Abstract
BACKGROUND
Atrial fibrillation (AF) occurs in many clinical contexts and is diagnosed and treated by clinicians across many specialties, which has been associated with treatment variation.
OBJECTIVES
We evaluated the association of treating specialty with AF outcomes among patients with newly-diagnosed AF.
METHODS
Using complete data of the TREAT-AF (The Retrospective Evaluation and Assessment of Therapies in AF) study from the Veterans Health Administration, we identified patients with newly diagnosed, nonvalvular AF between 2004-2012 and at least one outpatient encounter with primary care or cardiology within 90 days of AF diagnosis. Cox proportional hazards regression was used to evaluate association between treating specialty and AF outcomes.
RESULTS
Among 184,161 patients with newly-diagnosed AF (age 70±11; 1.7% women; CHADS-VASc 2.6±1.7), 40% received cardiology care and 60% received primary care only. After adjustment for covariates, cardiology care was associated with reductions in stroke (HR 0.91, 95% CI 0.86-0.96, p <0.001) and death (HR 0.89; 95% CI 0.88-0.91; p <0.0001) and increases in AF/SVT (HR 1.38; 95% CI 1.35-1.42; p <0.0001) and MI (HR 1.03; 95% CI 1.00-1.05; p <0.04). The propensity matched cohort had similar results. In mediation analysis, oral anticoagulation (OAC) prescription within 90 days of diagnosis may have mediated reductions in stroke but did not mediate reductions in survival.
CONCLUSIONS
In patients with newly-diagnosed AF, cardiology care was associated with improved outcomes, potentially mediated by early OAC prescription. Although hypothesis-generating, these data warrant serious consideration and study of health care system interventions at the time of new AF diagnosis.
Keywords: Atrial fibrillation, stroke
INTRODUCTION
Atrial fibrillation (AF), the second most common cardiovascular condition after hypertension,(1,2) requires management in diverse clinical contexts challenging providers of varied backgrounds with complex treatment decisions. Primary care providers and cardiologists, including cardiology subspecialties such as electrophysiology, are tasked with caring for the majority of AF patients and managing their increased risk of stroke, myocardial infarction (MI), heart failure, and death.(3–7) Oral anticoagulation (OAC), with warfarin or non-vitamin K oral anticoagulants (NOAC), has been shown to prevent stroke.(8,9) However, OAC prescription is not uniform across treating specialties, with cardiology care associated with higher odds of receipt of OAC.(10–13) Similarly, rates of antiarrhythmic prescription are higher in cardiology treated patients.(11,12) Association of treating specialty with other aspects of AF care (e.g., catheter ablation) have not been adequately studied.
Despite these data, there has been limited investigation into health care interventions, such as treating specialty assignment, on outcomes of AF.(14) Therefore, using observational data we sought to evaluate the association of treating specialty on clinical outcomes in AF.
METHODS
The Retrospective Evaluation and Assessment of Therapies in AF (TREAT-AF) study is a retrospective cohort study of patients with newly diagnosed AF treated in the Department of Veterans Affairs (VA) national health care system between October 1, 2003 and September 30, 2012 (VA fiscal years 2004-2012). Data sets used represent the claims data and electronic health records covering the full denominator of VA users. These include data from the VA National Patient Care Database,(15) the VA Decision Support System national pharmacy extract,(16) the VA Fee Basis Inpatient and Outpatient data sets,(16) the VA Laboratory Decision Support System extract,(17) and the VA Vital Status File, which contains validated combined mortality data from VA, Medicare, and Social Security Administration sources.(18,19) Methods for cohort creation have been previously described in detail.(11,20)
We defined patients with newly-diagnosed AF as having a primary or secondary diagnosis of AF (International Classification of Diseases, Ninth Revision [ICD-9], 427.31 or 427.32) associated with an inpatient or outpatient VA encounter, no prior diagnosis of AF within 4 years, and a second confirmatory diagnosis of AF between 30 and 365 days after index AF diagnosis. We excluded patients if 1) they were not seen in outpatient cardiology or primary care clinics within 90 days of index AF diagnosis; 2) they were not seen in outpatient cardiology or primary care clinics within the continental U.S.; 3) they did not receive any outpatient prescriptions within 90 days of index AF diagnosis; 4) they died within 120 days of index AF diagnosis; or 5) age and gender were not available.
The primary predictor was outpatient treating specialty. The methodology has been previously detailed and applied.(11) Patients seen in a cardiology clinic within 90 days of AF diagnosis, regardless of whether they were seen in primary care clinics, were categorized as having received cardiology care. Patients seen in primary care clinics within 90 days of AF diagnosis, who were not seen in a cardiology clinic within 90 days of AF diagnosis, were categorized as having received primary care only. For cardiology clinics, we included encounters from general and specialty cardiology clinics (e.g., heart failure, electrophysiology), which are classified by a single clinic type code, but not cardiac surgery or hypertension clinics as they are typically not staffed by cardiologists in the VA system. For primary care clinics, we only included encounters from clinics designated as general internal medicine or primary care.
