Abstract
Phorate is a highly toxic agricultural pesticide currently in use throughout the world. Like many other organophosphorus (OP) pesticides, the primary mechanism of the acute toxicity of phorate is acetylcholinesterase (AChE) inhibition mediated by its bioactivated oxon metabolite. AChE reactivation is a critical aspect in the treatment of acute OP intoxication. Unfortunately, very little is currently known about the capacity of various oximes to rescue phorate oxon (PHO)-inhibited AChE. To help fill this knowledge gap, we evaluated the kinetics of inhibition, reactivation, and aging of PHO using recombinant AChE derived from three species (rat, guinea pig and human) commonly utilized to study the toxicity of OP compounds and five oximes that are currently fielded (or have been deemed extremely promising) as anti-OP therapies by various nations around the globe: 2-PAM Cl, HI-6 DMS, obidoxime Cl2, MMB4-DMS, and HLö7 DMS. The inhibition rate constants (k i) for PHO were calculated for AChE derived from each species and found to be low (i.e., 4.8 × 103 to 1.4 × 104 M−1 min−1) compared to many other OPs. Obidoxime Cl2 was the most effective reactivator tested. The aging rate of PHO-inhibited AChE was very slow (limited aging was observed out to 48 hours) for all three species. Conclusions: (1) Obidoxime Cl2 was the most effective reactivator tested. (2) 2-PAM Cl, showed limited effectiveness in reactivating PHO-inhibited AChE, suggesting that it may have limited usefulness in the clinical management of acute PHO intoxication. (3) The therapeutic window for oxime administration following exposure to phorate (or PHO) is not limited by aging.
Keywords: phorate, acetylcholinesterase, kinetics, oxime
1.0 Introduction
Organophosphorus (OP) compounds include a widely used class of pesticides that present a significant health hazard to humans. For example, accidental occupational exposures and deliberate self-poisoning are significant problems in the developing world, with the latter accounting for up to an estimated 200,000 deaths each year [1]. Additionally, while the relative risk of exposure following an industrial accident or act of terrorism may be comparatively lower, it should not be ignored either. Similar to chemical warfare nerve agents (CWNAs), the general mechanism of action of OP pesticides is the irreversible inhibition of the enzyme acetylcholinesterase (AChE). OP pesticides and CWNAs differ in their intended applications, potency, response to conventional medical countermeasures, and most common routes of exposure (e.g., dermal, oral, inhalational), and understanding the various biochemical characteristics of individual OP compound is critical for risk mitigation and medical treatment. Consequently, it was the goal of the study reported herein to fill a knowledge gap regarding one particular OP pesticide, namely phorate.
Phorate (O, O-diethyl S-[ethylthiomethyl] phosphorodithioate; CAS number 298-02-2) is an OP pesticide that is commonly used to control sucking and biting insects and nematodes on a variety of field crops including corn, cotton, potatoes, sugar beets, and beans [2]. Phorate is highly toxic to birds, fish, and mammals (male rat oral LD50 for the metabolite phorate oxon = 0.88 mg/kg [3]) and accidental human exposures, resulting in death in some instances, have been reported [4–6]. It is globally available for agricultural purposes, including within the U.S., and is generally applied to the soil as dry granules. Runoff and leaching of phorate into water sources such as nearby lagoons and ponds as well as ground/drinking water have been reported [7, 8]. Despite phorate’s toxicity and the fact that it is used widely throughout the world, there is still relatively little known in the open literature about it compared to other OP pesticides or even CWNAs. Furthermore, it has been identified as a compound of particular interest to the National Institutes of Health Countermeasures Against Chemical Threats (NIH CounterACT) program because it has been identified by the U.S. Department of Homeland Security as a chemical threat of interest on the Chemical Terrorism Risk Assessment [9].
Many OP pesticides undergo a metabolic process in which the toxicity of the parent compound is enhanced through the formation of an oxon metabolite that is a potent inhibitor of AChE [2, 10]. Phorate is no exception; it is transformed by cytochrome P450-mediated desulfuration into an oxon metabolite (Fig. 1) and it is the inhibition of AChE, mediated primarily by the oxon metabolite phorate oxon (PHO; also called phoratoxon), that is presumed to be the primary mechanism of the acute toxicity of phorate. Inhibition of AChE leads to the accumulation of the neurotransmitter acetylcholine at neuronal synapses and neuromuscular junctions, resulting in continual stimulation of acetylcholine receptors. This continual activation can lead to cholinergic crisis that manifests as increased secretions, tremors, convulsions, seizures, and ultimately death due to respiratory paralysis and a buildup of secretions in the airway [11, 12]. Medical countermeasures for OP poisoning typically include a muscarinic acetylcholine receptor antagonist (e.g., atropine) along with an oxime AChE reactivator (e.g., pralidoxime; also known as 2-PAM) [13–15]. A benzodiazepine (e.g., diazepam) may also be indicated if the patient displays convulsive activity. Removal of the OP from AChE permits the enzyme to resume its normal physiologic function. Therefore, reactivation of OP-inhibited AChE is a critical aspect of the therapeutic strategy for acute OP intoxication.
Fig. 1.
Bioactivation of Phorate.
