Abstract
Environmental contamination with hexavalent chromium (CrVI) is a growing problem both in the U.S and developing countries. CrVI is a heavy-metal endocrine disruptor; women working in Cr industries exhibit an increased incidence of premature abortion and infertility. The current study was designed to understand the mechanism of CrVI toxicity on placental cell survival/death pathways. Pregnant mothers were treated with or without CrVI (50 ppm K2Cr2O7) through drinking water from gestational day (GD) 9.5–14.5, and placentas were analyzed on GD 18.5. Results indicated that CrVI increased apoptosis of trophoblasts, vascular endothelium of the metrial glands and yolk sac epithelium through caspase-3 and p53-dependent pathways. CrVI increased apoptosis in labyrinth and basal zones in a caspase-3-independent manner via AIF, and through an ATM-p53-NOXA-PUMA-p27 network. CrVI downregulated cell survival proteins Bcl-2, Bcl-XL and XIAP in the placenta. CrVI disrupts placental histoarchitecture and increases cell death by spatiotemporal modulation of apoptotic signaling.
Keywords: Hexavalent chromium, Placenta, Trophoblasts, Apoptosis
1. Introduction
In utero exposure to endocrine disrupting chemicals (EDCs) such as heavy metals, pharmaceutical drugs, and pesticides can adversely affect the development of the fetuses [1]. Retrospective epidemiological studies have shown that early adverse events during fetal development and intrauterine fetal growth restriction (IUGR) are linked to various adult disorders such as hypertension, coronary heart disease, stroke, and type 2 diabetes [2,3]. Environmental contamination with hexavalent chromium (CrVI) and the associated health effects of CrVI exposure in humans is a growing problem both in the U.S and developing countries. CrVI has been used in various industries such as leather and textiles, metallurgical, chemical and automobile [4,5]. Due to increased use and improper disposal of CrVI, levels in the water, soil and air continue to increase [6,7]. Significant contamination with CrVI has been found in drinking water sources of more than 30 cities in the U.S [8]. According to an Environmental Protection Agency (EPA) report from February 2014, well water from Midland, Texas contains 5.28 ppm Cr [9]. However, USEPA approved safety limit for Cr levels in drinking water is 0.1 ppm [10]. Occupational exposure to CrVI is found among approximately one-half million industrial workers in the U.S and several millions worldwide [11,12]. Epidemiological data indicate that women working in Cr industries such as textiles and tanneries have high Cr levels in their blood, urine, umbilical cord and placental tissue [13,14], experience abnormal menses [15,16], spontaneous abortion [17,18], and deliver children with developmental defects [19,20], and low birth weight [21].
The placenta is a multifaceted organ that grows rapidly and exhibits marked changes in histoarchitecture during fetal development [22,23]. It is the interface between the mothers and developing embryos/fetuses that performs a number of critical functions throughout gestation. Functions of placenta include anchoring the developing fetus to the uterine wall, mediating maternal immune tolerance, O2/CO2 exchange, providing nutrients for the fetus and removing waste products during embryonic development [24]. Placenta plays an important role as the gateway for the entry of EDCs into the fetal environment. Therefore, it is an important organ for the evaluation of risks for mothers and embryos/fetuses in toxicity screening. Mechanistic evaluation of placental toxicity has been scarce and incomplete in experimental animals. Rat placental models have been useful for evaluating the potential of EDCs that affect human reproductive development since there are several similarities (despite differences) between rats and humans in early placental development [25]. Both rat placenta and human placenta are hemochorial and share some unique features regarding uterine trophoblast invasion and spiral artery remodeling [26,27].
Apoptosis is a physiological process that is highly orchestrated at the molecular level by the activation of an aspartate-specific cysteine protease (caspase) cascade [28]. Whether apoptosis occurs naturally during the normal development of the placenta is a controversial subject. Some reports indicated that apoptotic cells in rat placenta rarely existed under normal physiological conditions in mid-late pregnancy [28,29]. In contrast, others have identified naturally occurring apoptosis in placentas at mid-and late pregnancy in humans and rodents. This is thought to play important roles in placental physiological processes such as growth, turnover, senescence, and parturition [30–33]. In human pregnancy, apoptosis is increased in placentas subjected to IUGR or other disorders [30,31,34,35]. In mice and rats, several developmental toxicants including cadmium induced excessive placental apoptosis as well as impaired fetal growth [36]. Apoptosis can be triggered by an increase in apoptosis-inducing signals and/or suppression of apoptosis-inhibiting signaling [37]. Bcl-2 family members that are both proapoptotic and antiapoptotic regulate mitochondrial pathways by controlling cytochrome c release from the mitochondria [38]. Our previous studies had shown that CrVI can activate mitochondria-mediated intrinsic apoptosis in the ovary through activation of caspase-3 (casp-3) [39].
The p53 tumor suppressor protein serves as a critical regulator of apoptosis to prevent the propagation of abnormal cells, especially after DNA damage [37,40]. P53 can bind the Bax promoter and regulate Bax transcription [41]. Phosphorylation activates and stabilizes the p53 protein, and activated p53 increases the transcription of target genes that can promote both the intrinsic and the extrinsic apoptotic pathways, and/or mediate cell cycle arrest and DNA repair [42,43]. Many types of cells are known to undergo apoptosis in a p53-dependent manner in response to cytotoxic stimuli [42,44–46]. Studies from our laboratory had clearly demonstrated potential roles for p53 pathway in CrVI-induced apoptosis through direct [39] and indirect [47] mechanisms in the ovary. A few studies have shown that more p53 protein is produced by the human placentas in abnormal pregnancies [48]. Thus, p53 may contribute to the pathogenesis of placental disorders through the induction of apoptosis [49–52]. Administration of the DNA-damaging agents ethylnitrosourea and cytosine arabinoside to pregnant rats induced apoptosis of trophoblasts in the placental labyrinth zone, with an upregulation of p53 protein [53,54]. However, little is known about the precise role of p53 in apoptosis of trophoblasts.
We have reported that exposure of pregnant rats to CrVI through drinking water resulted in Cr accumulation in placental tissue [47]. CrVI rapidly passes through cells by anionic transporters and is readily converted in cells and biological fluids into CrIII by antioxidants (AOX) such as ascorbic acid, glutathione, N-acetyl cysteine, and antioxidant enzymes such as superoxide dismutase and catalase. During this cellular reduction process, enormous amounts of free radicals are generated, which increases oxidative stress. CrIII directly binds with DNA and can cause mutations [55]. Exposure to EDCs (including CrVI), DNA damaging agents, cellular stress and ionizing radiations upregulates p53 expression in various cells. Therefore, determining the molecular pathways that regulate placental cell death during normal development and in response to EDC exposure is imperative to identify strategies to mitigate adverse effects on fetal development. In addition, while pre-term labor is a predominant health issue in women exposed to CrVI, the underlying mechanisms are not understood. Therefore, we hypothesize that maternal exposure to CrVI during early pregnancy disrupts placental development by activating apoptotic pathways and inhibiting cell survival pathway in a spatiotemporal pattern. The objectives were to determine the effects of maternal CrVI exposure on: (i) the histopathology of the maternal and fetal compartments; (ii) casp-3 and p53-mediated apoptotic pathways; and (iii) cell survival pathways in various compartments of the placenta.
2. Materials and methods
2.1. Materials
Reagents used in this study were purchased from Sigma-Aldrich, St. Louis, MO. Details of sources of antibodies, catalog numbers, dilutions, host species, immunogens, and homologies with rat/mouse are given in Table 1.
Table 1.
Details of antibodies showing the source, catalog number, dilution, host species and antigen sequences.
| S.No | Peptide/protein target | Antigen sequence | Name of Antibody | Manufacturer; catalog # | Antibody type | Dilution used |
|---|---|---|---|---|---|---|
| 1 | BCL2 | N terminal amino acids 1–18 of Human Bcl-2; AGRTGYDNREIVMKYIHY | Rabbit Anti-Bcl-2 | Abcam; ab7973 | Rabbit polyclonal | 1:300 |
| 2 | BCL-XL | Synthetic peptide corresponding to residues surrounding Asp61 of human Bcl-xL | Rabbit Anti-BCL-xl | Cell Signaling; 2762 | Rabbit polyclonal | 1:50 |
| 3 | XIAP | Synthetic peptide corresponding to a region surrounding serine 245 of human XIAP | Rabbit Anti-XIAP | Cell Signaling; 2045 | Rabbit monoclonal | 1:100 |
| 4 | BAX | Synthesized peptide derived from human BAX | Rabbit Anti-BAX | Sigma; SAB4502549 | Rabbit polyclonal | 1:1500 |
| 5 | AIF | Purified recombinant fragment of Human AIF expressed in E. Coli | Mouse Anti-AIF | Abcam; Ab119917 | Mouse monoclonal | 1:500 |
| 6 | Cleaved- Caspase-3 | Synthetic peptide corresponding to amino-terminal residues adjacent to (Asp175) in human caspase-3 | Rabbit Anti-Cleaved- Caspase-3 | Cell Signaling; 9661 | Rabbit polyclonal | 1:100 |
| 7 | ATM | Recombinant fragment, corresponding to amino acids 1980–2338 of Human ATM | Rabbit Anti-ATM | Abcam; Ab10941 | Rabbit polyclonal | 1:400 |
| 8 | P53 | Synthetic peptide within Human p53 aa 13–17 (PLSQE) conjugated to Keyhole Limpet Haemocyanin (KLH). | Rabbit Anti-P53 | Abcam; Ab131442 | Rabbit polyclonal | 1:2000 |
| 9 | PUMA | Synthetic peptide corresponding to C terminal amino acids 180–193 (PLPRGHR APEMEPN) of Human PUMA alpha | Rabbit Anti-PUMA | Abcam; Ab9643 | Rabbit polyclonal | 1:2000 |
| 10 | NOXA | Synthetic peptide corresponding to 17 amino acids at the amino terminus of mouse Noxa | Rabbit Anti-Noxa | Abcam; Ab36833 | Rabbit polyclonal | 1:1000 |
| 11 | P27 | Synthetic peptide corresponding to the sequence of mouse p27 | Rabbit Anti-P27 | Cell Signaling; 2552 | Rabbit polyclonal | 1:50 |
2.2. Animals
Timed pregnant Sprague-Dawley rats were purchased from Charles River Laboratories and maintained in AAALAC-approved animal facilities with a 12 h light/12 h dark regime at 23 – 25 °C, and provided with Teklad 4% mouse/rat diet and water ad libitum. Animal Use Protocols were performed in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals, and with the standards established by Guiding Principles in the Use of Animals in Toxicology and specific guidelines and standards of the Society for the Study of Reproduction, and approved by the Animal Care and Use Committee (IACUC) of Texas A&M University.
2.3. In vivo dosing and experimental design
The in vivo CrVI dosing used in this investigation was chosen based on Cr levels in drinking water in highly polluted regions in developing countries that range from 19 to 50 ppm [56–59]. Timed pregnant rats were divided into two groups: (1) Control (n = 5): rats received regular drinking water; (2) CrVI treatment (n = 5): rats received potassium dichromate (CrVI, 50 ppm) dissolved in drinking water from days post coitum (dpc) 9.5–14.5. On gestational day 18.5, the mothers were euthanized by CO2 asphyxiation followed by cervical dislocation. The uterus was opened for each mother and the complete uterus with the attached placenta was fixed in 10% neutral buffered formalin. After 24–48 h, the tissues were removed from the fixative and transferred to 70% ethanol and embedded in paraffin blocks using the routine histological procedures. The placentas were trimmed by a transverse cut in the center of the disc (parallel to the umbilical cord) in order to obtain a mid-sagittal section. Some sections were stained with hematoxylin and eosin (H&E) whereas unstained sections were used for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, and immunohistochemistry (IHC) of various proteins.
2.4. Terminal deoxynucleotidyl transferase dUTP nick end labeling assay
Paraffin-embedded tissue sections were deparaffinized and TUNEL assay was performed as we described recently [60,61]. The apoptosis index (AI) was calculated as the average percentage of TUNEL-positive cells from each zone of the placenta at a magnification of 400x.
