Abstract
Purpose
While several studies have evaluated the association of combined lifestyle factors on breast cancer-specific mortality, few have included Hispanic women. We constructed a “healthy behavior index” (HBI) and evaluated its associations with mortality in non-Hispanic White (NHW) and Hispanic women diagnosed with breast cancer from the southwestern U.S.
Methods
Diet and lifestyle questionnaires were analyzed for 837 women diagnosed with invasive breast cancer (1999–2004) in New Mexico as part of the 4-Corners Women’s Health Study. An HBI score ranging from 0 to 12 was based on dietary pattern, physical activity, smoking, alcohol consumption, and body size and shape, with increasing scores representing less healthy characteristics. Hazard ratios for mortality over 14 years of follow-up were estimated for HBI quartiles using Cox proportional hazards models adjusting for education and stratified by ethnicity and stage at diagnosis.
Results
A significant increasing trend was observed across HBI quartiles among all women, NHW women, and those diagnosed with localized or regional/distant stage of disease for all-cause (AC) mortality (p-trend = 0.006, 0.002, 0.03, respectively). AC mortality was increased >2-fold for all women and NHW women in HBI Q4 versus Q1 (HR = 2.18, 2.65, respectively). The association was stronger in women with regional/distant than localized stage of disease (HR = 2.62, 1.94, respectively). Associations for Hispanics or breast cancer-specific mortality were not significant.
Conclusions
These findings indicate the associations between the HBI and AC mortality, which appear to differ by ethnicity and stage at diagnosis. Interventions for breast cancer survivors should address the combination of lifestyle factors on prognosis.
Keywords: Breast cancer, Breast cancer-specific mortality, All-cause mortality, Lifestyle recommendations, Hispanic
Introduction
Despite advancements in detection and treatment, breast cancer remains the second leading cause of death for women in the US [1]. Modifiable lifestyle factors including diet [2–5], smoking status [6–8], physical activity [9–11], moderate/heavy alcohol consumption [12–14], as well as body size and shape [15–17] are associated with breast cancer risk, recurrence, and mortality. The American Cancer Society (ACS) Nutrition and Physical Activity Guidelines Advisory Committee has issued a comprehensive set of recommendations aimed at promoting health for cancer survivors through health behavior modifications [18].
Data suggest that many health behaviors occur in combination [19]; therefore, a composite variable may better capture how lifestyle factors act synergistically to influence breast cancer-specific and all-cause mortality. Consistent positive associations have been found in several studies between a composite lifestyle variable and both breast cancer risk [20–27] and all-cause mortality [20, 21, 28–31]. The combined effect of lifestyle factors on the inflammatory pathway may contribute to these associations [32–36]. Findings have been inconclusive, however, for breast cancer-specific mortality [20], and few studies have examined associations in racial/ethnic minorities. Evidence shows that Hispanic/Native Ameri-can (NA) women have a higher risk of breast cancer-specific mortality than non-Hispanic white (NHW) women [37–39] as well as less favorable prognostic factors [40–42].
The objective of this analysis was to construct a “healthy behavior index” (HBI) combining lifestyle and body size measures and to evaluate associations with all-cause and breast cancer-specific mortality in NHW and Hispanic women. Based on the findings of the previously described studies, we hypothesized that the HBI would be associated with breast cancer-specific and all-cause mortality in both racial/ethnic groups [2–17, 20, 21, 28–31], but that the association would be stronger in NHW than Hispanic women [37–42].
Methods
Study population
Data were from the New Mexico site of the 4-Corners Women’s Health Study (4-CWHS), a multi-site case–control study previously described in detail [43]. The main objective of 4-CWHS was to illuminate reasons for disparities in breast cancer incidence and survival between Hispanic and NHW women living in the US southwest. Controls were not included in the present analyses as the focus is on mortality in women diagnosed with breast cancer. Breast cancer cases were Hispanic and NHW women aged 25–79 years living in New Mexico with histologically confirmed first primary in situ (stage 0) or invasive (stages I–IIIA) breast cancer diagnosis between October 1999 and May 2004. Hispanic and NA women were combined for analysis due to the small number of women identifying as NA (n = 6). Cases were ascertained from the New Mexico SEER cancer registry with ethnicity verified as described previously [44]. Cases considered as outliers (n = 4) or missing information for risk factor/outcome variables or covariates (n = 98) were excluded from analyses. In situ cases (n = 151) were excluded because of their high five-year relative survival rate (98.8%) [1]. The final analytic sample included 837 invasive cases, of which 64.5% (n = 540) identified as NHW and 35.5% (n = 297) identified as Hispanic/NA.
Data collection
Data were collected via in-person interviewer-administered computerized questionnaires. Weight, height, and waist/hip circumference were measured. Participants reported information on dietary intake and lifestyle factors during the referent year, one year prior to breast cancer diagnosis. Stage at diagnosis was obtained from the New Mexico Tumor Registry (NMTR), as well as vital status and date and cause of death (via the National Death Index). Vital status information was available through December 31, 2009. Written informed consent was obtained prior to participation for all cases and the study was conducted under the approval of the Institutional Review Board at the University of Louisville.
Healthy behavior index
The HBI was constructed from smoking status, alcohol consumption, dietary pattern, vigorous physical activity, body mass index (BMI), and waist-to-hip ratio using the criteria shown in Table 1. BMI (kg/m2) was based on the ACS recommendation “be as lean as possible throughout life without being underweight” [18] and categorized using standard cut-points. Alcohol consumption (standard drinks/day) was categorized based on the ACS recommendation that women consume no more than one standard drink (approximately 14 g of alcohol)/day [18]. Vigorous physical activity (minutes/week) was defined as “activities generally using large muscle groups and resulting in faster heart rate, deeper and faster breathing, and sweating” [18]; it was assessed in accordance with the ACS recommendation of 75 min/week [18]. Continuous variables, dietary pattern and waist-to-hip ratio (inches), were categorized into tertiles (T) based on the distribution in controls. Dietary pattern was defined using a “Western diet” variable developed previously in the 4-CWHS [45] in which higher scores reflected a diet high in dairy fat, refined grains, red meat, sugar, and fast foods, but low in fresh fruits and vegetables. Ever smoking was defined as 100 cigarettes or more in a lifetime, and categorized into current, former, and never smoking status.
Table 1.
Summary of outcome and healthy behavior index variables
| Variable | Definition |
|---|---|
| All-cause mortality | Deceased any cause |
| Breast cancer-specific mortality | ICD C50 COD |
| Smoking status | 0 = Never |
| 1 = Former | |
| 2 = Current | |
| Body mass index (kg/m2) | 0 = Normal, <25 |
| 1 = Overweight, 25–30 | |
| 2 = Obese, ≥30 | |
| Waist-to-hip ratio (inches) | 0 = <0.775 |
| 1 = 0.775–0.84 | |
| 2 = ≥0.84 | |
| Alcohol consumption (std drinks/day) | 0 = ≤0.5 |
| 1 = 0.5–1 | |
| 2 = > 1 | |
| Dietary pattern* | 0 = T1 |
| 1 = T2 | |
| 2 = T3 | |
| Vigorous physical activity (min/week) | 0 = > 75 |
| 1 = ≤75 | |
| 2 = none | |
| Healthy behavior index | Q1 = 0–3 |
| Q2 = 4–5 | |
| Q3 = 6–7 | |
| Q4 = 8–12 |
Q quartiles, T tertiles, HBI healthy behavior index
High in dairy fat, refined grains, snacks, gravies and sauces, potatoes, bacon, beef, sugary drinks and desserts, prepared foods, and fast foods; low in fresh fruits and vegetables
Individual HBI components were assigned the scores of 0–2, with lower scores indicating a healthier lifestyle. Scores were assigned as defined in Table 1. An HBI summary score of 0–12 was calculated by summing the scores for the individual HBI components. The resulting HBI distribution was categorized into quartiles (Q) for analysis as follows: Q1 = 0–3, Q2 = 4–5, Q3 = 6–7, Q4 = 8–12 (Table 1).
