Abstract
Haplo-cord stem cell transplantation combines the infusion of CD34 selected hematopoietic progenitors from a haplo-identical donor with an umbilical cord blood graft from an unrelated donor and allows faster count recovery, with low rates of disease recurrence and chronic GVHD. But the contribution of the umbilical cord blood graft to long-term transplant outcome remains unclear.
We analyzed 39 recipients of haplo-cord transplants with AML and MDS, engrafted and in remission at 2 months. Median age was 66 (18-72) and all had intermediate, high, or very high risk disease. Less than 20% UCB chimerism in the CD33 lineage was associated with an increased rate of disease recurrence (54% vs 11% P<0.0001) and decrease in one year progression-free (20% vs 55%, P=0.004) and overall survival (30% vs 62%, P=0.02). Less than 100% UCB chimerism in the CD3 lineage was associated with increase rate of disease recurrence (46% vs 12%, P=0.007) Persistent haplo-chimerism in the CD3 lineage was associated with an increased rate of disease recurrence (40% vs 15%, P=0.009) Chimerism did not predict for treatment related mortality. The cumulative incidence of acute GVHD by day 100 was 43%. The cumulative incidence of moderate/severe chronic GVHD was only 5%.
Engraftment of the umbilical cord blood grafts provides powerful GVL effects which protect against disease recurrence and is associated with low risk of chronic GVHD. Engraftment of CD34 selected haplo-identical cells can lead to rapid development of circulating T-cells, but when these cells dominate, GVL-effects are limited and rates of disease recurrence are high.
Introduction
Haplo-cord stem cell transplantation combines the infusion of CD34 selected hematopoietic progenitor cells from a haplo-identical donor with an umbilical cord blood (UCB) graft from an unrelated donor.1–3 This combination allows faster count recovery than is observed after transplantation of single or double UBC by providing initial engraftment of the third party source, followed by permanent UCB engraftment.4;5 It also allows the use of smaller better HLA-matched UCB grafts thus leading to a low rate of acute and chronic graft-versus-host disease (GVHD). The haplo-cord strategy is based on the premise that long-term engraftment of an umbilical cord blood graft will provide superior outcomes to that of adult donor grafts in terms of graft-versus-leukemia (GVL)-effects and of GVHD. Supporting clinical evidence for this premise remains however scarce. After haplo-cord transplantation, initial engraftment of the haplo-identical donor is usually superseded by durable engraftment from the cord blood graft. But there are occasional cases where the cord blood graft fails and recipients are left with a permanent adult mismatched graft, or more commonly mixed chimerism.6 In a previous report, we analyzed causes of umbilical cord graft failure.6 Here we studied the implications of patterns of engraftment on disease recurrence in patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). To this purpose we focused on patients who were in remission with functioning grafts two months after transplant ensuring the study of durable patterns of chimerism.
Methods
Patients with hematologic malignancies in need of an allogeneic stem cell transplantation (SCT) for whom an HLA-identical related donor or HLA-identical URD donor could not be identified in a timely manner were offered haplo-cord transplant. Additional eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status ≤2, bilirubin ≤2 mg/dl, creatinine <1.5 times the upper limit of normal, preserved heart and lung function, and no evidence of chronic active hepatitis or cirrhosis. HIV negativity and negative pregnancy testing for females was required.
All the patients were enrolled in a prospective study of reduced intensity conditioning conducted by Weill Cornell Medical College. The primary objective of the study was to define the optimal cell dose of the UCB graft for haplo-cord transplantation, and if possible to match for inherited paternal antigens (IPA) and non-inherited maternal antigens (NIMA). The study was approved by the Institutional Review Board of Weill Cornell Medical College and all patients and donors provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki and registered on clinical trials.gov (NCT01810588). The present analysis focuses on the impact of graft dominance in a subset of patients - those with AML and MDS - accrued to this protocol.
Donors and stem cell processing
Cord blood
Cord blood units were selected based on HLA typing and cell count. Grafts were matched for at least 4 of 6 HLA loci by the standard cord criteria (ie, low resolution for HLA-AA and HLA-B and high resolution for HLA-DR)7 and contained a minimum cell count of 1.0 × 107 nucleated cells per kilogram of the recipient’s body weight before freezing. In contrast with common practice, we prioritized matching over cell dose. As of mid-2012 we utilized high resolution HLA typing for HLA-A, B, C and DR in the selection of cord blood grafts.8
Haploidentical donor
The haplo-identical donor was a relative in 38/39 cases. In one subject who did not have a haplo-identical relative, a partially matched unrelated donor was used. Donors underwent stem cell mobilization using filgrastim for four consecutive days +/− plerixafor on day 4. Apheresis was started on day 5 and continued daily until at least 5 × 106 CD34+ cells/recipient kg were collected. After collection and prior to cryopreservation, haplo-identical grafts were CD34-selected using the Miltenyi CliniMACS device under an Investigational New Device (IND) permit from the United States Food and Drug Agency.
The infused cell dose of the haplo-graft was fixed at a minimum of 3 × 106 CD34 cells/kg and a maximum of 5 × 106 CD34 cells/kg of recipient weight. For both UCB and haplo-identical grafts, donors targeted by pre-existing recipient HLA-antibodies (i.e, donor specific antibodies or DSA) were avoided or, when unavoidable, various strategies –including infusion of intravenous immunoglobulins, treatment with bortezomib, with rituximab or even plasma-exchange-were used to limit exposure of the graft to DSA.9;10
Preparative regimen
Patients received fludarabine 30mg/m2/d iv for 5 consecutive days (days −7, −6, −5, −4, −3), rabbit antithymocyte globulin (thymoglobulin, r-ATG) at 1.5 mg/kg every other day for 3 or 4 doses, and melphalan 140 mg/m2/d for 1 dose on day-2.11;12 Six patients were also given TBI 400 cGray because of concern over disease recurrence. The haplo-identical cells were infused on day 0 followed by UCB grafts later the same day or on day 1.