2.5. Immunohistochemistry
Paraffin-embedded tissue sections were deparaffinized and IHC was performed as previously described [60,61]. The intensity of staining for each protein was quantified using Image-ProPlus 6.3 image processing and analysis software according to the manufacturer’s instructions (Media Cybernetics, Inc.; Bethesda, MD). In brief, twenty five images from each mother for each zone of the placenta at 400 × magnification were captured randomly without bias. Integrated Optical Density (IOD) of immunostaining was quantified in the RGB mode with background staining normalized to isotype control immunoglobulins used at the same concentration as primary antibodies. Numerical data were expressed as least square mean ± SEM. Three-4 fold, 5–6 fold and >8 fold changes in expression levels were classified as slight increase, moderate increase, and high increase, respectively. Each of these changes is considered significant only if it is statistically significant at p < 0.05. This technique is more quantitative than conventional blind scoring systems and the validity of the quantification was reported previously by our group [62].
Definitions of the relevant structures and landmarks within the placentation site as well as the terminologies used to refer various cell types in the placenta vary from the laboratory to laboratory. This is also due to the complexity of the placental histoarchitecture and its uniqueness from species to species [63]. Rats have a hemotrichorial type of placenta [26]. In general, placenta is composed of the fetal part and maternal part [64]. The fetal components of the placenta consists of the labyrinth zone (LZ) and basal zone (BZ). In addition, yolk sac (YS), amnion and allantoic membranes are called as the fetal membranes [22,63,64]. The maternal components of the placenta consists of the decidua (DC) and metrial glands (MG) [22,64,65]. The LZ consists of trophoblasts, separating the maternal blood spaces or maternal vessels from the fetal blood vessels (FV). The outer trophectoderm, which comes into direct contact with the maternal vessels, is referred to as cytotrophoblasts with a microvillous surface. Two layers of syncytiotrophoblasts lie under this trophectoderm [22]. The BZ is comprised of three types of differentiated cells: (1) spongiotrophoblasts, (2) trophoblastic giant cells and (3) glycogen cells. The DC is comprised of the mesometrial decidual cells ultimately, and plays essential roles in the development of the vascularized decidual-placental interface. The MG is located in the mesometrial triangle of the pregnant uterus [22,65] and is composed of decidualized endometrial stromal cells, uterine natural killer cells, spiral-shaped arteries, trophoblasts originating from glycogen cells, and fibroblasts. The yolk sac (YS) is composed of epithelial cells and mesodermal cells [22].
2.6. Statistical analysis
Effects of CrVI on various parameters in the placental zones were analyzed and the results are expressed as mean ± SEM. Student t- test was used to compare groups and p values less than 0.05 were considered significant.
3. Results
3.1. Effects of CrVI on the histopathology of the placenta
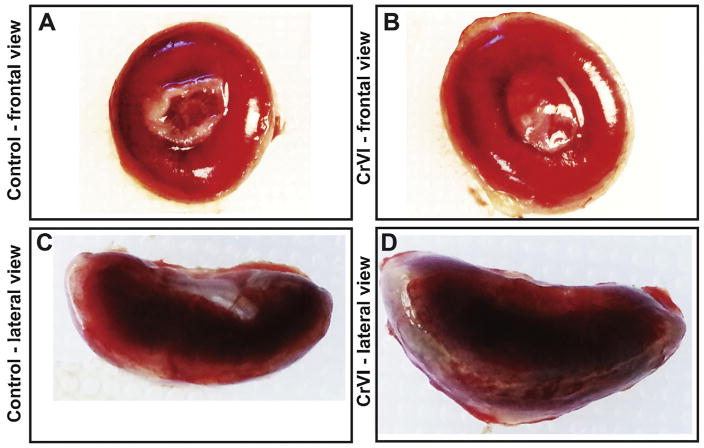
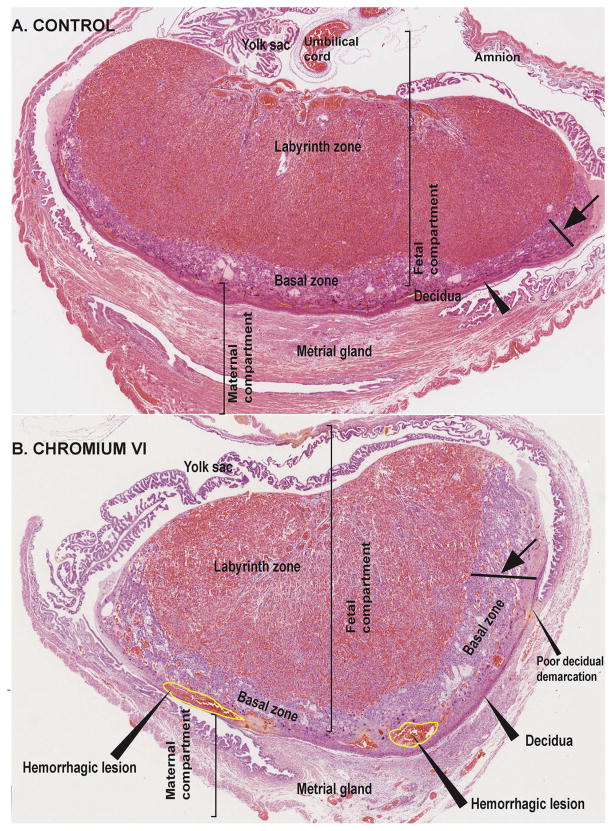
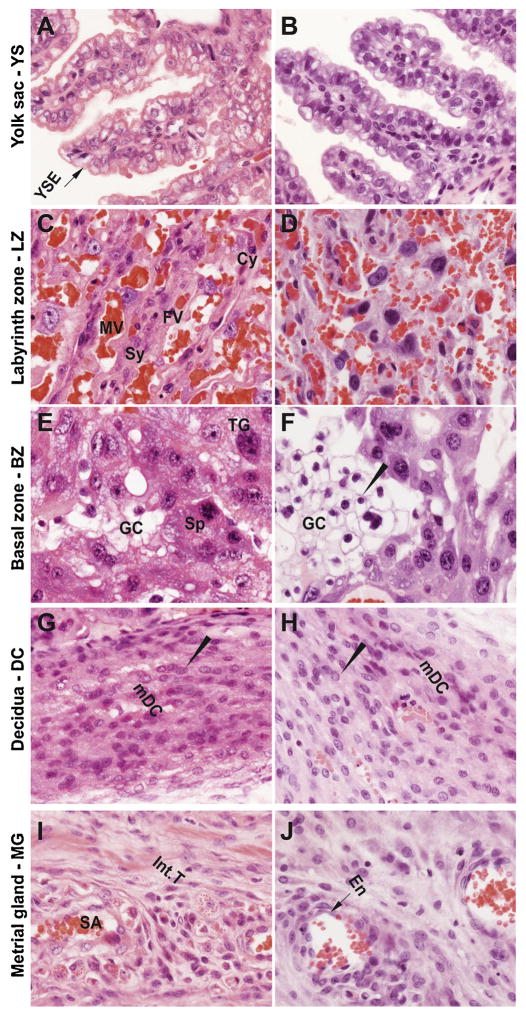
CrVI induced placental hypertrophy with an increase in placental diameter (Fig. 1B & D) and increased bazal zone (BZ) thickness compared to control (Fig. 2A & B). Within the BZ of CrVI-exposed placenta, hemorrhagic lesions were observed above the decidua which was very thin compared to control (Fig. 2B). Histopathology of the placenta showed that maternal vessels of the labyrinth zone (LZ) in CrVI-exposed mothers appeared narrow, fragmented and discontinuous with several pyknotic nuclei compared to control (Fig. 3D). Glycogen cells in the BZ were atrophied with pyknotic nuclei (Fig. 3F) compared to control.
Fig 1.
Effect of CrVI on the gross appearance of placenta on gestation day 18.5. Pregnant mothers (n = 5) received drinking water with or without CrVI (50 ppm) from gestational day (GD) 9.5–14.5, and placentas were separated on GD 18.5. Pictures of the placentas were captured after removing umbilical cord and fetuses. Frontal view (A) and lateral view (C) of the placenta from control mother; and frontal view (B) and lateral view (D) of the placenta from CrVI-treated mother are shown in the figure.
Fig. 2.
Effect of CrVI on the histopathology of whole placenta on gestation day 18.5. Pregnant mothers (n = 5) received drinking water with or without CrVI (50 ppm) from gestational day (GD) 9.5–14.5. Placentas were separated on GD 18.5 and processed for histology and sections were stained with H&E following standard protocol. H&E images of the placenta from control (A) and CrVI-treated (B) mothers, under low magnification are shown in the figure. CrVI increased hypertrophy of the basal zone (arrow); reduced the thickness of the decidual layer with poor demarcation, and caused hemorrhagic lesions above the decidual bed at the bottom layer of the basal zone, as shown in the image (arrow heads). YS – YS, LZ – labyrinth zone, BZ – basal zone and MG – MG.
Fig. 3.
Effect of CrVI on the histopathology of various zones of the placenta on gestation day 18.5. Pregnant mothers (n = 5) received drinking water with or without CrVI (50 ppm) from gestational day (GD) 9.5–14.5. Placentas were separated on GD 18.5 and processed for histology and sections were stained with H&E following standard protocol. H&E images were captured from the YS (A – control; B – CrVI), labyrinth zone (C – control; D – CrVI), basal zone (E – control; F – CrVI), decidua (G – control; H – CrVI) and MG (I – control; J – CrVI) at 400 x magnification. YSE – YS epithelium, MV – maternal vessel, FV – fetal vessel, Cy – cytotrophoblast, Sy – syncytiotrophoblast, TG – trophoblastic giant cell, GC – glycogen cell, Sp – spongiotrophoblast, mDC – mesometrial decidual cells, SA – spiral artery, En – vascular endothelial cells, Int.T – interstitial trophoblast. YS – YS, LZ – labyrinth zone, BZ – basal zone and MG – MG. Arrow heads point to individual cell types. Width of field for each image is 220 μm.
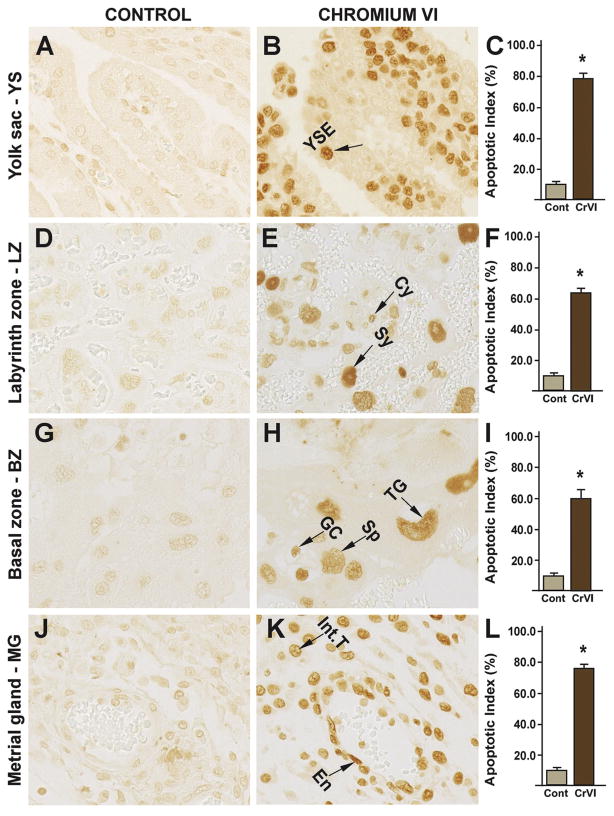
3.2. Effects of CrVI on the apoptosis of trophoblasts
CrVI significantly increased trophoblast apoptosis compared to control in: (i) yolk sac (YS) epithelium (Fig. 4B–C); (ii) cytotrophoblast and syncytiotrophoblast of the LZ (Fig. 4 E–F); (iii) spongiotrophoblast, trophoblastic giant cells and glycogen cells of the BZ (Fig. 4H–I); and (iv) endothelial cells of the spiral artery and interstitial trophoblast cells of the metrial gland (MG) (Fig. 4K–L).
Fig. 4.