Covariates, clinical characteristics, and outcome variables
Education was assessed as less than high school, high school/GED, and greater than high school. Women were classified as premenopausal if they were having periods or pregnant during the referent year. Women who reported natural menopause or menopause due to medical intervention were classified as postmenopausal. Race/ethnicity was self-reported as NHW or Hispanic. Stage at diagnosis was assessed as localized (stage I), regional (stage II), and distant (stage IIIA). All-cause mortality was defined as death due to any cause, and breast cancer-specific mortality was defined using International Classification of Disease (ICD) code 50 [46] (Table 1).
Statistical analysis
Descriptive statistics were calculated by HBI quartiles for age, survival time, race, education, menopausal status, smoking status, BMI, alcohol consumption, vigorous physical activity, dietary pattern, waist-to-hip ratio, and stage at diagnosis. Differences between quartiles were assessed using analysis of variance (ANOVA) for continuous variables and the Mantel–Haenszel Chi-square test for categorical variables. Kaplan–Meier curves were constructed for breast cancer-specific survival and overall survival in years by HBI quartiles, and log-rank p values were calculated for differences between quartiles. Cox proportional hazards regression modeling was used to evaluate the association between the HBI and breast cancer-specific and all-cause mortality controlling for pertinent covariates. Hazard ratios (HR) and 95% confidence intervals (CI) were estimated for HBI quartiles with Q1 as the referent group and months from diagnosis to the end of the study or censor date as the time scale. All models were adjusted for education. Other covariates were retained in the models if their inclusion produced a change of ≥10% in the HR for HBI. Effect modification by self-reported ethnicity and stage at diagnosis was evaluated. Regional and distant stages of disease were combined due to the small number of women with distant stage of disease. The linear trend across HBI quartiles was estimated (p-trend) by entering HBI as a continuous variable in the models. Participants were censored if they were lost to follow-up, or in the analysis of breast cancer-specific mortality, if they died of causes not related to breast cancer. The proportional hazards assumption was tested graphically and statistically using an interaction of main effects and covariates with the log of survival time. All analyses met the proportional hazards assumption. Analyses were conducted using the SAS statistical package (version 9.4, SAS Institute, Cary, NC).
Results
The study population was predominantly NHW women compared to Hispanic women (64.5% vs. 35.5%, respectively) (Table 2). Race/ethnicity differed significantly between HBI quartiles (p = 0.02); the highest proportion of NHW women were in Q2 (34.6%), while the highest proportion of Hispanic women were in Q3 (35.4%). The mean age of women in Q1 was three years younger than in Q4 (p = 0.02). Education was inversely associated with HBI (e.g., 76.4% > high school in Q1 versus 55.9% in Q4, p = 0.0002). The majority of women were post-menopausal; however, healthier quartiles had a larger proportion of premenopausal women (46.2% in Q1 vs. 29.4% in Q4, p = 0.005). Stage at diagnosis did not differ significantly between HBI quartiles (p = 0.53). In all quartiles, the largest proportion of women were diagnosed with localized stage of disease (67.3, 69.0, 65.4, and 63.7%, respectively). However, the percentage of women with regional/distant stage of disease was higher in Q4 than in Q1–Q3 (36.3% vs. 32.2, 30.7, and 34.2%, respectively). All component variables of the HBI differed significantly by HBI quartile (p <0.0001).
Table 2.
Descriptive statistics for demographic and prognostic variables by healthy behavior index quartiles, New Mexico site of 4-Corners Women’s Health Study (N = 837)
| Characteristic | Healthy behavior index
|
pa | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 (0–3) n = 208
|
Q2 (4–5) n = 284
|
Q3 (6–7) n = 243
|
Q4 (8–12) n = 102
|
||||||
| n | % | n | % | n | % | n | % | ||
| Age (years; mean ± SD) | 53.1 ± 11.3 | 56.2 ± 11.7 | 55.5 ± 12.1 | 56.1 ± 11.2 | 0.02 | ||||
| Survival (years; mean ± SD) | 10.3 ± 2.5 | 10.3 ± 2.7 | 10.1 ± 2.6 | 9.5 ± 3.4 | 0.046 | ||||
| Race | 0.02 | ||||||||
| Non-Hispanic White | 145 | 69.7 | 187 | 65.9 | 138 | 56.8 | 70 | 68.6 | |
| Hispanic | 63 | 30.3 | 97 | 34.2 | 105 | 43.2 | 32 | 31.4 | |
| Education | |||||||||
| <High school | 13 | 6.3 | 29 | 10.2 | 38 | 15.6 | 17 | 16.7 | 0.0002 |
| High school/GED | 36 | 17.3 | 70 | 24.7 | 70 | 28.8 | 28 | 27.5 | |
| >High school | 159 | 76.4 | 183 | 64.4 | 135 | 55.6 | 57 | 55.9 | |
| Menopausal status | 0.005 | ||||||||
| Premenopausal | 96 | 46.2 | 93 | 32.8 | 83 | 34.2 | 30 | 29.4 | |
| Postmenopausal | 112 | 53.9 | 191 | 67.3 | 160 | 65.8 | 72 | 70.6 | |