Post-transplant immunosuppression
Tacrolimus at 0.03 mg/kg/d iv was given by continuous infusion over 24 hours from day-2 until engraftment or when patient was able to take it orally. Tacrolimus was targeted to maintain levels of 5-15 ng/ml through day 180. Thereafter, tacrolimus was tapered by 20% every week unless there was evidence of acute GVHD (aGVHD) or chronic GVHD (cGVHD). Mycophenolate mofetil 1 gram po three times a day was given until day 28. A few patients who did not tolerate tacrolimus were –sometimes transiently-transitioned to sirolimus. Other aspects of supportive care and GVHD treatment were as previously reported.4
Chimerism Assessment
CD3 positive and CD33 positive cells were isolated from post-transplant peripheral blood (PB) samples using magnetically-labeled anti-human CD3+ or CD33+ antibodies (isotypes: mouse IgG1 and IgM kappa, respectively) and the fully automated RoboSep® cell separator (STEMCELL Technologies Inc., Vancouver, Canada). A purity of > 95% CD3- or CD-33 positive cells was routinely achieved using this procedure. DNA was extracted from both CD3 and CD33 positive cells using QiaAmpTM kits (Qiagen Inc., Valencia, CA). DNA concentration and purity were measured using a NanodropTM spectrophotometer. When WBC was lower than 2 ×109/L, isolation of CD3 or CD33 cells was not performed, instead whole blood chimerism was analyzed.
Chimerism was analyzed using short tandem repeat (STR) analysis using the PowerPlex® 16 HS System(Promega, Madison, WI), a multiplex PCR reaction which simultaneously amplifies 16 STR loci for the identification of recipient- and donor-specific loci, and to monitor engraftment, according to Rennert et al.13 Briefly, 2 ng of DNA were amplified in a total reaction volume of 10 uL and PCR products were separated by capillary electrophoresis on an ABI 3130 PRISM Genetic Analyzer (Applied Biosystems, Foster City, CA). Fragment sizes were determined with GeneMapper 4.0 software (ABI). Assessment of donor cell engraftment was performed by determining the ratio of peak areas for donor and recipient informative alleles. Using this method, the assay was shown to be linear for mixtures in the range of 5–95%. The analytical sensitivity of the test was 1%. Results were reported as percentage recipient and donor present in each DNA sample. Hence, a result of ≥95% was considered full donor chimerism (FDC).
PB samples were obtained at 30 days (± 3 days), 56 days (± 7 days), 100 days (± 7 days), 180 days (± 10 days), and 1 year (± 15 days), following HCT. Samples were then collected yearly thereafter, and at the time of disease recurrence.
In 5 of 39 cases, no chimerism sample was available for CD33 measurements on day 56, but both prior and subsequent samples were available and the mean of prior (usually day 28) and subsequent chimerism was used to impute the day 56 value. In two of these six cases, the day 28 WBC was below 2 ×10^9/L and D28 chimerism was tested on unfractionated cells. In one case the day 56 chimerism was unfractionated. (Supplemental figure A) In 9 of 39 cases, no chimerism sample was available for CD3 measurements on day 56. In six of these cases, but both prior and subsequent samples were available and the mean of prior (usually day 28) and subsequent chimerism was used to impute the day 56 value. (Supplemental figure B). In three patients, we could not with confidence impute day 56 CD3 chimerism. Further analysis relating to CD3 chimerism were based on 36 patients.
Statistical Methods
Descriptive statistics such as count, percentage, mean, standard deviation, median and range were used to describe study variables. A landmark analysis of patients alive and in remission at day 56 was conducted to determine the effect of CD33 and CD3 chimerism on the outcome of these patients. For this purpose, patients were initially divided in three groups: those in the lowest quartile of UCB engraftment, those with higher UCB engraftment but mixed chimerism, and those with full UCB chimerism. Disease recurrence rates were very similar between those in the lowest quartile of UCB CD3 chimerism and those with higher percentages of mixed UCB CD3 chimerism (Supplemental figure C). Therefore those two groups were combined and we compared 16 patients with mixed UCB CD3 chimerism with 20 who had full UCB CD3 chimerism. Ten of the patients with mixed CD3 chimerism had contributions from host, haplo donor and UCB donor. We compared outcomes of those with absent CD3 haplo-chimerism to those who had any degree of CD3 haplo-chimerism. Similarly we compared outcomes of those with absent CD3 host-chimerism to those who had any degree of CD3 host-chimerism.
Recurrence rates were high for those in the lowest quartile of UCB CD33 chimerism; however they were similar between those in the next quartile of UCB CD33 chimerism and those with full UCB CD33 chimerism. (Supplemental figure D). Therefore those two groups were combined and we compared 10 patients in the lowest quartile of UCB CD33 chimerism (0–20%) with 29 who had at least 25% UCB CD33 chimerism. Since there were only 2 patients with any host contribution to CD33 chimerism, the degree of CD33 haplo-chimerism was inversely proportional to CD33 UCB chimerism, obviating the need for separate analysis of CD33 haplo or host chimerism.
