Abstract
PD-(L)1 checkpoint blockade is revolutionizing cancer therapy, and biomarkers capable of predicting which patients are most likely to respond are highly desired. The detection of PD-L1 protein expression by immunohistochemistry can enrich for response to anti-PD-(L)1-blockade in a variety of tumor types, but is not absolute. Limitations of current commercial PD-L1 IHC assays and improvements anticipated in next generation PD-L1 testing are reviewed. Assessment of tumor infiltrating lymphocytes in conjunction with PD-L1 testing could improve specificity by distinguishing adaptive (interferon-gamma-driven, and cytotoxic T-lymphocyte-associated) from constitutive (non-immune-mediated) expression. The presence of a high tumor mutational burden also enriches for response to therapy and early data indicate that this may provide additive predictive value beyond PD-L1 IHC alone. As candidate biomarkers continue to emerge, the pathologist’s assessment of the tumor microenvironment on H&E combined with PD-L1 IHC remains a rapid and robust way to evaluate the tumor-immune dynamic.
Introduction
Immune checkpoint pathways can be co-opted by tumors to prevent tumor-specific immune responses. The finding that therapeutic monoclonal antibodies can be used to inhibit these checkpoint interactions, unleashing pre-existing anti-tumor immunity, has revolutionized cancer therapy. One of the best studied of these immune checkpoints is the PD-1/PD-L1 receptor-ligand pair. The PD-1 receptor is expressed on activated lymphocytes, including T cells, B cells and NK cells.1 Its ligand, PD-L1, can be expressed by an extended number of cell types, including tumor cells, lymphocytes, macrophages-lineage cells, endothelial cells, amongst others.2 The PD-1/PD-L1 immune inhibitory pathway was originally described in the setting of chronic viral infections, whereby it prevents destruction of host tissues by an unrestrained immune response. In the setting of cancer, however, this homeostatic mechanism prevents tumor cell clearance by the immune system.
The power of the PD-1/PD-L1 pathway to thwart antitumor immune activity has been confirmed by the durable clinical responses achieved with therapeutic anti-PD-(L)1-blockade in patients with melanoma, non-small cell lung cancer (NSCLC), urothelial carcinoma, classic Hodgkin lymphoma, head and neck squamous cell carcinoma (HNSCC), renal cell carcinoma (RCC), Merkel cell carcinoma, hepatocellular carcinoma, and gastric carcinomas. Consequently, an unprecedented number of FDA approvals have been secured over the past 3 years for agents in this class for these different indications.3 Among unselected patients with the eight solid tumor types listed, 10–40% show clinical response to anti-PD-(L)1 monotherapy and approximately 7–34% experience high-grade immune-related adverse events (irAEs).4,5 Given the cost and side effect profile of these therapies, biomarkers predictive of response are highly sought after for patient selection. In the context of the first clinical trials of PD-1 blockade, it was shown that immunohistochemical (IHC) detection of tumor cell PD-L1 expression was associated with therapeutic response.6–8 To date, PD-L1 expression in the pre-treatment tumor microenvironment (TME) represents the most well-studied potential biomarker of response to anti-PD-(L)1 therapy, though a number of other markers are currently under investigation.
PD-L1 IHC Assays
Different companion and complimentary PD-L1 IHC assays were developed for each anti-PD-(L)1 agent in association with early clinical trials, Table 1. Many parameters vary between these assays, including the primary antibody clone used. The interpretation guidelines also differ, both among assays and among indications for a given assay. The 22C3 and 28-8 assays center on tumor cell PD-L1 expression, except for the 22C3 assay in gastric cancer which also incorporates scoring immune cell PD-L1 expression. In contrast, the SP263 and SP142 assays include both tumor and immune cell PD-L1 expression for most tumor types, except for the SP142 assay in urothelial cancer, which exclusively scores immune cell PD-L1. Even for a given agent and tumor type, different assay scoring parameters have been called for in some instances, depending on the line of therapy, e.g. high PD-L1 expression (≥50%) is required for first-line pembrolizumab in NSCLC, while any PD-L1 expression (≥1%) is sufficient for use in the second-line setting.
Table 1. Companion/complementary PD-L1 IHC diagnostics by tumor type.
