Abstract
An increasing amount of data supports an inverse association between statin use and cancer risk. The findings for prostate cancer, particularly advanced disease, are the most promising of all cancers studied. Use of these agents seems to also be associated with improved prostate-cancer-specific survival, particularly in men undergoing radiotherapy, suggesting usefulness of statins in secondary and tertiary prevention. Some study results might be influenced by increased PSA screening and health-conscious behaviour in statin users but these factors are unlikely to completely account for observed beneficial effects. The epidemiological evidence is supported by preclinical studies that show that statins directly inhibit prostate cancer development and progression in cell-based and animal-based models. The antineoplastic effect of statins might arise from a number of cholesterol-mediated and non-cholesterol-mediated mechanisms that affect pathways essential for cancer formation and progression. Understanding these mechanisms is instrumental in drug discovery research for the development of future prostate cancer therapeutics, as well as in designing clinical trials to test a role for statins in prostate cancer prevention. Currently, sufficient data are lacking to support the use of statins for the primary prevention of prostate cancer and further research is clearly warranted. Secondary and tertiary prevention trials in men who have been diagnosed with prostate cancer might soon be performed.
Statins are a class of medications that effectively lower serum cholesterol levels by inhibiting 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase, which is the rate-limiting enzyme for cholesterol synthesis in the liver. Statins are becoming one of the most commonly prescribed medications in the USA, owing to the epidemic proportions of hyperlipidaemia in this country1. In 2012, more than one in four US adults aged ≥40 years reported using statins; simvastatin and atorvastatin were the two most commonly used agents (42% and 20% of all statin users, respectively)2. Unequivocal evidence exists that statins reduce the number of adverse cardiovascular events associated with hyperlipidaemia3, but during the past decade several reports have highlighted the potential of statins in chemoprevention of other diseases4–11. For example, statin use has been linked to reduced risk of several cancer types. The most promising data relate to the prevention of prostate cancer — in particular advanced disease6–11. However, advocating that all men start taking statins as a chemopreventive measure against prostate cancer would currently be premature, as not all data agree on the potential benefits of statins, especially in reducing the risk of prostate cancer of any stage (referred to as total prostate cancer)12,13. In this Review, we present the current evidence supporting and opposing a role for statins in the chemoprevention of prostate cancer. We review cell-based and animal-based pre-clinical studies that examined the molecular mechanisms of inhibitory effects of statins on prostate cancer growth and examine the most current data from studies in humans on associations between statin use and prostate cancer, with an emphasis on the accumulating evidence that supports an effect of statin use in preventing advanced prostate cancer and prostate cancer progression. We also discuss the current gaps in our understanding of how statins might modify prostate cancer risk, which require further work to better guide future research and funding strategies.
Statin medications: the basics
The ability of statins to reduce the number of adverse cardiovascular events associated with hyperlipidaemia by lowering total serum cholesterol and LDL cholesterol levels is well established. The underlying mechanism is based on restricting cholesterol synthesis in the liver via inhibition of the rate-limiting hepatic HMG-CoA reductase3. Statins can be classified as either hydrophilic or lipophilic, depending on their solubility14 (TABLE 1). Hydrophilic statins are more hepatoselective than lipophilic statins, as they are actively transported into the liver by members of the organic anion transporting poly-peptide family (also known as OATPs). By contrast, lipophilic statins enter the liver by passive diffusion. Relative to hydrophilic statins, lipophilic statins are taken up more easily by nonhepatic tissues that do not express dedicated transporters, such as the prostate15. Hence, lipophilic stat-ins have been hypothesized to have a greater influence on the prostate than hydrophilic statins, but this theory has not been corroborated by observational studies of statin use and prostate cancer risk (TABLE 2), in part because the number of men taking hydrophilic statins was low6,16–18.
Table 1.
| Statin type | Rate of use* (%) | Solubility | IC50 for HMG-CoA reductase (nM) | Systemic bioavailability (%) |
|---|---|---|---|---|
| Simvastatin | 42.0 | Lipophilic | 11.2 | <5 |
| Atorvastatin | 20.2 | Lipophilic | 8.2 | ~14 |
| Pravastatin | 11.2 | Hydrophilic | 44.1 | 17 |
| Rosuvastatin | 8.2 | Hydrophilic | 5.4 | ~20 |
| Lovastatin | 7.4 | Lipophilic | 2.7–11.1 | <5 |
| Pitavastatin | NR | Lipophilic | 6.8 | >60 |
| Fluvastatin | NR | Lipophilic | 27.6 | 24 |
Rate of use in US adults aged ≥40 years reporting to take a cholesterol-lowering medication in the past 30 days (2011–2012).
IC50, half maximal inhibitory concentration; NR, not reported.
Table 2.
Observational studies of statin use and prostate cancer risk
| Study | Design | Country | Participants (n) | Statin type | Follow- up duration |
Exposure definition for primary analysis |
Fully adjusted results |
Definition of advanced disease |
|---|---|---|---|---|---|---|---|---|
| Case-control studies | ||||||||
| Shannon et al. (2005)6 | Hospital-based case-control | USA |
|
>97% simvastatin or lovastatin | NA | Statin use vs nonuse |
|
Gleason score ≥7 |
| Graaf et al. (2004)7 | Population-based nested case-control | Netherlands |
|
|
Mean 7.2 years | Statin use vs nonuse | Total: OR 0.37 (95% CI 0.11–1.25) | NA |
| Murtola et al. (2007)10 | Population-based case-control | Finland | 24,723 case-control pairs (~3,700 advanced) |
|
NA | Statin use vs nonuse |
|
High disease stage (not defined) |
| Agalliu et al. (2008)16 | Population-based case-control | USA |
|
|
NA | Statin use vs nonuse |
|
High disease stage (not defined) |
| Jespersen et al. (2014)18 | Population-based case-control | Denmark |
|
|
NA | Current statin use vs nonuse |
|
Disease stage ≥3, N1, M1 |
| Blais et al. (2000)65 | Population-based nested case-control | Canada |
|
NR | Median 2.7 years | Statin use vs use of bile-acid-binding resins | Total: RR 0.74 (95% CI 0.36–1.51) | NA |
| Kaye et al. (2004)66 | Case-control | UK |
|
NR | NA | Current statin use vs nonuse (in men without hyper-lipidaemia) | Total: RR 1.3 (95% CI 1.0–1.9) | NA |
| Coogan et al. (2010)67 | Hospital-based case-control | USA |
|
Majority lipophilic | NA | Statin use vs nonuse |
|
Disease stage ≥3 |
| Chang et al. (2011)68 | Population-based case-control | Taiwan |
|
NR | NA | Statin use vs nonuse | Total: OR 1.55 (95% CI 1.09–2.19) | NA |
| Haukka et al. (2010)69 | Population-based nested case-control | Finland | 235,830 pairs of statin users and nonuser (n cases NR) |
|
Mean 8.8 years | Statin use vs nonuse | Total: RR 1.12 (95% CI 1.08–1.17) | NA |
| Tan et al. (2011)70 | Case-control | USA |
|
NR | NA | Current statin use vs nonuse |
|
Gleason score ≥7 |
| Fowke et al. (2011)89 | Cross-sectional case-control | USA |
|
|
NA | Current statin use vs nonuse | Advanced: OR 0.95 (95% CI 0.73–1.24) | Gleason score ≥7 |
| Retrospective cohort studies | ||||||||
| Friis et al. (2005)5 | Retrospective population-based cohort | Denmark |
|
Majority simvastatin | Mean 4 years | Statin use vs nonuse | Total: RR 0.87 (95% CI 0.61–1.23) | NA |
| Boudreau et al. (2008)17 | Retrospective cohort | USA |
|
Majority lovastatin and simvastatin | Median 5.7 years | Statin use vs nonuse |
|
Gleason score ≥8, or regional or distant stage |
| Farwell et al. (2011)71 | Retrospective cohort | USA |
|
|
Median 5.6 years | Statin use vs hypertension medication use |
|
Gleason score ≥4 + 3 |
| Lustman et al. (2014)74 | Retrospective population-based cohort | Israel |
|
NR | NR | Long-term statin use (≥5 years) vs nonuse | Total: HR 0.26(95% CI 0.22–0.31) | NA |
| Morote et al. (2014)75 | Retrospective cohort | Spain |
|
NR | NR | Long-term statin use (≥3 years) vs nonuse |
|
Gleason score ≥8 |
| Nordstrom et al. (2015)90 | Retrospective cohort | Sweden |
|
NR | NR | Statin use vs nonuse |
|
Gleason score ≥7 |
| Prospective cohort studies | ||||||||
| Platz et al. (2006)8 | Prospective cohort | USA |
|
NR | 376,939 person-years | Current statin use vs never or past statin use |
|
Disease stage ≥3b, N1, M1 or fatal |
| Jacobs et al. (2007)9 | Prospective cohort | USA |
|
NR | NR | Long-term statin use (≥5 years) vs nonuse |
|
Disease stage ≥3 or fatal with unknown stage at diagnosis |
| Flick et al. (2007)11 | Prospective cohort | USA |
|
|
Median 2.3 years | Statin use vs nonuse |
|
Disease stage ≥2 |
| Murtola et al. (2010)72 | Prospective cohort | Finland |
|
|
Median 6.9 years | Statin use vs nonuse |
|
Disease stage ≥3, N1, M1 |
| Breau et al. (2010)73 | Prospective cohort | USA |
|
NR | Median 15 years | Daily statin use vs nonuse |
|
Gleason score ≥7 |
| Kantor et al. (2015)76 | Prospective cohort | USA |
|
NR | Mean 5.2 years | Current statin use at baseline vs nonuse |
|
Gleason score ≥4 + 3 |
| Smeeth et al. (2009)78 | Prospective cohort | UK |
|
>50% simvastatin or atorvastatin | Median 4.4 years | Statin use vs nonuse | Total: HR 1.06 (95% CI 0.86–1.30) | NA |
| Hippisley-Cox et al. (2010)79 | Prospective cohort | UK |
|
|
NR | Statin use vs nonuse | Total: HR 1.05 (95% CI 0.98–1.13) | NA |
| Jacobs et al. (2011)80 | Prospective cohort | USA |
|
NR | NR | Long-term statin use (≥5 years) vs nonuse |
|
Disease stage ≥3 or fatal with unknown stage at diagnosis |
| Chan et al. (2012)81 | Prospective cohort | USA |
|
NR | Mean 7 years | Current statin use at baseline vs nonuse |
|
Gleason score ≥7 |
| Friedman et al. (2008)82 | Prospective cohort | USA |
|
|
Median 4.9 years | Statin use vs nonuse |
|
Disease stage ≥2 |
| Platz et al. (2014)88 | Prospective cohort | USA |
|
NR | 7 years | Statin use vs nonuse |
|
Gleason score ≥7 |
| Other study type | ||||||||
| Freedland et al. (2013)77 | Secondary analysis of prospective trial | Multi-national |
|
NR | Prostate biopsy at 2 years and 4 years | Current statin use at baseline vs nonuse |
|
Gleason score ≥7 |
HR, hazard ratio; NA, not applicable; NR, not reported; OR, odds ratio; RR, relative risk ratio; vs, compared with.