The primary outcome was ischemic stroke. Secondary outcomes included death and cardiovascular hospitalizations (transient ischemic attack (TIA), heart failure, AF or supraventricular tachycardia (SVT), MI). We determined incidence of stroke and other cardiovascular hospitalizations from inpatient claims and medical record data using validated and previously applied algorithms.(21) For ascertainment of death, we used the VA Vital Status file which has previously been shown to have 97.6% agreement and 98.3% sensitivity for detection of deaths identified by the National Death Index.(19) Because cause of death was not available, we also looked at deaths preceded by a cardiovascular hospitalization within 30 days. ICD-9 codes used to define cardiovascular death and hospitalizations are available in the supplemental material (Supplemental Table 1) and have also been previously detailed.(22)
We performed Cox regression to estimate hazard ratios for outcomes of interest, adjusting for patient demographics (age, patient distance to clinic/medical center, race, sex, VA priority status), patient comorbidities (Charlson and Selim comorbidity index, diabetes, glomerular filtration rate, heart failure, hypertension, MI, stroke/TIA,), cardiovascular non-AF medications (antiplatelet, ACE inhibitor/angiotensin receptor blocker, diuretics, niacin/fibrates, statins), and anticoagulation, rate control, and antiarrhythmic medications (amiodarone, anticoagulation [warfarin/NOAC], beta blockers, calcium channel blockers, class I agents, class III agents, digoxin).
In parallel, we also performed a propensity-matched analysis, applying multivariate logistic regression to develop a propensity score using all baseline covariates to predict the probability of evaluation in cardiology clinic. Cox regression was performed in a 1:1 matched cohort using nearest-neighbor matching without replacement. Methods for propensity matched cohort creation and analysis have been previously described in detail.(11,20)
Mediation analysis, using multivariate logistic regression, was performed to determine whether the association of cardiology care on outcomes was mediated by receipt of anticoagulation (OAC) within 90-days of AF diagnosis. Mediation was assessed for in a stepwise fashion using the Baron and Kenny approach.(23) All analyses were performed using SAS, version 9.2 (Cary, NC) and STATA, version 11.0 (College Station, TX). The study was approved by the local Institutional Review Board.
RESULTS
The full cohort included 184,161 newly-diagnosed AF patients (age 70±11; 1.7% women; CHADS-VASc 2.6±1.7) (Figure 1), of which, 69,901 received cardiology care and 114,260 received primary care only within 90-days of AF diagnosis. Cardiology-treated patients, compared to primary care only, lived closer to medical centers, were slightly younger, and, despite similar CHADS-VASc scores, had a higher baseline prevalence of hypertension, diabetes, coronary disease, MI, and stroke (Table 1).
Figure 1. Flow Diagram.
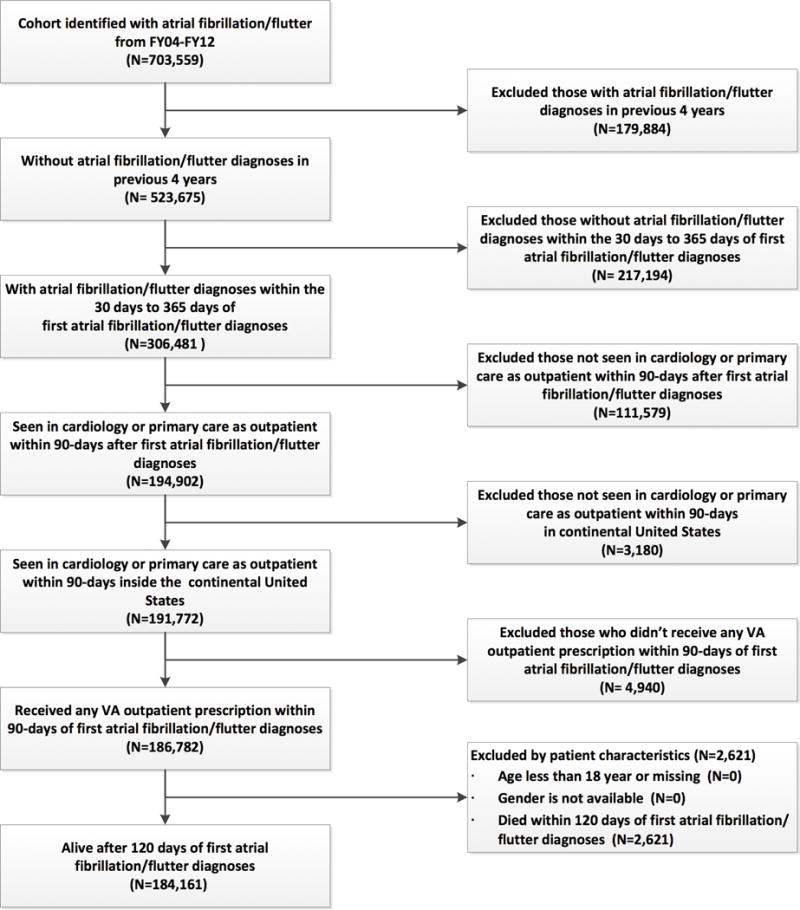
Inclusion and exclusion criteria used to select analysis cohort.
Table 1.