Many of the scoring parameters have the potential to change as number of patients examined and disease indications expand.
| Ab | Melanoma | NSCLC | Urothelial | HNSCC | Gastric | |
|---|---|---|---|---|---|---|
| Anti-PD-1 | ||||||
| Nivolumab (BMS) | 28-8 Dako | 1% (TC) | 1%, 5%, 10% (TC) | 1% (TC) | 1% (TC) | |
| Pembrolizumaba (Merck) | 22C3 Dako | 1%b, 50%c (TC) | 1% (TC/IC) | |||
| Anti-PD-L1 | ||||||
| Durvalumab (AstraZeneca) | SP263 Ventana | 25% (TC/IC) | ||||
| Atezolizumab (Roche/Genentech) | SP142 Ventana | 50% (TC)/10% (IC) | 5% (IC) |
FDA approved use requires PD-L1 positivity using the indicated assay and thresholds
Second-line
First-line
Abbreviations: (Ab) antibody; (NSCLC) non-small cell lung cancer; (HNSCC) head and neck squamous cell carcinoma; (TC) tumor cell PD-L1; (IC) immune cell PD-L1.
The approval of multiple assays associated with unique interpretation thresholds has made it difficult to directly compare results across clinical trials. It has also created many practical concerns regarding which test to use and the interchangeability of the results. Notwithstanding, the different PD-L1 antibodies and other assay reagents, interpretation thresholds, and anti-PD-(L)1 therapeutic agents tested, the detection of PD-L1 expression in the pretreatment tumor microenvironment by IHC consistently enriches for patients harboring multiple different tumor types who are likely to respond to PD-(L)1 blockade.9,10
Several studies have attempted to compare the different marketed PD-L1 IHC assays. The 22C3, 28-8, and SP263 assays have been shown to detect equivalent amounts of PD-L1 tumor cell staining in NSCLC specimens in multiple studies.11–13 In contrast, the SP142 assay has been shown to have a reduced sensitivity for the detection of tumor cell PD-L1 expression11,13 as well as decreased sensitivity for highlighting immune cell PD-L1 expression.11 Additionally, it was shown that there is a greater degree of variability in scoring of immune cell PD-L1 expression across all four assays, likely due to a lack of defined criteria and a lack of training, contributing to poor reproducibility amongst pathologists.11
It is important to note that a complete IHC assay system contains many components in addition to the antibody that is used in that assay. Studies have been performed to determine whether the 22C3, 28-8, SP263, and SP142 antibodies themselves differ in their ability to highlight PD-L1 expression on tumor cells and/or immune cells. It has been shown in both melanoma14 and NSCLC15 that the primary antibodies (including the SP142 clone) are no different in their capacity to stain for PD-L1 in these different cell types, when the other features of the assay are essentially held consistent. This finding indicates that it is other features of the assay system (antigen retrieval, concentration of primary antibody, amplification system, etc) that leads to the observed difference in performance of the SP142 assay and not the SP142 antibody. This has important implications for potential laboratory-developed tests that may be used by surgical pathologists for clinical care in the future.
Adaptive and Constitutive PD-L1 Expression
Current interpretation guidelines do not distinguish between adaptive and constitutive patterns of PD-L1 expression. Adaptive PD-L1 expression was first described in melanoma, and is a dynamic and geographically heterogeneous, interferon-gamma-driven mechanism of PD-L1 expression by tumor and immune cells at the host-tumor interface.16 In contrast, “constitutive” tumor cell PD-L1 expression is defined as a population of tumor cells expressing PD-L1 on their cell surface, independent of an immune infiltrate. Constitutive expression can be driven by numerous tumor-intrinsic mechanisms associated with genetic alterations.17,18 The frequency of constitutive PD-L1 expression appears to vary by tumor type, for example, melanoma demonstrates infrequent constitutive PD-L1 expression, i.e., ~1% of cases16 while cutaneous squamous cell carcinoma shows a notably higher proportion of pathology specimens with some degree of constitutive expression.19
It has been suggested that anti-PD-1 therapy exerts its effect by inhibiting the adaptive form of the PD-L1 immune resistance mechanism.20 It is possible that when PD-L1 expression occurs in the absence of a pre-existing anti-tumor immune response, it may not predict response to PD-1/PD-L1 blockade. This is one potential explanation for the proportion of patients that have tumors that are PD-L1+, but which do not respond to anti-PD-(L)1 therapy, Table 2. It also potentially explains the closely related finding that PD-L1 expression by immune cells is more predictive of response than tumor cell PD-L1 expression in squamous cell carcinomas of the head and neck,21 which tend to show high levels of constitutive PD-L1 expression. Further complicating the issue, however, is the fact that the adaptive and constitutive patterns of PD-L1 expression are not mutually exclusive. For example, murine and in vitro studies suggest tumor cell-intrinsic PD-L1 drives expression of genes related to a tumor initiating cell phenotype and interferon gamma sensitivity;22 elevated PD-L1 expression associated with a JAK3 mutation was associated with a response to anti-PD-L1;23 and histologic patterns of combined adaptive and constitutive PD-L1 expression have been observed in multiple tumor types.3
Table 2.
