Abstract
Background
Based on preclinical studies and a phase I trial of the combination of bortezomib and pegylated liposomal doxorubicin (PLD), which both showed activity in breast cancer, we conducted a phase II study of this regimen in patients with metastatic breast cancer.
Patients and Methods
Patients received bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11 of an every-21-day cycle, along with PLD 30 mg/m2 on day 4. The primary objective was to evaluate the response rate of this combination, while secondary objectives were to obtain further safety data about this combination, to evaluate the time to disease progression (TTP), and to evaluate response by the breast cancer subtype.
Results
One of 12 evaluable patients had a partial response (8%), while 3 (25%) had stable disease. At 26 months follow-up, the median overall survival was 4.3 months (95% CI, 1.2–26.2) and the median TTP was 1.3 months (95% CI, 0.8–14.0 months). The combination was well tolerated, with the most common events including low-grade nausea and vomiting, neutropenia, and neuropathy, and no cardiac toxicity was seen. Of the 7 tumors subtyped, no association was seen between intrinsic subtype or receptor status and response.
Conclusion
The combination of PLD and bortezomib was well tolerated but has minimal activity in heavily pretreated unselected metastatic breast cancer.
Keywords: Dexamethasone, Ondansetron, Liver metastases, Proteasome inhibitor, Pyridoxine, Ranitidine
Introduction
Bortezomib (VELCADE®; Millennium Pharmaceuticals, Inc., San Diego, CA and Johnson & Johnson Pharmaceuticals Research & Development, L.L.C., Raritan, NJ) is a dipeptide boronic acid derivative that specifically inhibits the chymotrypsin-like activity of the proteasome. The proteasome is a large, multicatalytic proteinase complex responsible for the degradation of many intracellular proteins, including targets such as IκB, p53, p21, and p27, and other proteins involved in the cell cycle and apoptosis-related signal transduction pathways.1 Proteasome inhibition has antineoplastic effects through several distinct mechanisms, including inhibition of cell growth signaling pathways, inhibition of angiogenesis, and induction of apoptosis. Bortezomib has shown antitumor activity in preclinical models and clinical trials, most notably against multiple myeloma,2,3 and is Food and Drug Association approved both for the treatment of multiple myeloma and mantle cell lymphoma. Activity in solid tumors has been seen as well, including in ovarian cancer,4 prostate cancer,5 renal cell carcinoma,6 and non-small-cell lung cancer.7 Preclinical studies in breast cancer models have demonstrated an antitumor effect of bortezomib as a single agent,8–11 but in the clinical arena bortezomib has not shown activity, as judged by the lack of objective responses seen in 2 studies with a total of 24 patients with breast cancer.12,13
Proteasome inhibition is also a rational strategy to achieve chemosensitization and overcome chemoresistance,14 and we therefore considered that a bortezomib-based combination regimen might show greater efficacy against breast cancer. This hypothesis was supported in part by preclinical studies, which suggested that bortezomib-containing combinations were more active preclinically.8–11 In particular, bortezomib has been found to suppress expression of proteins involved in DNA damage repair pathways, resulting in enhanced sensitivity to anthracyclines.15 Moreover, anthracyclines have been noted to suppress the induction of mitogen-activated protein kinase phosphatase (MKP)-1,16 which is an antiapoptotic stress-response protein induced by proteasome inhibitors.17 Indeed, bortezomib with pegylated liposomal doxorubicin (PLD) was shown to have synergistic activity in drug-naive models in vitro, and enhanced antitumor activity in vivo against breast cancer.18,19 Pegylated liposomal doxorubicin has demonstrated clinical activity in breast cancer. In a phase II study of PLD at 45–60 mg/m2 administered every 3–4 weeks to patients with anthracycline-naive breast cancer, the overall response rate (RR) was 31%.20 Though RRs are lower in anthracycline pretreated breast cancer patients, additive dosing of PLD may be less cardiotoxic than the parent drug, doxorubicin.21,22 The regimen of bortezomib and PLD has been extensively evaluated in patients with multiple myeloma, where it was shown to be both safe and effective in phase I,23,24 and superior to bortezomib alone in a phase III trial.25 Finally, in our previous phase I study of this combination targeting patients with solid tumors, we found that bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11, and PLD, 30 mg/m2 on day 4, could be safely administered on an every-21-day schedule.26 Evidence of a clinical benefit was seen in breast cancer patients in the phase I setting, and a phase II study was therefore designed to further evaluate the possible efficacy of this combination.
In the current report, we present the results of the phase II trial of bortezomib and PLD in breast cancer. The primary objective of this study was to evaluate the RR of this combination in metastatic breast cancer. Secondary objectives were to evaluate the time to progression (TTP) of disease in metastatic breast cancer patients undergoing treatment with this combination, to obtain further evidence of the safety of this combination in this population, and to compare any responses with the breast cancer intrinsic subtype.
