Beath SV, Davies P, Papadopoulou A, Khan AR, Buick RG, Corkery JJ, et al. Parenteral nutrition-related cholestasis in postsurgical neonates: multivariate analysis of risk factors. J Pediatr Surg. 1996;31:604–6. doi: 10.1016/s0022-3468(96)90507-2. [DOI] [PubMed] [Google Scholar]
- 28.Rangel SJ, Calkins CM, Cowles RA, Barnhart DC, Huang EY, Abdullah F, et al. Parenteral nutrition-associated cholestasis: an American Pediatric Surgical Association Outcomes and Clinical Trials Committee systematic review. J Pediatr Surg. 2012;47:225–40. doi: 10.1016/j.jpedsurg.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Beale EF, Nelson RM, Bucciarelli RL, Donnelly WH, Eitzman DV. Intrahepatic cholestasis associated with parenteral nutrition in premature infants. Pediatrics. 1979;64:342–7. [PubMed] [Google Scholar]
- 30.Nelson DK, Skinner D, Guarda S, Choudhury S, Sideris J, Barnum L, et al. Obtaining consent from both parents for pediatric research: what does “reasonably available” mean? Pediatrics. 2013;131:e223–9. doi: 10.1542/peds.2012-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hojsak I, Colomb V, Braegger C, Bronsky J, Campoy C, Domellof M, et al. ESPGHAN Committee on nutrition position paper. intravenous lipid emulsions and risk of hepatotoxicity in infants and children: a systematic review and meta-analysis. J Pediatr Gastroenterol Nutr. 2016;62:776–92. doi: 10.1097/MPG.0000000000001121. [DOI] [PubMed] [Google Scholar]
- 32.Ng K, Stoll B, Chacko S, Saenz de Pipaon M, Lauridsen C, Gray M, et al. Vitamin E in new-generation lipid emulsions protects against parenteral nutrition-associated liver disease in parenteral nutrition-fed preterm pigs. JPEN J Parenter Enteral Nutr. 2016;40:656–71. doi: 10.1177/0148607114567900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beken S, Dilli D, Fettah ND, Kabatas EU, Zenciroglu A, Okumus N. The influence of fish-oil lipid emulsions on retinopathy of prematurity in very low birth weight infants: a randomized controlled trial. Early Hum Dev. 2014;90:27–31. doi: 10.1016/j.earlhumdev.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 34.D’Ascenzo R, Savini S, Biagetti C, Bellagamba MP, Marchionni P, Pompilio A, et al. Higher docosahexaenoic acid, lower arachidonic acid and reduced lipid tolerance with high doses of a lipid emulsion containing 15% fish oil: a randomized clinical trial. Clin Nutr. 2014;33:1002–9. doi: 10.1016/j.clnu.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Savini S, D’Ascenzo R, Biagetti C, Serpentini G, Pompilio A, Bartoli A, et al. The effect of 5 intravenous lipid emulsions on plasma phytosterols in preterm infants receiving parenteral nutrition: a randomized clinical trial. Am J Clin Nutr. 2013;98:312–8. doi: 10.3945/ajcn.112.056556. [DOI] [PubMed] [Google Scholar]
- 36.Vlaardingerbroek H, Vermeulen MJ, Carnielli VP, Vaz FM, van den Akker CH, van Goudoever JB. Growth and fatty acid profiles of VLBW infants receiving a multicomponent lipid emulsion from birth. J Pediatr Gastroenterol Nutr. 2014;58:417–27. doi: 10.1097/MPG.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 37.Diamond IR, Grant RC, Pencharz PB, de Silva N, Feldman BM, Fitzgerald P, et al. Preventing the progression of intestinal failure-associated liver disease in infants using a composite lipid emulsion. JPEN J Parenter Enteral Nutr. 2016;41:866–77. doi: 10.1177/0148607115626921. [DOI] [PubMed] [Google Scholar]