Effect of CrVI on apoptosis of the placenta on gestation day 18.5. Pregnant mothers (n = 5) received drinking water with or without CrVI (50 ppm) from gestational day (GD) 9.5 – 14.5. Placentas were separated on GD 18.5 and processed for TUNEL as given in materials and methods. TUNEL images were captured from the YS (A – control; B –CrVI), labyrinth zone (D – control; E – CrVI), basal zone (G – control; H – CrVI), and MG (J – control; K – CrVI) at 400 x magnification. Histograms showing apoptotic indices are given for the respective regions of the placenta (YS – C, LZ – F, BZ – I and MG – L). YSE – YS epithelium, MV – maternal vessel, FV – fetal vessel, Cy – cytotrophoblast, Sy – syncytiotrophoblast, TG – trophoblastic giant cell, GC – glycogen cell, Sp – spongiotrophoblast, mDC – mesometrial decidual cells, SA – spiral artery, En – vascular endothelial cells, Int.T – interstitial trophoblast. YS – YS, LZ – labyrinth zone, BZ – basal zone and MG – MG. Arrow heads point to individual cell types. Each value represents mean ± SEM of 5 placentas. *Control vs CrVI, P<0.05. Width of field for each image is 220 μm.
3.3. Effects of CrVI on placental apoptosis: roles of casp-3 dependent and casp-3-independent pathways
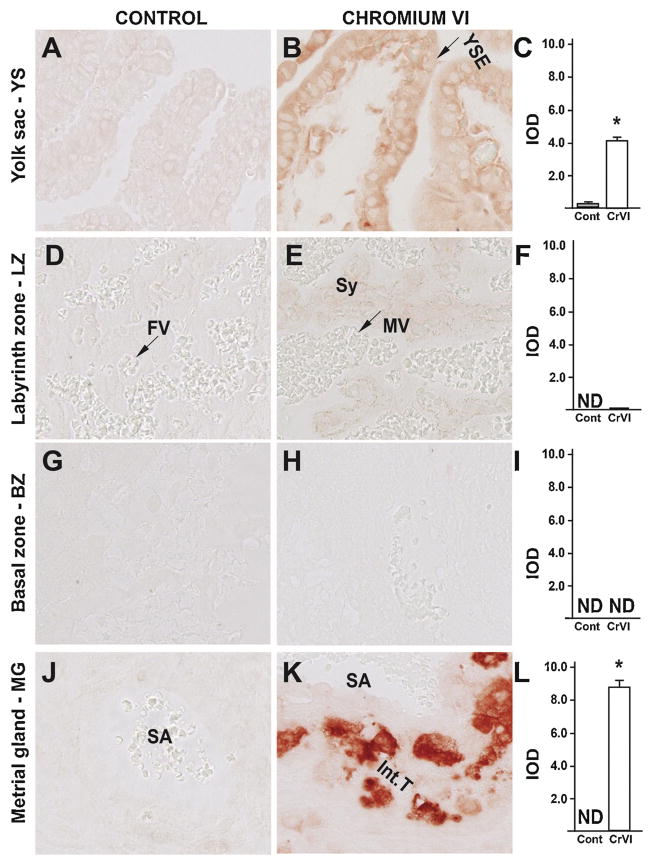
In order to understand the molecular mechanism of CrVI-induced placental apoptosis, the expression of cleaved casp-3 and Bax was examined. CrVI significantly increased expression of cleaved casp-3 in YS epithelium (Fig. 5B–C); and vascular endothelial cells of the spiral artery and interstitial trophoblast cells of the MG (Fig. 5K–L). CrVI had no significant effect on casp-3 expression in the LZ (Fig. 2D–F) or BZ (Fig. 3G–I). Casp-3 was undetectable in the LZ, BZ and MG of the control group (Fig. 5D, G and J).
Fig. 5.
Effect of CrVI on the expression of casp-3 protein in placenta on gestation day 18.5. Pregnant mothers (n = 5) received drinking water with or without CrVI (50 ppm) from gestational day (GD) 9.5 – 14.5. Placentas were separated on GD 18.5 and processed for IHC as given in materials and methods. Images were captured from the YS (A – control; B – CrVI), labyrinth zone (D – control; E – CrVI), basal zone (G – control; H – CrVI), and MG (J – control; K – CrVI) at 400 x magnification. Histograms showing integrated optical density (IOD) are given for the respective regions of the placenta (YS – C, LZ – F, BZ – I and MG – L). YSE – YS epithelium, MV – maternal vessel, FV –fetal vessel, Cy – cytotrophoblast, Sy – syncytiotrophoblast, TG – trophoblastic giant cell, GC – glycogen cell, Sp – spongiotrophoblast, mDC – mesometrial decidual cells, SA – spiral artery, En – vascular endothelial cells, Int.T – interstitial trophoblast. YS – YS, LZ – labyrinth zone, BZ – basal zone and MG – MG. Arrows point to individual cell types. Each value represents mean ± SEM of 5 placentas. *Control vs CrVI, P < 0.05. Width of field for each image is 220 μm.
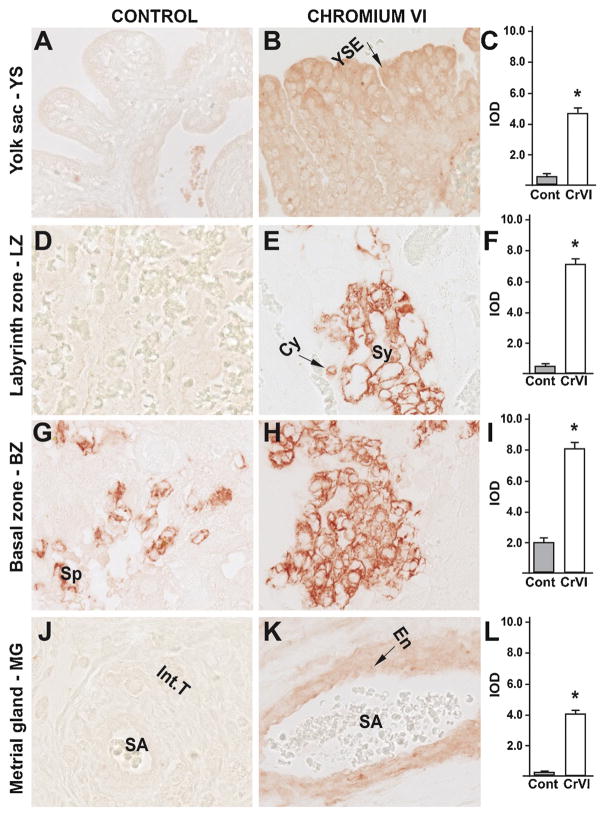
CrVI increased apoptosis of trophoblasts, YS epithelium and vascular endothelium of the MG, without changing casp-3 expression. Therefore, in order to delineate the apoptotic pathways involved, we determined the expression of apoptosis inducing factor (AIF), an important protein in the casp-3-independent apoptotic pathway. CrVI significantly increased expression of AIF in: (i) YS epithelium (Fig. 6B–C); (ii) cytotrophoblast and syncytiotrophoblast of the LZ (Fig. 6E–F); (iii) spongiotrophoblast, trophoblastic giant cells and glycogen cells of the BZ (Fig. 6H–I); and (iv) endothelial cells of the spiral artery and interstitial trophoblasts of MG (Fig. 6K–L). Thus, rVI may mediate cell death in the fetal compartment through casp-3-independent apoptosis, while it triggered both casp-3-dependent as well as casp-3-independent apoptosis in the maternal compartment. Casp-3-independent apoptosis was partly mediated through AIF.
Fig. 6.
Effect of CrVI on the expression of AIF protein in placenta on gestation day 18.5. Pregnant mothers (n = 5) received drinking water with or without CrVI (50 ppm) from gestational day (GD) 9.5 – 14.5. Placentas were separated on GD 18.5 and processed for IHC as given in materials and methods. Images were captured from the YS (A – control; B – CrVI), labyrinth zone (D – control; E – CrVI), basal zone (G – control; H – CrVI), and MG (J – control; K – CrVI) at 400 x magnification. Histograms showing integrated optical density (IOD) are given for the respective regions of the placenta (YS – C, LZ – F, BZ – I and MG – L). YSE – YS epithelium, MV – maternal vessel, FV –fetal vessel, Cy – cytotrophoblast, Sy – syncytiotrophoblast, TG – trophoblastic giant cell, GC – glycogen cell, Sp – spongiotrophoblast, mDC – mesometrial decidual cells, SA – spiral artery, En – vascular endothelial cells, Int.T – interstitial trophoblast. YS – YS, LZ – labyrinth zone, BZ – basal zone and MG – MG. Arrows point to individual cell types. Each value represents mean ± SEM of 5 placentas. *Control vs CrVI, P < 0.05. Width of field for each image is 220 μm.
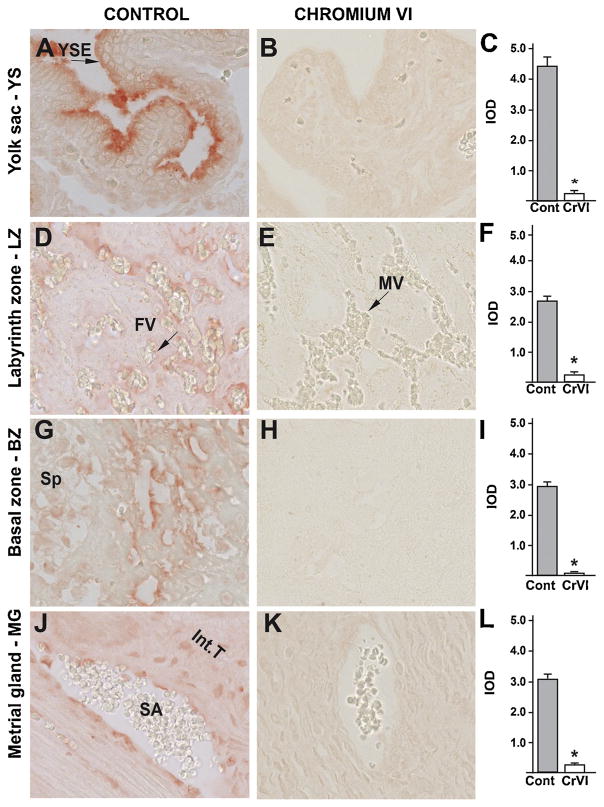
Bcl-2 family members Bcl-2, Bcl-XL, Bax and Bad proteins are the key mediators of intrinsic apoptotic pathway [66]. CrVI significantly increased expression of Bax in: (i) YS epithelium (Fig. 7B–C); (ii) cytotrophoblast and syncytiotrophoblast of the LZ (Fig. 7E–F); (iii) spongiotrophoblast and trophoblastic giant cells of the BZ (Fig. 7H–I); and (iv) endothelial cells of the spiral artery and interstitial trophoblast cells of the MG (Fig. 7K–L).
Fig. 7.
Effect of CrVI on the expression of BAX protein in placenta on gestation day 18.5. Pregnant mothers (n = 5) received drinking water with or without CrVI (50 ppm) from gestational day (GD) 9.5 – 14.5. Placentas were separated on GD 18.5 and processed for IHC as given in materials and methods. Images were captured from the YS (A – control; B – CrVI), labyrinth zone (D – control; E – CrVI), basal zone (G – control; H – CrVI), and MG (J – control; K – CrVI) at 400 x magnification. Histograms showing integrated optical density (IOD) are given for the respective regions of the placenta (YS – C, LZ – F, BZ – I and MG – L). YSE – YS epithelium, MV – maternal vessel, FV –fetal vessel, Cy – cytotrophoblast, Sy – syncytiotrophoblast, TG – trophoblastic giant cell, GC – glycogen cell, Sp – spongiotrophoblast, mDC – mesometrial decidual cells, SA – spiral artery, En – vascular endothelial cells, Int.T – interstitial trophoblast. YS – YS, LZ – labyrinth zone, BZ – basal zone and MG – MG. Arrows point to individual cell types. Each value represents mean ± SEM of 5 placentas. *Control vs CrVI, P < 0.05. Width of field for each image is 220 μm.