| Smoking status | <0.0001 | ||||||||
| Never | 153 | 73.6 | 164 | 57.8 | 125 | 51.4 | 17 | 16.7 | |
| Former | 48 | 23.1 | 86 | 30.3 | 70 | 28.8 | 42 | 41.2 | |
| Current | 7 | 3.4 | 34 | 12.0 | 48 | 19.8 | 43 | 42.2 | |
| Body mass index (kg/m2) | <0.0001 | ||||||||
| Normal, <25 | 165 | 79.3 | 136 | 47.9 | 50 | 20.6 | 11 | 10.8 | |
| Overweight, 25–30 | 41 | 19.7 | 113 | 39.8 | 96 | 39.5 | 35 | 34.3 | |
| Obese, ≥30 | 2 | 0.96 | 35 | 12.3 | 97 | 39.9 | 56 | 54.9 | |
| Alcohol consumption (std drinks/day) | <0.0001 | ||||||||
| ≤0.5 | 186 | 89.4 | 237 | 83.5 | 199 | 81.9 | 65 | 63.7 | |
| 0.5–1 | 19 | 9.1 | 26 | 9.2 | 19 | 7.8 | 13 | 12.8 | |
| >1 | 3 | 1.4 | 21 | 7.4 | 25 | 10.3 | 24 | 23.5 | |
| Vigorous physical activity (min/wk) | <0.0001 | ||||||||
| >75 | 110 | 52.9 | 68 | 23.9 | 32 | 13.2 | 2 | 2.0 | |
| ≤75 | 64 | 30.8 | 107 | 37.7 | 79 | 32.5 | 15 | 14.7 | |
| None | 34 | 16.4 | 109 | 38.4 | 132 | 54.3 | 85 | 83.3 | |
| Dietary pattern | <0.0001 | ||||||||
| T1 | 107 | 51.4 | 88 | 31.0 | 30 | 12.4 | 2 | 2.0 | |
| T2 | 82 | 39.4 | 118 | 41.6 | 90 | 37.0 | 26 | 25.5 | |
| T3 | 19 | 9.1 | 78 | 27.5 | 123 | 50.6 | 74 | 72.6 | |
| Waist-to-hip ratio (inches) | <0.0001 | ||||||||
| <0.775 | 130 | 62.5 | 70 | 24.7 | 24 | 9.9 | 3 | 2.9 | |
| 0.775–0.84 | 67 | 32.2 | 140 | 49.3 | 78 | 32.1 | 18 | 17.7 | |
| ≥0.84 | 11 | 5.3 | 74 | 26.1 | 141 | 58.0 | 81 | 79.4 | |
| Stage | 0.53 | ||||||||
| Localized | 140 | 67.3 | 196 | 69.0 | 159 | 65.4 | 65 | 63.7 | |
| Regional | 64 | 30.8 | 84 | 29.6 | 77 | 31.7 | 37 | 36.3 | |
| Distant | 3 | 1.4 | 3 | 1.1 | 6 | 2.5 | 0 | 0 | |
Q quartile, T tertile. Column percentages (%) may not add up to 100% due to rounding or missing observations. Column totals (n) may not add up to total due to missing observations: education (n = 2) stage (n = 3)
Comparisons between healthy behavior index quartiles; p values reported for Mantel–Haenszel Chi-square (categorical) and ANOVA (continuous)
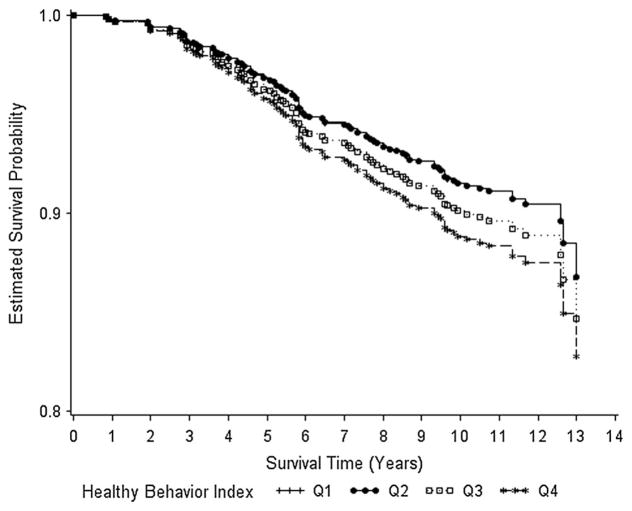
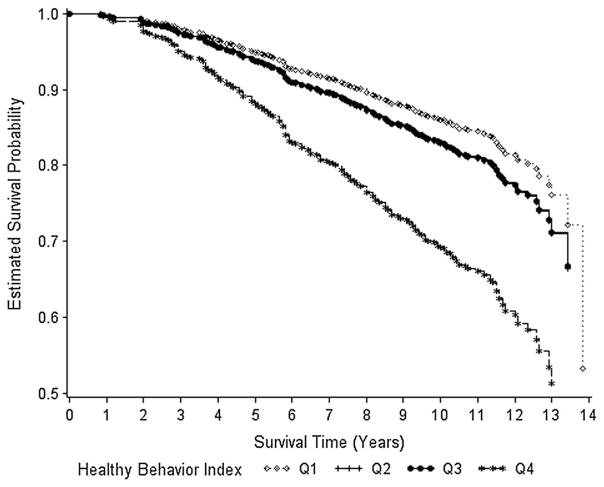
During the 14 years of follow-up, overall survival differed significantly by HBI quartile (p = 0.046) (Table 2). Comparing mean years of survival, women in HBI Q1 and Q2 lived 0.8 years longer than women in Q4. Survival rates did not significantly differ between quartiles for breast cancer survival (log-rank p value = 0.81) (Fig. 1). However, a decreasing trend in survival was observed as HBI quartile increased (84, 85, 86, and 77%, respectively). Survival rates significantly differed between quartiles of the HBI for overall survival (log-rank p value = 0.0005); the rate was similar across Q1–Q3 (70, 71, and 71%, respectively) but lower in Q4 (53%) (Fig. 2).
Fig. 1.
Breast cancer survival by healthy behavior index quartiles (Q). Survival curves adjusted for stage at diagnosis and education level; Q1 and Q2 overlap
Fig. 2.
Overall survival by healthy behavior index quartiles (Q). Survival curves adjusted for stage at diagnosis and education level; Q2 and Q3 overlap
We evaluated the association between the HBI and breast cancer-specific and all-cause mortality for all women and stratified by race/ethnicity, adjusting for education level and stage at diagnosis (Tables 3 and 4). An increased risk of breast cancer-specific mortality was present for HBI Q3–Q4 compared to Q1, but was not statistically significant overall by race/ethnicity in crude or adjusted models. A significant increasing trend across HBI quartiles for all-cause mortality was observed among all women and NHW women (p-trend = 0.006 and 0.002, respectively), but not among Hispanic women. A significant > 2-fold increased risk of all-cause mortality was observed for all women and NHW women in HBI Q4 compared to Q1. Adjusting for education level and stage at diagnosis attenuated the results slightly (HR = 2.18 in all women, HR = 2.65 in NHW). Despite the observed differences in associations in racial/ethnic groups, the interaction between HBI quartile and race/ethnicity was not statistically significant (p-interaction = 0.60).
Table 3.
Associations between healthy behavior index and breast cancer-specific and all-cause mortality among all women
| Healthy behavior index | Deaths/No | Crude HR (95% CI) | Adjusted HRa (95% CI) |
|---|---|---|---|
| Breast cancer-specific mortality | |||
| Q1 (0–3) | 23/207 | 1.00 | 1.00 |
| Q2 (4–5) | 31/282 | 1.00 (0.58–1.71) | 0.98 (0.57–1.68) |
| Q3 (6–7) | 31/242 | 1.19 (0.69–2.04) | 1.04 (0.60–1.81) |
| Q4 (8–12) | 13/102 | 1.27 (0.65–2.52) | 1.15 (0.58–2.28) |
| p-trend | 0.79 | 0.68 | |
| All-cause mortality | |||
| Q1 (0–3) | 36/207 | 1.00 | 1.00 |
| Q2 (4–5) | 63/283 | 1.28 (0.85–1.93) | 1.19 (0.79–1.80) |
| Q3 (6–7) | 51/242 | 1.27 (0.83–1.95) | 1.11 (0.72–1.72) |
| Q4 (8–12) | 39/102 | 2.44 (1.55–3.85) | 2.18 (1.37–3.44) |
| p-trend | 0.0008 | 0.006 | |
Q quartile, HR hazard ratio, CI confidence interval
Adjusted for education and stage at diagnosis
Table 4.