Baseline Characteristics
| Demographics | Full Cohort (N=184,161) |
Propensity Matched Cohort (N=106,080) |
||||
|---|---|---|---|---|---|---|
| Primary Care Only (N=114,260) |
Cardiology Care (N=69,901) |
P value | Primary Care Only (N=53,040) |
Cardiology Care (N=53,040) |
Standardized Difference* | |
| Age (mean±SD) | 71.6 ± 10.6 | 68.5 ± 10.5 | <0.0001 | 68.8 ± 10.4 | 68.6 ± 10.5 | 0.0276 |
| FemaleRace | 1,779 (1.6%) | 1,257 (1.8%) | <0.0001 | 925 (1.7%) | 967 (1.8%) | 0.0174 |
| White | 102,519 (90.5%) | 61,082 (88.2%) | <0.0001 | 46,899 (88.4%) | 46,864 (88.4%) | 0.0021 |
| Comorbidity Index | ||||||
| Charlson | 1.67 ± 1.52 | 1.87 ± 1.66 | <0.0001 | 1.90± 1.67 | 1.91± 1.68 | 0.0046 |
| Selim | 3.89 ± 2.48 | 4.29 ± 2.69 | <0.0001 | 4.35 ± 2.68 | 4.36± 2.70 | 0.0024 |
| CHADS2 Score Group | ||||||
| CHADS 0–1 | 57,337 (50.2%) | 34,209 (43.9%) | <0.0001 | 25,433 (48.0%) | 25,542 (48.2%) | 0.0041 |
| CHADS 2–3 | 48,973 (42.9%) | 30,354 (43.4%) | <0.0001 | 23,358 (44.0%) | 23,303 (43.9%) | 0.0021 |
| CHADS 4–6 | 7,950 (7.0%) | 5,338 (7.6%) | <0.0001 | 4,249 (8.0%) | 4,195 (7.9%) | 0.0038 |
| CHADS2 Score (mean±SD) | 1.59 ± 1.21 | 1.63 ± 1.24 | <0.0001 | 1.66 ± 1.25 | 1.65± 1.24 | 0.0024 |
| CHA2DS2-VASc Score (mean±SD) | 2.64 ± 1.65 | 2.61 ± 1.73 | 0.0037 | 2.66 ± 1.73 | 2.65 ± 1.73 | 0.0085 |
| Anemia | 21,771 (19.1%) | 13,774 (19.7%) | 0.0006 | 11,535 (21.8%) | 10,679 (20.1%) | 0.0391 |
| Heart Failure | 15,240 (13.3%) | 13,829 (19.8%) | <0.0001 | 10,126 (19.1%) | 10,715 (20.2%) | 0.028 |
| Hypertension | 70,101 (61.4%) | 47,704 (68.3%) | <0.0001 | 36,593 (69.0%) | 36,654 (69.1%) | 0.0025 |
| Prior MI | 33,896 (29.7%) | 24,915 (35.6%) | <0.0001 | 18,754 (35.4%) | 19,339 (36.5%) | 0.023 |
| Diabetes | 34,399 (30.1%) | 23,553 (33.7%) | <0.0001 | 18,291 (34.5%) | 18,177 (34.3%) | 0.0045 |
| Prior Stroke/TIA | 8,140 (7.1%) | 5,479 (7.8%) | <0.0001 | 4,280 (8.1%) | 4,242 (8.0%) | 0.0026 |
| Coronary Artery Disease | 33,318 (29.2%) | 24,384 (34.9%) | <0.0001 | 18,392 (34.7%) | 18,924 (35.7%) | 0.021 |
| Peripheral Artery Disease | 7,000 (6.1%) | 4,945 (7.1%) | <0.0001 | 4,111 (7.8%) | 3,868 (7.3%) | 0.0174 |
| Access to care (miles) | ||||||
| Distance to nearest outpatient facility within network boundary | 13.1 ± 13.1 | 12.8 ± 12.1 | <0.0001 | 13.2 ± 12.8 | 12.8 ± 12.2 | 0.0307 |
| Distance to nearest medical center facility within network boundary | 41.99 ± 45.3 | 34.2 ± 40.0 | <0.0001 | 35.0 ± 40.1 | 34.2 ± 40.2 | 0.0191 |
| Differential distance | 28.9 ± 42.4 | 21.4 ± 36.9 | <0.0001 | 21.8 ± 37.0 | 21.4 ± 37.1 | 0.0103 |
| Medication Use | ||||||
| Alpha Blockers | 3,051 (2.7%) | 1,895 (2.7%) | 0.5996 | 1,729 (3.3%) | 1,442 (2.7%) | 0.0318 |
| Beta Blockers | 71,070 (62.2%) | 54,473 (77.9%) | <0.0001 | 41,350 (78.0%) | 41.571 (78.4%) | 0.0101 |
| Calcium Channel Blockers | 40,890 (35.8%) | 27,589 (39.5%) | <0.0001 | 19,675 (37.1%) | 27,589 (39.5%) | 0.0521 |
| Rate Control Agents | 91,939 (80.5%) | 62,984 (90.1%) | <0.0001 | 47,334 (89.2%) | 47,944 (90.4%) | 0.038 |
| Amiodarone or Dronedarone | 7,881 (6.9%) | 9,731 (13.9%) | <0.0001 | 4,136 (7.8%) | 7,338 (13.8%) | 0.1953 |
| Digoxin | 23,283 (20.4%) | 14,923 (21.4%) | <0.0001 | 10,965 (20.7%) | 11,329 (21.4%) | 0.0168 |
| Class I Drugs | 1,625 (1.4%) | 1,983 (2.8%) | <0.0001 | 705 (1.3%) | 1,439 (2.7%) | 0.0985 |
| Class III Drugs | 3,309 (2.9 %) | 3,174 (4.5%) | <0.0001 | 1,385 (2.6%) | 2,390 (4.5%) | 0.1024 |
| Rhythm Control Agents | 12,611 (11.0%) | 14,557 (20.8%) | <0.0001 | 6,115 (11.5%) | 10,917 (20.6%) | 0.2485 |
| Anti-Platelet Agents | 32,676 (28.6%) | 29,774 (42.6%) | <0.0001 | 21,639 (40.8%) | 22,767 (42.9%) | 0.0431 |
| NOAC | 593 (0.5%) | 715 (1.0%) | <0.0001 | 230 (0.4%) | 524 (1.0%) | 0.066 |
| Warfarin | 66,648 (58.3%) | 48,583 (69.5%) | <0.0001 | 36,584 (69.0%) | 37,159 (70.1%) | 0.0236 |
| Anticoagulation (Warfarin or NOAC) | 67,142 (58.8%) | 49,138 (70.3%) | <0.0001 | 36,755 (69.3%) | 37,565 (70.8%) | 0.0333 |