Patients whose tumors are PD-L1+ and who do not respond to anti-PD-(L)1 as well as patients whose tumors are PD-L1- and who do respond may be attributable to numerous factors potentially limiting the sensitivity and specify of the available IHC assays.
| PD-L1(+) non-responders | PD-L1(−) responders |
|---|---|
| Assay performance and threshold for positivity | Assay performance and threshold for positivity |
| Exclusive constitutive PD-L1 expression | Exclusive immune cell PD-L1 expression |
| Spatial and temporal expression heterogeneity | Spatial and temporal expression heterogeneity |
| Limited sampling | Limited sampling |
| Alternate PD-1/PD-L1 ligand/receptor activity | Alternate PD-1/PD-L1 ligand/receptor activity |
| Other inhibitory checkpoints/mechanisms |
Potential limitations of PD-L1 IHC
In addition to the variability in PD-L1 assay performance and interpretation thresholds as well as the different physiologic implications of adaptive vs. constitutive PD-L1 expression described above, there are a number of other potential reasons why a PD-L1(+) patient may not respond to therapy and why a PD-L1(-) patient could show an objective response, Table 2. The first reason is simply the focal and heterogeneous expression of PD-L1. PD-L1 expression can also be dynamic, so it may change over time. Thus, PD-L1 expression levels in an archival, pre-treatment specimen could contribute to either a “false positive” or “false negative” value of PD-L1 expression. Similarly, there’s the possibility of limited tissue sampling, which can lead to test results that are not representative of the entire tumor.14,24,25 Lastly, in addition to sampling issues related to PD-L1 expression, there may be other resistance mechanisms present in the tumor, including alternate PD-1/PD-L1 ligand/receptor activity (PD-L2, B7-1/CD80) or other checkpoint molecules expression, e.g. LAG-3.26,27 Depending on the drug used (anti-PD-1 vs. anti-PD-L1), such mechanisms could be a factor underlying why some patients with PD-L1+ tumors fail to respond to anti-PD-1 therapy or some patients with PD-L1- tumors do respond.
Assessment of Tumor Infiltrating Lymphocytes (TIL)
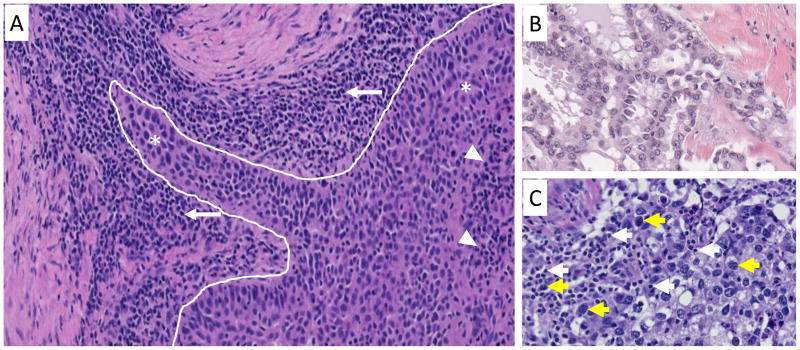
Tumor infiltrating lymphocytes indicating a “T-cell inflamed phenotype” have been assessed for their association with response to anti-PD-(L)1 therapy. Increased CD8+ T-cell densities at the tumor’s leading edge (Figure 1A) have been associated with response to anti-PD-1 from pre-treatment tumor specimens from patients with metastatic melanoma.20 Unfortunately, a threshold of CD8+ cell density in pre-treatment specimens is not evident that can clearly separate responders from non-responders. Similar results were seen when CD3 and CD45RO+ T-cell densities were assessed in pre-treatment melanoma specimens,28 and when parameters such as the CD4:CD8 ratio or PD-1 or CD3 expression were assessed in a semi-quantitative fashion in a cohort that included a number of different solid tumor types.29
Figure 1. Photomicrograph showing the geographic assessment of lymphocyte infiltrates in the tumor microenvironment.