Associations between healthy behavior index and breast cancer-specific and all-cause mortality, stratified by race
| Healthy behavior index | Non-Hispanic WhiteDS
|
Hispanic
|
||||
|---|---|---|---|---|---|---|
| Deaths/No | Crude HR (95% CI) | Adjusted HRa (95% CI) | Deaths/No | Crude HR (95% CI) | Adjusted HRa (95% CI) | |
| Breast cancer-specific mortality | ||||||
| Q1 (0–3) | 15/144 | 1.00 | 1.00 | 8/63 | 1.00 | 1.00 |
| Q2 (4–5) | 17/186 | 0.89 (0.44–1.78) | 0.91 (0.45–1.82) | 14/97 | 1.17 (0.49–2.79) | 1.13 (0.47–2.70) |
| Q3 (6–7) | 16/137 | 1.91 (0.59–2.41) | 1.19 (0.58–2.45) | 15/105 | 1.12 (0.47–2.64) | 0.97 (0.41–2.31) |
| Q4 (8–12) | 8/70 | 1.30 (0.55–3.06) | 1.25 (0.52–2.98) | 5/32 | 1.28 (0.42–3.91) | 1.27 (0.41–3.88) |
| p-trend | 0.43 | 0.50 | 0.73 | 0.89 | ||
| p-interactionb | 0.94 | |||||
| All-cause mortality | ||||||
| Q1 (0–3) | 26/144 | 1.00 | 1.00 | 10/63 | 1.00 | 1.00 |
| Q2 (4–5) | 40/186 | 1.21 (0.74–1.98) | 1.19 (0.72–1.95) | 23/97 | 1.51 (0.72–3.17) | 1.29 (0.61–2.74) |
| Q3 (6–7) | 27/137 | 1.20 (0.70–2.07) | 1.14 (0.66–1.98) | 24/105 | 1.42 (0.68–2.98) | 1.18 (0.56–2.50) |
| Q4 (8–12) | 30/70 | 2.90 (1.71–4.91) | 2.65 (1.54–4.55) | 9/32 | 1.75 (0.71–4.31) | 1.63 (0.66–4.03) |
| p-trend | 0.0005 | 0.002 | 0.2887 | 0.43 | ||
| p-interactionb | 0.60 | |||||
Q quartile, HR hazard ratio, CI confidence interval
Adjusted for education and stage at diagnosis
Interaction for HBI quartile and race
Results of analyses stratified by stage at diagnosis are shown in Table 5. HBI was not significantly associated with breast cancer-specific mortality in women with localized or regional/distant stage of disease. However, results differed by stage; women in Q2–Q4 with localized stage of disease had decreased risk compared to Q1, whereas women in Q2–Q4 with regional/distant stage of disease had increased risk compared to Q1. A significant increasing trend across HBI quartiles was observed among women diagnosed with regional/distant stage of disease for all-cause mortality (p-trend = 0.03). A significantly increased risk of all-cause mortality for HBI Q4 compared to Q1 was present for women with localized and regional/ distant stage of disease. However, this association was stronger in women with regional/distant than in localized stage of disease (HR = 2.49 and 1.94, respectively). The interaction between HBI quartile and stage of disease did not significantly influence risk of breast cancer-specific or all-cause mortality (p-interaction = 0.65 and 0.92, respectively).
Table 5.
Associations between healthy behavior index and breast cancer-specific and all-cause mortality, stratified by stage at diagnosis
| Healthy behavior index | Localized
|
Regional/distant
|
||||
|---|---|---|---|---|---|---|
| Deaths/No | Crude HR (95% CI) | Adjusted HRa (95% CI) | Deaths/No | Crude HR (95% CI) | Adjusted HRa (95% CI) | |
| Breast cancer-specific mortality | ||||||
| Q1 (0–3) | 10/140 | 1.00 | 1.00 | 13/67 | 1.00 | 1.00 |
| Q2 (4–5) | 11/196 | 0.79 (0.34–1.86) | 0.72 (0.30–1.70) | 20/87 | 1.21 (0.60–2.43) | 1.18 (0.57–2.39) |
| Q3 (6–7) | 11/159 | 0.98 (0.42–2.31) | 0.83 (0.35–2.00) | 20/83 | 1.33 (0.66–2.67) | 1.21 (0.59–2.48) |
| Q4 (8–12) | 3/65 | 0.68 (0.19–2.47) | 0.56 (0.15–2.05) | 10/37 | 1.66 (0.73–3.79) | 1.59 (0.70–3.65) |
| p-trend | 0.7349 | 0.48 | 0.2252 | 0.32 | ||
| p-interactionb | 0.65 | |||||
| All-cause mortality | ||||||
| Q1 (0–3) | 22/140 | 1.00 | 1.00 | 14/67 | 1.00 | 1.00 |
| Q2 (4–5) | 37/196 | 1.21 (0.72–2.06) | 1.09 (0.64–1.87) | 26/87 | 1.43 (0.75–2.75) | 1.38 (0.72–2.66) |
| Q3 (6–7) | 26/159 | 1.10 (0.62–1.94) | 0.97 (0.54–1.72) | 25/83 | 1.53 (0.79–2.94) | 1.36 (0.70–2.67) |
| Q4 (8–12) | 22/65 | 2.29 (1.27–4.14) | 1.94 (1.06–3.57) | 17/37 | 2.62 (1.29–5.31) | 2.49 (1.22–5.08) |
| p-trend | 0.0287 | 0.099 | 0.0130 | 0.03 | ||
| p-interactionb | 0.92 | |||||
Q quartile, HR hazard ratio, CI confidence interval
Adjusted for education
Interaction reported for HBI quartile and stage at diagnosis
Discussion
This analysis sought to evaluate the combined effect of lifestyle factors, in terms of a healthy behavior index, on breast cancer-specific and all-cause mortality in NHW and Hispanic women diagnosed with invasive breast cancer. We observed a statistically significant association between the HBI and all-cause mortality. An increased risk of breast cancer-specific mortality for HBI Q2–Q4 compared to Q1 was present, but was not statistically significant overall, by ethnicity, or by stage of disease. Risk was significantly increased >2-fold for HBI Q4 versus Q1 for all-cause mortality among all women and NHW women, but not in Hispanic women.
Few studies have investigated the combined impact of lifestyle factors on breast cancer-specific mortality. Our results agree with those from the Women’s Health Initiative (WHI) prospective cohort study, which did not find a significant association for a similar index with breast cancer-specific mortality [20]. However, a significant p-trend was reported in WHI for a positive association between adherence to ACS guidelines and breast cancer-specific mortality (p-trend = 0.049) [20], in contrast to our study. However, our study is smaller with less statistical power to detect small associations [20].