Progression-free survival (PFS) and overall survival were calculated using the Kaplan-Meier product-limit estimate and expressed as probabilities with a 95% confidence interval (CI). Comparisons used log-rank testing. For PFS subjects were considered treatment failures at the time of relapse or progression or death from any cause.14
The cumulative incidence of disease progression with death before progression as the competing risk, as well as cumulative incidence of treatment related mortality with non-treatment related death as the competing risk were also calculated.14 Comparisons were performed using Gray’s test. The incidence of aGVHD and cGVHD were calculated using the cumulative incidence function, with death, relapse, disease progression, and graft failure as competing risks. Statistical software (Statsoft) was used for most analyses. R software was used for calculations of cumulative incidence. All risk factor analysis was considered exploratory and Bonferroni or other corrections were not used for multiple comparisons.
Results
Patient characteristics
Fifty-five patients with AML or MDS were enrolled between January 2012 and February 2015. Sixteen patients were excluded from analysis because of early death (n=4), persistent disease/relapse before day 60 (n = 7), failure of both grafts with residual MDS (n = 2), or lack of chimerism measurements (n = 3).
Thirty-nine patients were analyzed and their clinical characteristics are shown in Table 1. All 39 were included in the analysis relating to CD33 chimerism, but only 36 were included in the analysis relating to CD3 chimerism. All patients had routine bone marrow biopsy and aspiration with molecular and cytogenetic studies at approximately day 28 post-transplant. None had morphologic or cytogenetic evidence of disease recurrence at that time, although one patient, previously FLT3-ITD positive, was found to have a new ASXL1 mutation on day 28, without morphologic or cytogenetic evidence of recurrence and without persistent FLT3 –ITD. No patient had any clinical evidence of disease recurrence at two months. Median age was 66 years (18–72 years). Twenty-two patients had an intermediate category disease risk index (DRI), fifteen had a high DRI, and two had a very high DRI.15 Twenty-three patients had HCT-CI ≥3.16–18 Conditioning was Flu Mel ATG (n=33) or Flu Mel TBI 400 ATG (n=6). Median follow up for survivors at the time of this analysis is 19 months (range 10-37 months)
Table 1.
Patients, Disease, Graft and Conditioning
| N | 39 |
| Median Age (range) | 66 (18-72) |
| Male/female | 19/20 |
| Median Weight | 80 (41-118) |
| Disease | |
| DRI Intermediate/High/Very High | 22/15/2 |
| KPS ≤ 80 vs >90-100 | 14/25 |
| HCTCI >3 vs ≤ 3 >3 | 15/24 |
| Haplo Graft | |
| HLA match 4-5/8 vs 6-7/8 | 35 vs 4 |
| DSA present | 4 |
| UCB Graft | |
| CD34 Dose collected median-range (×105.kg) | .66 (.13–4.3) |
| CBU viability at infusion | 97% (88–99) |
| TNC infused (×107.kg) | 2.1 (0.8–3.9) |
| HLA match 4-5-6/8 vs 7-8/8 | 16 vs 23 |
| DSA present | 6 |
| Flu Mel ATG vs Flu MEL ATG Low DBI | 33 vs 6 |
Patterns of Chimerism
The distribution of CD3 and CD33 chimerism by day 56 is shown in figures 1A and 1B. Sixteen patients (44%) had no or mixed UCB CD3 chimerism at a median percentage of 63% (range 0–98) and were compared to 20 patients (55 %) who were full UCB chimeras in the CD3 compartment (100% UCB CD3 chimerism).
Figure 1.
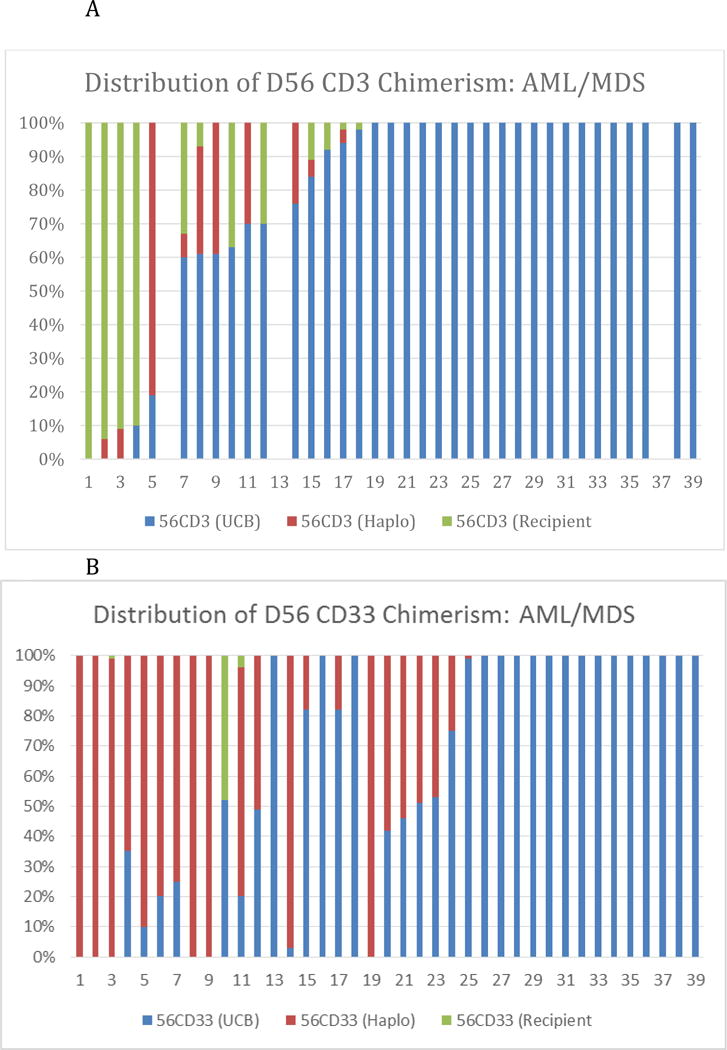
Distribution of D56 Chimerism-Each Individual Patient is Represented
A: Distributon of CD33 chimerism-B: Distribution of CD Chimerism
The ten patients in the lowest quartile of UCB CD33 chimerism (low UCB CD33 chimerism group) had a median UCB CD33 chimerism of 0% (range 0–20). There were an additional 12 patients (33%) with higher mixed UCB CD33 chimerism. with a median UCB CD33chimerism at 52% (range 25–99%) (Moderate UCB CD33 chimerism group). Fourteen patients (25%) were full UCB chimeras in the CD33 compartment (100% UCB CD33 chimerism).