The propensity for reactivation with an oxime varies among different OPs. Some OP-AChE conjugates are reactivated readily by a number of oximes, while others are resistant to reactivation by any known oxime [16]. In addition to the inherent resistance to reactivation observed with some OPs, the capacity for oxime mediated reactivation may also be limited by another phenomenon that varies between specific OPs, namely aging. The OP-AChE conjugate is susceptible to spontaneous dealkylation (i.e., aging), which results in an OP-AChE complex that cannot be reactivated by oximes [17]. As such, aging is a major consideration for effective oxime-mediated therapy for poisoning by some OPs, most notably the CWNA soman, which ages in a matter of minutes [18, 19]. Aging also occurs with OP pesticides and has been suggested to play a role in limiting the efficacy of certain oximes against dimethyl OP pesticides in particular [15, 20].
In the laboratory setting, the overall efficacy of a given oxime reactivator is dependent on a number of factors, including but not limited to the chemical structure of the OP, the route of OP exposure, the route and timing of oxime administration, and the species being treated. The resulting variability in oxime efficacy has contributed to something of a controversy in the field, with some investigators endorsing the use of oximes for the treatment of poisoning by certain OPs and others concluding that, regardless of the OP, oximes provide no therapeutic benefit at all (reviewed in [21, 22]). Unfortunately, little is known about oxime-assisted reactivation of AChE inhibited by PHO. To help fill this knowledge gap, we evaluated the kinetics of PHO inhibition and aging against recombinant AChE derived from three species (rat, guinea pig and human) and reactivation with five prominent oximes: 2-PAM Cl, HI-6 DMS, obidoxime Cl2, MMB4-DMS, and HLö7 DMS.
2.0 Materials and Methods
2.1 Materials
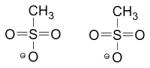
Phorate oxon was synthesized at Battelle (Columbus, OH) as previously described [3]. Sarin was procured from the Edgewood Chemical Biological Center (Aberdeen Proving Ground, MD). Acetylthiocholine (ATC), 5,5′-dithio-bis-[2-nitrobenzoic acid] (DTNB), 2-PAM Cl, obidoxime Cl2, and recombinant human AChE were obtained from Sigma-Aldrich (St. Louis, MO). Recombinant rat and guinea pig AChE were produced by Chesapeake PERL (Savage, MD). HI-6 DMS and HLö-7 DMS were synthesized by Southwest Research Institute (San Antonio, TX). MMB4 DMS was provided by the Medical Countermeasures Systems Joint Project Management Office, (Frederick MD). Additional information about the OPs and oximes is provided in Table 2.
Table 2.
Oximes and Organophosphorus Compounds
| Compound | CAS RN* | Synonym(s) | Structure |
|---|---|---|---|
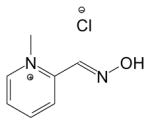
| 2-PAM Cl | 51-15-0 | Pralidoxime chloride, 2-hydroxyiminomethyl-1-methylpyridinium chloride |
|
| MMB4 DMS | None given | Methoxime dimethanesulfonate; 1,1-methylene bis[4(hydroxyimino) methyl]pyridinium) dimethanesulfonate |
|
| Obidoxime Cl2 | 114-90-9 | LüH-6, Toxogonin, oxo-[[1-[[4-(oxoazaniu mylmethylidene) pyridin-1-yl] methoxymethyl]pyridin-4-ylidene]methyl]azanium dichloride |
|
| HI-6 DMS | 34433-31-3 | Asoxime; 4-carbamoyl-1-[({2-[(E)-(hydroxyimino) methyl)]pyridinium-1-yl}methoxyl)methyl] pyridinium dimethanesulfonate |
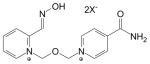
|
| HLö-7 DMS | 120103-35-7 | Pyridinium,1-(((4-(aminocarbonyl) pyridinio)methoxy) methyl)-2,4-bis((hydroxyimino)methyl dimethanesulfonate |
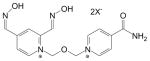
|
| Phorate oxon (PHO) | 2600-69-3 | Phoratoxon, O, O,-diethyl S-[(ethylsulfanyl)methyl] phosphorothioate |
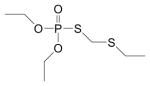
|
| Sarin | 107-44-8 | GB, isopropyl methylphosphonofluoridate |
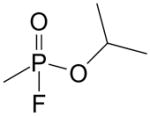
|
| Dimethane-sulfonate | NA | DMS |
|
Chemical Abstracts Services Registration Number
2.2 AChE Activity Determination
AChE activity was determined spectrophotometrically with a modified Ellman assay [23], using 1 mM ATC as the substrate and 0.5 mM DTNB as the indicator. Absorbance readings were captured at 412 nm using a BioTek® Synergy™ HTX Multi-Mode Microplate Reader. All experiments were performed at 37°C in phosphate buffered saline, pH 7.4 + 0.01% bovine serum albumin + 0.01% glycerol. For experiments that included oximes, background resulting from oximolysis was accounted for during calculations.
2.3 Kinetics of Inhibition
AChE was incubated with 5 μM PHO at 37°C for various time intervals (0–30 minutes). The inhibition rate constant, k i, was calculated as described previously [24].
