Abstract
Although dementia has been described in ancient texts over many centuries (e.g., “Be kind to your father, even if his mind fail him.” – Old Testament: Sirach 3:12), our knowledge of its underlying causes is little more than a century old. Alzheimer published his now famous case study only 110 years ago, and our modern understanding of the disease that bears his name, and its neuropsychological consequences, really only began to accelerate in the 1980s. Since then we have witnessed an explosion of basic and translational research into the causes, characterizations, and possible treatments for Alzheimer’s disease (AD) and other dementias. We review this lineage of work beginning with Alzheimer’s own writings and drawings, then jump to the modern era beginning in the 1970s and early 1980s and provide a sampling of neuropsychological and other contextual work from each ensuing decade. During the 1980s our field began its foundational studies of profiling the neuropsychological deficits associated with AD and its differentiation from other dementias (e.g., cortical vs. subcortical dementias). The 1990s continued these efforts and began to identify the specific cognitive mechanisms affected by various neuropathologic substrates. The 2000s ushered in a focus on the study of prodromal stages of neurodegenerative disease before the full-blown dementia syndrome (i.e., mild cognitive impairment). The current decade has seen the rise of imaging and other biomarkers to characterize preclinical disease before the development of significant cognitive decline. Finally, we suggest future directions and predictions for dementia-related research and potential therapeutic interventions.
Keywords: Neuropsychology, Cognition, Neuroscience, Alzheimer’s disease, Mild cognitive impairment, Neuroimaging, Biomarkers, Clinical trials
INTRODUCTION
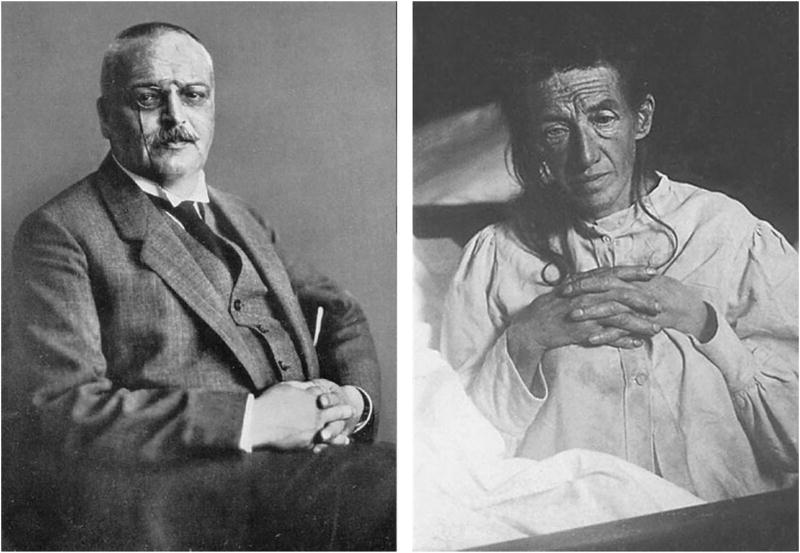
One of the great challenges faced by neuropsychologists over the past 50 years is to understand the cognitive and behavioral manifestations of dementia and their relationship to underlying brain pathology. This challenge has grown substantially over the years with the aging of the population and the age-related nature of many dementia-producing neurodegenerative diseases. Although the concept of dementia has existed for thousands of years (Mahandra, 1984), it is only early in the past century that the essential clinical syndrome and associated neurodegenerative changes were first discovered. In 1907, Aloysius “Alöis” Alzheimer carefully described the symptoms of a 51-year-old woman, Auguste Deter, who was under his care at the state asylum in Frankfurt Germany (Alzheimer, 1907; for an English translation, see Stelzmann et al., 1995) (Figure 1). Alzheimer’s description of her symptoms is almost certainly the first neuropsychological characterization of the disease:
“Her memory is seriously impaired. If objects are shown to her, she names them correctly, but almost immediately afterwards she has forgotten everything. When reading a test, she skips from line to line or reads by spelling the words individually, or by making them meaningless through her pronunciation. In writing she repeats separate syllables many times, omits others and quickly breaks down completely. In speaking, she uses gap-fills and a few paraphrased expressions (“milk-pourer” instead of cup); sometimes it is obvious she cannot go on. Plainly, she does not understand certain questions. She does not remember the use of some objects.”
Fig. 1.
Photographs of Alois Alzheimer (left) and his patient Auguste Deter (right).
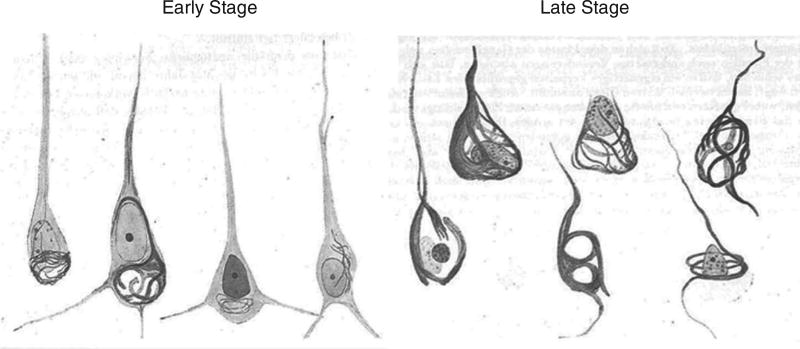
When Auguste Deter died, Alzheimer used the then-new silver staining histological technique to examine her brain microscopically. When he did so, he observed the neuritic plaques, neurofibrillary tangles, and amyloid angiopathy that were to become the hallmarks of the disease that now bears his name (as shown in Figure 2 from sketches of the histologic preparations in his 1911 paper). Alzheimer himself did not claim to have discovered “Alzheimer’s disease,” although his mentor Emil Kraepelin at the Munich Medical School rightly credited him with doing so by coining the term in his own Handbook of Psychiatry (Kraepelin, 1910). By 1911, the medical community was using Alzheimer’s depictions of the disease to diagnose patients both in Europe and the United States (Mauer & Mauer, 2003).
Fig. 2.
Sketches of Auguste Deter’s histopathologic preparations of early and late stage neurofibrillary tangle pathology as drawn by Alzheimer from his 1911 paper entitled “Über eigenartige Krankheitsfälle des späteren Alters.”
It was also during this time that Eugen Blueler in his study of schizophrenia coined the term “organic psychosyndrome” to refer to decrements in memory, judgment, perceptual discrimination and attention, emotional lability, and defective impulse control associated with chronic diffuse cortical damage. This classification was essentially adopted by the American Psychiatric Association (APA) to define dementia in the first two editions of their Diagnostic and Statistical Manual of Mental Disorders (DSM). Specifically, DSM-II defined “organic brain syndrome” as a “basic mental condition characteristically resulting from diffuse impairment of brain tissue function from whatever cause,” and which is manifested behaviorally as impairment in orientation, memory, intellectual functions, judgment, and affect (APA, 1968).
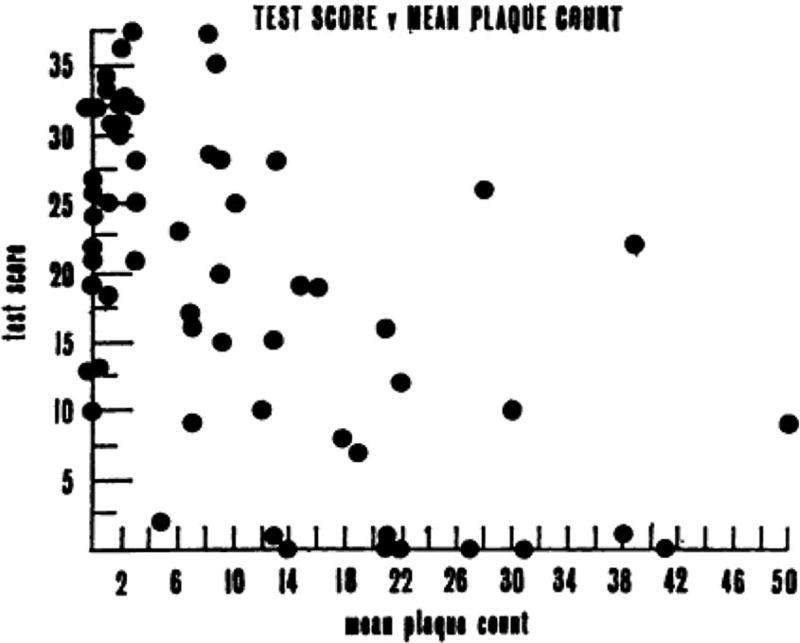
Armed with these uniform criteria and newly developed standardized bedside cognitive screening tests (Blessed, Tomlinson, & Roth, 1968; Folstein, Folstein, & McHugh, 1975), a handful of investigators began scientific studies of dementia, particularly focusing on dementia associated with Alzheimer’s disease (AD). Although dementia is associated with more than 70 different causes of brain dysfunction, AD is the most common cause accounting for roughly half of all cases (for review, see Cummings & Benson, 1992). One of the most important studies during this period showed that the degree of AD pathology in the brain was significantly correlated with performance on standardized cognitive tests shortly before death (Blessed, Tomlinson, & Roth, 1968). This was the first study to strongly link the clinical features of AD with the pathologic brain changes that Alzheimer had described (Figure 3).
