Highlights
-
•
An examination of mutations in PIK3CA signaling pathways and radioresistance.
-
•
PIK3CA mutation was found to be associated with local failure after lung SBRT.
-
•
This is a small study and there is need for further validation.
Abbreviations: PI3K, phosphatidylinositol-3-kinases; PIP2, phosphatidylinositol-4,5-biphosphate; PIP3, phosphatidylinositol-3,4,5-triphosphate; SBRT, stereotactic body radiation therapy; AKT, protein kinase B; CT, computed tomography; GTV, gross tumor volume; ITV, internal target volume; CTV, clinical target volume; PTV, planning target volume; BED, biologically effective dose; LF, local failure
Keywords: Lung stereotactic body radiation therapy (SBRT), PIK3CA, Radiation resistance, Local failure
Abstract
Objectives
Hyperactivation of the phosphatidylinositol-3-kinase (PI3K) pathway has been associated with radioresistance. It is unclear whether such mutations confer suboptimal local control for patients who receive lung stereotactic body radiation therapy (SBRT). Our objective was to examine whether mutations in the EGFR/AKT/PIK3CA signaling pathway are associated with local failure (LF) after lung SBRT.
Methods
We retrospectively reviewed 166 patients who underwent SBRT to primary or metastatic lung lesions from 2007 to 2015 for whom genetic testing data was available for EGFR, AKT, and PIK3CA genes. Association between clinical factors, including molecular mutation status, and LF was evaluated.
Results
Six patients (4%) had PIK3CA mutation, 36 patients (22%) had EGFR mutation, and one patient (0.6%) had AKT1 mutation. Median lesion size was 2.0 cm (range, 0.6–5.6 cm); median dose was 48 Gy in 4 fractions (range, 30–70 Gy in 3–10 fractions). Median follow-up for survivors was 27.3 months (range, 3.8–66.7 months). LF occurred in 16 patients (10%). On univariate analysis, PIK3CA mutation was associated with LF (HR 10.44 [95% CI 2.16–50.46], p = .003), while tumor histology, tumor size, primary tumor site, BED and EGFR mutation were not. At one year, probability of LF in lesions with PIK3CA mutation was 20.0% vs. 2.9% in lesions without mutation (p < .001 by log rank test).
Conclusion
Although the number of patients affected was small, PIK3CA mutation was significantly associated with higher risk of LF in patients undergoing lung SBRT. This association has not previously been reported for lung SBRT and indicates the need for further validation.
Introduction
Hyperactivation of the phosphatidylinositol-3-kinases (PI3K) pathway has been associated with radioresistance in vitro [1]. Following growth factor stimulation and activation of receptor tyrosine kinases such as epidermal growth factor receptor (EGFR), PI3K (consisting of regulatory and catalytic subunits) is recruited to the cellular membrane. The activated catalytic subunit of PIK3 converts phosphatidylinositol-4,5-biphosphate (PIP2) to phosphatidylinositol-3,4,5-triphosphate (PIP3) and provides a docking site for the activation of Protein Kinase B (AKT), leading to downstream pro-survival signaling events [2]. Mutations in PIK3CA, the gene that encodes the catalytic subunit of PI3K, have been reported in 2–5% of non-small cell lung cancers in 6–40% of other solid tumors such as breast, liver, and colon [3], [4]. It is unknown whether mutations in the PI3K pathway confer suboptimal local control for patients who receive lung stereotactic body radiation therapy (SBRT). Our objective was to examine whether mutations in the EGFR/AKT/PIK3CA signaling pathway are associated with local failure (LF) after lung SBRT.
Methods
Study population
All patients who received stereotactic body radiation therapy (SBRT) to the lung, for either a primary or a metastatic lung tumor, at our institution from 2007 to 2015 were reviewed following Institutional Review Board approval. There were 166 patients who met inclusion criteria: no prior in-field radiation, imaging follow-up available, and tumor genetic testing results for EGFR, AKT, and PIK3CA genes. For tumors of lung origin (n = 154), genetic testing data was required from the lung tumor undergoing SBRT. For metastatic tumors to lung (n = 12), genetic testing data from either a primary or metastatic tumor site was used (whichever was available). Genetic testing was performed either by Sequenom mass-spectrometry genotyping (n = 153) or Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) (n = 13). Medical records were reviewed and clinical outcomes including death and LF were collected.