| Statins | 66,371 (58.1%) | 45,836 (65.6%) | <0.0001 | 34,515 (65.1%) | 34.990 (66.0%) | 0.0188 |
| Niacin/Fibrates | 8,417 (7.4%) | 6,232 (8.9%) | <0.0001 | 4,703 (8.9%) | 4,771 (9.0%) | 0.0045 |
| ACE-I/ARB | 65,707 (57.5%) | 46,119 (66.0%) | <0.0001 | 34,610 (65.3%) | 35,211 (66.4%) | 0.0239 |
| Diuretics | 59,385 (52.0%) | 40,652 (58.1%) | <0.0001 | 30,695 (57.9%) | 31,120 (58.7%) | 0.0163 |
ACE-I: angiotensin-converting-enzyme inhibitor, ARB: angiotensin receptor blocker, MI: myocardial infarction, NOAC: non-vitamin K oral anticoagulant, SD: standard deviation, TIA: transient ischemic attack
Standardized differences were calculated by dividing the difference in mean outcome between groups by the standard deviation of outcome among participants
Cardiology-treated patients, compared to those treated only by primary care, had a substantially higher proportion of 90-day receipt of OAC (70.3% vs. 58.8%, p < 0.0001), AF rate control agents (90.1% vs. 80.5%, p < 0.0001), and AF rhythm control agents (20.8% vs. 11.0%, p < 0.0001) (Table 1). Cardiology treated patients also received higher proportion of receipt of anti-platelet agents (42.6% vs. 28.6%, p < 0.0001); statins (65.6% vs. 58.1%, p < 0.0001), and ACE-I/ARB (66.0% vs. 57.5%, p < 0.0001).
Patients that received cardiology care, as compared to primary care only, had lower unadjusted incidence rates of stroke (7.6 vs. 8.8 per 1,000 person-years, p < 0.0001), overall mortality (69.4 vs. 85.3 per 1,000 person-years, p < 0.0001), and mortality preceded by cardiovascular hospitalization within 30 days (15.5 vs. 18.8 per 1,000 person-years, p < 0.0001) (Table 2). However, cardiology treated patients had higher unadjusted incidence rates of hospitalizations for heart failure (53.4 vs. 44.1 per 1,000 person-years, p < 0.0001), AF/SVT (78.2 vs. 43.1 per 1,000 person-years, p < 0.0001), and MI (57.8 vs. 45.7 per 1,000 person-years, p < 0.0001). There were no differences in rates of TIA between cardiology and primary care treated patients. In the propensity matched cohort analysis, incidence rates were similar without substantial attenuation (Table 2). Primary cardiovascular discharge diagnoses for hospitalizations within 30 days of cardiovascular death are presented in Supplemental Table 2.
Table 2.
Incidence of Mortality and Adverse Events in Newly-Diagnosed AF by Treating Specialty
| Full Cohort | Total (N=184,161) |
Primary Care Only (N=114,260) |
Cardiology Care (N=69,901) |
||||
|---|---|---|---|---|---|---|---|
| N (%) | IR* | N (%) | IR* | N (%) | IR* | P value | |
| Stroke | 6,364 (3.5) | 8.4 | 4,230 (3.7) | 8.8 | 2,134 (3.1) | 7.6 | <0.0001 |
| Overall Mortality | 61,677 (33.5) | 79.5 | 41,977 (36.7) | 85.3 | 19,700 (28.2) | 69.4 | <0.0001 |
| Cardiovascular mortality! | 13,641 (7.4) | 17.6 | 9,240 (8.1) | 18.8 | 4,401 (6.3) | 15.5 | <0.0001 |
| TIA | 2,300 (1.3) | 3.0 | 1,462 (1.3) | 3.0 | 848 (1.2) | 3.0 | 0.87 |
| Heart Failure | 33,028 (17.9) | 47.4 | 19,614 (17.2) | 44.1 | 13,414 (19.2) | 53.4 | <0.0001 |
| AF/SVT | 36,068 (19.6) | 55.1 | 18,521 (16.2) | 43.1 | 17,547 (25.1) | 78.2 | <0.0001 |
| Myocardial Infarction | 33,640 (18.3) | 50.1 | 19,701 (17.2) | 45.7 | 13,939 (19.9) | 57.8 | <0.0001 |
|
| |||||||
| Propensity Matched Cohort |
Total (N=106,080) |
Primary Care Only (N=53,040) |
Cardiology Care (N=53,040) |
||||
| N (%) | IR* | N (%) | IR* | N (%) | IR* | P value | |
|
| |||||||
| Stroke | 3,540 (3.3) | 8.0 | 1,908 (3.6) | 8.4 | 1,596 (3.0) | 7.5 | 0.0003 |
| Overall Mortality | 33,308 (31.4) | 74.4 | 18,175 (34.3) | 78.5 | 15,133 (28.5) | 70.0 | <0.0001 |
| Cardiovascular mortality† | 7,432 (7.0) | 16.6 | 4,034 (7.6) | 17.4 | 3,398 (6.4) | 15.7 | <0.0001 |
| TIA | 1,371 (1.3) | 3.1 | 705 (1.3) | 3.1 | 666 (1.3) | 3.1 | 0.88 |
| Heart Failure | 20,572 (19.4) | 51.8 | 10,206 (19.2) | 49.5 | 10,366 (19.5) | 54.4 | <0.0001 |
| AF/SVT | 23,828 (22.5) | 65.0 | 10,245 (19.3) | 52.1 | 13,583 (25.6) | 79.9 | <0.0001 |