(A) A dense population of small lymphocytes (white arrows) is present in the peritumoral stroma along the leading edge of the tumor (white line). Although fewer in number, tumor infiltrating lymphocytes (white arrowheads) are also intermixed among larger tumor cells (asterisks) more centrally within the tumor. (B) A non-inflamed tumor composed almost exclusively of large tumor cells without admixed lymphocytes. (C) An inflamed tumor with numerous small lymphocytes (white arrows) among the larger tumor cells (yellow arrows). Hematoxylin and eosin (H&E) stain, Original magnification 200x.
Gene expression profiling has also been used to identify an inflamed tumor phenotype by using an interferon-gamma immune-related signature score, with similar results across multiple different tumor types.30 In fact, the receiver operating characteristic (R.O.C.) curves for the sensitivity and specificity for the IFN-gamma gene signature, CD8 density, and PD-L1 expression often overlap, indicating the connected nature of these biomarkers, Figure 2.31
Figure 2. Hive plot for demonstrating the relationship between PD-L1 expression, CYT (cytotoxic gene signature expression), and mutational load in multiple common solid tumor types.
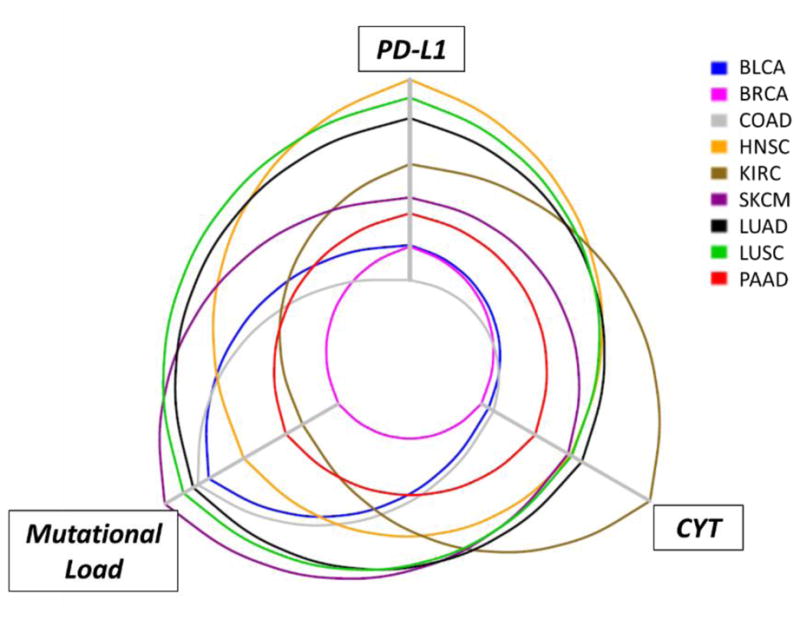
Comparisons of PD-L1 and CYT expression levels amongst different tumor types have been challenging due to the diverse assays used across studies. TCGA public datasets were used to determine the relative expression levels of PD-L1 and CYT (combined GZMA_PRF1 transcript levels, indicating cytolytic effector activity56). for nine solid tumor types (SKCM, melanoma; KIRC, renal cell carcinoma; LUSC, squamous cell carcinoma of the lung; LUAD, lung adenocarcinoma; COAD, colonic adenocarcinoma; HNSC, head and neck squamous cell carcinoma; PAAD, pancreatic adenocarcinoma; BRCA, breast carcinoma; BLCA, bladder carcinoma). Each axis increases in value as it extends from the center of the plot. The highest levels of median PD-L1 expression are seen in the tumor types with squamous differentiation, consistent with the known constitutive expression in these tumor types. Notably, PD-L1 expression levels and CYT track each other closely, while mutational density tends to be less aligned.
Most of the focus to date has been on predicting response to therapy in pre-treatment tumor specimens. It is worth noting that some studies have assessed TIL from patients who have already started therapy with impressive results. A marked difference has been reported in immune cell densities in on-treatment biopsies from responders vs. non-responders to anti-PD-1 therapy in patients with melanoma, including CD8, CD4, CD3, PD-1, PD-L1, and LAG-3 expression. When the location of CD8+ immune cells were assessed, the presence of a high density in the tumor center (Figure 1A) was associated with therapeutic response.28 New therapeutic approaches are predicated on changing a non-inflamed TME (Figure 1B) to an inflamed one (Figure 1C) with the aim of overcoming primary resistance to anti-PD-1. Most recently, a small cohort of melanoma patients was treated with oncolytic virotherapy with talimogene laherparepvec to alter the TME to a more inflamed phenotype, contributing to an increase in anti-PD-1 efficacy.32 Undoubtedly, many other strategies to change a non-inflamed TME to an inflamed one will be reported in the near future.