The relationship between a combination of lifestyle factors and all-cause mortality has been examined in six studies [20, 21, 28–31], although not all of these studies included women diagnosed with breast cancer. Consistent reductions in risk ranging from 27 to 68% over 10.5–24 years of follow-up were reported in those with the highest adherence to cancer prevention guidelines compared to the lowest adherence [20, 21, 28–31]. Similarly, a significant p-trend was reported as adherence to cancer prevention guidelines increased in several studies [20, 29, 30]. A large proportion of all-cause mortality in cancer patients consists of deaths due to cardiovascular and pulmonary diseases [47]. Since these outcomes have consistently been linked to individual lifestyle factors [48, 49], it is likely that they may also be associated with the combination of lifestyle factors. Several prospective cohort studies have reported a significantly reduced risk of death due to cardiovascular disease (HR = 0.21–0.59) among those displaying the greatest adherence to cancer prevention guidelines compared to those displaying the lowest adherence [28–31, 50].
Race/ethnicity is a significant predictor of both all-cause and breast cancer-specific mortality [51–54]. An unresolved question is that to which extent race/ethnicity modifies the associations of lifestyle factors with mortality. We detected no effect modification by race/ethnicity for breast cancer-specific mortality. To our knowledge, this is the first combined lifestyle factor study to evaluate race/ ethnicity as an effect modifier for breast cancer-specific mortality. We did detect effect modification by race/ethnicity for all-cause mortality, which is consistent with the findings of Thomson et al. [20]. However, there was a stronger association between all-cause mortality and adherence to ACS guidelines for Hispanic women than for NHW women (HR = 0.56 and 0.73, respectively) in their study, whereas a statistically significant association was found in NHW women but not in Hispanic women in the present study.
Stage at diagnosis is a strong predictor of breast cancer survival [1]. To our knowledge, this is the first combined lifestyle factor study to evaluate stage at diagnosis as an effect modifier for breast cancer-specific mortality or all-cause mortality. We detected no effect modification by stage at diagnosis for breast cancer-specific mortality. However, we did detect differences by stage at diagnosis for all-cause mortality; the association was stronger for regional/distant stage of disease than localized stage of disease, and a larger proportion of deaths took place in regional/distant than localized (33.8% vs. 45.9%). Comorbid conditions may have played a role in this relationship. Research suggests that women with comorbid conditions tend to be diagnosed with breast cancer at a later stage of disease [55] and have lower rates of cancer screening than women without comorbidities [56, 57]. Women with advanced disease may also receive more intensive treatment with chemotherapeutic drugs and radiotherapy that may exacerbate the underlying non-cancer diseases such as cardiovascular disease [58, 59].
Inconsistencies among studies could partially be explained by differences in the construction of the composite lifestyle variable. The majority of the studies used women displaying the poorest adherence to lifestyle recommendations as the referent group [20–27, 29–31, 50], while we used the quartile with the healthiest lifestyle factors. Several studies constructed the composite variable based on different sets of cancer prevention guidelines [22–24, 26, 27, 30, 50], which differed from our HBI as well as other indices constructed based on ACS guidelines [20, 21, 29]. Scoring for the individual components of the composite variable also differed between studies.
No studies have evaluated the mechanism by which the combination of lifestyle factors might affect breast cancer-specific mortality; however, these lifestyle factors may individually act through several mechanisms that could operate synergistically to increase risk of breast cancer-specific mortality. Obesity is associated with chronic inflammation, increased plasma estrogen, insulin-like growth factor 1 (IGF-1), and insulin levels [32, 60–63]. Obesity-induced chronic inflammation has been associated with increased tumor size, high tumor grade, and increased tumor metastasis [32]. Physical activity modulates serum estrogen levels and immune function [64, 65] as well as insulin levels [66, 67]. Diets low in fruits and vegetables and high in red meat have been linked to increased risk of weight gain and/or obesity [68, 69]. Additionally, diets high in fruits and vegetables may exert a protective effect against breast cancer-specific mortality [70], and some components of cooked meat may increase invasiveness of breast cancer cells [71, 72]. Alcohol consumption has been linked to increases in tumor progression, serum estrogen levels, and expression of growth factors that promote tumor angiogenesis [73–76]. Smoking is reported to increase the production of inflammatory mediators and suppress immune function [33, 77], and has been associated with larger tumor size [78], increased risk of cancer metastasis [78, 79], and hormone receptor-negative cancers [80].
These lifestyle factors also may influence risk of all-cause mortality through cardiovascular and pulmonary conditions. Obesity and increased waist-to-hip ratio are associated with increased cardiac demand of adipose tissue [81, 82] and metabolic syndrome [83], which includes such risk factors as hypertension, glucose intolerance, and dyslipidemia [34, 84]. Increased leptin and IGF-1 concentrations are associated with inflammation, prothrombotic state, endothelial damage, and vascular hypertrophy [85, 86]. Physical activity exerts a protective effect against cardiovascular disease by creating favorable changes in inflammatory markers, lipids, cholesterol, and hypertension [87]. It also contributes to “vascular conditioning” and improvements in the function of vessel walls [88]. Recent research has focused on the importance of dietary pattern in cardiometabolic risk [89, 90]. Diets high in fruits and vegetables and whole grains and low in animal fats have been linked to decreased risk of obesity and reduced cardiac events [69, 91], which may be explained by favorable effects on blood pressure, serum insulin levels, inflammation, blood lipoproteins and lipids, and endothelial function [35]. Although light alcohol consumption has been shown to exert a protective effect against all-cause mortality and cardiovascular disease [92], heavy drinking has been associated with hypertension [93] and increased risk of mortality due to stroke, cancer, cirrhosis, and alcoholic cardiomyopathy [94–96]. Oxidant chemicals found in cigarette smoke have been linked to inflammation, thrombosis, and endothelial dysfunction, while exposure to nicotine is associated with hypertension [36, 97]. An association has also been found between smoking and higher serum triglyceride, higher low-density lipoprotein (HDL), and lower high-density lipoprotein (LDL) [98]; it has been hypothesized that these changes may contribute to the development of insulin resistance [99].
Our study has a number of strengths. The population-based study design allowed for the recruitment of a large number of participants, and cases were ascertained through a SEER cancer registry. This study is one of the first to investigate the association of combined lifestyle factors on breast cancer-specific and all-cause mortality in a Hispanic population within the U.S. The collection of comprehensive diet and lifestyle data through in-person interviews allowed for all ACS guidelines to be included in the construction of the HBI. The 14-year follow-up period increased the likelihood for mortality events for both localized and regional/distant stages of disease. The likelihood of misclassification was reduced through ascertainment of vital status and cause of death data from the National Death Index and the New Mexico Tumor Registry.
There are several limitations related to our study. Statistical power was limited due to the low number of events within each quartile of the HBI, particularly in terms of breast cancer-specific mortality. Since data were collected retrospectively and self-reported, it is possible that the data could have been subject to recall bias. An additional vital status update could increase the number of deaths per quartile of the HBI, resulting in an increase in statistical power. The analysis did not account for lifestyle changes that may have taken place over the course of follow-up since HBI scores were based on the data reported for lifestyle in the referent year.
In summary, these data indicate that the combination of unhealthy lifestyle factors is significantly associated with risk of all-cause mortality and may be associated with risk of breast cancer-specific mortality. Further research is necessary on the association between the combination of lifestyle factors and breast cancer-specific mortality, particularly in racial/ethnic minorities. Since these factors are associated with poorer outcomes both individually and in combination, interventions for breast cancer survivors should address the combination of lifestyle factors and their effect on prognosis, recurrence, and second primaries.