Patients and Methods
Eligibility
This was an open label phase II trial of PLD and bortezomib in patients with histologically or cytologically confirmed metastatic breast cancer. Patients were required to have measurable disease by the Response Evaluation Criteria in Solid Tumors (RECIST).27 All patients gave written, informed consent according to federal and institutional guidelines before treatment, and this study received approval from the University of North Carolina at Chapel Hill Institutional Review Board, and was conducted in accordance with the Declaration of Helsinki. Patients were recruited from the University of North Carolina Multidisciplinary Breast Cancer Clinic. Eligibility criteria included: age ≥ 18; Karnofsky performance status > 60%; a life expectancy of ≥ 3 months; no other concurrent antineoplastic treatment; adequate hematologic (platelets > 100,000/mm3, hemoglobin ≥ 9.0 g/dL, and absolute neutrophil count ≥ 1500/mm3), hepatic (aspartate aminotransferase and alanine aminotransferase ≤ 2 times the upper limit of normal; alkaline phosphatase ≤ 2 times the upper limit of normal, except if attributed to tumor; and total bilirubin ≤ the upper limit of normal), and renal (creatinine ≤ 2.5 mg/dL) function. A normal left ventricular ejection fraction (LVEF), defined as > 50% within 42 days before the first dose of study drug, was necessary. Also, patients could not be pregnant or nursing, had to be amenable to the use of appropriate contraception, could not have symptomatic brain metastases, any treated brain metastases had to have been stable for more that 3 months and did not require chronic steroids, and, according to the study investigators, patients could have no other coexisting medical problems of sufficient severity to limit full study compliance or cause undue risk. Patients were ineligible if they had a previous cumulative exposure to doxorubicin > 300 mg/m2, or epirubicin > 540 mg/m2; previous anthracycline treatment within the last 6 months; New York Heart Association Class II or greater heart failure, or clinical evidence of congestive heart failure, or a myocardial infarction within 6 months before enrollment; uncontrolled angina; electrocardiographic evidence of acute ischemia or active conduction system abnormalities; hypersensitivity to PLD, bortezomib, boron, or mannitol; more than 2 previous chemotherapy regimens for metastatic disease; or grade 2 peripheral neuropathy within 14 days before enrollment.
Treatment Plan
Pegylated liposomal doxorubicin from commercial stock was prepared as described in the package insert, and a dose of 30 mg/m2 administered as a 60 to 90 minute infusion 1 hour after bortezomib administration on day 4 of each cycle. Bortezomib, a sterile, lyophilized powder in vials with mannitol, was reconstituted with normal saline to a drug concentration of 1 mg/mL. A dose of 1.3 mg/m2 was administered by intravenous push over 3–5 seconds on days 1, 4, 8, and 11 of an every-21-day cycle. For grade 2 or higher neutropenia or thrombocytopenia, or hand-foot syndrome (HFS), or stomatitis, or for grade 3 or higher other nonhematologic toxicity, doses were held until recovery to grade 0–1. Held doses within a cycle were skipped and not delayed. In the case of toxicity observed on the first day of a new cycle, doses were delayed for up to 2 weeks. If grade 2 or higher bilirubin levels were seen, the PLD was dose reduced by 50% and the bortezomib continued at full dose. No inter- or intra-patient dose escalation was allowed.
All patients received pyridoxine at 200 mg orally daily beginning up to 1 week before the initiation of therapy. As premedications, patients received ondansetron 24 mg orally, ranitidine 50 mg intravenous (I.V.), dexamethasone 20 mg orally, and diphenhydramine 50 mg orally before dosing of PLD. Alternative and/or additional antiemetics could be used at the discretion of one of the study investigators. No premedications were administered before or after dosing with bortezomib.
Toxicity Assessment
Complete blood cell counts, serum chemistries, and adverse events were evaluated on day 1 of each cycle, and were graded according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 3.0. If during treatment the LVEF fell to ≤ 45%, or the LVEF fell by 20% from the baseline level, PLD was discontinued. For patients with cumulative doxorubicin dosing of < 350 mg/m2, or of epirubicin < 540 mg/m2, cardiac monitoring was performed at the physician’s discretion. For patients with cumulative doxorubicin dosing of ≥ 350 mg/m2, or epirubicin of ≥ 540 mg/m2, cardiac monitoring was performed every 2 cycles.
Response Criteria
Tumor assessments were performed every 6 weeks (2 cycles), or at the follow-up visit if the patient was removed or withdrew from therapy in mid-cycle. Response was evaluated using the RECIST criteria.