Radiation therapy
Patients were immobilized in an alpha cradle with arms up, or if not tolerated, in an Aquaplast mold with arms down. Radiation simulation was performed with four-dimensional computed tomography (4D-CT) to account for tumor respiratory motion. Gross tumor volume (GTV) was delineated on a free-breathing CT scan (with aid from positron emission tomography (PET) scan when available), and an internal target volume (ITV) was created based on GTV motion on the 4D-CT. The clinical target volume (CTV) was a 2–3 mm expansion of the ITV to account for microscopic disease. The CTV was expanded by 5 mm to create the planning target volume (PTV). Dose was prescribed to the 100% isodose line, which surrounded the PTV. Treatments were with coplanar intensity modulated 6 MV fields, and plans were calculated with inhomogeneity correction. The median dose prescribed was 48 Gy (range, 30–70 Gy), and median number of fractions was 4 (range, 3–10). Treatments were image-guided with cone-beam CT to verify positioning.
Statistical analysis
Patients were generally followed with CT scans every 3–4 months for the first two years, then every six months thereafter. Outcomes were measured from the initiation of SBRT until last follow up or death. LF was defined as unequivocal radiographic recurrence within the treatment target, based on CT and PET scans. Association between clinical factors including presence or absence of gene mutations and outcomes were tested using univariate and multivariate Cox regression analysis. The Kaplan-Meier method was used to assess actuarial LF and overall survival. Analyses were performed with SAS release 9.4 (SAS Institute, Carry NC), R version 3.1 package survival, and IBM SPSS Statistics.
Results
Patient and tumor characteristics are summarized in Table 1. The most common tumor histology in the cohort was adenocarcinoma (92%). Six patients (4%) had PIK3CA mutation, 36 patients (21%) had EGFR mutation, and one patient (0.6%) had AKT mutation. Median lesion size was 2.0 cm (range, 0.6–5.6 cm). Median follow-up for survivors was 27.3 months (range, 3.8–66.7 months). LF was observed for 16 patients (10%).
Table 1.
Patient, tumor, and radiation characteristics.
| No. (%), n = 166 | Median (range) | |
|---|---|---|
| Age, years | 75 (23–95) | |
| Sex | ||
| Male | 68 (41.0) | |
| Female | 98 (59.0) | |
| Primary tumor site | ||
| Lung | 154 (92.8) | |
| Gastrointestinal | 8 (4.8) | |
| Thyroid | 1 (0.6) | |
| Genitourinary | 3 (1.8) | |
| Histology | ||
| Adenocarcinoma | 152 (91.6) | |
| Squamous cell carcinoma | 9 (5.4) | |
| Other | 5 (3.0) | |
| Tumor size, cm | 2.0 (0.6–5.6) | |
| <2.0 cm | 82 (49.7) | |
| ≥2.0 cm or <3.0 cm | 42 (25.5) | |
| ≥3.0 cm or <4.0 cm | 25 (15.2) | |
| ≥4.0 cm | 16 (9.7) | |
| AKT1 mutation status | ||
| Present | 1 (0.6) | |
| Absent | 165 (99.4) | |
| EGFR mutation status | ||
| Present | 35 (21.1) | |
| Absent | 131 (78.9) | |
| PIK3CA mutation status | ||
| Present | 6 (3.6) | |
| Absent | 160 (96.4) | |
| SBRT total dose, Gy | 48 (30–70) | |
| SBRT total fractions | 4 (3–10) | |
| BED,*Gy | 106 (48–180) | |
| <100 Gy | 41 (24.7) | |
| ≥100 Gy | 125 (75.3) | |
| Local failure status | ||
| No | 150 (90.4) | |
| Yes | 16 (9.6) | |
| Survival status | ||
| Alive | 114 (68.7) | |
| Dead | 52 (31.3) | |
Abbreviations: BED = biologically effective dose; Gy = Gray; SBRT = stereotactic body radiation therapy.