3.4. Effects of CrVI on the placental apoptosis: role of p53 pathway
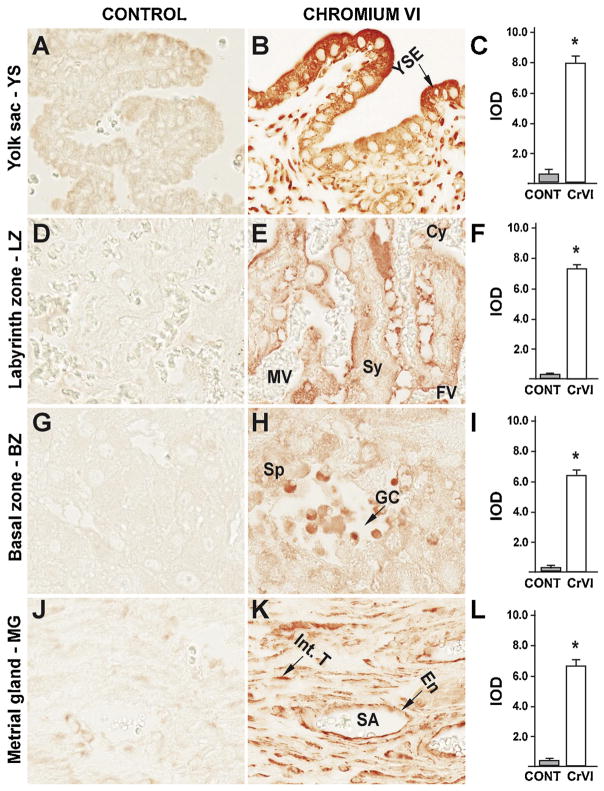
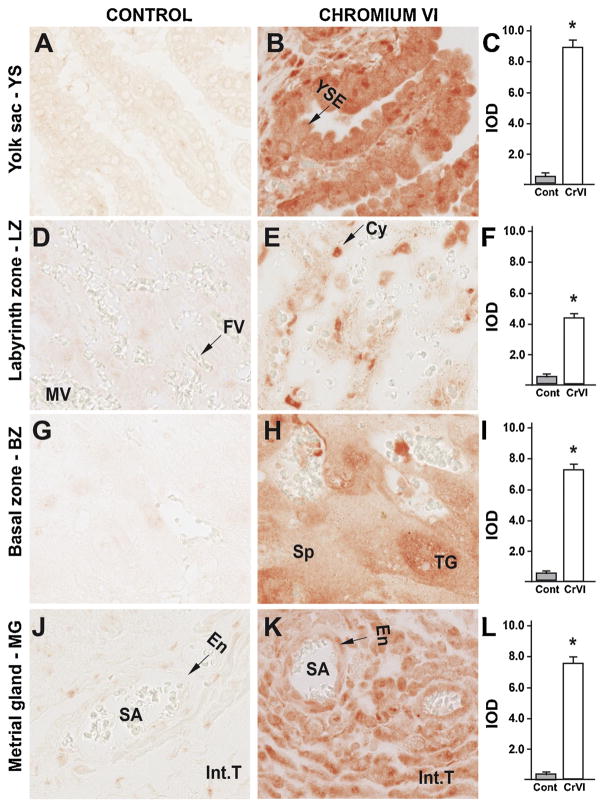
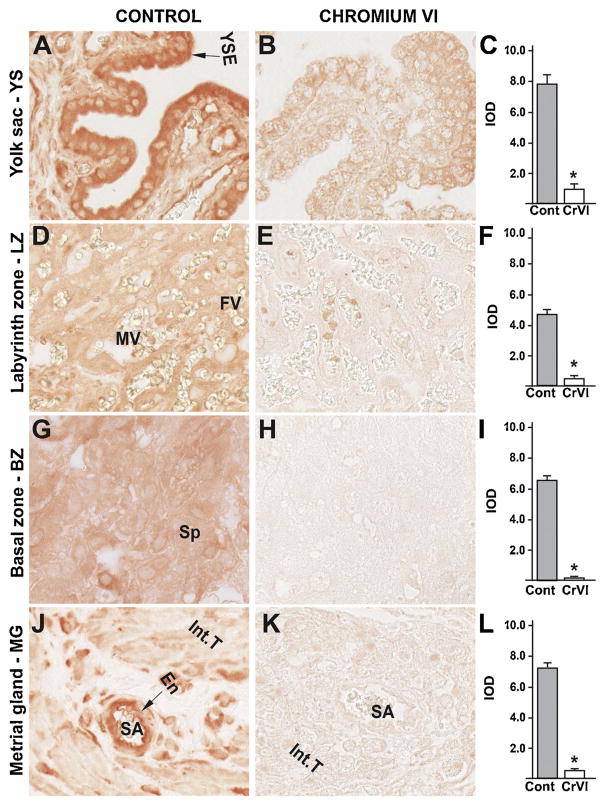
Our previous findings have documented an important role for the p53 pathway in CrVI-induced ovarian toxicity and apoptosis of the granulosa cells and the oocyte, as well as fetal ovarian germ cell death [39,61]. In order to evaluate if a similar mechanism exists in the placenta, the expression of ataxia-telangiectasia-mutated kinase (ATM), p53, and the p53-inducible proteins with apoptotic activity p27, PUMA and NOXA were evaluated. ATM is a protein kinase that phosphorylates the serine 15 residue of p53 protein. CrVI significantly increased expression of ATM in: (i) YS epithelium (Fig. 8B–C); (ii) trophoblasts of the LZ and BZ (Fig. 9E–F); (iii) spongiotrophoblast of the BZ (Fig. 8H–I); and (iv) endothelial cells of the spiral artery of the MG (Fig. 6K–L). In particular, ATM was highly up regulated in the trophoblasts of the LZ and BZ, compared to YS and MG.
Fig. 8.
Effect of CrVI on the expression of ATM protein in placenta on gestation day 18.5. Pregnant mothers (n = 5) received drinking water with or without CrVI (50 ppm) from gestational day (GD) 9.5 – 14.5. Placentas were separated on GD 18.5 and processed for IHC as given in materials and methods. Images were captured from the YS (A – control; B – CrVI), labyrinth zone (D – control; E – CrVI), basal zone (G – control; H – CrVI), and MG (J – control; K – CrVI) at 400 x magnification. Histograms showing integrated optical density (IOD) are given for the respective regions of the placenta (YS – C, LZ – F, BZ – I and MG – L). YSE – YS epithelium, MV – maternal vessel, FV –fetal vessel, Cy – cytotrophoblast, Sy – syncytiotrophoblast, TG – trophoblastic giant cell, GC – glycogen cell, Sp – spongiotrophoblast, mDC – mesometrial decidual cells, SA – spiral artery, En – vascular endothelial cells, Int.T – interstitial trophoblast. YS – YS, LZ – labyrinth zone, BZ – basal zone and MG – MG. Arrows point to individual cell types. Each value represents mean ± SEM of 5 placentas. *Control vs CrVI, P < 0.05. Width of field for each image is 220 μm.
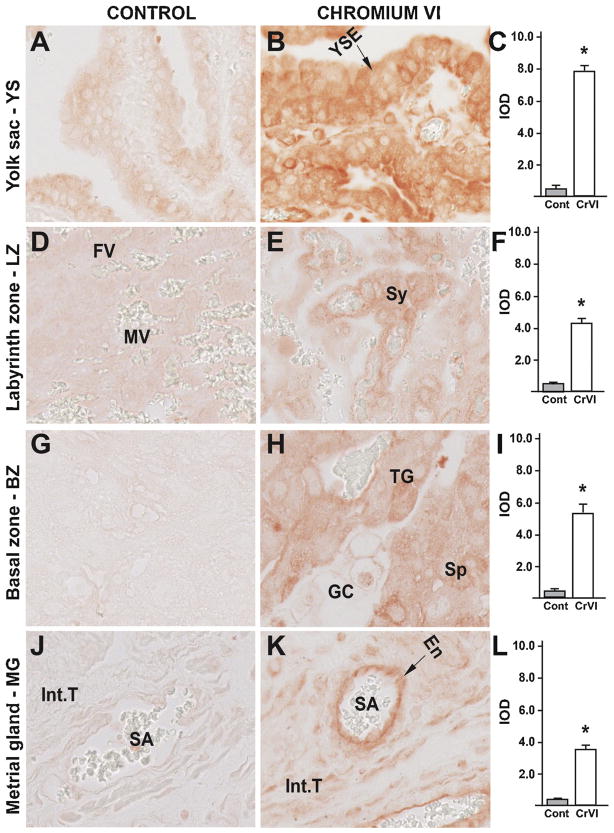
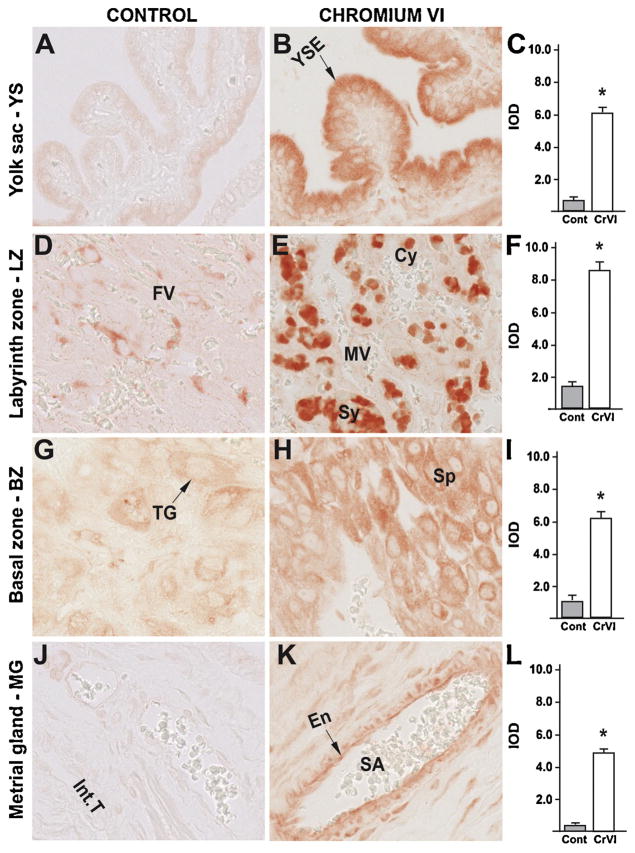
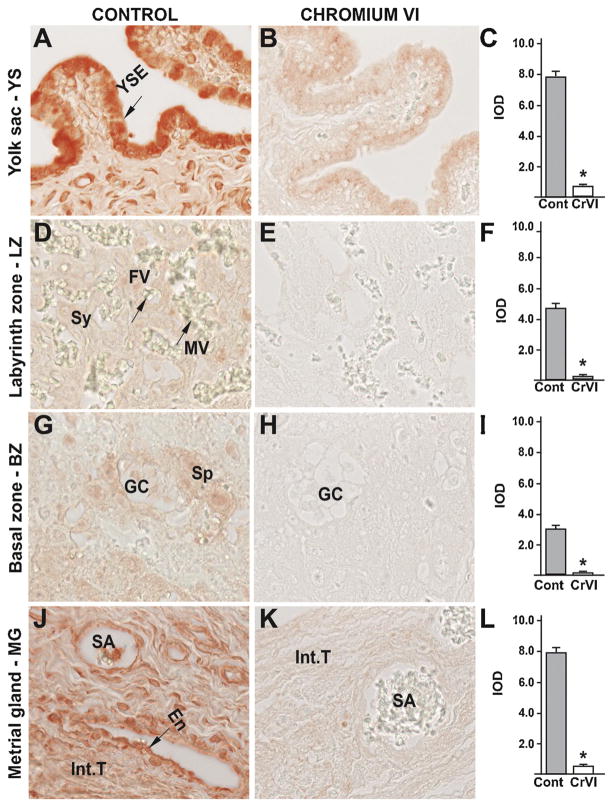
Fig. 9.
Effect of CrVI on the expression of p53 protein in placenta on gestation day 18.5. Pregnant mothers (n = 5) received drinking water with or without CrVI (50 ppm) from gestational day (GD) 9.5 – 14.5. Placentas were separated on GD 18.5 and processed for IHC as given in materials and methods. Images were captured from the YS (A – control; B – CrVI), labyrinth zone (D – control; E – CrVI), basal zone (G – control; H – CrVI), and MG (J – control; K – CrVI) at 400 x magnification. Histograms showing integrated optical density (IOD) are given for the respective regions of the placenta (YS – C, LZ – F, BZ – I and MG – L). YSE – YS epithelium, MV – maternal vessel, FV –fetal vessel, Cy – cytotrophoblast, Sy – syncytiotrophoblast, TG – trophoblastic giant cell, GC – glycogen cell, Sp – spongiotrophoblast, mDC – mesometrial decidual cells, SA – spiral artery, En – vascular endothelial cells, Int.T – interstitial trophoblast. YS – YS, LZ – labyrinth zone, BZ – basal zone and MG – MG. Arrows point to individual cell types. Each value represents mean ± SEM of 5 placentas. *Control vs CrVI, P < 0.05. Width of field for each image is 220 μm.