Mixed chimerism in CD3 cells was composed of variable percentages of host, donor and recipient CD3 cells. Host CD3 chimerism was present in twelve cases and ranged from 2% to 100% (median 30%). Haplo CD3 chimerism was present in ten cases and ranged from 4% to 81% (median 30%)
Mixed chimerism in CD33 cells was usually composed of a mixture of umbilical cord and haplo-grafted cells. In two cases there was a component of host CD33 chimerism (4% and 44%).
Chimerism and Disease Recurrence (Table 2)
Table 2.
Day 56 Chimerism and Transplant outcomes and 95% CI-Significant differences highlighted
| D56 UCB CD33 Low (n=10) |
D56 UCB CD33 Moderate/full (n=29) |
P value | D56 UCB CD3 Mixed (n=16) |
D56 UCB CD3 Full (n=20) |
P value | D56 haplo CD3 Mixed (n=10) |
D56 haplo CD3 None (n=26) |
P value | D56 host CD3 Mixed (n=11) |
D56 host CD3 None (n=25) |
P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CI Relapse @ 1 year | 54% | 11% | 0.0001 | 46% | 12% | 0.007 | 40% | 15% | 0.009 | 33% | 16% | 0.09 |
| CI TRM@ 1 Year | 34% | 12% | 0.23 | 18 | 36 | 0.24 | 20% | 34% | 0.37 | 17% | 37% | 0.16 |
| KM PFS @ 1 year | 20% | 55% | 0.004 | 30% | 55% | 0.21 | 33% | 52% | 0.10 | 46% | 48% | 0.78 |
| KM OS@ 1 year | 30% | 62% | 0.02 | 50% | 48% | 0.92 | 44% | 55% | 0.48 | 61% | 48% | 0.30 |
| CI AGVHD gr II-IV@ d100 | 50% | 41% | 0.88 | 27% | 64% | 0.09 | 50% | 53% | 0.8 | 33% | 62% | 0.08 |
| CI cGVHD | 0% | 7% | 0.33 | 0% | 8% | 0.35 | 0% | 8% | 0.38 | 0% | 8% | 0.31 |
The cumulative incidence of disease progression at one year was 29% (95% CI 15-43). The median time to disease progression was day 179 after transplant (range day 79-day 641). There was a strong association between UCB CD3 chimerism on day 56 and disease progression. The cumulative risk of disease relapse in subjects with mixed CD3 UCB chimerism was 46% at one year vs 12% for those with full CD3 UCB chimerism (p=0.007). (Figure 2A) The ten subjects with partial haplo CD3 chimerism had a much increased risk for disease recurrence (P=0.000) compared to those who did not have residual haplo chimerism. But presence of host CD3 chimerism in eleven cases was not significantly associated with disease recurrence.
Figure 2.
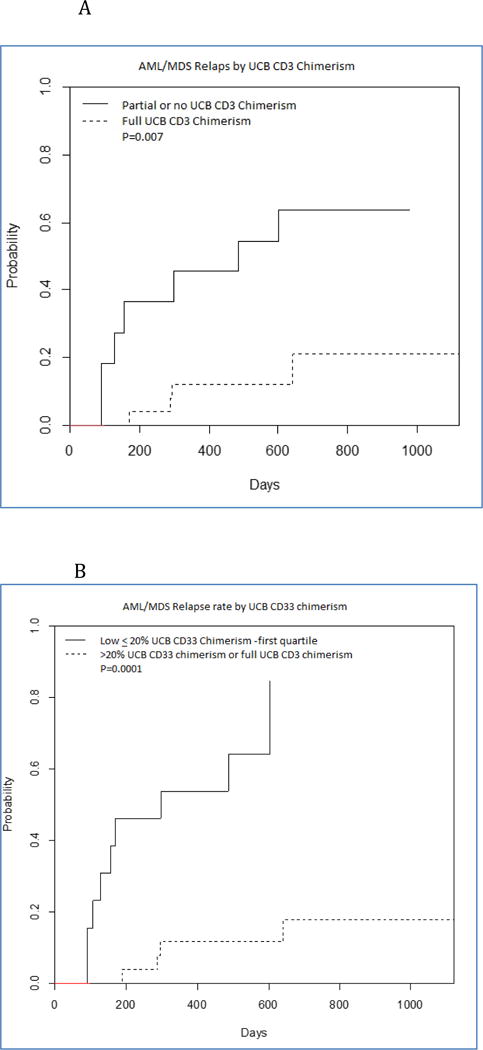
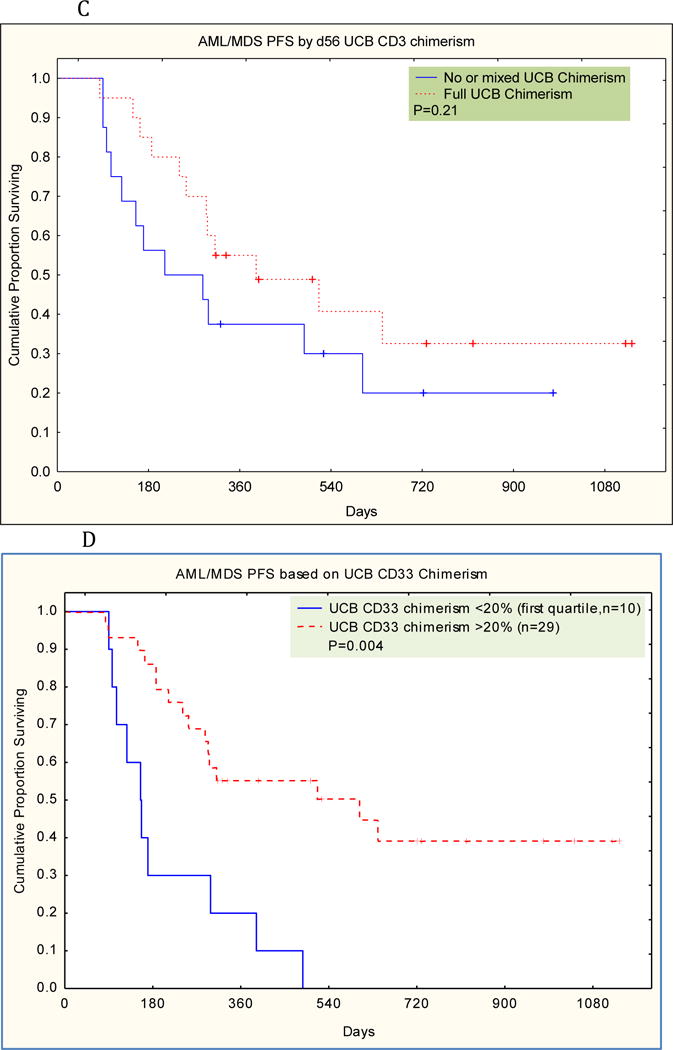
A. Cumulative Incidence of Relapse based on D56 UCB CD3 Chimerism AML-MDS
B. Cumulative Incidence of Relapse based on D56 UCB CD33 Chimerism AML-MDS
C. Progression Free Survival based on D56 UCB CD3 Chimerism-AML-MDS
D. Progression Free Survival based on D56 UCB CD33 Chimerism-AML-MDS