| Myocardial Infarction | 21,315 (20.1) | 55.9 | 10,530 (19.9) | 53.1 | 10,785 (20.3) | 58.9 | <0.0001 |
AF: atrial fibrillation, IR: incidence rate, SVT: supraventricular tachycardia, TIA: transient ischemic attack
Incidence per 1,000 person-years,
Death preceded by cardiovascular hospitalization within 30 days
After adjustment for covariates, cardiology care was associated with lower risk of stroke (HR 0.91; 95% CI 0.86-0.96; p 0.001), overall mortality (HR 0.89; 95% CI 0.88-0.91; p < 0.0001), and mortality preceded by cardiovascular hospitalization within 30 days (HR 0.88; 95% CI 0.84-0.91; p < 0.0001). Cardiology care was associated with increased risk of hospitalization for AF/SVT (HR 1.38; 95% CI 1.35-1.42; p < 0.0001) and MI (HR 1.03; 95% CI 1.00-1.05; p < 0.04). There was no significant association with risk of heart failure hospitalization or TIA. In the propensity matched cohort, results were similar to the full cohort with the exception of cardiology care being associated with a small, but significant, increased risk of heart failure hospitalization (HR 1.07; 95% CI 1.04-1.10; p < 0.0001) (Table 3).
Table 3.
Association of Cardiology Care to Mortality and Adverse Events in Newly-Diagnosed AF
| Full Cohort
|
Propensity Matched Cohort
|
|||||
|---|---|---|---|---|---|---|
| Univariate
|
Multivariate*
|
|||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Stroke | 0.86 (0.82–0.91) | <0.0001 | 0.91 (0.86–0.96) | 0.001 | 0.88 (0.83–0.94) | 0.0003 |
| Overall Mortality | 0.82 (0.81–0.84) | <0.0001 | 0.89 (0.88–0.91) | <0.0001 | 0.90 (0.88–0.92) | <0.0001 |
| Cardiovascular mortality† | 0.82 (0.79–0.85) | <0.0001 | 0.88 (0.84–0.91) | <0.0001 | 0.90 (0.86–0.94) | <0.0001 |
| TIA | 0.99 (0.87–0.93) | 0.87 | 0.95 (0.87–1.04) | 0.27 | 0.99 (0.89–1.10) | 0.88 |
| Heart Failure | 1.18 (1.16–1.21) | <0.0001 | 1.02 (1.00–1.05) | 0.088 | 1.07 (1.04–1.10) | <0.0001 |
| AF/SVT | 1.71 (1.67–1.74) | <0.0001 | 1.38 (1.35–1.42) | <0.0001 | 1.44 (1.41–1.48) | <0.0001 |
| Myocardial Infarction | 1.23 (1.20–1.26) | <0.0001 | 1.03 (1.00–1.05) | 0.04 | 1.08 (1.05–1.11) | <0.0001 |
AF: atrial fibrillation, HR: hazard ratio, SVT: supraventricular tachycardia), TIA: transient ischemic attack
Multivariate model includes: demographics (age, patient distance to clinic/medical center, race, sex, and VA priority status), comorbidities (Charlson comorbidity index, diabetes, heart failure, hypertension, Selim comorbidity index, stroke/transient ischemic attack, and glomerular filtration rate), non AF medications (antiplatelet, ACE inhibitor/angiotensin receptor blocker, diuretics, niacin/fibrates, and statins), and AF medications (amiodarone, anticoagulation [warfarin/novel oral anticoagulant], beta blockers, calcium channel blockers, class I agents, class III agents, digoxin),
Death preceded by cardiovascular hospitalization within 30 days
In mediation analysis, there was a statistical trend for partial mediation of the association of cardiology care to reduced risk of stroke by 90-day OAC receipt (indirect effect 0.957, 95% CI 0.909-1.007, p = 0.09) (Supplemental Table 3). Results were similar in the propensity-matched cohort (indirect effect 0.932, 95% CI 0.868-1.001, p = 0.0538). Mediation effects were nonsignificant for death or death preceded by cardiovascular hospitalization within 30 days.
DISCUSSION
In patients with newly-diagnosed AF in the VA health care system, cardiology care, as compared to primary care only, within 90 days of AF diagnosis was associated with reduced risk of stroke, death, and death preceded by cardiovascular hospitalization within 30 days. However, there was increased risk of hospitalization for AF/SVT and MI. Early OAC prescription (within 90 days of AF diagnosis) was substantially higher in cardiology treated patients (70.3% vs. 58.8%, p <0.0001), which may have partially mediated reductions in stroke but did not mediate reduction in death.