2.4 Oxime Reactivation
AChE was incubated at 37°C with the experimentally determined IC90 of PHO for 15 minutes. To remove any unbound PHO, the reaction was dialyzed against reaction buffer overnight with buffer exchanges at 2 hours and 4 hours. Following dialysis, PHO-inhibited AChE was incubated at 37°C with varying concentrations of oximes and absorbance measurements were recorded each minute over a period of 30 minutes.
2.5 Kinetics of Reactivation
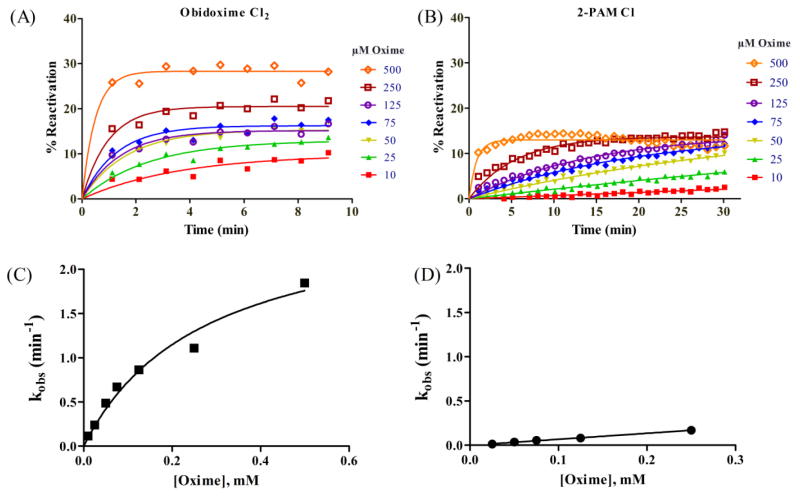
The first-order reactivation rate constant, k obs, was determined for multiple concentrations of each oxime by plotting the percent reactivation vs. time and analyzing the curves with one-phase association as described previously [24, 25]. Representative plots for obidoxime Cl2 and 2-PAM Cl are presented in Fig. 2A–B. Plotting k obs vs. [oxime] enabled the calculation of the reaction rate constant, k r, and the dissociation constant, KD, using Michaelis-Menten kinetics (Fig. 2C–D). An oxime’s affinity for the PHO-AChE complex is reflected by KD, and an oxime’s capacity for removing PHO and restoring AChE function is reflected by k r. The second-order reactivation rate constant, k r2, was calculated using the equation k r2 = k r/KD [24, 25]. In some cases, k r and KD could not be determined because the plot of k obs vs. [oxime] was linear (e.g., Fig. 2D) rather than a saturation curve (e.g., Fig. 2C), and k r2 was estimated from the slope of the line as described by Luo and colleagues [25].
Fig. 2.
Representative plots for the reactivation kinetics of PHO-inhibited recombinant guinea pig AChE reactivated by obidoxime Cl2 (A, C) and PHO-inhibited recombinant human AChE reactivated by 2-PAM Cl (B, D).
2.6 Aging
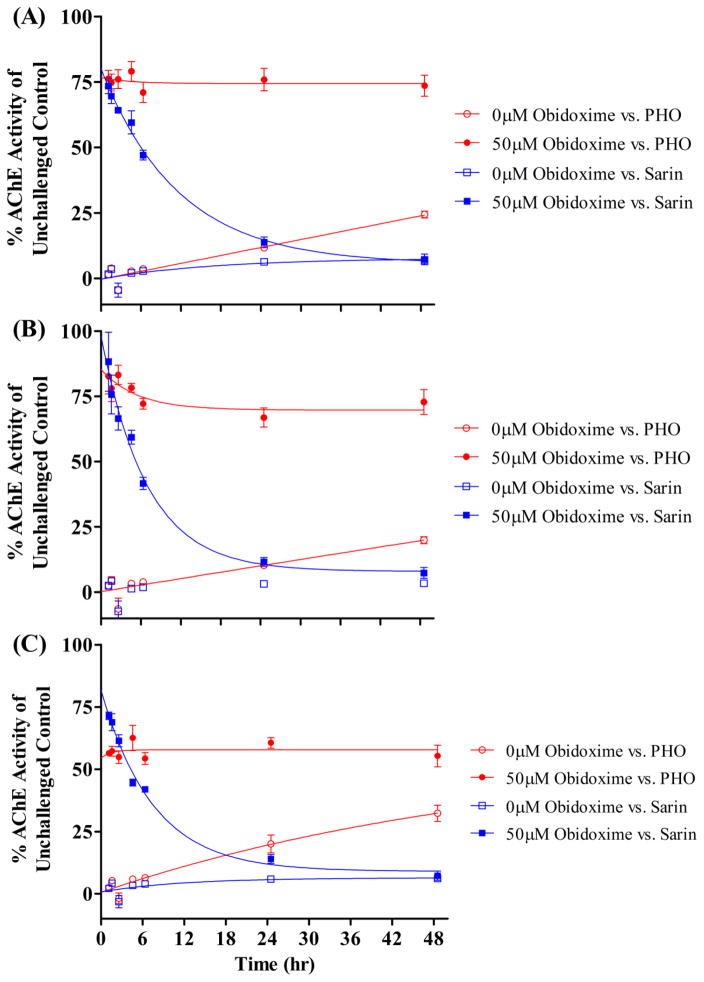
AChE was incubated at 37°C with the experimentally determined IC90 of PHO or sarin (GB) for 15 minutes. Unbound PHO or sarin was removed by passing the reaction mixture through 7K MWCO Zeba spin desalting columns (Thermo Scientific; Waltham, MA). Next, the inhibited AChE was incubated at 37°C for various time intervals (0–48 hr) before obidoxime Cl2 (50 μM) was added and AChE activity was determined. Obidoxime Cl2 was selected for the aging experiments because it was shown to be the most effective reactivator of PHO-inhibited AChE of the oximes tested, and it has been shown to reactivate sarin-inhibited AChE from all three species tested [26]. The rate constants for aging (k a) and spontaneous reactivation (k s) were calculated using a nonlinear regression model [24].