Fig. 3.
Mean plaque count plotted against the summary cognitive test score constructed by Blessed, Tomlinson, & Roth (1968). The “Blessed” test score was computed from “a number of simple psychological tests of orientation, remote memory, recent memory, and concentration,” resulting in a total score ranging from 0 (complete failure) to 37 (perfect score). The scatterplot resulted in a highly significant correlation coefficient of −0.59 (p <.001) (from Blessed, Tomlinson, & Roth, 1968).
Neuropsychological studies of dementia and AD during this period were rare and largely limited to presenile dementia with onset before the age of 65. A notable exception was a series of studies by Edgar Miller who showed that the main behavioral feature of presenile AD is a memory disorder in which recently acquired information fails to reach long-term memory storage due to both an abnormally rapid loss of material from short-term storage (perhaps due to encoding inefficiency) and difficulty in transferring information between short-term and long-term storage systems (Miller, 1971, 1973). He also suggested that inefficient retrieval of information from long-term storage may contribute to the memory deficit in presenile AD (Miller, 1975, 1978). These early studies set the stage for countless subsequent studies that examined the nature of memory dysfunction in AD in the decades to follow (for reviews, see Salmon & Bondi, 2009; Smith & Bondi, 2013).
A major sea-change in the study of dementia occurred in 1976 when Robert Katzman summarized data showing that senile and presenile AD were histopathologically identical and suggested that, based on epidemiological data, AD was the fourth leading cause of death in the elderly (Katzman, 1976). Suddenly, AD dementia went from a relatively rare condition to a major public health issue. This led to greater attention to the disease by the public and at the National Institutes of Health, which established the National Alzheimer’s Disease Research Center program to study the cause, neuropathology, and clinical characteristics of AD. At this time, the diagnostic criteria for dementia were refined in the DSM-III (American Psychiatric Association, 1980) and International Statistical Classification of Diseases and Related Health Problems, 10th Revision (World Health Organization, 1992), and specific research diagnostic criteria for AD were established (McKhann et al., 1984).
Also notable at this time was a growing realization that various dementing disorders are associated with patterns of relatively preserved and impaired cognitive abilities that vary depending upon the etiology and neuropathology of the underlying disease. Martin Albert and his colleagues (Albert, Feldman, & Willis, 1974) referred to the pattern of cognitive dysfunction observed in patients with progressive supranuclear palsy as a “subcortical dementia” characterized by forgetfulness, slowness of thought processes, altered personality with apathy or depression, and impaired ability to manipulate acquired knowledge. Similar cognitive changes were noted in patients with Huntington’s disease (McHugh & Folstein, 1975). This pattern of impairment was contrasted with the cortical dementia (e.g., frank amnesia, aphasia, and agnosia) observed in AD. Subsequent studies further delineated qualitative differences in the cognitive deficits associated with so-called “cortical” and “subcortical” dementing disorders (Huber, Shuttleworth, Paulson, Bellchambers, & Clapp, 1986; Salmon, Kwo-on-Yuen, Heindel, Butters, & Thal, 1989), and several investigators suggested that these two forms of dementia should be recognized as distinct clinical syndromes (for reviews, see Cummings & Benson, 1992; Cummings, 1990).
THE 1990s: NEUROPSYCHOLOGICAL CHARACTERIZATION OF ALZHEIMER’S DISEASE AND RELATED DISORDERS
The new criteria for dementia and AD adopted in the 1980s improved the reliability of the clinical diagnosis and allowed group studies of mildly demented patients to be carried out with a reasonable degree of accuracy. Many of these studies applied the theories and methods of cognitive psychology to study the cognitive consequences of AD. By using this approach, these studies characterized the component cognitive processes underlying the neuropsychological deficits observed in AD, and showed that cognitive changes attributable to AD and other dementing disorders could have important implications for existing theories of brain–behavior relationships underlying normal cognition.
Several studies at this time showed that episodic memory impairment (i.e., amnesia) is usually the earliest and most salient aspect of the AD dementia syndrome. These findings were consistent with neuropathologic studies that showed extensive AD pathology occurs earliest in medial temporal lobe (MTL) structures (e.g., hippocampus, entorhinal cortex) important for episodic memory (Hyman et al., 1984). The memory deficit was shown to reflect an inability to effectively encode and store new information since patients with very early AD were particularly impaired on measures of delayed recall (i.e., have abnormally rapid forgetting), exhibited an abnormal serial position effect with attenuation of the primacy effect (i.e., recall of words from the beginning of a list), and remained impaired even if retrieval demands were reduced by the use of recognition testing (e.g., Delis et al., 1991).
Semantic encoding was found to be less effective in improving the episodic memory performance of patients with AD than normal elderly individuals (Buschke, Sliwinski, Kuslansky, & Lipton, 1997). In addition, patients with AD more often produced intrusion errors (i.e., previously learned information is produced during the attempt to recall new material) on both verbal and non-verbal memory tests, presumably due to increased sensitivity to interference and/or decreased inhibitory processes (Butters, Granholm, Salmon, Grant, & Wolfe, 1987; Jacobs, Salmon, Tröster, & Butters, 1990). This pattern of memory deficits was shown to differ from the pattern exhibited by patients with subcortical dementia who had difficulty learning new information, but retained what was learned well and showed improved performance with retrieval aids (e.g., cueing or recognition formats) (Cummings, 1990). These findings provided evidence of differential roles of MTL and fronto-striatal brain structures in memory performance.
Studies also showed that, as the neuropathology of AD spreads beyond MTL structures to adjacent temporal, parietal, and frontal association cortices, several higher order cognitive abilities became affected. A deficit in language abilities (i.e., aphasia) was observed relatively early in the course of AD, with deficits in confrontation naming, verbal fluency (particularly from semantic categories), semantic categorization, and a reduced ability to recall over-learned facts (e.g., the number of days in a year) (Hodges & Patterson, 1995; Nebes, 1989). Patients were highly consistent in the individual items they missed across different semantic memory tests that used unique modes of access and output (e.g., fluency versus confrontation naming; Chertkow & Bub, 1990; Hodges, Salmon, & Butters, 1992), or within the same test across serial evaluations (Norton, Bondi, Salmon, & Goodglass, 1997).
These findings demonstrated that AD results in a true loss of semantic knowledge (i.e., general knowledge and the meanings of words) rather than only an impaired ability to retrieve information from intact semantic memory stores (also see Salmon, Heindel, & Lange, 1999). A similar loss of knowledge was thought to contribute to the severe deficit patients with AD exhibited in the ability to remember past events that were successfully remembered before the onset of the disease (i.e., retrograde amnesia) (Squire, 1987). Patients with subcortical dementia or fronto-temporal dementia, in contrast, retained semantic knowledge well, but had difficulty in systematic retrieval from semantic memory stores (Rosser & Hodges, 1994; Rascovsky, Salmon, Hansen, Thal, & Galasko, 2007).
Deficits in “executive” functions responsible for concurrent mental manipulation of information, concept formation, problem solving, and cue-directed behavior were found to develop in the course of AD (Bondi, Monsch, Butters, Salmon, & Paulsen, 1993; Lefleche & Albert, 1995; Perry & Hodges, 1999). Attention deficits were also found to occur and were usually evident on dual-processing tasks, tasks that require the disengagement and shifting of attention, and working memory tasks that depend upon the control of attentional resources (for reviews, see Parasuraman & Haxby, 1993; Perry & Hodges, 1999). Deficits in working memory were relatively mild and primarily characterized by disruption of the “central executive” with relative sparing of immediate memory (Baddeley, Bressi, Della Sala, Logie, Spinnler, 1991; Collette, Van der Linden, Bechet, Salmon, 1999). Executive dysfunction and deficits in attention played a less prominent role in the AD dementia syndrome than in the subcortical dementia syndrome associated with fronto-striatal dysfunction.
Several studies showed that visuospatial deficits occurred in patients with AD (for review, see Cronin-Golomb & Amick, 2001), but these deficits were usually less salient than other cognitive deficits in the early stages of the disease (Storandt Botwinick, Danziger, Berg, Hughes, 1984). Visuospatial tasks that were sensitive to early AD often involved not only visuoperceptual and constructional aspects of performance, but also required conceptual knowledge (e.g., Clock Drawing; Rouleau, Salmon,, Butters, Kennedy, & McGuire, 1992) or planning ability (e.g., Block Design).