BED based on alpha/beta of 10.
On univariate analysis, PIK3CA mutation was associated with increased LF (HR 10.44 [95% CI 2.16–50.46]), p = .003 (and remained p < .05 after Bonferroni multiple testing correction), while tumor histology (adenocarcinoma vs. other), tumor size (≤2 cm vs. >2 cm), primary tumor site (lung vs. other), prescription biologically effective dose (BED) (<100 Gy versus ≥100 Gy) and EGFR mutation presence were not, Table 2. There were too few events for multivariate analysis, but all tumors with PIK3CA mutation were ≤3.2 cm in size and all but one of the tumors with PIK3CA mutation received BED >100 Gy.
Table 2.
Univariate analysis for local failure after lung SBRT.
| Hazard Ratio (95% CI) | p-value | |
|---|---|---|
| Patient age (continuous variable) | 1.04 (0.99–1.10) | .15 |
| Patient sex (female vs male) | 0.56 (0.21–1.50) | .25 |
| Histology (adenocarcinoma vs other) | 0.61 (0.14–2.70) | .52 |
| Primary tumor site (lung vs other) | 0.35 (0.08–1.52) | .16 |
| Tumor size (≥2.0 cm vs <2.0 cm) | 0.94 (0.35–2.53) | .90 |
| BED* of SBRT (≥100 Gy vs <100 Gy) | 0.95 (0.31–2.96) | .93 |
| EGFR mutation (present vs absent) | 0.77 (0.22–2.71) | .68 |
| PIK3CA mutation (present vs absent) | 10.44 (2.16–50.46) | .004 |
Abbreviations: BED = biologically effective dose; Gy = Gray; SBRT = stereotactic body radiation therapy.
Note: For each binary variable, the second category following “vs” was the reference level (e.g. for sex, male was the reference), and the hazard ratio presents the risk of developing local failure for the first category vs the reference category (e.g. comparing to male the risk of developing local failure for female was 56%).
BED based on alpha/beta of 10.
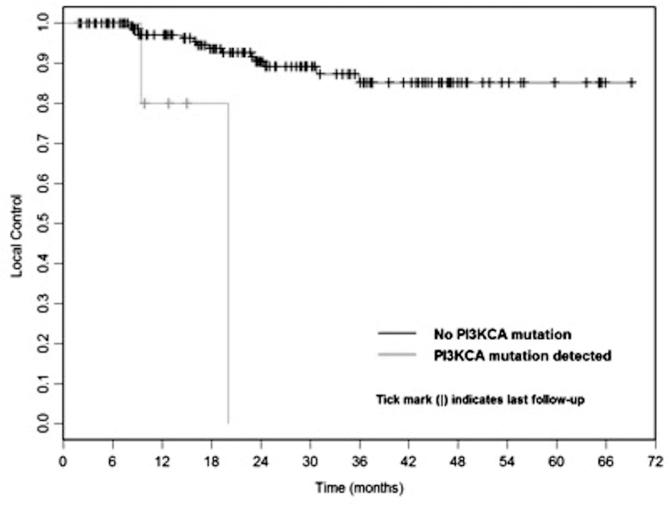
For all patients, actuarial local control at 1 year was 91.8% and at 2 years was 81.6%. At one year, probability of LF in lesions with PIK3CA mutations was 20.0% vs. 2.9% in lesions without mutations (p < .001 by log-rank test), Fig. 1.
Fig. 1.
Kaplan-Meier curve for local control following lung SBRT for primary or metastatic tumors to lung based on PIK3CA mutation status (p < .001).
For all patients, overall survival at 1 year was 89.9% and at 2 years was 77.1% with a median survival of 54.3 months. PIK3CA or EGFR mutation status was not significantly associated with overall survival.
Discussion
PIK3CA mutations are an attractive area of study due to the opportunity for pharmacokinetic inhibition. Results of a phase 1 dose-escalation study of an oral PI3K inhibitor taselisib recently reported a 36% response rate for PIK3CA-mutant tumor patients with measurable disease [5]. Therapeutic strategies combining PI3K inhibition with radiation may be a topic of future investigation.