3.0 Results
3.1 Inhibition kinetics
The inhibition rate constant (k i) values for the inhibition of each AChE by PHO are summarized in Table 2. The inhibition kinetics of PHO were similar between rat and guinea pig AChE. The k i value determined for human AChE was approximately two- to threefold higher than the k i values determined for rats or guinea pigs. Literature k i values determined for other OPs using similar experimental conditions are provided for comparison (Table 3). The k i values determined for PHO in the current study were more similar to the previously reported value for the pesticide methamidophos than to the values determined for the more potent CWNAs sarin and VX, which were several orders of magnitude higher.
Table 3.
Rate constants for the inhibition of AChE by OP (k i)
| OP | AChE | k i (M−1 min−1)a |
|---|---|---|
| PHO | Recombinant guinea pig | 4.8 ± 0.2 × 103 |
| PHO | Recombinant rat | 6.1 ± 0.6 × 103 |
| PHO | Recombinant human | 1.4 ± 0.05 × 104 |
| Methamidophosb | Human erythrocyte | 1.9 ± 0.1 × 103 |
| DFPb | Human erythrocyte | 1.3 ± 0.04 × 105 |
| Paraoxon-ethylc | Human erythrocyte | 2.2 × 106 |
| Paraoxon-methyld | Human erythrocyte | 1.2 × 106 |
| Sarinb | Human erythrocyte | 2.7 ± 0.1 × 107 |
| VXb | Human erythrocyte | 1.2 ± 0.002 × 108 |
3.2 Reactivation kinetics
The reactivation kinetics results are summarized in Table 4. Obidoxime Cl2 was the most effective reactivator of PHO-inhibited AChE in all three species, as reflected by its relatively high k r values. HI-6 DMS was the least effective reactivator against PHO, with values unable to be determined in the guinea pig and human due to low reactivation. The k r2 values that were determined for 2-PAM Cl (the oxime currently approved for use against OP cholinesterase inhibitors in the U.S.) were much lower than those determined for obidoxime Cl2. In fact, only HI-6 DMS was a less effective reactivator of PHO-inhibited AChE than 2-PAM Cl.
Table 4.
Reactivation rate constants
| 2-PAM Cl | MMB4 DMS | Obidoxime Cl2 | HI-6 DMS | HLö-7 DMS | ||
|---|---|---|---|---|---|---|
| Rat | k r (min−1) | * | * | 11.06 | * | * |
| KD (mM) | * | * | 0.21 | * | * | |
| k r2 (mM−1 min−1) | 0.68 | 2.93 | 51.80 | 0.25 | 1.58 | |
| Guinea Pig | k r (min−1) | * | 1.05 | 2.72 | ND | * |
| KD (mM) | * | 0.86 | 0.27 | ND | * | |
| k r2 (mM−1 min−1) | 0.91 | 1.22 | 9.93 | ND | 2.96 | |
| Human | k r (min−1) | * | * | * | ND | * |
| KD (mM) | * | * | * | ND | * | |
| k r2 (mM−1 min−1) | 0.24 | 0.51 | 16.89 | ND | 2.05 |
Values represent the average of two independent experiments, each run in triplicate.
Value could not be determined due to the linear relationship of k obs vs. [oxime].
ND – value could not be determined due to low reactivation.
Limitations of the experimental approach for reactivation are worth considering. In general, reactivation did not approach 100% in these experiments, which suggests that re-inhibition of reactivated AChE may have occurred during the incubation with oximes. Excess PHO was removed by dialysis, but re-inhibition of AChE by free oxime and/or phospho-oxime cannot be ruled out. These are well known challenges associated with oxime reactivation kinetics experiments (reviewed in [29]) that are difficult to eliminate completely. In addition, rapid reactivation was observed with some oximes, and a modified approach that permitted the evaluation of reactivation at shorter time points may have enabled more accurate estimation of the rate constants for some of the oximes evaluated in this study. However, the experimental approach was based on previously reported methods and was more than sufficient to draw conclusions about the relative effectiveness of each oxime as a reactivator of PHO-inhibited AChE.
