Abstract
Activating transcription factor 3 (ATF3) is a highly regulated protein that is implicated in a wide range of pathological conditions including inflammation and transformation. Transcription from the ATF3 gene is induced by several stress-induced signaling pathways, including amino acid limitation (amino acid response, AAR) and ER stress (unfolded protein response, UPR). Induction of ATF3 transcription by these pathways is mediated by ATF4 and cJUN recruitment to enhancer elements within the ATF3 gene. Although a canonical promoter (promoter A) has been studied by numerous laboratories, a second promoter activity (promoter A1), 43 kb upstream of the first, has been reported to respond to stress-induced signaling and to be critical for ATF3 expression in certain transformed cells. The results of the present study show that in normal human hepatocytes and HepG2 human hepatoma cells both basal as well as AAR- and UPR-induced transcription occurs almost exclusively from promoter A. This selectivity between the two promoters correlated with increased binding of ATF4, recruitment of RNA polymerase II, and the expected histone modifications in the promoter A region of the gene. Time course studies of ATF3 transcription activity revealed that the temporal kinetics for ATF3 induction differ between the AAR and UPR, with the former being more transient than the latter. Collectively, the results document that ATF3 expression in normal and transformed human liver originates from the canonical promoter A that responds to multiple stress signals.
Keywords: liver, ATF4, transcription, histone modification, AAR, UPR
1. Introduction
Mammalian cells respond to dietary insufficiency of protein or amino acids (AA) through activation of several signaling cascades collectively referred to as the amino acid response (AAR) [reviewed in 1]. The best characterized AAR pathway senses uncharged tRNA levels which activate the general control non-derepressible-2 (GCN2) kinase. The primary substrate for GCN2 is the alpha subunit of the eukaryotic translation initiation factor elF2. Phospho-eIF2 (p-eIF2) leads to a partial suppression of global protein synthesis, but also causes increased translation of selected mRNA species, including that for activating transcription factor 4 (ATF4) [2]. Expression array analysis has shown that AA deprivation, via ATF4 action, leads to induction of hundreds of genes that mediate a spectrum of cellular processes [3–7]. There are three other eIF2 kinases that are activated by a wide range of cellular stresses [8]. For example, endoplasmic reticulum (ER) stress triggers ATF4 synthesis through activation of the eIF2 kinase PKR-like endoplasmic reticulum kinase (PERK) [9, 10]. ATF4 mediates an increase in transcription from genes that contain an enhancer sequence composed of a half-site for CAAT enhancer binding protein (C/EBP) family members and a half-site for the ATF family of transcription factors [11, 12]. These enhancer sequences are referred to as a C/EBP-ATF response element (CARE). Most, but not all [13], functional CARE sites respond to ATF4 regardless of which eIF2 kinase was activated [reviewed in 1] and collectively, the p-eIF2-ATF4 dependent pathways are often referred to as the Integrated Stress Response (ISR). The products of these CARE-containing genes control a broad range of physiological processes. Characterization of individual genes as well as chromatin immunoprecipitation (ChIP)-sequencing has led to the identification of a consensus CARE sequence (5′-TGATGXAAX-3′) [14]. As might be expected given the commonality of ATF4 action during AA and ER stress, the transcriptional programs activated have extensive overlap, but significant differences are also observed [15].
The ATF3 gene is subject to complicated transcriptional regulatory mechanisms from a spectrum of stress signals [16, 17]. The AAR and UPR both increase ATF3 expression via p-eIF2-ATF4 signaling [18, 19]. In a feedback mechanism, the increased ATF3 expression functions as a suppressor of ATF4 action [20]. Whereas most of the published ATF3 gene analysis studies have focused on control from a canonical promoter (herein termed promoter A), evidence has been reported that a second promoter (promoter A1) exists about 43 kb upstream of promoter A [21–23]. Miyazaki et al. showed that in HCT116 cells, ER stress [triggered by thapsigargin (Tg) or tunicamycin (Tm)] induced expression from both promoters, and in some transformed cells promoter A1 was the primary source of ATF3 expression [22]. Both promoters are also functional in adult T-cell leukemia cells, as reported by Hagiya et al. [23]. In previous reports, we have characterized AAR- and UPR-induced transcription from the human ATF3 gene in HepG2 human hepatoma cells, but, like most other laboratories, measured activity from promoter A only [19, 24–26].
The present study addresses the following questions. 1) Do both promoters A1 and A contribute to regulation of the ATF3 gene in response to the AAR and UPR pathways in human hepatocytes and HepG2 human hepatoma cells? 2) Do the enhancer-specific factors ATF4 and cJUN contribute to promoter A1 activity? 3) Are there differences in the epigenetic changes at the two promoters associated with stimulation of the ATF3 gene by these two stress pathways? The results indicate that the 24 h time course of transcription from the ATF3 gene is different after activation of the AAR or UPR pathway. The AAR induction is transient, whereas the activation following UPR signaling remains elevated for the entire 24 h period studied. In primary human hepatocytes and HepG2 hepatoma cells, only promoter A appears to be functional, neither the AAR nor the UPR increased transcription from promoter A1. This lack of promoter A1 activity was supported by ChIP analysis that revealed ATF4, RNA polymerase II, and transcription-associated histone changes at promoter A, but not at promoter A1. Collectively, the data indicate that for human liver-derived cells transcriptional regulation of the ATF3 gene by pathways belonging to the ISR occurs largely by promoter A and that unknown regulatory factors contribute to differential temporal control of the gene depending on which of the ISR pathways is activated.