For breast cancer patients treated on this or the previous phase I study who had archival tumor biopsy specimens available, the Prediction Analysis of Microarray 50-gene classifier (PAM50) intrinsic subtyping assay was performed on RNA from formalin-fixed paraffin-embedded (FFPE) tissue blocks, as previously described.28
Statistical Analysis
Response rate was the primary endpoint. For this patient population, we used as the benchmark for PLD response the 10% RR seen in the previous phase II study of the single agent in previously anthracycline treated patients.21 The null hypothesis was that the RR would be 10% or less, with a RR of interest being 30%. A two-stage approach with early stopping rules was used with 12 patients enrolled in stage I.29 The accrual goal was 40 patients with at least 35 eligible and evaluable patients.
Using the Simon optimal 2-stage design, if only 1 of the first 12 evaluable patients responded (complete response [CR] or partial response [PR]), the trial would be terminated based on the failure to reject the null hypothesis. If 2 or more of the first 12 patients responded, then an additional 23 patients would have been enrolled at stage II. The null hypothesis would be rejected if 6 or more of the 35 patients responded. This 2-stage design yielded an overall α level of 0.10; the power is 90% when the true RR is 30%. For RR assessment, only patients who completed an entire first cycle of therapy were considered evaluable for response.
Overall survival (OS) was defined from the date of enrollment to the date of death from any cause, or censored at the date of last follow-up. Time to progression (TTP) was defined as the time from the date of enrollment to the date of clinically or radiographically evident progression. The survival analysis was based on intention-to-treat principle and included all patients (intent to treat [ITT] cohort, n = 13). Analysis of toxicities was based on the ITT cohort using adverse events thought to be possibly, probably, or likely related to the therapy. The OS and TTP curves were computed by the Kaplan-Meier method. The hazard ratios for the corresponding 95% CIs were calculated by Cox proportional hazards methods. The statistical software package used was SAS version 9.2 (SAS Institute, Inc., Cary, NC).
Results
Patients
Thirteen patients were enrolled and received an average of 2.8 cycles per patient (range, 1–7 cycles). Of the 10 patients who received 2 or more cycles, 8 missed 1 or more doses of bortezomib for an average dose intensity of 83% in the first 2 cycles for these patients. Table 1 lists the patient characteristics. The median age was 51 years, with the range between 40–66 years of age. This population was heavily pretreated, with 31% having received 2 lines of chemotherapy in the metastatic setting. A significant percentage of tumors (39%) were human epidermal growth factor receptor 2 (HER2) overexpressing, and 23% of patients were “triple negative,” or estrogen receptor (ER), progesterone receptor (PgR), and HER2 negative. All of the patients had invasive ductal carcinoma, and none had invasive lobular carcinoma.
Table 1.
Baseline Patient Characteristics
| Characteristic, n (%) | Value |
|---|---|
| Age, Years | |
| Median | 51 |
| Range | 40–66 |
| Race | |
| Caucasian | 10 (77) |
| Black or African-American | 2 (15) |
| American Indian | 1 (8) |
| ECOG Performance Status | |
| 0 | 5 (38) |
| 1 | 8 (62) |
| Number of Previous Chemotherapy Lines (Metastatic) | |
| 0 | 3 (23) |
| 1 | 6 (46) |
| 2 | 4 (31) |
| Previous anthracycline | 10 (77) |
| Previous taxane | 12 (92) |
| Metastatic Sites | |
| Liver | 11 (85) |
| Lung | 10 (77) |
| Bone | 9 (69) |
| Lymph nodes | 3 (23) |
| Other (skin, peritoneum) | 2 (15) |
| ER/PgR positive | 9 (69) |
| ER/PgR negative | 4 (31) |
| HER2/neu overexpressed | 5 (39) |
| Triple negative | 3 (23) |
Abbreviations: ECOG = Eastern Cooperative Oncology Group; ER = estrogen receptor; PgR = progesterone receptor
Efficacy
Of the first 13 patients, 1 did not receive a complete cycle of therapy because of progression of an unrelated disease, and she was therefore considered unevaluable for response and was replaced. One of 12 evaluable patients had a PR (8%), and 3 of 12 had stable disease (SD; 25%). Of the patients with SD, 1 remained stable for 20 weeks, and 2 for 8 weeks. All others had progressive disease on treatment (66%). The 1 responding patient had hormone receptor positive, HER2 not overexpressed breast cancer, and had been treated previously with an anthracycline. She remained on study for 6 months, and was discontinued because of an inability to comply with frequent visits, and a desire to discontinue intravenous therapy.