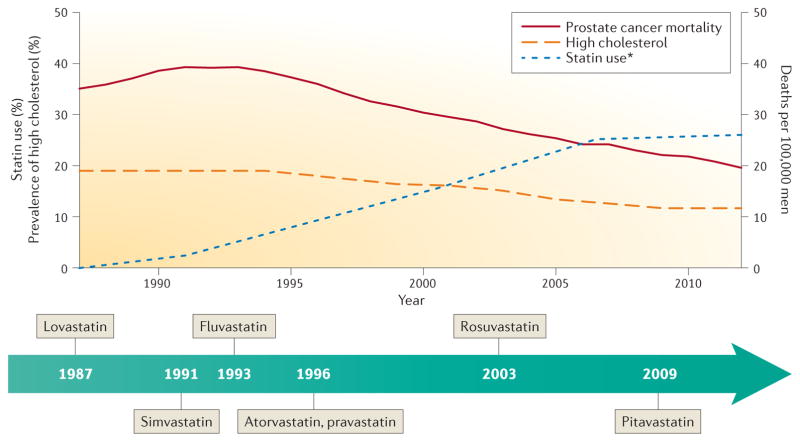
Statins are generally well tolerated, with the most common adverse effects being hepatic dysfunction and muscle myopathies. A meta-analysis of 35 clinical trials in patients with hyperlipidaemia receiving a statin or a placebo drug concluded that statin therapy is associated with a small excess risk of hepatic dysfunction but not of myalgias, rhabdomyolysis or elevation of creatine kinase levels, which is a marker of myopathy19. Another meta-analysis of 13 clinical trials showed that statin use was associated with a slightly elevated risk of new-onset diabetes, but this risk was offset by the cardiovascular benefits of statins20. Owing to the good efficacy and safety profile of statins, following the FDA approval of lovastatin in 1987, market introduction of six other statins was not surprising and statin use has been increasing continuously21,22 (FIG. 1). Interestingly, cardiovascular benefits of taking statins have also been observed in users who do not have elevated cholesterol levels23, suggesting that statin use has non-cholesterol-mediated effects. These findings of pleiotropic effects of statins lend support to the rationale to examine whether statins might modify cancer risk.
Figure 1. Statin use, high cholesterol and prostate cancer deaths in the USA.
Age-adjusted US prostate cancer-specific mortality peaked in 1993 at 39 deaths per 100,000 men and has since been declining114. The percentage of US men ≥20 years of age with high total serum cholesterol (≥240 mg/dl per National Cholesterol Education Program guidelines115) has also declined, from 19% in 1987 to 12% in 2012(REFS 21,116). This reduction coincided with increasing prevalence of statin use (~26% of US adults ≥40 years of age in 2011–2012)2,116. Currently, seven statin drugs are being marketed in the USA. Lovastatin was the first agent to be approved by the FDA in 1987. The newest agent, pitavastatin, was approved in 2009. *Data of statin use before 2011–2012 relates to US adults aged ≥45 years116, data for 2011–2012 relates to US adults aged ≥40 years2.
Interestingly, early observations in rodents indicated that cholesterol-lowering drugs could cause cancer, albeit at doses exceeding those administered to humans24. Similar results in human populations were subsequently attributed to reverse causality, caused by accumulation of cholesterol from the serum in tumours, resulting in a drop in serum cholesterol levels25. Since the publication of these findings, the majority of epidemiological data have indeed shown a protective effect of statins against cancer4–11.
Mechanisms of prostate cancer prevention
As evidence from studies in humans that supports a role for statins in modifying prostate cancer risk is accumulating, investigation of the underlying molecular mechanisms, using established cell-based and animal-based preclinical models becomes essential. Much data from these models have already been published, demonstrating that statins can inhibit prostate cancer growth through cholesterol-mediated and non-cholesterol-mediated mechanisms that affect many pathways essential for cancer formation and progression. Specifically, statins have been shown to inhibit prostate cancer inflammation26, angiogenesis27, cell proliferation28, migration and/or adhesion29 and invasion30, and to promote apoptosis31. In addition, inhibition of HMG-CoA reductase by stat-ins lowers the concentration of mevalonate (FIG. 2) and, consequently, levels of downstream, isoprenylated intermediates believed to be essential in signalling pathways that support cancer formation and progression32.
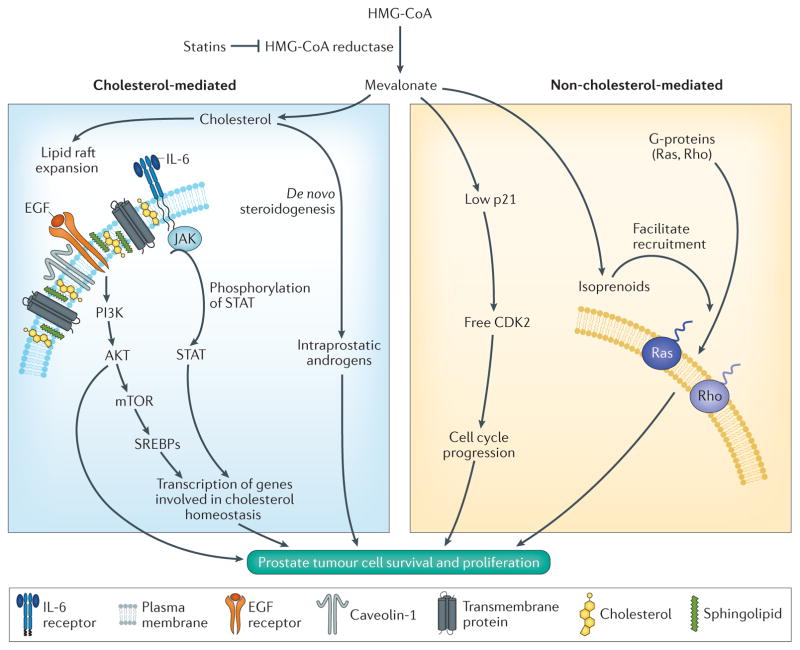
Figure 2. Mechanisms of prostate cancer growth affected by the mevalonate pathway.
Statins inhibit HMG-CoA reductase, the rate-limiting enzyme in the mevalonate pathway that results in the synthesis of cholesterol and isoprenoids. Cholesterol is the sole precursor for sex steroid biosynthesis and has been shown to increase tumour androgen signalling and stimulate tumour growth in mouse models of prostate cancer. In addition, cholesterol is a key component of lipid rafts, which facilitate intracellular signalling processes by serving as organizing centres for the assembly of signalling molecules, such as the epidermal growth factor (EGF) and IL-6. EGF and IL-6 activate the PI3K AKT and JAK–STAT pathways, respectively, enhancing the transcription of genes involved in cholesterol homeostasis. The mevalonate pathway can also support prostate tumour growth via non-cholesterol-mediated mechanisms. For example, resulting isoprenoids, such as farnesyl pyrophosphate and geranyl pyrophosphate, facilitate recruitment of G-proteins Ras and Rho to the plasma membrane. High mevalonate levels suppress levels of the cyclin-dependent kinase inhibitor 1 (p21), thereby promoting cell cycle progression via activation of cyclin-dependent kinase 2 (CDK2) activity. SREBPs, sterol-regulatory-element-binding proteins.