Acknowledgments
This research was supported by the University of Louisville Cancer Education Program, National Institute of Health (NIH)/National Cancer Institute (NCI) R25-CA134283, NIH/NCI R01-CA78762, the University of Louisville School of Public Health and Information Sciences, and the James Graham Brown Cancer Center.
Footnotes
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.Ethical standards
This study complies with the current laws of the country in which it was performed.
References
- 1.Howlader N, et al. SEER Cancer Statistics Review, 1975–2010. National Cancer Institute; Bethesda, MD: Apr, 2013. 2013. http://seer.cancer.gov/csr/1975_2010/, Based on November 2012 SEER data submission, posted to the SEER web site. [Google Scholar]
- 2.Ingram D. Diet and subsequent survival in women with breast cancer. Br J Cancer. 1994;69(3):592–595. doi: 10.1038/bjc.1994.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saxe GA, et al. Diet and risk for breast cancer recurrence and survival. Breast Cancer Res Treat. 1999;53(3):241–253. doi: 10.1023/a:1006190820231. [DOI] [PubMed] [Google Scholar]
- 4.Jain M, Miller AB. Tumor characteristics and survival of breast cancer patients in relation to premorbid diet and body size. Breast Cancer Res Treat. 1997;42(1):43–55. doi: 10.1023/a:1005798124538. [DOI] [PubMed] [Google Scholar]
- 5.Hebert JR, Hurley TG, Ma Y. The effect of dietary exposures on recurrence and mortality in early stage breast cancer. Breast Cancer Res Treat. 1998;51(1):17–28. doi: 10.1023/a:1006056915001. [DOI] [PubMed] [Google Scholar]
- 6.Boone SD, et al. Active and passive cigarette smoking and mortality among Hispanic and non-Hispanic white women diagnosed with invasive breast cancer. Ann Epidemiol. 2015;25(11):824–831. doi: 10.1016/j.annepidem.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pierce JP, et al. Lifetime cigarette smoking and breast cancer prognosis in the After Breast Cancer Pooling Project. J Natl Cancer Inst. 2014;106(1):djt359. doi: 10.1093/jnci/djt359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Passarelli MN, et al. Cigarette smoking before and after breast cancer diagnosis: mortality from breast cancer and smoking-related diseases. J Clin Oncol. 2016;34(12):1315–1322. doi: 10.1200/JCO.2015.63.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmes MD, et al. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 10.Holick CN, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(2):379–386. doi: 10.1158/1055-9965.EPI-07-0771. [DOI] [PubMed] [Google Scholar]
- 11.Irwin ML, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol. 2008;26(24):3958–3964. doi: 10.1200/JCO.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feigelson HS, et al. Alcohol consumption increases the risk of fatal breast cancer (United States) Cancer Causes Control. 2001;12(10):895–902. doi: 10.1023/a:1013737616987. [DOI] [PubMed] [Google Scholar]
- 13.Kwan ML, et al. Postdiagnosis alcohol consumption and breast cancer prognosis in the after breast cancer pooling project. Cancer Epidemiol Biomarkers Prev. 2013;22(1):32–41. doi: 10.1158/1055-9965.EPI-12-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwan ML, et al. Alcohol consumption and breast cancer recurrence and survival among women with early-stage breast cancer: the life after cancer epidemiology study. J Clin Oncol. 2010;28(29):4410–4416. doi: 10.1200/JCO.2010.29.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dal Maso L, et al. Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer. 2008;123(9):2188–2194. doi: 10.1002/ijc.23747. [DOI] [PubMed] [Google Scholar]
- 16.Connor AE, et al. Obesity and risk of breast cancer mortality in Hispanic and Non-Hispanic white women: the New Mexico Women’s Health Study. J Womens Health (Larchmt) 2013;22(4):368–377. doi: 10.1089/jwh.2012.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borugian MJ, et al. Waist-to-hip ratio and breast cancer mortality. Am J Epidemiol. 2003;158(10):963–968. doi: 10.1093/aje/kwg236. [DOI] [PubMed] [Google Scholar]
- 18.Kushi LH, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62(1):30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 19.Spring B, et al. Fostering multiple healthy lifestyle behaviors for primary prevention of cancer. Am Psychol. 2015;70(2):75–90. doi: 10.1037/a0038806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomson CA, et al. Nutrition and physical activity cancer prevention guidelines, cancer risk, and mortality in the women’s health initiative. Cancer Prev Res. 2014;7(1):42–53. doi: 10.1158/1940-6207.CAPR-13-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabat GC, et al. Adherence to cancer prevention guidelines and cancer incidence, cancer mortality, and total mortality: a prospective cohort study. Am J Clin Nutr. 2015;101(3):558–569. doi: 10.3945/ajcn.114.094854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hastert TA, et al. Adherence to the WCRF/AICR cancer prevention recommendations and cancer-specific mortality: results from the Vitamins and Lifestyle (VITAL) Study. Cancer Causes Control. 2014;25(5):541–552. doi: 10.1007/s10552-014-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makarem N, et al. Concordance with World Cancer Research Fund/American Institute for Cancer Research (WCRF/ AICR) guidelines for cancer prevention and obesity-related cancer risk in the Framingham Offspring cohort (1991–2008) Cancer Causes Control. 2015;26(2):277–286. doi: 10.1007/s10552-014-0509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris HR, Bergkvist L, Wolk A. Adherence to the World Cancer Research Fund/American Institute for Cancer Research recommendations and breast cancer risk. Int J Cancer. 2016;138(11):2657–2664. doi: 10.1002/ijc.30015. [DOI] [PubMed] [Google Scholar]
- 25.Catsburg C, Miller AB, Rohan TE. Adherence to cancer prevention guidelines and risk of breast cancer. Int J Cancer. 2014;135(10):2444–2452. doi: 10.1002/ijc.28887. [DOI] [PubMed] [Google Scholar]
- 26.Romaguera D, et al. Is concordance with World Cancer Research Fund/American Institute for Cancer Research guidelines for cancer prevention related to subsequent risk of cancer? Results from the EPIC study. Am J Clin Nutr. 2012;96(1):150–163. doi: 10.3945/ajcn.111.031674. [DOI] [PubMed] [Google Scholar]
- 27.Nomura SJ, et al. WCRF/AICR recommendation adherence and breast cancer incidence among postmenopausal women with and without non-modifiable risk factors. Int J Cancer. 2016;138(11):2602–2615. doi: 10.1002/ijc.29994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khaw KT, et al. Combined impact of health behaviours and mortality in men and women: the EPIC-Norfolk prospective population study. PLoS Med. 2008;5(1):e12. doi: 10.1371/journal.pmed.0050012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCullough ML, et al. Following cancer prevention guidelines reduces risk of cancer, cardiovascular disease, and all-cause mortality. Cancer Epidemiol Biomarkers Prev. 2011;20(6):1089–1097. doi: 10.1158/1055-9965.EPI-10-1173. [DOI] [PubMed] [Google Scholar]
