Abstract
What came first: oxygen-producing photosynthesis, or compounds that protect cells from oxygen-induced damage? It emerges that one such compound might have been produced in microbes before Earth’s oxygenation.
Living cells are highly susceptible to injury induced by oxygen and the more reactive products of its metabolism. Organisms therefore have the ability to produce or acquire antioxidant compounds that help to mitigate the deleterious effects of living in Earth’s highly oxidizing environments. But Earth was an oxygen-free (anaerobic) planet until microbes evolved the ability to produce molecular oxygen (O2) through photosynthesis, raising a chicken-and-egg question: what evolved first, photosynthesis or antioxidant systems?1 Writing in Angewandte Chemie, Burn et al.2 cast fresh light on the matter by reporting that the anaerobic bacterium Chlorobium limicola might produce the putative antioxidant ergothioneine (Fig. 1) through an enzymatic reaction that differs from all other known pathways. This means that at least some naturally occurring compounds might have had an alternative role in early anaerobic environments, only to be repurposed as antioxidants as the environment became more oxidizing.
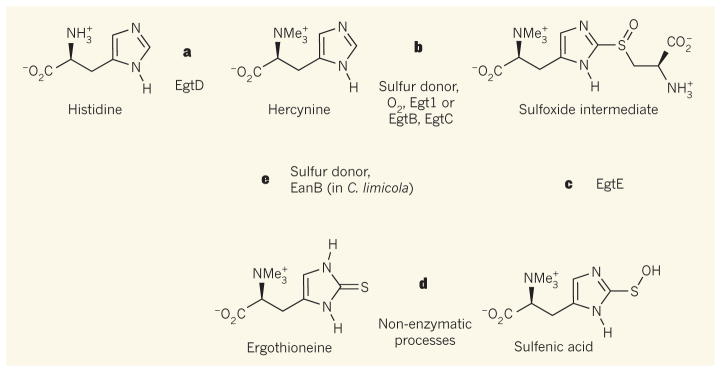
Figure 1. Biosynthetic pathways to ergothioneine.
Some fungi and bacteria produce ergothioneine, a putative antioxidant. a, In the first step, the enzyme EgtD converts the amino acid histidine into hercynine. Me, methyl group. b, The enzyme Egt1 (in fungi) or the pair of enzymes EgtB and EgtC (in bacteria) catalyses the reaction of hercynine with a sulfur donor, producing a sulfoxide intermediate. Molecular oxygen (O2) is needed for the reactions to occur. c, The sulfoxide intermediate produced in b is transformed to a sulfenic acid by the enzyme EgtE. d, The sulfenic acid is finally converted into ergothioneine non-enzymatically. e, Burn et al.2 report that the bacterium Chlorobium limicola encodes an enzyme, EanB, that can convert hercynine directly into ergothioneine in the presence of a suitable sulfur donor, without oxygen. Cofactors and side products of reactions are not shown.
The biosynthesis of ergothioneine was previously thought to require an aerobic environment. Its assembly occurs through a series of chemical transformations, beginning with the amino acid histidine3–7. The first step in this pathway adds three methyl groups to the amino group of histidine, to form an intermediate called hercynine (Fig. 1a). In fungal ergot-hioneine producers, such as Neurospora crassa, the enzyme Egt1 then catalyses the oxidation of hercynine and the amino acid cysteine, and combines them to form a sulfoxide intermediate (Fig. 1b); the cysteine is the source of the sulfur atom that ends up in ergothioneine. A similar oxidative process is catalysed by the enzymes EgtB and EgtC in bacteria such as Mycobacterium smegmatis, with the peptide γ-glutamylcysteine serving as the sulfur donor. In both fungi and bacteria, molecular oxygen is needed to accept the four electrons that are liberated during these oxidation reactions — which means that the conversion of hercynine to the sulfoxide requires an aerobic environment.
The final step of the pathway is decomposition of the sulfoxide, a process catalysed by the enzyme EgtE (Fig. 1c). The resulting sulfenic acid is converted directly to ergothioneine (Fig. 1d) in the presence of suitable reductants. Alternatively, in a more-oxidizing environment, the sulfenic acid can react with itself non-enzymatically, forming ergothioneine and regenerating hercynine in the process.
Burn and co-workers, however, have found that the genome of the anaerobe C. limicola does not possess a gene homologous with egtB (that is, a gene that shares ancestry with egtB, as inferred from the genes’ DNA sequences), despite having a homologue of egtD. They instead discovered that the bacterium has an enzyme called EanB, which can catalyse the generation of ergothioneine directly from hercynine and a sulfur donor such as thiosulfate (S2O32–) in the absence of molecular oxygen (Fig. 1e).