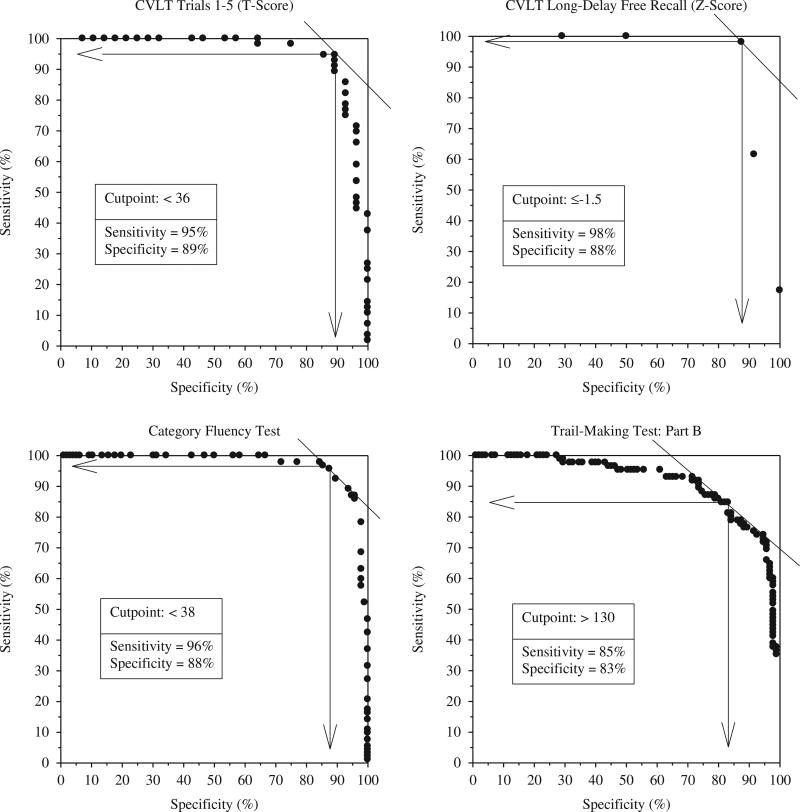
The advances made in characterizing the neuropsychological deficits associated with AD had a major impact on the ability to accurately diagnose the disease in its early stages. This clinical utility was demonstrated in a study that compared the ability of several sensitive measures of learning and memory, executive abilities, language, and visuospatial abilities to differentiate between mild AD and matched normal control subjects (Salmon et al., 2002). Results showed excellent sensitivity and specificity for learning and delayed recall measures from the California Verbal Learning Test (CVLT) (sensitivity: 95–98%, specificity: 88–89%), category fluency (sensitivity: 96%, specificity: 88%), and Trail-Making Part B (sensitivity: 85%, specificity: 83%). The best-fitting combination of category fluency and delayed recall accurately classified 96% of the patients with AD and 93% of the control subjects (see Figure 4). This study also illustrated that the pattern of cognitive deficits typically associated with AD is characterized by prominent deficits in episodic and semantic memory, with additional, although somewhat less prominent, deficits in executive functions, visuospatial abilities, and attention.
Fig. 4.
Receiver operating characteristic curves demonstrating excellent sensitivity and specificity for the accurate diagnosis of early AD achieved with neuropsychological tests of memory (California Verbal Learning Test), language (category fluency: animals, fruits, and vegetables) and executive functions (Trail-Making Test: Part B) (adapted from Salmon et al., 2002).
There are, however, somewhat rare instances, particularly in younger patients (e.g., less than 65 years old), where AD initially presents with dementia dominated by higher-order visual dysfunction, executive dysfunction or deficits in language. Posterior cortical atrophy (PCA) occurs when there is disproportionate atrophy and deposition of neurofibrillary tangles and neuritic plaques in the occipital cortex and posterior parietal cortex relative to other cortical association areas (Hof, Vogt, Bouras, & Morrison, 1997; Renner et al., 2004). Patients with PCA usually have prominent visual agnosia (sometimes including prosopagnosia) and constructional apraxia, and exhibit many or all of the features of Balint’s syndrome, including optic ataxia, gaze apraxia, and simultanagnosia (i.e., can detect visual details of an object but cannot organize them into a meaningful whole) (Caine, 2004; Mendez et al., 2002; Renner et al., 2004). PCA is associated with posterior cortical hypometabolism with particular involvement of the dorsal visual stream (Nestor, Caine, Fryer, Clarke, & Hodges, 2003), and with a posterior distribution of amyloid deposition revealed by positron emission tomography (PET) imaging using Pittsburgh compound-B ([11C]-PIB) (Tenovuo, Kemppainen, Aalto, Nagren, & Rinne, 2008).
A frontal variant of AD was identified in a subgroup of patients with autopsy-confirmed AD who initially presented with disproportionately severe deficits on neuropsychological tests of frontal lobe functioning (Johnson, Head, Kim, Starr, & Cotman, 1999). These patients had a significantly higher burden of neurofibrillary tangles, but not neuritic plaques, in the frontal cortex than a matched group of patients with a typical clinical presentation of AD. A subset of patients with primary progressive aphasia (PPA) was found to have AD pathology. These patients usually presented with logopenic PPA (PPA-L), which is characterized by hesitant, grammatically correct speech and spared language comprehension (Gorno-Tempini et al., 2004). PPA-L is most often associated with AD pathology disproportionately distributed in language-related cortical areas (Mesulam et al., 2008).
The existence of these AD “variants” has complicated the clinical and neuropsychological differentiation of AD from other neurodegenerative diseases that may have a different underlying focal pathology such as frontotemporal lobe dysfunction (FTLD), dementia with Lewy bodies (DLB), or PPA. However, considerable work has been done to identify how the neuropsychological presentations of these disorders differs from that of typical AD, and this information has been incorporated into the most recent clinical diagnostic criteria for behavioral variant FTLD (Rascovsky et al., 2011), DLB (McKeith et al., 2017), and PPA (Gorno-Tempini et al., 2011).
During the 1990s and early 2000s, important advances were also made in identifying genetic risks for AD. Mutations on three separate genes were identified in large families that displayed an autosomal dominant inheritance pattern of an early-onset form of AD (i.e., onset generally before the age of 60): the amyloid precursor protein gene on chromosome 21, the presenilin 1 gene on chromosome 14, and the presenilin 2 gene on chromosome 1 (for review, see Bird, 1999). These forms of familial AD are rare and account for only approximately 1 to 2% of all cases of the disease. A far more common genetic risk factor for sporadic, late-onset AD was identified as the type ε4 allele of the gene for apolipoprotein E (APOE), a low density lipoprotein cholesterol carrier (Strittmatter et al., 1993). Located on chromosome 19, the APOE ε4 allele was found to be present in 50 to 60% of patients with AD (compared to 20 to 25% of healthy older adults), regardless of whether or not they have a family history of dementia (Strittmatter, et al., 1993). Unlike the genes associated with early-onset familial AD, the APOE ε4 allele is not deterministic, but confers an approximately three-fold risk of developing AD if one copy of the ε4 allele is present, and an eight-fold risk if two copies are present (Katzman & Kawas, 1994).
The identification of the APOE ε4 risk led to a new approach to examining potential decrements in learning and memory during a “preclinical” phase of AD. The performance of non-demented older adults who have an increased risk for developing the disease due to an APOE ε4 genotype could be compared to that of individuals who do not have this risk factor with the presumption that more individuals with the ε4 genotype are in a preclinical stage of the disease. In one such study, Bondi, Salmon, Galasko, Thomas, and Thal (1999) compared the neuropsychological test performances of non-demented elderly individuals with or without at least one APOE ε4 allele. Although the groups did not differ significantly in age, education, or global cognitive status, the ε4+ subjects performed significantly worse than the ε4− subjects on measures of delayed recall, but not on tests of other cognitive abilities.
Cox proportional hazards analysis showed that APOE ε4 status and measures of delayed recall were significant independent predictors of subsequent progression to AD, suggesting that poor recall is an early sensitive neuropsychological marker of AD and not a cognitive phenotype of the ε4 genotype (also see Bondi et al., 1995; Petersen et al., 1995; Reed et al., 1994). Although ApoE remains the most potent susceptibility gene, the advent of genome wide association studies have identified 25 loci known to associate with late-onset sporadic AD, and the advent of polygenic risk scores are now available and will further refine our understanding of genetic contributions to AD progression (for review, see Sims & Williams, 2016).
THE 2000s: “MILD COGNITIVE IMPAIRMENT”
Although in the 1990s a few investigators had begun to systematically study individuals at risk for dementia to determine whether cognitive declines could be detected before diagnosis (Bondi et al., 1994, 1999; La Rue, Matsuyama, McPherson, Sherman, & Jarvik, 1992; Small, Fratiglioni, Viitanen, Winblad, & Bäckman, 2000; Snowdon et al., 1996), following the turn of this century, the focus of the field heavily shifted to the study of prodromal stages of AD that precede the full-blown dementia syndrome. Characterization of such early phases was largely crystallized by Ron Petersen, Glenn Smith, and colleagues from the Mayo Clinic who introduced of the concept of “mild cognitive impairment” (MCI) (Petersen et al., 1999).
MCI was defined as a condition in which individuals experience memory loss to a greater extent than one would expect for age, yet do not meet criteria for dementia. The specific clinical criteria for MCI they originally put forth were: (1) subjective memory complaint, (2) objective memory impairment for age, (3) relatively preserved general cognition, (4) essentially intact activities of daily living, and (5) not demented (Petersen et al., 1999). This classification scheme was subsequently broadened to include “amnestic MCI” or “non-amnestic MCI” subtypes, and “single domain” or “multiple domain” conditions to indicate the number of cognitive domains affected (Petersen, 2004; Winblad et al., 2004). It was proposed that these MCI subtypes correspond to various etiologies, with “amnestic MCI” being most indicative of AD and “non-amnestic MCI” suggesting other neurodegenerative conditions such as FTLD or DLB (Petersen & Morris, 2005; see also Smith & Bondi, 2013, for review).