- 38.Jobe AH. Mechanisms of lung injury and bronchopulmonary dysplasia. Am J Perinatol. 2016;33:1076–8. doi: 10.1055/s-0036-1586107. [DOI] [PubMed] [Google Scholar]
- 39.Kapoor V, Glover R, Malviya MN. Alternative lipid emulsions versus pure soy oil based lipid emulsions for parenterally fed preterm infants. Cochrane Database Syst Rev. 2015;(12):CD009172. doi: 10.1002/14651858.CD009172.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collins CT, Makrides M, McPhee AJ, Sullivan TR, Davis PG, Thio M, et al. Docosahexaenoic acid and bronchopulmonary dysplasia in preterm infants. N Engl J Med. 2017;376:1245–55. doi: 10.1056/NEJMoa1611942. [DOI] [PubMed] [Google Scholar]
- 41.Woldesenbet M, Rosenfeld CR, Ramilo O, Johnson-Welch S, Perlman JM. Severe neonatal hypoxic respiratory failure correlates with histological chorioamnionitis and raised concentrations of interleukin 6 (IL6), IL8 and C-reactive protein. Arch Dis Child Fetal Neonatal Ed. 2008;93:F413–7. doi: 10.1136/adc.2007.124503. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez A, Sosenko IR, Chandar J, Hummler H, Claure N, Bancalari E. Influence of infection on patent ductus arteriosus and chronic lung disease in premature infants weighing 1000 grams or less. J Pediatr. 1996;128:470–8. doi: 10.1016/s0022-3476(96)70356-6. [DOI] [PubMed] [Google Scholar]
- 43.Smith LE. Through the eyes of a child: understanding retinopathy through ROP the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2008;49:5177–82. doi: 10.1167/iovs.08-2584. [DOI] [PubMed] [Google Scholar]
- 44.Brion LP, Bell EF, Raghuveer TS. Vitamin E supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2003;(4):CD003665. doi: 10.1002/14651858.CD003665. [DOI] [PubMed] [Google Scholar]
- 45.Pawlik D, Lauterbach R, Walczak M, Hurkala J, Sherman MP. Fish-oil fat emulsion supplementation reduces the risk of retinopathy in very low birth weight infants: a prospective, randomized study. JPEN J Parenter Enteral Nutr. 2014;38:711–6. doi: 10.1177/0148607113499373. [DOI] [PubMed] [Google Scholar]
- 46.Vayalthrikkovil S, Bashir RA, Rabi Y, Amin H, Spence JM, Robertson HL, et al. Parenteral fish-oil lipid emulsions in the prevention of severe retinopathy of prematurity: a systematic review and meta-analysis. Am J Perinatol. 2016;34:705–15. doi: 10.1055/s-0036-1597131. [DOI] [PubMed] [Google Scholar]
- 47.Schlotzer E, Kanning U. Elimination and tolerance of a new parenteral lipid emulsion (SMOF)—a double-blind cross-over study in healthy male volunteers. Ann Nutr Metab. 2004;48:263–8. doi: 10.1159/000080461. [DOI] [PubMed] [Google Scholar]
- 48.Cooke RJ, Ainsworth SB, Fenton AC. Postnatal growth retardation: a universal problem in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2004;89:F428–30. doi: 10.1136/adc.2001.004044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calder PC. Docosahexaenoic Acid. Ann Nutr Metab. 2016;69(Suppl 1):7–21. doi: 10.1159/000448262. [DOI] [PubMed] [Google Scholar]
- 50.Zhao Y, Wu Y, Pei J, Chen Z, Wang Q, Xiang B. Safety and efficacy of parenteral fish oil-containing lipid emulsions in premature neonates. J Pediatr Gastroenterol Nutr. 2015;60:708–16. doi: 10.1097/MPG.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 51.Carlson SE, Werkman SH, Peeples JM, Cooke RJ, Tolley EA. Arachidonic acid status correlates with first year growth in preterm infants. Proc Natl Acad Sci USA. 1993;90:1073–7. doi: 10.1073/pnas.90.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]