2. Materials and Methods
2.1 Cell culture
Primary human hepatocytes were purchased from Corning Life Sciences (Tewksbury, MA). Both the primary hepatocytes and the HepG2 human hepatoma cells (purchased from American Type Tissue Culture) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) as described previously [27]. For all experiments, to achieve a nutritional/stress “basal state”, fresh DMEM medium was supplied about 14 h before experimental treatments began.
2.2 RNA isolation and RT-qPCR
HepG2 cells were seeded at a density of 1 × 106 per 60-mm dish and the primary hepatocytes were purchased as pre-plated monolayers and used directly. Three plates were used per condition and per time point. Cells were treated with 2.5 mM histidinol (HisOH) or 50 nM thapsigargin (Tg) to activate the AAR and UPR pathways, respectively. Total RNA was isolated at the times indicated in each experiment and converted to cDNA as described [27]. To measure GAPDH mRNA, ATF3 mRNA, and ATF3 hnRNA, real time-quantitative PCR (RT-qPCR) was performed using SYBR Green (ThermoFisher Scientific) and a CFX-Connect Real-Time System (Bio-Rad) [27]. To detect steady state ATF3 mRNA, primers that spanned exon A and B were used, whereas the primers used to detect the non-spliced hnRNA spanned intron 1 and exon B (Table I). Transcription activity was assayed by measuring the abundance of the short-lived ATF3 hnRNA [28].
Table 1.
PCR primers.
| Primer | Sequences |
|---|---|
| GAPDH, mRNA | Forward: 5′-TTGGTATCGTGGAAGGACTC-3′ |
| Reverse: 5′-ACAGTCTTCTGGGTGGCAGT-3′ | |
| ATF3, mRNA | Forward: 5′-CAGTCACTGTCAGCGACAGACCC-3′ |
| Reverse: 5′-TCTTCTTCAGGGGCTACCTCGG-3′ | |
| ATF3, hnRNA, promoter A1 | Forward: 5′-GGGGACGATGGCAGAAGCACT-3′ |
| Reverse: 5′-GGAGGCTTCCTGACCAAACACCT-3′ | |
| ATF3, hnRNA, promoter A | Forward: 5′-CATCACAAAAGCCGAGGTGGGG-3′ |
| Reverse: 5′-CAGTGGCTGCGAGCGAAACA-3′ | |
| ATF3 P1, chip assay | Forward: 5′-CTGTCTTTTCCTCTTCTTCTAAGGGCAC-3′ |
| Reverse: 5′-CAGCCTTTGAGAGATCATTAGGTTTGG-3′ | |
| ATF3 P2, chip assay | Forward: 5′-ATCAGTGTCAAGCCCCTCACTCAG-3′ |
| Reverse: 5′-GCTTCCCTTCGAGCCATCATCTA-3′ | |
| ATF3 P3, chip assay | Forward: 5′-GATGGGATCAGATGGGAAGATGTGA-3′ |
| Reverse: 5′-TTGGGGCAAGGTGCTGAAAATC-3′ | |
| ATF3 P4, chip assay | Forward: 5′-CCGTTCCAAAGCGAAGAAGTAGGT-3′ |
| Reverse: 5′-CTGTATTCGTGCCCAGAATGCTAGA-3′ | |
| ATF3 P5, chip assay | Forward: 5′-GTTCCTTGGTTCTGCCGCTCTC-3′ |
| Reverse: 5′-TCCGAGATTCGAGCTGAGACCTC-3′ | |
| ATF3 P6, chip assay | Forward: 5′-GACTTTGGACACCTTCCCCACAC-3′ |
| Reverse: 5′-TGGTCATTTTCTGGAGCTTCAGGA-3′ | |
| ATF3 P7, chip assay | Forward: 5′-TGAGGGCTATAAAAGGGGTGATGC-3′ |
| Reverse: 5′-GCGAGAGAAGAGAGCTGTGCAGTG-3′ | |
| ATF3 P8, chip assay | Forward: 5′-ACTTCTTCTAAGCCACCGCTGCTC-3′ |
| Reverse: 5′-GACCTCCGTCACCAGGAACCTTT-3′ | |
| ATF3 P9, chip assay | Forward: 5′-CACAATGCAGTGGTTGGACCAGAT-3′ |
| Reverse: 5′-TGGCTCCTTTTCTCCCCACTACAC-3′ | |
| ATF3 P10, chip assay | Forward: 5′-AAGGTGGGGGGATCTGAGAGAATA-3′ |
| Reverse: 5′-TGACATCACCACTACCAACAGGAGAC-3′ |
2.3 Chromatin immunoprecipitation (ChIP)
HepG2 cells were seeded at a density of 8–10 × 106 cells per 150 mm dish, cultured for 24 h, and then incubated with DMEM containing no treatment, 2.5 mM HisOH, or 50 nM Tg. Treatment continued for either 4 or 16 h before cross-linking cells with formaldehyde and performing ChIP assays, as described previously [27]. Primers used to detect specific regions of the ATF3 promoter are described in Table I. Antibodies against ATF3 (sc-188), RNA Pol II (sc-899), cJUN (sc-1694), and rabbit anti-IgG (sc-2027) were purchased from Santa Cruz Biotechnology. Antibodies against total H3 (ab1791), H4K8-Ac (ab15823), and H3K4me3 (ab8580) were purchased from Abcam. The antibody for p-Ser5 Pol II was purchased from Chromotek and the antibody for ATF4 was produced as described [29].