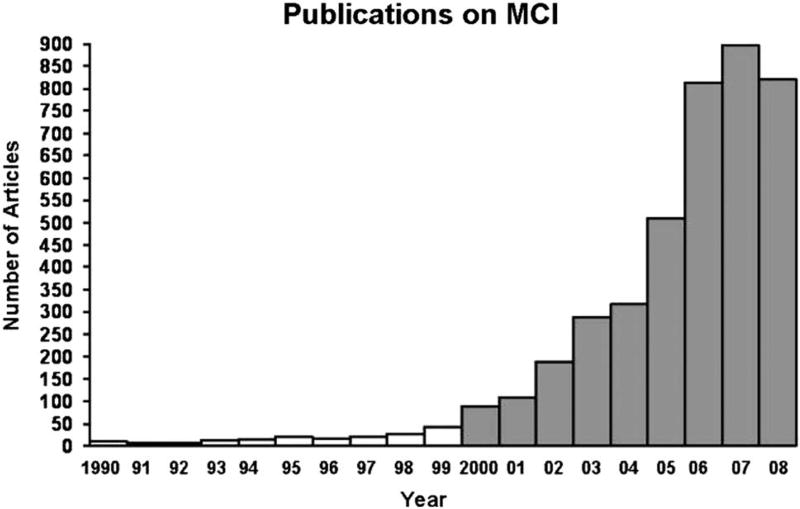
With the advent of these new criteria, the study of MCI became widespread during the 2000s. To illustrate this increasing attention and productivity, Petersen and colleagues (2009) noted that in 1999 fewer than 50 papers were published in the medical literature on the topic of MCI, whereas by 2007, this number approached 900 peer-reviewed studies in that year alone (see Figure 5). He rightly concluded that the increased awareness and study of MCI had been extremely valuable for the field by enhancing our understanding of the early neuropsychological manifestations of AD and improving the ability to identify those at risk for progression to dementia.
Fig. 5.
A representative number of publications with the search term “mild cognitive impairment” in the title or abstract from 1990 through part of 2008. Note the exponential rate of increase in the numbers of publications during the 2000s (from Petersen et al., 2009).
Detection and characterization of prodromal AD continued to be a vibrant area of research moving into the 2010s. In 2011, the National Institute on Aging and Alzheimer’s Association (NIA-AA) published updated diagnostic guidelines for MCI (Albert et al., 2011) and introduced research criteria for “preclinical” AD (Sperling et al., 2011). The new guidelines for MCI largely retained the criteria developed by Petersen and colleagues, but expanded the subjective cognitive complaint criterion to allow the complaint to come from either the patient, an informant or a skilled clinician, and incorporated the use of biomarkers into the diagnosis (discussed below). Research began on the potential of subjective cognitive complaints alone to accurately signal the development of underlying AD pathology (for review, see Jessen et al., 2014). Criteria for “preclinical” AD were developed to identify at-risk individuals at a stage of disease when they were still considered “asymptomatic” (i.e., had no significant cognitive impairment in the presence of one or more positive biomarkers for AD).
Although the criteria for MCI have been widely adopted, recent research has demonstrated limitations in the way the criteria were operationalized for clinical trials (e.g., Petersen et al., 2005) and large-scale natural history studies (e.g., the Alzheimer’s Disease Neuroimaging Initiative or ADNI; Weiner et al., 2013). These studies operationalized MCI as subjective complaints about memory, normal performance on simple cognitive screens, marginal memory ratings on scales based on clinical judgment, and impaired performance on a single memory test. Unfortunately, this method appears to be highly susceptible to false positive diagnostic errors (Bondi et al., 2014; Clark et al., 2013; Edmonds, Delano-Wood, Clark, et al., 2015).
This susceptibility was demonstrated by Edmonds, Delano-Wood, Clark, et al. (2015) who applied cluster-analytic statistical techniques to the neuropsychological test scores of participants in the ADNI cohort who had been classified as MCI using the conventional criteria. Despite their MCI diagnosis, approximately one-third of these participants performed within normal limits on this more extensive cognitive testing and showed a low rate of progression to dementia. Given these limitations in the conventional diagnostic criteria, Jak, Bondi, and colleagues (Jak et al., 2009, Bondi et al., 2014) developed an actuarial neuropsychological diagnostic method to identify individuals with MCI. Rather than using a single memory test, a diagnosis of MCI is established on the basis of scores achieved on multiple objective neuropsychological tests that assess a range of cognitive domains without reference to subjective complaints or clinical judgment. This actuarial method was shown to produce greater diagnostic stability than the conventional method (i.e., individuals classified as MCI did not revert to “normal” cognition after 1 year; Jak et al., 2009), and revealed stronger relationships between cognition, biomarkers, and rates of progression to dementia in patients classified as MCI in this way (Bondi et al., 2014).
THE 2010s: THE ERA OF BIOMARKERS
Over the past 20 years great progress was made in identifying in vivo biological markers of AD. Several investigators refined the ability to detect and measure cerebrospinal fluid levels of Aβ (the main constituent of the plaque) and tau protein (a constituent of the neurofibrillary tangle) that were indicative of AD pathology in the brain. Klunk and colleagues (see Mathis et al., 2003) developed Pittsburgh compound-B ([11C]-PIB), an agent that binds to Aβ, for use with PET imaging to reveal deposition of amyloid in the brain. Tau-binding agents that can be used with PET imaging have also been recently developed (for review, see Brosch, Farlow, Risacher, & Apostolova, 2017).
Neuroimaging measures of hippocampal, cortical, and general brain atrophy were developed and applied to detect early neurodegenerative changes associated with AD (for review, see Frisoni, Fox, Jack, Scheltens, & Thompson, 2010). Other advanced structural and functional neuroimaging methodologies, including resting-state functional MRI and diffusion tensor imaging, have been used to detect pathological changes associated with AD and to create algorithms for classifying AD and MCI (for review, see Rathore, Habes, Iftikhar, Shacklett, & Davatzikos, 2017). All of these biomarkers have greatly increased the accuracy with which AD pathology in the brain can be detected before the onset of cognitive symptoms, and improved the ability to differentiate AD from other pathologies that lead to dementia.
In the current decade, several large-scale longitudinal studies have examined the relationship between various AD biomarkers and the development of cognitive decline and dementia (e.g., ADNI, Australian Imaging, Biomarkers, and Lifestyle study). Based on results from these studies, Jack and colleagues (2010) proposed a hypothetical model of dynamic biomarker changes in the development of AD. Their model, consistent with the amyloid cascade hypothesis, proposed that amyloid deposition related to abnormal processing of the amyloid precursor protein (i.e., amyloidosis) drives the formation of abnormal tau aggregates. This in turn leads to tangle-mediated neuronal injury and neurodegeneration, which then produces cognitive and functional impairment (see Jack et al., 2010, 2013, for discussion).
Many biomarker studies align with this temporal sequence of pathophysiologic changes, particularly in early-onset autosomal dominant mutation carriers (e.g., Bateman et al., 2012). The model has been very influential in the development of treatment strategies for AD because it posits that, if the preclinical build-up of amyloid can be blocked or built-up, amyloid can be cleared and the cascade of events that leads to cognitive decline and dementia can be prevented (for review, see Musiek & Holtzman, 2015). The hypothesis also provided the framework for revised diagnostic criteria for AD (McKhann et al., 2011), MCI (Albert et al., 2011), and preclinical AD (Sperling et al., 2011).
Despite its wide influence, there is increasing evidence that calls the amyloid cascade hypothesis into question, especially with regard to its invariant temporal sequence of pathological events (Drachman, 2014). Several studies, for example, have shown that neurodegeneration (measured by tau biomarkers or neuroimaging measures of atrophy) can occur before amyloidosis in individuals with prodromal AD (Braak, Zetterberg, Del Tredici, & Blennow, 2013; Knopman et al., 2013; Ryan et al., 2013; Sheline et al., 2010; Wirth et al., 2013). Neurodegeneration in the face of normal amyloid levels was evident in 23% of the original sample of Jack et al. (2010) (and in an even higher percentage in Edmonds, Delano-Wood, Galasko, et al., 2015). Axonal injury (Ryan et al., 2013) and tau lesions in late-myelinating regions (Braak et al., 2011) have been shown to predate amyloid deposition in prodromal AD.
In addition, a growing number of studies have shown that cognitive measures can be as sensitive as physical biomarkers in predicting progression to dementia (Gomar et al., 2014; Heister et al., 2011; Jedynak et al., 2012; Landau et al., 2010; Richard, Schmand, Eikelenboom, Van Gool; Alzheimer’s Disease Neuroimaging Initiative, 2013). Taken together, these findings strongly suggest that the neurodegeneration of AD may not depend upon prior amyloidosis (Knopman et al., 2013, but cf. Jack, Knopman, et al., 2016).