Previous studies have investigated the effect of treating specialty on OAC prescription, a cornerstone of AF management and plausible mediator for improved outcomes. In a previous analysis of the TREAT-AF cohort using data from 2004-2008, cardiology care was associated with a higher rate of warfarin prescription after adjustment for covariates and site-level factors (OR 2.05; 95% CI 1.99-2.11; p < 0.0001).(11) Similar results were obtained in AF patients with prior stroke (CHADS-VASc ≥ 2) in the AFNET registry, which found non-cardiology care associated with a lower rate of warfarin prescription after adjustment for covariates (OR 0.40; 95 % CI 0.21-0.77; p < 0.001).(13) The ORBIT-AF registry found a non-significant trend towards non-cardiology, as compared to general cardiology, treated patients having a lower rate of OAC prescription (OR 0.61; 95% CI 0.32-1.13; p 0.12).(12) Notably, ORBIT-AF stratified electrophysiologists and general cardiologists reducing power and excluding cardiologists with the highest rate of OAC prescription from comparison with non-cardiology treated patients.
Effect of treating specialty on cardiovascular outcomes has previously been investigated for other disease states, most notably for heart failure. Cardiology treated patients were found to have reduced rates of readmission and mortality.(24–26) In an analysis more reflective of contemporary treatment models, the effect of, and interaction between, treating specialty on outcomes for Medicare patients hospitalized for heart failure found treatment by primary and cardiology care, as compared to primary or cardiology care, had lower readmission rates and performed better with respect to heart failure quality of care measures.(27) These studies did not evaluate subgroups or outcomes related to AF.
In our analysis, stroke, the most frequent major adverse outcome associated with AF, was reduced 9%, after controlling for covariates, in patients who received cardiology care, with cardiology treated patients substantially more likely to receive early OAC prescription. Mediation analyses demonstrated indirect effects of borderline statistical significance, which may still plausibly demonstrate partial mediation by early OAC treatment. These findings warrant serious consideration of care pathways for AF patients soon after diagnosis, identification of additional mediators of improved outcomes and exploration into the scalability of these interventions across health care settings, and innovative health care delivery models. Investigation, using both qualitative and quantitative methods, would be informative to determine barriers to appropriate cardiology care. Even so, these data show notable differences and health care system interventions may be warranted. AF specialty clinics, focusing on patient education and employing decision support software based on AF guidelines, have been evaluated in small observational and randomized studies with promising reductions in cardiovascular mortality, hospitalization, and stroke.(14,28) Additionally, these care models have been shown to be cost effective.(29) Point of care or pragmatic trials evaluating AF specialty clinics and novel care models for newly-diagnosed AF should be considered as a next step.
We also found reduced mortality in cardiology treated patients (11% after controlling for covariates). There are several plausible mechanisms that may explain this observation. Central to the care of AF patients is appropriate OAC prescription, which in addition to preventing stroke has been associated with reductions in all cause mortality and stroke related mortality.(30–32) Although, we did not find that OAC prescription mediated cardiology treated patients reduced mortality, which may be attributable to limitations in our mediator variable (does not capture duration and adequacy of anticoagulation). Lack of mediation of mortality preceded by cardiovascular hospitalization within 30 days, may also be due to anticoagulation only being protective against specific cardiovascular mortality subtypes.
In addition to reducing mortality through AF care, cardiology care may lower mortality through improved care of non-AF cardiovascular conditions. Cardiology treated patients in our cohort had higher rates of statin prescription and anti-platelet agents, which can improve survival.(33,34) Also, survival advantages might be conferred from collaborative cardiology and primary care (as opposed to cardiology care only) with primary care able to focus limited resources on optimal management of non-cardiovascular conditions when AF (and non-AF cardiovascular conditions) are managed by cardiology.
Despite cardiology treated patients having reduced overall mortality and mortality preceded by cardiovascular hospitalization within 30 days we saw a paradoxical increase in hospitalization for AF/SVT and MI in both cohorts and heart failure in the propensity matched cohort. Explanations for increased risk of hospitalization include differential practice patterns and confounding by severity, with highly symptomatic AF patients more likely to be referred to cardiology care (independent of other baseline characteristics). Regarding practice patterns, cardiologists may be more aggressive in utilizing hospitalization to intervene on disease progression (e.g., heart failure optimization), potentially improving long-term outcomes. Additionally, increased use of anti-arrhythmic drugs by cardiology clinicians may result in increased hospitalization for drug initiation. Notably, increased rates of MI and heart failure in cardiology treated patients, although statistically significant, may not be clinically significant (MI: full cohort HR 1.03; propensity matched cohort HR 1.08. heart failure: propensity matched cohort HR 1.07).
These data have significant limitations owing to the observational design. Assignment to cardiology care is likely not random, as evidenced by differences in distance to care. Even after accounting for observed baseline differences through two sets of techniques, there is substantial risk of unidentified confounders, which could include patient motivation (or medication and behavioral adherence), self-efficacy, or health-user effect of specialty care. Cardiology care may be a marker, rather than a cause, of improved patient health status, including lifestyle factors such as diet and exercise. Also, we could not ascertain non-VA specialty care, which could lead to misclassification of primary care only patients if they were receiving cardiology care using private insurance, for example. As such, these data remain hypothesis generating.
In conclusion, in patients with newly-diagnosed AF, cardiology care was associated with a reduced risk of stroke, death, and death preceded by cardiovascular hospitalization within 30 days and increased risk of hospitalization for AF/SVT and MI. Early OAC prescription was substantially higher in cardiology treated patients, which may have mediated reductions in risk of stroke but not mortality. These data warrant serious consideration and study of health care system interventions at the time of new AF diagnosis.