2.4 Protein isolation and immunoblotting
For protein analysis, HepG2 cells were seeded at a density of 1 × 106 per 60 mm dish and after 24 h of growth, cells were incubated with DMEM containing no treatment, 2.5 mM HisOH, or 50 nM Tg. At the times indicated in each experiment, cells were harvested using 300 μl of RIPA buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.5% sodium deoxycholate, 0.1% SDS, and 1% triton X-100) with protease and phosphatase inhibitors (ThermoFisher Scientific). Nuclear protein extracts were obtained using the NE-PER Extraction Kit (ThermoFisher Scientific) per the manufacturer’s protocol. Immunoblotting was performed as described previously [27], using the antibodies described above.
3. Results
3.1 Induction of the ATF3 gene by the AAR and UPR pathways
To contrast the kinetics of ATF3 gene induction by the AAR and UPR pathways, HepG2 cell were incubated with 2.5 mM HisOH (AAR) or 50 nM Tg (UPR) and the transcription activity of the gene measured by analysis of the short-lived hnRNA (Fig. 1A), as described previously [19]. Interestingly, although the magnitude of the increase in transcription from both pathways was similar, after 8 h ATF3 transcription in the HisOH-treated cells declined back to basal levels whereas it remained elevated in the Tg-treated cells. This pattern was observed in many repeated experiments with independent batches of cells. We have documented that following AAR activation the ATF3 mRNA is subject to post-transcriptional stabilization involving HuR and AUF1 RNA binding proteins [26]. When steady state ATF3 mRNA levels were measured in response to the two stress pathways, there was no difference between the pathways at 12 and 24 h (Fig. 1B). It was possible that the decline in transcription in the HisOH-treated cells was due to degradation of the AA alcohol, so two independent approaches were used to address this question. First, cells were either incubated in HisOH from time 0 with no media adjustments, as in Fig. 1A, or the medium was supplemented with fresh HisOH after 4 h (Fig. 1C). Adding fresh HisOH did not prevent the decline in transcription activity. Second, the HepG2 cells were incubated in DMEM lacking histidine to activate the AAR, instead of the HisOH treatment (Fig. 1D). A peak of transcription and then a decline back toward basal was once again observed, suggesting that this pattern was associated with the AAR pathway rather than the mechanism by which it was activated.
Fig. 1.
Temporal analysis of ATF3 transcription activity during the AAR and UPR. HepG2 cells were cultured in DMEM (Control) ± 2.5mM HisOH or±50 nM Tg for 0–24 h. Total RNA was isolated at the indicated times and RT-qPCR was performed to analyze the ATF3 transcriptional activity as measured by hnRNA (A) and steady-state mRNA levels of ATF3 (B). To verify that the decreased ATF3 transcriptional activity in DMEM+HisOH was not the result of HisOH degradation, transcriptional activity was measured in HepG2 cells in which the media in one half of the cells was supplemented with fresh 2.5mM HisOH at the 4 h time period (C) or in cells incubated in DMEM±histidine for 0–24 h (D). In all panels, GAPDH mRNA, which is not affected by the AAR or UPR, was used as an internal control. The results shown are the means±S.D. of at least three replicates. Each experiment was repeated to ensure reproducibility between batches of cells.
3.2 AAR or UPR induction of the ATF3 gene in human liver cells is mediated by one promoter
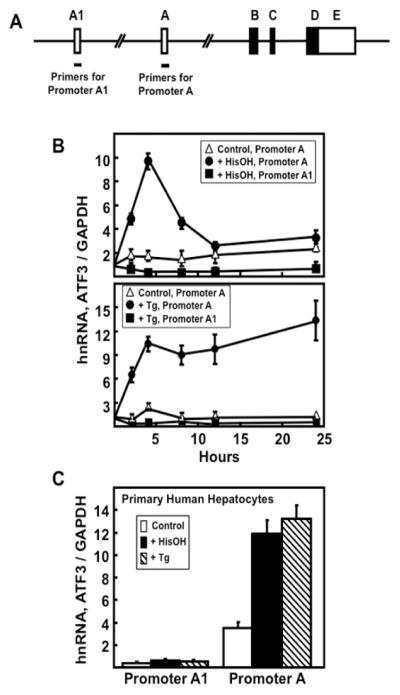
Miyazaka et al. [22] presented evidence for a second promoter (promoter A1) within the human ATF3 gene that was about 43 kb upstream of the canonical promoter (promoter A) (Fig. 2A). Using HCT116 colorectal carcinoma cells, Miyazaka et al. showed that ER stress induced expression from both promoters. Hagiya et al. [23] showed that both promoters were active in several adult T-cell leukemia cell lines. Neither of those studies investigated promoter usage in response to the AAR. To determine promoter activity in human liver-derived cells, we treated HepG2 hepatoma cells with either HisOH (Fig. 2B) or Tg (Fig. 2B) and measured hnRNA production from either promoter A1 or A using primers specific for an exon/intron junction unique for each. The results indicated that regardless of the stress pathway activated transcription from promoter A was increased, whereas there was little or no transcription from promoter A1. To determine if this result applied to non-transformed human liver, primary human hepatocytes were subjected to HisOH and Tg treatment for 4 h and then transcription activity from promoters A1 and A assayed (Fig. 2C). The results showed that for these primary human liver cells, only promoter A responded to the two stress pathways to a significant degree.
Figure 2.