Cholesterol-mediated pathways
A positive correlation between cholesterol accumulation in prostatic tissues and the presence of prostate cancer was already reported in 1981 (REF. 33). Several mechanisms have since been shown to contribute to dysregulation of cholesterol homeostasis in prostate tumours. One study found that hypermethylation of the ABCA1 promoter resulted in reduced expression of the encoded cholesterol efflux transporter, decreased cholesterol efflux rates and elevated intracellular cholesterol levels in prostate cancer cell lines, and that the presence of this epigenetic alteration was associated with high-grade prostate cancer34. In addition, the mTOR pathway is important in regulating sterol-regulatory-element-binding proteins (also called SREBPs), which are transcription factors that control lipid and cholesterol homeostasis35. One study reported that intracellular accumulation of cholesteryl ester in lipid droplets was driven by loss of expression of the tumour suppressor PTEN and subsequent activation of the PI3K–AKT–mTOR signalling pathway, and that intracellular accumulation of cholesteryl ester was associated with high-grade prostate cancer in humans36.
One of the major cholesterol-mediated mechanisms through which statins inhibit tumour growth involves specialized cholesterol-rich regions of the cell membrane known as lipid rafts37. These domains facilitate membrane-initiated signalling events in the cell through compartmentalization of signalling pathways, which can enhance tumour growth. Cell signalling pathways implicated in prostate cancer development and progression that might be affected by lipid raft cholesterol composition include pathways involving the androgen receptor38, the epidermal growth factor receptor (EGFR)39 and the luteinizing hormone receptor40. Statins, through their effect on intracellular cholesterol homeostasis, are thought to disrupt the organization of lipid rafts and, thus, interfere with these or other downstream intracellular signalling pathways41.
The EGFR pathway is one example of the direct effect that reduced cholesterol content of the rafts can have on membrane-initiated signalling. EGFR is a cell-membrane-bound receptor that associates with lipid rafts in prostate cancer cells39. EGFR activation leads to activation of protein kinase B (AKT, encoded by AKT1), which promotes the growth of several solid tumour types, including prostate cancer42. Treatment of prostate cancer cells with cholesterol binders can disrupt lipid raft organization and interfere with EGFR signalling39.
In addition, one study found that activation of cholesterol efflux through treatment with a liver X receptor agonist induced apoptosis through disruption of lipid rafts and subsequent downregulation of AKT signalling in LNCaP in vitro and in vivo models43. Other signalling pathways implicated in the development of prostate cancer and castration resistance, such as IL-6-activated JAK–STAT3 (Janus kinase–signal transducer and activator of transcription 3) signalling, have also been found to be affected by lipid raft organization and are, therefore, potentially influenced by lipid raft cholesterol concentrations44. The importance of cholesterol in prostate cancer development has also been seen in a mouse model. In one study, mice were either fed a high-fat, high-cholesterol diet or a low-fat, low-cholesterol diet45. After subcutaneous injection of LNCaP cells, elevated cholesterol levels in the serum of mice that were fed the high-fat, high-cholesterol diet promoted xenograft tumour growth and reduced apoptosis, in part by increasing activity of AKT. Inhibition of cholesterol synthesis with a statin disrupted lipid rafts in the tumours and induced apoptosis via attenuation of AKT signalling45.
In addition, cholesterol levels might also affect prostate cancer development via androgen signalling pathways, as cholesterol is the precursor of androgens. Lowering cholesterol levels using statins might reduce prostate cancer growth by reducing serum or intratumoural levels of androgens. However, the effect of statins on serum androgen levels is unclear. Some studies have suggested that statins reduce serum testosterone levels46–48, but these reductions were small or caused by statin doses that were higher than commonly used in clinical practice. Other observational studies49,50 and two clinical trials51,52 found no association between statin use and serum androgen levels. A study in 1,812 men in the Boston Area Community Health Survey cohort of which 237 men were statin users found no association between statin use and serum androgen levels53. Emerging evidence suggests that intratumoural levels of androgens remain high even when castrate levels of androgens are reached in the serum of patients with prostate cancer, possibly owing to de novo androgen synthesis in the tumour cell54–56. Thus, statins might conceivably be able to lower intratumoural androgen levels by lowering intratumoural cholesterol levels. Indeed, a study in noncastrated mice with hyper-cholesterolaemia induced by a high-fat, high-cholesterol diet found increased intratumoural levels of androgens in LNCaP xenografts without an effect on androgen levels in serum, suggesting that hypercholesterolaemia induces intratumoural de novo steroidogenesis57.
Non-cholesterol-mediated pathways
Statins inhibit the conversion of HMG-CoA to mevalonate, thereby reducing cellular mevalonate concentrations. Mevalonate is a precursor for a class of compounds called isoprenoids, such as farnesyl pyrophosphate and geranyl pyrophosphate (FIG. 2). Farnesyl pyrophosphate and geranyl pyrophosphate facilitate the recruitment of signalling proteins, such as G-proteins of the Ras and Rho superfamilies, by bridging their attachment to the plasma membranes, where their signalling activities can promote prostate cancer cell survival and proliferation58,59. Thus, statins, by reducing mevalonate and downstream isoprenoids, might inhibit cancer cell proliferation.
Furthermore, statins seem to directly induce apoptosis in cancer cells independently of their effect on cholesterol levels32. For example, in prostate cancer, statins can inhibit cyclin-dependent kinase 2 and stimulate cell cycle arrest60, or activate specific proteases that themselves can activate apoptosis61. Statins also have direct anti-inflammatory and antiangiogenic properties that, conceivably, might also inhibit cancer growth and progression32. One study in a cohort of men undergoing radical prostatectomy found that statin users were 69% less likely to have inflammation within their prostate tumours than nonusers (P=0.047), as assessed by pathological evaluation of tumour sections stained with haematoxylin and eosin62.
Epidemiological evidence
In the past few years, interest in the use of statins for prostate cancer prevention has increased63. It has even been suggested in a study by Colli and Amling64 that stat-ins might be partially responsible for the steep decline in the prostate cancer death rate in the USA during the past 15 years (see the American Cancer Society Cancer Facts & Figures 2016), as it paralleled the market introduction and distribution of statins (FIG. 1). Evaluation of epidemiological studies and secondary analyses of randomized controlled trials seems to show that most evidence supports the hypothesis that statin use reduces prostate cancer risk, with the strongest evidence to date supporting that statins might selectively lower the risk of advanced prostate cancer. In addition to data supporting an inverse association between statin use and risk of advanced prostate cancer, evidence also exists that statins might affect prostate cancer progression at multiple stages of the disease course, including biochemical recurrence after primary therapy, development of castration resistance following androgen deprivation therapy, as well as prostate cancer-specific mortality (FIG. 3).
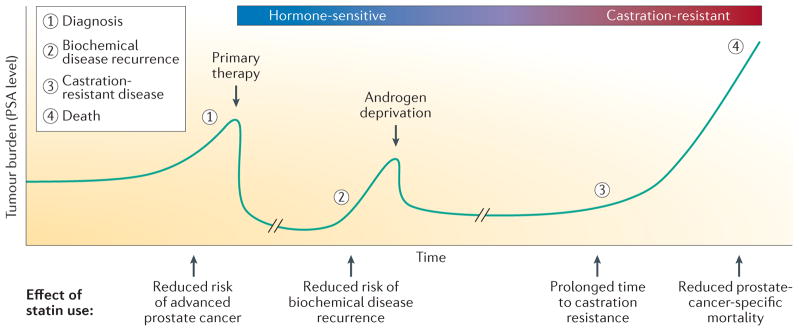
Figure 3. Effects of statin use during the clinical course of prostate cancer.
The clinical course of prostate cancer can be followed using measurements of serum PSA levels, serving as a marker of tumour burden. Rising PSA levels indicate prostate cancer growth and clinical diagnosis. Primary therapy (for example, surgery or radiation) causes a rapid drop in PSA level, showing tumour removal or eradication. Prostate cancer recurrence is detected by rising PSA level after primary treatment. Subsequent androgen deprivation therapy initially results in a reduction in tumour burden and PSA level but most patients eventually develop castration-resistant prostate cancer. Currently, castration-resistant disease cannot be cured and these patients will eventually die of their disease. Statins have been shown to have a protective role at various stages of the clinical course of prostate cancer.
Total prostate cancer
More than 30 observational studies have examined the link between statin use and total prostate cancer risk with encouraging though conflicting results (TABLE 2). A number of case-control studies reported no associations7,16,65–67, but three reported an elevated risk of total prostate cancer in statin users10,68,69. The investigators of one of these studies suggested that the positive association between statin use and total prostate cancer risk is potentially attributable to bias arising from increased surveillance in men initiating statin treatment10. Indeed, one study in Finland found that the elevated prostate cancer risk in new statin users disappeared with increasing duration of statin use, supporting this possible explanation69. Other case-control studies have reported inverse associations between statin use and risk of total prostate cancer6,18,70, including one study in 4,204 men undergoing prostate biopsy that reported a significant 8% reduced risk of total prostate cancer in statin users in comparison with nonusers (RR 0.92; 95% CI 0.85–0.98)70. The largest population-based case-control study to date, a Danish study that included >40,000 patients with any stage of prostate cancer and >200,000 controls, reported a significant 6% reduction in risk of total prostate cancer in statin users (adjusted OR 0.94; 95% CI 0.91–0.97)18.