- 30.Petersen KE, et al. The combined impact of adherence to five lifestyle factors on all-cause, cancer and cardiovascular mortality: a prospective cohort study among Danish men and women. Br J Nutr. 2015;113(5):849–858. doi: 10.1017/S0007114515000070. [DOI] [PubMed] [Google Scholar]
- 31.van Dam RM, et al. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ. 2008;337:a1440. doi: 10.1136/bmj.a1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simpson ER, Brown KA. Obesity and breast cancer: role of inflammation and aromatase. J Mol Endocrinol. 2013;51(3):T51–T59. doi: 10.1530/JME-13-0217. [DOI] [PubMed] [Google Scholar]
- 33.Slattery ML, et al. Diet and lifestyle factors modify immune/inflammation response genes to alter breast cancer risk and prognosis: the Breast Cancer Health Disparities Study. Mutat Res. 2014;770:19–28. doi: 10.1016/j.mrfmmm.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malik S, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110(10):1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 35.Mozaffarian D. Nutrition and cardiovascular and metabolic disease. In: Bonow RO, Mann DL, Zipes DP, Libby P, editors. Braunwald’s heart disease: a textbook of cardiovascular medicine. Elsevier Saunders; Philadelphia: 2015. [Google Scholar]
- 36.Benowitz NL. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Prog Cardiovasc Dis. 2003;46(1):91–111. doi: 10.1016/s0033-0620(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 37.Boone SD, et al. The joint contribution of tumor phenotype and education to breast cancer survival disparity between Hispanic and non-Hispanic white women. Cancer Causes Control. 2014;25(3):273–282. doi: 10.1007/s10552-013-0329-3. [DOI] [PubMed] [Google Scholar]
- 38.Hill DA, et al. Method of detection and breast cancer survival disparities in Hispanic women. Cancer Epidemiol Biomarkers Prev. 2010;19(10):2453–2460. doi: 10.1158/1055-9965.EPI-10-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163(1):49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 40.Hines LM, et al. Ethnic disparities in breast tumor phenotypic subtypes in Hispanic and non-Hispanic white women. J Womens Health. 2011;20(10):1543–1550. doi: 10.1089/jwh.2010.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Connor AE, et al. Differences between Hispanic and non-Hispanic white women with breast cancer for clinical characteristics and their correlates. Ann Epidemiol. 2013;23(4):227–232. doi: 10.1016/j.annepidem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banegas MP, Li CI. Breast cancer characteristics and outcomes among Hispanic Black and Hispanic White women. Breast Cancer Res Treat. 2012;134(3):1297–1304. doi: 10.1007/s10549-012-2142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slattery ML, et al. Body size, weight change, fat distribution and breast cancer risk in Hispanic and non-Hispanic white women. Breast Cancer Res Treat. 2007;102(1):85–101. doi: 10.1007/s10549-006-9292-y. [DOI] [PubMed] [Google Scholar]
- 44.Sweeney C, et al. Recruiting Hispanic women for a population-based study: validity of surname search and characteristics of nonparticipants. Am J Epidemiol. 2007;166(10):1210–1219. doi: 10.1093/aje/kwm192. [DOI] [PubMed] [Google Scholar]
- 45.Murtaugh MA, et al. Diet composition and risk of overweight and obesity in women living in the southwestern United States. J Am Diet Assoc. 2007;107(8):1311–1321. doi: 10.1016/j.jada.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 46.WHO. International statistical classification of diseases and related health problems (10th Revision) World Health Organization; Geneva: 1992. [Google Scholar]
- 47.Hoyert DL. 75 years of mortality in the United States, 1935–2010. NCHS Data Brief. 2012;88:1–8. [PubMed] [Google Scholar]
- 48.Kant S, Gupta B. Role of lifestyle in the development of chronic obstructive pulmonary disease: a review. Lung India. 2008;25(2):95–101. doi: 10.4103/0970-2113.59591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kromhout D, et al. Prevention of coronary heart disease by diet and lifestyle: evidence from prospective cross-cultural, cohort, and intervention studies. Circulation. 2002;105(7):893–898. doi: 10.1161/hc0702.103728. [DOI] [PubMed] [Google Scholar]
- 50.Vergnaud AC, et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research guidelines and risk of death in Europe: results from the European Prospective Investigation into Nutrition and Cancer cohort study 1,4. Am J Clin Nutr. 2013;97(5):1107–1120. doi: 10.3945/ajcn.112.049569. [DOI] [PubMed] [Google Scholar]
- 51.Grann V, et al. Regional and racial disparities in breast cancer-specific mortality. Soc Sci Med. 2006;62(2):337–347. doi: 10.1016/j.socscimed.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 52.Komenaka IK, et al. Race and ethnicity and breast cancer outcomes in an underinsured population. J Natl Cancer Inst. 2010;102(15):1178–1187. doi: 10.1093/jnci/djq215. [DOI] [PubMed] [Google Scholar]
- 53.Borrell LN, Lancet EA. Race/ethnicity and all-cause mortality in US adults: revisiting the Hispanic paradox. Am J Public Health. 2012;102(5):836–843. doi: 10.2105/AJPH.2011.300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harper S, et al. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987–2005) Cancer Epidemiol Biomarkers Prev. 2009;18(1):121–131. doi: 10.1158/1055-9965.EPI-08-0679. [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez EC, et al. Comorbid illness and the early detection of cancer. South Med J. 2001;94(9):913–920. [PubMed] [Google Scholar]
- 56.Burack RC, Liang J. The acceptance and completion of mammography by older black women. Am J Public Health. 1989;79(6):721–726. doi: 10.2105/ajph.79.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kiefe CI, et al. Chronic disease as a barrier to breast and cervical cancer screening. J Gen Intern Med. 1998;13(6):357–365. doi: 10.1046/j.1525-1497.1998.00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Darby SC, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 59.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 60.Grossmann ME, et al. Obesity and breast cancer: status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev. 2010;29(4):641–653. doi: 10.1007/s10555-010-9252-1. [DOI] [PubMed] [Google Scholar]
- 61.Lautenbach A, et al. Obesity and the associated mediators leptin, estrogen and IGF-I enhance the cell proliferation and early tumorigenesis of breast cancer cells. Nutr Cancer. 2009;61(4):484–491. doi: 10.1080/01635580802610115. [DOI] [PubMed] [Google Scholar]
- 62.Cleary MP. Impact of obesity on development and progression of mammary tumors in preclinical models of breast cancer. J Mammary Gland Biol Neoplasia. 2013;18(3–4):333–343. doi: 10.1007/s10911-013-9300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rock CL, Demark-Wahnefried W. Nutrition and survival after the diagnosis of breast cancer: a review of the evidence. J Clin Oncol. 2002;20(15):3302–3316. doi: 10.1200/JCO.2002.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McTiernan A, et al. Effect of exercise on serum estrogens in postmenopausal women: a 12-month randomized clinical trial. Cancer Res. 2004;64(8):2923–2928. doi: 10.1158/0008-5472.can-03-3393. [DOI] [PubMed] [Google Scholar]