Supplementary Material
Central Illustration. Cardiology Care in Newly-Diagnosed Atrial Fibrillation.
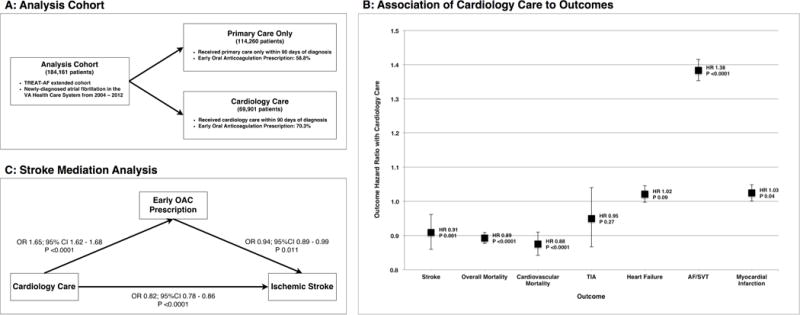
(A) Description of analysis cohort and treatment specialty groups and OAC treatment variation by specialty. (B) Hazard ratios and 95% confidence intervals for AF outcomes (stroke, overall mortality, cardiovascular mortality, TIA, heart failure, AF/SVT, myocardial infarction) in patients who received cardiology care within 90 days of newly-diagnosed AF. (C) Mediation analysis of association between cardiology care and reduced risk of stroke with OAC prescription within 90-days of newly-diagnosed AF as mediator variable. AF: atrial fibrillation, OAC: oral anticoagulation, SVT: supraventricular tachycardia, TIA: transient ischemic attack.
CLINICAL PERSPECTIVES.
Systems-Based Practice
For newly-diagnosed atrial fibrillation, there is differential outcomes based on treatment specialty with reductions in stroke and overall mortality for patients who received cardiology care within 90 days of diagnosis. Consideration of care pathways for patients with newly-diagnosed atrial fibrillation may be warranted.
Translational Outlook
To efficiently manage a rapidly growing population of patients with atrial fibrillation, high value interventions and approaches utilized by cardiologist must be identified and scaled across health care settings. To this end, point of care or pragmatic trials evaluating atrial fibrillation specialty clinics and novel care models are needed.
Acknowledgments
None
Funding Sources: Dr. Turakhia is supported by a Veterans Health Services Research & Development Career Development Award (CDA09027-1), and an American Heart Association National Scientist Development Grant (09SDG2250647).
ABBREVIATIONS LIST
- AF
Atrial fibrillation
- ICD-9
International Classification of Diseases, Ninth Revision
- MI
Myocardial infarction
- NOAC
Non-vitamin K oral anticoagulants
- OAC
Oral anticoagulation
- SVT
Supraventricular tachycardia
- TIA
Transient ischemic attack
- VA
Veterans Affairs
Footnotes
Disclosures: A.C. Perino: None. J. Fan: None. S. Schmitt: None. M. Askari: None. D.W. Kaiser: None. A. Deshmukh: None. P.A. Heidenreich: None. C. Swan: None. S. Narayan: Ownership Interest; Abbott. P.J. Wang: Research Grant; Modest; Medtronic, Inc., Siemens, Cardiofocus, ARCA. Other Research Support; Modest; Medtronic, Inc., St Jude Medical, Boston Scientific Corp, Biosense Webster. Honoraria; Modest; Janssen Pharmaceuticals, St Jude Medical, Medtronic, Inc., Amgen. Ownership Interest; Modest; Vytronus. Consultant/Advisory Board; Modest; Janssen Pharmaceuticals, St Jude Medical, Medtronic, Inc., Amgen. M. Turakhia: Research Grant; Modest; Medtronic, Inc., Research Grant; Significant; Janssen Pharmaceuticals, Inc. Consultant/Advisory Board; Modest; Medtronic, Inc., St Jude Medical, Abbott.