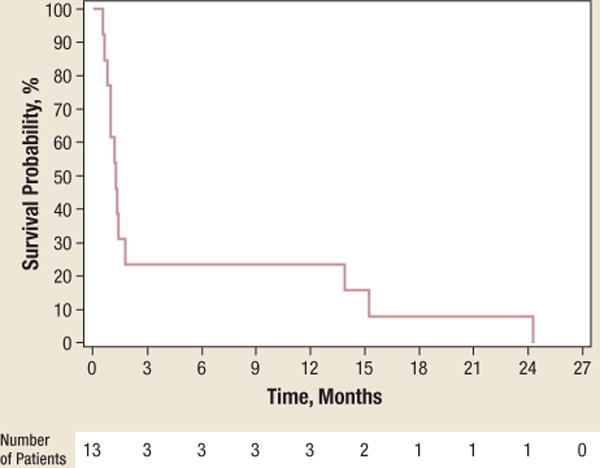
At a follow-up of 26 months, 9 of the 13 patients on study had died, for a median OS of 4.3 months (95% CI, 1.2–26.2). The median TTP was 1.3 months (95% CI, 0.8–14). Figure 1 shows the Kaplan-Meier curves for OS and TTP.
Figure 1.
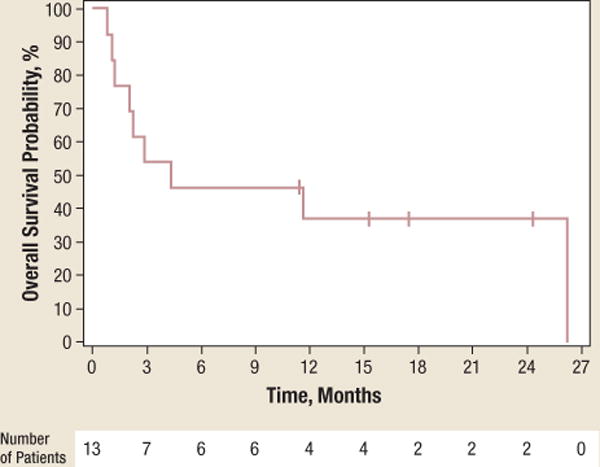
a The Kaplan-Meier Curves for Overall Survival With the Number of Patients at Risk
At a follow-up of 26 months, the median overall survival was 4.3 months (95% CI, 1.2–26.2).
b The Kaplan-Meier Curves for Time to Progression With the Number of Patients at Risk
At a follow-up of 26 months, the median time to progression was 1.3 months (95% CI, 0.8–14).
Toxicity
No grade 4 adverse events (AEs) were seen. Of the grade 3 events thought to be possibly, probably, or likely related to therapy, thrombocytopenia and neutropenia were seen in 2 patients, and hypoalbuminemia, nausea, diarrhea, anemia, lymphopenia, and hand-foot syndrome were seen in 1 patient each. As shown in Table 2, the most frequent toxicities of any grade were nausea, neuropathy, vomiting, neutropenia, diarrhea, fatigue, rash, and thrombocytopenia, which were all seen in > 30% of patients. All 13 patients had at least 1 AE; however, 5 of 13 (38%) had a grade 3 AE. No cardiac toxicity was observed.
Table 2.
Drug-Related Toxicities
| Adverse Event | All Grades | Grade 3 |
|---|---|---|
| Number (%) | Number (%) | |
| Nausea | 6 (46) | 1 (8) |
| Neuropathy | 6 (46) | 0 |
| Diarrhea | 5 (39) | 1 (8) |
| Vomiting | 5 (39) | 0 |
| Neutropenia | 4 (31) | 2 (15) |
| Thrombocytopenia | 4 (31) | 2 (15) |
| Fatigue | 4 (31) | 0 |
| Rash | 4 (31) | 1 (8) |
| Lymphopenia | 3 (23) | 1 (8) |
| Constipation | 3 (23) | 0 |
| Arthralgias/Myalgias | 3 (23) | 0 |
| Headache | 2 (15) | 0 |
| Oral Sores | 2 (15) | 0 |
| Abdominal Pain | 2 (15) | 0 |
| Thrush | 1 (8) | 0 |
| Taste Alteration | 1 (8) | 0 |
| Anorexia | 1 (8) | 0 |
| Anemia | 1 (8) | 1 (8) |
| Leukopenia | 1 (8) | 0 |
| Pruritus | 1 (8) | 0 |
| Hot Flashes | 1 (8) | 0 |
| Shingles | 1 (8) | 0 |
| Redness at Injection Site | 1 (8) | 0 |
| Hand-Foot Skin Reaction | 1 (8) | 0 |
| Skin Hyperpigmentation | 1 (8) | 0 |
| Allergic Rhinitis | 1 (8) | 0 |
| Cardiac Palpitations | 1 (8) | 0 |
| Hypertension | 1 (8) | 0 |
| Fever | 1 (8) | 0 |
| Dyspnea | 1 (8) | 0 |
| Cough | 1 (8) | 0 |
| Blurred Vision | 1 (8) | 0 |
| Red Eyes | 1 (8) | 0 |
| Elevated Creatinine | 1 (8) | 0 |
| Hypoalbuminemia | 1 (8) | 1 (8) |
| Proteinuria | 1 (8) | 0 |
Intrinsic Subtype Prediction
Archival tumor tissue from 5 patients in this study and 2 patients treated in the phase I trial were available to be subtyped (Table 3). Of the 7 typed, none of the basal breast cancers (typically, ER/PgR/HER2 negative) responded.