CrVI highly increased the expression of p53 in YS epithelium (Fig. 9B–C); trophoblasts and trophoblastic giant cells of the BZ (Fig. 9H–I); and vascular endothelial cells and interstitial trophoblasts of the spiral artery in the MG (Fig. 9K–L). CrVI, moderately, but significantly increased p53 expression in the cytotrophoblasts of the LZ (Fig. 9E–F).
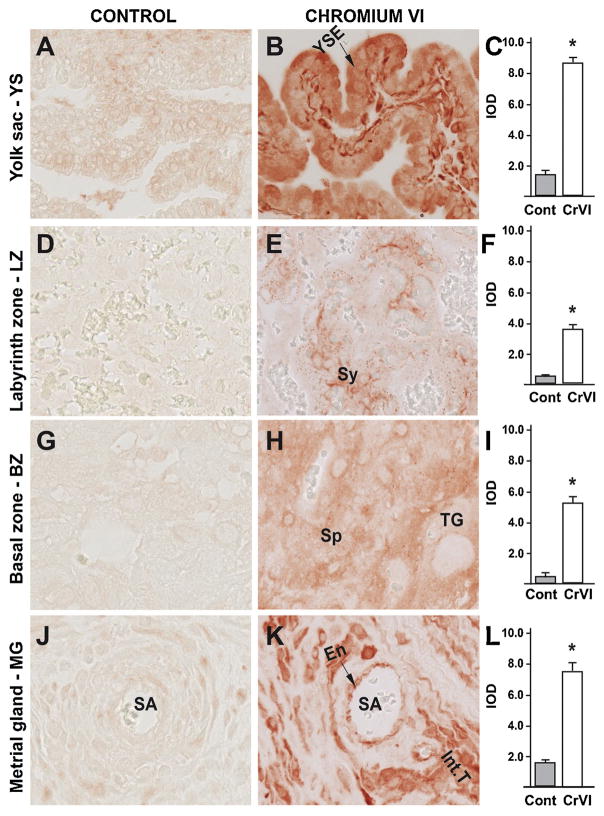
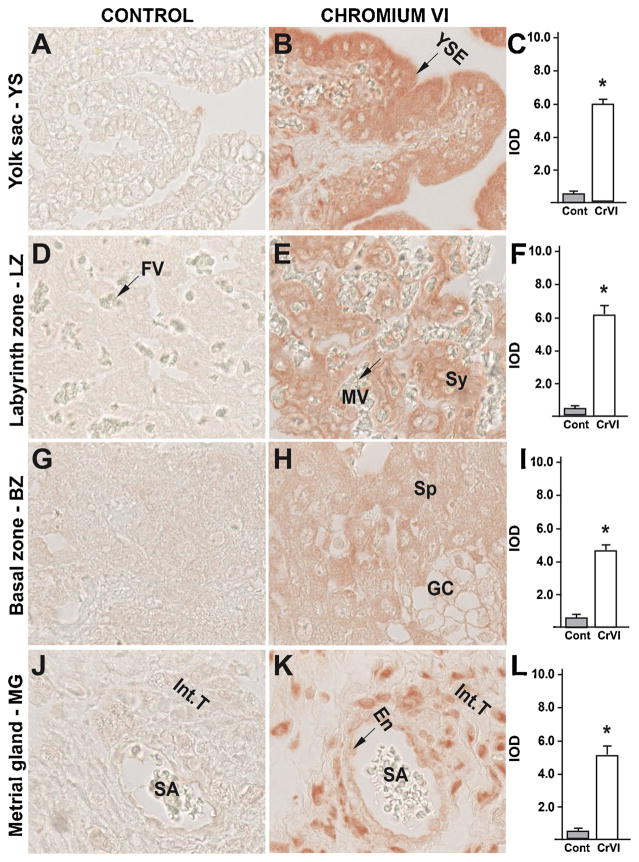
In order to study the downstream modulators of the p53 pathway in apoptosis, we studied the expression of PUMA, NOXA and p27. CrVI highly upregulated NOXA in cytotrophoblast and syn-cytiotrophoblast of LZ (Fig. 10E–F) and moderately upregulated NOXA in YS epithelium (Fig. 10B–C), in spongiotrophoblasts of BZ (Fig. 10H–I), and in vascular endothelium and interstitial trophoblasts of MG (Fig. 10K–L). CrVI highly upregulated PUMA in YS epithelium (Fig. 11B–C), vascular endothelium and interstitial trophoblasts in MG (Fig. 11K–L), moderately upregulated PUMA in spongiotrophoblasts and trophoblastic giant cells of BZ (Fig. 10H–I); and syncytiotrophoblasts of LZ (Fig. 11E–F). CrVI upregulated p27 in YS epithelium (Fig. 12B–C), syncytiotrophoblasts of LZ (Fig. 12E–F), spongiotrophoblasts in BZ (Fig. 12H–I), and vascular endothelium and interstitial trophoblasts in MG (Fig. 12K–L). Taken together, these data suggested (based on the expression level) that downstream of p53, PUMA may play an effector role in YS and MG, while NOXA play an effector role in the LZ. P27 was significantly upregulated in all four zones, and exhibited a uniform distribution. Thus, downstream effectors of p53 are spatially distinct in placental tissues.
Fig. 10.
Effect of CrVI on the expression of NOXA protein in placenta on gestation day 18.5. Pregnant mothers (n = 5) received drinking water with or without CrVI (50 ppm) from gestational day (GD) 9.5 – 14.5. Placentas were separated on GD 18.5 and processed for IHC as given in materials and methods. Images were captured from the YS (A – control; B – CrVI), labyrinth zone (D – control; E – CrVI), basal zone (G – control; H – CrVI), and MG (J – control; K – CrVI) at 400 x magnification. Histograms showing integrated optical density (IOD) are given for the respective regions of the placenta (YS – C, LZ – F, BZ – I and MG – L). YSE – YS epithelium, MV – maternal vessel, FV –fetal vessel, Cy – cytotrophoblast, Sy – syncytiotrophoblast, TG – trophoblastic giant cell, GC – glycogen cell, Sp – spongiotrophoblast, mDC – mesometrial decidual cells, SA – spiral artery, En – vascular endothelial cells, Int.T – interstitial trophoblast. YS – YS, LZ – labyrinth zone, BZ – basal zone and MG – MG. Arrows point to individual cell types. Each value represents mean ± SEM of 5 placentas. *Control vs CrVI, P < 0.05. Width of field for each image is 220 μm.
Fig. 11.
Effect of CrVI on the expression of PUMA protein in placenta on gestation day 18.5. Pregnant mothers (n = 5) received drinking water with or without CrVI (50 ppm) from gestational day (GD) 9.5 – 14.5. Placentas were separated on GD 18.5 and processed for IHC as given in materials and methods. Images were captured from the YS (A – control; B – CrVI), labyrinth zone (D – control; E – CrVI), basal zone (G – control; H – CrVI), and MG (J – control; K – CrVI) at 400 x magnification. Histograms showing integrated optical density (IOD) are given for the respective regions of the placenta (YS – C, LZ – F, BZ – I and MG – L). YSE – YS epithelium, MV – maternal vessel, FV –fetal vessel, Cy – cytotrophoblast, Sy – syncytiotrophoblast, TG – trophoblastic giant cell, GC – glycogen cell, Sp – spongiotrophoblast, mDC – mesometrial decidual cells, SA – spiral artery, En – vascular endothelial cells, Int.T – interstitial trophoblast. YS – YS, LZ – labyrinth zone, BZ – basal zone and MG – MG. Arrows point to individual cell types. Each value represents mean ± SEM of 5 placentas. *Control vs CrVI, P < 0.05. Width of field for each image is 220 μm.
Fig. 12.
Effect of CrVI on the expression of p27 protein in placenta on gestation day 18.5. Pregnant mothers (n = 5) received drinking water with or without CrVI (50 ppm) from gestational day (GD) 9.5 – 14.5. Placentas were separated on GD 18.5 and processed for IHC as given in materials and methods. Images were captured from the YS (A – control; B – CrVI), labyrinth zone (D – control; E – CrVI), basal zone (G – control; H – CrVI), and MG (J – control; K – CrVI) at 400 x magnification. Histograms showing integrated optical density (IOD) are given for the respective regions of the placenta (YS – C, LZ – F, BZ – I and MG – L). YSE – YS epithelium, MV – maternal vessel, FV –fetal vessel, Cy – cytotrophoblast, Sy – syncytiotrophoblast, TG – trophoblastic giant cell, GC – glycogen cell, Sp – spongiotrophoblast, mDC – mesometrial decidual cells, SA – spiral artery, En – vascular endothelial cells, Int.T – interstitial trophoblast. YS – YS, LZ – labyrinth zone, BZ – basal zone and MG – MG. Arrows point to individual cell types. Each value represents mean ± SEM of 5 placentas. *Control vs CrVI, P < 0.05. Width of field for each image is 220 μm.
3.5. Effects of CrVI on the expression of cell survival proteins in the placenta
Our previous findings in the ovary indicated that CrVI-induced apoptosis is not only mediated through an elevation in the expression of apoptotic protein machinery, but also through a downregulation of cell survival proteins such as Bcl-2, Bcl-XL and XIAP [47]. In order to understand the effects of CrVI on the cell survival proteins in the placenta, we localized Bcl-2, Bcl-XL and XIAP. Bcl-2 was moderately expressed in all the four zones of the placenta, namely, YS, LZ, BZ and MG (Fig. 13A, D, G & J). CrVI significantly downregulated Bcl-2 in YS (Fig. 13B & C), LZ (Fig. 13E & F), BZ (Fig. 13H & I) and MG (Fig. 13K & L). Bcl-XL was highly expressed in YS, BZ and MG (Fig. 14A, G & J), and moderately expressed in LZ (Fig. 14 D). CrVI significantly downregulated Bcl-XL in YS (Fig. 14B &C), LZ (Fig. 14E & F), BZ (Fig. 14H & I) and MG (Fig. 14K & L). XIAP was highly expressed in YS and MG (Fig. 15A & J), and moderately expressed in LZ (Fig. 15D) and BZ (Fig. 15G). CrVI significantly downregulated XIAP in YS (Fig. 15B & C), LZ (Fig. 15E & F), BZ (Fig. 15H & I) and MG (Fig. 15K & L).
Fig. 13.
Effect of CrVI on the expression of Bcl-2 protein in placenta on gestation day 18.5. Pregnant mothers (n = 5) received drinking water with or without CrVI (50 ppm) from gestational day (GD) 9.5 – 14.5. Placentas were separated on GD 18.5 and processed for IHC as given in materials and methods. Images were captured from the YS (A – control; B – CrVI), labyrinth zone (D – control; E – CrVI), basal zone (G – control; H – CrVI), and MG (J – control; K – CrVI) at 400 x magnification. Histograms showing integrated optical density (IOD) are given for the respective regions of the placenta (YS – C, LZ – F, BZ – I and MG – L). YSE – YS epithelium, MV – maternal vessel, FV –fetal vessel, Cy – cytotrophoblast, Sy – syncytiotrophoblast, TG – trophoblastic giant cell, GC – glycogen cell, Sp – spongiotrophoblast, mDC – mesometrial decidual cells, SA – spiral artery, En – vascular endothelial cells, Int.T – interstitial trophoblast. YS – YS, LZ – labyrinth zone, BZ – basal zone and MG – MG. Arrows point to individual cell types. Each value represents mean ± SEM of 5 placentas. *Control vs CrVI, P < 0.05. Width of field for each image is 220 μm.
Fig. 14.
Effect of CrVI on the expression of Bcl-XL protein in placenta on gestation day 18.5. Pregnant mothers (n = 5) received drinking water with or without CrVI (50 ppm) from gestational day (GD) 9.5 – 14.5. Placentas were separated on GD 18.5 and processed for IHC as given in materials and methods. Images were captured from the YS (A – control; B – CrVI), labyrinth zone (D – control; E – CrVI), basal zone (G – control; H – CrVI), and MG (J – control; K – CrVI) at 400 x magnification. Histograms showing integrated optical density (IOD) are given for the respective regions of the placenta (YS – C, LZ – F, BZ – I and MG – L). YSE – YS epithelium, MV – maternal vessel, FV –fetal vessel, Cy – cytotrophoblast, Sy – syncytiotrophoblast, TG – trophoblastic giant cell, GC – glycogen cell, Sp – spongiotrophoblast, mDC – mesometrial decidual cells, SA – spiral artery, En – vascular endothelial cells, Int.T – interstitial trophoblast. YS – YS, LZ – labyrinth zone, BZ – basal zone and MG – MG. Arrows point to individual cell types. Each value represents mean ± SEM of 5 placentas. *Control vs CrVI, P < 0.05. Width of field for each image is 220 μm.