References
- 1.Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–9. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 3.Wolf PA, Dawber TR, Thomas HE, Jr, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology. 1978;28:973–7. doi: 10.1212/wnl.28.10.973. [DOI] [PubMed] [Google Scholar]
- 4.Hinton RC, Kistler JP, Fallon JT, Friedlich AL, Fisher CM. Influence of etiology of atrial fibrillation on incidence of systemic embolism. Am J Cardiol. 1977;40:509–13. doi: 10.1016/0002-9149(77)90064-9. [DOI] [PubMed] [Google Scholar]
- 5.Soliman EZ, Safford MM, Muntner P, et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174:107–14. doi: 10.1001/jamainternmed.2013.11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–64. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 7.Conen D, Chae CU, Glynn RJ, et al. Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA. 2011;305:2080–7. doi: 10.1001/jama.2011.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003;290:2685–92. doi: 10.1001/jama.290.20.2685. [DOI] [PubMed] [Google Scholar]
- 9.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 10.Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123:638–645.e4. doi: 10.1016/j.amjmed.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 11.Turakhia MP, Hoang DD, Xu X, et al. Differences and trends in stroke prevention anticoagulation in primary care vs cardiology specialty management of new atrial fibrillation: The Retrospective Evaluation and Assessment of Therapies in AF (TREAT-AF) study. Am Heart J. 2013;165:93–101.e1. doi: 10.1016/j.ahj.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Fosbol EL, Holmes DN, Piccini JP, et al. Provider specialty and atrial fibrillation treatment strategies in United States community practice: findings from the ORBIT-AF registry. J Am Heart Assoc. 2013;2:e000110. doi: 10.1161/JAHA.113.000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haeusler KG, Gerth A, Limbourg T, et al. Use of vitamin K antagonists for secondary stroke prevention depends on the treating healthcare provider in Germany - results from the German AFNET registry. BMC Neurol. 2015;15:129. doi: 10.1186/s12883-015-0371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hendriks JM, de Wit R, Crijns HJ, et al. Nurse-led care vs. usual care for patients with atrial fibrillation: results of a randomized trial of integrated chronic care vs. routine clinical care in ambulatory patients with atrial fibrillation Euro Heart J. 2012;33:2692–9. doi: 10.1093/eurheartj/ehs071. [DOI] [PubMed] [Google Scholar]
- 15.Cowper DC, Hynes DM, Kubal JD, Murphy PA. Using administrative databases for outcomes research: select examples from VA Health Services Research and Development. J Med Syst. 1999;23:249–59. doi: 10.1023/a:1020579806511. [DOI] [PubMed] [Google Scholar]
- 16.Smith MW, Joseph GJ. Pharmacy data in the VA health care system. Med Care Res Rev. 2003;60:92S–123S. doi: 10.1177/1077558703256726. [DOI] [PubMed] [Google Scholar]
- 17.VA Information Resource Center. VIReC Research User Guide: VHA Decision Support System Clinical National Data Extracts. 2nd. Hines, IL: U.S. Dept. of Veterans Affairs, Health Services Research and Development Service, VA Information Resource Center; 2009. [Google Scholar]
- 18.Arnold N, Sohn M-W, Maynard C, et al. VIReC Technical Report 2: VANDI Mortality Data Merge Project. VA Information Resource Center; Hines, IL: 2006. [Google Scholar]
- 19.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turakhia MP, Santangeli P, Winkelmayer WC, et al. Increased mortality associated with digoxin in contemporary patients with atrial fibrillation: findings from the TREAT-AF study. J Am Coll Cardiol. 2014;64:660–8. doi: 10.1016/j.jacc.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turakhia MP, Ziegler PD, Schmitt SK, et al. Atrial Fibrillation Burden and Short-Term Risk of Stroke: Case-Crossover Analysis of Continuously Recorded Heart Rhythm From Cardiac Electronic Implanted Devices. Circ Arrhythm Electrophysiol. 2015;8:1040–7. doi: 10.1161/CIRCEP.114.003057. [DOI] [PubMed] [Google Scholar]
- 22.Turakhia MP, Solomon MD, Jhaveri M, et al. Burden, timing, and relationship of cardiovascular hospitalization to mortality among Medicare beneficiaries with newly diagnosed atrial fibrillation. Am Heart J. 2013;166:573–80. doi: 10.1016/j.ahj.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 24.Jong P, Gong Y, Liu PP, Austin PC, Lee DS, Tu JV. Care and outcomes of patients newly hospitalized for heart failure in the community treated by cardiologists compared with other specialists. Circulation. 2003;108:184–91. doi: 10.1161/01.CIR.0000080290.39027.48. [DOI] [PubMed] [Google Scholar]
- 25.Reis SE, Holubkov R, Edmundowicz D, et al. Treatment of patients admitted to the hospital with congestive heart failure: specialty-related disparities in practice patterns and outcomes. J Am Coll Cardiol. 1997;30:733–8. doi: 10.1016/s0735-1097(97)00214-3. [DOI] [PubMed] [Google Scholar]
- 26.Selim AM, Mazurek JA, Iqbal M, Wang D, Negassa A, Zolty R. Mortality and readmission rates in patients hospitalized for acute decompensated heart failure: a comparison between cardiology and general-medicine service outcomes in an underserved population. Clin Cardiol. 2015;38:131–8. doi: 10.1002/clc.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed A, Allman RM, Kiefe CI, et al. Association of consultation between generalists and cardiologists with quality and outcomes of heart failure care. Am Heart J. 2003;145:1086–93. doi: 10.1016/S0002-8703(02)94778-2. [DOI] [PubMed] [Google Scholar]
- 28.Tran HN, Tafreshi J, Hernandez EA, Pai SM, Torres VI, Pai RG. A multidisciplinary atrial fibrillation clinic. Curr Cardiol Rev. 2013;9:55–62. doi: 10.2174/157340313805076287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendriks J, Tomini F, van Asselt T, Crijns H, Vrijhoef H. Cost-effectiveness of a specialized atrial fibrillation clinic vs. usual care in patients with atrial fibrillation Europace. 2013;15:1128–35. doi: 10.1093/europace/eut055. [DOI] [PubMed] [Google Scholar]
- 30.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–67. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 31.Hylek EM, Go AS, Chang Y, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349:1019–26. doi: 10.1056/NEJMoa022913. [DOI] [PubMed] [Google Scholar]
- 32.Johnsen SP, Svendsen ML, Hansen ML, Brandes A, Mehnert F, Husted SE. Preadmission oral anticoagulant treatment and clinical outcome among patients hospitalized with acute stroke and atrial fibrillation: a nationwide study. Stroke. 2014;45:168–75. doi: 10.1161/STROKEAHA.113.001792. [DOI] [PubMed] [Google Scholar]
- 33.Berger JS, Brown DL, Becker RC. Low-dose aspirin in patients with stable cardiovascular disease: a meta-analysis. Am J Med. 2008;121:43–9. doi: 10.1016/j.amjmed.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