Table 3.
Intrinsic Breast Cancer Subtype and Response
| Study Phase | Subtype | Cycles Received | Response |
|---|---|---|---|
| II | Basal | 1 | PD |
| II | Luminal A | 7 | PR |
| II | Luminal B | 6 | SD |
| II | Basal | 2 | PD |
| II | Basal | 2 | PD |
| I | Basal | 4 | PD |
| I | Basal | 2 | PD |
Abbreviations: PD = progressive disease; PR = partial response; SD = stable disease
Discussion
The primary goal of this study was to evaluate the RR of the combination of bortezomib and PLD in metastatic breast cancer, with the secondary objectives being to estimate the TTP of disease with this treatment, and its tolerability. In our phase I study, RECIST measurable disease was not required, but 2 of 19 breast cancer patients (11%) had either a CR or PR, and 2 others showed clinical benefit.26 With only 1 of the 12 patients responding in this phase II study, the trial was terminated according to the designed early-stopping rules, given the failure to reject the null hypothesis. This study suggests that the combination of PLD and bortezomib is no more efficacious than single-agent PLD or single-agent bortezomib in metastatic breast cancer.12,13,30,31
Other combination trials conducted concurrently have yielded mixed efficacy results. A phase I combination of bortezomib with weekly paclitaxel that enrolled 3 patients with metastatic breast cancer did not show evidence to suggest enhanced efficacy above what would be expected of single-agent paclitaxel.30 Similarly, a phase I/II study of bortezomib with capecitabine in metastatic breast cancer showed a 15% overall RR.31 However, a phase I/II study combining docetaxel with bortezomib in metastatic breast cancer was more promising, and revealed a RR of 38% at the maximum tolerated dose.32
In this phase II study, our population was heavily pretreated, and the majority had received a previous anthracycline. Furthermore, this was an unselected population that had a higher percentage of HER2 overexpression than the anticipated 20%.33 These factors may have contributed to the lower RR, especially because of the activity of the p44/42 mitogen-activated protein kinase pathway, which is induced by HER2 signaling, has been shown to oppose proteasome inhibitor-mediated cell death.17 The low-dose intensity may also have contributed to the poor RR, and also comments on the tolerability of this combination. Interestingly, of the subtyped tumors, none of the patients with basal breast cancers showed evidence of a response to this combination. Basal breast cancer is typically “triple negative,” and the hormone receptor–negative subtypes traditionally have a worse prognosis.34–36 Basal breast cancer may be associated with breast and ovarian cancer susceptibility (BRCA) 1 pathway dysfunction, and also dysregulation of intracellular proteolysis given the role of BRCA1 as an E3 ubiquitin ligase.37,38 It is thus tempting to speculate that, because such tumors appear to already be tolerating some dysfunction of the ubiquitin-proteasome pathway, they may be less sensitive to other approaches targeting this protein turnover mechanism.
Unfortunately, our encouraging preclinical studies were not predictive of clinical activity for this drug regimen in this case. This may have been in part a result of the use of drug-naive breast cancer models in vitro, which were likely more intrinsically drug-sensitive. In contrast, our heavily pretreated population likely had breast cancer in which multiple drug resistance pathway were activated, reducing the likelihood that a 2-drug regimen would be active, especially in those patients who had previously received anthracyclines. Finally, proteasome inhibitors like bortezomib activate apoptosis through the c-Jun-N-terminal kinase (JNK), but they also induce MKP-1, which inactivates JNK and protects tumors from programmed cell death.17,18,39–44 MKP-1 may also play a role in tumorigenesis, and its overexpression has shown to be associated with poor patient outcomes in a number of malignancies, including breast cancer.45–47 Unfortunately, a specific, clinically-relevant inhibitor of MKP-1 is not available, and though anthracyclines suppress MKP-1 preclinically,16 it is likely that the doses used in our study were not sufficient to suppress the promoter of this phosphatase. Combined with the known baseline overexpression of MKP-1 in many breast cancers,48,49 this inability to suppress MKP-1 may also have contributed to the lack of activity of this regimen clinically. If this is the case, it may indicate that, once targeted MKP-1 inhibitors are available, future studies of these agents in combination with chemotherapeutics may be warranted.