- 65.McTiernan A, et al. Physical activity and cancer etiology: associations and mechanisms. Cancer Causes Control. 1998;9(5):487–509. doi: 10.1023/a:1008853601471. [DOI] [PubMed] [Google Scholar]
- 66.Chlebowski RT, et al. Insulin, physical activity, and caloric intake in postmenopausal women: breast cancer implications. J Clin Oncol. 2004;22(22):4507–4513. doi: 10.1200/JCO.2004.04.119. [DOI] [PubMed] [Google Scholar]
- 67.Kaaks R, Lukanova A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc Nutr Soc. 2001;60(1):91–106. doi: 10.1079/pns200070. [DOI] [PubMed] [Google Scholar]
- 68.He K, et al. Changes in intake of fruits and vegetables in relation to risk of obesity and weight gain among middle-aged women. Int J Obes Relat Metab Disord. 2004;28(12):1569–1574. doi: 10.1038/sj.ijo.0802795. [DOI] [PubMed] [Google Scholar]
- 69.Mozaffarian D, et al. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fink BN, et al. Dietary flavonoid intake and breast cancer survival among women on Long Island. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2285–2292. doi: 10.1158/1055-9965.EPI-07-0245. [DOI] [PubMed] [Google Scholar]
- 71.Lauber SN, Gooderham NJ. The cooked meat-derived mammary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine promotes invasive behaviour of breast cancer cells. Toxicology. 2011;279(1–3):139–145. doi: 10.1016/j.tox.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 72.Papaioannou MD, Koufaris C, Gooderham NJ. The cooked meat-derived mammary carcinogen 2-amino-1-methyl-6-phenylim-idazo[4,5-b]pyridine (PhIP) elicits estrogenic-like microRNA responses in breast cancer cells. Toxicol Lett. 2014;229(1):9–16. doi: 10.1016/j.toxlet.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 73.Purohit V. Moderate alcohol consumption and estrogen levels in postmenopausal women: a review. Alcohol Clin Exp Res. 1998;22(5):994–997. doi: 10.1111/j.1530-0277.1998.tb03694.x. [DOI] [PubMed] [Google Scholar]
- 74.Endogenous H, et al. Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. Br J Cancer. 2011;105(5):709–722. doi: 10.1038/bjc.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eliassen AH, Hankinson SE. Endogenous hormone levels and risk of breast, endometrial and ovarian cancers: prospective studies. Adv Exp Med Biol. 2008;630:148–165. [PubMed] [Google Scholar]
- 76.Gu JW, et al. Ethanol stimulates tumor progression and expression of vascular endothelial growth factor in chick embryos. Cancer. 2005;103(2):422–431. doi: 10.1002/cncr.20781. [DOI] [PubMed] [Google Scholar]
- 77.United States. Public Health Service. Office of the Surgeon General. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General. Rockville, MD. Washington, DC: U.S. Dept. of Health and Human Services, Public Health Service; 2010. p. 704. [Google Scholar]
- 78.Daniell HW. Increased lymph node metastases at mastectomy for breast cancer associated with host obesity, cigarette smoking, age, and large tumor size. Cancer. 1988;62(2):429–435. doi: 10.1002/1097-0142(19880715)62:2<429::aid-cncr2820620230>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 79.Murin S, Inciardi J. Cigarette smoking and the risk of pulmonary metastasis from breast cancer. Chest. 2001;119(6):1635–1640. doi: 10.1378/chest.119.6.1635. [DOI] [PubMed] [Google Scholar]
- 80.Manjer J, et al. Smoking associated with hormone receptor negative breast cancer. Int J Cancer. 2001;91(4):580–584. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1091>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 81.Lesser GT, Deutsch S. Measurement of adipose tissue blood flow and perfusion in man by uptake of 85Kr. J Appl Physiol. 1967;23(5):621–630. doi: 10.1152/jappl.1967.23.5.621. [DOI] [PubMed] [Google Scholar]
- 82.Collis T, et al. Relations of stroke volume and cardiac output to body composition: the strong heart study. Circulation. 2001;103(6):820–825. doi: 10.1161/01.cir.103.6.820. [DOI] [PubMed] [Google Scholar]
- 83.Mathew B, et al. Obesity: effects on cardiovascular disease and its diagnosis. J Am Board Fam Med. 2008;21(6):562–568. doi: 10.3122/jabfm.2008.06.080080. [DOI] [PubMed] [Google Scholar]
- 84.Shamsuzzaman AS, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105(21):2462–2464. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 85.Lundgren CH, et al. Elaboration of type-1 plasminogen activator inhibitor from adipocytes. A potential pathogenetic link between obesity and cardiovascular disease. Circulation. 1996;93(1):106–110. doi: 10.1161/01.cir.93.1.106. [DOI] [PubMed] [Google Scholar]
- 86.Steppan CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 87.Mora S, et al. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116(19):2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thijssen DH, et al. Impact of inactivity and exercise on the vasculature in humans. Eur J Appl Physiol. 2010;108(5):845–875. doi: 10.1007/s00421-009-1260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jacobs DR, Jr, Tapsell LC. Food, not nutrients, is the fundamental unit in nutrition. Nutr Rev. 2007;65(10):439–450. doi: 10.1111/j.1753-4887.2007.tb00269.x. [DOI] [PubMed] [Google Scholar]
- 90.Mozaffarian D, Ludwig DS. Dietary guidelines in the 21st century–a time for food. JAMA. 2010;304(6):681–682. doi: 10.1001/jama.2010.1116. [DOI] [PubMed] [Google Scholar]
- 91.Mozaffarian D, Appel LJ, Van Horn L. Components of a cardioprotective diet: new insights. Circulation. 2011;123(24):2870–2891. doi: 10.1161/CIRCULATIONAHA.110.968735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bradley KA, Donovan DM, Larson EB. How much is too much? Advising patients about safe levels of alcohol consumption. Arch Intern Med. 1993;153(24):2734–2740. doi: 10.1001/archinte.153.24.2734. [DOI] [PubMed] [Google Scholar]
- 93.Klatsky AL, et al. Alcohol consumption and blood pressure Kaiser-Permanente multiphasic health examination data. N Engl J Med. 1977;296(21):1194–1200. doi: 10.1056/NEJM197705262962103. [DOI] [PubMed] [Google Scholar]
- 94.Diamond I. Alcoholic myopathy and cardiomyopathy. N Engl J Med. 1989;320(7):458–460. doi: 10.1056/NEJM198902163200709. [DOI] [PubMed] [Google Scholar]
- 95.Stampfer MJ, et al. A prospective study of moderate alcohol consumption and the risk of coronary disease and stroke in women. N Engl J Med. 1988;319(5):267–273. doi: 10.1056/NEJM198808043190503. [DOI] [PubMed] [Google Scholar]
- 96.Pearson TA. Alcohol and heart disease. Circulation. 1996;94(11):3023–3025. doi: 10.1161/01.cir.94.11.3023. [DOI] [PubMed] [Google Scholar]
- 97.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43(10):1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 98.Craig WY, Palomaki GE, Haddow JE. Cigarette smoking and serum lipid and lipoprotein concentrations: an analysis of published data. BMJ. 1989;298(6676):784–788. doi: 10.1136/bmj.298.6676.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reaven G, Tsao PS. Insulin resistance and compensatory hyperinsulinemia: the key player between cigarette smoking and cardiovascular disease? J Am Coll Cardiol. 2003;41(6):1044–1047. doi: 10.1016/s0735-1097(02)02982-0. [DOI] [PubMed] [Google Scholar]