Induction of the human liver ATF3 gene by the AAR and UPR stress pathways is associated with transcription from promoter A only. (A) Schematic showing ATF3 gene structure and primer locations for analysis of both promoters. (B) HepG2 hepatoma cells were cultured in DMEM (Control) and ATF3 transcription activity from promoter A1 or A was analyzed by measurement of hnRNA after treatment ± 2.5 mM HisOH or ± 50 nM Tg for 0–24 h. (C) Primary human hepatocytes were incubated with 2.5 mM HisOH or 50 nM Tg for 4 h and then ATF3 transcription was measured from promoters A1 and A. In all panels, GAPDH mRNA, which is not affected by the AAR or UPR, was used an internal control. The results shown are the means ± S.D. of at least three replicates and each experiment was repeated to ensure reproducibility between batches of cells.
3.3 Characterization of transcription factor recruitment to the ATF3 gene
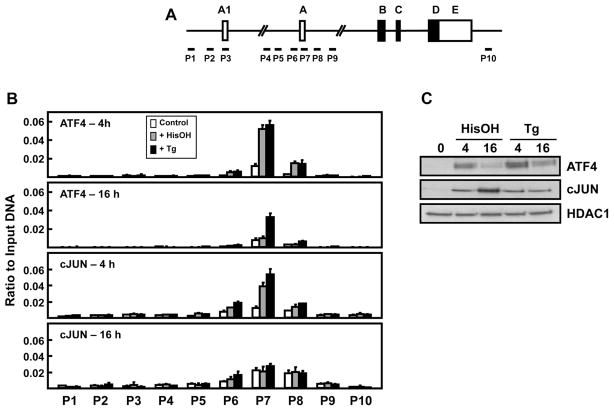
Following activation of either the AAR or UPR pathway, induction of the ATF3 gene is coincident with recruitment of specific transcription factors, among which are ATF4 and cJUN [19, 25]. These proteins interact with two enhancer elements, a CARE and a cyclic AMP response element (CRE) sequence, located immediately upstream of promoter A. Association of these factors was not tested in the work by Miyazaka et al. [22] or Hagiya et al. [23], so their contribution to expression driven by promoter A1 was unknown. To address this question, their binding at specific loci across the ATF3 gene was assessed using chromatin immunoprecipitation (ChIP), with particular attention to the A1 and A promoter regions (Fig. 3A). Cells were treated with either HisOH or Tg for 4 or 16 h to also gain insight into the temporal difference in transcription, as illustrated in Fig. 1. The data revealed that the gene association of ATF4, the primary AAR or UPR transcriptional activator of ATF3, is highly increased at 4 h, but only at promoter A (Fig. 3B). Little or no binding was observed near the promoter A1 region. Interestingly, at 16 h the abundance of ATF4 bound had returned to near the control level in the HisOH-treated cells, whereas it was still elevated in the Tg-treated cells. This difference parallels the decline in ATF3 gene transcription observed by hnRNA analysis for the AAR pathway (Fig. 1A). The changes in ATF4 bound at promoter A were relatively similar to the total abundance of nuclear ATF4 protein content as measured by immunoblotting, consistent with translational regulation of its synthesis and function (Fig. 3C). Collectively, these data document that ATF4 is not recruited to the region surrounding promoter A1 during either stress pathway and that in contrast to the UPR pathway, during the AAR there is transient ATF4 binding at the ATF3 gene.
Figure 3.
ATF4 and cJUN transcription factor binding across the ATF3 gene locus during activation of the AAR and UPR pathways. (A) Schematic showing ATF3 gene structure and primer locations for ChIP analysis at specific regions of the gene locus. (B) HepG2 cells were cultured in DMEM ± 2.5 mM HisOH or 50 nM Tg for 4 h and 16 h and then the cells were subjected to ChIP analysis with antibodies specific for either ATF4 or cJUN. The data are plotted as the ratio to input DNA values and are means ± S.D. for at least three replicates. (C) Immunoblot analysis of ATF4 and cJUN protein content was performed using nuclear extracts of HepG2 cells incubated in DMEM ± 2.5 mM HisOH or 50 nM Tg for 4 h and 16 h. Each experiment was repeated to ensure reproducibility between batches of cells.
A cJUN:ATF2 complex binds to a CRE enhancer immediately upstream of promoter A during activation of the AAR pathway [25]. ChIP analysis was used to investigate the association of cJUN with the ATF3 gene and the results indicate that there is no association of cJUN surrounding the promoter A1 region (Fig. 3B). Both stress pathways caused an increase in cJUN binding at promoter A after 4 h of treatment that returned to the control value by 16 h. Despite the lack of increased gene association at 16 h for cJUN, immunoblots showed elevated protein levels in the nucleus (Fig. 3C). These observations indicate that cJUN is likely not responsible for the observed temporal difference in ATF3 transcription at 16 h.