Fig. 15.
Effect of CrVI on the expression of XIAP protein in placenta on gestation day 18.5. Pregnant mothers (n = 5) received drinking water with or without CrVI (50 ppm) from gestational day (GD) 9.5 – 14.5. Placentas were separated on GD 18.5 and processed for IHC as given in materials and methods. Images were captured from the YS (A – control; B – CrVI), labyrinth zone (D – control; E – CrVI), basal zone (G – control; H – CrVI), and MG (J – control; K – CrVI) at 400 x magnification. Histograms showing integrated optical density (IOD) are given for the respective regions of the placenta (YS – C, LZ – F, BZ – I and MG – L). YSE – YS epithelium, MV – maternal vessel, FV –fetal vessel, Cy – cytotrophoblast, Sy – syncytiotrophoblast, TG – trophoblastic giant cell, GC – glycogen cell, Sp – spongiotrophoblast, mDC – mesometrial decidual cells, SA – spiral artery, En – vascular endothelial cells, Int.T – interstitial trophoblast. YS – YS, LZ – labyrinth zone, BZ – basal zone and MG – MG. Arrows point to individual cell types. Each value represents mean ± SEM of 5 placentas. *Control vs CrVI, P < 0.05. Width of field for each image is 220 μm.
4. Discussion
Most adverse pregnancy outcomes can trace their origin to the placenta. Preeclampsia and IUGR are disorders that are rooted in defects of placental development [67]. The current study shows that CrVI induces toxic lesions in the LZ and BZ of the placenta. Several EDCs have been reported to cause lesions in the placenta such as ketoconazole (hypertrophy) [68], cadmium and lead (necrosis) [69], 6-mercaptopurine (cystic degeneration of glycogen cells) [70], and busulfan (apoptosis of trophoblasts and endothelial cells) [22] [71]. CrVI increased hypertrophy of the BZ and induced hem- orrhagic lesions in BZ above the decidual bed (Fig. 2B). Decidual diameter was decreased in certain regions. Placental hypertrophy has been reported as a compensatory reaction to intrauterine growth restriction (IUGR) under an unfavorable maternal environment such as maternal hemorrhage [72], uterine vessel ligation [73], and indomethacin [74] or ethanol exposure [75].
Ultrastructure of the endoderm cells of the visceral YS epithelium, with its apical invaginations and, vesicles revealed that they are highly specialized for absorption of substances from the uterine lumen [76]. Thus, YS is an important organ of maternal-embryonic exchange of amino acids [77], transferrin [78], vitamin B12 [79], calcium [80] and other ions [81]. In rodents, visceral YS is also the route by which immunoglobulins are passed to the embryo [82]. Agents that cause visceral YS dysfunction during organogenesis can result in embryo toxicity and its damage leads to embryonic malformation [83]. Rodent YS also produces cholesterol de novo [84,85] besides the transfer of maternal cholesterol [86]. Cholesterol is an essential component of development, including cell membrane composition affecting signaling cascades, steroidogenesis and development of the central nervous system [86,87]. Thus the role of YS is critical throughout gestation, influencing implantation as well as embryonic and fetal growth [81]. CrVI increased apoptosis of the YS by upregulating casp-3, Bax and AIF. Thus, our data suggests that CrVI-induced cell death in YS may be mediated through casp-3-dependent and –independent pathways. CrVI also decreased cell survival proteins Bcl2, Bcl-XL, and XIAP in YS.
Placental apoptosis is increased in spontaneous abortion, preeclamptic pregnancy and pregnancy with IUGR [88,89]. Epidemiological data indicate that women working in Cr industries or living in Cr-contaminated environments suffer from several gynecological complications including spontaneous abortion [17,18,90]. Occupational exposure to CrVI during pregnancy decreased intrauterine growth of the fetuses, resulting in low birth weight [91]. Therefore, in order to understand the effects of CrVI on the placenta, we evaluated placental apoptosis as well as expression of apoptotic proteins. CrVI increased apoptosis in YS, LZ, BZ and MG. CrVI increased cleaved casp-3 only in the YS and MG, and not in the LZ and BZ. Therefore, we further evaluated the expression of AIF which mediates apoptosis through a casp-3-independent pathway. CrVI upregulated AIF in YS, MG, LZ and BZ. These data indicate that CrVI induced apoptosis through casp-3-dependent and/or casp-3-independent mechanisms in a spatiotemporal manner, resulting in a region-dependent response to CrVI toxicity. Such a trend in placental tissue has previously been reported by several authors under various toxic, physiological and pathological conditions across mammalian species [22,70,89,92,93].
In vertebrate animals, cellular responses to aberrations of DNA structure and replication are initiated by activation of DNA damage transducers such as ATM, ataxia-telangiectasia and Rad3 related (ATR), and DNA-dependent protein kinase catalytic subunit (DNA-PKCs), which phosphorylate a number of cell cycle checkpoint and effector proteins [94,95]. The serine 15 residue of p53 protein is a target of the ATM and ATR kinases, in addition to various protein kinases that can also phosphorylate p53 protein in response to diverse forms of cellular stress [43,96]. Our data showed that CrVI significantly increased protein levels of ATM and p53; and the downstream regulators of p53 such as NOXA, PUMA and p27. It is suggested that CrVI may have induced apoptosis of YS through three key pathways: (i) ATM-p53-PUMA-NOXA-p27-mediated pathway, (ii) casp-3-dependent pathway, and (iii) casp-3-independent pathway through AIF. However, further mechanistic studies are needed to prove these pathways.
Trophoblasts of the placenta are known as the common target for placental toxicity [22]. Trophoblasts are highly proliferative and constitute the major structural component of the placenta, as shown in Fig. 3. Several EDCs and anticancer drugs are known to increase trophoblast apoptosis [53,54]. In the current study, CrVI increased apoptosis of cytotrophoblasts and syncytiotrophoblasts of the LZ; and spongiotrophoblasts and trophoblastic giant cells of the BZ. Interestingly, CrVI-induced apoptosis in the LZ and BZ was casp-3-independent as well as p53-dependent. In order to understand the p53 downstream pathway we determined NOXA, PUMA and p27. Even though all the three proteins were upregulated by CrVI, there was a region-specific variation in terms of abundance of these proteins: expression of NOXA was very high in LZ, while PUMA was significantly high in BZ, and p27 expression was moderately upregulated in both LZ and BZ. Thus, even though CrVI upregulated p53, the downstream effectors that mediate apoptosis seem to be region and cell type-specific.
In the rat placenta, there is an increase in the size of the cytotrophoblast cells in the LZ zone over gestation. By E18.5, most of the cytotrophoblast cells have partially transformed into trophoblastic giant (TG) cells [97] which express placental lactogen II [98]. On the other hand, basement membranes of trophoblasts and fetal capillaries are closely apposed and may even fuse together [99]. Within the LZ, fetal and maternal blood travel in opposite directions, in order to facilitate and maximize the umbilical venous oxygen delivery to the fetus. Factors from the trophoblast cells in the LZ regulate feto-placental vascular development [100]. In the mouse placenta, differentiated glycogen cells support breakdown of glycogen to glucose [101], providing embryonic cells with an energy source [99]. Thus, trophoblasts and glycogen cells are critical for maintaining placental growth, circulation, differentiation and function, ultimately facilitating the survival of the fetus. CrVI increased fragmentation of maternal blood vessels in BZ (Fig. 3), cytolysis of glycogen cells, and apoptosis of the trophoblasts with a zone specific alterations in apoptotic machinery.
Further, we examined the effects of CrVI on the MG. MGs are aggregates of heterogeneous tissue that develop in the mesometrial triangle in the uterine wall of rats and mice during pregnancy [22]. These glands are important in fetal growth and successful placentation [102,103]. CrVI increased apoptosis of the interstitial trophoblasts cells and endothelial cells of the spiral artery and apoptosis of uNK cells by up regulating casp-3, AIF and Bax. Unlike in the LZ and BZ, CrVI increased apoptosis of the MG through casp-3-dependent as well as –independent mechanisms. CrVI downregulated XIAP, Bcl-XL and Bcl2 in MG. In addition, CrVI significantly upregulated ATM and p53 in MG. Even though ATM phosphorylates p53, there are several kinases activates p53 by phosphorylation based on the cellular stress and DNA damage as well as other toxic responses [104,105]. We suggest that p53 could be upregulated by various kinases in a region-specific manner. Moreover, p53 downstream candidates PUMA, NOXA and p27 showed a spatiotemporal expression pattern.
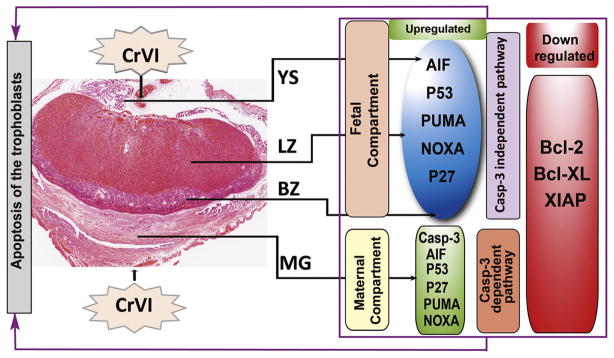
Thus the effects of CrVI on the cell survival and apoptotic pathway effector proteins vary in the maternal vs the fetal compartments. Based on the literature, the pathogenesis of placental lesions show various complex features, because the constitutive cells of the placenta originate from embryonic and maternal tissue; and induced lesions are histopathologically different depending upon the toxicant and exposure period. Taken together, as illustrated in Fig. 16, data from the current study revealed that CrVI upregulated proapoptotic proteins such as casp-3, AIF, Bax, and ATM-p53-Noxa-PUMA-p27 network, specific to the region in the placenta. Our ongoing studies are expected to reveal more molecular mechanism(s) of CrVI on the multifaceted development and function of the placenta, as well as on the phenotype of different cells within the uteroplacental compartment.
Fig. 16.