Conclusion
The combination of PLD and bortezomib had minimal activity in heavily pretreated, unselected metastatic breast cancer, with a RR of 8%. The regimen is tolerable and the toxicities in this study are commensurate with those seen in our previous phase I study.26 Further studies of PLD/bortezomib in this population are not warranted, but the ubiquitin-proteasome pathway remains an interesting target, and studies of patients earlier in their disease course, or with novel, irreversible proteasome inhibitors, may uncover a role for this class of drugs in our armamentarium against breast cancer.50–52
Acknowledgments
The authors thank Rachel Phipps, RN, for research nursing, Mary O’Dwyer for regulatory assistance, Judy Fogleman and Paul Jones for help with data collection and management, and most importantly the patients who participated in this study and their families for their courage and contribution to the evaluation of new cancer therapies.
This study was supported in part by grants from Ortho Biotech Products, L.P., Millennium Pharmaceuticals, Inc., the General Clinical Research Centers Program of the Division of Research Resources, National Institutes of Health (RR00046), and the National Cancer Institute SPORE in Breast Cancer (5-P50-CA58223).
Footnotes
This summary may include the discussion of investigational and/or unlabeled uses of drugs and/or devices that may not be approved by the FDA.
Electronic forwarding or copying is a violation of US and International Copyright Laws.
Authorization to photocopy items for internal or personal use, or the internal or personal use of specific clients, is granted by CIG Media Group, LP, ISSN #1526-8209, provided the appropriate fee is paid directly to Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923 USA. www.copyright.com 978-750-8400.
Disclosures
Charles Perou has received research support from Bristol-Myers Squibb Company, Eli Lilly and Company, and Roche Pharmaceuticals. He has served as Board of Directors or other leadership position with University Genomics. He has served as a paid consultant or member of the advisory board for AstraZeneca, Bristol-Myers Squibb Company, Eli Lilly and Company, GlaxoSmithKline, PharmaMar USA, Inc. and Roche Pharmaceuticals. He also has stock or equity ownership in Bioclassifier, LLC. Philip S. Bernard has served as Board of Directors or other leadership position with Bioclassifier, LLC. He has served as a paid consultant or member of the advisory board for Bioclassifier, LLC. He also has stock or equity ownership in Bioclassifier, LLC. Robert Z. Orlowski has received research support from Millennium Pharmaceuticals, Inc., and Johnson & Johnson Services, Inc. All other authors report that they have no relevant relationships to disclose.
References
- 1.Ciechanover A. Intracellular protein degradation from a vague idea through the lysosome and the ubiquitin-proteasome system and on to human diseases and drug targeting: Nobel Lecture, December 8, 2004. Ann N Y Acad Sci. 2007;1116:1–28. doi: 10.1196/annals.1402.078. [DOI] [PubMed] [Google Scholar]
- 2.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–17. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 3.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–98. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 4.Aghajanian C, Soignet S, Dizon DS, et al. A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res. 2002;8:2505–11. [PubMed] [Google Scholar]
- 5.Papandreou CN, Daliani DD, Nix D, et al. Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J Clin Oncol. 2004;22:2108–21. doi: 10.1200/JCO.2004.02.106. [DOI] [PubMed] [Google Scholar]
- 6.Kondagunta GV, Drucker B, Schwartz L, et al. Phase II trial of bortezomib for patients with advanced renal cell carcinoma. J Clin Oncol. 2004;22:3720–5. doi: 10.1200/JCO.2004.10.155. [DOI] [PubMed] [Google Scholar]
- 7.Fanucchi MP, Fossella FV, Belt R, et al. Randomized phase II study of bortezomib alone and bortezomib in combination with docetaxel in previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2006;24:5025–33. doi: 10.1200/JCO.2006.06.1853. [DOI] [PubMed] [Google Scholar]
- 8.Cooper C, Liu GY, Niu YL, et al. Intermittent hypoxia induces proteasome-dependent down-regulation of estrogen receptor alpha in human breast carcinoma. Clin Cancer Res. 2004;10:8720–7. doi: 10.1158/1078-0432.CCR-04-1235. [DOI] [PubMed] [Google Scholar]