The Society for Immunotherapy of Cancer (SITC) immune biomarker taskforce has highlighted the need for “standardized protocols for the histopathologic assessment of TIL and effector cell populations.”33 Proposals have been made by the International Immuno-Oncology Biomarkers Working Group.34,35 Such protocols are expected to become key as TIL assessments likely make their way into routine surgical pathology practice as predictive and prognostic markers.
Combination PD-L1 IHC and TIL assessment
The next generation of IHC assays will likely include both PD-L1 and TIL assessments. A TIL score combined with PD-L1 IHC would greatly improve our ability to categorize a tumor into one that is infiltrated, i.e., TIL+, and “immunoactive”, i.e. PD-L1+; infiltrated, but by non-activated or exhausted TIL (TIL+/PD-L1-); or one that is non-inflamed (TIL-/PD-L1- or TIL-/PD-L1+, the latter by constitutive PD-L1 expression).4,16,36 Such findings could have therapeutic implications. For example, patients whose tumors show PD-L1 expression in association with TIL may be more likely to benefit from anti-PD-(L)1 monotherapy, while those whose tumors are not inflamed or harboring non-activated or exhausted TIL may be more likely benefit from a combinatorial therapeutic approach. The assessment of PD-L1 and TIL could be performed using currently available PD-L1 IHC assays, simply by adding an additional parameter into the interpretation, and therefore could be a fast and inexpensive way to improve upon the currently available technology.
Other possible next generation assays combining PD-L1 with a TIL assessment require assaying for a second parameter. For example, PD-L1 protein expression and an IFN-gamma gene signature have been combined in the assessment of pre-treatment specimens from patients with NSCLC. Preliminary results suggest that this combination of markers is more predictive than either of those markers alone.37 Another example of combining PD-L1 expression with a measure of TIL status is assaying the proximity between PD-1+ and PD-L1+ cells, i.e., the dominant interaction that is ostensibly being blocked by anti-PD-1 therapy. For patients with both Merkel cell carcinoma and melanoma, the expression of these two parameters in close proximity to each other is more predictive than PD-L1 IHC expression alone.38,39 Detailed quantitative assessments of the TME may soon be possible via multiplex immunohistochemical/immunofluorescence technologies.40–42 This approach would have many advantages compared to traditional IHC, but streamlined assays necessary for routine use have yet to be clinically validated.
Mutational Burden
Another candidate biomarker that has been associated with a response to anti-PD-1 is the mutational load of a patient’s tumor. Patients with NSCLC tumors that had a higher number of non-synonymous mutations were more likely to demonstrate an initial response to therapy as well as an increase in progression free survival.43 Perhaps the most striking example of the correlation between mutational density and response to anti-PD-1 therapies is the differential response rate between patients with mismatch repair-deficient colorectal carcinomas and those with mismatch repair-proficient tumors (78% vs. 11%, respectively).44 Further proof of principle is the fact that patients with microsatellite unstable tumors, regardless of the tumor’s site of origin, are likely to demonstrate a response to anti-PD-1. A clinical trial of anti-PD-1 in patients with 12 different mismatch repair-deficient tumor types showed a 53% response rate, including 21% of patients with a compete response.45 This latter finding led to an unprecedented FDA approval for a tumor-type agnostic indication, i.e., pembrolizumab (anti-PD-1) for all microsatellite-unstable tumors.
The reason that mutational load is thought to be a predictor of response to anti-PD-1 is that mutations are thought to serve as the source of immunogenic neoantigens. It is recognized that some mutations are more immunogenic than others, and that increasing mutational density likely increases the chances that one or more of the antigens will elicit a notable anti-tumor immune response. Factors beyond simple immunogenicity of mutations are also important. Clonal neoantigens, i.e., those that occur early in the evolution of the tumor and are present in a large population of tumor cells, are more strongly associated with response than subclonal neoantigens.46 Neoantigen loss is also a mechanism of secondary resistance to PD-1 blockade.47
While mutational burden may be associated with the potential of a tumor-specific immune response, TIL/CD8 densities and PD-L1 expression are more direct measures of whether an immune response is currently present in given tumor. When principal component analysis was performed on measures of cytotoxic (CYT) gene expression, PD-L1 expression, and mutational burden from melanoma tumor specimens in The Cancer Genome Atlas dataset, PD-L1 expression and CYT were closely related and formed one variable, while mutational load formed a separate, independent variable.31 These relationships can be visualized on a hive plot, Figure 2, where it can be observed that the median values for PD-L1 and CYT expression profiles tend to track each other closely across multiple different solid tumor types, while mutational density does not parallel the other two parameters. Ultimately, a combinatorial biomarker representing 1) whether an immunoactive infiltrate is present within the tumor and 2) the genomic features of the tumor, is likely to have improved predictive value over either metric alone.