EanB is similar to rhodanese, an enzyme that detoxifies cyanide, and so its reaction might proceed in a manner akin to that of rhodanese8,9, through the formation of a per-sulfide (SSH) group from a cysteine residue in the enzyme’s active site. The persulfide then probably attacks hercynine to generate a ‘mixed disulfide’ intermediate that subsequently decomposes to produce ergothioneine. Consequently, C. limicola might produce ergothioneine despite living in completely anaerobic waters, where it subsists on sulfur sources through a type of photosynthesis that does not produce molecular oxygen as a by-product.
But just because an organism has the genes for anaerobic ergothioneine production does not mean that it will actually produce the compound. However, the authors found that the aerobic bacterium Salinibacter ruber — which is more easily cultivated than C. limicola, and possesses only the genes for the anaerobic ergothioneine pathway — does indeed produce ergothioneine at levels comparable to those seen in bacteria that rely on the aerobic pathway. It therefore seems likely that ergothioneine is produced not only in aerobic bacteria and fungi, but also in anaerobic bacteria.
Ergothioneine has been found in the cells and tissues of plants and mammals, which must have acquired it from the environment10. The cellular accumulation of ergothioneine in mammals requires a specific transporter protein11, but such accumulation does not seem to be crucial for an animal’s survival. For example, failure to accumulate ergothioneine leaves mice largely unaffected, except for an increased susceptibility to oxidative stress10,12. Ergothioneine therefore probably serves mainly as a protective antioxidant, alongside molecules such as glutathione and ascorbic acid (vitamin C), although this is still an open question.
If the primary role of ergothioneine is as an antioxidant, then what do anaerobes such as C. limicola need it for? Burn et al. carried out a phylogenetic analysis of organisms capable of producing ergothioneine, which revealed that these microbes split into two mostly distinct branches depending on whether their genomes encode aerobic EgtB or anaerobic EanB. Although it remains to be seen whether ergothioneine is actually produced by anaerobic organisms, the authors suggest that evolutionary divergence between the anaerobic and aerobic pathways probably occurred sometime in the distant past. It is therefore possible that ergothioneine had an important, as-yet unknown role under the anaerobic conditions of early Earth, only to have been co-opted later as a biological antioxidant.
Burn and colleagues’ findings show that the ergothioneine biosynthetic pathway is worth further investigation — not only for the unusual enzymatic reactions it entails, but also to gain insight into its possible functions. Future lines of investigation will include determining whether anaerobic microbes do indeed produce ergothioneine, how the enzymes responsible work, and, ultimately, the role of ergothioneine in the biochemistry of these organisms. The newly discovered pathway also casts light on the evolution of natural products in general, and of the biosynthetic pathways responsible for their production.
Contributor Information
MARK W. RUSZCZYCKY, Division of Chemical Biology and Medicinal Chemistry, College of Pharmacy, University of Texas at Austin, Austin, Texas 78712, USA
HUNG-WEN LIU, Division of Chemical Biology and Medicinal Chemistry, College of Pharmacy, University of Texas at Austin, Austin, Texas 78712, USA. Also in the Department of Chemistry, University of Texas at Austin.
References
- 1.Kornas A, KuŸniak E, Œlesak I, Miszalski Z. Acta Biochim Polon. 2010;57:143–151. [PubMed] [Google Scholar]
- 2.Burn R, Misson L, Meury M, Seebeck FP. Angew Chem Int Edn. 2017;56:12508–12511. doi: 10.1002/anie.201705932. [DOI] [PubMed] [Google Scholar]
- 3.Seebeck FP. J Am Chem Soc. 2010;132:6632–6633. doi: 10.1021/ja101721e. [DOI] [PubMed] [Google Scholar]
- 4.Hu W, et al. Org Lett. 2014;16:5382–5385. doi: 10.1021/ol502596z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goncharenko KV, Vit A, Blankenfeldt W, Seebeck FP. Angew Chem Int Edn. 2015;54:2821–2824. doi: 10.1002/anie.201410045. [DOI] [PubMed] [Google Scholar]
- 6.Song H, et al. Sci Rep. 2015;5:11870. doi: 10.1038/srep11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faponle AS, Seebeck FP, de Visser SP. J Am Chem Soc. 2017;139:9259–9270. doi: 10.1021/jacs.7b04251. [DOI] [PubMed] [Google Scholar]
- 8.Westley J. Adv Enzymol. 1973;39:327–368. doi: 10.1002/9780470122846.ch5. [DOI] [PubMed] [Google Scholar]
- 9.Cipollone R, Ascenzi P, Visca P. IUBMB Life. 2007;59:51–59. doi: 10.1080/15216540701206859. [DOI] [PubMed] [Google Scholar]
- 10.Cheah IK, Halliwell B. Biochim Biophys Acta. 2012;1822:784–793. doi: 10.1016/j.bbadis.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Gründemann D, et al. Proc Natl Acad Sci USA. 2005;102:5256–5261. doi: 10.1073/pnas.0408624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato Y, et al. Pharm Res. 2010;27:832–840. doi: 10.1007/s11095-010-0076-z. [DOI] [PubMed] [Google Scholar]