These case-control studies reported varying findings for associations between statin use and total prostate cancer risk, but a number of cohort studies have also been conducted. A retrospective study of data from a cohort of >55,000 men in the Veterans Affairs New England Healthcare System found that statin users were 31% less likely to be diagnosed with total prostate cancer than men who did not use statins71. Two prospective cohort studies including 6,692 and 634 men taking statins and undergoing PSA screening found a 25% and 64% reduced prostate cancer risk, respectively72,73. A retrospective cohort study in Israel of 37,645 men taking statins found a 74% reduced risk of total prostate cancer in long-term statin users, defined as >5 years of statin use, in comparison with nonusers74. Other studies found weaker but still inverse associations between statin use and total prostate cancer risk75,76, including a population-based study in Washington, USA, that found that statin users had a nonsignificant 12% lower prostate cancer risk17.
Despite these promising data, other observational studies found no link between statin use and total prostate cancer risk, including a secondary analysis of a randomized trial in men with a negative prostate biopsy who underwent repeat biopsies at 2 years and 4 years77, in addition to several cohort studies5,8,9,11,78–82. Overall, individual case-control and cohort studies had conflicting findings, but the most recent meta-analysis of these studies from 2012 reported a significant 7% reduction in risk of total prostate cancer in statin users in comparison with nonusers (P= 0.03)83.
Three meta-analyses of randomized controlled trials of statin use for the primary and secondary prevention of adverse cardiovascular outcomes reported no association between statin use and total prostate cancer risk84–86. However, trial participants do not represent the general population. For example, all trials of statin use incorporated dietary interventions in both statin and placebo groups and all trial participants had a history of cardiovascular disease3. Furthermore, although the most commonly used statin in the USA is simvastatin2 (TABLE 1), participants in the majority of clinical trials were randomized to receive pravastatin, which inhibits HMG-CoA reductase more weakly than simvastatin and has reduced cholesterol-lowering efficacy87. Finally, randomized controlled trials have relatively short follow-up periods: the median follow-up duration was 4.8 years for the 27 statin trials performed to date84. Together, these factors could explain differences in associations between statin use and total prostate cancer risk reported by observational studies and randomized trials.
Advanced prostate cancer
Overall, the data from studies examining associations between statin use and total prostate cancer are inconclusive, with the majority of studies showing no effect of statin use on total prostate cancer risk. However, increasing data indicate that statin use might selectively lower the risk of advanced prostate cancer (defined using varying levels of Gleason grade, clinical stage or a combination of both variables; TABLE 2).
Six large, prospective studies all found that statin users had a reduced risk of advanced prostate cancer without any reduction, or with an attenuated reduction, in total prostate cancer risk8,9,11,76,80,82. In all six studies, bias was minimized by controlling for potential confounding variables, for example, concomitant diseases, such as diabetes, use of antidiabetic drugs or other treatments and cardiovascular risk factors that are associated with prostate cancer risk, such as age, race and body mass index.
In a report from The Health Professionals Follow-up Study, data on cholesterol-lowering drug use for the period 1990–2002 from 34,989 men without a cancer diagnosis in 1990 were analysed8. Statin use was significantly associated with a 49% reduced risk of advanced prostate cancer (RR 0.51; 95% CI 0.30–0.86) and a 61% reduced risk of metastatic or fatal prostate cancer (RR 0.39; 95% CI 0.19–0.77), but it was not associated with a reduced risk of total prostate cancer. In men who took statins for ≥5 years, the risk of advanced prostate cancer was significantly reduced by 74% (RR 0.26; 95% CI 0.08–0.83). Investigators of another prospective cohort, using data from the Cancer Prevention Study II Nutrition Survey (n= 55,454)9, found that men who took statins for ≥5 years had a 40% reduced risk of advanced prostate cancer but these findings were only just statistically significant (RR 0.60; 95% CI 0.36–1.00) and did not reach significance in a follow-up analysis of this data-set80. Analyses of data from the California Men’s Health Study (n= 69,047)11, Southern Community Cohort Study (n = 32,091)76 and Kaiser Permanente Medical Care Program (n= 2,097,474)82 found 20%, 38% and 17% reductions, respectively, in the risk of advanced prostate cancer among statin users; however, only the findings from Kaiser Permanente, the largest of these studies, were statistically significant (HR 0.83; 95% CI 0.72–0.96).
Three small prospective studies have also been conducted, one of which found a 75% significantly reduced risk of advanced prostate cancer among daily statin users (HR 0.25; 95% CI 0.11–0.58)73; findings of the other two studies were not significant81,88. A large retrospective cohort study71 that included men in the Veterans Affairs New England Healthcare System found a significant 60% reduction in risk of advanced prostate cancer among statin users (HR 0.40; 95% CI 0.24–0.65), but results from two other retrospective cohorts were not significant17,75.
Among published case-control studies, some reported no associations between statin use and risk of advanced prostate cancer7,65,66,68,69,89, but one small study6 and two large studies70,18 reported a significantly reduced risk of advanced prostate cancer in statin users (76%, 24% and 10%, respectively).
In summary, despite some contradicting reports, the majority of evidence supports an inverse association between statin use and risk of advanced prostate cancer. This finding is also demonstrated by the most recent meta-analysis, conducted using data from 27 observational studies published before 2012, which found that statin use was associated with only a modest reduction in total prostate cancer risk (7%; P = 0.03) but a more pronounced reduction in advanced disease risk (20%; RR 0.80; 95% CI 0.70–0.90; P<0.001) 83. Eight studies were published in 2012 or later18,74–77,81,88,90 and were, therefore, not included in this meta-analysis. Seven of these studies did not find statin use to be associated with reduced risk of advanced prostate cancer74–77,81,88,90. However, the study18 including the highest number of men with advanced prostate cancer of all studies to date (n = 12,412) reported a 10% significantly reduced risk of advanced prostate cancer in statin users (OR 0.90; 95% CI 0.85–0.96), in line with the findings of the meta-analysis83.
Prostate cancer mortality
Understanding associations between statin use and prostate-cancer-specific mortality is important, as not all men with advanced prostate cancer die from their disease. An analysis of 1,001 men with prostate cancer of whom 289 men were statin users reported a hazard ratio of 0.19 (95% CI 0.06–0.56) for prostate-cancer-specific death in statin users compared with nonusers91. A registry-based study in a Danish population of 27,752 men with prostate cancer of whom 10,542 died of this disease, and who commenced statin use before diagnosis of any type of cancer, found that statin users had significantly lower prostate-cancer-specific mortality than nonusers (HR 0.81; 95% CI 0.75–0.88)92. A study that had been designed to assess the association between β-blocker use and prostate-cancer-specific mortality and analysed use of statins as a potential confounding factor found that statin use was inversely associated with lethal prostate cancer among 3,561 men with the disease with a median follow-up period of 39 months (HR 0.70; 95% CI 0.56–0.88; P= 0.03)93. Finally, an analysis of a population-based electronic database in the UK, containing data from 11,772 men with prostate cancer and 1,791 deaths from prostate cancer during a mean follow-up period of 52 months, found that use of statins was associated with a lower risk of death from prostate cancer. The reduction in risk was larger in men who had commenced statin use before prostate cancer diagnosis (HR 0.55; 95% CI 0.41–0.74) compared with those who started taking statins after diagnosis (HR 0.82; 95% CI 0.71–0.96)94.
Statin use and PSA levels
PSA testing is the most widely used method for prostate cancer screening. If statin use affects PSA levels, a systematic bias would be inherent in all studies that evaluated participants with PSA-based prostate cancer diagnoses (TABLE 2). Indeed, a pilot study in 15 men demonstrated that statin use caused a 42% decline in PSA levels over a period of 5 years95.
One cross-sectional study in 323,426 men aged ≥65 years who had a screening PSA test in 2003 investigated how statin use affects PSA levels at the time of prostate cancer screening. Statin use was associated with a reduced probability of having an abnormal screening PSA result for each of the commonly-used PSA thresholds of >2.5 ng/ml, >4.0 ng/ml and >6.5 ng/ml (REF. 96). Another study examined the effect of use of statins, thiazide diuretics and nonsteroidal anti-inflammatory drugs on PSA levels in a cohort of 1,864 men ≥40 years of age from the National Health and Nutrition Examination Survey that had no history of prostate cancer, prostatitis or recent prostate manipulations. The investigators found that statin use was inversely correlated to PSA levels (P = 0.01) and that men who had been using statins for ≥5 years had a 13% reduction in PSA levels97.
The observation that statin use results in reduced PSA levels at screening seems to indicate that these reduced PSA levels would diminish biopsy rates in statin users and use of statins would, therefore, be associated with a decreased incidence of total prostate cancer. If this hypothesis was true, prostate cancer diagnoses would be delayed and statin users would have an increased incidence of advanced prostate cancer. However, as the vast majority of studies found a reduced risk of advanced disease in statin users, a substantial bias introduced by the effect of statins on PSA levels is unlikely.