Other Candidate Biomarker Approaches
Numerous reports nominating additional candidate biomarkers predictive of response or resistance to PD-1/PD-L1 blockade therapy are emerging with increasing frequency. This complex and evolving landscape has been recently covered in detail elsewhere.36,48,49 Effective T cell antigen recognition, priming, tumor infiltration, and cytotoxic effector function are all necessary steps for tumor elimination and potential targets of therapeutic intervention. Moreover, tumor heterogeneity and evolution under the pressure of therapy add to the complexity of assessing the value of emerging biomarker candidates. In addition to tumor tissue, other sources of biospecimens are being explored in the quest to predict and monitor response to therapy in a given patient. Tumor-involved lymph nodes,50 circulating immune cells,50,51 circulating tumor DNA,52 and microbiome reservoirs such as stool53,54 have all showed early promise in predicting response, resistance, or toxicity with checkpoint blockade therapy.
Conclusions and Future Directions
The SITC immune biomarkers taskforce has proposed that for routine use, “evaluation of a novel [biomarker] should be routine, feasible, simple, rapid, robust, reproducible, objective, specific, quantitative, standardized, powerful, and preferentially IHC-based.”33 While the current value and limitations of PD-L1 IHC are widely acknowledged, the power of a pathologist’s assessment of the TME on H&E combined with PD-L1 IHC has the potential to provide more information about the state of the tumor-immune dynamic than an assessment of PD-L1 expression as simply “positive” or “negative”. In the future, it is anticipated that PD-L1 IHC will be incorporated into a multiplex IHC format that includes a number of potential immunotherapeutic targets or checkpoint molecules, e.g., IDO55 or LAG-327, likely accompanied by spatial metrics. Measures of gene expression profiles and genomic studies are also likely to provide unique information, leading to multimodality assessments of the TME. These approaches will have to be integrated and prioritized to inform testing strategies and allow for a more rational approach that consumes less time, money, and tissue. As our therapeutic arsenal and associated biomarkers continue to evolve, such an approach is essential to maximize the yield of information from a finite amount of tissue, and thus most productively guide treatment strategy for an individual patient.
Acknowledgments
The authors would like to thank Ludmila Danilova, PhD for preparation of the hive plot figure. This work was supported by the Melanoma Research Alliance (JMT); Harry J. Lloyd Trust (JMT); Emerson Collective (JMT); Sidney Kimmel Cancer Center Core Grant P30 CA006973 (JMT); the National Cancer Institute NIH Grant R01 CA142779 (JMT); NIH Grant T32 CA193145 (TRC); The authors were also supported by the Bloomberg-Kimmel Institute for Cancer Immunotherapy and a Stand Up To Cancer–Cancer Research Institute Cancer Immunology Translational Cancer Research Grant (SU2C-AACR-DT1012). Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research.
Footnotes
Disclosures: JMT serves as a consultant/advisory board member for Bristol-Myers Squibb, Merck, and Astra Zeneca. She also receives investigator-initiated research funding from Bristol-Myers Squibb.