Schematic diagram of the effects of CrVI on the expression of various apoptotic and cell survival proteins in placenta on gestation day 18.5. Briefly, CrVI induced apoptosis of the trophoblasts in the fetal compartments (LZ and BZ) through a casp-3-independent and AIF/p53-dependent mechanisms. Whereas, CrVI induced apoptosis in the maternal compartment through a casp-3-dependent and AIF/p53-dependent mechanisms.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Environmental Health Sciences (NIEHS) grants ES020561-01 and ES025234-01A1 (S.K.B.) and the Center for Translational Environmental Health Research (CTEHR, P30ES023512) and CTEHR pilot project grant (S.K.B., R.C.B). Microscopy was performed in the Texas A&M University College of Veterinary Medicine & Biomedical Sciences Image Analysis Laboratory, supported by an NIH-NCRR Shared Instrumentation Grant (R.C.B.) (1 S10 RR22532-01).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.reprotox.2016.07.006.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol Metab TEM. 2002;13:364–368. doi: 10.1016/s1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- 3.Ward AM, Syddall HE, Wood PJ, Chrousos GP, Phillips DI. Fetal programming of the hypothalamic-pituitary-adrenal (HPA) axis: low birth weight and central HPA regulation. J Clin Endocrinol Metab. 2004;89:1227–1233. doi: 10.1210/jc.2003-030978. [DOI] [PubMed] [Google Scholar]
- 4.Banu SK. Heavy metals and the ovary. In: Hoyer PB, editor. Ovarian Toxicology. 2. CRC Press; 2013. pp. 191–228. [Google Scholar]
- 5.Barceloux DG. Chromium. J Toxicol Clin Toxicol. 1999;37:173–194. doi: 10.1081/clt-100102418. [DOI] [PubMed] [Google Scholar]
- 6.Shanker AK, Venkateswarlu B. Chromium Environmental Pollution, Health Effects and Mode of Action. In: Nriagu JO, editor. Encyclopedia of Environmental Health. Elsevier Publishers; 2011. [Google Scholar]
- 7.Pellerin C, Booker SM. Reflections on hexavalent chromium: health hazards of an industrial heavyweight. Environ Health Perspect. 2000;108:A402–7. doi: 10.1289/ehp.108-a402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutton R. In: Chromium-6 in U S Tap Water. Houlihan J, Sharp R, Bruzelius N, editors. Washington, DC: 2010. pp. 1–23. [Google Scholar]
- 9.Malott V. EPA, editor. West County Road 112 Ground Water Plume. Environmental Protection Agency; 2014. pp. 1–7. [Google Scholar]
- 10.USEPA, National Primary Drinking Water Regulations. EPA 816-F-09-004. United States Environmental Protection Agency; Washington, D.C: 2009. pp. 1–6. [Google Scholar]
- 11.OSHA. Occupational exposure to hexavalent chromium Final rule. Federal register. 2006;71:10099–10385. [PubMed] [Google Scholar]
- 12.Zhitkovich A. Chromium Exposure, Toxicity and Biomonitoring Approaches, in Biomarkers of Environmentally Associated Disease: Technologies, Concepts, and Perspectives. In: Wilson SH, Suk WA, editors. Biomarkers of environmentally associated disease: Technologies, concepts, and perspectives. CRC Press; New York: 2002. pp. 269–287. [Google Scholar]
- 13.Shmitova LA. Content of hexavalent chromium in the biological substrates of pregnant women and puerperae engaged in the manufacture of chromium compounds. Gig Tr Prof Zabol. 1980:33–35. [PubMed] [Google Scholar]
- 14.Li Y, Xu X, Liu J, Wu K, Gu C, Shao G, et al. The hazard of chromium exposure to neonates in Guiyu of China. Sci Total Environ. 2008;403:99–104. doi: 10.1016/j.scitotenv.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 15.Li-min R. Effect of hexavalent chromium on reproductive system in exposing workers. Mod Med Health. 2011;8:1–8. [Google Scholar]
- 16.Greene LE, Riederer AM, Marcus M, Lkhasuren O. Associations of fertility and pregnancy outcomes with leather tannery work in Mongolia. Int J Occup Environ Health. 2010;16:60–68. doi: 10.1179/107735210800546100. [DOI] [PubMed] [Google Scholar]
- 17.Hemminki K, Kyyronen P, Niemi ML, Koskinen K, Sallmen M, Vainio H. Spontaneous abortions in an industrialized community in Finland. Am J Public Health. 1983;73:32–37. doi: 10.2105/ajph.73.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Liu H, Xiang XH, Liu FY. Outline of occupational chromium poisoning in China. Bull Environ Contam Toxicol. 2013;90:742–749. doi: 10.1007/s00128-013-0998-3. [DOI] [PubMed] [Google Scholar]
- 19.Stern RM, Berlin A, Fletcher A, Hemminki K, Jarvisalo J, Peto J. International conference on health hazards and biological effects of welding fumes and gases. Int Arch Occup Environ Health. 1986;57:237–246. [Google Scholar]
- 20.Das AP, Mishra S. Hexavalent chromium (VI): Environment pollutant and health hazard. J Environ Res Dev. 2008;2 [Google Scholar]
- 21.Quansah R, Jaakkola JJK. Paternal and maternal exposure to welding fumes and metal dusts or fumes and adverse pregnancy outcomes. Int Arch Occup Environ Health. 2008;82:529–537. doi: 10.1007/s00420-008-0349-6. [DOI] [PubMed] [Google Scholar]
- 22.Furukawa S, Hayashi S, Usuda K, Abe M, Hagio S, Ogawa I. Toxicological pathology in the rat placenta. J Toxicol Pathol. 2011;24:95–111. doi: 10.1293/tox.24.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burton GJ, Fowden AL. The placenta: a multifaceted, transient organ. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140066. doi: 10.1098/rstb.2014.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauer MK, Harding JE, Bassett NS, Breier BH, Oliver MH, Gallaher BH, et al. Fetal growth and placental function. Mol Cell Endocrinol. 1998;140:115–120. doi: 10.1016/s0303-7207(98)00039-2. [DOI] [PubMed] [Google Scholar]
- 25.Pijnenborg R, Bland JM, Robertson WB, Dixon G, Brosens I. The pattern of interstitial trophoblastic invasion of the myometrium in early human pregnancy. Placenta. 1981;2:303–316. doi: 10.1016/s0143-4004(81)80027-6. [DOI] [PubMed] [Google Scholar]
- 26.Soares MJ, Chakraborty D, Rumi MAK, Konno T, Renaud SJ. Rat placentation: an experimental model for investigating the hemochorial maternal-fetal interface. Placenta. 2012;33:233–243. doi: 10.1016/j.placenta.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Rijk EP, Van Esch E, Flik G. Pregnancy dating in the rat: placental morphology and maternal blood parameters. Toxicol Pathol. 2002;30:271–282. doi: 10.1080/019262302753559614. [DOI] [PubMed] [Google Scholar]
- 28.Chauhan M, Yallampalli U, Reed L, Yallampalli C. Adrenomedullin 2 antagonist infusion to rats during midgestation causes fetoplacental growth restriction through apoptosis. Biol Reprod. 2006;75:940–947. doi: 10.1095/biolreprod.106.053322. [DOI] [PubMed] [Google Scholar]
- 29.Erboga M, Kanter M. Effect of cadmium on trophoblast cell proliferation and apoptosis in different gestation periods of rat placenta. Biol Trace Elem Res. 2016;169:285–293. doi: 10.1007/s12011-015-0439-8. [DOI] [PubMed] [Google Scholar]
- 30.Halperin R, Peller S, Rotschild M, Bukovsky I, Schneider D. Placental apoptosis in normal and abnormal pregnancies. Gynecol Obstet Invest. 2000;50:84–87. doi: 10.1159/000010287. [DOI] [PubMed] [Google Scholar]
- 31.Jurisicova A, Detmar J, Caniggia I. Molecular mechanisms of trophoblast survival: from implantation to birth Birth defects research Part C. Embryo today: reviews. 2005;75:262–280. doi: 10.1002/bdrc.20053. [DOI] [PubMed] [Google Scholar]
- 32.Levy R, Nelson DM. To be, or not to be, that is the question. Apoptosis in human trophoblast. Placenta. 2000;21:1–13. doi: 10.1053/plac.1999.0450. [DOI] [PubMed] [Google Scholar]
- 33.Mu J, Kanzaki T, Tomimatsu T, Fukuda H, Wasada K, Fujii E, et al. Expression of apoptosis in placentae from mice lacking the prostaglandin F receptor. Placenta. 2002;23:215–223. doi: 10.1053/plac.2001.0759. [DOI] [PubMed] [Google Scholar]
- 34.Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol. 2002;186:158–166. doi: 10.1067/mob.2002.119176. [DOI] [PubMed] [Google Scholar]
- 35.Huppertz B, Kingdom JC. Apoptosis in the trophoblast–role of apoptosis in placental morphogenesis. J Soc Gynecol Investig. 2004;11:353–362. doi: 10.1016/j.jsgi.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Wang H, Xu ZM, Ji YL, Chen YH, Zhang ZH, et al. Cadmium-induced teratogenicity: association with ROS-mediated endoplasmic reticulum stress in placenta. Toxicol Appl Pharmacol. 2012;259:236–247. doi: 10.1016/j.taap.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- 38.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 39.Banu SK, Stanley JA, Lee J, Stephen SD, Arosh JA, Hoyer PB, et al. Hexavalent chromium-induced apoptosis of granulosa cells involves selective sub-cellular translocation of Bcl-2 members, ERK1/2 and p53. Toxicol Appl Pharmacol. 2011;251:253–266. doi: 10.1016/j.taap.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haupt S, Berger M, Goldberg Z, Haupt Y. Apoptosis-the p53 network. J Cell Sci. 2003;116:4077–4085. doi: 10.1242/jcs.00739. [DOI] [PubMed] [Google Scholar]
- 41.Toshiyuki M, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 42.Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22:9030–9040. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]
- 43.Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 44.Chipuk JE, Green DR. Dissecting p53-dependent apoptosis. Cell Death Differ. 2006;13:994–1002. doi: 10.1038/sj.cdd.4401908. [DOI] [PubMed] [Google Scholar]
- 45.D’Sa-Eipper C, Leonard JR, Putcha G, Zheng TS, Flavell RA, Rakic P, et al. DNA damage-induced neural precursor cell apoptosis requires p53 and caspase 9 but neither Bax nor caspase 3. Development (Cambridge, England) 2001;128:137–146. doi: 10.1242/dev.128.1.137. [DOI] [PubMed] [Google Scholar]
- 46.Norbury CJ, Zhivotovsky B. DNA damage-induced apoptosis. Oncogene. 2004;23:2797–2808. doi: 10.1038/sj.onc.1207532. [DOI] [PubMed] [Google Scholar]
- 47.Sivakumar KK, Stanley JA, Arosh JA, Pepling ME, Burghardt RC, Banu SK. Prenatal exposure to chromium induces early reproductive senescence by increasing germ cell apoptosis and advancing germ cell cyst breakdown in the F1 offspring. Dev Biol. 2014;388:22–34. doi: 10.1016/j.ydbio.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdul-Mohaymen N, Asmaa’B A-O, Rasheed T. High expression of P53 protein in recurrent pregnancy loss could play a role in the pathology. J Fac Med Baghdad. 2011;53:194–198. [Google Scholar]
- 49.Fulop V, Mok SC, Genest DR, Gati I, Doszpod J, Berkowitz RS. p53, p21, Rb and mdm2 oncoproteins Expression in normal placenta, partial and complete mole, and choriocarcinoma. J Reprod Med. 1998;43:119–127. [PubMed] [Google Scholar]
- 50.Qiao S, Nagasaka T, Harada T, Nakashima N. p53, Bax and Bcl-2 expression, and apoptosis in gestational trophoblast of complete hydatidiform mole. Placenta. 1998;19:361–369. doi: 10.1016/s0143-4004(98)90075-3. [DOI] [PubMed] [Google Scholar]
- 51.Levy R, Smith SD, Yusuf K, Huettner PC, Kraus FT, Sadovsky Y, et al. Trophoblast apoptosis from pregnancies complicated by fetal growth restriction is associated with enhanced p53 expression. Am J Obstet Gynecol. 2002;186:1056–1061. doi: 10.1067/mob.2002.122250. [DOI] [PubMed] [Google Scholar]
- 52.Hu C, Smith SD, Pang L, Sadovsky Y, Nelson DM. Enhanced basal apoptosis in cultured term human cytotrophoblasts is associated with a higher expression and physical interaction of p53 and Bak. Placenta. 2006;27:978–983. doi: 10.1016/j.placenta.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Katayama K, Ueno M, Takai H, Ejiri N, Uetsuka K, Nakayama H, et al. Ethylnitrosourea induces apoptosis and growth arrest in the trophoblastic cells of rat placenta. Biol Reprod. 2002;67:431–435. doi: 10.1095/biolreprod67.2.431. [DOI] [PubMed] [Google Scholar]