One could also argue that statin users might be more health conscious and might make more frequent visits to their health care provider compared with nonusers. This behaviour might make statin users more likely to be diagnosed with prostate cancer of an early stage compared with nonusers. Early detection of prostate cancer and subsequent early treatment is associated with less frequent progression to advanced disease stages. Overall, these relationships might explain the reduced risk of advanced prostate cancer observed in statin users. However, a number of studies reported that adjusting for the intensity of PSA screening did not affect the association between statin use and risk of advanced disease76,98,99. The most recent meta-analysis, published in 2012, reported that the findings of studies which controlled statistical models for potential confounding introduced by differing rates of PSA screening between statin users and nonusers did not greatly differ from the findings of studies that did not consider differences in PSA screening83 In addition, PSA testing is performed much more rarely in Europe compared with the USA, which makes the case-control studies set in Denmark18 and Finland10 relatively free from this potential bias100. Yet, these studies also observed a significant reduction in the risk of advanced prostate cancer, similar to the studies from the USA. Hence, although the potential for screening-related detection biases should be considered, studies taking into account differences in PSA screening frequency between statin users and nonusers (in addition to studies in populations with coherently different PSA screening frequencies) support a true association between statin use and reduced risk of advanced prostate cancer.
Combination with prostate cancer therapies
In addition to the potential chemopreventive effect of statins, investigators are beginning to study whether statin use can improve the outcome of patients receiving established prostate cancer therapies.
One study in 938 men treated with brachytherapy compared the outcomes of 191 men taking statins with those of nonusers101. Statin users had smaller prostate volumes, lower PSA values and lower tumour volume in their biopsy specimens compared with nonusers. A trend was found that statin use was associated with improved prostate-cancer-specific and overall survival, but this association was not statistically significant. A different study in 1,171 men with stage T1–3 prostate cancer treated with radiotherapy included 382 men who were taking a statin at the time of diagnosis and found that statin use was a significant predictor of improved 5-year PSA-failure-free survival (P= 0.002)102. Oh et al.103 retrospectively examined the association between use of statins and risk of biochemical recurrence in 247 men with prostate cancer treated with permanent 125I brachy-therapy, with a median follow-up period of 51 months. In this study, statin use was associated with a significant delay in biochemical disease recurrence (P= 0.03). Furthermore, a meta-analysis of 13 studies that examined the effect of statin use on biochemical recurrence following local treatment with radical prostatectomy or radiotherapy found that statin use was associated with a statistically significant improvement in recurrence-free survival in patients who underwent radiotherapy (six studies; HR 0.68; 95% CI 0.49–0.93), but not in patients who underwent radical prostatectomy (seven studies; HR 1.05; 95% CI 0.90–1.24)104.
Taken together, these results suggest that statin use slows the progression of prostate cancer in men undergoing radiation treatment, possibly by sensitizing the cells to radiotherapy, but further research is needed to confirm these findings. One hypothesis states that statins might radiosensitize prostate tumour cells by causing cell cycle arrest in the late G1 phase, which is the stage at which cells are most sensitive to radiation-induced cell death105.
In addition, some evidence suggests that a beneficial effect of statin use in men who have received treatment might not be limited to radiotherapy. A retrospective study with a median follow-up period of 76 months in a cohort of 1,146 men that had never received statins before radical prostatectomy found that postoperative use of statins was associated with a 36% reduction in the risk of PSA recurrence (P=0.004)106. Furthermore, a study comparing pre-operative and postoperative use of statins in 2,137 Korean men who underwent radical prostatectomy between 1998 and 2011 found that postoperative statin use prolonged recurrence-free survival over a median follow-up period of 32 months, especially in patients with high-risk disease (Gleason score ≥7; HR 0.27; 95% CI 0.13–0.59; P= 0.001), but preoperative statin use did not change pathological outcomes107. Finally, one study with a median follow-up time of 70 months reported that statin use significantly prolonged time to progression in 926 men receiving androgen deprivation therapy, even after adjusting for known prognostic factors such as biopsy-based Gleason score, type of primary therapy and presence of metastases at initiation of androgen deprivation (HR 0.83; 95% CI 0.69–0.99; P= 0.04)108.
Statins have also been evaluated for their ability to reduce common adverse effects of local prostate cancer treatment, such as erectile dysfunction. Investigators prospectively examined the effect of statins on recovery of erectile function after radical retropubic prostatectomy in a randomized controlled trial including 50 men without hypercholesterolaemia who never used statins109. They found that postoperative treatment with a statin resulted in accelerated recovery of erectile function. Statin users had a significantly improved score in the 5-item International Index of Erectile Function (IIEF-5) tool at 6 months after surgery compared with nonusers (P = 0.003), and 55% of statin users versus 26% of nonusers had recovered erectile function by this time point109. This result is in agreement with a meta-analysis published in 2014 of 11 prospective randomized clinical trials that found that randomization of men without prostate cancer to receive statins resulted in a clinically relevant improvement in erectile function, indicated by a 3.4-point improvement on the IIEF-5 scale (P = 0.0001), even after adjusting for two potential confounding factors (average age of study participants and level of LDL cholesterol)110.
Future perspective
Successful completion of the Prostate Cancer Prevention Trial (PCPT), the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) and the Selenium and Vitamin E Cancer Prevention Trial (SELECT) demonstrates that participants can be recruited for large prostate cancer primary prevention trials. Evidence is accumulating that supports a role for statins in reducing prostate cancer risk, especially advanced prostate cancer. Thus, the question arises whether a trial of similar size should be launched to test the efficacy of statins in the primary prevention of prostate cancer. We strongly believe that a trial of this nature should not yet be initiated.
First, our understanding of the many potential molecular mechanisms through which statins might prevent development and progression of cancer is still far from complete. Deciphering these mechanisms will help guide statin clinical trials with appropriate intermediate end points, as well as enable us to identify novel anticancer pathways that could inform the development of next-generation prostate cancer therapeutics. In addition, understanding the mechanisms that link cholesterol and prostate cancer will lead to the identification of tumour biomarkers that can indicate response to statins and enable clinicians to prescribe statin therapy to those patients who are predicted to show a tumour response.
Second, which type of statin would be most appropriate for use in a clinical trial is currently unclear. Simvastatin is the most commonly used statin in the vast majority of epidemiological studies that report an inverse association between statin use and risk of advanced prostate cancer, potentially supporting the use of simvastatin in prostate cancer trials. Future epidemiological studies with sufficient sample size should investigate the effects of different statin types on prostate cancer risk and progression or, at least, report the frequency of the use of different statin types in their populations.
Two major obstacles to a primary prevention statin trial are readily foreseeable. First, as the prevalence of statin use is so great, finding eligible nonusers who would enrol in such a trial and stay in the placebo arm without becoming statin users at later stages would be a considerable challenge. Second, diagnosis of advanced prostate cancer is a relatively rare occurrence in the current era of PSA screening. As statins seem to be most strongly linked with a reduced risk of this form of the disease, the number of men that would need to be randomized and the duration of follow-up monitoring required to detect a difference in advanced disease incidence would be very high.
Much can be learned without launching an expensive, large and time-consuming primary prevention trial. For example, a strong impetus exists to begin analysing the role of statins in secondary and tertiary prevention. Whether statin use improves outcomes in men who have already been diagnosed with prostate cancer is not fully elucidated. Statin use seems to not affect the risk of localized prostate cancer. However, epidemiological evidence supports an effect of statin use in delaying disease recurrence and reducing prostate-cancer-specific mortality, regardless of disease characteristics at diagnosis. These findings provide a rationale for secondary prevention trials in all men with prostate cancer. Late stage castration-resistant or metastatic prostate cancer is a disease of short duration and outcome events occur in a time span of a few months to 1–2 years. Accordingly, from an epidemiological standpoint, more meaningful results from much smaller sample sizes and after shorter trial durations can be extrapolated from studying the effect of statin use at this disease stage in comparison with early-stage prostate cancer. In this setting, much could be learned about the biological actions of statins.
Earlier in the development of prostate cancer, studying men who undergo primary treatment would also yield information on how statins interact with current treatment modalities and might identify factors that predict response, for example, changes in lipid profiles following the start of statin use. In men on active surveillance protocols, particularly those at highest risk of disease progression, statins could be tested as an adjuvant therapy to reduce or delay the need for subsequent treatment, and tumour response could be monitored using tumour imaging111. Indeed, targeting cancer prevention at populations at high-risk of disease has been suggested as a way to improve the risk:benefit ratio of giving medications with potential adverse effects as preventive agents112; however, statins are considered to be well tolerated drugs with few major adverse effects.