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–87. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taube JMGJ, Sholl LM, Rodig SJ, Cottrell TR, Giraldo NA, Baras AS, Patel SS, Anders RA, Rimm DL, Cimino-Mathews A. Implications of the tumor immune microenvironment for staging and therapeutics. Modern Pathology. doi: 10.1038/modpathol.2017.156. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrone KA, Ying W, Naidoo J. Immune-Related Adverse Events From Immune Checkpoint Inhibitors. Clin Pharmacol Ther. 2016;100:242–51. doi: 10.1002/cpt.394. [DOI] [PubMed] [Google Scholar]
- 6.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taube JM. Unleashing the immune system: PD-1 and PD-Ls in the pre-treatment tumor microenvironment and correlation with response to PD-1/PD-L1 blockade. Oncoimmunology. 2014;3:e963413. doi: 10.4161/21624011.2014.963413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sunshine J, Taube JM. PD-1/PD-L1 inhibitors. Curr Opin Pharmacol. 2015;23:32–8. doi: 10.1016/j.coph.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khunger M, Hernandez AV, Pasupuleti V, et al. Programmed Cell Death 1 (PD-1) Ligand (PD-L1) Expression in Solid Tumors As a Predictive Biomarker of Benefit From PD-1/PD-L1 Axis Inhibitors: A Systematic Review and Meta-Analysis. JCO Precision Oncology. 2017:1–15. doi: 10.1200/PO.16.00030. [DOI] [PubMed] [Google Scholar]
- 11.Rimm DL, Han G, Taube JM, et al. A Prospective, Multi-institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non-Small Cell Lung Cancer. JAMA Oncol. 2017;3:1051–8. doi: 10.1001/jamaoncol.2017.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ratcliffe MJ, Sharpe A, Midha A, et al. Agreement between Programmed Cell Death Ligand-1 Diagnostic Assays across Multiple Protein Expression Cutoffs in Non-Small Cell Lung Cancer. Clin Cancer Res. 2017;23:3585–91. doi: 10.1158/1078-0432.CCR-16-2375. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017;12:208–22. doi: 10.1016/j.jtho.2016.11.2228. [DOI] [PubMed] [Google Scholar]
- 14.Sunshine JC, Nguyen PL, Kaunitz GJ, et al. PD-L1 Expression in Melanoma: A Quantitative Immunohistochemical Antibody Comparison. Clin Cancer Res. 2017;23:4938–44. doi: 10.1158/1078-0432.CCR-16-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaule P, Smithy JW, Toki M, et al. A Quantitative Comparison of Antibodies to Programmed Cell Death 1 Ligand 1. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24. 1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–77. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lastwika KJ, Wilson W, 3rd, Li QK, et al. Control of PD-L1 Expression by Oncogenic Activation of the AKT-mTOR Pathway in Non-Small Cell Lung Cancer. Cancer Res. 2016;76:227–38. doi: 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
- 19.Yanik EL, Kaunitz GJ, Cottrell TR, et al. Association of HIV Status With Local Immune Response to Anal Squamous Cell Carcinoma: Implications for Immunotherapy. JAMA Oncol. 2017;3:974–8. doi: 10.1001/jamaoncol.2017.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HR, Ha SJ, Hong MH, et al. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Sci Rep. 2016;6:36956. doi: 10.1038/srep36956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta HB, Clark CA, Yuan B, et al. Tumor cell-intrinsic PD-L1 promotes tumor-initiating cell generation and functions in melanoma and ovarian cancer. Signal Transduct Target Ther. 2016:1. doi: 10.1038/sigtrans.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Allen EM, Golay HG, Liu Y, et al. Long-term Benefit of PD-L1 Blockade in Lung Cancer Associated with JAK3 Activation. Cancer Immunol Res. 2015;3:855–63. doi: 10.1158/2326-6066.CIR-15-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ilie M, Long-Mira E, Bence C, et al. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol. 2016;27:147–53. doi: 10.1093/annonc/mdv489. [DOI] [PubMed] [Google Scholar]
- 25.McLaughlin J, Han G, Schalper KA, et al. Quantitative Assessment of the Heterogeneity of PD-L1 Expression in Non-Small-Cell Lung Cancer. JAMA Oncol. 2016;2:46–54. doi: 10.1001/jamaoncol.2015.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yearley JH, Gibson C, Yu N, et al. PD-L2 Expression in Human Tumors: Relevance to Anti-PD-1 Therapy in Cancer. Clin Cancer Res. 2017;23:3158–67. doi: 10.1158/1078-0432.CCR-16-1761. [DOI] [PubMed] [Google Scholar]