3.4 Association of RNA polymerase II with the ATF3 gene
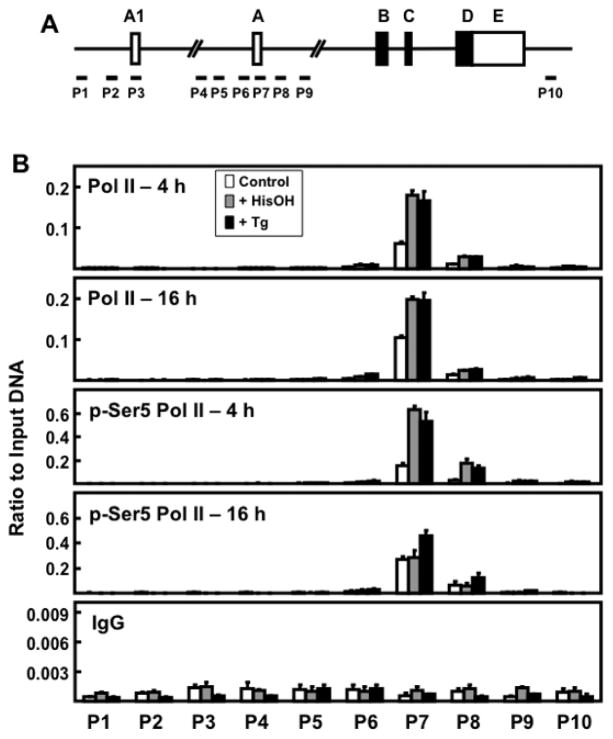
It is known that following AAR activation, there is de novo recruitment and increased association of RNA Pol II with the ATF3 gene [19]. ChIP analysis was employed to determine if this association occurred at both promoters (Fig. 4A) and if the amount of Pol II phosphorylated at serine 5 (p-Ser5 Pol II), which is associated with release of paused Pol II from the TSS region [30], was consistent with the transcription activity measured by hnRNA. The data revealed that Pol II was recruited to promoter A, not promoter A1, in response to activation of either the AAR or the UPR pathway (Fig. 4B). Total Pol II did not decline in amount from 4 to 16 h and was not different in abundance regardless of whether the stimulus was the AAR or the UPR (Fig. 4B). This result likely reflects that there is a sufficient amount of paused Pol II at promoter A to maintain transcription [30]. Consistent with the hnRNA measurements showing increased transcription from the ATF3 gene at 4 h, both stress pathways resulted in an increase in p-Ser5 Pol II at promoter A. However, in parallel with the decline in ATF3 transcription observed at 16 h for the AAR pathway (Fig. 1A), the abundance of p-Ser5 Pol II returned to the control value at 16 h during AAR activation, while the amount remained elevated for the UPR pathway (Fig. 4B). These results document that Pol II is not associated with promoter A1 in HepG2 cells for either stress pathway and that the abundance of the p-Ser5 Pol II form that signals release from the promoter reflects the temporal difference between the two pathways.
Figure 4.
RNA Pol II association across the ATF3 gene locus during AAR and UPR pathway activation. (A) Schematic showing ATF3 gene structure and primer locations for ChIP analysis at specific regions of the gene locus. (B) HepG2 cells were cultured in DMEM ± 2.5 mM HisOH or ± 50 nM Tg for 4 h or 16 h. The levels of total Pol II, p-Ser5 Pol II, and non-specific IgG were analyzed by ChIP at the ATF3 gene regions indicated. The data, plotted as the ratio to input DNA, are the means ± S.D. for at least three replicates and each experiment was repeated to ensure reproducibility between batches of cells.
3.5 Histone abundance and modification across the ATF3 gene locus
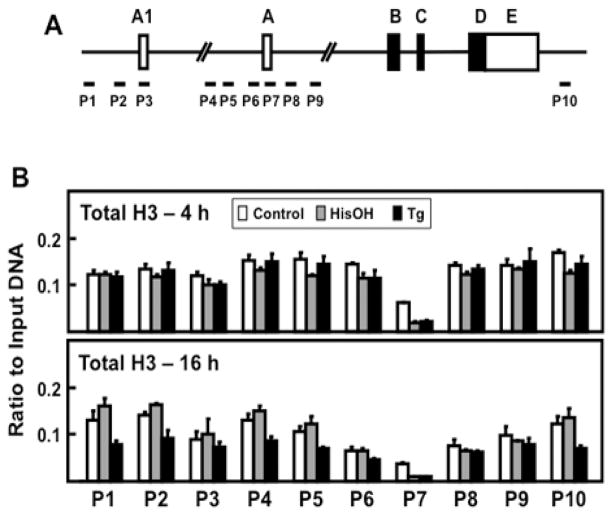
Histone proteins make up the nucleosomes that modulate the accessibility of protein regulatory factors to genomic DNA [31]. Chromatin-associated total histone H3 protein reflects nucleosome content and general location. There is a reduction of one or more nucleosomes at the TSS and specific histone modifications are associated with increased transcription. ChIP analysis was used to determine if changes in chromatin structure and activating histone modifications were associated with ATF3 promoter A1 or promoter A, and if they reflected the time course difference between the two stress signaling pathways. Analysis of the ATF3 gene locus for total H3 protein showed that the nucleosome abundance at promoter A1 was largely the same as that for non-promoter regions of the gene (Fig. 5B). In contrast, the nucleosome content was less at promoter A and it was reduced even further after activation of the AAR or UPR pathways (Fig. 5B). These observations are consistent with the current concept that the TSS is largely a nucleosome-free region of the gene and that any nucleosomes that may exist in this region can be repositioned during gene activation [31].
Figure 5.
Analysis of total H3 abundance across the ATF3 gene locus during activation of the AAR and UPR pathways. (A) Schematic showing ATF3 gene structure and primer locations for ChIP analysis at specific regions of the gene locus. (B) HepG2 cells were cultured in DMEM ± 2.5 mM HisOH or ± 50 nM Tg for 4 h or 16 h. The association of total histone H3, a reflection of nucleosome abundance, was assessed by ChIP analysis. The data, plotted as the ratio to input DNA, are the means ± S.D. for at least three replicates. Each experiment was repeated to ensure reproducibility between batches of cells.