Future drugs against prostate cancer could be used separately or in combination with statins to reduce prostate cancer mortality and/or morbidity. Clinical trials to investigate treatment with statins in combination with other agents are warranted, particularly combinations with compounds that show synergy with statins in animal models and whose mechanism of synergistic activity is known. Indeed, a study published in 2015 in 767 diabetic men with prostate cancer undergoing radical prostatectomy found that combined treatment with statins and metformin, but neither statin nor metformin use alone, resulted in a significantly reduced risk of biochemical recurrence during a follow-up period of 27 months (P = 0.037 for combined statin and metformin use, P = 0.676 for statin use alone and P= 0.117 for metformin use alone)113. Liver X receptor agonists are a group of other potential candidates for combination treatments with statins. These agents stimulate cholesterol efflux from cancer cells, thus, reducing intracellular cholesterol levels and inducing apoptosis43. Through this mechanism, these agents might act synergistically with statins to inhibit prostate cancer growth.
Conclusions
Increasing evidence is being published that supports the hypothesis that statin use is associated with a reduced risk of advanced prostate cancer. Determining causality from observational studies is difficult, but these epidemiological data are also supported by a multitude of preclinical studies that show that statins directly inhibit prostate cancer development and progression in cell-based and animal-based models. Thus, ample justification exists to proceed with further population-based and basic research. The results from these studies will bolster the current rationale for a primary prevention trial as well as targeted clinical trials with mechanistic end points. At present, we still need to further elucidate the benefits of statins before we can advocate that all men at risk of prostate cancer start statins regardless of their cholesterol profile. However, the use of statins in secondary and tertiary prevention to improve therapeutic outcomes in men who have already been diagnosed with prostate cancer might become reality in the not too distant future.
Key points.
Statins are a commonly prescribed class of medications that lower serum cholesterol levels by inhibiting HMG-CoA reductase, the rate-limiting enzyme for cholesterol synthesis in the liver
Preclinical research shows that statins can inhibit prostate cancer growth through cholesterol-mediated and non-cholesterol-mediated mechanisms (for example, lipid-raft-mediated and Ras signalling, respectively) that affect pathways essential for cancer formation and progression
Of >30 observational studies on statin use and prostate cancer risk published to date, most support the hypothesis that statin use reduces the risk of advanced prostate cancer
Increased PSA screening and health-conscious behaviour in statin users might bias some findings but are unlikely to fully explain the inverse association between statin use and prostate cancer risk
Statin use also seems to be associated with improved prostate-cancer-specific survival, particularly in men undergoing radiotherapy, suggesting a role for statins in secondary and tertiary prostate cancer prevention
Before conducting primary prevention trials, further research into the mechanisms contributing to reported inverse associations is required; however, secondary and tertiary prevention trials in men diagnosed with prostate cancer might soon be performed
Acknowledgments
E.H.A. receives funding support from the American Institute for Cancer Research and S.J.F. receives funding support from the National Institutes of Health Award 1K24CA160653.
Footnotes
Author contributions
M.A.A. and E.H.A. researched data for the article. E.H.A., R.J.H. and S.J.F. made a substantial contribution to discussion of content. M.A.A., E.H.A. and S.J.F. wrote the article. E.H.A., R.J.H., M.R.F. and S.J.F. reviewed and/or edited the article before submission.
Competing interests statement
The authors declare no competing interests.
FURTHER INFORMATION
American Cancer Society Cancer Facts & Figures 2016: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Wong ND, Lopez V, Tang S, Williams GR. Prevalence, treatment, and control of combined hypertension and hypercholesterolemia in the United States. Am J Cardiol. 2006;98:204–208. doi: 10.1016/j.amjcard.2006.01.079. [DOI] [PubMed] [Google Scholar]
- 2.Gu Q, Paulose-Ram R, Burt VL, Kit BK. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003–2012. NCHS Data Brief. 2014;177:1–8. [PubMed] [Google Scholar]
- 3.Baigent C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 4.Poynter JN, et al. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352:2184–2192. doi: 10.1056/NEJMoa043792. [DOI] [PubMed] [Google Scholar]
- 5.Friis S, et al. Cancer risk among statin users: a population-based cohort study. Int J Cancer. 2005;114:643–647. doi: 10.1002/ijc.20758. [DOI] [PubMed] [Google Scholar]
- 6.Shannon J, et al. Statins and prostate cancer risk: a case-control study. Am J Epidemiol. 2005;162:318–325. doi: 10.1093/aje/kwi203. [DOI] [PubMed] [Google Scholar]
- 7.Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ. The risk of cancer in users of statins. J Clin Oncol. 2004;22:2388–2394. doi: 10.1200/JCO.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Platz EA, et al. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98:1819–1825. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs EJ, et al. Cholesterol-lowering drugs and advanced prostate cancer incidence in a large U. S. cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:2213–2217. doi: 10.1158/1055-9965.EPI-07-0448. [DOI] [PubMed] [Google Scholar]
- 10.Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Cholesterol-lowering drugs and prostate cancer risk: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2007;16:2226–2232. doi: 10.1158/1055-9965.EPI-07-0599. [DOI] [PubMed] [Google Scholar]
- 11.Flick ED, et al. Statin use and risk of prostate cancer in the California Men’s Health Study cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:2218–2225. doi: 10.1158/1055-9965.EPI-07-0197. [DOI] [PubMed] [Google Scholar]
- 12.Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis. JAMA. 2006;295:74–80. doi: 10.1001/jama.295.1.74. [DOI] [PubMed] [Google Scholar]
- 13.Browning DR, Martin RM. Statins and risk of cancer: a systematic review and metaanalysis. Int J Cancer. 2007;120:833–843. doi: 10.1002/ijc.22366. [DOI] [PubMed] [Google Scholar]
- 14.McKenney JM. Pharmacologic characteristics of statins. Clin Cardiol. 2003;26:32–38. doi: 10.1002/clc.4960261507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gazzerro P, et al. Pharmacological actions of statins: a critical appraisal in the management of cancer. Pharmacol Rev. 2012;64:102–146. doi: 10.1124/pr.111.004994. [DOI] [PubMed] [Google Scholar]
- 16.Agalliu I, Salinas CA, Hansten PD, Ostrander EA, Stanford JL. Statin use and risk of prostate cancer: results from a population-based epidemiologic study. Am J Epidemiol. 2008;168:250–260. doi: 10.1093/aje/kwn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boudreau DM, Yu O, Buist DS, Miglioretti DL. Statin use and prostate cancer risk in a large population-based setting. Cancer Causes Control. 2008;19:767–774. doi: 10.1007/s10552-008-9139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jespersen CG, Nørgaard M, Friis S, Skriver C, Borre M. Statin use and risk of prostate cancer: a Danish population-based case-control study, 1997–2010. Cancer Epidemiol. 2014;38:42–47. doi: 10.1016/j.canep.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Kashani A, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114:2788–2797. doi: 10.1161/CIRCULATIONAHA.106.624890. [DOI] [PubMed] [Google Scholar]
- 20.Sattar N, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 21.Carroll MD, Kit BK, Lacher DA, Shero ST, Mussolino ME. Trends in lipids and lipoproteins in US adults, 1988–2010. JAMA. 2012;308:1545–1554. doi: 10.1001/jama.2012.13260. [DOI] [PubMed] [Google Scholar]
- 22.Siegel D, Lopez J, Meier J. Use of cholesterol-lowering medications in the United States from 1991 to 1997. Am J Med. 2000;108:496–499. doi: 10.1016/s0002-9343(00)00319-3. [DOI] [PubMed] [Google Scholar]
- 23.LaRosa JC. Pleiotropic effects of statins and their clinical significance. Am J Cardiol. 2001;88:291–293. doi: 10.1016/s0002-9149(01)01643-5. [DOI] [PubMed] [Google Scholar]
- 24.Newman TB, Hulley SB. Carcinogenicity of lipid-lowering drugs. JAMA. 1996;275:55–60. [PubMed] [Google Scholar]
- 25.Law MR, Thompson SG. Low serum cholesterol and the risk of cancer:an analysis of the published prospective studies. Cancer Causes Control. 1991;2:253–261. doi: 10.1007/BF00052142. [DOI] [PubMed] [Google Scholar]
- 26.Youssef S, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 27.Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- 28.Rao S, et al. Lovastatin-mediated G1 arrest is through inhibition of the proteasome, independent of hydroxymethyl glutaryl-CoA reductase. Proc Natl Acad Sci USA. 1999;96:7797–7802. doi: 10.1073/pnas.96.14.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nubel T, Dippold W, Kleinert H, Kaina B, Fritz G. Lovastatin inhibits Rho-regulated expression of E-selectin by TNF-α and attenuates tumor cell adhesion. FASEB J. 2004;18:140–142. doi: 10.1096/fj.03-0261fje. [DOI] [PubMed] [Google Scholar]