There was also a very strong correlation between the day 56 degree of UCB chimerism in the CD33 lineage and risk for disease recurrence. (Figure 2B).The cumulative risk of disease relapse in subjects with low D56 CD33 UCB chimerism was 54% at one year vs 11% for those with moderate/full D56 CD33 UCB chimerism (p=0.0001).
Chimerism and Treatment Related Mortality, Progression Free Survival and GVHD (Table 2)
The cumulative incidence of treatment related mortality at one year was 27% (95% CI 13-41). There was no statistically significant relation between UCB CD3 chimerism, haplo CD3 chimerism, host CD3 chimerism or UCB CD33 chimerism and risk for treatment related mortality.
The Kaplan-Meier estimate of one year progression free survival was 44% (95% CI 28-60). As a result of the much decreased rate of disease recurrence, progression free survival was significantly superior in those with high CD33 UCB chimerism. (Figure 2C)
The cumulative incidence of aGVHD (grade II–IV) was 42% at day 100. Most acute GVHD was grade III characterized by gut GVHD and reversible. There was only one case of fatal acute GVHD. Neither degree of UCB CD3 nor UCB CD33 chimerism were associated with the risk for acute GVHD. There were only two case of moderate chronic GVHD, both in patients with high CD3 chimerism, for a cumulative incidence of five percent (Table 2 and figure 3B). One of them had been lost to follow-up and had discontinued immunosuppression prematurely. There was no statistically significant relation between chimerism and risk for acute or chronic GVHD.
Figure 3.
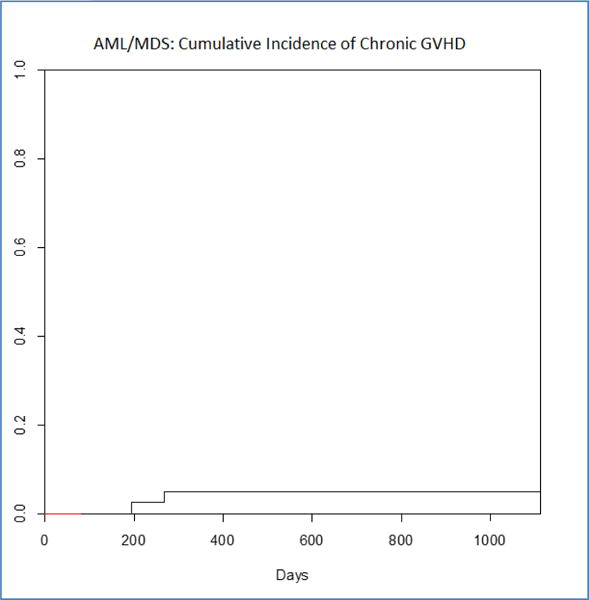
Cumulative Incidence of Chronic GVHD
Discussion
The haplo-cord procedure with infusion of grafts from different sources provides an opportunity to directly assess the relative immune competence of umbilical cord blood and adult haplo-identical grafts. Here we show that predominance of the umbilical cord blood graft is associated with much reduced rates of disease recurrence and improved progression-free survival, indicating a protective effect of cord chimerism on prevention of disease recurrence. Among those with high rates of UCB donor-derived chimerism, rates of disease recurrence were remarkably low at approximately eleven percent at one year. The protective effect of umbilical cord blood engraftment was not associated with an increased rate of acute GVHD, and the rates of chronic GVHD were extraordinarily low. The difference in recurrence rates is unlikely due to differences in disease characteristics which –as measured by DRI- were similar in those with high vs low UCB chimerism.