Our prior work (Edmonds, Delano-Wood, Galasko, et al., 2015) in this area confirms that biomarker development in most individuals with preclinical/prodromal AD does not follow the temporal order proposed by the amyloid cascade hypothesis. We have shown that cognitively normal individuals who later progressed to MCI or AD, and had only one abnormal biomarker at baseline, were most likely to have neurodegeneration (i.e., P-tau positivity) as that abnormal biomarker rather than either amyloidosis alone or subtle cognitive deficit alone. In fact, neurodegeneration in isolation was 2.5 times more common than amyloidosis alone.
Jack, Bennett, and colleagues (2016) have recently acknowledged these and similar findings and proposed a more descriptive classification scheme for AD biomarkers that is agnostic to the temporal ordering of mechanisms underlying AD pathogenesis. This new model, known as the A/T/N system (“A” refers to Aβ, “T” to tau, and “N” to neurodegeneration), makes no assumptions about temporal ordering of biomarkers or their putative causal relationships. This “agnosticism” concurs with the notion of a simple tallying of biomarker risks as previously suggested by Edmonds, Delano-Wood, Galasko, et al. (2015).
Such a dramatic shift away from the strictures of the amyloid cascade model toward a more equipotential conceptualization of AD biomarker risks espoused by our tally system and by the A/T/N classification system fits well with a continuum hypothesis proposed by Braak and colleagues. An original Braak staging theory proposed that progression of neurofibrillary tangle pathology proceeds along well-defined predilection sites beginning in the MTL and then expands to adjacent association cortices and beyond (Braak & Braak, 1991). Amyloid plaque pathology, in contrast, accumulates more diffusely across neocortex.
This theory was recently updated to suggest that the pathogenic process actually starts with the formation of pretangle material in the lower brainstem with the first visible pathologic changes occurring in the locus coeruleus (Braak et al., 2011). Tangle pathology then spreads (possibly through cell-to-cell propagation; Iba et al., 2015) to MTL through specific projections from the locus coeruleus. It is postulated that this begins well before amyloidosis. Braak and Del Tredici (2015) proposed that the initial tau pathology in locus coeruleus and its axonal projections may not result in outright neuronal death, but may restrict neuronal function. Thus, a central role of neuropsychology in the coming decades may be to provide sophisticated measurement of functionality of affected neural systems in preclinical/prodromal AD.
Critics of the continuum theory argue that tau aggregation confined to brainstem structures and MTL, in the context of little to no amyloid deposition, should be considered an independent pathological process that is not integral to the developmental continuum of sporadic AD. They have termed this condition primary age-related tauopathy (PART; Crary et al., 2014). In this view, the pathological diagnosis of AD requires the presence of amyloid pathology. Braak and Del Tredici (2014) counter this argument by suggesting that amyloid plaques may develop after neurofibrillary tangle pathology develops in sites associated with AD (e.g., MTL); therefore, the “absence of Aβ deposits is not an adequate rationale for excluding tau-only cases from the developmental spectrum of the AD-related process.” They further argue that requiring a minimum threshold level of amyloid deposition for a neuropathologic diagnosis of AD (as in the PART criteria) may be justified only when applied in cases with clinically evident dementia, but not when applied to non-demented individuals.
As we move toward the end of the current decade, it is clear that the dogma that “amyloidosis is AD” is giving way to a broader conceptualization of the disease. This is evident in the adoption of biomarker staging systems that are agnostic to the temporal order of their occurrence (e.g., a tally system or the A/T/N system), and with the acceptance of new evidence that brainstem tauopathy and its propagation to the MTL may occur before amyloidosis associated with late-onset sporadic AD. This new understanding of AD may drive fundamental shifts in biomarker strategies, drug discovery, and therapeutics.
THE FUTURE
Neuropsychology has played a critical role in characterizing the cognitive changes associated with AD and related dementing disorders. This has improved the ability to accurately diagnose AD and differentiate it from other dementing disorders, to identify subtle cognitive changes that occur in the preclinical/prodromal phase of disease, and to track progression of the disease over the aging-MCI-AD continuum. Recent advances in AD biomarker development will alter this role. Increasingly, diagnosticians and investigators will be asked to use an array of available biomarkers to identify the neuropathologic determinants underlying cognitive changes within a given individual, and to detect neuropathology in its earliest stages before the onset of significant cognitive change.
That is not to say, however, that neuropsychology will cease to play an important role in dementia assessment and research. Regardless of the underlying pathology, it remains a critical function to identify the onset and nature of the earliest cognitive deficits that might impact someone’s life, to be able to predict the course of cognitive decline, and to measure the cognitive outcome of future treatments. These functions will likely be enhanced by integrating biomarker information into assessments. The use of such a “precision medicine” approach might bring increased specificity to the study of dementia in the future.
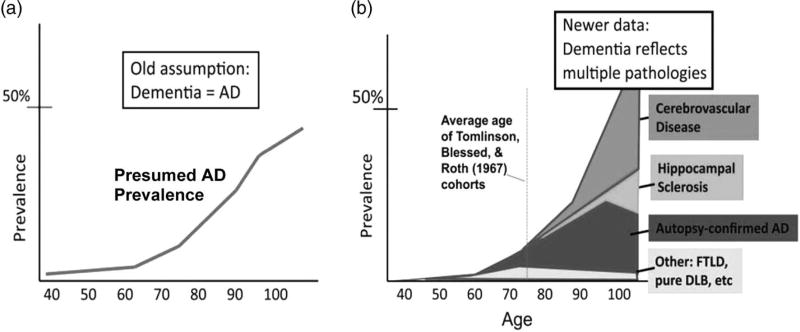
It has also become clear that, as age increases, there is increasing heterogeneity in the neuropathology underlying what is clinically diagnosed as “AD dementia.” Nelson and colleagues (2011) showed that the prevalence of AD pathology increases with age but reaches a plateau at approximately age 90; however, the prevalence of dementia and other pathologies, such as cerebrovascular disease or hippocampal sclerosis (arteriolosclerosis more generally), continue to increase with age (see Figure 6). This observation suggests that, in some cases, “AD dementia” appears only following the addition of other pathologies to a sub-threshold level of AD pathology. Such pathological heterogeneity leads to neuropsychological heterogeneity, making dementia characterization and differential diagnosis more difficult.
Fig. 6.
Nelson et al.’s (2011) contrasting depictions of the epidemiology of dementia. Panel (a) is the schematic representation of the prevailing view of Alzheimer neuropathology by age, whereas panel (b) depicts distinct brain diseases other than AD that may contribute to cognitive impairment in late life (adapted from Nelson et al., 2011).
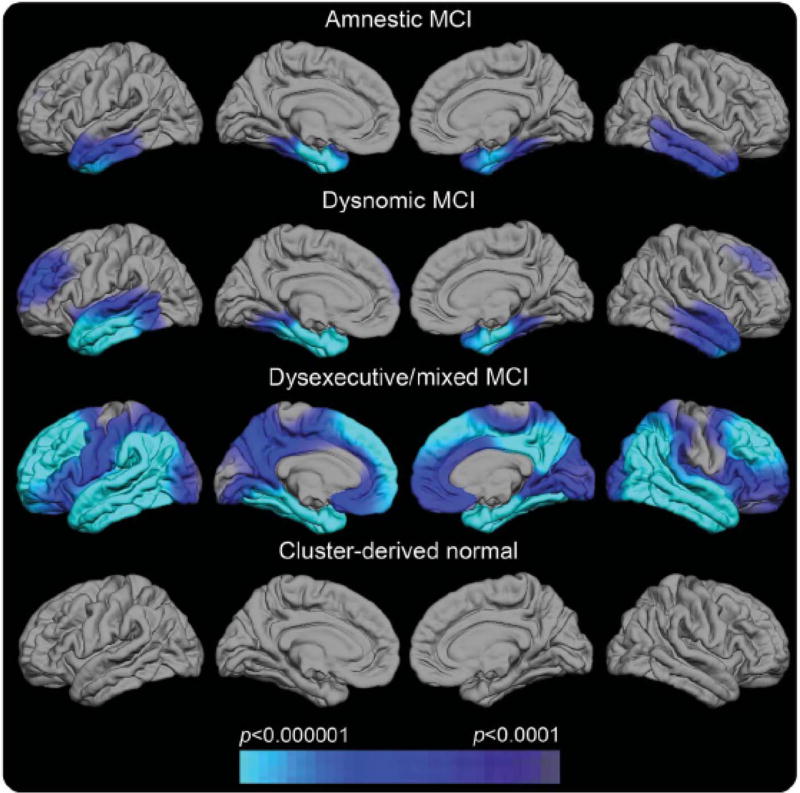
In the future, a precision medicine approach will allow multiple biomarkers to target distinct pathologies to show which pathologies are present, a genetic analysis will allow polygenic risk for various disorders to be assessed, and neuropsychological assessment will identify distinct patterns of deficits that reflect the differential impact of distinct pathologies on the dementia syndrome. Movement toward this goal is illustrated in a recent study which showed that individuals diagnosed as amnestic MCI in the ADNI cohort had great heterogeneity in the pattern of cognitive deficits they exhibited and that their deficits coincided well with specific regions of cortical thinning on neuroimaging (see Figure 7; Edmonds et al., 2016). These results demonstrate the potential utility of a combination of neuropsychological assessment and neuroimaging biomarkers to help explain a heterogeneous presentation of prodromal AD.