- 30.Brown M, et al. The differential effects of statins on the metastatic behaviour of prostate cancer. Br J Cancer. 2012;106:1689–1696. doi: 10.1038/bjc.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoque A, Chen H, Xu XC. Statin induces apoptosis and cell growth arrest in prostate cancer cells. Cancer Epidemiol Biomarkers Prev. 2008;17:88–94. doi: 10.1158/1055-9965.EPI-07-0531. [DOI] [PubMed] [Google Scholar]
- 32.Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 33.Schaffner CP. Prostatic cholesterol metabolism: regulation and alteration. Prog Clin Biol Res. 1981;75A:279–324. [PubMed] [Google Scholar]
- 34.Lee BH, et al. Dysregulation of cholesterol homeostasis in human prostate cancer through loss of ABCA1. Cancer Res. 2013;73:1211–1218. doi: 10.1158/0008-5472.CAN-12-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porstmann T, et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8:224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yue S, et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014;19:393–406. doi: 10.1016/j.cmet.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 38.Freeman MR, Cinar B, Lu ML. Membrane rafts as potential sites of nongenomic hormonal signaling in prostate cancer. Trends Endocrinol Metab. 2005;16:273–279. doi: 10.1016/j.tem.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62:2227–2231. [PubMed] [Google Scholar]
- 40.Smith SM, et al. Luteinizing hormone receptors translocate to plasma membrane microdomains after binding of human chorionic gonadotropin. Endocrinology. 2006;147:1789–1795. doi: 10.1210/en.2005-1046. [DOI] [PubMed] [Google Scholar]
- 41.Lawrence JC, Saslowsky DE, Edwardson JM, Henderson RM. Real-time analysis of the effects of cholesterol on lipid raft behavior using atomic force microscopy. Biophys J. 2003;84:1827–1832. doi: 10.1016/s0006-3495(03)74990-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toren P, Zoubeidi A. Targeting the PI3K/Akt pathway in prostate cancer: challenges and opportunities (review) Int J Oncol. 2014;45:1793–1801. doi: 10.3892/ijo.2014.2601. [DOI] [PubMed] [Google Scholar]
- 43.Pommier AJ, et al. Liver X Receptor activation downregulates AKT survival signaling in lipid rafts and induces apoptosis of prostate cancer cells. Oncogene. 2010;29:2712–2723. doi: 10.1038/onc.2010.30. [DOI] [PubMed] [Google Scholar]
- 44.Horinaga M, et al. Clinical and pathologic significance of activation of signal transducer and activator of transcription 3 in prostate cancer. Urology. 2005;66:671–675. doi: 10.1016/j.urology.2005.03.066. [DOI] [PubMed] [Google Scholar]
- 45.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115:959–968. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dobs AS, et al. Effects of high-dose simvastatin on adrenal and gonadal steroidogenesis in men with hypercholesterolemia. Metabolism. 2000;49:1234–1238. doi: 10.1053/meta.2000.7716a. [DOI] [PubMed] [Google Scholar]
- 47.Hyyppa MT, Kronholm E, Virtanen A, Leino A, Jula A. Does simvastatin affect mood and steroid hormone levels in hypercholesterolemic men? A randomized double-blind trial. Psychoneuroendocrinology. 2003;28:181–194. doi: 10.1016/s0306-4530(02)00014-8. [DOI] [PubMed] [Google Scholar]
- 48.Smals AG, Weusten JJ, Benraad TJ, Kloppenborg PW. The HMG-CoA reductase inhibitor simvastatin suppresses human testicular testosterone synthesis in vitro by a selective inhibitory effect on 17-ketosteroid-oxidoreductase enzyme activity. J Steroid Biochem Mol Biol. 1991;38:465–468. doi: 10.1016/0960-0760(91)90333-z. [DOI] [PubMed] [Google Scholar]
- 49.Segarra A, Chacon P, Vilardell M, Piera LL. Prospective case control study to determine the effect of lovastatin on serum testosterone and cortisol concentrations in hyperlipidemic nephrotic patients with chronic renal failure. Nephron. 1996;73:186–190. doi: 10.1159/000189038. [DOI] [PubMed] [Google Scholar]
- 50.Bernini GP, et al. Effects of long-term simvastatin treatment on testicular and adrenal steroidogenesis in hypercholesterolemic patients. J Endocrinol Invest. 1994;17:227–233. doi: 10.1007/BF03348962. [DOI] [PubMed] [Google Scholar]
- 51.Bohm M, Herrmann W, Wassmann S, Laufs U, Nickenig G. Does statin therapy influence steroid hormone synthesis? Z Kardiol. 2004;93:43–48. doi: 10.1007/s00392-004-1003-2. [DOI] [PubMed] [Google Scholar]
- 52.Dobs AS, et al. Effects of simvastatin and pravastatin on gonadal function in male hypercholesterolemic patients. Metabolism. 2000;49:115–121. doi: 10.1016/s0026-0495(00)90938-7. [DOI] [PubMed] [Google Scholar]
- 53.Hall SA, et al. Do statins affect androgen levels in men? Results from the Boston area community health survey. Cancer Epidemiol Biomarkers Prev. 2007;16:1587–1594. doi: 10.1158/1055-9965.EPI-07-0306. [DOI] [PubMed] [Google Scholar]
- 54.Mohler JL, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 55.Page ST, et al. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metab. 2006;91:3850–3856. doi: 10.1210/jc.2006-0968. [DOI] [PubMed] [Google Scholar]
- 56.Pelletier G, Luu-The V, El-Alfy M, Li S, Labrie F. Immunoelectron microscopic localization of 3β-hydroxysteroid dehydrogenase and type 5–17β-hydroxysteroid dehydrogenase in the human prostate and mammary gland. J Mol Endocrinol. 2001;26:11–19. doi: 10.1677/jme.0.0260011. [DOI] [PubMed] [Google Scholar]
- 57.Mostaghel EA, Solomon KR, Pelton K, Freeman MR, Montgomery RB. Impact of circulating cholesterol levels on growth and intratumoral androgen concentration of prostate tumors. PLoS ONE. 2012;7:e30062. doi: 10.1371/journal.pone.0030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Graaf MR, Richel DJ, van Noorden CJ, Guchelaar HJ. Effects of statins and farnesyltransferase inhibitors on the development and progression of cancer. Cancer Treat Rev. 2004;30:609–641. doi: 10.1016/j.ctrv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 59.Weber MJ, Gioeli D. Ras signaling in prostate cancer progression. J Cell Biochem. 2004;91:13–25. doi: 10.1002/jcb.10683. [DOI] [PubMed] [Google Scholar]
- 60.Lee SJ, et al. Inhibition of the 3-hydroxy-3-methylglutaryl-coenzyme A reductase pathway induces p53-independent transcriptional regulation of p21(WAF1/CIP1) in human prostate carcinoma cells. J Biol Chem. 1998;273:10618–10623. doi: 10.1074/jbc.273.17.10618. [DOI] [PubMed] [Google Scholar]
- 61.Marcelli M, et al. Caspase-7 is activated during lovastatin-induced apoptosis of the prostate cancer cell line LNCaP. Cancer Res. 1998;58:76–83. [PubMed] [Google Scholar]
- 62.Banez LL, et al. Association between statins and prostate tumor inflammatory infiltrate in men undergoing radical prostatectomy. Cancer Epidemiol Biomarkers Prev. 2010;19:722–728. doi: 10.1158/1055-9965.EPI-09-1074. [DOI] [PubMed] [Google Scholar]
- 63.Mucci LA, Stampfer MJ. Mounting evidence for prediagnostic use of statins in reducing risk of lethal prostate cancer. J Clin Oncol. 2014;32:1–2. doi: 10.1200/JCO.2013.53.2770. [DOI] [PubMed] [Google Scholar]
- 64.Colli JL, Amling CL. Exploring causes for declining prostate cancer mortality rates in the United States. Urol Oncol. 2008;26:627–633. doi: 10.1016/j.urolonc.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 65.Blais L, Desgagne A, LeLorier J. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and the risk of cancer: a nested case-control study. Arch Intern Med. 2000;160:2363–2368. doi: 10.1001/archinte.160.15.2363. [DOI] [PubMed] [Google Scholar]
- 66.Kaye JA, Jick H. Statin use and cancer risk in the General Practice Research Database. Br J Cancer. 2004;90:635–637. doi: 10.1038/sj.bjc.6601566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coogan PF, Kelly JP, Strom BL, Rosenberg L. Statin and NSAID use and prostate cancer risk. Pharmacoepidemiol Drug Saf. 2010;19:752–755. doi: 10.1002/pds.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang CC, Ho SC, Chiu HF, Yang CY. Statins increase the risk of prostate cancer: a population-based case-control study. Prostate. 2011;71:1818–1824. doi: 10.1002/pros.21401. [DOI] [PubMed] [Google Scholar]
- 69.Haukka J, et al. Incidence of cancer and statin usage — record linkage study. Int J Cancer. 2010;126:279–284. doi: 10.1002/ijc.24536. [DOI] [PubMed] [Google Scholar]
- 70.Tan N, Klein EA, Li J, Moussa AS, Jones JS. Statin use and risk of prostate cancer in a population of men who underwent biopsy. J Urol. 2011;186:86–90. doi: 10.1016/j.juro.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 71.Farwell WR, D’Avolio LW, Scranton RE, Lawler EV, Gaziano JM. Statins and prostate cancer diagnosis and grade in a veterans population. J Natl Cancer Inst. 2011;103:885–892. doi: 10.1093/jnci/djr108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murtola TJ, et al. Prostate cancer and PSA among statin users in the Finnish prostate cancer screening trial. Int J Cancer. 2010;127:1650–1659. doi: 10.1002/ijc.25165. [DOI] [PubMed] [Google Scholar]