Our data are consistent with other clinical studies that consistently show lower rates of recurrence of AML after UCB transplantation compared to transplant from adult unrelated donors, suggesting that they mediate superior GVL effects.19;20 These clinical observations are supported by animal data.21 Using a NSG model, mice with Burkitt’s lymphoma were transplanted with mismatched UCB cells or mismatched adult PBSC. The UCB grafts mediated far superior GVL-effects and less GVHD than the adult cells. The mechanism by which UCB cells mediate their GVL effects remain only partially understood. Fetal adaptive immunity is fundamentally geared toward tolerance and a preponderance of T-regulatory cells.22 But recent evidence also suggests that they are more adaptive and that, in a tumor environment UCB T-cells can rapidly transform into Th-17 cells with effector capacity.21 Other proposed mechanisms of UCB mediated GVL effects relate to contamination with maternal cells sensitized to inherited paternal antigens.23
By contrast, adult haplo-identical donor cells do not seem to provide superior GVL effects in myeloid malignancies. We found that CD3 chimerism form the haplo-donor was associated with a much increased risk of disease recurrence. These findings again are consistent with numerous other clinical observations. Ringden et al found no difference in recurrence rate between sibling transplant and haplo-identical transplant.24. In a parallel study of RIC umbilical cord blood and haplo-identical transplant, Brunstein et al found much increased rates of disease recurrence after haplo-identical transplantation.25. Ciurea et al compared haplo-identical with unrelated donor transplantation and found an increased rate of disease recurrence after haplo-identical transplant with reduced intensity conditioning that reached 42% at one year.26 Kasamon et al reported on 65 patients with AML undergoing T-replete haplo-identical RIC conditioning and found a one year rate of disease recurrence of 42%.27 These rates of disease recurrence contrast sharply with those observed in our patients with full UCB engraftment, particularly since in both the Ciurea and Kasamon studies, the percentage of patients with a high disease risk index was only 16% as opposed to 45% in our study.
While intuitively it might be thought that the HLA-mismatch provides an additional target for GVL effects, several mechanisms might contribute to escape from GVL effects after haplo-identical transplantation.24 Most interestingly and possibly specific to AML is the observation that leukemia cells escape GVL effects directed against the host HLA haplo-type through genomic loss of the HLA locus.28;29
Residual host T-lymphoid chimerism present in approximately one third of cases, and often thought of as a harbinger of disease relapse30;31, was not related to disease progression. It was associated with a moderate (not statistically significant) reduction in acute GVHD, consistent with prior observations in adult donor transplantation using in-vivo T-cell depletion32;33.
Our results are based on a small series and have several additional limitations. First, the distribution of chimerism patterns did not follow a normal distribution, therefore analysis as a continuous variable did not seem advisable and any categorical division has an arbitrary aspect.34 Second, use of ATG has been controversial in umbilical cord blood transplantation, because of delays in immune reconstitution.35;36 It may however be necessary in the haplo-cord procedure to avoid fulminant rejection of the haplo-graft with associated toxicity.37 It is uncertain if the results obtained with ATG in conditioning, can be extrapolated to other conditioning regimens and/or methods of GVHD prophylaxis. A third limitation consists of the fact that the population we analyzed was restricted to those surviving in remission for more than 60 days. Relapse and/or graft failure frequently occurs earlier, but the distinction between disease persistence and graft failure can be challenging in such cases. Fourth, in chimerism, distinguishing predictors of relapse from early diagnosis of disease recurrence is difficult. The fact that host chimerism was hardly ever present in the CD33 fraction of our patients, strongly argues against early recurrence as an explanation for our findings.
For all these reasons, our results can only be hypothesis generating and require confirmation in further prospective studies. Still, they were achieved in a group of older adults with a median age of 66 and with very high risk disease. This group is quite representative of those most in need of stem cell transplant. Survival benefit from allogeneic transplantation is most pronounced in older patients,38 but the lack of suitable HLA-identical siblings is a major limitation for this group.39 Concerns over acute and chronic GVHD are deterrents40 and avoidance of chronic GVHD is of great importance given its severe toxicity, its detrimental impact on long-term survival and its lack of impact on recurrence rates in AML.41–43 The extremely low rates of chronic GVHD using the haplo-cord platform are therefore of major interest in this older patient population.