Fig. 7.
Regional cortical thickness maps of the left and right lateral and medial pial surfaces for each neuropsychological MCI subtype relative to normal control (NC) participants (Edmonds et al., 2016). The scale indicates group differences in cortical thickness at p< .0001. The cyan/blue shades represent areas where the MCI subgroup has significantly thinner cortex than the NC group. Cluster-derived normal (CDN) = those participants who performed normally across the neuropsychological tests but whom ADNI diagnosed as MCI. Their maps show no areas of cortical thinning relative to the NC group, suggesting they are false-positive diagnostic errors. Our prior work showing the CDN subgroup to have normal CSF AD biomarkers and low progression rates adds to the inference that they received false-positive MCI diagnoses (Bondi et al., 2014).
Neuropathological heterogeneity in AD could also have important implications for future therapeutic approaches to the disease. Given the shift away from the amyloid cascade model toward a more equipotential conceptualization of AD, it is not surprising that the recent singular focus on anti-amyloid treatments has led to disappointing results (Cummings, Morstorf, & Zhong, 2014). In an equipotential model of AD, other aspects of AD related pathology may already exist, continue to develop, and adversely affect cognition even if amyloid pathology is removed. If patients in anti-amyloid trials are positive for significant levels of amyloid, the anti-amyloid agent engages and clears amyloid, yet there is no clinical or cognitive benefit, it is reasonable to presume that pathology other than amyloid needs to be targeted.
Since tau pathology is more firmly associated with clinical and cognitive decline than is amyloid pathology, and may accumulate in susceptible regions earlier than that of amyloid, tau-altering pharmacologic interventions would seem worthwhile. Specific therapeutics may also be needed for other underlying pathologies (e.g., arteriolosclerosis, blood–brain barrier dysfunction, α-synuclein) that could be interacting with abnormal amyloid and tau in older individuals with sporadic “AD dementia.” Such agents could be used in a “precision medicine” context, where aberrant biomarkers coupled with a specific pattern of neuropsychological deficits could specify a particular treatment regimen within a prevention framework. Such a framework would also be accommodative of the specter of multiple biomarker abnormalities occurring concurrently.
SUMMARY
Over the past century since Alzheimer’s original publication, we have witnessed an explosion of work in the neuropsychology of dementia, and we have much work yet to complete. To borrow from another prominent psychologist who spoke of his perspective to better understand schizophrenia nearly 2 decades ago, Irving Gottesman (2001) pointedly suggested that no discipline committed to understanding any of the major disorders (insert Alzheimer’s disease in this example) has a monopoly on the amounts of uncertainty that remain for current and future generations of investigators. By joining forces across disciplines and assembling the most certain and important facts, investigators can launch new initiatives not previously imagined. Such an effort will be required to solve the complex puzzle of Alzheimer’s disease.
Acknowledgments
This work was supported by National Institutes of Health grants P50 AG05131 (M.W.B., D.P.S.), R01 AG049810 (M.W.B.) and K24 AG026431 (M.W.B.), and the U.S. Department of Veterans Affairs Clinical Sciences Research and Development Service (Career Development Award-2 1IK2 CX001415-01A1 to E.C.E.). Dr. Salmon serves as a consultant for Bristol-Myers Squibb. Dr. Bondi serves as a consultant for Novartis and Eisai and receives royalties from Oxford University Press.
Footnotes
The other authors report no disclosures.
References
- Albert ML, Feldman RG, Willis AL. The ‘subcortical dementia’ of progressive supranuclear palsy. Journal of Neurology, Neurosurgery, and Psychiatry. 1974;37:121–130. doi: 10.1136/jnnp.37.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer A. über eine eigenartige Erkankung der Hirnrinde. Allgemeine Zeitschrift fur Psychiatrie under Psychisch-Gerichtliche Medizin. 1907;64:146–148. [Google Scholar]
- Alzheimer A. Über eigenartige Krankheitsfälle des späteren Alters. Zeitschrift für die Gesamte Neurologie und Psychiatrie. 1911;4:356–385. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 2. Washington, DC: American Psychiatric Association; 1968. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-III) Washington, DC: American Psychiatric Association; 1980. Task force on nomenclature and statistics. [Google Scholar]
- Baddeley AD, Bressi S, Della Sala S, Logie R, Spinnler H. The decline of working memory in Alzheimer’s disease: A longitudinal study. Brain. 1991;114:2521–2542. doi: 10.1093/brain/114.6.2521. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Morris JC Dominantly Inherited Alzheimer Network. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. The New England Journal of Medicine. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird TD. Clinical genetics of familial Alzheimer’s disease. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer disease. Philadelphia: Lippincott Williams & Wilkens; 1999. pp. 57–66. [Google Scholar]
- Blessed G, Tomlinson B, Roth M. The association between quantitative measures of dementia and of senile changes in the cerebral grey matter of elderly subjects. British Journal of Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, Salmon DP. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and prediction of progression. Journal of Alzheimer’s Disease. 2014;42:275–289. doi: 10.3233/JAD-140276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Monsch AU, Butters N, Salmon DP, Paulsen JS. Utility of a modified version of the Wisconsin Card Sorting Test in the detection of dementia of the Alzheimer type. Clinical Neuropsychologist. 1993;7:161–170. doi: 10.1080/13854049308401518. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Monsch AU, Galasko D, Butters N, Salmon DP, Delis DC. Preclinical cognitive markers of dementia of the Alzheimer’s type. Neuropsychology. 1994;8:374–384. [Google Scholar]
- Bondi MW, Salmon DP, Galasko D, Thomas RG, Thal LJ. Neuropsychological function and apolipoprotein E qgenotype in the preclinical detection of Alzheimer’s disease. Psychology and Aging. 1999;14:295–303. doi: 10.1037//0882-7974.14.2.295. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Salmon DP, Monsch AU, Galasko D, Butters N, Klauber MR, Saitoh T. Episodic memory changes are associated with the ApoE-ε4 allele in nondemented older adults. Neurology. 1995;45:2203–2206. doi: 10.1212/wnl.45.12.2203. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Are cases with tau pathology occurring in the absence of Aβ deposits part of the AD-related pathological process? Acta Neuropathologica. 2014;128:767–772. doi: 10.1007/s00401-014-1356-1. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain. 2015;138:2814–2833. doi: 10.1093/brain/awv236. [DOI] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. Journal of Neuropathology & Experimental Neurology. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- Braak H, Zetterberg H, Del Tredici K, Blennow K. Intraneuronal tau aggregation precedes diffuse plaque deposition, but amyloid-β changes occur before increases of tau in cerebrospinal fluid. Acta Neuropathologica. 2013;126:631–641. doi: 10.1007/s00401-013-1139-0. [DOI] [PubMed] [Google Scholar]
- Brosch JR, Farlow MR, Risacher SL, Apostolova LG. Tau imaging in Alzheimer’s disease diagnosis and clinical trials. Neurotherapeutics. 2017;14:62–68. doi: 10.1007/s13311-016-0490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschke H, Sliwinski MJ, Kuslansky G, Lipton RB. Diagnosis of early dementia by the double memory test. Neurology. 1997;48:989–997. doi: 10.1212/wnl.48.4.989. [DOI] [PubMed] [Google Scholar]
- Butters N, Granholm E, Salmon DP, Grant I, Wolfe J. Episodic and semantic memory: A comparison of amnesic and demented patients. Journal of Clinical and Experimental Neuropsychology. 1987;9:479–497. doi: 10.1080/01688638708410764. [DOI] [PubMed] [Google Scholar]
- Caine D. Posterior cortical atrophy: a review of the literature. Neurocase. 2004;10:382–385. doi: 10.1080/13554790490892239. [DOI] [PubMed] [Google Scholar]
- Chertkow H, Bub D. Semantic memory loss in dementia of Alzheimer’s type. Brain. 1990;113:397–417. doi: 10.1093/brain/113.2.397. [DOI] [PubMed] [Google Scholar]
- Clark LR, Delano-Wood L, Libon DJ, McDonald CR, Nation DA, Bangen KJ, Bondi MW. Are empirically derived subtypes of mild cognitive impairment consistent with conventional subtypes? Journal of the International Neuropsychological Society. 2013;19:635–645. doi: 10.1017/S1355617713000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collette F, Van der Linden M, Bechet S, Salmon E. Phonological loop and central executive functioning in Alzheimer’s disease. Neuropsychologia. 1999;37:905–918. doi: 10.1016/s0028-3932(98)00148-1. [DOI] [PubMed] [Google Scholar]
- Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Nelson PT. Primary age-related tauopathy (PART): A common pathology associated with human aging. Acta Neuropathologica. 2014;128:755–766. doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin-Golomb A, Amick M. Spatial abilities in aging, Alzheimer’s disease, and Parkinson’s disease. In: Boller F, Cappa SF, editors. Handbook of Neuropsychology. Aging and dementia. 2. Vol. 6. Amsterdam: Elsevier; 2001. pp. 119–143. [Google Scholar]