3.3 Aging kinetics
No significant aging of PHO-inhibited AChE was detected over a 48-hour incubation for any of the three species tested (Fig. 3). Sarin-inhibited AChE was also tested as a control condition, where significant aging was observed in all three species (Fig. 3). Higher levels of reactivation were observed in these experiments compared to the reactivation experiments, presumably due to differences in the experimental design (esp. the use of desalting columns vs. overnight dialysis to remove excess OP). Rate constants for aging (k a) could not be calculated for PHO because no significant aging was observed under the conditions tested. The k a values and aging half-times that were calculated for sarin are reported in Table 5. The aging half-time of sarin-inhibited human AChE was comparable to the previously reported value of ~5 hours [30]. For sarin-inhibited rat AChE, the previously reported aging half-time of 5.8 hours [31] was within ~30% of the value determined in the present study (i.e., 8.1 hr). The small difference could easily be due to variations between the experimental methods between the two studies.
Fig. 3.
Activity of PHO-inhibited rat (A), guinea pig (B), or human (C) AChE in the presence or absence of obidoxime Cl2. PHO- or sarin-inhibited AChE was incubated at 37°C until obidoxime was added and the AChE activity measured at the times indicated. Data represent average ± SEM from at least two independent experiments, each performed in triplicate.
Table 5.
Rate constants for aging (k a) and aging half-times of OP-inhibited AChE
| Species | OP | ka (h−1)1 | Aging t1/2 (h) |
|---|---|---|---|
| Rat | Sarin | 0.09 ± 0.01 | 8.1 |
| PHO | ND | ND | |
|
| |||
| Guinea Pig | Sarin | 0.15 ± 0.03 | 4.8 |
| PHO | ND | ND | |
|
| |||
| Human | Sarin | 0.13 ± 0.01 | 5.2 |
| PHO | ND | ND | |
Data represent average ± SEM of two independent experiments, each performed in triplicate.
ND – value could not be determined because the inhibited AChE was reactivated comparably at all time points (i.e., aging did not occur).
4.0 Discussion
The inhibition of AChE by OPs differs widely depending on the identity of the OP and the species in which it is evaluated. Differences in the rates of bioactivation of the parent compound and detoxification of the active metabolite are likely to be important drivers of species differences in the acute toxicity of OP pesticides [32]. However, differences in the inhibitory potency of OPs on AChE may also contribute to interspecies differences. In fact, the determination of k i values using isolated AChE in previous studies have revealed differences between species. Human and rhesus macaque AChE was shown to be more susceptible to inhibition by the OP pesticide metabolite paraoxon than rat, guinea pig, rabbit, or swine AChE [33]. In another study that evaluated the inhibition kinetics of the OP CWNAs VX, Russian VX, and Chinese VX, the k i values for all three agents that were determined using human AChE were approximately two-fold higher than those determined with swine AChE [34]. The results reported herein were similar; human AChE was more susceptible to inhibition with PHO than rat or guinea pig AChE (Table 2).
The species differences in the ability of oximes to reactivate AChE in vitro and the efficacy of oximes in animal studies are well documented and such differences complicate efforts to extrapolate the results of animal studies to humans. In one study, the reactivation kinetics of obidoxime, 2-PAM, HI-6, and HLö-7 were determined using rabbit, rat, guinea pig, and human AChE inhibited by the CWNAs sarin, cyclosarin, and VX [26]. The performance of each oxime varied between species, and the highest reactivation efficiency for all four oximes was found with human AChE. This is consistent with the results reported by Luo and colleagues, who found consistently higher reactivation constants with human AChE compared to guinea pig AChE when they evaluated the inhibition of both enzymes by three CWNAs (soman, cyclosarin, Russian VX) and reactivation with three oximes (HI-6, HLö-7, ICD-585) [25]. In a subsequent study that used the same CWNAs with the oximes 2-PAM, MMB4, HI-6, and HLö-7, the authors determined reactivation kinetics using AChE from humans and rhesus, cynomolgus, and African green monkeys [35]. In this study, human AChE did not consistently yield the highest reactivation efficiency, but the primate AChE results were still generally more similar to human AChE than those found with other species. Worek and colleagues reported moderate differences in reactivation constants between human, rhesus monkey, swine, rabbit, rat, and guinea pig AChE inhibited by paraoxon and reactivated with 2-PAM, MMB4, obidoxime, or HI-6 [33]. Interestingly, the reactivation constants determined for rat AChE were higher than the human values for most oximes, which is consistent with the results of the current study (Table 2). Obidoxime was found to be the most effective reactivator of paraoxon-inhibited AChE from humans, rats, and guinea pigs [33], which is also consistent with the PHO results reported herein (Table 2). The results of both studies support the assertion that obidoxime is one of the more effective reactivators for AChE inhibited by OP pesticides [36].