- 27.Taube JM, Young GD, McMiller TL, et al. Differential Expression of Immune-Regulatory Genes Associated with PD-L1 Display in Melanoma: Implications for PD-1 Pathway Blockade. Clin Cancer Res. 2015;21:3969–76. doi: 10.1158/1078-0432.CCR-15-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen PL, Roh W, Reuben A, et al. Analysis of Immune Signatures in Longitudinal Tumor Samples Yields Insight into Biomarkers of Response and Mechanisms of Resistance to Immune Checkpoint Blockade. Cancer Discov. 2016;6:827–37. doi: 10.1158/2159-8290.CD-15-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–74. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayers M, Lunceford J, Nebozhyn M, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127:2930–40. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danilova L, Wang H, Sunshine J, et al. Association of PD-1/PD-L axis expression with cytolytic activity, mutational load, and prognosis in melanoma and other solid tumors. Proc Natl Acad Sci U S A. 2016;113:E7769–E77. doi: 10.1073/pnas.1607836113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ribas A, Dummer R, Puzanov I, et al. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell. 2017;170:1109–19. e10. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gnjatic S, Bronte V, Brunet LR, et al. Identifying baseline immune-related biomarkers to predict clinical outcome of immunotherapy. J Immunother Cancer. 2017;5:44. doi: 10.1186/s40425-017-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hendry S, Salgado R, Gevaert T, et al. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in Melanoma, Gastrointestinal Tract Carcinomas, Non-Small Cell Lung Carcinoma and Mesothelioma, Endometrial and Ovarian Carcinomas, Squamous Cell Carcinoma of the Head and Neck, Genitourinary Carcinomas, and Primary Brain Tumors. Adv Anat Pathol. 2017;24:311–35. doi: 10.1097/PAP.0000000000000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hendry S, Salgado R, Gevaert T, et al. Assessing Tumor-infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method From the International Immunooncology Biomarkers Working Group: Part 1: Assessing the Host Immune Response, TILs in Invasive Breast Carcinoma and Ductal Carcinoma In Situ, Metastatic Tumor Deposits and Areas for Further Research. Adv Anat Pathol. 2017;24:235–51. doi: 10.1097/PAP.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–30. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 37.Higgs BW, Robbins PB, Blake-Haskins JA, et al. 15LBA High tumoral IFNγ mRNA, PD-L1 protein, and combined IFNγ mRNA/PD-L1 protein expression associates with response to durvalumab (anti-PD-L1) monotherapy in NSCLC patients. 2015;51:S717. [Google Scholar]
- 38.Bordeaux JJD, Sosman J, Kim JY, Vaupel C, Dabbas B, Cates J, Hall J, Lameh J, Tangri S, Dakappagari N. Abstract 853: Novel quantitative multiplexed PD-1/PD-L1 immunohistochemistry test provides superior prediction of treatment response in melanoma patients. 107th Annual Meeting of the American Association for Cancer Research; 2016 July; New Orleans (LA): AACR; 2016. p. pp.853. [Google Scholar]
- 39.Giraldo NAKG, Cottrell TR, Berry S, Sunshine JC, Nguyen P, Xu H, Orgutsova A, Church CD, Miller N, Yearley JH, Lipson EJ, Danilova L, Nghiem PT, Topalian SL, Taube JM. Abstract 662: The differential association of PD-1, PD-L1, and CD8+ cells with response to pembrolizumab and presence of Merkel cell polyomavirus (MCPyV) in patients with Merkel cell carcinoma (MCC) 2017;77:662. [Google Scholar]
- 40.McKinley ET, Sui Y, Al-Kofahi Y, et al. Optimized multiplex immunofluorescence single-cell analysis reveals tuft cell heterogeneity. JCI Insight. 2017:2. doi: 10.1172/jci.insight.93487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerdes MJ, Sevinsky CJ, Sood A, et al. Highly multiplexed single-cell analysis of formalin-fixed, paraffin-embedded cancer tissue. Proc Natl Acad Sci U S A. 2013;110:11982–7. doi: 10.1073/pnas.1300136110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Remark R, Merghoub T, Grabe N, et al. In-depth tissue profiling using multiplexed immunohistochemical consecutive staining on single slide. Sci Immunol. 2016;1:aaf6925. doi: 10.1126/sciimmunol.aaf6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–13. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–9. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anagnostou V, Smith KN, Forde PM, et al. Evolution of Neoantigen Landscape during Immune Checkpoint Blockade in Non-Small Cell Lung Cancer. Cancer Discov. 2017;7:264–76. doi: 10.1158/2159-8290.CD-16-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spranger S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int Immunol. 2016;28:383–91. doi: 10.1093/intimm/dxw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017;168:707–23. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacquelot N, Roberti MP, Enot DP, et al. Predictors of responses to immune checkpoint blockade in advanced melanoma. Nat Commun. 2017;8:592. doi: 10.1038/s41467-017-00608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Subudhi SK, Aparicio A, Gao J, et al. Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc Natl Acad Sci U S A. 2016;113:11919–24. doi: 10.1073/pnas.1611421113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cabel L, Riva F, Servois V, et al. Circulating tumor DNA changes for early monitoring of anti-PD1 immunotherapy: a proof-of-concept study. Ann Oncol. 2017;28:1996–2001. doi: 10.1093/annonc/mdx212. [DOI] [PubMed] [Google Scholar]
- 53.Vetizou M, Pitt JM, Daillere R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–84. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–9. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]