- 54.Yamauchi H, Katayama K, Ueno M, Uetsuka K, Nakayama H, Doi K. Involvement of p53 in 1-beta-D-arabinofuranosylcytosine-induced trophoblastic cell apoptosis and impaired proliferation in rat placenta. Biol Reprod. 2004;70:1762–1767. doi: 10.1095/biolreprod.103.026252. [DOI] [PubMed] [Google Scholar]
- 55.Wise SS, Wise JP., Sr Chromium and genomic stability. Mutat Res. 2012;733:78–82. doi: 10.1016/j.mrfmmm.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma P, Bihari V, Agarwal SK, Verma V, Kesavachandran CN, Pangtey BS, et al. Groundwater contaminated with hexavalent chromium [Cr (VI)]: a health survey and clinical examination of community inhabitants (Kanpur, India) PLoS One. 2012;7:e47877. doi: 10.1371/journal.pone.0047877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Armienta-Hernandez A, Rodriguez-Castillo R. Environmental exposure to chromium compounds in the valley of Leon, Mexico. Environ Health Perspect. 1995;103:47–51. doi: 10.1289/ehp.103-1519325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rao GT, Rao VG, Ranganathan K, Surinaidu L, Mahesh J, Ramesh G. Assessment of groundwater contamination from a hazardous dump site in Ranipet, Tamil Nadu, India. Hydrol J. 2011;19:1587–1598. [Google Scholar]
- 59.Dubey C, Sahoo B, Nayak N. Chromium (VI) in waters in parts of Sukinda chromite valley and health hazards, Orissa, India. Bull Environ Contam Toxicol. 2001;67:541–548. doi: 10.1007/s001280157. [DOI] [PubMed] [Google Scholar]
- 60.Banu SK, Stanley JA, Sivakumar KK, Arosh JA, Barhoumi R, Burghardt RC. Identifying a novel role for X-prolyl aminopeptidase (Xpnpep) 2 in CrVI-induced adverse effects on germ cell nest breakdown and follicle development in rats. Biol Reprod. 2015;92:1–18. doi: 10.1095/biolreprod.114.125708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stanley JA, Arosh JA, Burghardt RC, Banu SK. A fetal whole ovarian culture model for the evaluation of CrVI-induced developmental toxicity during germ cell nest breakdown. Toxicol Appl Pharmacol. 2015;289:58–69. doi: 10.1016/j.taap.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Banu SK, Lee J, Stephen SD, Nithy TK, Arosh JA. Interferon tau regulates PGF2α release from the ovine endometrial epithelial cells via activation of novel JAK/EGFR/ERK/EGR-1 pathways. Mol Endocrinol. 2010;24:2315–2330. doi: 10.1210/me.2010-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Furukawa S, Kuroda Y, Sugiyama A. A comparison of the histological structure of the placenta in experimental animals. J Toxicol Pathol. 2014;27:11–18. doi: 10.1293/tox.2013-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Furukawa S, Hayashi S, Abe M, Hagio S, Irie K, Kuroda Y, et al. Background data on developmental parameters during the gestation period in rats. J Toxicol Pathol. 2013;26:83–88. doi: 10.1293/tox.26.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fonseca BM, Correia-da-Silva G, Teixeira NA. The rat as an animal model for fetoplacental development: a reappraisal of the post-implantation period. Reprod Biol. 2012;12:97–118. doi: 10.1016/s1642-431x(12)60080-1. [DOI] [PubMed] [Google Scholar]
- 66.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 67.Ilekis JV, Tsilou E, Fisher S, Abrahams VM, Soares MJ, Cross JC, et al. Placental origins of adverse pregnancy outcomes: potential molecular targets: an executive workshop summary of the eunice kennedy shriver national institute of child health and human development. Am J Obstet Gynecol. 2016 doi: 10.1016/j.ajog.2016.03.001. http://dx.doi.org/10.1016/j.ajog.2016.03.001. [DOI] [PMC free article] [PubMed]
- 68.Furukawa S, Hayashi S, Usuda K, Abe M, Ogawa I. Histopathological effect of ketoconazole on rat placenta. J Vet Med Sci. 2008;70:1179–1184. doi: 10.1292/jvms.70.1179. [DOI] [PubMed] [Google Scholar]
- 69.Parizek J. Vascular changes at sites of oestrogen biosynthesis produced by parenteral injection of cadmium salts: the destruction of placenta by cadmium salts. J Reprod Fertil. 1964;7:263–265. doi: 10.1530/jrf.0.0070263. [DOI] [PubMed] [Google Scholar]
- 70.Furukawa S, Usuda K, Abe M, Hayashi S, Ogawa I. Effect of 6-mercaptopurine on rat placenta. J Vet Med Sci. 2008;70:551–556. doi: 10.1292/jvms.70.551. [DOI] [PubMed] [Google Scholar]
- 71.Fuentes M, Torregrosa A, Mora R, Götzens V, Corbellla J, Domingo JL. Placental effects of lead in mice. Placenta. 1996;17:371–376. doi: 10.1016/s0143-4004(96)90063-6. [DOI] [PubMed] [Google Scholar]
- 72.Bruce NW, Cabral DA. Effects of maternal blood loss on embryonic and placental development in the rat. J Reprod Fertil. 1975;45:349–365. doi: 10.1530/jrf.0.0450349. [DOI] [PubMed] [Google Scholar]
- 73.Bruce NW. The effect of ligating a uterine artery on fetal and placental development in the rat. Biol Reprod. 1976;14:246–247. doi: 10.1095/biolreprod14.3.246. [DOI] [PubMed] [Google Scholar]
- 74.Wellstead JR, Bruce NW, Rahima A. Effects of indomethacin on spacing of conceptuses within the uterine horn and on fetal and placental growth in the rat. Anat Rec. 1989;225:101–105. doi: 10.1002/ar.1092250204. [DOI] [PubMed] [Google Scholar]
- 75.Akay MT, Kockaya EA. The effects of alcohol on rat placenta. Cell Biochem Funct. 2005;23:435–445. doi: 10.1002/cbf.1175. [DOI] [PubMed] [Google Scholar]
- 76.King BF, Enders AC. The fine structure of the guinea pig visceral yolk sac placenta. Am J Anat. 1970;127:397–413. doi: 10.1002/aja.1001270405. [DOI] [PubMed] [Google Scholar]
- 77.Beckman DA, Brent RL, Lloyd JB. Sources of amino acids for protein synthesis during early organogenesis in the rat: 4. Mechanisms before envelopment of the embryo by the yolk sac. Placenta. 1996;17:635–641. doi: 10.1016/s0143-4004(96)80082-8. [DOI] [PubMed] [Google Scholar]
- 78.McArdle HJ, Priscott PK. Uptake and metabolism of transferrin and albumin by rat yolk sac placenta. Am J Physiol. 1984;247:C409–14. doi: 10.1152/ajpcell.1984.247.5.C409. [DOI] [PubMed] [Google Scholar]
- 79.Padykula HA, Deren JJ, Wilson TH. Development of structure and function in the mammalian yolk sac: i. Developmental morphology and vitamin B12 uptake of the rat yolk sac. Dev Biol. 1966;13:311–348. doi: 10.1016/0012-1606(66)90053-4. [DOI] [PubMed] [Google Scholar]
- 80.Delorme AV, Cassier P, Geny B, Mathieu H. Immunocytochemical localization of vitamin D-dependent calcium-binding protein in the yolk sac of the rat. Placenta. 1983;4:263–270. doi: 10.1016/s0143-4004(83)80005-8. [DOI] [PubMed] [Google Scholar]
- 81.King B, Enders A. Comparative development of the mammalian yolk sac. In: Nogales F, editor. The Human Yolk Sac and Yolk Sac Tumors. Springer-Verlag; Berlin: 1993. pp. 1–32. [Google Scholar]
- 82.Merad Z, Wild AE. The route of maternal igm transport to the rabbit fetus. Placenta. 1992;13:291–304. doi: 10.1016/0143-4004(92)90044-t. [DOI] [PubMed] [Google Scholar]
- 83.Beckman DA, Koszalka TR, Jensen M, Brent RL. Experimental manipulation of the rodent visceral yolk sac. Teratology. 1990;41:395–404. doi: 10.1002/tera.1420410405. [DOI] [PubMed] [Google Scholar]
- 84.Plonne D, Winkler L, Franke H, Dargel R. The visceral yolk sac–an important site of synthesis and secretion of apolipoprotein B containing lipoproteins in the feto-placental unit of the rat. Biochim Biophys Acta. 1992;1127:174–185. doi: 10.1016/0005-2760(92)90275-z. [DOI] [PubMed] [Google Scholar]
- 85.Plonné D, Stacke A, Weber K-U, Endisch U, Dargel R. The pattern of apolipoprotein B100 containing lipoprotein subclasses produced by the isolated visceral rat yolk sac depends on developmental stage and fatty acid availability. Biochim Biophys Acta (BBA) - Lipids Lipid Metab. 1996;1299:54–66. doi: 10.1016/0005-2760(95)00189-1. [DOI] [PubMed] [Google Scholar]
- 86.Woollett LA. Maternal cholesterol in fetal development: transport of cholesterol from the maternal to the fetal circulation. Am J Clin Nutr. 2005;82:1155–1161. doi: 10.1093/ajcn/82.6.1155. [DOI] [PubMed] [Google Scholar]
- 87.Orth M, Bellosta S. Cholesterol: its regulation and role in central nervous system disorders. Cholesterol. 2012;2012:292598. doi: 10.1155/2012/292598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith SC, Baker PN, Symonds EM. Increased placental apoptosis in intrauterine growth restriction. Am J Obstet Gynecol. 1997;177:1395–1401. doi: 10.1016/s0002-9378(97)70081-4. [DOI] [PubMed] [Google Scholar]
- 89.Erel CT, Dane B, Calay Z, Kaleli S, Aydinli K. Apoptosis in the placenta of pregnancies complicated with IUGR. Int J Gynecol Obstet. 2001;73:229–235. doi: 10.1016/s0020-7292(01)00373-3. [DOI] [PubMed] [Google Scholar]
- 90.Hemminki K, Niemi ML, Koskinen K, Vainio H. Spontaneous abortions among women employed in the metal industry in Finland. Int Arch Occup Environ Health. 1980;47:53–60. doi: 10.1007/BF00378328. [DOI] [PubMed] [Google Scholar]
- 91.Quansah R, Jaakkola JJ. Paternal and maternal exposure to welding fumes and metal dusts or fumes and adverse pregnancy outcomes. Int Arch Occup Environ Health. 2009;82:529–537. doi: 10.1007/s00420-008-0349-6. [DOI] [PubMed] [Google Scholar]
- 92.Goodman DR, James RC, Harbison RD. Placental toxicology. Food Chem Toxicol. 1982;20:123–128. doi: 10.1016/s0278-6915(82)80018-5. [DOI] [PubMed] [Google Scholar]
- 93.Magnarelli G, Guiñazú N. Placental Toxicology of Pesticides. Intech Open Access Publisher; 2012. [Google Scholar]
- 94.Niida H, Nakanishi M. DNA damage checkpoints in mammals. Mutagenesis. 2006;21:3–9. doi: 10.1093/mutage/gei063. [DOI] [PubMed] [Google Scholar]
- 95.Bartek J, Lukas J. DNA damage checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol. 2007;19:238–245. doi: 10.1016/j.ceb.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 96.Lavin MF, Gueven N. The complexity of p53 stabilization and activation. Cell Death Differ. 2006;13:941–950. doi: 10.1038/sj.cdd.4401925. [DOI] [PubMed] [Google Scholar]
- 97.Davies J, Glasser SR. Histological and fine structural observations on the placenta of the rat. Acta Anat (Basel) 1968;69:542–608. doi: 10.1159/000143100. [DOI] [PubMed] [Google Scholar]
- 98.Soares MJ, Chapman BM, Rasmussen CA, Dai G, Kamei T, Orwig KE. Differentiation of trophoblast endocrine cells. Placenta. 1996;17:277–289. doi: 10.1016/s0143-4004(96)90051-x. [DOI] [PubMed] [Google Scholar]
- 99.Coan PM, Ferguson-Smith AC, Burton GJ. Ultrastructural changes in the interhaemal membrane and junctional zone of the murine chorioallantoic placenta across gestation. J Anat. 2005;207:783–796. doi: 10.1111/j.1469-7580.2005.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2:538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- 101.Coan PM, Conroy N, Burton GJ, Ferguson-Smith AC. Origin and characteristics of glycogen cells in the developing murine placenta. Dev Dyn. 2006;235:3280–3294. doi: 10.1002/dvdy.20981. [DOI] [PubMed] [Google Scholar]
- 102.Greenwood J, Minhas K, Di Santo J, Makita M, Kiso Y, Croy B. Ultrastructural studies of implantation sites from mice deficient in uterine natural killer cells. Placenta. 2000;21:693–702. doi: 10.1053/plac.2000.0556. [DOI] [PubMed] [Google Scholar]
- 103.Stewart I, Peel S, Webster A. A study of natural cytotoxic (NC) activity in the metrial glands of rats and mice. Scand J Immunol. 1996;44:394–400. doi: 10.1046/j.1365-3083.1996.d01-325.x. [DOI] [PubMed] [Google Scholar]
- 104.Maclaine NJ, Hupp TR. The regulation of p53 by phosphorylation: a model for how distinct signals integrate into the p53 pathway. Aging (Albany, NY) 2009;1:490–502. doi: 10.18632/aging.100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sakaguchi K, Herrera JE, Si Saito, Miki T, Bustin M, Vassilev A, et al. DNA damage activates p53 through a phosphorylation–acetylation cascade. Genes Dev. 1998;12:2831–2841. doi: 10.1101/gad.12.18.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.