Two recent studies evaluated the efficacy of eight oximes against either a subcutaneous challenge or a percutaneous challenge with PHO in guinea pigs. In the subcutaneous challenge study, 2-PAM Cl, MMB4 DMS, obidoxime Cl2, and HLö-7 all significantly reduced PHO-induced mortality when administered approximately one minute after challenge [37]. However, no oxime significantly enhanced survival following a percutaneous challenge with PHO, when oxime administration was withheld until the onset of clinical signs (average oxime administration was approximately 5 hr after challenge) [38]. One obvious difference between the study designs is the timing of the oxime administration, which was almost concurrent with PHO challenge in the subcutaneous study but was delayed for several hours in the percutaneous study in order to mimic a real-world scenario, where a patient might not be aware of a small percutaneous exposure without the presence of clinical signs or symptoms. Given the difference in the timing of oxime administration, one plausible explanation for the disagreement of oxime efficacy results between the two studies that was proposed by the authors was aging of the PHO-AChE complex, which would make the inhibited AChE refractory to oxime reactivation [38]. Diethyl OPs like PHO are known to be generally less prone to aging than dimethyl OPs [15] and the results reported herein confirmed that PHO-inhibited AChE from any of the three species tested does not undergo significant aging within 48 hr (Table 4). Therefore, it is unlikely that aging was a factor in the observed difference in overall survival. Another possible explanation that was proposed by Snider and colleagues is that the oximes had the opportunity to interact directly with PHO in circulation and/or with target proteins in a blocking fashion in the subcutaneous challenge model [38]. This possibility was not evaluated in the current study, since the in vitro reactivation and aging experiments were conducted with purified, PHO-inhibited AChE in the absence of other receptors and proteins. Phorate is known to be transformed in vivo into a number of other metabolites in addition to PHO (summarized in [39]) and it is plausible that that one or more of these metabolites contributed to the lack of oxime efficacy observed in the percutaneous challenge study conducted by Snider and colleagues. The biological effects of other metabolites of phorate were not evaluated in the current study; it is recommended to evaluate their contribution to the toxicity of phorate in future studies.
In the present study, the kinetics of inhibition, reactivation, and aging were evaluated using AChE derived from humans, rats, and guinea pigs inhibited with PHO and reactivated with 2-PAM Cl, HI-6 DMS, obidoxime Cl2, MMB4-DMS, and HLö7 DMS. Obidoxime Cl2 was the most effective reactivator tested, which is consistent with the results of an oxime efficacy study conducted in guinea pigs, in which obidoxime treatment rescued all of the animals that received an LD85 subcutaneous PHO challenge [37]. 2-PAM Cl showed similar efficacy to obidoxime Cl2 in the aforementioned guinea pig study, which is interesting in light of the fact that 2-PAM Cl showed limited effectiveness in reactivating PHO-inhibited AChE in the current study. However, no oxime tested (including 2-PAM Cl and obidoxime Cl2) was efficacious against a percutaneous PHO challenge when treatment was delayed until onset of signs [38]. The ineffectiveness of delayed treatment with oximes against a percutaneous PHO challenge suggests that the therapeutic window for oxime administration following exposure is limited and the results reported herein indicate that this lack of efficacy is likely not due to aging of the PHO-AChE complex.
Taken together, the findings of the current and previous PHO studies underscore the need to evaluate the effects of individual OPs and subsequent interactions with individual oximes in relevant species in order to interpret the results of animal studies and ultimately inform the development of medical countermeasures and treatment strategies.
Highlights.
Obidoxime Cl2 is an effective reactivator of PHO-inhibited AChE.
2-PAM Cl showed limited effectiveness in reactivating PHO-inhibited AChE.
For PHO, the therapeutic window for oxime administration is not limited by aging.
Acknowledgments
The authors wish to thank Dr. Jill Harvilchuck for reviewing the manuscript and the following Battelle staff members for excellent technical assistance: Julianne Bartz, Erin Lemmon, Beth Reed, and Tyson Winters. We are also grateful to the kind generosity of the late Dr. Douglas M. Cerasoli (U.S. Army Medical Research Institute of Chemical Defense) for providing the recombinant rat AChE.
Funding:
This work was supported by the NIH Office of the Director through an interagency agreement (OD#: Y1- OD-0387-01) between NIAID and Department of Defense (DoD) and prepared under the auspices of the NIH, NIAID, NINDS, and the DoD Defense Technical Information Center (DTIC) under the CBRNIAC program, Contract No. SP0700-00-D-3180, Delivery Order Number 0794, CBRNIAC Task 689/CB-13-0689. The authors have no known conflicts of interest. The views expressed in this article are those of the authors and do not reflect the official policy of the NIH, HHS, DoD, or the U.S. Government. No official support or endorsement of this article by the NIAID, NINDS, or NIH is intended or should be inferred. The sponsor developed the concept of the study, contributed to its design, and the interpretation of the data as well as the preparation of the manuscript and the decision to submit it for publication. The sponsor also made similar contributions to other studies occurring at Battelle during the same time frame.