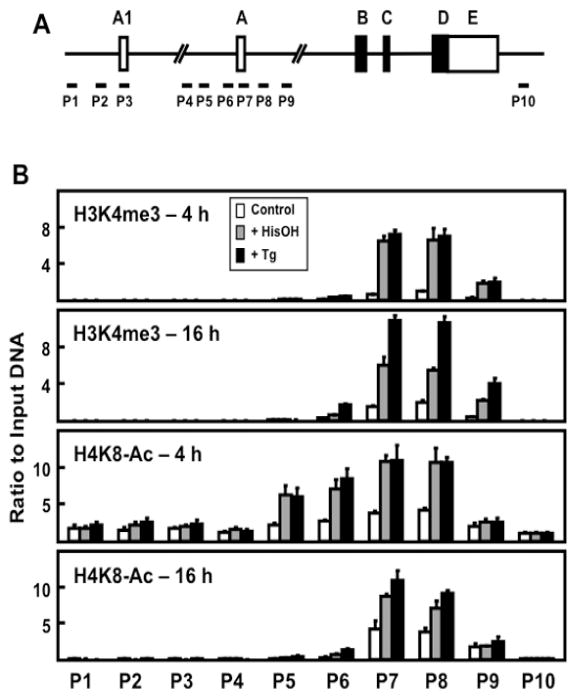
There are specific histone modifications associated with increased transcription. ChIP analysis for two of these, H3K4me3 and H4K8-Ac, was performed to scan across the ATF3 gene locus after activation of the AAR and UPR pathways in HepG2 cells (Fig. 6). The data reveal that neither modification is significantly detectable at promoter A1, but both are highly enriched at promoter A. Furthermore, both modifications were increased significantly at 4 h after treating the cells with either HisOH or Tg. Consistent with the transient nature of transcriptional activation by the AAR compared to the UPR (Fig. 1A), at 16 h the level of H3K4me3 and H4K8-Ac was less in HisOH-treated cells relative to Tg-treated cells (Fig. 6B).
Figure 6.
Analysis of H3K4me3 and H4K8Ac modifications across the ATF3 gene locus during the AAR and UPR pathways. (A) Schematic showing ATF3 gene structure and primer locations for ChIP analysis at specific regions of the gene locus. (B) HepG2 cells were cultured in DMEM ± 2.5 mM HisOH or ± 50 nM Tg for 4 or 16 h. The histone modifications H3K4me3 and H4K8-Ac were analyzed by ChIP at the ATF3 gene regions indicated. The data are plotted as the ratio to input DNA and the values for histone modifications were normalized to the signal obtained for total histone H3 at the respective regions (Figure 5). Values are means ± S.D. for at least three replicates and each experiment was repeated to ensure reproducibility between batches of cells.
DISCUSSION
The results presented in this report document three novel observations concerning stress-associated regulation of the human ATF3 gene in liver cells. 1) After AA deprivation, activation of ATF3 transcription is transient, peaking during the first 8 h and then returning toward the basal level, whereas after ER stress the transcription activity remains elevated for at least 24 h. 2) For both the AAR and the UPR stress pathways, there are specific transcription factor recruitment (ATF4 and cJUN) and epigenetic changes associated with promoter A that are not observed for the upstream promoter A1 locus. 3) Based on transcription activity and Pol II association, promoter A, appears to be responsible for ATF3 mRNA expression in primary human hepatocytes and HepG2 human hepatoma cells, both in the basal state as well as after induction of the gene by the AAR or UPR pathways.
Regulation of the ATF3 gene has been extensively investigated given the importance of ATF3 as a critical transcription factor in numerous disease related states [17]. Most of these studies have focused on the canonical promoter activity (termed promoter A in this report) located immediately upstream of Exon A [32]. However, Miyazaki et al. provided evidence that there is a second promoter (referred to as promoter A1 in this report) about 43 kb upstream of promoter A [22]. Those authors showed that the two promoters were differentially activated by cell type, drug treatment, transformation, and cell stress. Although they concluded that ER stress activated both promoters in HCT116 colorectal carcinoma cells, neither the AAR pathway nor liver tissue were reported in their studies. The present experiments show that in primary human hepatocytes and HepG2 human hepatoma cells, the upstream promoter A1 does not make a significant contribution to ATF3 expression. This conclusion is reached for the both the basal state as well as after activation of the AAR and UPR stress pathways.
RNA polymerase II (Pol II)-mediated transcription can be controlled through initiation, elongation, or termination [33]. Previously published data documented that ATF4 recruitment to the CARE immediately upstream of promoter A triggers de novo assembly of an active initiation complex [19]. In the present study, promoter A was activated by both the AAR and UPR pathways in HepG2 cells, but analysis of transcription activity versus steady state mRNA revealed an unexpected observation. Although the induced transcription after AA limitation was transient and only lasted about 8–12 h, expression from the gene after ER stress remained elevated for at least 24 h. ChIP analysis revealed that the difference in ATF3 transcription activity between the AAR and UPR after 16 h was paralleled by the degree of ATF4 association. ATF4 is known to activate the ATF3 gene by binding to a CARE enhancer sequence within promoter A (nt −23/−15) [19, 20]. cJUN binding was also increased within the promoter A region 4 h after activation of either the AAR or UPR pathways, but by 16 h the cJUN association decreased for both pathways equally. This result suggests that cJUN is not responsible for the continued activation by the UPR at 16 h. The abundance of total Pol II within promoter A was increased by both pathways and remained elevated even after 16 h. However, the abundance of p-Ser5 Pol II, the form associated with promoter release of Pol II to mediate transcription initiation [34], decreased after 16 h of AA limitation, but not after Tg treatment. Thus, the p-Ser5 form of Pol II also paralleled the hnRNA readout of ATF3 transcription activity supporting the interpretation that the two stress pathways exhibit different kinetics with regard to activation of the gene.