- 73.Breau RH, et al. The association between statin use and the diagnosis of prostate cancer in a population based cohort. J Urol. 2010;184:494–499. doi: 10.1016/j.juro.2010.03.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lustman A, Nakar S, Cohen AD, Vinker S. Statin use and incident prostate cancer risk: does the statin brand matter? A population-based cohort study. Prostate Cancer Prostatic Dis. 2014;17:6–9. doi: 10.1038/pcan.2013.34. [DOI] [PubMed] [Google Scholar]
- 75.Morote J, et al. Role of serum cholesterol and statin use in the risk of prostate cancer detection and tumor aggressiveness. Int J Mol Sci. 2014;15:13615–13623. doi: 10.3390/ijms150813615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kantor ED, et al. Statin use and risk of prostate cancer: results from the Southern Community Cohort Study. Prostate. 2015;75:1384–1393. doi: 10.1002/pros.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Freedland SJ, et al. Statin use and risk of prostate cancer and high-grade prostate cancer: results from the REDUCE study. Prostate Cancer Prostatic Dis. 2013;16:254–259. doi: 10.1038/pcan.2013.10. [DOI] [PubMed] [Google Scholar]
- 78.Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol. 2009;67:99–109. doi: 10.1111/j.1365-2125.2008.03308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197. doi: 10.1136/bmj.c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jacobs EJ, Newton CC, Thun MJ, Gapstur SM. Long-term use of cholesterol-lowering drugs and cancer incidence in a large United States cohort. Cancer Res. 2011;71:1763–1771. doi: 10.1158/0008-5472.CAN-10-2953. [DOI] [PubMed] [Google Scholar]
- 81.Chan JM, et al. Statin use and risk of prostate cancer in the prospective Osteoporotic Fractures in Men (MrOS) Study. Cancer Epidemiol Biomarkers Prev. 2012;21:1886–1888. doi: 10.1158/1055-9965.EPI-12-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Friedman GD, et al. Screening statins for possible carcinogenic risk: up to 9 years of follow-up of 361,859 recipients. Pharmacoepidemiol Drug Saf. 2008;17:27–36. doi: 10.1002/pds.1507. [DOI] [PubMed] [Google Scholar]
- 83.Bansal D, Undela K, D’Cruz S, Schifano F. Statin use and risk of prostate cancer: a meta-analysis of observational studies. PLoS ONE. 2012;7:e46691. doi: 10.1371/journal.pone.0046691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cholesterol Treatment Trialists’ Collaboration et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–1405. doi: 10.1016/S0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 85.Bonovas S, Filioussi K, Sitaras NM. Statin use and the risk of prostate cancer: a metaanalysis of 6 randomized clinical trials and 13 observational studies. Int J Cancer. 2008;123:899–904. doi: 10.1002/ijc.23550. [DOI] [PubMed] [Google Scholar]
- 86.Kuoppala J, Lamminpaa A, Pukkala E. Statins and cancer: a systematic review and meta-analysis. Eur J Cancer. 2008;44:2122–2132. doi: 10.1016/j.ejca.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 87.Solomon KR, Freeman MR. The complex interplay between cholesterol and prostate malignancy. Urol Clin North Am. 2011;38:243–259. doi: 10.1016/j.ucl.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Platz EA, et al. Statin drug use is not associated with prostate cancer risk in men who are regularly screened. J Urol. 2014;192:379–384. doi: 10.1016/j.juro.2014.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fowke JH, et al. The associations between statin use and prostate cancer screening, prostate size, high-grade prostatic intraepithelial neoplasia (PIN), and prostate cancer. Cancer Causes Control. 2011;22:417–426. doi: 10.1007/s10552-010-9713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nordström T, Clements M, Karlsson R, Adolfsson J, Grönberg H. The risk of prostate cancer for men on aspirin, statin or antidiabetic medications. Eur J Cancer. 2015;51:725–733. doi: 10.1016/j.ejca.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 91.Geybels MS, et al. Statin use in relation to prostate cancer outcomes in a population-based patient cohort study. Prostate. 2013;73:1214–1222. doi: 10.1002/pros.22671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367:1792–1802. doi: 10.1056/NEJMoa1201735. [DOI] [PubMed] [Google Scholar]
- 93.Grytli HH, Fagerland MW, Fossa SD, Tasken KA. Association between use of β-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur Urol. 2014;65:635–641. doi: 10.1016/j.eururo.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 94.Yu O, et al. Use of statins and the risk of death in patients with prostate cancer. J Clin Oncol. 2014;32:5–11. doi: 10.1200/JCO.2013.49.4757. [DOI] [PubMed] [Google Scholar]
- 95.Cyrus-David MS, Weinberg A, Thompson T, Kadmon D. The effect of statins on serum prostate specific antigen levels in a cohort of airline pilots: a preliminary report. J Urol. 2005;173:1923–1925. doi: 10.1097/01.ju.0000158044.94188.88. [DOI] [PubMed] [Google Scholar]
- 96.Shi Y, et al. Statin medications are associated with a lower probability of having an abnormal screening prostate-specific antigen result. Urology. 2014;84:1058–1065. doi: 10.1016/j.urology.2014.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chang SL, Harshman LC, Presti JC., Jr Impact of common medications on serum total prostate-specific antigen levels: analysis of the National Health and Nutrition Examination Survey. J Clin Oncol. 2010;28:3951–3957. doi: 10.1200/JCO.2009.27.9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Allott EH, et al. Statin use and prostate cancer aggressiveness: results from the population-based North Carolina–Louisiana Prostate Cancer Project. Cancer Epidemiol Biomarkers Prev. 2016;25:670–677. doi: 10.1158/1055-9965.EPI-15-0631. [DOI] [PubMed] [Google Scholar]
- 99.Mondul AM, Caffo B, Platz EA. Minimal detection bias in the inverse association between statin drug use and advanced prostate cancer risk: a simulation study. Cancer Epidemiol. 2011;35:e6–e11. doi: 10.1016/j.canep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ciatto S, et al. Contamination by opportunistic screening in the European Randomized Study of Prostate Cancer Screening. BJU Int. 2003;92(Suppl 2):97–100. doi: 10.1111/j.1464-410x.2003.04407.x. [DOI] [PubMed] [Google Scholar]
- 101.Moyad MA, et al. Statins, especially atorvastatin, may improve survival following brachytherapy for clinically localized prostate cancer. Urol Nurs. 2006;26:298–303. [PubMed] [Google Scholar]
- 102.Kollmeier MA, et al. Improved biochemical outcomes with statin use in patients with high-risk localized prostate cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:713–718. doi: 10.1016/j.ijrobp.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 103.Oh DS, et al. Statin use is associated with decreased prostate cancer recurrence in men treated with brachytherapy. World J Urol. 2015;33:93–97. doi: 10.1007/s00345-014-1281-x. [DOI] [PubMed] [Google Scholar]
- 104.Park HS, et al. Statins and prostate cancer recurrence following radical prostatectomy or radiotherapy: a systematic review and meta-analysis. Ann Oncol. 2013;24:1427–1434. doi: 10.1093/annonc/mdt077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res. 2003;9:10–19. [PubMed] [Google Scholar]
- 106.Allott EH, et al. Postoperative statin use and risk of biochemical recurrence following radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. BJU Int. 2014;114:661–666. doi: 10.1111/bju.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Song C, et al. Statin use after radical prostatectomy reduces biochemical recurrence in men with prostate cancer. Prostate. 2015;75:211–217. doi: 10.1002/pros.22907. [DOI] [PubMed] [Google Scholar]
- 108.Harshman LC, et al. Statin use at the time of initiation of androgen deprivation therapy and time to progression in patients with hormone-sensitive prostate cancer. JAMA Oncol. 2015;1:495–504. doi: 10.1001/jamaoncol.2015.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hong SK, Han BK, Jeong SJ, Byun SS, Lee SE. Effect of statin therapy on early return of potency after nerve sparing radical retropubic prostatectomy. J Urol. 2007;178:613–616. doi: 10.1016/j.juro.2007.03.132. [DOI] [PubMed] [Google Scholar]
- 110.Kostis JB, Dobrzynski JM. The effect of statins on erectile dysfunction: a meta-analysis of randomized trials. J Sex Med. 2014;11:1626–1635. doi: 10.1111/jsm.12521. [DOI] [PubMed] [Google Scholar]
- 111.Futterer JJ, et al. Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur Urol. 2015;68:1045–1053. doi: 10.1016/j.eururo.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 112.Meyskens FL, Jr, et al. Cancer prevention: obstacles, challenges and the road ahead. J Natl Cancer Inst. 2016;108:djv309. doi: 10.1093/jnci/djv309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Danzig MR, et al. Synergism between metformin and statins in modifying the risk of biochemical recurrence following radical prostatectomy in men with diabetes. Prostate Cancer Prostatic Dis. 2015;18:63–68. doi: 10.1038/pcan.2014.47. [DOI] [PubMed] [Google Scholar]
- 114.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 115.National Cholesterol Education Program. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 116.National Center for Health Statistics. Health, United States, 2010: with special feature on death and dying. CDC; 2011. http://www.cdc.gov/nchs/data/hus/hus10.pdf. [PubMed] [Google Scholar]