Abbreviations
- AChE
acetylcholinesterase
- ATC
acetylthiocholine
- CAS
Chemical Abstracts Service
- CWNA
chemical warfare nerve agent
- DTNB
5,5′-dithio-bis-[2-nitrobenzoic acid]
- OP
organophosphorus
- PHO
phorate oxon
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eddleston M, Buckley NA, Eyer P, Dawson AH. Management of acute organophosphorus pesticide poisoning. Lancet. 2008;371:597–607. doi: 10.1016/S0140-6736(07)61202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.W.H. Organization. Toxicological. World Health Organization; 2006. Pesticide Residues in Food - 2004: Evaluations 2004. [Google Scholar]
- 3.Snider TH, McGarry KG, Jr, Babin MC, Jett DA, Platoff GE, Jr, Yeung DT. Acute toxicity of phorate oxon by oral gavage in the Sprague-Dawley rat. Fundam Toxicol Sci. 2016;3:195–204. doi: 10.2131/fts.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young RJ, Jung FP, Ayer HE. Phorate intoxication at an insecticide formulating plant. American Industrial Hygiene Association journal. 1979;40:1013–1016. doi: 10.1080/15298667991430631. [DOI] [PubMed] [Google Scholar]
- 5.Kashyap SK, Jani JP, Saiyed HN, Gupta SK. Clinical effects and cholinesterase activity changes in workers exposed to Phorate (Thimet) Journal of environmental science and health. Part. B, Pesticides, food contaminants, and agricultural wastes. 1984;19:479–489. doi: 10.1080/03601238409372445. [DOI] [PubMed] [Google Scholar]
- 6.Khatiwada S, Tripathi M, Pokharel K, Acharya R, Subedi A. Ambiguous phorate granules for sesame seeds linked to accidental organophosphate fatal poisoning. JNMA J Nepal Med Assoc. 2012;52:49–51. [PubMed] [Google Scholar]
- 7.Bavili Tabrizi A, Abdollahi A. Determination of Organothiophosphate Insecticides in Environmental Water Samples by a Very Simple and Sensitive Spectrofluorimetric Method. Bulletin of environmental contamination and toxicology. 2015;95:536–541. doi: 10.1007/s00128-015-1612-7. [DOI] [PubMed] [Google Scholar]
- 8.Lari SZ, Khan NA, Gandhi KN, Meshram TS, Thacker NP. Comparison of pesticide residues in surface water and ground water of agriculture intensive areas. J Environ Health Sci Eng. 2014;12:11. doi: 10.1186/2052-336X-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox JA. Developing and Using Risk Based Requirements (Lessons from the Chemical Terrorism Risk Assessment). Keynote lecture delivered at the 10th Annual CounterACT Network Research Symposium; Davis, CA. June 16, 2016. [Google Scholar]
- 10.Chambers JE, Meek EC. Mammalian Toxicology of Insecticides. The Royal Society of Chemistry; 2012. Chapter 2 Mammalian Metabolism of Insecticides; pp. 14–36. [Google Scholar]
- 11.Taylor P. Anticholinesterase Agents. In: Brunton LL, editor. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. McGraw-Hill; New York: 2011. pp. 239–254. [Google Scholar]
- 12.Sidell FR, Newmark J, McDonough JH. Nerve Agents. In: Tuorinsky SD, editor. Medical Aspects of Chemical Warfare. Office of the Surgeon General; Washington, D.C: 2008. pp. 155–220. [Google Scholar]
- 13.Cannard K. The acute treatment of nerve agent exposure. J Neurol Sci. 2006;249:86–94. doi: 10.1016/j.jns.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Thiermann H, Worek F, Kehe K. Limitations and challenges in treatment of acute chemical warfare agent poisoning. Chemico-biological interactions. 2013;206:435–443. doi: 10.1016/j.cbi.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Eyer P. The role of oximes in the management of organophosphorus pesticide poisoning. Toxicological reviews. 2003;22:165–190. doi: 10.2165/00139709-200322030-00004. [DOI] [PubMed] [Google Scholar]
- 16.Worek F, Thiermann H, Szinicz L. Reactivation and aging kinetics of human acetylcholinesterase inhibited by organophosphonylcholines. Archives of toxicology. 2004;78:212–217. doi: 10.1007/s00204-003-0533-0. [DOI] [PubMed] [Google Scholar]
- 17.Fleisher JH, Harris LW. Dealkylation as a mechanism for aging of cholinesterase after poisoning with pinacolyl methylphosphonofluoridate. Biochemical pharmacology. 1965;14:641–650. doi: 10.1016/0006-2952(65)90082-1. [DOI] [PubMed] [Google Scholar]
- 18.Talbot BG, Anderson DR, Harris LW, Yarbrough LW, Lennox WJ. A comparison of in vivo and in vitro rates of aging of soman-inhibited erythrocyte acetylcholinesterase in different animal species. Drug and chemical toxicology. 1988;11:289–305. doi: 10.3109/01480548809017884. [DOI] [PubMed] [Google Scholar]
- 19.de Jong LP, Wolring GZ. Stereospecific reactivation by some Hagedorn-oximes of acetylcholinesterases from various species including man, inhibited by soman. Biochemical pharmacology. 1984;33:1119–1125. doi: 10.1016/0006-2952(84)90523-9. [DOI] [PubMed] [Google Scholar]
- 20.Eddleston M, Eyer P, Worek F, Mohamed F, Senarathna L, von Meyer L, Juszczak E, Hittarage A, Azhar S, Dissanayake W, Sheriff MH, Szinicz L, Dawson AH, Buckley NA. Differences between organophosphorus insecticides in human self-poisoning: a prospective cohort study. Lancet. 2005;366:1452–1459. doi: 10.1016/S0140-6736(05)67598-8. [DOI] [PubMed] [Google Scholar]