- Cummings JL. Subcortical dementia. New York: Oxford University Press; 1990. [Google Scholar]
- Cummings JL, Benson DF. Dementia: A clinical approach. Boston: Butterworth-Heinemann; 1992. [Google Scholar]
- Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimer’s Research & Therapy. 2014;6:37. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Massman PJ, Butters N, Salmon DP, Cermak LS, Kramer JH. Profiles of demented and amnesic patients on the California verbal learning test: Implications for the assessment of memory disorders. Psychological Assessment. 1991;3:19–26. [Google Scholar]
- Drachman DA. The amyloid hypothesis, time to move on: Amyloid is the downstream result, not cause, of Alzheimer’s disease. Alzheimer’s & Dementia. 2014;10:372–380. doi: 10.1016/j.jalz.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Edmonds EC, Delano-Wood L, Clark LR, Jak AJ, Nation DA, McDonald CR, Bondi MW. Susceptibility of the conventional criteria for mild cognitive impairment to false-positive diagnostic errors. Alzheimer’s & Dementia. 2015;11:415–424. doi: 10.1016/j.jalz.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, Bondi MW. Subtle cognitive decline and biomarker staging in preclinical Alzheimer’s disease. Journal of Alzheimer’s Disease. 2015;47:231–242. doi: 10.3233/JAD-150128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds EC, Eppig J, Bondi MW, Leyden KM, Goodwin B, Delano-Wood L, McDonald CR. Heterogeneous cortical atrophy patterns not captured by conventional diagnostic criteria. Neurology. 2016;87:2108–2116. doi: 10.1212/WNL.0000000000003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Minimental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Fox NC, Jack CR, Jr, Scheltens P, Thompson PM. The clinical use of structural MRI in Alzheimer’s disease. Nature Reviews Neurology. 2010;6:67–77. doi: 10.1038/nrneurol.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomar JJ, Conejero-Goldberg C, Davies P, Goldberg TE Alzheimer’s Disease Neuroimaging Initiative. Extension and refinement of the predictive value of different classes of markers in ADNI: Four-year follow-up data. Alzheimer’s & Dementia. 2014;10:704–712. doi: 10.1016/j.jalz.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Miller BL. Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II. Psychopathology through a life span-genetic prism. American Psychologist. 2001;56:867–878. doi: 10.1037/0003-066x.56.11.867. [DOI] [PubMed] [Google Scholar]
- Heister D, Brewer JB, Magda S, Blennow K, McEvoy LK Alzheimer’s Disease Neuroimaging Initiative. Predicting MCI outcome with clinically available MRI and CSF biomarkers. Neurology. 2011;77:1619–1628. doi: 10.1212/WNL.0b013e3182343314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR, Patterson K. Is semantic memory consistently impaired early in the course of Alzheimer’s disease? Neuroanatomical and diagnostic implications. Neuropsychologia. 1995;33:441–459. doi: 10.1016/0028-3932(94)00127-b. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Salmon DP, Butters N. Semantic memory impairment in Alzheimer’s disease: Failure of access or degraded knowledge? Neuropsychologia. 1992;30:301–314. doi: 10.1016/0028-3932(92)90104-t. [DOI] [PubMed] [Google Scholar]
- Hof PR, Vogt BA, Bouras C, Morrison JH. Atypical form of Alzheimer’s disease with prominent posterior cortical atrophy: A review of lesion distribution and circuit disconnection in cortical visual pathways. Vision Research. 1997;37:3609–3625. doi: 10.1016/S0042-6989(96)00240-4. [DOI] [PubMed] [Google Scholar]
- Huber SJ, Shuttleworth EC, Paulson GW, Bellchambers MJ, Clapp LE. Cortical vs subcortical dementia. Neuropsychological differences. Archives of Neurology. 1986;43:392–394. doi: 10.1001/archneur.1986.00520040072023. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Damasio AR, Van Hoesen GW, Barnes CL. Alzheimer’s disease: cell specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Iba M, McBride JD, Guo JL, Zhang B, Trojanowski JQ, Lee VMY. Tau pathology spread in PS19 tau transgenic mice following locus coeruleus (LC) injections of synthetic tau fibrils is determined by the LC’s afferent and efferent connections. Acta Neuropathologica. 2015;130:349–362. doi: 10.1007/s00401-015-1458-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, Dubois B. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–347. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Chetelat G, Dickson D, Fagan AM, Frisoni GB, Vos SJB. Suspected non-Alzheimer disease pathophysiology – Concept and controversy. Nature Reviews Neurology. 2016;12:117–124. doi: 10.1038/nrneurol.2015.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurology. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurology. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs D, Salmon DP, Tröster AI, Butters N. Intrusion errors in the figural memory of patients with Alzheimer’s and Huntington’s disease. Archives of Clinical Neuropsychology. 1990;5:49–57. [PubMed] [Google Scholar]
- Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, Delis DC. Quantification of five neuropsychological approaches to defining mild cognitive impairment. American Journal of Geriatric Psychiatry. 2009;17:368–375. doi: 10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedynak BM, Lang A, Liu B, Katz E, Zhang Y, Wyman BT Alzheimer’s Disease Neuroimaging Initiative. A computational neurodegenerative disease progression score: Method and results with the ADNI cohort. NeuroImage. 2012;63:1478–1486. doi: 10.1016/j.neuroimage.2012.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G Subjective Cognitive Decline Initiative (SCD-I) Working Group. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s & Dementia. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JK, Head E, Kim R, Starr A, Cotman CW. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Archives of Neurology. 1999;56:1233–1239. doi: 10.1001/archneur.56.10.1233. [DOI] [PubMed] [Google Scholar]
- Katzman R. The prevalence and malignancy of Alzheimer disease: A major killer. Archives of Neurology. 1976;33:217–218. doi: 10.1001/archneur.1976.00500040001001. [DOI] [PubMed] [Google Scholar]
- Katzman R, Kawas C. The epidemiology of dementia and Alzheimer disease. In: Terry RD, Katzman R, Bick KL, editors. Alzheimer disease. New York: Raven Press; 1994. pp. 105–122. [Google Scholar]
- Knopman DS, Jack CR, Jr, Wiste HJ, Weigand SD, Vemuri P, Lowe VJ, Petersen RC. Brain injury biomarkers are not dependent on β-amyloid in normal elderly. Annals of Neurology. 2013;73:472–480. doi: 10.1002/ana.23816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E. Psychiatrie: Ein Lehrbuch fur studierende und artzte. In: Kraepelin E, editor. Handbook of psychiatry. 8. Leipzig: Barth; 1910. pp. 593–632. [Google Scholar]
- Lefleche G, Albert MS. Executive function deficits in mild Alzheimer’s disease. Neuropsychology. 1995;9:313–320. [Google Scholar]
- Landau SM, Harvey D, Madison CM, Reiman EM, Foster NL, Aisen PS Alzheimer’s Disease Neuroimaging Initiative. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rue A, Matsuyama SS, McPherson S, Sherman J, Jarvik LF. Cognitive performance in relatives of patients with probable Alzheimer disease: An age at onset effect? Journal of Clinical and Experimental Neuropsychology. 1992;14:533–538. doi: 10.1080/01688639208402842. [DOI] [PubMed] [Google Scholar]
- Mahandra B. Dementia: A survey of the syndrome of dementia. Lancaster, England: MTP; 1984. [Google Scholar]
- Mathis CA, Wang Y, Holt DP, Huang G-F, Debnath ML, Klunk WE. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. Journal of Medicinal Chemistry. 2003;46:2740–2754. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- Maurer K, Maurer U. Alzheimer: The life of a physician and career of a disease. New York: Columbia University Press; 2003. [Google Scholar]
- McHugh PR, Folstein MF. Psychiatric symptoms of Huntington’s chorea: A clinical and phenomenologic study. In: Benson DF, Blumer D, editors. Psychiatric aspects of neurological disease. New York: Raven Press; 1975. pp. 267–285. [Google Scholar]
- McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, Kosaka K. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89:88–100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan M. Clinical diagnosis of Alzheimer’s disease: report of the NINCD-ADRDA work group. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging – Alzheimer’s Association workgroup. Alzheimer’s & Dementia. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF, Ghajarania M, Perryman KM. Posterior cortical atrophy: Clinical characteristics and differences compared to Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 2002;14:33–40. doi: 10.1159/000058331. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, Bigio EH. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Annals of Neurology. 2008;63:709–719. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. On the nature of the memory disorder in presenile dementia. Neuropsychologia. 1971;9:75–81. doi: 10.1016/0028-3932(71)90064-9. [DOI] [PubMed] [Google Scholar]
- Miller E. Short- and long-term memory in patients with presenile dementia (Alzheimer’s disease) Psychological Medicine. 1973;3:221–224. doi: 10.1017/s003329170004856x. [DOI] [PubMed] [Google Scholar]