- 9.Codony-Servat J, Tapia MA, Bosch M, et al. Differential cellular and molecular effects of bortezomib, a proteasome inhibitor, in human breast cancer cells. Mol Cancer Ther. 2006;5:665–75. doi: 10.1158/1535-7163.MCT-05-0147. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso F, Durbecq V, Laes JF, et al. Bortezomib (PS-341, Velcade) increases the efficacy of trastuzumab (Herceptin) in HER-2-positive breast cancer cells in a synergistic manner. Mol Cancer Ther. 2006;5:3042–51. doi: 10.1158/1535-7163.MCT-06-0104. [DOI] [PubMed] [Google Scholar]
- 11.Teicher BA, Ara G, Herbst R, et al. The proteasome inhibitor PS-341 in cancer therapy. Clin Cancer Res. 1999;5:2638–45. [PubMed] [Google Scholar]
- 12.Yang CH, Gonzalez-Angulo AM, Reuben JM, et al. Bortezomib (VELCADE) in metastatic breast cancer: pharmacodynamics, biological effects, and prediction of clinical benefits. Ann Oncol. 2006;17:813–7. doi: 10.1093/annonc/mdj131. [DOI] [PubMed] [Google Scholar]
- 13.Engel RH, Brown JA, Von Roenn JH, et al. A phase II study of single agent bortezomib in patients with metastatic breast cancer: a single institution experience. Cancer Invest. 2007;25:733–7. doi: 10.1080/07357900701506573. [DOI] [PubMed] [Google Scholar]
- 14.Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res. 2008;14:1649–57. doi: 10.1158/1078-0432.CCR-07-2218. [DOI] [PubMed] [Google Scholar]
- 15.Mitsiades N, Mitsiades CS, Richardson PG, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101:2377–80. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- 16.Small GW, Somasundaram S, Moore DT, et al. Repression of mitogen-activated protein kinase (MAPK) phosphatase-1 by anthracyclines contributes to their anti-apoptotic activation of p44/42-MAPK. J Pharmacol Exp Ther. 2003;307:861–9. doi: 10.1124/jpet.103.055806. [DOI] [PubMed] [Google Scholar]
- 17.Orlowski RZ, Small GW, Shi YY. Evidence that inhibition of p44/42 mitogen-activated protein kinase signaling is a factor in proteasome inhibitor-mediated apoptosis. J Biol Chem. 2002;277:27864–71. doi: 10.1074/jbc.M201519200. [DOI] [PubMed] [Google Scholar]
- 18.Small GW, Shi YY, Edmund NA, et al. Evidence that mitogen-activated protein kinase phosphatase-1 induction by proteasome inhibitors plays an antiapoptotic role. Mol Pharmacol. 2004;66:1478–90. doi: 10.1124/mol.104.003400. [DOI] [PubMed] [Google Scholar]
- 19.Shi YY, Small GW, Orlowski RZ. Proteasome inhibitors induce a p38 mitogen-activated protein kinase (MAPK)-dependent anti-apoptotic program involving MAPK phosphatase-1 and Akt in models of breast cancer. Breast Cancer Res Treat. 2006;100:33–47. doi: 10.1007/s10549-006-9232-x. [DOI] [PubMed] [Google Scholar]
- 20.Ranson MR, Carmichael J, O’Byrne K, et al. Treatment of advanced breast cancer with sterically stabilized liposomal doxorubicin: results of a multicenter phase II trial. J Clin Oncol. 1997;15:3185–91. doi: 10.1200/JCO.1997.15.10.3185. [DOI] [PubMed] [Google Scholar]
- 21.Rivera E, Valero V, Esteva FJ, et al. Lack of activity of stealth liposomal doxorubicin in the treatment of patients with anthracycline-resistant breast cancer. Cancer Chemother Pharmacol. 2002;49:299–302. doi: 10.1007/s00280-001-0405-3. [DOI] [PubMed] [Google Scholar]
- 22.Lyass O, Uziely B, Ben-Yosef R, et al. Correlation of toxicity with pharmacokinetics of pegylated liposomal doxorubicin (Doxil) in metastatic breast carcinoma. Cancer. 2000;89:1037–47. doi: 10.1002/1097-0142(20000901)89:5<1037::aid-cncr13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 23.Orlowski RZ, Voorhees PM, Garcia RA, et al. Phase 1 trial of the proteasome inhibitor bortezomib and pegylated liposomal doxorubicin in patients with advanced hematologic malignancies. Blood. 2005;105:3058–65. doi: 10.1182/blood-2004-07-2911. [DOI] [PubMed] [Google Scholar]
- 24.Biehn SE, Moore DT, Voorhees PM, et al. Extended follow-up of outcome measures in multiple myeloma patients treated on a phase I study with bortezomib and pegylated liposomal doxorubicin. Ann Hematol. 2007;86:211–6. doi: 10.1007/s00277-006-0220-3. [DOI] [PubMed] [Google Scholar]
- 25.Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 26.Dees EC, O’Neil BH, Lindley CM, et al. A phase I and pharmacologic study of the combination of bortezomib and pegylated liposomal doxorubicin in patients with refractory solid tumors. Cancer Chemother Pharmacol. 2008;63:99–107. doi: 10.1007/s00280-008-0716-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 28.Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–7. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 30.Cresta S, Sessa C, Catapano CV, et al. Phase I study of bortezomib with weekly paclitaxel in patients with advanced solid tumours. Eur J Cancer. 2008;44:1829–34. doi: 10.1016/j.ejca.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 31.Schmid P, Kuhnhardt D, Kiewe P, et al. A phase I/II study of bortezomib and capecitabine in patients with metastatic breast cancer previously treated with taxanes and/or anthracyclines. Ann Oncol. 2008;19:871–6. doi: 10.1093/annonc/mdm569. [DOI] [PubMed] [Google Scholar]