In summary we provide preliminary evidence that umbilical cord blood grafts mediate superior GVL effects in AML and MDS patients and that umbilical cord blood engraftment results in improved progression free survival, without excessive acute GVHD and with a very low risk of chronic GVHD. We continue efforts to enhance UCB engraftment in this setting by limiting the cell dose of the haplo-identical graft,4;6 avoiding haplo-grafts which are matched to the recipient at more than 5 of 8 HLA-alleles,44 by avoiding grafts targeted by donor specific antibodies and by selecting appropriate cord blood units.9
Supplementary Material
References
- 1.Lindemans CA, van Besien K. Topping it up: methods to improve cord blood transplantation outcomes by increasing the number of CD34+ cells. Cytotherapy. 2015;17:723–729. doi: 10.1016/j.jcyt.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Liu H, van Besien K. Alternative donor transplantation–“mixing and matching”: the role of combined cord blood and haplo-identical donor transplantation (haplo-cord SCT) as a treatment strategy for patients lacking standard donors? Curr Hematol Malig Rep. 2015;10:1–7. doi: 10.1007/s11899-014-0245-y. [DOI] [PubMed] [Google Scholar]
- 3.Kwon M, Bautista G, Balsalobre P, et al. Haplo-cord transplantation using CD34+ cells from a third-party donor to speed engraftment in high-risk patients with hematologic disorders. Biol Blood Marrow Transplant. 2014;20:2015–2022. doi: 10.1016/j.bbmt.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 4.Liu H, Rich ES, Godley L, et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood. 2011;118:6438–6445. doi: 10.1182/blood-2011-08-372508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Besien K, Hari P, Zhang MJ, et al. Reduced intensity haplo plus single cord transplant compared to double cord transplant: improved engraftment and survival free of progression and GVHD (GRFS) Haematol. 2016 doi: 10.3324/haematol.2015.138594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai SB, Liu H, Shore T, et al. Frequency and Risk Factors Associated with Cord Graft Failure (CGF) after Transplant with Single Unit Umbilical Cord Cells Supplemented by Haploidentical Cells (Haplo-Cord) with Reduced-Intensity Conditioning. Biol Blood Marrow Transplant. 2016 doi: 10.1016/j.bbmt.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker JN, Byam C, Scaradavou A. How I treat: the selection and acquisition of unrelated cord blood grafts. Blood. 2011;117:2332–2339. doi: 10.1182/blood-2010-04-280966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eapen M, Klein JP, Ruggeri A, et al. Impact of allele-level HLA matching on outcomes after myeloablative single unit umbilical cord blood transplantation for hematologic malignancy. Blood. 2014;123:133–140. doi: 10.1182/blood-2013-05-506253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshihara S, Taniguchi K, Ogawa H, Saji H. The role of HLA antibodies in allogeneic SCT: is the ‘type-and-screen’ strategy necessary not only for blood type but also for HLA? Bone Marrow Transplant. 2012;47:1499–1506. doi: 10.1038/bmt.2011.249. [DOI] [PubMed] [Google Scholar]
- 10.Gergis U, Mayer S, Gordon B, et al. A strategy to reduce donor-specific HLA Abs before allogeneic transplantation. Bone Marrow Transplant. 2014;49:722–724. doi: 10.1038/bmt.2014.11. [DOI] [PubMed] [Google Scholar]
- 11.Van Besien KW, Demuynck H, LeMaistre CF, Bogaerts MA, Champlin RE. High dose melphalan allows durable engraftment of allogeneic bone marrow. Bone Marrow Transplant. 1995;15:321–323. [PubMed] [Google Scholar]
- 12.Giralt S, Thall PF, Khouri II, et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood. 2001;97:631–637. doi: 10.1182/blood.v97.3.631. [DOI] [PubMed] [Google Scholar]
- 13.Rennert H, Leonard DG, Cushing M, Azurin C, Shore T. Avoiding pitfalls in bone marrow engraftment analysis: a case study highlighting the weakness of using buccal cells for determining a patient’s constitutional genotype after hematopoietic stem cell transplantation. Cytotherapy. 2013;15:391–395. doi: 10.1016/j.jcyt.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Klein JP, Rizzo JD, Zhang MJ, Keiding N. Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part 2: Regression modeling. Bone Marrow Transplant. 2001;28:1001–1011. doi: 10.1038/sj.bmt.1703271. [DOI] [PubMed] [Google Scholar]
- 15.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123:3664–3671. doi: 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armand P, Kim HT, Cutler CS, et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2007;109:4586–4588. doi: 10.1182/blood-2006-10-054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorror M, Storer B, Sandmaier BM, et al. Hematopoietic cell transplantation-comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer. 2008;112:1992–2001. doi: 10.1002/cncr.23375. [DOI] [PubMed] [Google Scholar]
- 18.Artz AS, Wickrema A, Dinner S, et al. Pretreatment C-reactive protein is a predictor for outcomes after reduced-intensity allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:1209–1216. doi: 10.1016/j.bbmt.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponce DM, Hilden P, Devlin SM, et al. High Disease-Free Survival with Enhanced Protection against Relapse after Double-Unit Cord Blood Transplantation When Compared with T Cell-Depleted Unrelated Donor Transplantation in Patients with Acute Leukemia and Chronic Myelogenous Leukemia. Biol Blood Marrow Transplant. 2015;21:1985–1993. doi: 10.1016/j.bbmt.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verneris MR, Brunstein CG, Barker J, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 2009;114:4293–4299. doi: 10.1182/blood-2009-05-220525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiwarkar P, Qasim W, Ricciardelli I, et al. Cord blood T cells mediate enhanced antitumor effects compared with adult peripheral blood T cells. Blood. 2015;126:2882–2891. doi: 10.1182/blood-2015-06-654780. [DOI] [PubMed] [Google Scholar]