- Miller E. Impaired recall and the memory disturbance in presenile dementia. British Journal of Social and Clinical Psychology. 1975;14:73–79. doi: 10.1111/j.2044-8260.1975.tb00151.x. [DOI] [PubMed] [Google Scholar]
- Miller E. Retrieval from long-term memory in presenile dementia: two tests of an hypothesis. British Journal of Social and Clinical Psychology. 1978;17:143–148. doi: 10.1111/j.2044-8260.1978.tb00256.x. [DOI] [PubMed] [Google Scholar]
- Musiek ES, Holtzman DM. Three dimensions of the amyloid hypothesis: Time, space and ‘wingmen’. Nature Neuroscience. 2015;18:800–806. doi: 10.1038/nn.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebes R. Semantic memory in Alzheimer’s disease. Psychological Bulletin. 1989;106:377–394. doi: 10.1037/0033-2909.106.3.377. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Head E, Schmitt FA, Davis PR, Neitner JH, Jicha GA, Scheff SW. Alzheimer’s disease is not “brain aging”: Neuropathological, genetic, and epidemiological human studies. Acta Neuropathologica. 2011;121:571–587. doi: 10.1007/s00401-011-0826-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PJ, Caine D, Fryer TD, Clarke J, Hodges JR. The topography of metabolic deficits in posterior cortical atrophy (the visual variant of Alzheimer’s disease) with FDG-PET. Journal of Neurology, Neurosurgery, and Psychiatry. 2003;74:1521–1529. doi: 10.1136/jnnp.74.11.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton LE, Bondi MW, Salmon DP, Goodglass H. Deterioration of generic knowledge in patients with Alzheimer’s disease: Evidence from the Number Information Test. Journal of Clinical and Experimental Neuropsychology. 1997;19:857–866. doi: 10.1080/01688639708403766. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Haxby JV. Attention and brain function in Alzheimer’s disease. Neuropsychology. 1993;7:242–272. [Google Scholar]
- Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer’s disease: A critical review. Brain. 1999;122:383–404. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Early diagnosis of Alzheimer disease: Is MCI too late? Current Alzheimer Research. 2009;6:324–330. doi: 10.2174/156720509788929237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, Smith GE, Jack CR., Jr Mild cognitive impairment: Ten years later. Archives of Neurology. 2009;66:1447–1455. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Archives of Neurology. 2005;62:1160–1163. doi: 10.1001/archneur.62.7.1160. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Ivnik RJ, Tangalos EG, Schaid DJ, Thibodeau SN, Kurland LT. Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. Journal of the American Medical Association. 1995;273:1274–1278. [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Thal LJ. Vitamin E and donepezil for the treatment of mild cognitive impairment. New England Journal of Medicine. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Salmon DP, Hansen LA, Thal LJ, Galasko D. Disparate phonemic and semantic fluency deficits in autopsy-confirmed frontotemporal dementia and Alzheimer’s disease. Neuropsychology. 2007;21:20–30. doi: 10.1037/0894-4105.21.1.20. [DOI] [PubMed] [Google Scholar]
- Rathore S, Habes M, Iftikhar MA, Shacklett A, Davatzikos C. A review on neuroimaging-based classification studies and associated feature extraction methods for Alzheimer’s disease and its prodromal stages. NeuroImage. 2017;155:530–548. doi: 10.1016/j.neuroimage.2017.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed T, Carmelli D, Swan GE, Breitner JCS, Welsh KA, Jarvik GP, Auwerx J. Lower cognitive performance in normal older adult male twins carrying the apolipoprotein E ε4 allele. Archives of Neurology. 1994;51:1189–1192. doi: 10.1001/archneur.1994.00540240033012. [DOI] [PubMed] [Google Scholar]
- Renner JA, Burns JM, Hou CE, McKeel DW, Jr, Storandt M, Morris JC. Progressive posterior cortical dysfunction: a clinicopathologic series. Neurology. 2004;63:1175–1180. doi: 10.1212/01.wnl.0000140290.80962.bf. [DOI] [PubMed] [Google Scholar]
- Richard E, Schmand BA, Eikelenboom P, Van Gool WA Alzheimer’s Disease Neuroimaging Initiative. MRI and cerebrospinal fluid biomarkers for predicting progression to Alzheimer’s disease in patients with mild cognitive impairment: a diagnostic accuracy study. BMJ Open. 2013;3:e002541. doi: 10.1136/bmjopen-2012-002541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser A, Hodges JR. Initial letter and semantic category fluency in Alzheimer’s disease, Huntington’s disease, and progressive supranuclear palsy. Journal of Neurology, Neurosurgery, and Psychiatry. 1994;57:1389–1394. doi: 10.1136/jnnp.57.11.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouleau I, Salmon DP, Butters N, Kennedy C, McGuire K. Quantitative and qualitative analyses of clock drawings in Alzheimer’s and Huntington’s disease. Brain and Cognition. 1992;18:70–87. doi: 10.1016/0278-2626(92)90112-y. [DOI] [PubMed] [Google Scholar]
- Ryan NS, Keihaninejad S, Shakespeare TJ, Lehmann M, Crutch SJ, Malone IB, Fox NC. Magnetic resonance imaging evidence for presymptomatic change in thalamus and caudate in familial Alzheimer’s disease. Brain. 2013;136:1399–1414. doi: 10.1093/brain/awt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon DP, Bondi MW. Neuropsychological assessment of dementia. Annual Review of Psychology. 2009;60:257–282. doi: 10.1146/annurev.psych.57.102904.190024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon DP, Heindel WC, Lange KL. Differential decline in word generation from phonemic and semantic categories during the course of Alzheimer’s disease: Implications for the integrity of semantic memory. Journal of the International Neuropsychological Society. 1999;5:692–703. doi: 10.1017/s1355617799577126. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Kwo-on-Yuen PF, Heindel W, Butters N, Thal LJ. Differentiation of Alzheimer’s disease and Huntington’s disease with the Dementia Rating Scale. Archives of Neurology. 1989;46:1204–1208. doi: 10.1001/archneur.1989.00520470060028. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Thomas RG, Pay MM, Booth A, Hofstetter CR, Thal LJ, Kaltzman R. Alzheimer’s disease can be accurately diagnosed in very mildly impaired individuals. Neurology. 2002;59:1022–1028. doi: 10.1212/wnl.59.7.1022. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Morris JC, Snyder AZ, Price JL, Yan Z, D’Angelo G, Mintun MA. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. Journal of Neuroscience. 2010;30:17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims R, Williams J. Defining the genetic architecture of Alzheimer’s disease: Where next? Neurodegenerative Diseases. 2016;16:6–11. doi: 10.1159/000440841. [DOI] [PubMed] [Google Scholar]
- Small BJ, Fratiglioni L, Viitanen M, Winblad B, Bäckman L. The course of cognitive impairment in preclinical Alzheimer disease: three- and six-year follow-up of a population based sample. Archives of Neurology. 2000;57:839–844. doi: 10.1001/archneur.57.6.839. [DOI] [PubMed] [Google Scholar]
- Smith GE, Bondi MW. Mild cognitive impairment and dementia: Definitions, diagnosis, and treatment. New York: Oxford University Press; 2013. [Google Scholar]
- Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Markesbery WR. Linguistic ability in early life and cognitive function and Alzheimer’s disease in late life. Findings from the Nun Study. Journal of the American Medical Association. 1996;275:528–532. [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and brain. New York: Oxford University Press; 1987. [Google Scholar]
- Stelzmann RA, Schnitzlein N, Murtagh FR. An English translation of Alzheimer’s paper, “über eine eigenartige Erkankung der Hirnrinde”. Clinical Anatomy. 1995;8:429–431. doi: 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]
- Storandt M, Botwinick J, Danziger WL, Berg L, Hughes CP. Psychometric differentiation of mild senile dementia of the Alzheimer type. Archives of Neurology. 1984;41:497–499. doi: 10.1001/archneur.1984.04050170043013. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein-E – High-avidity binding to B-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:9649–9653. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenovuo O, Kemppainen N, Aalto S, Nagren K, Rinne JO. Posterior cortical atrophy: A rare form of dementia with in vivo evidence of amyloid-beta accumulation. Journal of Alzheimer’s Disease. 2008;15:351–355. doi: 10.3233/jad-2008-15301. [DOI] [PubMed] [Google Scholar]
- Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, Trojanowski JQ. The Alzheimer’s Disease Neuroimaging Initiative: A review of papers published since its inception. Alzheimer’s & Dementia. 2013;9:e111–e194. doi: 10.1016/j.jalz.2013.05.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Petersen RC. Mild cognitive impairment–beyond controversies, towards a consensus: Report of the Inter-national Working Group on Mild Cognitive Impairment. Journal of Internal Medicine. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Wirth M, Madison CM, Rabinovici GD, Oh H, Landau SM, Jagust WJ. Alzheimer’s disease neurodegenerative biomarkers are associated with decreased cognitive function but not β-Amyloid in cognitively normal older individuals. Journal of Neuroscience. 2013;33:5553–5563. doi: 10.1523/JNEUROSCI.4409-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. International Classification of Diseases. 10. Geneva, Switzerland: WHO; 1992. [Google Scholar]