- 32.Awada A, Albanell J, Canney PA, et al. Bortezomib/docetaxel combination therapy in patients with anthracycline-pretreated advanced/metastatic breast cancer: a phase I/II dose-escalation study. Br J Cancer. 2008;98:1500–7. doi: 10.1038/sj.bjc.6604347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5:63–9. doi: 10.3816/cbc.2004.n.011. [DOI] [PubMed] [Google Scholar]
- 34.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–23. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–74. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 37.Hashizume R, Fukuda M, Maeda I, et al. The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. J Biol Chem. 2001;276:14537–40. doi: 10.1074/jbc.C000881200. [DOI] [PubMed] [Google Scholar]
- 38.Foulkes WD, Stefansson IM, Chappuis PO, et al. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst. 2003;95:1482–5. doi: 10.1093/jnci/djg050. [DOI] [PubMed] [Google Scholar]
- 39.Meriin AB, Gabai VL, Yaglom J, et al. Proteasome inhibitors activate stress kinases and induce Hsp72. Diverse effects on apoptosis. J Biol Chem. 1998;273:6373–9. doi: 10.1074/jbc.273.11.6373. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Ikezoe T, Saito T, et al. Proteasome inhibitor PS-341 induces growth arrest and apoptosis of non-small cell lung cancer cells via the JNK/c-Jun/AP-1 signaling. Cancer Sci. 2004;95:176–80. doi: 10.1111/j.1349-7006.2004.tb03200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hideshima T, Mitsiades C, Akiyama M, et al. Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood. 2003;101:1530–4. doi: 10.1182/blood-2002-08-2543. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Gorospe M, Yang C, et al. Role of mitogen-activated protein kinase phosphatase during the cellular response to genotoxic stress. Inhibition of c-Jun N-terminal kinase activity and AP-1-dependent gene activation. J Biol Chem. 1995;270:8377–80. doi: 10.1074/jbc.270.15.8377. [DOI] [PubMed] [Google Scholar]
- 43.Franklin CC, Srikanth S, Kraft AS. Conditional expression of mitogen-activated protein kinase phosphatase-1, MKP-1, is cytoprotective against UV-induced apoptosis. Proc Natl Acad Sci USA. 1998;95:3014–9. doi: 10.1073/pnas.95.6.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo YL, Kang B, Williamson JR. Inhibition of the expression of mitogen-activated protein phosphatase-1 potentiates apoptosis induced by tumor necrosis factor-alpha in rat mesangial cells. J Biol Chem. 1998;273:10362–6. doi: 10.1074/jbc.273.17.10362. [DOI] [PubMed] [Google Scholar]
- 45.Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 2008;27:253–61. doi: 10.1007/s10555-008-9123-1. [DOI] [PubMed] [Google Scholar]
- 46.Liao Q, Guo J, Kleeff J, et al. Down-regulation of the dual-specificity phosphatase MKP-1 suppresses tumorigenicity of pancreatic cancer cells. Gastroenterology. 2003;124:1830–45. doi: 10.1016/s0016-5085(03)00398-6. [DOI] [PubMed] [Google Scholar]
- 47.Rojo F, Gonzalez-Navarrete I, Bragado R, et al. Mitogen-activated protein kinase phosphatase-1 in human breast cancer independently predicts prognosis and is repressed by doxorubicin. Clin Cancer Res. 2009;15:3530–9. doi: 10.1158/1078-0432.CCR-08-2070. [DOI] [PubMed] [Google Scholar]
- 48.Loda M, Capodieci P, Mishra R, et al. Expression of mitogen-activated protein kinase phosphatase-1 in the early phases of human epithelial carcinogenesis. Am J Pathol. 1996;149:1553–64. [PMC free article] [PubMed] [Google Scholar]
- 49.Wang HY, Cheng Z, Malbon CC. Overexpression of mitogen-activated protein kinase phosphatases MKP1, MKP2 in human breast cancer. Cancer Lett. 2003;191:229–37. doi: 10.1016/s0304-3835(02)00612-2. [DOI] [PubMed] [Google Scholar]
- 50.Chauhan D, Catley L, Li G, et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell. 2005;8:407–19. doi: 10.1016/j.ccr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 51.Demo SD, Kirk CJ, Aujay MA, et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67:6383–91. doi: 10.1158/0008-5472.CAN-06-4086. [DOI] [PubMed] [Google Scholar]
- 52.Kuhn DJ, Chen Q, Voorhees PM, et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110:3281–90. doi: 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]