Associations between PIK3CA mutations and outcomes have shown conflicting results in the literature. PIK3CA mutations have demonstrated association with improved overall survival in squamous cell lung cancer and breast cancer [6], [7] yet decreased progression-free survival in lung adenocarcinoma [8]. In a study by Boros et al. [9], next generation sequencing was performed on 57 patients with locally advanced NSCLC for EGFR, KRAS, BRAF, PIK3CA, NRAS, and ALK with 1 patient (2%) expressing a PIK3CA mutation. Boros et al. reported that following chemoradiation, overall survival was not significantly different among the three groups of wild-type versus EGFR-ALK versus other mutations. In our study, PIK3CA mutation status was not associated with overall survival following lung SBRT.
We found PIK3CA mutation was significantly associated with higher risk of LF in patients undergoing lung SBRT. Although the number of patients affected was small, the magnitude of the effect was marked, and most affected patients developed LF despite having small tumors receiving high-BED radiation. Association between PIK3CA mutation and outcome after lung SBRT has not previously been reported. Our preliminary results should be verified by larger studies and suggest that the interaction of PIK3CA and radiation response is worthy of further investigation.
Conflict of interest statement
AW reports grant from CivaTech Oncology, Inc., outside the submitted work. AR reports grants from Varian Medical Systems and Boehringer Ingelheim, outside the submitted work. The other authors report no conflicts of interest.
Summary
Hyperactivation of the PI3K pathway has been associated with radioresistance in vitro; it is unclear whether PIK3CA mutations confer increased local failure following lung SBRT. We found PIK3CA mutation was associated with higher local failure following lung SBRT. This is a novel finding that needs further validation in larger cohorts.
Acknowledgements
This work was supported in part by NIH/NCI Memorial Sloan-Kettering Cancer Center Support Grant [grant number P30 CA008748]. We acknowledge the Molecular Diagnostics Service in the Department of Pathology and the Marie-Josée and Henry R. Kravis Center for Molecular Oncology.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ctro.2017.11.002.
Appendix A. Supplementary data
References
- 1.Gupta A.K., Bakanauskas V.J., Cerniglia G.J. The Ras radiation resistance pathway. Cancer Res. 2001;61:4278–4282. [PubMed] [Google Scholar]
- 2.Liu P., Cheng H., Roberts T.M., Zhao J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heavey S., O’Byrne K.J., Gately K. Strategies for co-targeting the PI3K/AKT/mTOR pathway in NSCLC. Cancer Treat Rev. 2014;40:445–456. doi: 10.1016/j.ctrv.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Karakas B., Bachman K.E., Park B.H. Mutation of the PIK3CA oncogene in human cancer. Br J Cancer. 2006;94:455–459. doi: 10.1038/sj.bjc.6602970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juric D., Krop I., Ramanathan R.K. Phase I dose-escalation study of taselisib, an oral PI3K inhibitor, in patients with advanced solid tumors. Cancer Discov. 2017;7:704–715. doi: 10.1158/2159-8290.CD-16-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGowan M., Hoven A.S., Lund-Iversern M. PIK3CA mutations as prognostic factor in squamous cell lung carcinoma. Lung Cancer. 2017;103:52–57. doi: 10.1016/j.lungcan.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Kalinsky K., Jacks L.M., Heguy A. PIK3CA mutation associates with improved outcome in breast cancer. Clin Cancer Res. 2009;15:5050–5059. doi: 10.1158/1078-0432.CCR-09-0632. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L., Shi L., Zhao X., Wang Y., Yue W. PIK3CA gene mutation associated with poor prognosis of lung adenocarcinoma. Oncol Targets Ther. 2013;6:497–502. doi: 10.2147/OTT.S41643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boros A., Lacroix L., Lacas B., Adam J., Caramella C., Planchard D. Prognostic value of tumor mutations in radically treated locally advanced non-small cell lung cancer patients. Oncotarget. 2017;8:25189–25199. doi: 10.18632/oncotarget.15966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.