- 22.Mold JE, McCune JM. Immunological Tolerance During Fetal Development Advances in Immunology. Elsevier; 2012. pp. 73–111. [DOI] [PubMed] [Google Scholar]
- 23.van Rood JJ, Scaradavou A, Stevens CE. Indirect evidence that maternal microchimerism in cord blood mediates a graft-versus-leukemia effect in cord blood transplantation. Proceedings of the National Academy of Sciences. 2012;109:2509–2514. doi: 10.1073/pnas.1119541109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ringden O, Labopin M, Ciceri F, et al. Is there a stronger graft-versus-leukemia effect using HLA-haploidentical donors compared with HLA-identical siblings? Leukemia. 2016;30:447–455. doi: 10.1038/leu.2015.232. [DOI] [PubMed] [Google Scholar]
- 25.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciurea SO, Zhang MJ, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126:1033–1040. doi: 10.1182/blood-2015-04-639831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasamon YL, Bolanos-Meade J, Prince GT, et al. Outcomes of Nonmyeloablative HLA-Haploidentical Blood or Marrow Transplantation With High-Dose Post-Transplantation Cyclophosphamide in Older Adults. J Clin Oncol. 2015;33:3152–3161. doi: 10.1200/JCO.2014.60.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crucitti L, Crocchiolo R, Toffalori C, et al. Incidence, risk factors and clinical outcome of leukemia relapses with loss of the mismatched HLA after partially incompatible hematopoietic stem cell transplantation. Leukemia. 2015;29:1143–1152. doi: 10.1038/leu.2014.314. [DOI] [PubMed] [Google Scholar]
- 29.Vago L, Toffalori C, Ciceri F, Fleischhauer K. Genomic loss of mismatched human leukocyte antigen and leukemia immune escape from haploidentical graft-versus-leukemia. Semin Oncol. 2012;39:707–715. doi: 10.1053/j.seminoncol.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Koreth J, Kim HT, Nikiforow S, et al. Donor chimerism early after reduced-intensity conditioning hematopoietic stem cell transplantation predicts relapse and survival. Biol Blood Marrow Transplant. 2014;20:1516–1521. doi: 10.1016/j.bbmt.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee HC, Saliba RM, Rondon G, et al. Mixed T Lymphocyte Chimerism after Allogeneic Hematopoietic Transplantation Is Predictive for Relapse of Acute Myeloid Leukemia and Myelodysplastic Syndromes. Biol Blood Marrow Transplant. 2015;21:1948–1954. doi: 10.1016/j.bbmt.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Besien K, Dew A, Lin S, et al. Patterns and kinetics of T-cell chimerism after allo transplant with alemtuzumab-based conditioning: mixed chimerism protects from GVHD, but does not portend disease recurrence. Leuk Lymphoma. 2009;50:1809–1817. doi: 10.3109/10428190903200790. [DOI] [PubMed] [Google Scholar]
- 33.Lim ZY, Pearce L, Ho AY, et al. Delayed attainment of full donor chimaerism following alemtuzumab-based reduced-intensity conditioning haematopoeitic stem cell transplantation for acute myeloid leukaemia and myelodysplastic syndromes is associated with improved outcomes. Br J Haematol. 2007;138:517–526. doi: 10.1111/j.1365-2141.2007.06676.x. [DOI] [PubMed] [Google Scholar]
- 34.Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Nat Cancer Inst. 1994;86:829–835. doi: 10.1093/jnci/86.11.829. [DOI] [PubMed] [Google Scholar]
- 35.Pascal L, Tucunduva L, Ruggeri A, et al. Impact of ATG-containing reduced-intensity conditioning after single- or double-unit allogeneic cord blood transplantation. Blood. 2015;126:1027–1032. doi: 10.1182/blood-2014-09-599241. [DOI] [PubMed] [Google Scholar]
- 36.Lindemans CA, Chiesa R, Amrolia PJ, et al. Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcome. Blood. 2014;123:126–132. doi: 10.1182/blood-2013-05-502385. [DOI] [PubMed] [Google Scholar]
- 37.Lindemans CA, Te Boome LC, Admiraal R, et al. Sufficient Immunosuppression with Thymoglobulin Is Essential for a Successful Haplo-Myeloid Bridge in Haploidentical-Cord Blood Transplantation. Biol Blood Marrow Transplant. 2015;21:1839–1845. doi: 10.1016/j.bbmt.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Kurosawa S, Yamaguchi T, Uchida N, et al. Comparison of allogeneic hematopoietic cell transplantation and chemotherapy in elderly patients with non-M3 acute myelogenous leukemia in first complete remission. 2011;17:401–411. doi: 10.1016/j.bbmt.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Estey E, de LM, Tibes R, et al. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS) Blood. 2007;109:1395–1400. doi: 10.1182/blood-2006-05-021907. [DOI] [PubMed] [Google Scholar]
- 40.Kurosawa S, Yamaguchi T, Miyawaki S, et al. A Markov decision analysis of allogeneic hematopoietic cell transplantation versus chemotherapy in patients with acute myeloid leukemia in first remission. 2010;117:2113–2120. doi: 10.1182/blood-2010-05-285502. [DOI] [PubMed] [Google Scholar]
- 41.Boyiadzis M, Arora M, Klein JP, et al. Impact of Chronic Graft-versus-Host Disease on Late Relapse and Survival on 7,489 Patients after Myeloablative Allogeneic Hematopoietic Cell Transplantation for Leukemia. Clin Cancer Res. 2015;21:2020–2028. doi: 10.1158/1078-0432.CCR-14-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Besien K. Allogeneic transplantation for AML and MDS: GVL versus GVHD and disease recurrence. Hematology Am Soc Hematol Educ Program. 2013;2013:56–62. doi: 10.1182/asheducation-2013.1.56. [DOI] [PubMed] [Google Scholar]
- 43.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110:3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koshy N, Mayer S, Phillips A, et al. Predictors and GVL Effects of UCB Chimerism after Haplo-Cord Transplant. Blood. 2015;126(Suppl 1):4385. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