- 21.Eddleston M, Szinicz L, Eyer P, Buckley N. Oximes in acute organophosphorus pesticide poisoning: a systematic review of clinical trials. QJM. 2002;95:275–283. doi: 10.1093/qjmed/95.5.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Worek F, Thiermann H, Wille T. Oximes in organophosphate poisoning: 60 years of hope and despair. Chemico-biological interactions. 2016;259:93–98. doi: 10.1016/j.cbi.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 23.Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical pharmacology. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 24.Worek F, Thiermann H, Szinicz L, Eyer P. Kinetic analysis of interactions between human acetylcholinesterase, structurally different organophosphorus compounds and oximes. Biochemical pharmacology. 2004;68:2237–2248. doi: 10.1016/j.bcp.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 25.Luo C, Tong M, Chilukuri N, Brecht K, Maxwell DM, Saxena A. An in vitro comparative study on the reactivation of nerve agent-inhibited guinea pig and human acetylcholinesterases by oximes. Biochemistry. 2007;46:11771–11779. doi: 10.1021/bi701002f. [DOI] [PubMed] [Google Scholar]
- 26.Worek F, Reiter G, Eyer P, Szinicz L. Reactivation kinetics of acetylcholinesterase from different species inhibited by highly toxic organophosphates. Archives of toxicology. 2002;76:523–529. doi: 10.1007/s00204-002-0375-1. [DOI] [PubMed] [Google Scholar]
- 27.Worek F, Backer M, Thiermann H, Szinicz L, Mast U, Klimmek R, Eyer P. Reappraisal of indications and limitations of oxime therapy in organophosphate poisoning. Human & experimental toxicology. 1997;16:466–472. doi: 10.1177/096032719701600808. [DOI] [PubMed] [Google Scholar]
- 28.Worek F, Diepold C, Eyer P. Dimethylphosphoryl-inhibited human cholinesterases: inhibition, reactivation, and aging kinetics. Archives of toxicology. 1999;73:7–14. doi: 10.1007/s002040050580. [DOI] [PubMed] [Google Scholar]
- 29.Worek F, Eyer P, Thiermann H. Determination of acetylcholinesterase activity by the Ellman assay: a versatile tool for in vitro research on medical countermeasures against organophosphate poisoning. Drug testing and analysis. 2012;4:282–291. doi: 10.1002/dta.337. [DOI] [PubMed] [Google Scholar]
- 30.Sidell FR, Groff WA. The reactivatibility of cholinesterase inhibited by VX and sarin in man. Toxicology and applied pharmacology. 1974;27:241–252. doi: 10.1016/0041-008x(74)90195-1. [DOI] [PubMed] [Google Scholar]
- 31.Harris LW, Fleisher JH, Clark J, Cliff WJ. Dealkylation and loss of capacity for reactivation of cholinesterase inhibited by sarin. Science. 1966;154:404–407. doi: 10.1126/science.154.3747.404. [DOI] [PubMed] [Google Scholar]
- 32.Chambers JE, Carr RL. Biochemical mechanisms contributing to species differences in insecticidal toxicity. Toxicology. 1995;105:291–304. doi: 10.1016/0300-483x(95)03225-5. [DOI] [PubMed] [Google Scholar]
- 33.Worek F, Aurbek N, Wille T, Eyer P, Thiermann H. Kinetic analysis of interactions of paraoxon and oximes with human, Rhesus monkey, swine, rabbit, rat and guinea pig acetylcholinesterase. Toxicology letters. 2011;200:19–23. doi: 10.1016/j.toxlet.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Aurbek N, Thiermann H, Szinicz L, Eyer P, Worek F. Analysis of inhibition, reactivation and aging kinetics of highly toxic organophosphorus compounds with human and pig acetylcholinesterase. Toxicology. 2006;224:91–99. doi: 10.1016/j.tox.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 35.Luo C, Tong M, Maxwell DM, Saxena A. Comparison of oxime reactivation and aging of nerve agent-inhibited monkey and human acetylcholinesterases. Chemico-biological interactions. 2008;175:261–266. doi: 10.1016/j.cbi.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 36.Worek F, Eyer P, Aurbek N, Szinicz L, Thiermann H. Recent advances in evaluation of oxime efficacy in nerve agent poisoning by in vitro analysis. Toxicology and applied pharmacology. 2007;219:226–234. doi: 10.1016/j.taap.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Wilhelm CM, Snider TH, Babin MC, Jett DA, Platoff GE, Jr, Yeung DT. A comprehensive evaluation of the efficacy of leading oxime therapies in guinea pigs exposed to organophosphorus chemical warfare agents or pesticides. Toxicology and applied pharmacology. 2014;281:254–265. doi: 10.1016/j.taap.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snider TH, Wilhelm CM, Babin MC, Platoff GE, Jr, Yeung DT. Assessing the therapeutic efficacy of oxime therapies against percutaneous organophosphorus pesticide and nerve agent challenges in the Hartley guinea pig. The Journal of toxicological sciences. 2015;40:759–775. doi: 10.2131/jts.40.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.INCHEM, [Internet] [accessed October 3, 2017];International Programme on Chemical Safety. c2017 pp. Available from http://www.inchem.org/documents/jmpr/jmpmono/v96pr10.htm.