Active transcription is associated with characteristic changes in histone abundance and modification. Nucleosomes are positioned along the locus of a gene, but for active genes the area proximal to the TSS is usually devoid of nucleosomes [35]. For the ATF3 gene in HepG2 cells, the region surrounding promoter A1 was enriched in histone H3, whereas the promoter A region was depleted of histone H3. Furthermore, two histone modifications associated with active transcription, H3K4me3 and H4K8-Ac, were also enriched at promoter A, but not at promoter A1. Collectively, the results indicate that promoter A is responsible for both basal and induced transcription after activation of the AAR and UPR pathways in normal human hepatocytes and HepG2 hepatoma cells. These results are also consistent with the interpretation that mechanistically, ATF4 recruitment to the CARE within promoter A by either the AAR or UPR pathway is responsible for transcriptional control of the ATF3 gene via assembly of an active initiation complex.
HIGHLIGHTS.
AAR activation of ATF3 transcription is transient relative to activation by the UPR
In contrast to ATF3 promoter A, promoter A1 has minimal activity in human liver cells
Only promoter A exhibits stress-induced transcription factor and epigenetic changes
Acknowledgments
The authors thank the other members of the laboratory for helpful discussions. This research was supported by a grant to MSK from the National Cancer Institute (CA203565).
Abbreviations
- AA
amino acid
- AAR
AA response
- ATF
activating transcription factor
- C/EBP
CAAT/enhancer binding protein
- CARE
C/EBP-ATF response element
- CRE
cyclic AMP response element
- IF2
eukaryotic initiation factor 2
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GCN2
general control non-derepressible 2
- HisOH
histidinol
- ISR
integrated stress response
- PERK
PKR-like endoplasmic reticulum kinase
- RT-qPCR
real time quantitative PCR
- Tg
thapsigargin
- TSS
transcription start site
- UPR
unfolded protein response
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kilberg MS, Shan J, Su N. ATF4-Dependent Transcription Mediates Signaling of Amino Acid Limitation. Trends Endocrinol Metab. 2009;20:436–443. doi: 10.1016/j.tem.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 3.Endo Y, Fu Z, Abe K, Arai S, Kato H. Dietary protein quantity and quality affect rat hepatic gene expression. J Nutr. 2002;132:3632–3637. doi: 10.1093/jn/132.12.3632. [DOI] [PubMed] [Google Scholar]
- 4.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 5.Lee JI, Dominy JE, Jr, Sikalidis AK, Hirschberger LL, Wang W, Stipanuk MH. HepG2/C3A cells respond to cysteine-deprivation by induction of the amino acid deprivation/integrated stress response pathway. Physiol Genomics. 2008;33:218–229. doi: 10.1152/physiolgenomics.00263.2007. [DOI] [PubMed] [Google Scholar]
- 6.Deval C, Chaveroux C, Maurin AC, Cherasse Y, Parry L, Carraro V, Milenkovic D, Ferrara M, Bruhat A, Jousse C, Fafournoux P. Amino acid limitation regulates the expression of genes involved in several specific biological processes through GCN2-dependent and GCN2-independent pathways. FEBS J. 2009;276:707–718. doi: 10.1111/j.1742-4658.2008.06818.x. [DOI] [PubMed] [Google Scholar]
- 7.Shan J, Lopez MC, Baker HV, Kilberg MS. Expression profiling after activation of the amino acid deprivation response in HepG2 human hepatoma cells. Physiol Genomics. 2010;41:315–327. doi: 10.1152/physiolgenomics.00217.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 9.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 10.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 11.Wolfgang CD, Chen BP, Martindale JL, Holbrook NJ, Hai T. gadd153/Chop10, a potential target gene of the transcriptional repressor ATF3. Mol Cell Biol. 1997;17:6700–6707. doi: 10.1128/mcb.17.11.6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fawcett TW, Martindale JL, Guyton KZ, Hai T, Holbrook NJ. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J. 1999;339:135–141. [PMC free article] [PubMed] [Google Scholar]
- 13.Gjymishka A, Palii SS, Shan J, Kilberg MS. Despite increased ATF4 binding at the C/EBP-ATF composite site following activation of the unfolded protein response, system A transporter 2 (SNAT2) transcription activity is repressed in HepG2 cells. J Biol Chem. 2008;283:27736–27747. doi: 10.1074/jbc.M803781200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, Kilberg MS, Sartor MA, Kaufman RJ. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nature Cell Biol. 2013;15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dang Do AN, Kimball SR, Cavener DR, Jefferson LS. eIF2alpha kinases GCN2 and PERK modulate transcription and translation of distinct sets of mRNAs in mouse liver. Physiol Genomics. 2009;38:328–341. doi: 10.1152/physiolgenomics.90396.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson MR, Xu D, Williams BR. ATF3 transcription factor and its emerging roles in immunity and cancer. J Mol Med. 2009;87:1053–1060. doi: 10.1007/s00109-009-0520-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hai T, Wolford CC, Chang YS. ATF3, a hub of the cellular adaptive-response network, in the pathogenesis of diseases: is modulation of inflammation a unifying component? Gene Expr. 2010;15:1–11. doi: 10.3727/105221610x12819686555015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang HY, Wek SA, McGrath BC, Lu D, Hai TW, Harding HP, Wang XZ, Ron D, Cavener DR, Wek RC. Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol Cell Biol. 2004;24:1365–1377. doi: 10.1128/MCB.24.3.1365-1377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan YX, Chen H, Thiaville MM, Kilberg MS. Activation of the ATF3 gene through a co-ordinated amino acid-sensing response programme that controls transcriptional regulation of responsive genes following amino acid limitation. Biochem J. 2007;401:299–307. doi: 10.1042/BJ20061261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan YX, Chen H, Siu F, Kilberg MS. Amino acid deprivation and endoplasmic reticulum stress induce expression of multiple ATF3 mRNA species which, when overexpressed in HepG2 cells, modulate transcription by the human asparagine synthetase promoter. J Biol Chem. 2003;278:38402–38412. doi: 10.1074/jbc.M304574200. [DOI] [PubMed] [Google Scholar]
- 21.Kimura K, Wakamatsu A, Suzuki Y, Ota T, Nishikawa T, Yamashita R, Yamamoto J, Sekine M, Tsuritani K, Wakaguri H, Ishii S, Sugiyama T, Saito K, Isono Y, Irie R, Kushida N, Yoneyama T, Otsuka R, Kanda K, Yokoi T, Kondo H, Wagatsuma M, Murakawa K, Ishida S, Ishibashi T, Takahashi-Fujii A, Tanase T, Nagai K, Kikuchi H, Nakai K, Isogai T, Sugano S. Diversification of transcriptional modulation: large-scale identification and characterization of putative alternative promoters of human genes. Genome Res. 2006;16:55–65. doi: 10.1101/gr.4039406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyazaki K, Inoue S, Yamada K, Watanabe M, Liu Q, Watanabe T, Adachi MT, Tanaka Y, Kitajima S. Differential usage of alternate promoters of the human stress response gene ATF3 in stress response and cancer cells. Nucleic Acids Res. 2009;37:1438–1451. doi: 10.1093/nar/gkn1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagiya K, Yasunaga J, Satou Y, Ohshima K, Matsuoka M. ATF3, an HTLV-1 bZip factor binding protein, promotes proliferation of adult T-cell leukemia cells. Retrovirology. 2011;8:19. doi: 10.1186/1742-4690-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu L, Balasubramanian M, Shan J, Dudenhausen EE, Kilberg MS. Auto-activation of c-JUN gene by amino acid deprivation of hepatocellular carcinoma cells reveals a novel c-JUN-mediated signaling pathway. J Biol Chem. 2011;286:36724–36738. doi: 10.1074/jbc.M111.277673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu L, Kilberg MS. Elevated cJUN expression and an ATF/CRE site within the ATF3 promoter contribute to activation of ATF3 transcription by the amino acid response. Physiol Genomics. 2013;45:127–137. doi: 10.1152/physiolgenomics.00160.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan YX, Chen H, Kilberg MS. Interaction of RNA-binding Proteins HuR and AUF1 with the Human ATF3 mRNA 3′-Untranslated Region Regulates Its Amino Acid Limitation-induced Stabilization. J Biol Chem. 2005;280:34609–34616. doi: 10.1074/jbc.M507802200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shan J, Fu L, Balasubramanian MN, Anthony T, Kilberg MS. ATF4-Dependent Regulation of the JMJD3 Gene During Amino Acid Deprivation Can be Rescued in Atf4-Deficient Cells by Inhibition of Deacetylation. J Biol Chem. 2012;287:36393–36403. doi: 10.1074/jbc.M112.399600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipson KE, Baserga R. Transcriptional activity of the human thymidine kinase gene determined by a method using the polymerase chain reaction and an intron-specific probe. Proc Natl Acad Sci USA. 1989;86:9774–9777. doi: 10.1073/pnas.86.24.9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su N, Pan YX, Zhou M, Harvey RC, Hunger SP, Kilberg MS. Correlation between asparaginase sensitivity and asparagine synthetase protein content, but not mRNA, in acute lymphoblastic leukemia cell lines. Pediatr Blood Cancer. 2008;50:274–279. doi: 10.1002/pbc.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jonkers I, Lis JT. Getting up to speed with transcription elongation by RNA polymerase II. Nat Rev Mol Cell Biol. 2015;16:167–177. doi: 10.1038/nrm3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai WKM, Pugh BF. Understanding nucleosome dynamics and their links to gene expression and DNA replication. Nat Rev Mol Cell Biol. 2017 doi: 10.1038/nrm.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang G, Wolfgang CD, Chen BP, Chen TH, Hai T. ATF3 gene. Genomic organization, promoter, and regulation. J Biol Chem. 1996;271:1695–1701. doi: 10.1074/jbc.271.3.1695. [DOI] [PubMed] [Google Scholar]
- 33.Adelman K, Lis JT. Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans. Nat Rev Genet. 2012;13:720–731. doi: 10.1038/nrg3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harlen KM, Churchman LS. The code and beyond: transcription regulation by the RNA polymerase II carboxy-terminal domain. Nat Rev Mol Cell Biol. 2017;18:263–273. doi: 10.1038/nrm.2017.10. [DOI] [PubMed] [Google Scholar]
- 35.Lorch Y, Kornberg RD. Chromatin-remodeling and the initiation of transcription. Q Rev Biophys. 2015;48:465–470. doi: 10.1017/S0033583515000116. [DOI] [PubMed] [Google Scholar]